Abstract
Atypical trajectory of brain growth in autism spectrum disorders (ASDs) has been recognized as a potential etiology of an atypical course of behavioral development. Numerous neuroimaging studies have focused on childhood to investigate atypical age-related change of brain structure and function, because it is a period of neuron and synapse maturation. Recent studies, however, have shown that the atypical age-related structural change of autistic brain expands beyond childhood and constitutes neural underpinnings for lifelong difficulty to behavioral adaptation. Thus, we examined effects of aging on neurochemical aspects of brain maturation using 3-T proton magnetic resonance spectroscopy (1H-MRS) with single voxel in the medial prefrontal cortex (PFC) in 24 adult men with non-medicated high-functioning ASDs and 25 age-, IQ- and parental-socioeconomic-background-matched men with typical development (TD). Multivariate analyses of covariance demonstrated significantly high N-acetylaspartate (NAA) level in the ASD subjects compared with the TD subjects (F=4.83, P=0.033). The low NAA level showed a significant positive correlation with advanced age in the TD group (r=−0.618, P=0.001), but was not evident among the ASD individuals (r=0.258, P=0.223). Fisher's r-to-z transformation showed a significant difference in the correlations between the ASD and TD groups (Z=−3.23, P=0.001), which indicated that the age–NAA relationship was significantly specific to people with TD. The current 1H-MRS study provided new evidence that atypical age-related change of neurochemical aspects of brain maturation in ASD individuals expands beyond childhood and persists during adulthood.
Keywords: anterior cingulate, Asperger syndrome, autistic disorder, case–control, human, pervasive developmental disorder
Introduction
Atypical growth trajectory has been recognized in individuals with autism spectrum disorders (ASDs), both at the behavioral and neural levels. Previous meta-analyses have repeatedly reported that overall brain size is slightly reduced at birth, dramatically increases within the first year of life, but then gradually plateaus into adulthood.1, 2 It has been suggested that there is a dynamic curve of atypical trajectory during the first year of life, and the latest longitudinal studies have shown different developmental curves in brain structure between infants with ASDs and typical development (TD) during this period.3, 4 The aberrant brain growth has been shown to occur in many brain areas, but particularly in the frontal lobe.1, 5, 6 Several studies employing neuroimaging have shown that among many other functions,7, 8 the PFC is critically important in mentalizing,9 empathy,10 irony comprehension,11 social judgment12 and self-referencing,13, 14 which are impaired and constitute a core feature of ASDs.
Although the period of dynamic change in infantile autistic brain has been investigated so far, cumulative data from structural and functional neuroimaging studies have demonstrated that those atypical age-related changes expand beyond childhood. Emerging studies that recruited adolescents with ASDs showed different aging effects on brain structure between subjects with ASDs and TD.15 Furthermore, several studies have demonstrated atypical age-related changes in brain structure and function, especially in the frontal lobe16, 17, 18 and tract involving the frontal lobe,19 even during adulthood in people with ASDs. Although these atypical age-related changes at the neural level may constitute substrates for lifelong behavioral difficulty in people with ASDs,20 such changes during adulthood have rarely been reported on brain neurochemical aspects.
1H-magnetic resonance spectroscopy (1H-MRS) is a non-invasive neuroimaging technique that estimates specific chemical metabolite measures in vivo.21 Although what the measured metabolite levels reflect is an area of debate, previous studies have used 1H-MRS to quantify glutamine/glutamate (referred to collectively as ‘Glx'); N-acetylaspartate (NAA), a marker of neuronal density, plasticity and function; choline-containing compounds (Cho), a measure primarily reflecting the constituents of cell membranes; creatine and phosphocreatine (Cre), a measure of cellular energy metabolism; and myo-inositol (mI), a major osmolite precursor for phosphoinositides involved in the second messenger system.22, 23
Consistent with atypical age-related brain morphological change in people with ASDs, our recent meta-analysis of 1H-MRS studies involving ASD individuals also robustly demonstrated age-related diminishment of NAA reduction in the frontal lobe.24 The meta-analysis showed a significant frontal NAA reduction in groups with ASDs compared with TD during childhood, and further demonstrated a significant linear correlation between older age and a smaller magnitude of the frontal NAA decrease. Thus, the meta-analysis showed no significant difference in NAA level between ASD and TD subjects during adulthood. However, because of a lack of sufficient number of studies recruiting adults with ASDs in the meta-analysis, it remains unclear whether this atypical relationship between NAA and age continues during adulthood. In contrast, in people with TD, age-dependent NAA reduction in the frontal lobe during adulthood has been revealed by a number of cross-sectional 1H-MRS studies25, 26, 27, 28, 29 and a meta-analysis of 1H-MRS studies.30
Taken together, it was expected that adults with ASD would show atypical age–NAA relationship (that is, lack of age-related decrease in frontal NAA) in the PFC. As a result, an absence of decrease, or even increase, in prefrontal NAA level would be predicted in adults with ASD compared with the matched TD controls, while previous studies have demonstrated a significant decrease in the prefrontal NAA of children with ASD.24 To test these hypotheses with minimizing potential confounders, the present study utilized 3-T 1H-MRS to examine differences in the medial prefrontal NAA levels between non-medicated high-functioning adult males with ASD and age-, IQ- and parental-socioeconomic (SES)-background-matched TD male subjects. Then, we examined correlations between the medial prefrontal NAA levels and age in the diagnostic groups separately. The statistical significance of diagnostic difference in the correlation coefficients was further examined.
