Abstract
Morphine and related µ-opioid receptor (MOR) agonists remain among the most effective drugs known for acute relief of severe pain. A major problem in treating painful conditions is that tolerance limits the long-term utility of opioid agonists. Considerable effort has been expended on developing an understanding of the molecular and cellular processes that underlie acute MOR signaling, short-term receptor regulation, and the progression of events that lead to tolerance for different MOR agonists. Although great progress has been made in the past decade, many points of contention and controversy cloud the realization of this progress. This review attempts to clarify some confusion by clearly defining terms, such as desensitization and tolerance, and addressing optimal pharmacological analyses for discerning relative importance of these cellular mechanisms. Cellular and molecular mechanisms regulating MOR function by phosphorylation relative to receptor desensitization and endocytosis are comprehensively reviewed, with an emphasis on agonist-biased regulation and areas where knowledge is lacking or controversial. The implications of these mechanisms for understanding the substantial contribution of MOR signaling to opioid tolerance are then considered in detail. While some functional MOR regulatory mechanisms contributing to tolerance are clearly understood, there are large gaps in understanding the molecular processes responsible for loss of MOR function after chronic exposure to opioids. Further elucidation of the cellular mechanisms that are regulated by opioids will be necessary for the successful development of MOR-based approaches to new pain therapeutics that limit the development of tolerance.
I. Introduction
Opioids have been used for pain relief and their psychotropic effects since antiquity. As a drug class they remain the most effective analgesics known for many types of pain but their clinical utility is limited by tolerance and fear of addiction. Since the isolation of morphine in the early 19th century and introduction of heroin in 1898, medicinal chemistry efforts have yielded thousands of morphine analogs and structurally distinct opioids, resulting in a rich pharmacology (Corbett et al., 2006). One motivation was to develop nonaddictive analgesics, based on the idea that morphine might produce its desired and undesired effects by binding to different receptor subtypes. This hypothesis is not supported by data from μ-opioid receptor (MOR) knockout mice, (Matthes et al., 1996) demonstrating that most morphine-induced actions require the MOR and a truly nonaddictive opioid agonist has not been identified. It is interesting to note, for example, that heroin was one of the first morphine derivatives claimed to have nonaddictive properties. The in vivo effects of heroin result from breakdown and metabolism to 6-acetylmorphine and morphine, a rapid process in aqueous solution as well as in brain and peripheral tissues (Umans and Inturrisi, 1981).
Considerable study at the molecular, cellular, and systems levels has been devoted to understanding processes that underlie tolerance to opioids. The identification of reduced opioid responsiveness in ex vivo preparations derived from tolerant animals, together with seminal studies demonstrating reductions in opioid sensitivity in cultured cells, led to the interest in opioid tolerance as a cell biologic problem. To determine the mechanisms involved in the development of tolerance, one approach has been to determine the acute actions of opioids and short-term regulation of the MOR with the aim to identify processes leading to tolerance. There are numerous reviews on the acute actions of opioids and short-term plasticity of MOR function that may be precursors to the development of tolerance (Williams et al., 2001; von Zastrow et al., 2003; Connor et al., 2004; Waldhoer et al., 2004; Bailey and Connor, 2005; Koch and Höllt, 2008). A second approach is to induce tolerance to opioids, with chronic treatment of cultured cells or animals, and then determine the adaptations that result from that treatment. A third approach is to perturb candidate mechanisms, with pharmacological or genetic manipulation, and examine effects on opioid tolerance in cell culture, tissue preparations, or intact animals. Together, these approaches have led to the identification of a cornucopia of mechanisms potentially contributing to opioid tolerance. So far, however, they have failed to identify any single regulatory mechanism that can account for the degree of opioid tolerance typically observed in the intact animal (Christie, 2008). A prevalent idea is that opioid tolerance is a complex, multifaceted process that likely involves the interplay of multiple regulatory mechanisms occurring both at the level of individual opioid-responsive cells and at the level of neural circuits.
A contributing factor to the complexity of in vivo administration of opioid drugs is the number of clinically important differences among opioids, including differences in pharmacokinetics, potency, and efficacy. Methadone and buprenorphine have long-lasting actions in humans, a property that has made these drugs favored for use in treating chronic painful conditions and management of opioid dependence (Pergolizzi et al., 2008). Fentanyl, on the other hand, is sequestered rapidly into body fat following acute administration, making this drug advantageous in the operating room and certain outpatient procedures. There are also significant differences among opioids in potency and efficacy, which influence drug choice for various clinical indications and distinguish the safety of various opioids. Buprenorphine has lower intrinsic efficacy at MORs and thus produces less respiratory depression, making it safer than methadone for management in opioid addicts. Nevertheless, long-term use of all MOR agonists produces adverse effects that include the development of tolerance (Williams et al., 2001; von Zastrow et al., 2003; Connor et al., 2004; Waldhoer et al., 2004; Bailey and Connor, 2005; Christie, 2008; Koch and Höllt, 2008; Morgan and Christie, 2011). Differences in agonist efficacies between opioids also have important implications for tolerance: Agonists of low efficacy will occupy and engage a larger fraction of the available receptors to produce their effects than agonists with high efficacy. The relative efficiency of initiating signaling events is correlated with relative efficiency for initiating steps leading to receptor desensitization.
This review examines a number of the receptor regulatory mechanisms affected by opioids at the cellular level that are likely to contribute to opioid tolerance in vivo. The evidence for biased agonism (defined below) for each process is considered. The extent to which these regulatory mechanisms are consistent with findings of opioid-receptor function in opioid-tolerant animals will be considered, and candidate mechanisms that mediate these perturbations in signaling will be discussed.
II. Definition of Terms
When comparing the ability of MOR agonists to induce a given response it is important to have a measure of their efficacy. Efficacy is defined as the ability of an agonist to evoke a response through a given receptor in a specific tissue. As such it is governed by both the receptor and the tissue. It is common to study the relative efficacy of a group of agonists at a receptor on one type of tissue, thus removing the influence of how efficacy changes between tissue types. Intrinsic efficacy is the amount of signaling response (“stimulus”) produced by each drug-receptor-binding event, whereas intrinsic activity is the fraction of maximal response evoked by a receptor-saturating concentration of drug in that test system. The latter does not discriminate between possible differences in intrinsic efficacies of full agonists, as by definition full agonists all produce the same maximum response. A measure of intrinsic efficacy can be achieved using either the operational model of Black and Leff (1983) or by the method of Ehlert (1985). In the method of Black and Leff, the concentration-response curve of an agonist is fit to the operational model (see for example McPherson et al., 2010). In contrast, in the method of Ehlert, the concentration-response curve of the agonist is measured before and after removing any receptor reserve with an irreversible antagonist. The advantage of the latter method is that it does not require an accurate measure of the affinity of agonist binding, which may be different under physiologic conditions from those used in radioligand-binding studies and may also differ from tissue to tissue. For more detailed information on agonist intrinsic efficacy see Kenakin (1997).
Many features of opioid tolerance can be viewed in terms of the concept of homeostasis, where opioid-responsive neurons adapt to the prolonged presence of opioid receptor activation to normalize net activity. We consider it important to define the terms highlighted in the following, and outlined in the general scheme of MOR regulation in Fig. 1, to avoid confusion with varying (but often strictly correct) usage in different studies. Adaptations refer to regulatory processes that directly reduce opioid response or sensitivity. Counter-adaptations, sometimes called opponent processes, refer to processes that effectively reduce opioid responses by engaging opposing or compensatory regulatory mechanisms or signaling pathways. By comparing the acute and chronic effects of opioid drugs, a number of adaptations and counter-adaptations have been identified that may be important to the opioid-tolerant state. The contribution of counter-adaptations/opponent processes to tolerance have been reviewed elsewhere (Waldhoer et al., 2004; Christie, 2008) and will not be considered further. However, these compensatory changes caused by sustained opioid receptor activation are unmasked when drug administration stops and are responsible for the withdrawal signs frequently noted in opiate users (goose flesh, dysphoria, hyperalgesia, and gut hypermotility).
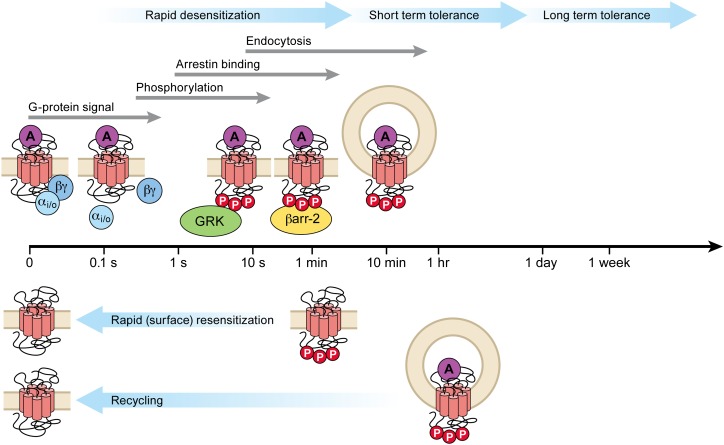
Fig. 1.
General scheme of MOR regulation following binding of an efficacious agonist such as [Met]5enkephalin. The time scales for each process are shown (log scale). Phosphorylation by G protein receptor kinase (GRK) is very rapid, saturating in less than 20 seconds. Arrestin binding saturates in several minutes, and desensitization reaches steady state in approximately 5 minutes. The steady state of rapid desensitization represents the equilibrium between the forward desensitizing process, presumably phosphorylation and arrestin binding (other kinases may be involved, see Section V.D–V.G) and dephosphorylation at the cell surface (see Sections I, V, and VI). Endocytosis reaches steady state in approximately 30 minutes and recycling over approximately 60 minutes, although this varies for different splice variants. The present review defines desensitization as the rapid process preceding significant endocytosis (approximately 2–5 minutes); short-term tolerance includes endocytosis and other mechanisms (up to 1 day); and long-term tolerance (greater than 1 day) presumably involves multiple regulatory processes.
