Abstract
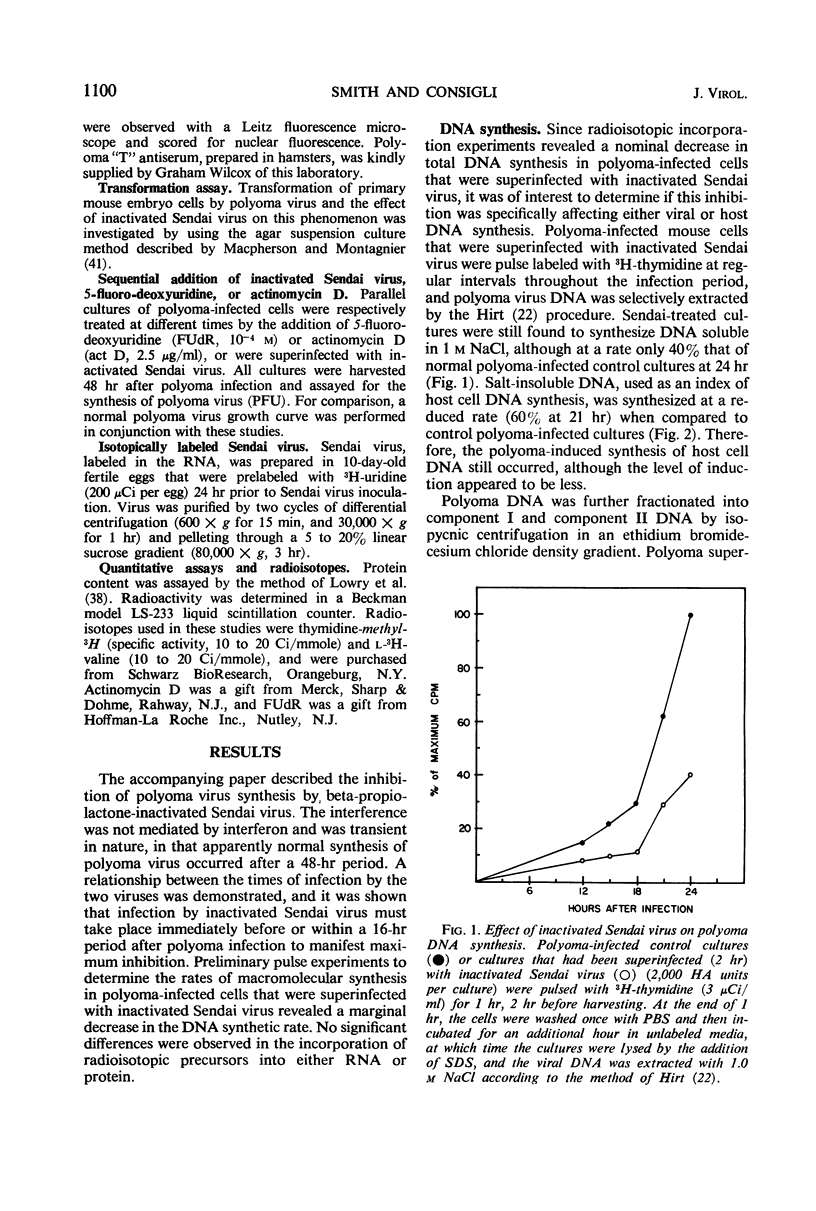
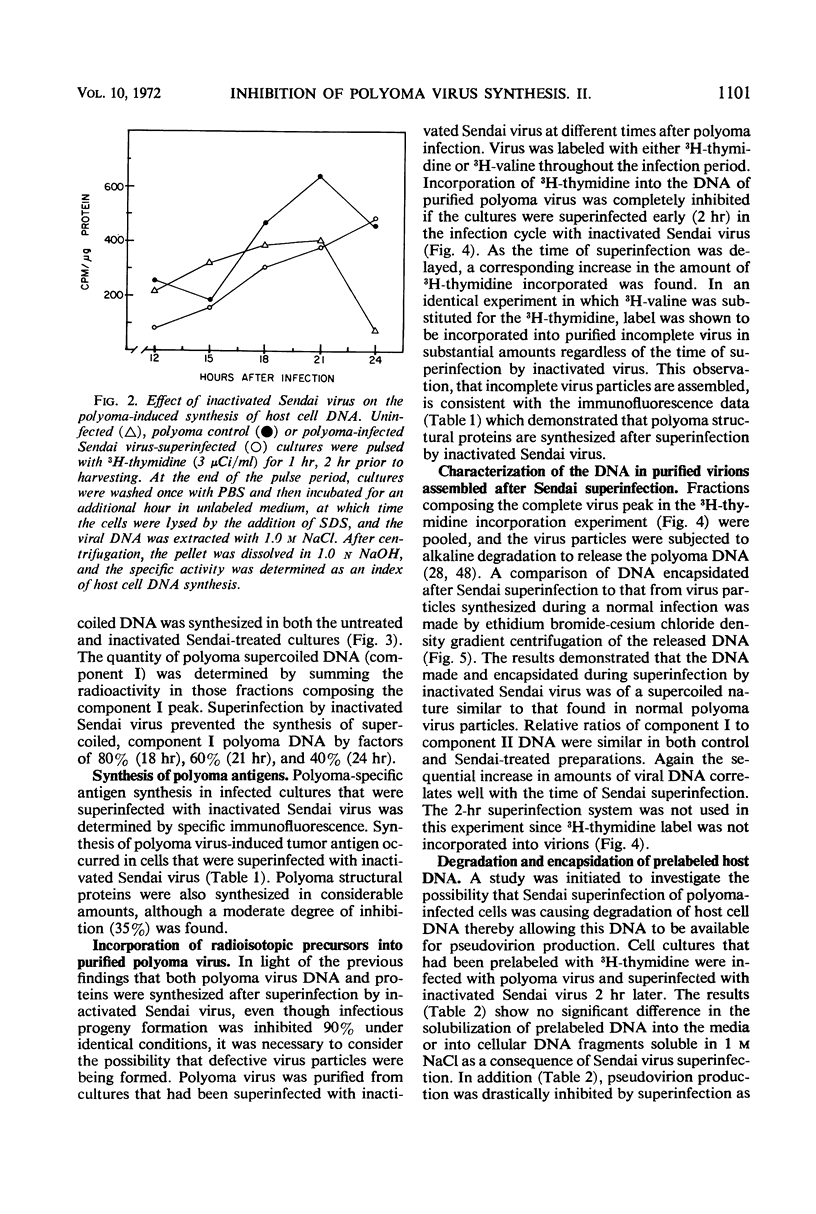
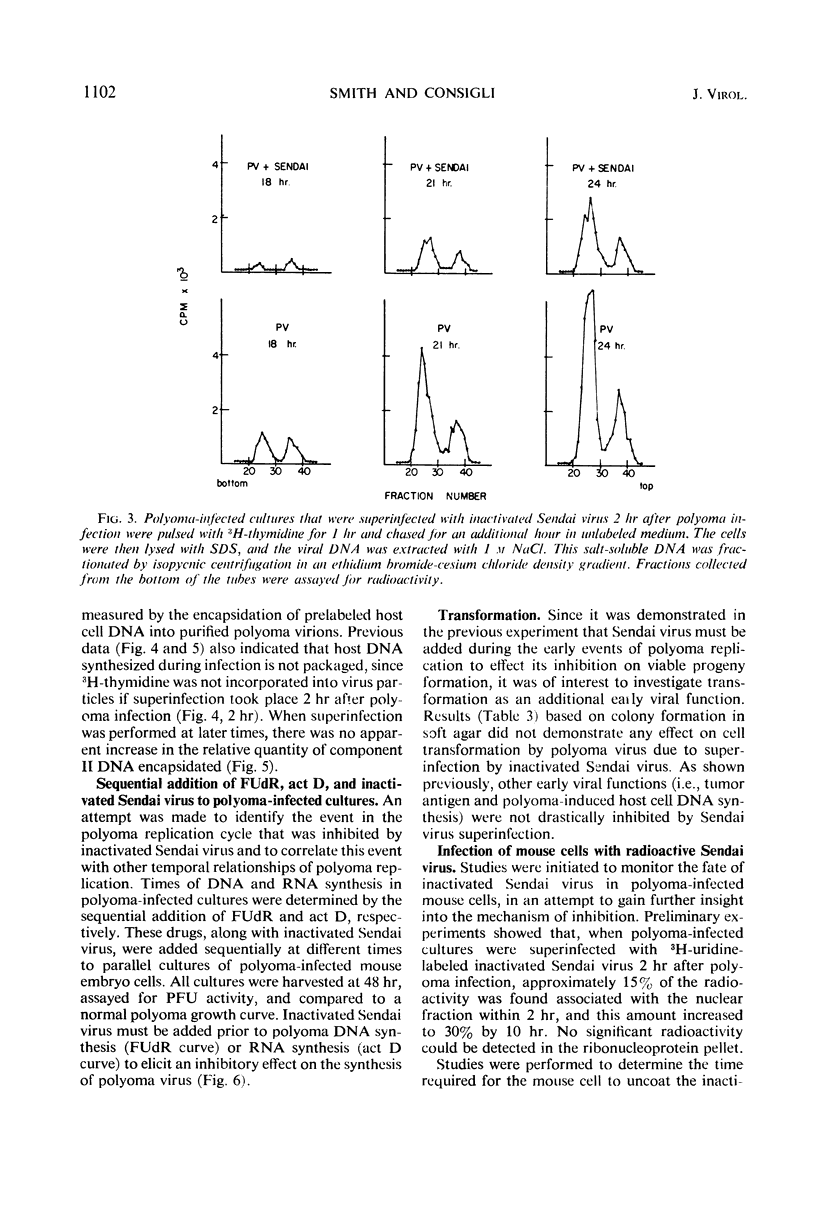
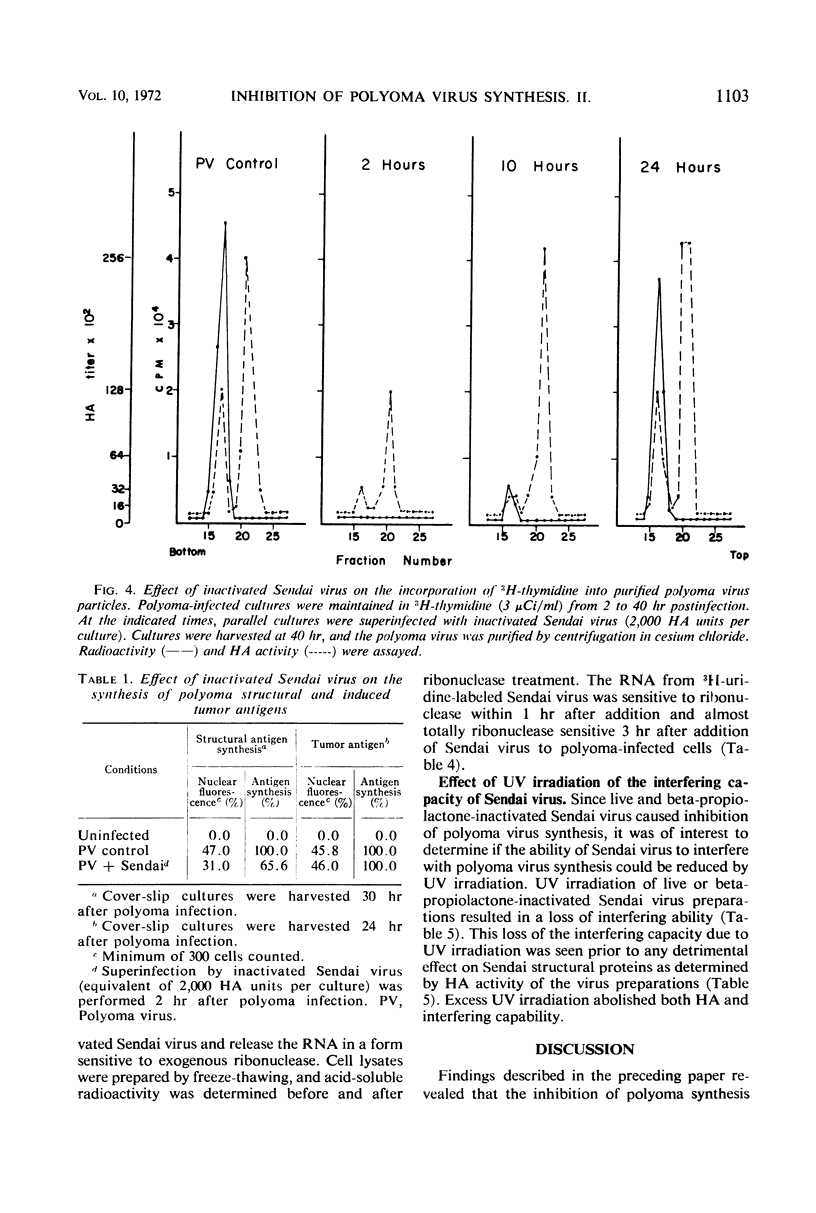
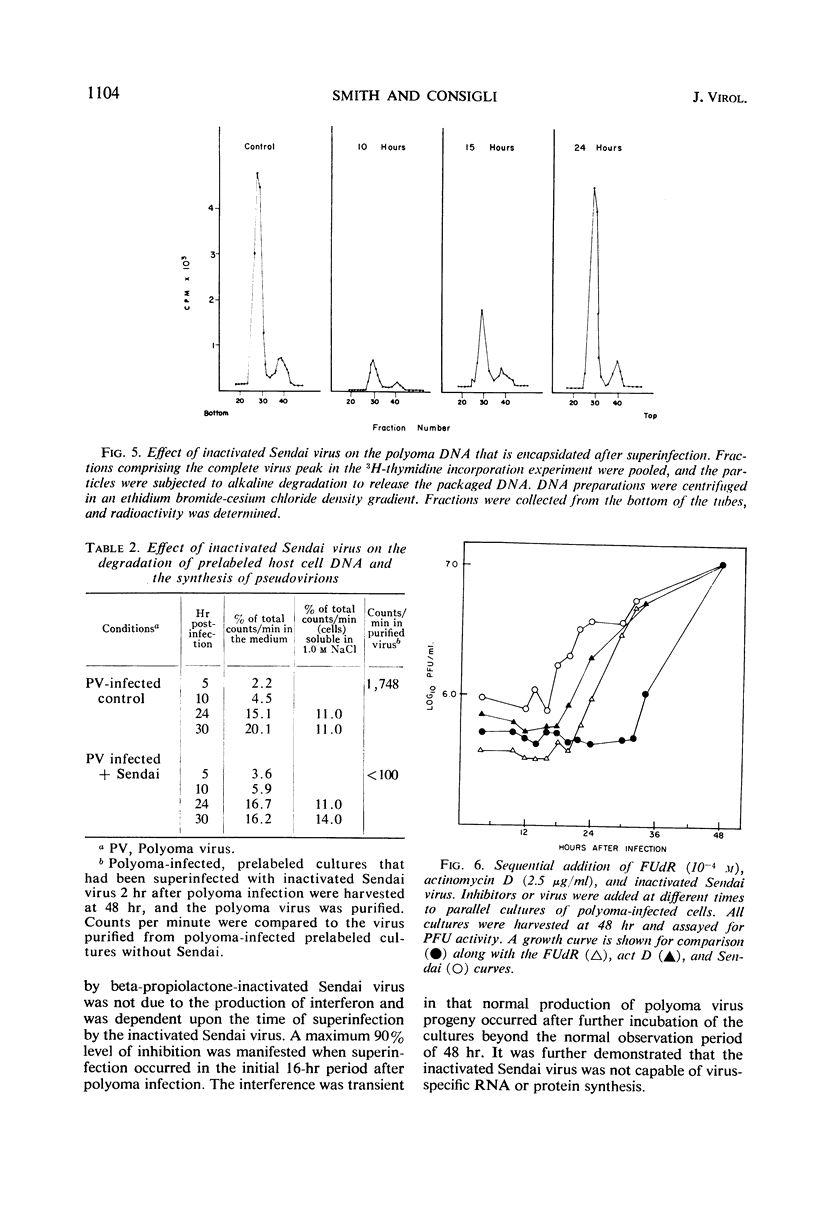
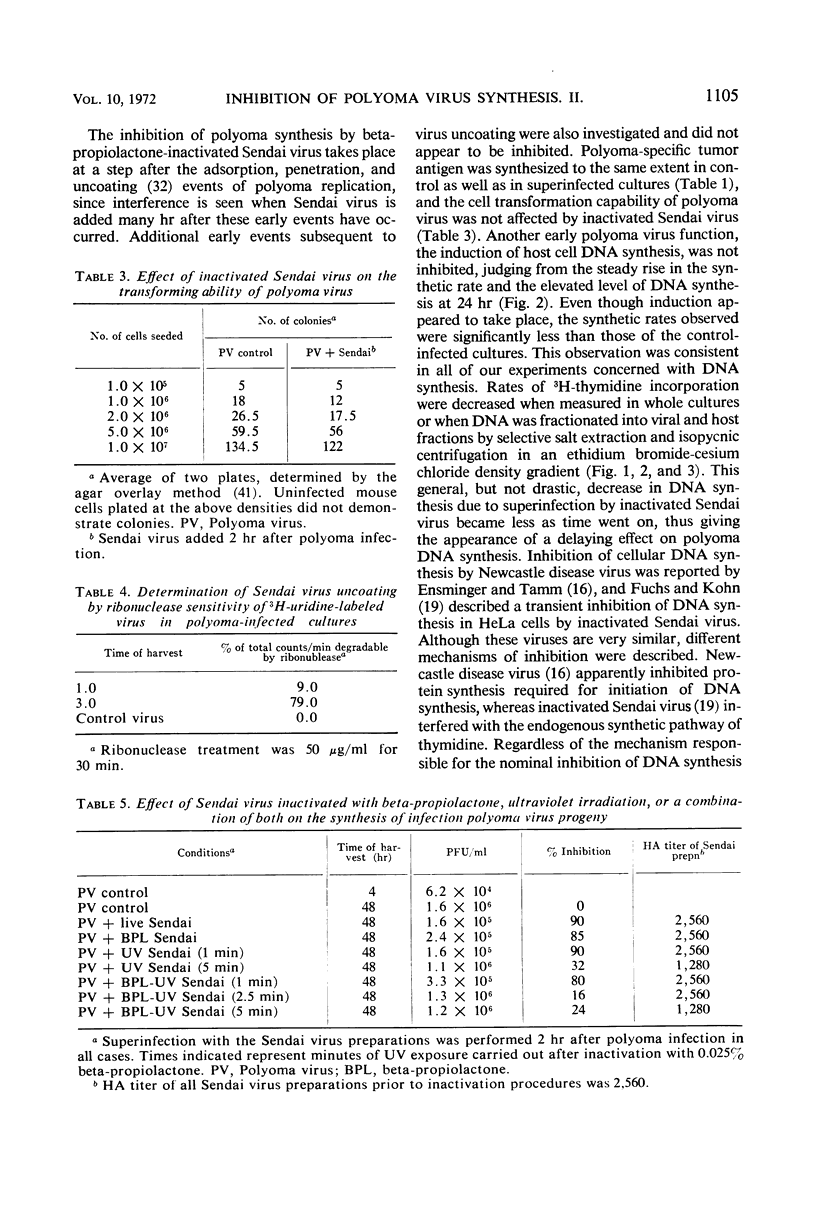
The mechanism of the transient inhibition of polyoma virus synthesis by betapropiolactone-inactivated Sendai virus was studied. Polyoma virus early functions did not appear to be affected, although deoxyribonucleic acid (DNA) and structural protein synthesis were inhibited 60 and 35% respectively. The inhibition of macromolecular synthesis was not sufficient to account for the 90% inhibition of infectious progeny formation. Encapsidation of polyoma DNA into mature virions appears to be completely inhibited after superinfection by beta-propiolactone-inactivated Sendai virus. Ultraviolet irradiation of live or beta-propiolactone-inactivated Sendai virus preparations abolishes the interfering capacity, indicating that a functional Sendai virus ribonucleic acid molecule is the interfering component.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., Guir J., Kirn A. The inhibition of vaccinia virus DNA synthesis in KB cells infected with frog virus 3. J Gen Virol. 1970 Aug;8(2):105–111. doi: 10.1099/0022-1317-8-2-105. [DOI] [PubMed] [Google Scholar]