Materials and methods
Participants
The inclusion and exclusion criteria, diagnostic protocols and clinical assessments of study participants were the same as our previous study.12 In all, 24 adult males (mean age=29.5, range=20–44 years) with a clinical diagnosis of high-functioning ASDs were recruited from the outpatient services of The University of Tokyo Hospital. The ASD participants met the following criteria to be included: no psychotropic medication and IQ >80. The ASD participants were diagnosed according to the strict criteria included in the Diagnostic and Statistical Manual-Revision IV, requiring consensus based on more than 2 months of longitudinal follow-up examinations by at least two trained child–adolescent psychiatrists with more than 10 years of clinical experience (HY and NK). The diagnoses were further confirmed using the validated Japanese version of Autism Diagnostic Interview-Revised (ADI-R) by another trained child adolescent psychiatrist (HK).31, 32 Of the participants not reaching the threshold in the ADI-R social domain, the Childhood Autism Rating Scale33 was employed to confirm the diagnosis of ASDs. Previous studies have suggested that individuals classified as autistic according to both ADI-R and Childhood Autism Rating Scale had significantly lower IQ than those classified with the Childhood Autism Rating Scale only.34 It is reasonable that the current high-functioning ASD participants who did not meet the ASD criteria based on ADI-R, which was rated based on descriptive information by caregivers, could be classified as ASDs based on the Childhood Autism Rating Scale, which was rated by clinicians based on direct observations on behavior.35 All of the ASD participants were interviewed by a trained psychiatrist (HY) to screen for the presence or absence of comorbid neuropsychiatric disorders using the Structured Clinical Interview for Diagnostic and Statistical Manual-Revision IV axis I disorder. In all, 25 age-, IQ- and parental-SES-matched, TD adult males were employed as controls. The ethics committee of The University of Tokyo Hospital approved this study (No. 397). After a complete explanation of the study to the subjects, written informed consent was obtained from every participant.
The exclusion criteria for both groups were: current or past neurological comorbidity, traumatic brain injury with any known cognitive consequences or loss of consciousness for more than 5 min, a history of electroconvulsive therapy and substance abuse or addiction. An additional exclusion criterion for the control group was a history of psychiatric disease in the subjects themselves or a family history of axis I disorder in their first-degree relatives.
To detect the diagnostic difference in age–NAA relationship, power was based on previous MRS studies comparing correlation between NAA level in volume of interest (VOI) at the similar location and its functional or behavioral substrates of adults with ASDs with those with TD.36, 37 Since the effect size q for difference between Pearson's correlation coefficient of these previous studies ranged from 0.75 to 1.26, the required total sample sizes for 80% power at a 0.05 level of significance ranged from 26 to 62. Thus, in the current study, we collected data from more than 44 individuals, which is the median of the calculated range.
Questionnaire measures
Handedness was determined using the Edinburgh Handedness Inventory,38 with a laterality index of >0.5 used as the cutoff for right-handedness. Participants whose laterality index score ranged from −0.5 to 0.5 were defined as mixed-handedness. All of the ASD and TD participants completed valid Japanese translations39 of the 50-item Autism-Spectrum Quotient.40 The maximum total score of Autism-Spectrum Quotient was 24 in the controls, while the cutoff threshold was defined as 34 points.40 The IQ of the TD controls was estimated using the Japanese version of the National Adult Reading Test.41 Although the National Adult Reading Test can represent the full IQ in TD participants, it is problematic for ASD participants because of their well-known imbalanced intellectual abilities. Therefore, IQ was evaluated using the full scale of the Wechsler Adult Intelligence Scale Revised Japanese version42 for participants with ASDs. The SES of participants and their parents were assessed using the Hollingshead Scale.43
MRI acquisition
Magnetic resonance imaging (MRI) data were obtained using a 3-T scanner (GE Signa HDxt, Waukesha, WI, USA). All the participants from both the diagnostic groups were scanned during the same period, between January 2010 and November 2011. There was no upgrade of MRI scanner or software version in this period. An 8-channel brain-phased array coil was used for both MRI and 1H-MRS. A sagittal localizer scan was obtained first, followed by the axial T2-weighted images (echo time (TE)=82.32 ms, repetition time (TR)=4400 ms, field of view=240 × 240 mm2, matrix=256 × 256, slice thickness=2.5 mm, number of axial slices=62) for positioning the voxel of interest (VOI). Three-dimensional fast-spoiled gradient recalled acquisition with steady state (TE=1.94 ms, TR=6.80 ms, field of view =240 × 240 mm2, matrix=256 × 256, flip angle=20°, slice thickness=1.0 mm, number of axial slices=176) was acquired for tissue segmentation correction. Trained neuroradiologists (OA, WG, HS, MM, MK, HT or YN) evaluated the MRI scans and found no gross abnormalities in any of the subjects.
