Abstract
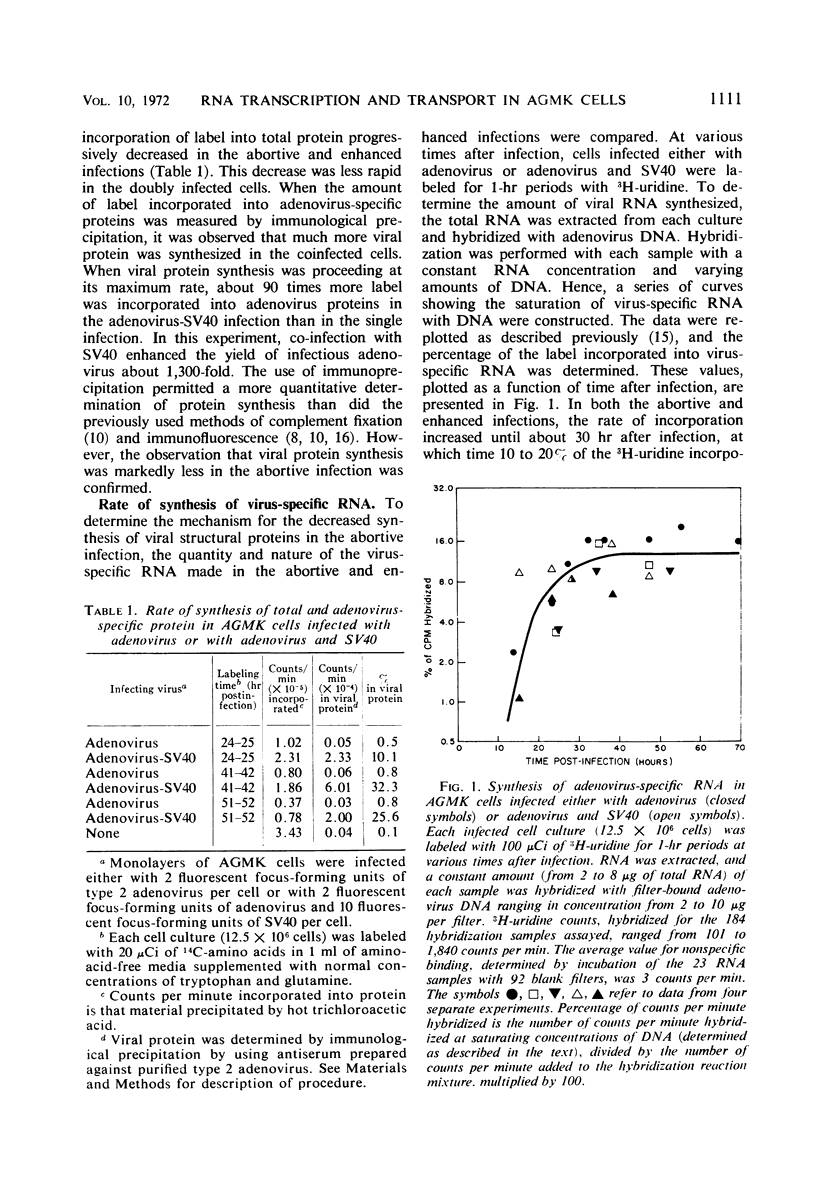
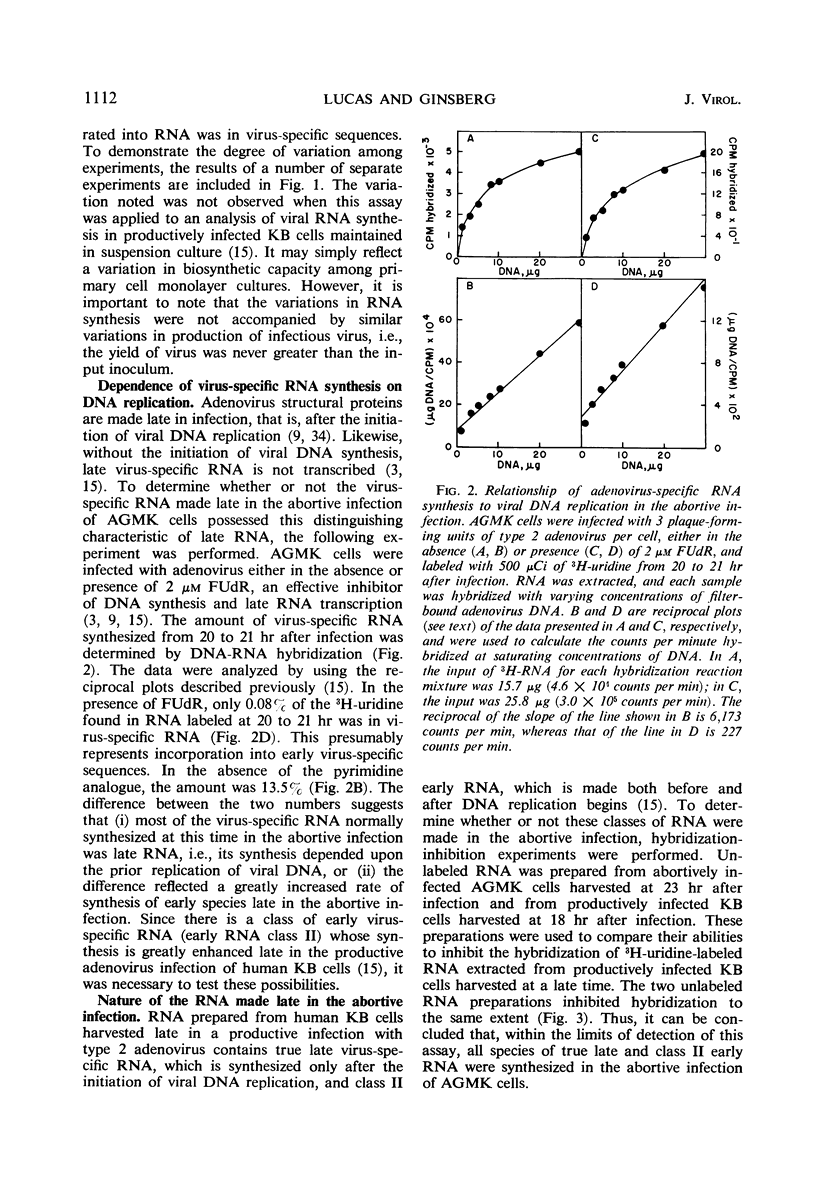
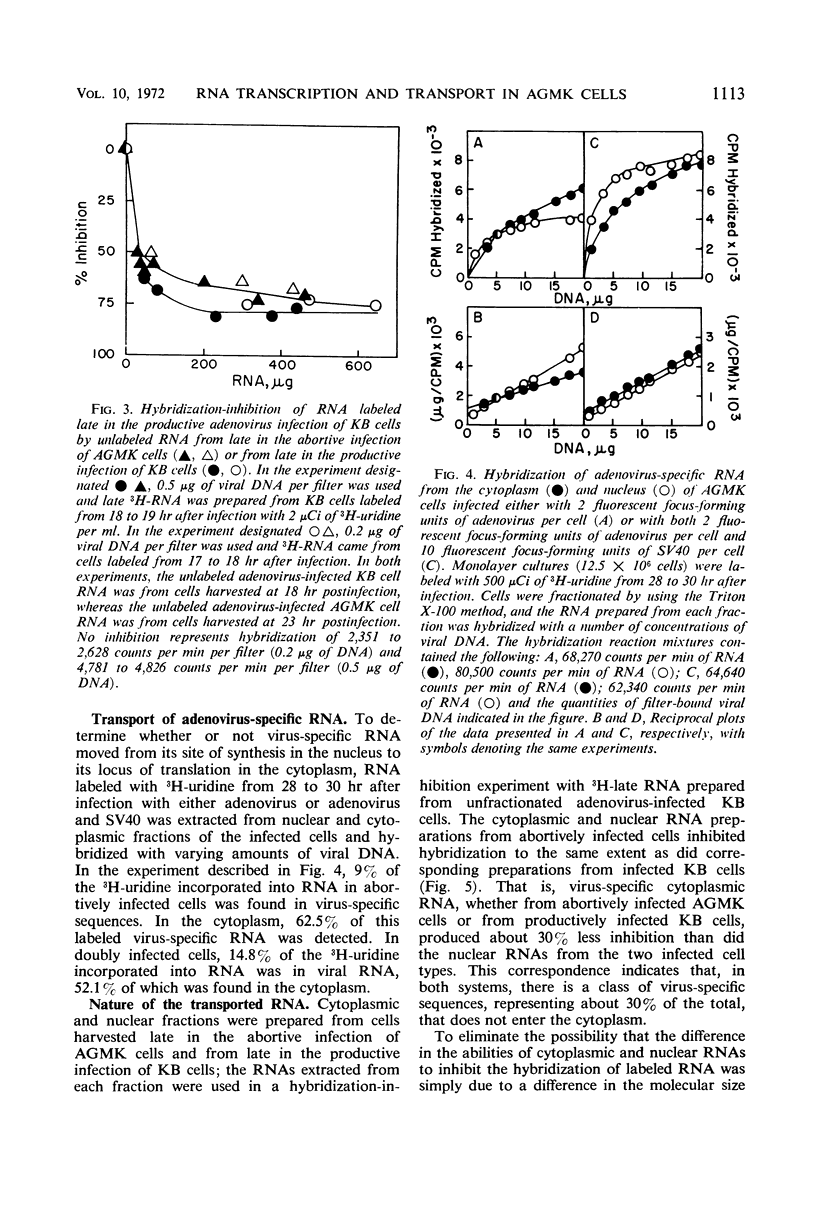
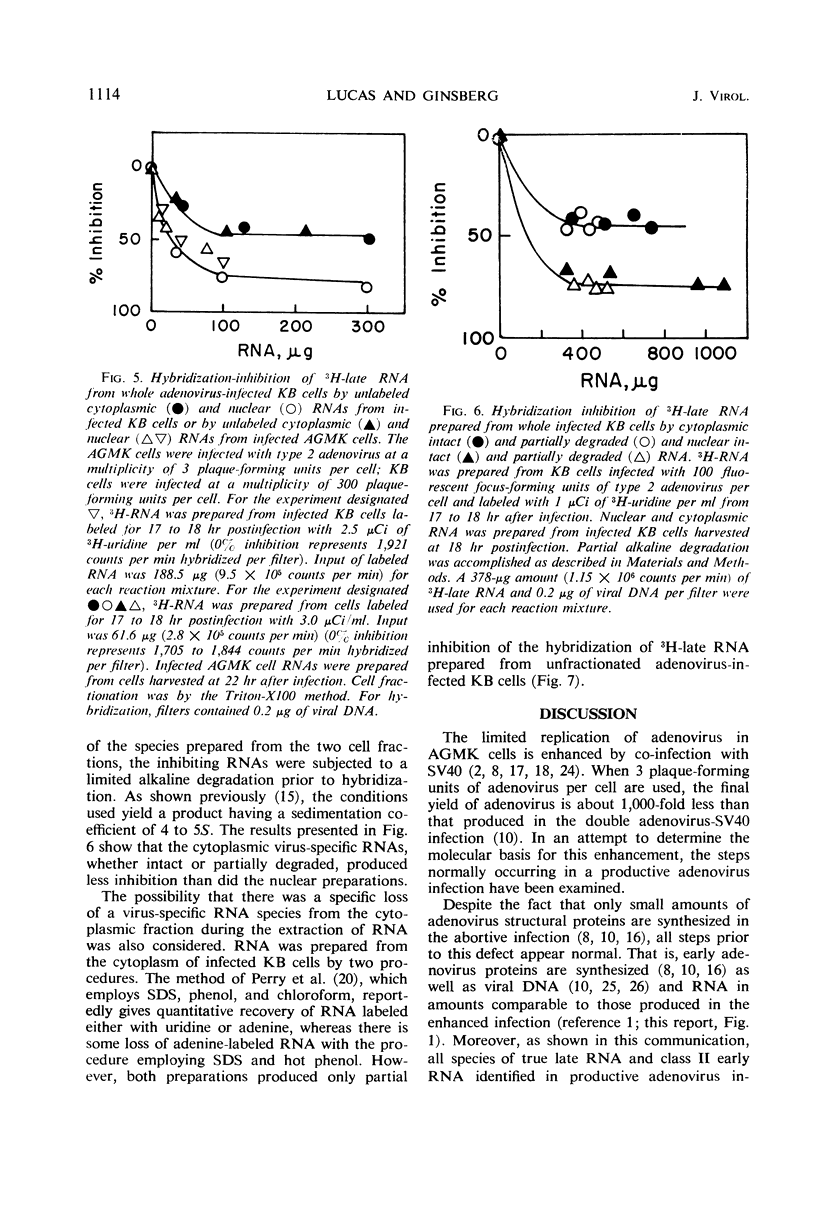
The techniques of deoxyribonucleic acid-ribonucleic acid (DNA-RNA) hybridization and immunological precipitation were used to compare the synthesis of adenovirus-specific macromolecules in African green monkey kidney (AGMK) cells infected with adenovirus, an abortive infection, and coinfected with both adenovirus and simian virus 40 (SV40), which renders the cells permissive for adenovirus replication. When viral protein synthesis was proceeding at its maximum rate, the incorporation of 14C-amino acids into adenovirus structural proteins was about 90 times greater in the doubly infected cells than in cells infected only with adenovirus. However, the rates of synthesis of virus-specific ribonucleic acid appeared to be comparable in the two infections at all times measured. A time-dependent increase in the rate of RNA synthesis observed late in the abortive infection was dependent upon the prior replication of viral DNA. Moreover, all virus-specific RNA species that are normally made late in a productive adenovirus infection (i.e., the true late and class II early RNA species) were also detected in the abortive infection. Adenovirus-specific RNA was detected by molecular hybridization in both the cytoplasm and nuclei of abortively infected cells. Comparable amounts of viral