The terms tolerance and desensitization are often used interchangeably to describe the loss of receptor activity following continued or intermittent agonist treatment. However, it is necessary to mechanistically distinguish these two terms. Drug tolerance is defined as a loss of responsiveness to an agonist after continued exposure, without necessarily specifying the cellular or molecular mechanisms responsible. It is evident in whole-animal studies where the underlying cellular and molecular mechanisms are difficult to resolve, and it is often studied in isolated tissues where tolerance can be measured as a rightward shift in the dose-response curve that may also be associated with a reduction in the maximum response. Downregulation traditionally refers to a reduced number of functional receptors present in cells, usually detected by reduced Bmax in radioligand-binding assays of tissue extracts, which occurs as a result of enhanced degradation and/or reduced biosynthesis of receptors (Tsao and von Zastrow, 2000). In contrast, desensitization usually refers to molecular changes at the level of receptor signaling and can be homologous (reduced effects restricted to agonists acting through a specific receptor) or heterologous (reduced effects of agonists acting at other receptors that share a component of the signaling cascade, Lefkowitz et al., 1983). However, common use of the term in different experimental contexts can be confusing because the mechanisms regulating MOR function during short-term agonist exposure may or may not differ from mechanisms initiated during or following long-term agonist exposure. Here we suggest that desensitization be used only to describe acute loss of MOR-effector coupling that occurs within seconds to minutes after initiation of exposure to opioid agonists. The same term has been applied to measurements of acute MOR-effector coupling occurring in vitro after intermediate (for several hours; Tan et al., 2009) or long-term opioid exposure (days; Bohn et al., 1999, 2002), but we prefer to use the term tolerance for such prolonged exposure, qualified as “acute” (several hours, Cox et al., 1968) or “long-term” (days) to avoid confounding the mechanisms of rapid desensitization of MOR with potentially different mechanisms of tolerance.
When homologous desensitization is identified, direct inferences can be drawn concerning the coupling mechanisms of the stimulated receptor. For example, coupling of MORs to G protein-gated inwardly rectifying potassium [GIRK, GIRK isoform (Kir3)] channels in the presence of an efficacious agonist such as [Met]5enkephalin (ME) leads to homologous desensitization, because the sensitivity of closely related G protein-coupled receptors (GPCRs) such as the α2-adrenoceptor, somatostatin, or orphanin-FQ (ORL) receptors is unaffected. These receptors activate the same population of GIRK channels, indicating that loss of sensitivity is restricted to MOR-coupling mechanisms. Where this has been examined in neurons, homologous desensitization has usually been reported (e.g., Harris and Williams, 1991; Fiorillo and Williams, 1996; Bailey et al., 2004, 2009,b; Dang et al., 2009, 2011). However, heterologous desensitization of the α2-adrenoceptor-dependent current after activation of MOR in locus coeruleus neurons has been reported (Blanchet and Luscher, 2002; Blanchet et al., 2003).
It is important to note that desensitization and endocytosis are not mechanistically or functionally equivalent, and assays with poor temporal resolution are likely to lump both together (Connor et al., 2004). For example, biochemical assays for desensitization, such as inhibition of adenylyl cyclase that requires more than 5 minutes of sustained opioid exposure (most assays take 10–20 minutes, e.g., Law et al., 2000, or longer, e.g., Koch et al., 2005), measure the combined effects of rapid desensitization at the cell surface plus endocytosis or recovery from desensitization. Robust desensitization generally precedes endocytosis and can occur when endocytosis is absent or prevented (Johnson et al., 2005; Arttamangkul et al., 2006; Dang et al., 2009). While it is clear that arrestin-dependent internalization can sequester the opioid receptors in compartments that reduce the efficiencies of certain forms of signaling (e.g., Gβγ activation of Kir3 channels), it is now equally clear that arrestin binding does not inactivate all receptor signaling. Thus, while arrestin binding will sterically block G protein activation and prevent some forms of signaling (membrane-delimited ion channel regulation), arrestin has a scaffolding function that enables MOR activation of the ERK1/2 MAPK pathway (Macey et al., 2006; Miyatake et al., 2009).
Internalization has also been widely considered as the first step in receptor recovery from desensitization, leading to re-insertion of nondesensitized/reactivated receptors in the plasma membrane. This has been postulated to explain differences in the levels of desensitization between different MOR splice variants (Koch et al., 1998). However, more recent evidence has established that internalization is not necessary for dephosphorylation or recovery from desensitization of MOR (Dang et al., 2011; Doll et al., 2011; Quillinan et al., 2011).
Cross-tolerance to opioids refers to the property of tolerance that has developed following chronic exposure to one opioid that generalizes to a second opioid. If the extent of tolerance to the challenge opioid drug is similar to the induction opioid then cross-tolerance is symmetric, if not it is asymmetric. Asymmetric cross-tolerance among selective agonists has often been interpreted as suggesting that tolerance is mediated by different splice variants of MOR (Pasternak, 2001) but there are alternative interpretations. Opioids differ in their degrees of receptor selectivity and none are absolutely specific, particularly at the high doses used to generate tolerance. Given the multiplicity of sites that can mediate opioid analgesia (peripheral, spinal, brain stem, and cortical sites), it is likely that differences in physical and pharmacokinetic properties of different agonists, including hydrophobicity, could result in bias with respect to regions of receptor availability. In addition, cross-tolerance can appear asymmetric if the two opioids act at the same receptor but have very different intrinsic efficacies. For example, repeated administration of highly efficacious μ-opioids (e.g., sufentanyl, etonitazine, etorphine, or fentanyl) produced less analgesic tolerance than low-efficacy MOR agonists (e.g., morphine or buprenorphine; Sosnowski and Yaksh, 1990; Duttaroy and Yoburn, 1995; Walker and Young, 2001; Grecksch et al., 2006). Moreover, symmetric cross-tolerance will be evident even if the receptor is desensitized by different molecular mechanisms. For example, fentanyl produces short-term analgesic tolerance in the mouse tail-flick assay through a G protein receptor kinase (GRK)3-dependent, c-Jun N-terminal kinases (JNK)-independent mechanism, whereas acute analgesic tolerance to morphine is JNK-dependent and GRK3-independent. Pretreatment with fentanyl reduced morphine sensitivity and vice versa (Melief et al., 2010). As described elsewhere in this review, it remains to be established whether these mechanistic insights can be used to develop opioid agonists or treatment paradigms that do not produce analgesic tolerance. The striking differences between the tolerance produced by different opioids suggests that rational design of better therapeutic drugs is at least theoretically plausible.
III. Structure and Function of μ-Opioid Receptors
A. Why Focus on μ-Opioid Receptors?
Opioid drugs exert nearly all of their clinically relevant actions through stimulation of MORs. The molecular biology of endogenous opioid peptides and receptors has been extensively reviewed (Evans, 2004). Pharmacologically distinct MORs, δ (DOR)-, and κ (KOR)-opioid receptors are encoded by distinct structural genes with regions of extensive homology, and each opioid-receptor gene encodes a predicted seven-transmembrane G protein-coupled receptor. Specific opioid receptors are expressed in many neuronal populations, with a distribution in the central nervous system (CNS) that closely corresponds to sites of opioid action deduced from the effects of local agonist and antagonist infusion. Morphine binds with highest affinity to MORs encoded by the MOR-1 gene, and all physiologic actions of morphine, including analgesia and tolerance, are absent in MOR1 knockout mice (Matthes et al., 1996; Le Merrer et al., 2009).
B. Primary Structure and Structural Diversity of μ-Opioid Receptors
While genetic knockout studies have definitively established that MORs are encoded by a single structural gene (OPRM1), there is evidence for variation of this genetic structure based on alternatively spliced variants of the receptor mRNA, and a number of polymorphisms may impinge on receptor regulation. Several of the described splice variants (Pasternak, 2001) have no known cellular activity, although other variants have been described that affect the structure of the carboxyl-terminal cytoplasmic tail. These variants can clearly produce functional opioid receptors, and there is reasonably strong evidence that some of these variants are expressed at significant levels in vivo. The MOR1B variant, in particular, has been detected at the mRNA and protein levels and shown to have differential expression in brain relative to the predominant MOR1 isoform (Oldfield et al., 2008).
The splice variants, MOR1C and MOR1D, differ in their endocytic membrane trafficking properties, affecting ligand-dependent regulation of opioid signaling in transfected non-neuronal cells (Koch et al., 1998, 2001, 2006; Oldfield et al., 2008; Tanowitz et al., 2008). There is some evidence that splice variants may also underlie different behaviors. For example, it has been reported that MOR1D mediates morphine-induced scratching, whereas the MOR1 is the only isoform required for morphine-induced analgesia (Liu et al., 2011). However, no convincing evidence for the presence of MOR1C or MOR1D receptors in rat CNS has been reported (Oldfield et al., 2008). Given the confusion over the mere presence of splice variants, except MOR1B, the extent to which different splice variants contribute to tolerance in different populations of neurons is unknown.
There is also evidence for variation in genetic structure of opioid receptors by polymorphisms in the human population. One polymorphism, a single-nucleotide polymorphism producing a single-residue substitution in the amino-terminal extracellular domain (A118G), has been studied in some detail and shown to specifically affect receptor activation by β-endorphin relative to enkephalin (Bond et al., 1998). However, the initial report of this variant affecting β-endorphin potency has not been replicated (Beyer et al., 2004; Kroslak et al., 2007). More consistent has been the finding that the A118G allele results in attenuated MOR expression, although the mutation does not appear to reduce MOR in all brain regions (Wang et al., 2012). An interesting recent study, based on complete exon sequencing, identified a surprisingly high rate of other OPRM1 variants. When studied in cultured cells, one of the variants completely lacked functional activity while others differed in ligand-dependent regulation and membrane trafficking (Ravindranathan et al., 2009). These polymorphisms may contribute to sensitivity to opioids or tolerance development, but this has not yet been determined.
C. Tertiary Structure and Conformational States
X-ray crystal structures of the MOR, DOR, and KOR have been recently reported (Granier et al., 2012; Manglik et al., 2012; Wu et al., 2012), as was a structure of the orphanin FQ receptor (Thompson et al., 2012). All were determined in the presence of bound antagonist or inverse agonist, and in the absence of associated G protein, so likely represent inactive conformations. Opioid receptors are similar in overall helical organization to other GPCRs but possess a remarkably deep ligand-binding pocket, and the MOR crystallizes as a biologically plausible dimer. While the opioid receptor structures already provide detailed information about ligand-binding specificity, it remains to be determined how receptor activation is achieved. Pioneering studies of rhodopsin (Choe et al., 2011) and the β2-adrenergic receptor (Rasmussen et al., 2011; Rosenbaum et al., 2011) provide interesting clues. Briefly, activation of these GPCRs involves displacement of transmembrane domain 6 away from the helical bundle, and extension of the cytoplasmic end of transmembrane domain 5. Studies of the β2-receptor–G protein complex are also beginning to reveal how conformational changes in the receptor are allosterically coupled to those occurring in the Gα subunit, resulting in displacement of its N-terminal α-helical domain and opening of the nucleotide-binding pocket (Chung et al., 2011; Westfield et al., 2011).