1H-MRS acquisition
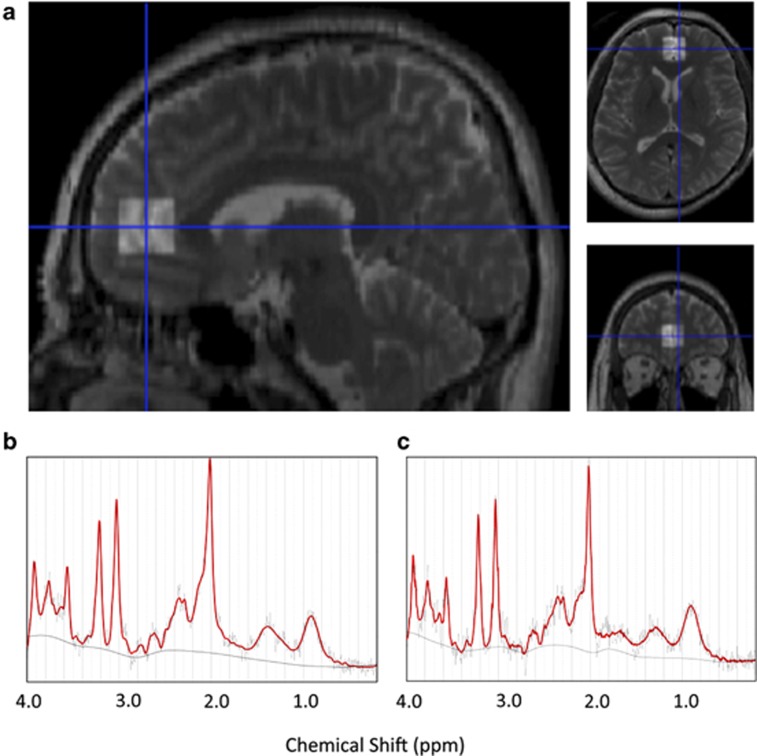
The stimulated echo acquisition mode (TR=3000 ms, TE=15 ms, mixing time=13.7 ms, 128 water-suppressed and 8 water-unsuppressed averages) was applied to obtain proton MR spectrum. The definition of positioning of the VOI was based on our previous study.44 Briefly, in the mid-sagittal slice based on the T2-weighted image, the VOI (20 × 20 × 20 mm3) was placed closest to the most anterior part of the genu of the corpus callosum with the center of the VOI, containing predominantly the gray matter of the medial PFC (mainly anterior cingulate and paracingulate gyri) bilaterally (Figure 1a).
Figure 1.
Location of volume of interest (VOI) and representative spectra of 3-T proton magnetic resonance spectroscopy (1H-MRS). (a) A T2-weighted brain image in orthogonal slices in a control subject. The square indicates the VOI (20 × 20 × 20 mm3) voxel in the medial prefrontal cortex, including mainly the pregenual anterior cingulate and paracingulate gyri. (b and c) Representative medial prefrontal 1H-MRS spectra of autism spectrum disorder (ASD) subject (b) or typical development (TD) subject (c) as fit by the LCModel.
Spectrum quantification
All spectra were quantified with an LCModel (ver. 6.1-4F). The raw spectral data were read into an LCMgui by which spectrum processing were performed automatically. Based on the comparison of in vitro spectra with its measurements analyzed with the LCModel basis set, the absolute levels for 17 metabolites, such as NAA, N-acetylaspartylglutamate, alanine, γ-aminobutyric acid, aspartate, choline, creatine (total), glutamate, glutamine, glutathione, glycerophosphocholine, glycine, myo-inositol, scyllo-inositol, lactate, phosphocholine and taurine, were estimated from in vivo spectra. Among the 17 metabolites, the current study focused on NAA, Cre, Glx (glutamine plus glutamine), mI and Cho (glycerophosphocholine plus phosphocholine). Representative spectra of ASD and TD are shown in Figures 1b and c.
Spectrum quality
All of the metabolite spectra that showed %s.d.>20% were excluded from the analysis. In addition, full-width at half-maximum <0.16 p.p.m. and signal-to-noise ratio (S/N) >3 were used as determinants of spectrum quality required for inclusion. For all the participants, all five metabolites satisfied the criteria for spectrum quality.
Tissue segmentation
Three-dimensional fast-spoiled gradient recalled acquisition with steady state images were used to calculate the volumes of different tissue types (gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF)) by the new segmentation tool of SPM8 (www.fil.ion.ucl.ac.uk/spm). By the tag information of the header of each spectrum file, the center point of VOI was identified. Then, the VOI was reconstructed in the fast-spoiled gradient recalled acquisition with steady state images by tracing the center point. Using SPM8, we co-registered T2-weighted and three-dimensional fast-spoiled gradient recalled acquisition with steady state images, and calculated the volume of GM, WM and CSF. Subsequently, to obtain tissue-contamination-corrected metabolite intensities, each metabolite value was corrected for the CSF content of the VOI using the formula: corrected level=uncorrected level/(1−C), where C was the fractional CSF content of the VOI.45
Statistical method
All statistical analyses were conducted using SPSS 18.0 (SPSS, Chicago, IL, USA). Demographic variables, including age, height, body weight, self-SES, parental-SES, handedness, total Autism-Spectrum Quotient score and IQ, volumes of each tissue component within the VOI (GM, WM and CSF volumes) and indices representing MRS quality (that is, full-width at half-maximum, %s.d. and S/N ratio), were compared using independent two-tailed t-tests between ASD and TD subjects. To assess strictly the effect of potential confounders or MRS quality control, significance level was set at P<0.05 without correction for multiple comparisons.