RNA were found in the cytoplasmic fractions of AGMK cells infected either with adenovirus or with both adenovirus and SV40. The results of hybridization-inhibition experiments clearly showed that there was a class of virus-specific RNA molecules, representing about 30% of the total, in the nucleus that was not transported to the cytoplasm. This class of RNA was also identified in similar amounts in productively infected human KB cells. The difference in the abilities of cytoplasmic and nuclear RNA to inhibit the hybridization of virus-specific RNA from whole cells was shown not to be due to a difference in the molecular size of the RNA species from the two cell fractions or to the specific loss of a cytoplasmic species during RNA extraction procedures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum S. G., Wiese W. H., Reich P. R. Studies on the mechanism of enhancement of adenovirus 7 infection in African green monkey cells by simian virus 40: formation of adenovirus-specific RNA. Virology. 1968 Feb;34(2):373–376. doi: 10.1016/0042-6822(68)90253-5. [DOI] [PubMed] [Google Scholar]
- Beardmore W. B., Havlick M. J., Serafini A., McLean I. W., Jr Interrelationship of adenovirus (type 4) and papovavirus (SV-40) in monkey kidney cell cultures. J Immunol. 1965 Sep;95(3):422–435. [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Relationship between deoxyribonucleic acid-like ribonucleic acid synthesis and inhibition of host protein synthesis in type 5 adenovirus-infected KB cells. J Virol. 