- Aula P., Nichols W. W. Lysosomes and virus induced chromosomes breaage. Exp Cell Res. 1968 Aug-Sep;51(2-3):595–601. doi: 10.1016/0014-4827(68)90147-x. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A. Homologous interference by ultraviolet-inactivated Newcastle disease virus. Virology. 1957 Aug;4(1):72–96. doi: 10.1016/0042-6822(57)90044-2. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A. Loss of viral receptors in homologous interference by ultraviolet-irradiated Newcastle disease virus. Virology. 1959 Mar;7(3):315–327. doi: 10.1016/0042-6822(59)90201-6. [DOI] [PubMed] [Google Scholar]
- BUKRINSKAYA A. G., ZHDANOV V. M. SHORTENING BY ACTINOMYCIN D OF LATENT PERIOD OF MULTIPLICATION OF SENDAI VIRUS. Nature. 1963 Nov 30;200:920–921. doi: 10.1038/200920a0. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Is a specific protein responsible for the supercoiling of polyoma DNA? Nature. 1972 Jan 14;235(5333):105–107. doi: 10.1038/235105a0. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Rubin H. Specific interference among strains of Newcastle disease virus. II. Comparison of interference by active and inactive virus. Virology. 1968 Jul;35(3):381–394. doi: 10.1016/0042-6822(68)90217-1. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Zhdanov V. M., Vorkunova G. K. Fate of Sendai virus ribonucleoprotein in virus-infected cells. J Virol. 