For the group comparison of metabolite levels, we employed multivariate analyses of covariance, treated corrected level of each metabolite as a dependent variable (NAA, Cre, Glx, mI and Cho) and diagnosis as a main factor. As the CSF components had already been accounted for by calculating the corrected level, GM and WM components were additionally treated as covariates in the multivariate analyses of covariances to account for the significant difference in the GM within VOI and WM tissue water content between ASD and TD subjects.46, 47 The level of statistical significance was defined as P<0.05. As we have a priori hypothesis that NAA level in ASDs is deviated from that in TD in adulthood, we did not apply correction for multiple comparisons.
Associations between NAA level and age were analyzed with Pearson's correlation analysis in ASD and TD groups, respectively, and differences in correlations between groups were examined with the Fisher r-to-z transformation. The significance was reported when P-value was <0.05.
The associations between clinical scores, such as ADI-R scores and NAA levels, which showed significant effect of diagnosis, were explored for significance using Pearson's correlation coefficient in the ASD subjects. The significance level was set at P<0.05. In addition, Pearson's correlation coefficient between the demographical information (height, body weight, self-SES, parental-SES and IQ) and the NAA levels was also calculated in each group separately. We considered P<0.05 as denoting statistical significance to detect effects of potential confounders.
Results
Group difference in demographic characteristics
There were no significant differences between the ASD and control groups in age, body weight, parental-SES and IQ, although the ASD group had significantly lower self-SES and shorter height than controls. The ASD subjects had significantly higher GM (P=0.006) and lower CSF (P=0.003) contaminations within the VOI (Table 1).
Table 1. Demographic characteristics of the participants.
| Subjects with ASD (N=24) | TD controls (N=25) | T-test | ||||
|---|---|---|---|---|---|---|
|
Variables |
Mean |
s.d. |
Mean |
s.d. |
t-Value |
P-value |
| Age (range) (years) | 29.5 (20–44) | 6.9 | 29.4 (20–41) | 6.2 | −0.10 | 0.923 |
| Height (cm) | 170.3 | 5.0 | 174.0 | 6.1 | 2.29 | 0.027 |
| Body weight (kg) | 67.6 | 13.2 | 67.6 | 10.5 | 0.00 | 0.999 |
| SESa | 2.8 | 1.1 | 1.6 | 0.5 | −5.13 | 0.000 |
| Parental-SESa | 2.3 | 0.7 | 2.2 | 0.4 | −1.08 | 0.284 |
| Handedness: right/mixed/left | 19/3/2 | 25/0/0 | χ2 | 0.032 | ||
| IQ | ||||||
| FIQ | 104.2 | 11.6 | 108.5 | 7.5 | 1.53 | 0.134 |
| VIQ | 111.3 | 14.0 | ||||
| PIQ | 91.3 | 14.6 | ||||
| HFA**/Asperger/PDD-NOS | 24/1/0 | |||||
| Autism Diagnostic Interview-Revised | ||||||
| Social | 15.0 | 5.9 | ||||
| Communication | 12.4 | 3.3 | ||||
| Repetitive | 4.7 | 2.3 | ||||
| Autism spectrum quotient | 39.0 | 5.2 | 15.2 | 5.0 | −15.76 | 0.000 |
| Gray matter volume within VOI | 5.2 | 0.3 | 4.9 | 0.4 | −2.87 | 0.006 |
| White matter volume within VOI | 0.6 | 0.3 | 0.6 | 0.3 | −0.25 | 0.801 |
| Cerebrospinal fluid within VOI | 2.2 | 0.3 | 2.5 | 0.3 | 3.14 | 0.003 |
Abbreviations: ASD, autism spectrum disorder; FIQ, full IQ; HFA, high-functioning autism; IQ, intelligence quotient; PDD-NOS, pervasive developmental disorder not otherwise specified; PIQ, performance IQ; SES, socioeconomic status; TD, typical development; VIQ, verbal IQ; VOI, volume of interest.
Socioeconomic status, assessed using the Hollingshead index. Higher scores indicate lower status.
Diagnostic differences in spectral quality and metabolite level
The quality of the spectra obtained from 1H-MRS was good, with a mean (s.d.) S/N ratio reported by the LCModel at 9.96 (2.85) and 11.12 (2.67) in the ASD and TD groups, respectively. Full-width at half-maximums recorded by the LCModel in subjects with ASDs and TD were 0.075 (0.003) and 0.064 (0.019), respectively. %s.d.'s reported by the LCModel in individuals with ASDs and TD were 4.04 (0.93) and 4.67 (1.20) in Cre, 5.84 (1.65) and 6.46 (1.61) in mI, 5.08 (2.23) and 5.08 (1.79) in NAA, 4.08 (1.22) and 4.04 (0.75) in Cho and 7.40 (2.04) and 7.46 (1.56) in Glx, respectively. Independent t-tests demonstrated that there were no significant differences in spectral quality between the ASD and TD groups in S/N ratio (P=0.147) and full-width-at-half-maximum (P=0.107). With regard to %s.d.'s, independent t-test demonstrated no significant difference in mI (P=0.192), NAA (P=0.995), Cho (P=0.896) and Glx (P=0.911). Conversely, independent t-test showed a significant difference in %s.d. of Cre between ASD individuals and TD subjects (P=0.047).