Presumably opioid receptor activation involves generally similar conformational changes but, considering the remarkably deep solvent-exposed binding pocket that is characteristic of opioid receptors, one might anticipate interesting surprises in the effects of structurally distinct agonists. The structural flexibility of GPCRs, as a class, suggests the possible existence of expanded selectivity of drug action (Galandrin et al., 2007; Weis and Kobilka 2008; Steyaert and Kobilka 2011). Indeed, there is abundant evidence for “functional selectivity” or “ligand-biased signaling” among opioids (Pineyro and Archer-Lahlou, 2007; Martini and Whistler, 2007; Christie, 2008; Koch and Höllt, 2008; Berger and Whistler, 2010; von Zastrow, 2010). However, the precise structural basis for diversity of functional opioid effects remains to be elucidated.
D. Higher-Order Structure of Opioid Receptors
Another important, but unresolved topic is the higher-order structure of opioid receptors and how receptors are organized in native neurons with other signaling proteins. Crystallized MOR had a twofold symmetrical dimer through transmembrane segments 5 and 6, with a sufficiently large contact area between protomers (>1000 square angstroms) that could potentially stabilize dimers in vivo (Manglik et al., 2012). Functional reconstitution of individual receptors in high-density lipoprotein nanoparticles provides elegant and definitive evidence that opioid receptors can also mediate ligand-induced activation of G proteins as monomers (Kuszak et al., 2009). Some GPCRs form stable oligomers and there is also evidence that other GPCRs form transient oligomers (Hern et al., 2010). Single-particle tracking studies of two GPCRs, the M1 muscarinic receptor (Hern et al., 2010) and formyl peptide receptor (Kasai et al., 2011), indicate that receptor dimers can form and dissociate with remarkably rapid kinetics (∼100 milliseconds to several seconds). Similar approaches have not yet been applied to the MOR so stability of oligomers remains uncertain. A number of reviews on opioid receptor oligomers summarize this evolving area and it will not be discussed further here (Agnati et al., 2003; Smith and Milligan, 2010; Costantino et al., 2012; Stockton and Devi, 2012).
E. Microdomains and Compartmentalization
Another type of organization that has been reported for opioid receptors is the association in compositionally specialized domains of the plasma membrane. Single-particle tracking studies of many membrane-associated receptors suggest that receptors are normally confined in microdomains of the plasma membrane but can “hop” between these confined areas of the membrane (Daumas et al., 2003; Suzuki et al., 2005). Using fluorescence recovery after photobleaching (FRAP), the movement of MORs was found to be agonist-dependent (Saulière-Nzeh et al., 2010). Morphine-bound receptors were more restricted, whereas [d-Ala2, N-MePhe4,Gly-ol]enkephalin (DAMGO)-bound receptors either moved freely or were restricted, possibly to clathrin-coated pits. One hypothesis is that lipid rafts, regions of the plasma membrane that are enriched in sphingolipids and cholesterol, are sites that influence MOR signaling. Reports on the localization of agonist-bound MORs vary. In one study, morphine-bound receptors remained within the rafts but etorphine-bound receptors diffused out of raft domain (Zheng et al., 2008a), but another study found that DAMGO-bound MORs moved into rafts (Gaibelet et al., 2008). The lipid environment can have substantial effects on agonist binding (Lazar and Medzihradsky, 1992) and MOR activity. Cholesterol stabilizes MOR in a high-affinity state (Lagane et al., 2000; Gaibelet et al., 2008; Levitt et al., 2009), but removal of cholesterol has variable actions on the activation of MOR (increases, Huang et al., 2007; decreases, Gaibelet et al., 2008; Zheng et al., 2008a; Levitt et al., 2009). Change in the diffusion of receptors was ligand- and G protein-dependent (Gaibelet et al., 2008; Zheng et al., 2008a) and receptor-arrestin interactions are also dependent on the content of membrane cholesterol (Qiu et al., 2011). Thus, agonist binding and signaling results in changes in the diffusion pattern(s) of MORs. Although there is some confusion as to the precise movement of receptors at the plasma membrane, the specific location of MORs could have a potent influence on the association with effectors and downstream modulators. The dynamic regulation of MORs at the plasma membrane following acute and chronic administration of opioids may therefore direct downstream signaling.
F. Cellular and Subcellular Compartments
MORs localized in different parts of the cell—soma, dendrites, and terminals—have distinctly different functional actions. The activation of receptors localized in the somatodendritic compartment decrease excitability, whereas terminally localized receptors inhibit transmitter release. The inhibition of transmitter release can decrease downstream excitation or result in an indirect excitation through disinhibition of inhibitory transmission (Williams et al., 2001). The greatest amount of work has focused on desensitization, tolerance, and trafficking of MORs located in the somato-dendritic compartment, as these events can be directly observed by standard electrophysiological recording methods. However, studies measuring presynaptic inhibition induced by MORs on terminals differ distinctly from those measuring the postsynaptic regulation of MORs. The most striking difference is the inability to induce acute desensitization of MORs in the presynaptic compartment (Fyfe et al., 2010; Pennock and Hentges, 2011). In addition, most studies report that morphine lacks the ability to induce efficient internalization (Keith et al., 1996, 1998; Sternini et al., 1996). More recently morphine-induced internalization has been reported in dendrites but not cell bodies of nucleus accumbens neurons, suggesting different regulatory mechanisms in different neuronal membrane structures (Haberstock-Debic et al., 2003, 2005). In spite of the inability to induce acute desensitization, repeated or chronic administration of morphine results in substantial tolerance to MOR inhibition of transmitter release (North and Vitek, 1980; Schulz et al., 1980; Williams et al., 2001; Hack et al., 2003; Fyfe et al., 2010). In addition, following chronic morphine treatment, there are many examples of counter-adaptations in transmitter release that normalize transmitter release even in the continued presence of morphine (Ingram et al.,1998; Williams et al., 2001; Hack et al., 2003). The differences in the acute and chronic regulation of pre- and postsynaptic MORs is an interesting mechanistic problem that remains unsolved and is of significant functional relevance.
IV. μ-Opioid Receptor Regulation
A. Desensitization and Tolerance Are Both Associated with Reduction of Functional Receptors
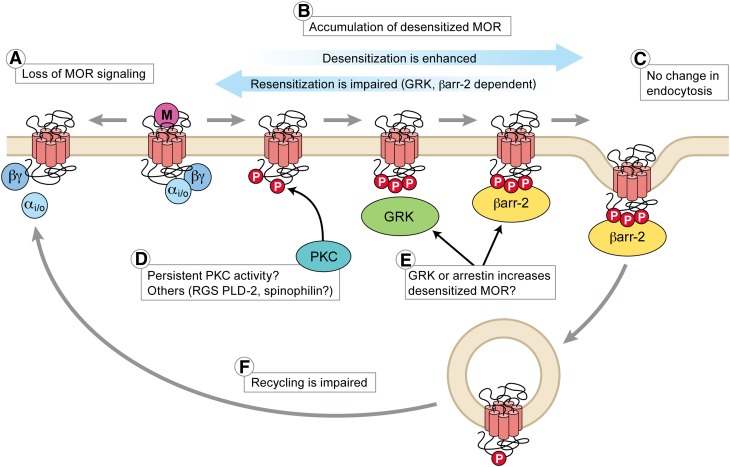
Quantitative models (Operational models or Furchgott analysis) used to quantify the loss of functional MOR-effector coupling associated with rapid desensitization of MOR, short-term tolerance, or long-term tolerance to morphine have all calculated that a loss of 80% or more (up to 95%) of functional surface MOR is required to account for the observed shift in morphine concentration-response curves (Chavkin and Goldstein, 1984; Christie et al., 1987; Osborne and Williams, 1995; Bailey et al., 2009a). Similar estimates of loss of MOR function between these studies might be interpreted to suggest that tolerance at the level of MOR represents nothing more than desensitization. However qualitatively there are important differences. Recovery from rapid desensitization occurs in approximately 1 hour (Harris and Williams, 1991; Dang and Williams, 2004; Virk and Williams, 2008). After long-term treatment with morphine MOR function recovers in two phases. One phase recovers within 2 hours after removal of morphine and is thought to be recovery from desensitization as it is produced by either agonist removal or inhibition of protein kinase C (PKC) (Bailey et al., 2009a). The second component persists for many hours and represents tolerance (Christie et al., 1987). Interestingly, two phases of tolerance reversal have also been observed in vivo on cessation of chronic opioid administration (Cox et al., 1968).
Studies using physiologic end-points (direct Gβγ interactions with ion channels) in single opioid-sensitive neurons have also reported similar impaired MOR-effector coupling in a range of neuronal cell types from animals chronically treated with morphine in vivo, including rat and mouse periaqueductal gray (Bagley et al., 2005b; Ingram et al., 2008), rat and mouse locus coeruleus (Christie et al., 1987; Connor et al., 1999; Dang and Williams, 2004; Bailey et al., 2009a; Dang et al., 2011; Quillinan et al., 2011), and mouse trigeminal ganglion neurons (Johnson et al., 2005). Similar results have also been reported for inhibition of GABAergic synaptic transmission in nerve terminals in periqueductal gray taken from animals treated chronically with morphine (Fyfe et al., 2010; Hack et al., 2003). Importantly, some of these studies showed that the loss of MOR function after chronic opioid treatment was selective because there was no change in the sensitivity to agonists at other GPCRs known to couple to the same effectors (Christie et al., 1987; Connor et al., 1999; Bailey et al., 2009a). These studies have usually used chronic treatment with morphine, but similar results have been reported after chronic methadone treatment in locus coeruleus neurons (Quillinan et al., 2011). Assay of 35S-labeled guanosine-5′-O-(3-thio)triphosphate ([35S]GTPγS) binding to tissue sections, a method for estimating receptor–G protein coupling in situ, indicates that chronic heroin treatment produced pronounced reductions in MOR activity in several brain regions with an upregulation in total opioid receptor number (Sim-Selley et al., 2000).
The mechanisms responsible for loss of functional MORs have not been determined. One process that could, in principle, reduce receptor reserve is receptor downregulation. While some opioids (such as etorphine) can produce substantial downregulation of MORs in vivo (Stafford et al., 2001), chronic morphine produces little net change in most brain regions (Stafford et al., 2001; reviewed by Koch and Höllt, 2008). Thus, a potential role of receptor downregulation in morphine tolerance has been generally discounted. Furthermore, as mentioned above, chronic heroin treatment of rats can reduce agonist-stimulated GTPγS binding while upregulating total MOR number.