1969 Feb;3(2):106–113. doi: 10.1128/jvi.3.2.106-113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Pène J. J., Darnell J. E. Studies on HeLa cell nuclear DNA-like RNA by RNA-DNA hybridization. Proc Natl Acad Sci U S A. 1967 Jul;58(1):320–327. doi: 10.1073/pnas.58.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffier H., Raskas H. J., Parsons T. J., Green M. Initiation of mammalian viral protein synthesis. Nat New Biol. 1971 Feb 24;229(8):239–241. doi: 10.1038/newbio229239a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Ribonucleic acids from animal cells. Bacteriol Rev. 1968 Sep;32(3):262–290. doi: 10.1128/br.32.3.262-290.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLANAGAN J. F., GINSBERG H. S. Synthesis of virus-specific polymers in adenovirus-infected cells; effect of 5-fluorodeoxyuridine. J Exp Med. 1962 Aug 1;116:141–157. doi: 10.1084/jem.116.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. A., Butel J. S., Rapp F. Interaction of a simian papovavirus and adenoviruses. I. Induction of adenovirus tumor antigen during abortive infection of simian cells. J Bacteriol. 1966 Feb;91(2):813–818. doi: 10.1128/jb.91.2.813-818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. P., Lyons M. J., Ginsberg H. S. Biochemical consequences of type 2 adenovirus and Simian virus 40 double infections of African green monkey kidney cells. J Virol. 