The multivariate analyses of covariances controlling the effect of structural differences between the ASD and TD subjects showed that the medial prefrontal NAA level was significantly higher in the ASD individuals than in the TD subjects (F=4.832, P=0.033). Moreover, the statistical conclusions did not change when the mixed- and/or left-handed subjects were excluded from the analysis. No significant difference was found in the other metabolite measures (Table 2).
Table 2. Metabolite concentrations of participants.
| MANCOVAs | ||||
|---|---|---|---|---|
|
Metabolites |
d.f. |
Effect size (f) |
F-value |
P-value |
| N-acetylaspartate | 47 | 1.26 | 4.83 | 0.033 |
| Creatine | 47 | 0.71 | 0.33 | 0.570 |
| Choline-containing compounds | 47 | 0.73 | 0.39 | 0.534 |
| Glutamine+glutamate | 47 | 1.00 | 1.82 | 0.184 |
| Myo-inositol | 47 | 0.73 | 0.39 | 0.534 |
Abbreviations: MANCOVA, multivariate analyses of covariance;d.f., degrees of freedom.
*Statistically significant after Bonferroni correction.
Diagnostic differences in the relationships between age and NAA levels
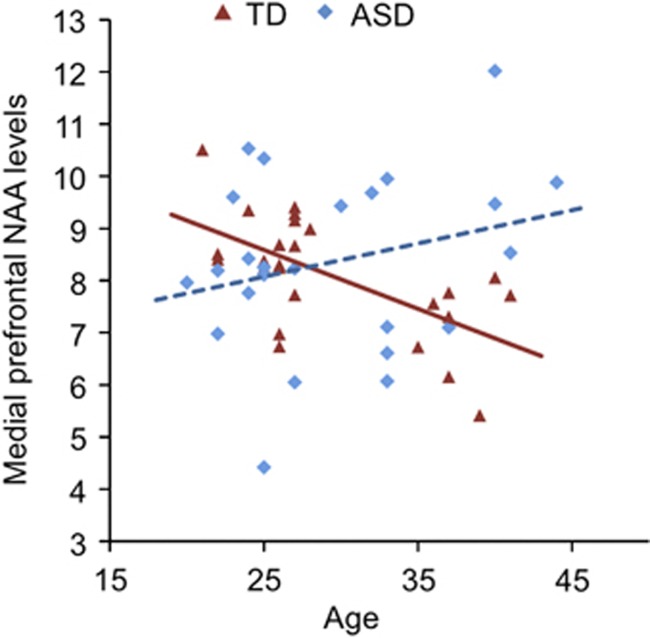
A significant negative correlation between age and NAA levels was demonstrated in the TD group (r=−0.618, P=0.001), whereas no significant correlation between these indices was shown in the ASD individuals (r=0.258, P=0.223). These correlations were significantly different between the ASD and TD groups (Z=−3.23, P=0.001), indicating that the typical relationship between NAA level and age was absent in the ASD individuals (Figure 2). Furthermore, these statistical conclusions were totally preserved when the mixed- and/or left-handed subjects were excluded from the analysis.
Figure 2.
Relationships between age and N-acetylaspartate (NAA) level in the autism spectrum disorder (ASD) and typical development (TD) subjects. Scatter plots depicting correlations between frontal NAA levels and age of participants in individuals with ASDs and TD. The diagnostic difference of these correlations was statistically significant (Fisher's r-to-z transformation; Z=−3.23, P=0.001).
Correlations with clinical indices
No significant correlation between the NAA and the ADI-R scores was found in the ASD group. There was also no significant correlation between the NAA level and height, body weight, SES, parental-SES and IQ.
Discussion
The present study, utilizing 3-T 1H-MRS, demonstrated significantly high medial prefrontal NAA in the non-medicated high-functioning adult males with ASDs compared with the demographically matched TD male subjects. Of note, while a significant negative correlation between age and medial prefrontal NAA levels was found in the TD group, such a correlation was absent in the ASD group. The difference in age–NAA relationships between the ASD and TD groups was reached at the statistically significant level.
The currently found atypical age-related NAA change in adult ASD subjects is in line with previous structural and functional imaging and post-mortem studies, which have reported different age-related trajectory in brain maturation between ASDs and TD during adulthood.16, 17, 19, 48, 49, 50 In particular, Murphy and his co-researchers, by their well-designed sequential studies, have repeatedly demonstrated an atypical relationship between age and brain structure, such as whole-brain GM volume,49 cortical volume and cortical thickness (CT),17 hippocampus volume50 and streamline measured by diffusion tensor imaging,19 in adolescence and adults with ASDs compared with TD. Regarding functional aspects of brain aging, a functional MRI study demonstrated that brain activity during face processing task augments with age in adults with ASDs, but it decreases with age in TDs, and that the age-related increase in activity was correlated with changes in gaze behavior and improvements in social functioning.18 One post-mortem study demonstrated atypical age-related change of microglial density both in GM and WM regions of brains in subjects with ASDs compared with TD.48 Thus, there are accumulated evidences that have demonstrated atypical brain structural and functional age-related changes in individuals with ASDs during adulthood, which might underlie well-established age-related autistic symptom changes beyond childhood.51, 52, 53, 54, 55 The current study further added new evidence that showed absence of age-related frontal NAA decline in people with ASDs during adulthood.