1969 Aug;4(2):141–146. doi: 10.1128/jvi.4.2.141-146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Holmes K. V. Replication of SV5 RNA and the effects of superinfection with poliovirus. Virology. 1967 Nov;33(3):442–451. doi: 10.1016/0042-6822(67)90119-5. [DOI] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. Multiplication of polyoma virus. II. Source of constituents for viral deoxyribonucleic acid and protein synthesis. J Bacteriol. 1966 Sep;92(3):789–791. doi: 10.1128/jb.92.3.789-791.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consigli R. A., Minocha H. C., Abo-Ahmed H. The effect of 5-fluoro-deoxyuridine on the replication of viral DNA and synthesis of polyoma capsid protein. J Gen Virol. 1968 May;2(3):437–441. doi: 10.1099/0022-1317-2-3-437. [DOI] [PubMed] [Google Scholar]
- Dales S., Silverberg H. Controlled double infection with unrelated animal viruses. Virology. 1968 Mar;34(3):531–543. doi: 10.1016/0042-6822(68)90072-x. [DOI] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Virus-induced interference in heterologously infected HeLa cells. J Virol. 1972 Jan;9(1):22–28. doi: 10.1128/jvi.9.1.22-28.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Ensminger W. D., Tamm I. The step in cellular DNA synthesis blocked by Newcastle disease or mengovirus infection. Virology. 1970 Jan;40(1):152–165. doi: 10.1016/0042-6822(70)90387-9. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Freda C. E., Buck C. A. System of double infection between vaccinia virus and mengovirus. J Virol. 1971 Sep;8(3):293–302. doi: 10.1128/jvi.8.3.293-302.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P., Kohn A. Nature of transient inhibition of deoxyribonucleic acid synthesis in HeLa cells by parainfluenza virus 1 (Sendai). J Virol. 1971 Nov;8(5):695–700. doi: 10.1128/jvi.8.5.695-700.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno R., Kates J. R. Mechanism of inhibition of vaccinia virus replication in adenovirus-infected HeLa cells. J Virol. 1971 Feb;7(2):208–213. doi: 10.1128/jvi.7.2.208-213.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. INHIBITION OF HOST CELL MACROMOLECULAR SYNTHESIS BY HIGH MULTIPLICITIES OF POLIOVIRUS UNDER CONDITIONS PREVENTING VIRUS SYNTHESIS. J Mol Biol. 1964 Apr;8:574–581. doi: 10.1016/s0022-2836(64)80012-7. [DOI] [PubMed] [Google Scholar]
- Hancock R., Weil R. Biochemical evidence for induction by polyoma virus of replication of the chromosomes of mouse kidney cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1144–1150. doi: 10.1073/pnas.63.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Inhibition of cellular RNA synthesis by nonreplicating vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1579–1584. doi: 10.1073/pnas.54.