A potential limitation of receptor binding studies is insensitivity to redistribution between cellular compartments. A reduction in receptor reserve could occur, in the absence of net reduction in total receptor number detected by typical ligand-binding assays, if there is redistribution of receptors from the plasma membrane to internal membranes. There are a limited number of studies in which the cellular distribution of MORs was examined at the electron microscopic level following the chronic morphine treatment. In the adrenergic neurons of the medulla, chronic treatment with morphine (3 × 75 mg morphine pellets) resulted in a dramatic redistribution of MORs from the plasma membrane to intracellular compartments (Drake et al., 2005). This is an important observation that may be one explanation for the decrease in effector activation that has been reported in physiologic studies discussed above. However, in a similar anatomical study of neurons in the locus coeruleus little or no loss of receptors was found after chronic morphine treatment (Van Bockstaele and Commons, 2001), but acute administration of morphine did induce internalization in the dendrites of neurons in the nucleus accumbens when measured with immuno-electron microscopy (Haberstock-Debic et al., 2003). Thus, depending on the treatment protocol, the selectivity profile of the antibodies used for detection and the neurons under study, the cellular localization of receptors may vary. It remains a significant challenge to develop methods to determine whether surface membrane receptor expression changes after chronic exposure to morphine and the extent to which this is brain-region specific.
B. Biased Agonism and μ-Opioid Receptor Regulation
The literature contains numerous examples of “agonist-selective” regulation of opioid receptors and this has been reviewed previously (Koch and Hölt, 2008; Martini and Whistler, 2007; Piñeyro and Archer-Lahlou, 2007; Raehal et al., 2011; Rajagopal et al., 2011). The concept of agonist selectivity, however, remains confused and the physical basis by which individual opioids produce different regulatory effects is poorly understood. Biased agonism refers to the ability of different agonists to either differentially activate signaling cascades or regulatory events, including differences in receptor trafficking. The concept suggests the formation of different protein complexes (containing signaling proteins, arrestins, GRKs, and other kinases) selected by ligand binding to the receptor, thereby triggering different downstream events. As shown in Fig. 2, biased signaling potentially arises at the level of biased association with different G proteins, phosphorylation by different kinases and interacting proteins (and subsequent endocytosis), and distinct G protein-independent signaling interactions. While the evidence for biased association with different G proteins remains relatively limited for MOR (reviewed by Piñeyro and Archer-Lahlou, 2007; Raehal et al., 2011; Audet et al., 2012) and will not be discussed further here, a large body of evidence suggests that different agonists exhibit bias for G protein interaction versus phosphorylation by different kinases and endocytosis as discussed below.
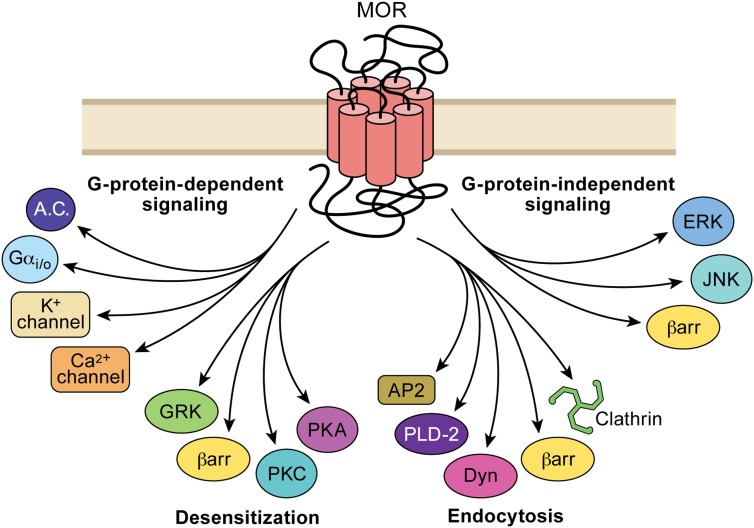
Fig. 2.
Agonist binding to MORs can result in the activation of multiple downstream pathways. Different agonists can selectively activate one or a number of these pathways that give rise to agonist-selective signaling through a single receptor subtype. G protein-dependent processes include the regulation of ion channels and inhibition of adenylyl cylase. Mechanisms involved in desensitization may involve selective activation of one or another kinase dependent pathway. G protein-independent processes, including the steps leading to endocytosis and interactions with scaffolding molecules and kinases, may influence MOR signaling by both direct and potentially indirect mechanisms.
However, the crux of the issue for MOR is whether opioids differ only in their relative efficacy for producing a single biochemical receptor form, or whether opioids can exhibit additional selectivity by supporting the production of functionally distinct biochemical receptor forms. The confusion is compounded by the fact that the ability of morphine to induce endocytosis appears to be different in different cell types and under different experimental conditions. Morphine fails to induce MOR endocytosis in spinal cord in vivo (Trafton and Basbaum, 2004) and locus coeruleus neurons in vitro (Arttamangkul et al., 2008) but quite efficiently induces endocytosis in the dendrites of medium spiny striatal neurons (Haberstock-Debic et al., 2003, 2005; Yu et al., 2009, 2010). Using rectifying potassium channel (FRET) to study arrestin-MOR interactions, Frölich et al. (2011) observed that three morphine metabolites, normorphine, 6-acetylmorphine, and morphine-6-glucuronide, had lower potencies for G protein activation but higher potencies and efficacies for β-arrestin recruitment than morphine itself, suggesting that they are biased toward β-arrestin pathways. Furthermore, some opioid drugs exhibit bias toward recruitment of different isoforms of arrestin. Morphine recruited β-arrestin2, whereas DAMGO recruited both β-arrestin1 and β-arrestin2 in mouse embryonic fibroblasts (Groer et al., 2011). Given that in recombinant expression systems the ability of morphine to induce endocytosis appears to be enhanced by over-expression of GRKs and arrestins (Whistler and von Zastrow, 1998; Zhang et al., 1998; Bohn et al., 2004), one explanation for differences between different neuronal preparations could be that morphine has a low, but not zero, ability to induce endocytosis and that morphine-induced receptor endocytosis becomes apparent if the levels of GRK and/or arrestin expression are higher as might occur in a different areas within the CNS or cellular infrastructure.
In this regard it is important when examining agonists for bias to have a measure of their relative intrinsic efficacies for each response rather than their intrinsic activities, as the latter do not discriminate between full agonists (see Definition of Terms). Using an encyclopedic series of opioid ligands together with multiple measures of ligand-receptor-effector interaction in cell lines, two recent studies correlated agonist efficacy with the ability to induce receptor internalization (McPherson et al., 2010; Rivero et al., 2012). The results demonstrated that for a wide range of agonists including morphine there was a good correlation between their ability to promote receptor activation and β-arrestin2 binding. From these studies, it was suggested that the efficacy (operational efficacy) of many agonists could be used as a predictor of multiple steps leading to receptor internalization. Interestingly, several important outliers, most notably endomorphin-2, produced much greater phosphorylation of Ser375 on MOR, arrestin recruitment, and endocytosis than would have been predicted from its efficacy to induce GTPγS binding. By contrast, a similar study by Molinari et al. (2010) reported a hyperbolic relationship between intrinsic activity of opioid agonists for G protein activation and β-arrestin2 translocation, potentially suggesting significant ligand bias. In addition, another study by Borgland et al. (2003) reported that the intrinsic signaling efficacy measured using the inhibition of calcium current by some agonists, most notably morphine, did not predict efficacy for endocytosis. One obvious difference between the study by Molinari et al. (2010) and that of McPherson et al. (2010) is in the method of data analysis. Molinari et al. (2010), but not Borgland et al. (2003), used the maximum of the concentration-response curve to estimate relative intrinsic activity, whereas McPherson et al. (2010) estimated relative intrinsic efficacy by applying an operational model to the concentration-response curves. Intrinsic activity is by definition the same for all full agonists, which would produce a hyperbolic relationship. While under the appropriate conditions, resonance energy transfer could measure intrinsic efficacy, the FRET-based study focused on intrinsic activity (Molinari et al., 2010). The intrinsic activity estimates obtained from the FRET measurements showed a good correlation with those obtained from parallel GTPγS studies, which measured intrinsic activity, not intrinsic efficacy. Other possible explanations for the discrepancy could be the different methods used to determine G protein activation and arrestin translocation [GTPγS binding and an arrestin complementation assay by McPherson et al. (2010), resonance energy transfer by Molinari et al. (2010)] and potentially different expression levels of the modified proteins used in the two different assay systems.
As one means to address the question of proportionality between the signaling and regulatory effects of opioids, a simple ratio of the two processes was proposed. Estimates of efficacy for eliciting receptor-mediated signaling as a function of the ability to promote receptor endocytosis was called “RAVE” (for “relative activation versus endocytosis”). According to this, opioids that drive receptor signaling and endocytosis in direct proportion would have an identical RAVE value, whereas those falling off the correlation line would have a higher or lower RAVE value. It was also proposed that agonists with high RAVE values (those compounds that do not induce efficient internalization) may have increased potential to produce opioid tolerance and dependence (Whistler et al., 1999; Finn and Whistler, 2001). The RAVE hypothesis has heuristic value but does not offer any advantage over more formal correlation analyses of intrinsic efficacy as described above. Further, different RAVE values do not account for many aspects of opioid action, and the exact values obtained are dependent both on the cell type and signaling pathway that is examined. For example, both morphine and DAMGO have high efficacy in inhibition of calcium channels in dorsal root ganglion neurons, whereas DAMGO but not morphine has high efficacy for p38 mitogen-activated protein kinase activation that regulates endocytosis in these neurons (Tan et al., 2009). Estimates of agonist efficacy to determine RAVE values will be dependent on the effector under study. In addition, secondary actions of agonists such as the block of potassium channels by high concentrations of methadone will affect accurate determinations of efficacy (Rodriguez-Martin et al., 2008; Matsui and Williams, 2010). Likewise, the development of tolerance varies considerably with the assay. Finally, a major limitation of the RAVE hypothesis is that it equates endocytosis with tolerance. Recent studies have documented that MORs can be desensitized without undergoing endocytosis and that endocytosis initiates other signaling events (for example, ERK-kinase activation). A possible relationship between endocytosis, tolerance, and addiction risk is still being resolved (Berger and Whistler, 2010).