1970 May;5(5):586–597. doi: 10.1128/jvi.5.5.586-597.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. SYNTHESIS OF RNA IN L CELLS INFECTED WITH MENGO VIRUS. J Cell Physiol. 1963 Oct;62:179–192. doi: 10.1002/jcp.1030620207. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. Ribosomal subunits of Landschütz ascites cells during changes in polysome distribution. Biochim Biophys Acta. 1968 Nov 20;169(1):129–138. doi: 10.1016/0005-2787(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Ginsberg H. S. Synthesis of virus-specific ribonucleic acid in KB cells infected with type 2 adenovirus. J Virol. 1971 Aug;8(2):203–214. doi: 10.1128/jvi.8.2.203-214.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren R. A., Rabson A. S., Carney P. G., Paul F. J. Immunofluorescence of green monkey kidney cells infected with adenovirus 12 and with adenovirus 12 plus simian virus 40. J Bacteriol. 1966 Jan;91(1):262–265. doi: 10.1128/jb.91.1.262-265.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., BEREZESKY I. K., PAUL F. J. MIXED INFECTION WITH SIMIAN VIRUS 40 AND ADENOVIRUS 12. J Natl Cancer Inst. 1963 Oct;31:903–917. [PubMed] [Google Scholar]
- O'CONOR G. T., RABSON A. S., MALMGREN R. A., BEREZESKY I. K., PAUL F. J. MORPHOLOGIC OBSERVATIONS OF GREEN MONKEY KIDNEY CELLS AFTER SINGLE AND DOUBLE INFECTION WITH ADENOVIRUS 12 AND SIMIAN VIRUS 40. J Natl Cancer Inst. 1965 May;34:679–693. [PubMed] [Google Scholar]