Although what NAA reflects is an area of debate, one possible explanation for the current findings is based on the notion that NAA, at least partially, reflects structural aspects such as neuron density or volume.56 As hippocampal NAA reduction was reported to be correlated with volume reduction in adults with TD,57 the current findings that NAA was negatively correlated with age in TDs, but not in ASDs, are partially concordant with a previous study that reported significant age-related frontal cortical volume and cortical thickness reduction in TDs, and less such relationship in the ASD group.17 However, another well-designed study reported that CT in the temporal and parietal lobes, but not in the frontal lobes, was negatively correlated with age in the ASD group but not in the TD group.15 Thus, the presence or absence of age–CT relationship in diagnostic groups and regions of brain are controversial among the various studies.15, 17 One potential explanation for the discrepancy is the influence of differences in ranges of ages between the studies (that is, ages from 12 to 24 years; Wallace et al.15 vs 10 to 60 years; Raznahan et al.17). Although there are compelling pieces of evidence that frontal and temporal lobes have a key role in abnormal brain growth trajectory in ASD,16, 58 abnormal enlargement and subsequent normalization of frontal and temporal lobes in ASDs were suggested to not occur simultaneously.16, 24, 58 Thus, the two previous studies may yield inconsistent results because of the different age range of participants as well as different brain areas examined.
Based on the notion that NAA also reflects functional aspects of neuronal tissues, one potential interpretation for the relationship between age and NAA is the age-related change of mitochondrial function. As NAA is synthesized from aspartate and acetyl-coenzyme A in mitochondria of neurons, and inhibitors of the mitochondrial respiratory chain decrease NAA levels, NAA has also been recognized as a marker of mitochondrial activity.59, 60 A recent meta-analysis demonstrated that biomarkers of mitochondrial dysfunction, such as lactate, pyruvate, carnitine and ubiquinone, were significantly deviated in individuals with ASDs from those with TD, and that the values of some of these markers correlated with autistic symptom severity.61 In addition, prevalence of mitochondrial disease was shown to be much higher in ASD (approximately 5%) than in TDs (<0.01%).61 Post-mortem studies have shown that mitochondrial respiratory chain activity was increased in brains of ASDs compared with TD, which may result in an increase of NAA).62, 63 Overall, this evidence supports the idea that mitochondrial dysfunction is associated with ASDs (reviewed in Rossignol and Frye).61 The concept of aging in mitochondrial function in TD individuals is well established by previous studies.64 Taken together, age-related NAA reduction in TD subjects can reflect age-related decline of mitochondrial function, and ASD individuals may show atypical age-related change in frontal NAA levels due to dysfunctional mitochondria.
The current results seem to be at least partially different from one previous 1H-MRS study, which revealed age-related NAA reduction in the amygdala–hippocampus complex in children and adults with ASDs but not in TD.37 The discrepancy in the findings between the previous and current studies can be explained by considering the difference in age range of the study participants. The previous study examined the relationship between age and NAA levels in the combined group consisting of child and adult subgroups.37 Age-related NAA decrease in TD subjects was not found in their study participants including children and adults. Thus, the previous study successfully examined age–NAA relationship during a period from childhood to adulthood, but they did not address the age-related change of NAA during adulthood.
The current study also identified significantly high frontal NAA levels of ASD individuals compared with the TD controls, which is concordant with results from the previous 1H-MRS studies that located VOIs in the similar location in adults with ASDs.65, 66 Increased NAA level can result from not only increased synthesis but also decreased metabolism of NAA.67 As NAA is synthesized in the mitochondria of neurons, neurons have been recognized to contribute significantly to NAA levels. ‘NAA trapping theory' also suggests that NAA may increase even with dysfunction of metabolism of astrocytes or oligodendrocytes,67 as recent studies have revealed that both astrocytes and oligodendrocytes are also involved in metabolism of NAA.68 For example, patients with Canavan's disease, which is caused by mutations in the gene that codes for the enzyme aspartoacylase, which is necessary for NAA metabolism, demonstrates increased NAA without increased neuron density.69 In addition, one experimental study showed that intravenous ethanol infusion, which enhances the activity of acetylaspartylase, a glial enzyme that degrades NAA, dynamically decreases cortical NAA measured by 1H-MRS.70 Thus, both increased NAA synthesis (for example, increased mitochondrial function in neuron) and decreased NAA metabolism (for example, depressed function of glia cell) can increase NAA in adults with ASD.