Agonist-selective effects have even been observed using the same peptide agonist, dermorphin, linked to two different fluorescent ligands. Dermorphin is an opioid peptide agonist originally isolated from frog skin (Erspamer et al., 1981) that is potent and highly selective for MORs. The properties of this peptide agonist were markedly changed with the conjugation of two different fluorescent molecules (Arttamangkul et al., 2000). The more hydrophobic, dermorphin-Bodipy Texas Red (derm-BTR) molecule was not a full agonist when measuring the activation of potassium currents in locus coeruleus neurons but was very efficient at causing internalization in HEK293 cells. Dermorphin–Alexa 488, on the other hand, was a full agonist but did not result in marked internalization (Arttamangkul et al., 2000; Alvarez et al., 2002). Thus, by changing the physicochemical properties of a single agonist a marked difference in “agonist-selective” properties were obtained.
Taken together, these studies make a strong case for biased signaling for different MOR agonists between G protein activation and arrestin recruitment, at least for some agonists. This has been reconsidered recently with a systematic analysis of a more limited set of agonists using the operational model to define operational efficacy for a range of end-points that reflect G protein activation or arrestin translocation (Rivero et al., 2012). A spectrum of signaling bias was demonstrated for different MOR agonists as shown in Fig. 3.
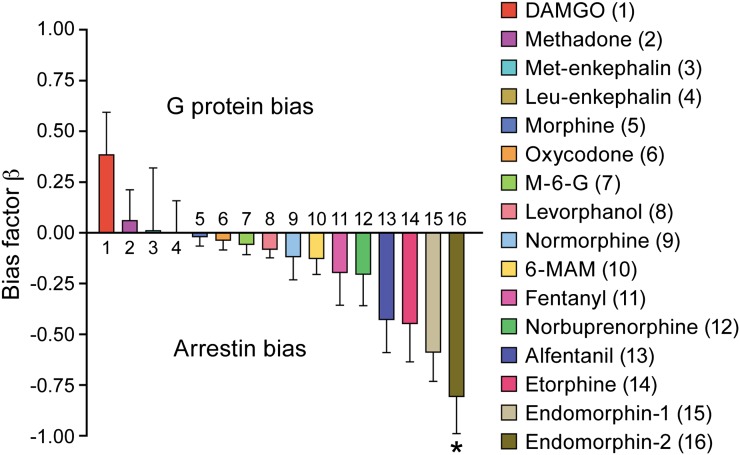
Fig. 3.
Ligand bias at MOR. The intrinsic efficacies (operational model) of a range of structurally dissimilar MOR agonists to activate [35S]GTPγS binding and arrestin recruitment was determined and the bias factor (β) calculated according to the method of Rajagopal et al. (2011). Reproduced from Rivero et al. (2012).
The idea that distinct ligands can selectively affect multiple receptor states is not unique to opioids. Indeed, biased agonist effects have been reported independently for a number of GPCRs (Gay et al., 2004; Kenakin, 2011). For example, in studies of the β2-adrenergic receptor, an inverse agonist not only suppressed the constitutive activity of the receptors but also activated MAPK via a β-arrestin2-dependent mechanism (Azzi et al., 2003). Also, fluorescence lifetime measurements suggested that agonists differ in their ability to induce or stabilize different conformational transitions upon binding to the receptor in vitro (Swaminath et al., 2005). Moreover, receptors purified from intact cells were found to exist in a complex mixture of phosphorylated forms, with agonist-selective effects on phosphorylation of a region in the cytoplasmic tail that controls desensitization and endocytosis (Trester-Zedlitz et al., 2005). There is accumulating evidence for biased phosphorylation and subsequent events such as arrestin binding and MAPK (ERK1/2, JNK, p38) activation (reviewed by Bruchas and Chavkin, 2010). There is also evidence supporting the existence of multiple phosphorylated forms of MOR in intact cells, and pronounced differences in the phosphorylation of MORs in response to morphine and peptide agonists such as DAMGO and endomorphin 2 (Yu et al., 1997; McPherson et al., 2010; Grecksch et al., 2011, Lau et al., 2011; Rivero et al., 2012). However, it remains unknown whether observed differences in the functional regulation of MOR, or other GPCRs, result specifically from agonist-selective stabilization of discrete receptor conformations. The following sections review what is known about these regulatory mechanisms for MOR and their potential impact on differential functional outcomes, including biased desenstization and tolerance.
V. Phosphorylation and μ-Opioid Receptor Regulation
The mechanisms that terminate MOR-G protein signaling following exposure to highly efficacious agonists for several minutes (rapid desensitization) are still not thoroughly understood. Phosphorylation of specific residues in the intracellular domains of MOR is widely accepted to precede and perhaps cause desensitization. It is not yet known whether phosphorylation per se desensitizes G protein activation and signaling or it produces desensitization by initiating β-arrestin (and other protein) binding that sterically occludes access of G proteins to MOR. Coexpression studies in Xenopus laevis oocytes showed that the desensitization of MOR activation of Kir3 currents required both GRK and arrestin expression (Kovoor et al., 1997) suggesting that receptor phosphorylation was not sufficient in this functional assay system. MOR phosphorylation may also contribute to both short-term and long-term tolerance (see below). Many of these observations were made using expression studies in cell lines and primary neuronal cultures, but their importance in native neurons and whether differences are dependent on neuronal compartments are not yet well established. An emerging concept is that some agonists such as DAMGO regulate MOR by selectively engaging GRK-arrestin mechanisms, while others such as morphine selectively engage non-GRK-arrestin mechanisms. The consequences of such differential regulatory process for tolerance are still not well understood but are likely to be the basis for biased agonism for arrestin recruitment.
As shown in Fig. 4, phosphorylation of around 20 potential sites in the intracellular regions of MOR could contribute to receptor desensitization and endocytosis, particularly to putative GRK phosphorylation sites near the C-terminal (reviewed in Connor et al., 2004; Koch and Höllt, 2008) and are summarized below. MORs are also phosphorylated by non-GRK kinases such as JNK, PKC, protein kinase A, calcium-calmodulin kinase (CaMKII), and MAPK (reviewed in Liu and Anand, 2001; Koch and Höllt, 2008; Fig. 5), which may contribute to both heterologous and homologous desensitization of receptors as well as tolerance. It is unclear whether each of these kinases acts directly on MOR or whether they act sequentially. Some kinases may phosphorylate other non-kinase proteins [e.g., regulator of G protein signaling (RGS) proteins] that are involved in MOR desensitization.
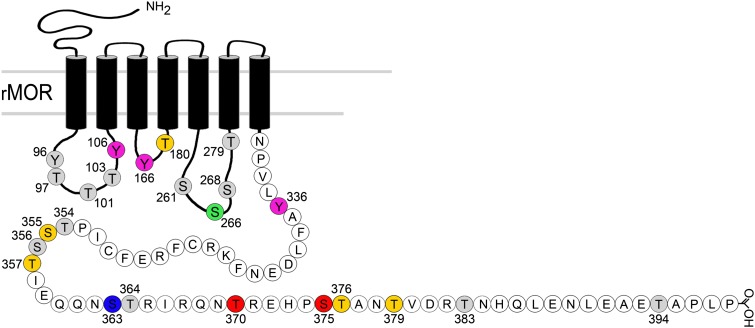
Fig. 4.
Summary of putative (shaded) and established agonist-induced phosphorylation sites on the MOR and associated kinases. There is moderate to strong evidence for the colored residues as follows: Tyr106, Tyr166, probably phosphorylated by tyrosine kinase (McLaughlin and Chavkin, 2001); Thr180, probably phosphorylated by GRK3 (Celver et al., 2001); Ser266, probably phosphorylated by CaMKII (Koch et al., 1997, 2000); Tyr336, phosphorylated by Src kinase (Zhang et al., 2009); Ser355, Thr357, one or both phosphorylated (Wang et al., 2002; Lau et al., 2011) by GRK2 (Wang 2000); Ser363, constitutive phosphorylation (Doll et al., 2011) by PKC (Feng et al., 2011); Thr370, Ser375, phosphorylation directly shown (Schulz et al., 2004; Doll et al., 2011); Thr376, Thr379, one or both phosphorylated (Lau et al., 2011); Thr383, Thr394, phosphoylation was predicted but not directly shown (Pak et al., 1997), and not observed (El Kouhen et al., 2001; Lau et al., 2011).
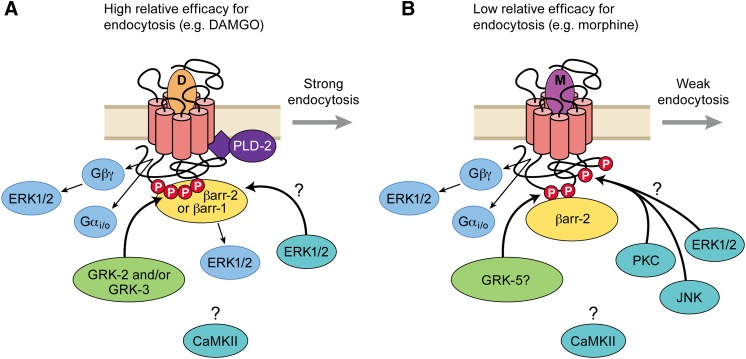
Fig. 5.
Summary of MOR phosphorylation and enzyme interactions leading to desensitization and endocytosis. (A) For agonists with high relative efficacy for endocytosis, G protein dissociation and conformational changes favorable to GRK phosphorylation drive desensitization and endocytosis. GRK2 and GRK3 appear to be the major isoforms involved (see Desensitization and Tolerance Are Both Associated with Reduction of Functional Receptors). Arrestin binding requires GRK phosphorylation, and both β-arrestin2 and β-arrestin1 can interact with MOR to promote endocytosis (see Biased Agonism and μ-Opioid Receptor Regulation; Groer et al., 2011). It is not certain if phosphorylation events, β-arrestin binding, or both produce uncoupling of MOR signaling (desensitization), but the time course appears to follow arrestin binding more closely. Strong internalizing agonists activate phospholipase D2 (Section V.G), but it is not certain whether this is required for endocytosis (Arttamangkul et al., 2012). There is some evidence that ERK1/2-dependent mechanisms may desensitize MOR by both arrestin-independent (Gβγ) and arrestin-dependent mechanisms. Other kinases may also be important, including CAMKII and PKC. GRK phosphorylation is rapidly reversible at the cell surface, but the rate of reversal at other potential phosphorylation sites or their requirements for endocytosis is unknown (see Phosphorylation and μ-Opioid Receptor Regulation). (B) Agonists with relatively low efficacy for endocytosis weakly and slowly phosphorylate GRK substrates on MOR and induce weak association with β-arrestin2 (see Desensitization and Tolerance Are Both Associated with Reduction of Functional Receptors and Biased Agonism and μ-Opioid Receptor Regulation). However, there is good evidence that PKC-dependent mechanisms, possibly via direct phosphorylation of MOR at Thr370 (Doll et al., 2011), contribute to desensitization by agonists such as morphine (Section V.D). PKC also appears to recruit JNK-dependent desensitization mechanisms for agonists such as morphine but not for high endocytosis efficacy agonists (Melief et al., 2010). It is tempting to speculate for agonists with low efficacy for endocytosis that PKC and other kinases can readily interact with the intracellular domain of MOR when it is not occluded either by G proteins or arrestins. Whether these events are rapidly reversible (as is GRK dephosphorylation) is not known.