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata A., Consigli R. A. Effect of phleomycin on polyoma virus synthesis in mouse embryo cells. J Virol. 1971 Jan;7(1):29–40. doi: 10.1128/jvi.7.1.29-40.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Sandberg A. A. Cellular phase of chromosome pulverization induced by Sendai virus. J Natl Cancer Inst. 1968 Nov;41(5):1125–1131. [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Cytologic studies in polyoma virus-infected mouse embryo cells. Am J Vet Res. 1967 Sep;28(126):1527–1536. [PubMed] [Google Scholar]
- Khare G. P., Consigli R. A. Multiplication of Polyoma Virus I. Use of Selectively Labeled (H) Virus to Follow the Course of Infection. J Bacteriol. 1965 Sep;90(3):819–821. doi: 10.1128/jb.90.3.819-821.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoobyarian N., Fischinger P. J. Role of heated adenovirus 2 in viral interference. Proc Soc Exp Biol Med. 1965 Nov;120(2):533–538. doi: 10.3181/00379727-120-30582. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A. On the genesis of incomplete Sendai virions. Virology. 1970 Dec;42(4):872–879. doi: 10.1016/0042-6822(70)90336-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine A. J., Ginsberg H. S. Mechanism by which fiber antigen inhibits multiplication of type 5 adenovirus. J Virol. 1967 Aug;1(4):747–757. doi: 10.1128/jvi.1.4.747-757.