Several limitations and methodological considerations could be noted in the current study. First, though the current study was able to address the relationship between age and NAA measure across age span of 24 years (from 20 to 44 years), which is too wide to conduct a longitudinal study, the current cross-sectional study design could not directly address the individual aging effect. Second, compared with previous cross-sectional studies (for example, from 10 to 60 years; Raznahan et al.17 and O'Brien et al.37), the age range of the current study participants was relatively narrow; thus, the current findings might not be generalized beyond young adulthood. As the pathophysiology of ASDs can be varied among different life stages, confinement to young adulthood in the current study participants increased homogeneity of the neural mechanisms underlying ASDs. However, future studies should examine different stages during adulthood. Third, although the single VOI model yields high S/N,71 the current study with no control VOI could not conclude whether this atypical age-related change in NAA is specific to medial PFC or was general to other brain regions. Fourth, although recruiting only male subjects in the current study decreased the heterogeneity of study participants and increased the reliability of results, it is unclear whether typical age–NAA relationship in TD subjects or absent of such relationship in ASD people can be generalized to female subjects.
In conclusion, the present findings demonstrated an absence of typical age-related medial prefrontal NAA decrement in ASD individuals during adulthood. Such an atypical relationship between age and NAA levels might contribute to a significant NAA increase in the ASD subjects compared with the TD adults. Although future studies should examine potential localization of atypical age-related NAA change and longitudinal course of autistic NAA abnormality, the current study has provided a new suggestion with regard to a role of atypical age-related NAA changes in the pathophysiology of ASD.
Acknowledgments
This study was supported in part by CREST (Japan Science and Technology Agency), and was also supported by KAKENHI (22689034 to HY; 20591378 to NY), the ‘Development of biomarker candidates for social behavior' project carried out under the Strategic Research Program for Brain Sciences by the MEXT and the Global Center of Excellence (COE) Program ‘Comprehensive Center of Education and Research for Chemical Biology of the Diseases' (NY).
The authors declare no conflict of interest.
References
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci USA. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Aoki A, Suwa H. Reduction of N-acetylaspartate in the medial prefrontal cortex correlated with symptom severity in obsessive-compulsive disorder: meta-analyses of 1H-MRS studies. Transl Psychiatry. 2012;2:e153. doi: 10.1038/tp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Yahata N, Abe O, Kuwabara H, Inoue H, Takano Y, et al. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS One. 2012;7:e39561. doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Sadek SA, Pasco G, Wheelwright SJ, et al. Atypical neural self-representation in autism. Brain. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–3754. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011;1380:138–145. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Nanetti L, van der Gaag C, Ketelaars C, Minderaa R, et al. Age-related increase in inferior frontal gyrus activity and social functioning in autism spectrum disorder. Biol Psychiatry. 2011;69:832–838. doi: 10.1016/j.biopsych.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Pugliese L, Catani M, Ameis S, Dell'Acqua F, Thiebaut de Schotten M, Murphy C, et al. The anatomy of extended limbic pathways in Asperger syndrome: a preliminary diffusion tensor imaging tractography study. NeuroImage. 2009;47:427–434. doi: 10.1016/j.neuroimage.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam KSL, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. J Autism Dev Disord. 2009;39:57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Inubushi T, Kato N. Magnetic resonance spectroscopy in affective disorders. J Neuropsychiatry Clin Neurosci. 1998;10:133–147. doi: 10.1176/jnp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Friedman S, Shaw D, Artru A, Richards T. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, et al. In vivo1H-magnetic resonance spectroscopy study of amygdala–hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry. 2012;2:e69–12. doi: 10.1038/tp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WE, Gass A, Glodzik L, Babb JS, Hirsch J, Sollberger M, et al. Whole brain N-acetylaspartate concentration is conserved throughout normal aging. Neurobiol Aging. 2012;33:2440–2447. doi: 10.1016/j.neurobiolaging.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Torres A, Pujol J, Soriano-Mas C, Deus J, Iranzo A, Santamaria J. Age-related metabolic changes in the upper brainstem tegmentum by MR spectroscopy. Neurobiol Aging. 2005;26:1051–1059. doi: 10.1016/j.neurobiolaging.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Gruber S, Pinker K, Riederer F, Chmelík M, Stadlbauer A, Bittsanský M, et al. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur J Radiol. 2008;68:320–327. doi: 10.1016/j.ejrad.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Roberts N, Kemp GJ, Gosney MA, Lye M, Whitehouse GH. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11:598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- Raininko R, Mattsson P. Metabolite concentrations in supraventricular white matter from teenage to early old age: a short echo time 1H magnetic resonance spectroscopy (MRS) study. Acta Radiol. 2010;51:309–315. doi: 10.3109/02841850903476564. [DOI] [PubMed] [Google Scholar]
- Haga KK, Khor YP, Farrall A, Wardlaw JM. A systematic review of brain metabolite changes, measured with 1H magnetic resonance spectroscopy, in healthy aging. Neurobiol Aging. 2009;30:353–363. doi: 10.1016/j.neurobiolaging.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KJ, Matsumoto K, Yagi A, Inada N, Kuroda M, Inokuchi E, et al. Reliability and validity of the autism diagnostic interview-revised—Japanese version J Autism Dev DisordPMID: 22806002 (in press). [DOI] [PubMed]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood autism rating scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Saemundsen E, Magnússon P, Smári J, Sigurdardóttir S. Autism Diagnostic Interview-Revised and the Childhood Autism Rating Scale: convergence and discrepancy in diagnosing autism. J Autism Dev Disord. 2003;33:319–328. doi: 10.1023/a:1024410702242. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shulman C, Dover R. The autism diagnostic interview-revised and the childhood autism rating scale: differences between diagnostic systems and comparison between genders. J Autism Dev Disord. 1998;28:143–151. doi: 10.1023/a:1026092632466. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Schweinsburg BC, Cohen DN, Müller R-A, Courchesne E. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien FM, Page L, O'Gorman RL, Bolton P, Sharma A, Baird G, et al. Maturation of limbic regions in Asperger syndrome: a preliminary study using proton magnetic resonance spectroscopy and structural magnetic resonance imaging. Psychiatry Res. 2010;184:77–85. doi: 10.1016/j.pscychresns.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi A, Baron-Cohen S, Wheelwright S, Tojo Y. The autism-spectrum quotient (AQ) in Japan: a cross-cultural comparison. J Autism Dev Disord. 2006;36:263–270. doi: 10.1007/s10803-005-0061-2. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders-Clinician Version (SCID-CV) American Psychiatric Press: Washington, DC; 1997. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation: New York; 1981. [Google Scholar]
- Hollingshead AB. Two-Factor Index of Social Position. 1957.