A. Phosphorylation by G Protein Receptor Kinase
GRK-mediated phosphorylation of GPCRs is thought to require a ligand activated conformation of the receptor because GRK2/3 requires Gβγ activation, and GαgdpGβγ binding to the receptor may sterically reduce access to the receptor phosphorylation sites (Krupnick and Benovic, 1998). Phosphorylation of MOR by GRK results in binding of nonvisual β-arrestin or β-arrestin2 and leads to homologous receptor desensitization. Multiple isoforms of GRK have been identified (Premont and Gainetdinov, 2007), and both GRK2 and GRK3 have been implicated in MOR desensitization in vitro (Kovoor et al., 1998; Zhang et al., 1998). The GRK2 knockout is embryonic lethal, whereas GRK3 knockout mice are viable (Peppel et al., 1997), and homozygous GRK3−/− mice show reduced analgesic tolerance to some opioid agonists in certain assay paradigms (Terman et al., 2004; Melief et al., 2010). Although GRK3 appears to mediate acute fentanyl-induced antinociceptive responses in the hotplate and tail-flick assays, the GRK2 isoform may be involved in MOR regulation in other neural circuits (Gabra et al., 2008; Bailey et al., 2009b; Dang et al., 2011). However, resolving the relative contributions of GRK2 or GRK3 is complicated by the lack of confirmation of the selectivity of the genetic knockdown experiments.
Phosphorylation of MOR at putative GRK sites is rapid (Doll et al., 2011), apparently saturating within 1–2 minutes (Thr370, Ser375) when MORs are stimulated by highly efficacious peptide agonists, such as DAMGO. The time course of β-arrestin2 association and rapid desensitization are slower than this, reaching saturation in 3–5 minutes (Oakley et al., 2000; McPherson et al., 2010; Molinari et al., 2010). This is a general paradigm by which GPCRs are functionally uncoupled from heterotrimeric G proteins and then endocytosed within minutes after receptor activation (Carman and Benovic, 1998; Goodman et al., 1998). Endocytosis appears somewhat slower than desensitization with time constants generally in the order of ≥5 minutes and reaching steady state in less than 30 minutes (Law et al., 2000; Borgland et al., 2003; Tanowitz and von Zastrow, 2003; Arttamangkul et al., 2006, 2008; Johnson et al., 2006; Tanowitz et al., 2008). The earliest studies of MOR phosphorylation established that the receptor is phosphorylated more efficiently following activation by high-efficacy agonists, such as DAMGO or etorphine, than by low-efficacy agonists such as morphine in cell lines (Yu et al., 1997; Zhang et al., 1998; Whistler et al., 1999; Schulz et al., 2004; Johnson et al., 2006). Although morphine weakly stimulates the phosphorylation of MOR, this is greatly enhanced by overexpression of GRK2 (Zhang et al., 1998). This could reflect distinct phosphorylation patterns or simply differences in overall efficacy to mobilize G proteins and phosphorylate the same residues. This issue has not yet been resolved. The relative importance of particular residues has been approached by site-directed mutagenesis of single residues or groups of residues, development of specific phosphosite antibodies and, more recently, mass spectrometric analyses of phosphorylated residues. These studies are beginning to indicate that agonists that strongly promote arrestin binding very efficiently phosphorylate clusters of residues in the C-terminal region of the receptor in the vicinity of Thr370 through Thr379.
Lower efficacy agonists, notably morphine, quite inefficiently phosphorylate some residues in the same C-terminal region when compared with DAMGO (Doll et al., 2011; Lau et al., 2011). For example, in these studies morphine-induced phosphorylation of Thr370 was either undetectable (using a phosphopeptide antibody) or observed at relatively low levels (by LC-MS). Morphine was particularly weak in its ability to promote multisite phosphorylation within the Thr370 through Thr379 region. Overall, it appears from these studies that residues in the middle region of the C-terminal tail are important GRK phosphorylation sites and function in the control of receptor internalization. Thr394, in the distal C-terminal region, may also be phosphorylated and its mutation affects internalization (Wolf et al., 1999). A direct correlation between the efficiency of receptor phosphorylation and an agonist’s efficacy is consistent with evidence suggesting that Gβγ is a cofactor necessary for maximal GRK activation (DebBurman et al., 1996), although it is also likely that differences in agonist-induced conformational changes affect the accessibility of specific phosphorylation sites in the receptor.
Construction of truncated MORs (Burd et al., 1998; Qiu et al., 2003) and site-directed mutagenesis (El-Kouhen et al., 2001) have also established that two major clusters of serine and threonine residues in the C-terminal region of MOR are likely to be GRK targets when expressed in cultured cells. Additional support for GRK phosphorylation of these sites comes from methods that measure pan-phosphorylation combined with disruption of β-arrestin2 translocation to the surface membrane and/or disruption of endocytosis after exposure to highly efficacious agonists such as DAMGO or etorphine. El-Kouhen et al. (2001) largely eliminated DAMGO-induced pan-phosphorylation when all serines and threonines from Ser363 to Thr376 were mutated to alanines. Inclusion of T394A to these residues produced a small additional effect. The most effective point mutation for reducing pan-phosphorylation was Ser375. This also had the largest impact on reducing the rate of DAMGO-induced endocytosis suggesting that Ser375 may be a crucial residue for GRK phosphorylation, arrestin recruitment, and endocytosis. Similar results using the S375A mutant were reported by Schulz et al. (2004). Lau et al. (2011) further verified this result, and found that mutations of other sites (Thr376 and Ser379) also inhibited arrestin recruitment and endocytosis. This suggests that Ser375 functions as part of a motif (375STANT379) whose multisite phosphorylation is required for efficient receptor internalization. Similar mutational approaches have also implicated Thr394 (Wolf et al., 1999; Deng et al., 2000) and Ser355/Thr357 (Wang et al., 2002) in DAMGO-induced pan-phosphorylation of MOR. Attempts to understand the involvement of these sites in desensitization are complicated because long assay durations may include the elimination of receptors through endocytosis. Indeed the same studies did show that mutations disrupting phosphorylation also disrupted endocytosis of MOR.
These mutational studies have established potential agonist-induced phosphorylation sites, but thorough understanding of phosphorylation patterns is still developing and will not be resolved until direct phosphorylation of specific residues is established. Phosphosite-specific antibodies have provided some information. Schulz et al. (2004) demonstrated that DAMGO induces robust phosphorylation of Ser375 in MOR-transfected HEK293 cells and primary neuronal cultures using a phospho-Ser375-specific antibody. Morphine weakly induced phosphorylation of this site but it was enhanced by overexpression of GRK2. Phosphospecific antibodies have also been used to detect MOR phosphorylation at Thr370 and Ser375 following sustained release of endogenous β-endorphin that was associated with morphine tolerance in vivo (Petraschka et al., 2007). More recently, Doll et al. (2011) have developed phospho-antibodies for Ser363, Thr370, and Ser375 and demonstrated that in transfected HEK293 cells DAMGO very rapidly (less than 2 minutes at 37°) phosphorylated Ser375, but morphine phosphorylated the same site more slowly. DAMGO also efficiently phosphorylated Thr370, but morphine did not. Notably, in their HEK293 cell line, Ser363 was constitutively phosphorylated but showed no increase with opioid agonists, and Thr370 could be phosphorylated by stimulation of PKC activity (see below) in addition to DAMGO. By use of GRK overexpression and knockdown methods in HEK293 cells, the same group recently reported DAMGO-induced phosphorylation of Thr370 and Ser375 is catalyzed by GRK2 and GRK3, but morphine-induced phosphorylation of Ser375 is predominantly mediated by GRK5 (Doll et al., 2012). Phosphorylation of Ser375 was also examined by McPherson et al. (2010) for a wide range of opioids in parallel with GTPγS activation assays and β-arrestin2 recruitment. There was a weak positive relationship between G protein activation and Ser375 phosphorylation and a stronger correlation with arrestin translocation and endocytosis. However there were notable outliers such as endomorphin-2, which is a relatively weak G protein activator but very effectively stimulates the phosphorylation of Ser375. So far only Ser375 phosphorylation has been shown to occur in vivo in intact mouse brain in an agonist-dependent manner (Grecksch et al., 2011).
These studies suggest biased agonism of GRK phosphorylation and consequent arrestin translocation at MOR, but the information will remain limited until more complete patterns of phosphorylation by different agonists are known. It is well established that overexpression of GRKs or arrestins can profoundly enhance induction of endocytosis by morphine (Whistler and von Zastrow, 1998; Zhang et al., 1998; Bohn et al., 2004; Doll et al., 2012), so it may be the case that morphine more weakly stimulates phosphorylation of the internalization-controlling middle portion of the C terminal receptor tail consistent with its weak efficacy to activate G proteins. However, the effects of agonists such as endomorphin-2 and to a lesser extent endomorphin-1 (McPherson et al., 2010; Rivero et al., 2012) are not consistent with this interpretation because they have a G protein-signaling efficacy comparable to morphine but phosphorylate Ser375 (and translocate arrestin) as efficiently as the most efficacious agonists (e.g., DAMGO). One possibility, because efficient endocytosis also requires MOR phosphorylation at Thr376 and/or Thr379 (Lau et al., 2011), is that endomorphins are less efficacious for producing multisite phosphorylation involving these additional residues. Further development of multiple phosphosite-specific antibodies, as well as application of mass spectrometry–based approaches (Feng et al., 2011; Lau et al., 2011), have the potential to elucidate agonist-biased phosphorylation of opioid receptors in vitro and in vivo. Immunohistochemical analyses using brain tissue will also be important to determine whether phosphorylation patterns vary in opioid-sensitive neurons throughout the nervous system because arrestin-dependent endocytosis by low-efficacy agonists is known to be nonuniform. Morphine fails to induce MOR endocytosis in spinal cord in vivo (Trafton and Basbaum, 2004) but it efficiently induces endocytosis in medium spiny striatal neurons (Haberstock-Debic et al., 2003, 2005; Yu et al., 2010).