- Parsons J. T., Gardner J., Green M. Biochemical studies on adenovirus multiplication, XIX. Resolution of late viral RNA species in the nucleus and cytoplasm. Proc Natl Acad Sci U S A. 1971 Mar;68(3):557–560. doi: 10.1073/pnas.68.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E., Clarkson K., Kossman C. R., Stonington O. G. Synthesis of ribosomal RNA on a protein-DNA complex isolated from bacteria: a comparison of ribosomal RNA synthesis in vitro and in vivo. J Mol Biol. 1970 Sep 14;52(2):281–300. doi: 10.1016/0022-2836(70)90031-8. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Ordered and preferential initiation of ribosomal RNA synthesis in vitro. Nat New Biol. 1972 Feb 16;235(59):204–206. doi: 10.1038/newbio235204a0. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Darnell J. E. Competition hybridization by "pre-saturation" of HeLa cell DNA. J Mol Biol. 1969 Sep 28;44(3):551–562. doi: 10.1016/0022-2836(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Green M. Biochemical studies on adenovirus multiplication. XV. Transcription of the adenovirus type II genome during productive infection. Virology. 1969 Oct;39(2):205–210. doi: 10.1016/0042-6822(69)90040-3. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. Protein synthesis in type 5 adenovirus-infected cells. Effect of p-flourophenylalanine on synthesis of protein. nucleic acids, and infectious virus. Virology. 1963 Jun;20:269–280. doi: 10.1016/0042-6822(63)90115-6. [DOI] [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