- Yamasue H, Fukui T, Fukuda R, Yamada H, Yamasaki S, Kuroki N, et al. 1H-MR spectroscopy and gray matter volume of the anterior cingulate cortex in schizophrenia. NeuroReport. 2002;13:2133–2137. doi: 10.1097/00001756-200211150-00029. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- Hendry J, DeVito T, Gelman N, Densmore M, Rajakumar N, Pavlosky W, et al. White matter abnormalities in autism detected through transverse relaxation time imaging. NeuroImage. 2006;29:1049–1057. doi: 10.1016/j.neuroimage.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Friedman SD, Shaw DWW, Artru AA, Dawson G, Dager SR. Gray matter abnormalities in autism spectrum disorder revealed by T2 relaxation. Neurology. 2006;67:632–636. doi: 10.1212/01.wnl.0000229923.08213.1e. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Daly E, Kumari V, Critchley HD, Van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger's syndrome. Brain. 2002;125:1594–1606. doi: 10.1093/brain/awf150. [DOI] [PubMed] [Google Scholar]
- Murphy CM, Deeley Q, Daly EM, Ecker C, O'Brien FM, Hallahan B, et al. Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: an in vivo magnetic resonance imaging study of Asperger syndrome. Autism Res. 2012;5:3–12. doi: 10.1002/aur.227. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J Am Acad Child Adolesc Psychiatry. 1996;35:523–529. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. J Autism Dev Disord. 2003;33:565–581. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord. 2006;37:1735–1747. doi: 10.1007/s10803-006-0307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MA, McMahon WM, Fombonne E, Jenson WR, Miller J, Gardner M, et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Res. 2009;2:109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt SN. An MRI study of the corpus callosum in autism. Am J Psychiatry. 1997;154:1051–1056. doi: 10.1176/ajp.154.8.1051. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Lowry M, Horsman A. Unchanged basal ganglia N-acetylaspartate and glutamate in idiopathic Parkinson's disease measured by proton magnetic resonance spectroscopy. Mov Disord. 1997;12:297–301. doi: 10.1002/mds.870120306. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend DL, Knowlton R, Norman D, Fein G, Weiner MW. Age-related metabolite changes and volume loss in the hippocampus by magnetic resonance spectroscopy and imaging. Neurobiol Aging. 1999;20:279–285. doi: 10.1016/s0197-4580(99)00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. NeuroReport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2011;17:290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, Sacco R, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Napolioni V, Persico AM, Porcelli V, Palmieri L. The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: physiological links and abnormalities in autism. Mol Neurobiol. 2011;44:83–92. doi: 10.1007/s12035-011-8192-2. [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DGM, Critchley HD, Schmitz N, McAlonan G, Van Amelsvoort T, Robertson D, et al. Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59:885–891. doi: 10.1001/archpsyc.59.10.885. [DOI] [PubMed] [Google Scholar]
- Oner O, Devrimci-Ozguven H, Oktem F, Yagmurlu B, Baskak B, Munir KM. Proton MR spectroscopy: higher right anterior cingulate N-acetylaspartate/choline ratio in Asperger syndrome compared with healthy controls. Am J Neuroradiol. 2007;28:1494–1498. doi: 10.3174/ajnr.A0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baslow MH. Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain's ‘operating system': how NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids. 2010;39:1139–1145. doi: 10.1007/s00726-010-0656-6. [DOI] [PubMed] [Google Scholar]
- Janson CG, McPhee SWJ, Francis J, Shera D, Assadi M, Freese A, et al. Natural history of Canavan disease revealed by proton magnetic resonance spectroscopy (1H-MRS) and diffusion-weighted MRI. Neuropediatrics. 2006;37:209–221. doi: 10.1055/s-2006-924734. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qayyum A. MR spectroscopy of the liver: principles and clinical applications. Radiographics. 2009;29:1653–1664. doi: 10.1148/rg.296095520. [DOI] [PMC free article] [PubMed] [Google Scholar]