B. G Protein Receptor Kinase Phosphorylation, Arrestin Binding, and Desensitization
Many studies indicate that the rapid desensitization of MOR function induced by agonists such as DAMGO that strongly induce GRK phosphorylation, arrestin translocation, and endocytosis is greatly attenuated when GRK or arrestin function is disrupted. Although this was suggested by many early studies, the time course of measurements (many minutes to hours) could not distinguish rapid desensitization from the loss of function produced by endocytosis. In X. laevis oocyte gene expression studies, homologous desensitization (without endocytosis) of MOR activation of Kir3 potassium currents was readily observed to require coexpression of both GRK3 and β-arrestin2 (Kovoor et al., 1997). More recently, Johnson et al. (2006) blocked rapid DAMGO-induced (but not morphine) desensitization in HEK293 cells using a dominant negative GRK2 mutant. Chu et al. (2008) reported that DAMGO-induced rapid desensitization (but not morphine) was blocked in mouse embryonic fibroblast (MEF) cells from β-arrestin2 or β-arrestin1 and 2 knockout mice. Li and Wang (2001) reported that intracellular perfusion of rostral ventromedial medulla neurons with a GRK2 inhibitory peptide blunted DAMGO-induced desensitization. Bailey et al. (2009a) also reported that in vivo viral expression of a dominant negative mutant GRK2 in locus coeruleus neurons attenuated DAMGO-induced rapid desensitization (but not morphine; however, see Quillinan et al., 2011). In a GRK3 knockout mouse, Terman et al. (2004) reported that desensitization of fentanyl-induced population spike facilitation in hippocampal dentate gyrus was greater than that produced by morphine. Together, these studies suggest that desensitization for agonists that strongly promote GRK-dependent phosphorylation and arrestin binding is blunted when these pathways are blocked, but agonists that do not engage this mechanism efficiently (notably morphine) still produce MOR desensitization.
Other studies have reported that efficient desensitization of MOR by highly efficacious agonists persists when GRK phosphorylation, arrestin binding, or endocytosis are disrupted. Walwyn et al. (2007) showed that DAMGO-induced desensitization of MOR coupling (Gβγ-mediated) to voltage-gated calcium current inhibition in sensory neurons was unaffected in the β-arrestin2 knockout and this was substantiated in locus coeruleus neurons from the knockout mice (Arttamangkul et al., 2008). More recent studies in locus coeruleus neurons established that desensitization induced by ME can be mediated by at least two distinct mechanisms independently involving ERK1/2 activity and GRK2–β-arrestin2 (Dang et al., 2009). Blocking the GRK mechanism alone with a GRK2 inhibitory peptide or using a β-arrestin2 knockout was not sufficient to inhibit desensitization, nor did ERK1/2 inhibition alone prevent desensitization. However, blocking both processes nearly abolished MOR desensitization. Another study testing a combination of kinase inhibitors, including staurosporine and heparin, observed that acute desensitization to ME persisted, although there was a significant qualitative change in the pattern of receptor trafficking (Arttamangkul et al., 2012). The reason for persistence of desensitization in these studies when others examining the same neurons have observed greatly attenuated desensitization is not known. However, as discussed further below, there is evidence that highly efficacious MOR agonists can engage other mechanisms of phosphorylation and desensitization.
C. Phosphorylation-Independent Actions of G Protein Receptor Kinase
Raveh et al. (2010) suggested that ME-induced MOR desensitization resulted from sequestration by GRK of the free Gβγ subunits required to activate the downstream signaling through GIRK channels in HEK293 cells. However this does not explain why overexpression of a GRK dominant negative reduced DAMGO desensitization (Johnson et al., 2006; Bailey et al., 2009b). Nor does it explain why arrestin expression was required for desensitization of DAMGO-activated Kir3 currents (Kovoor et al., 1997). GRK sequestration of free G protein βγ subunits would occur downstream of the receptor and is likely to result in heterologous desensitization with other GPCRs utilizing the same effector pathway. However, the majority of studies of MOR desensitization have reported only homologous desensitization (Harris and Williams, 1991; Fiorillo and Williams, 1996; Connor et al., 1999; Bailey et al., 2004, 2009a,b; Dang et al., 2009, 2011). There are examples in the literature where MOR agonists induce heterologous desensitization (Blanchet and Lüscher, 2002; Blanchet et al., 2003; Tan et al., 2003, 2009; Walwyn et al., 2006) and the sequestration of Gβγ subunits could be a possible mechanism. However, it should be noted that the heterologous desensitization reported in some of these studies involved incubation with agonists for up to 24 hours (Walwyn et al., 2006), which would be defined here as short-term tolerance. The Gβγ/GRK2 sequestration is an unlikely mechanism to explain heterologous desensitization in dorsal root ganglion cells because heterologous desensitization or “short-term tolerance” is not observed in the absence of β-arrestin2 or blockade of p38 mitogen-activated protein kinases (Tan et al., 2009).
D. Role of Protein Kinase C in Phosphorylation and Desensitization
There has been considerable interest in the role of PKC phosphorylation in the regulation of MOR by morphine (Kelly et al., 2007) since the demonstration that morphine-induced MOR desensitization but not DAMGO-induced desensitization is reduced by PKC inhibition in HEK293 cells (Johnson et al., 2006) and that activation of PKC enhances the rapid desensitization induced by morphine and ME (but not DAMGO) in native locus coeruleus neurons (Bailey et al., 2004, 2009a). With regard to the role of PKC phosphorylation in MOR desensitization, a number of separate but related questions still need to be answered. Can PKC phosphorylate MOR? Does agonist activation of MOR induce PKC phosphorylation of MOR? Does PKC phosphorylation of MOR induce desensitization? Does PKC phosphorylate and thus inhibit other components of MOR signaling downstream of the receptor? While a number of studies by various groups have already attempted to address these questions (see below), the picture still remains somewhat confused.
Use of phosphosite-specific antibodies and matrix-assisted laser desorption-ionization time-of-flight mass spectrometry analyses have established that PKCε can directly phosphorylate Ser363 in C-terminal constructs of MOR in vitro (Feng et al., 2011). The same authors also used a phospho-Ser363 antibody to show that stimulation of PKC in intact CHO cells phosphorylated MOR on Ser363. In contrast, studies using phosphosite antibodies by Doll et al. (2011) in HEK293 cells found that PKC stimulation phosphorylates MOR at Thr370 but Ser363 was constitutively phosphorylated. Although HEK293 cells express a range of PKC isoforms (Atwood et al., 2011), the variant responsible for phosphorylation was not identified by Doll et al. (2011). Johnson et al. (2006) observed that PKC inhibition decreased the basal level of MOR phosphorylation in HEK293 cells, implying that the receptor is prephosphorylated by PKC rather than in response to agonist activation.
Both PKC-mediated homologous and heterologous desensitization of MOR have been reported in different cell types. In locus coeruleus neurons, the PKC-enhanced rapid desensitization by ME was homologous (Bailey et al., 2004) and even after 6 hours exposure to morphine in vitro the desensitization was homologous (Bailey et al., 2009a). In both cases the α2-adrenoceptor-dependent current was not reduced by opioid exposure (Bailey et al., 2004, 2009a,b) indicating no loss of the ability of the effector ion channel (GIRK) to signal. By contrast, activation of PKC by morphine acting on MOR appears to induce heterologous desensitization of CB1 cannabinoid receptors expressed in HEK293 cells (Chu et al., 2010). Chu et al. (2010) reported that PKCε activation and translocation was required for morphine-induced but not DAMGO-induced desensitization of MOR function in HEK293 cells. Morphine (but not DAMGO) induced PKCε-dependent phosphorylation of Giα2 in HEK293 cells that was abolished when the PKC phosphorylation sites on the Giα2 were mutated to alanines (Chu et al., 2010). These findings in HEK293 cells suggest that in this cell type involvement of PKC activation in morphine-induced desensitization may involve phosphorylation of other proteins in addition to direct phosphorylation of MOR. This is consistent with the earlier finding (Chu et al., 2008) that inclusive mutations of Ser363, Thr370, and Ser375 on MOR had little or no effect on morphine-induced desensitization.
While it is difficult to reconcile the differences between the studies in locus coeruleus neurons with those in HEK293 cells, one possible explanation may relate to the MOR-effector coupling studied in each cell type. In locus coeruleus neurons where desensitization appears to be homologous, Gβγ subunit activation of GIRK was used as the read out of receptor activation (Harris and Williams, 1991; Osborne and Williams, 1995; Fiorillo and Williams, 1996; Connor et al., 1996; Alvarez et al., 2002; Bailey et al., 2004, 2009b). The experiments on HEK293 cells by Chu et al. (2008) measured the potentiation of ADP stimulation of intracellular calcium release. The mechanism by which this is produced remains obscure (Samways and Henderson, 2006) but could be sensitive to modification of the Gα subunit.
E. c-Jun N-Terminal Kinase
Similar to other studies showing that PKC-dependent processes are more closely associated with morphine-induced MOR desensitization, Melief et al. (2010) reported that JNK inhibitors attenuated morphine-induced acute short-term analgesic tolerance but not the fentanyl-induced short-term tolerance (the latter was sensitive to GRK3 knockout). Furthermore, morphine-induced short-term tolerance was absent in JNK-2 knockout mice. The JNK mechanism for acute analgesic tolerance was shared by buprenorphine and morphine-6-glucuronide (agonists that do not strongly recruit GRK-arrestin) but not by fentanyl or oxycodone. The mechanism of JNK activation by morphine-like opioids was not fully defined, but agonist increases in phospho-JNK were blocked by the selective PKC inhibitor Gö6976 [12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole], suggesting that PKC activation was required (Melief et al., 2010). Details of this regulatory mechanism are still to be defined, however it is interesting to suggest that activation of PKC may cause JNK-dependent receptor inactivation through a GRK-arrestin–independent pathway.