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Smith W. R. Deoxyribonucleic acid synthesis in FV-3-infected mammalian cells. J Virol. 1968 Oct;2(10):1006–1015. doi: 10.1128/jvi.2.10.1006-1015.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick W., Penman S. Replication of mengovirus in HeLa cells preinfected with nonreplicating poliovirus. J Virol. 1968 Sep;2(9):859–864. doi: 10.1128/jvi.2.9.859-864.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekler L. B., Shlyankevich M. A., Shevliaghyn V. J. Sendai virus RNA as messenger RNA determining the synthesis of early virus specific proteins. Arch Gesamte Virusforsch. 1970;30(4):309–315. doi: 10.1007/BF01258361. [DOI] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Miles C. P. Virus-induced chromosome pulverization in human diploid cells. Proc Soc Exp Biol Med. 1970 Jul;134(3):825–830. doi: 10.3181/00379727-134-34892. [DOI] [PubMed] [Google Scholar]
- PEREIRA H. G. A virus inhibitor produced in HeLa cells infected with adenovirus. Virology. 1960 Jul;11:590–602. doi: 10.1016/0042-6822(60)90102-1. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P., COOPER P. D. INTERFERENCE BETWEEN POLIOVIRUSES INDUCED BY STRAINS THAT CANNOT MULTIPLY. Virology. 1965 Mar;25:350–357. doi: 10.1016/0042-6822(65)90054-1. [DOI] [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]
- Petric M., Prevec L. Vesicular stomatitis virus--a new interfering particle, intracellular structures, and virus-specific RNA. Virology. 1970 Aug;41(4):615–630. doi: 10.1016/0042-6822(70)90427-7. [DOI] [PubMed] [Google Scholar]
- Portner A., Kingsbury D. W. Homologous interference by incomplete Sendai virus particles: changes in virus-specific ribonucleic acid synthesis. J Virol. 1971 Oct;8(4):388–394. doi: 10.1128/jvi.8.4.388-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilaginès R., McAuslan B. R. Interference with viral messenger RNA and DNA synthesis by superinfection with a heterologous deoxyvirus. Virology. 1970 Dec;42(4):1043–1053. doi: 10.1016/0042-6822(70)90352-1. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Youngner J. S. Inhibition of protein synthesis in L cells infected with vesicular stomatitis virus. J Virol. 1972 Jan;9(1):85–89. doi: 10.1128/jvi.9.1.85-89.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Robbins E. Histone synthesis in polyoma- and SV40-infected cells. Virology. 1970 Feb;40(2):307–315. doi: 10.1016/0042-6822(70)90406-x. [DOI] [PubMed] [Google Scholar]
- Yaoi Y., Amano M. Inhibitory effect of ultraviolet-inactivated vesicular stomatitis virus on initiation o DNA synthesis in cultured chick embryo cells. J Gen Virol. 1970 Oct;9(1):69–75. doi: 10.1099/0022-1317-9-1-69. [DOI] [PubMed] [Google Scholar]