There are some differences in the literature that may depend on assay conditions. Oxycodone (like morphine) very weakly recruited GRK-arrestin in transfected HEK293 cells in one study (McPherson et al., 2010). Oxycodone was able to efficiently evoke MOR internalization in HEK293 cells (Melief et al., 2010), but not in locus coeruleus neurons in Flag-MOR transgenic mice (Arttamangkul et al., 2008). Oxycodone did not induce any desensitization in locus coeruleus neurons, whereas acute analgesic tolerance was profound in wild type mice and blocked in the GRK3-knockout mice (Melief et al., 2010). These apparent discrepancies are likely a consequence of differences in the cell types and responses measured. For example, the relationship between actions of oxycodone on single cells in the locus coeruleus and the analgesic actions in vivo is not clear and differences in cell-type specific receptor regulation are plausible. One additional explanation that may account for different results is that the metabolism of oxycodone to oxymorphone may be more efficient in vivo than in brain slices. Oxymorphone causes desensitization but causes little or no internalization. A second confounding action of oxycodone could be that it is an agonist of KOR; however, there are conflicting reports. The antinociceptive action of oxycodone with i.c.v. or i.t. administration was blocked after pretreatment with nor-binaltorphimine dihydrochloride (norBNI) (Ross and Smith, 1997). In contrast, analgesia produced by systemic administration of oxycodone was blocked by naloxone but not nor-binaltorphimine dihydrochloride (Lemberg et al., 2006). In addition, oxycodone produced profound analgesia in the tail-flick assay (10 seconds latencies), whereas KOR agonists only produce 2–3-second increases in latencies, suggesting a dominant role of MORs in this assay (Melief et al., 2010). Interpretation of analgesic tolerance mechanisms is complicated for drugs affecting multiple receptors simultaneously. Additional studies are required to fully understand these molecular mechanisms of MOR regulation that underlie acute analgesic tolerance, but the current results suggest that opioids can either use a GRK-arrestin–dependent mechanism or a PKC-JNK dependent mechanism to produce MOR desensitization and short-term analgesic tolerance.
F. Extracellular Signal-Regulated Kinase
Opioid activation of the ERK1/2 signaling pathway has been studied extensively in vitro and in vivo following chronic morphine administration. Macey et al. (2006) found that acute administration of morphine does not activate ERK1/2, whereas fentanyl activates ERK1/2 in a β-arrestin-dependent manner. These results along with the observation that inhibition of ERK1/2 together with GRK-arrestin mechanisms blunts rapid desensitization of MOR in locus coeruleus neurons suggests that ERK1/2 mechanisms may be important for regulation of MOR (Dang et al., 2009). MOR desensitization, internalization, and phosphorylation have all been reported to be prevented by ERK1/2 inhibition in some heterologous expression systems (Polakiewicz et al., 1998; Schmidt et al., 2000). As is likely for the initial events of the GRK-β-arrestin2 interaction, signaling by ERK1/2 may prevent coupling of MOR to effectors by phosphorylation of MOR at sites not occupied by Gα-subunits (Schmidt et al., 2000). Alternatively, ERK1/2 may act indirectly to mediate desensitization via phosphorylation of Gα-interacting protein (GAIP), a regulator of G protein signaling (RGS), by potentiating the rate of GTP hydrolysis, as has been reported in some cell types (Ogier-Denis et al., 2000). It is also clear that morphine induces a different pattern of ERK1/2 activation from DAMGO (Zheng et al., 2008b) that might involve PKC. In several cell lines, ERK1/2 activation by morphine (presumably by a Gβγ-dependent mechanism) was found to be blocked by PKC inhibition and did not involve nuclear translocation of activated ERK1/2. Arrestin-recruiting agonists produced nuclear translocation of phospho-ERK1/2 that was not dependent on endocytosis (Zheng et al., 2008b).
ERK1/2 has a large range of potential substrates, including transcription factors controlling gene expression; thus it is likely that ERK activation may contribute to tolerance at both proximal (receptor-signaling–specific) and distal downstream events that regulate the behavioral responses following MOR activation. Understanding how these effects contribute to the compensatory behavioral adaptations to chronic opiates is not yet resolved. Animals treated with chronic morphine show enhanced ERK1/2 activation in many brain areas (Ortiz et al., 1995; Narita et al., 2002; Macey et al., 2009), although there are also areas of decreased activation (Eitan et al., 2003; Muller and Unterwald, 2004). Since the time course of the changes in ERK1/2 activity generally correlate with the development of morphine tolerance, it has been assumed (perhaps prematurely) that ERK1/2 activation plays a role in morphine tolerance. However, there are several conflicting reports that inhibition of ERK1/2 can either enhance or diminish measures of morphine tolerance. For example, disruption of ERK1/2 signaling enhances both the development and expression of morphine tolerance with microinjections of the MEK inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene] into the periaqueductal gray area (Macey et al., 2009). Others have reported that i.p. injections of an ERK inhibitor, SL327 [α-[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile], did not alter acute morphine tolerance (Melief et al., 2010) or tolerance following chronic morphine treatment (Mouledous, et al., 2007; but see Chen et al., 2008). These results suggest that ERK1/2 activation in different areas and under different conditions may lead to different effects (Eitan et al., 2003). One overall difficulty in interpretation of these in vivo studies is the fact that sometimes the ERK1/2 activation occurs in opioid-sensitive neurons and other times is indirect via disinhibition of other neurons or perhaps by activation of ERK1/2 signaling pathways in glia or astrocytes (Eitan et al., 2003; Wang, et al., 2009). ERK1/2 activation has also been implicated in the upregulation of pain-promoting mechanisms, such as transient receptor potential cation channel subfamily V member 1 (TRPV1) channels and calcitonin gene–related peptide (CGRP) in peripheral tissues (Ma et al., 2001; Chen et al., 2008; Wang et al., 2009, 2011), which could interact with behavioral measures of morphine tolerance. Thus, it is clear that ERK1/2 activation occurs, but how it contributes to tolerance is not yet defined.
G. Involvement of Other Kinases and Enzymes
Much less is known about the role of the many other potential phosphorylation sites on MOR and either direct or indirect actions of kinases on MOR function for desensitization and regulation of MOR during tolerance development (Fig. 4).
1. Thr180.
Celver et al. (2004) established in X. laevis oocytes and AtT20 cells that mutation of T180A in the second intracellular loop of MOR reduced desensitization induced by DAMGO (no desensitization was found following exposure to morphine in these experiments). Receptor desensitization in this assay required GRK3 and arrestin expression, and mutation of T180A blocked desensitization. To assess whether MOR tolerance in vivo also involved phosphorylation at this residue, a phosphoselective antibody (pT180) was generated and characterized (Petraschka et al., 2007). Although fentanyl treatment increased phosphorylation at serines 370 and 375 in striatum, there was no evidence that Thr180 was phosphorylated. Thus, a possible role for phosphorylation of this residue in analgesic tolerance is not yet clear.
2. Tyr166.
Relatively little attention has been paid to potential tyrosine phosphorylation sites in MOR. Clayton et al. (2010) using a novel phosphoselective antibody for pY166 in the Asp-Arg-Tyr motif of MOR, showed increased phosphorylation of this site only when the receptor was coactivated both by DAMGO and stress (H2O2) or the tyrosine kinase agonist epithelial growth factor. Coincident activation and phosphorylation of Tyr166 completely abolished the ability of DAMGO to activate the receptor as measured by GTPγS binding. Mutation of Y166P to prevent tyrosine phosphorylation greatly impaired the ability of coactivation to prevent DAMGO signaling. It would be of interest to determine whether coactivation with morphine and epithelial growth factor can induce the phosphorylation of this site and whether it increases with chronic morphine. These results suggest that signaling through MOR may be desensitized through a heterologous mechanism by receptor tyrosine kinase activation and that this mechanism may have important implications for pain control following tissue injury.
3. Calcium-Calmodulin Kinase.
There is limited evidence that calcium-calmodulin kinase (CaMKII) might phosphorylate and modulate MOR function (Mestek et al., 1995; Koch et al., 1997). Koch et al. reported that expression of mutants of a putative CaMKII site in the third intracellular loop of MOR (S261A/S266A) in X. laevis oocytes decreased the rate of slow desensitization (over several hours) by DAMGO. Whether this involved uncoupling, internalization, or receptor insertion is unknown.
4. Phospholipase D and p38 Mitogen-Activated Protein Kinase.
Recent studies in cell lines revealed that in addition to β-arrestin, phospholipase D (PLD) is another MOR-interacting protein that plays a role in the regulation of acute desensitization. PLD catalyzes the hydrolysis of phosphatidylcholine to form choline and phosphatidic acid, a signaling lipid that can be converted to diacylglycerol (DAG). One isoform of PLD (PLD2) has been shown to be largely associated with the plasma membrane (Liscovitch et al., 1999). A number of GPCRs (McCulloch et al., 2001), including the CB1 cannabinoid receptor (Koch et al., 2006), and MOR and DOR (Koch et al., 2003, 2004, 2006), activate PLD to affect exocytosis, cytoskeletal reorganization, and cellular proliferation (Ghosh et al., 2003; Jenkins and Frohman, 2005; Liscovitch et al., 2000; McDermott et al., 2004; Zouwail et al., 2005). There is also growing evidence that PLD facilitates membrane trafficking of MORs (Koch et al., 2003; Yang et al., 2010). In fact, it was demonstrated that only PLD2-activating opioids (like DAMGO, β-endorphin, methadone, piritramide, fentanyl, sufentanil, and etonitazene) strongly induce MOR endocytosis, whereas agonists that do not activate PLD2 (like morphine, buprenorphine, hydromorphone, and oxycodone) failed to activate MOR internalization (Koch et al., 2009). Furthermore, it was recently shown that opioid receptor-induced PLD2 activation after treatment with DAMGO is dependent on ADP-ribosylation factor 6 (ARF6) but not PKC (Rankovic et al., 2009). A mechanistic basis for the PLD-mediated endocytosis was speculated to result from altering membrane curvature (reviewed, Donaldson, 2009). In addition, it was shown that the PLD2-phosphatidic acid-DAG pathway is involved in the opioid receptor-mediated activation of p38 MAPK that is essential for MOR endocytosis (Yang et al., 2010). Activation of p38 MAPK regulates MOR endocytosis by phosphorylating the Rab5 effector early endosome antigen 1 (EEA1) (Macé et al., 2005; Yang et al., 2010). These results are in line with previous findings demonstrating an important role of p38 MAPK activation in the induction of endocytotic receptor trafficking (Cavalli et al., 2001; Macé et al., 2005; McLaughlin et al., 2006; Vergarajauregui et al., 2006). It should be noted, however, that inhibition of p38 MAPK in mouse locus coeruleus neurons blocked neither desensitization nor endocytosis of MOR but did increase the rate of recovery from desensitization (Arttamangkul et al., 2012).
In addition to the key role in the induction of receptor internalization, PLD-derived phosphatidic acid regulates NADH/NADPH oxidase activity leading to a production of reactive oxygen species (ROS) (Bellavite et al., 1988; Bonser et al., 1989; Rossi et al., 1990; Bauldry et al., 1991; McPhail et al., 1995; Waite et al., 1997; Touyz and Schiffrin, 1999). Only PLD2-activating and MOR-internalizing agonists such as DAMGO, β-endorphin, methadone, piritramide, fentanyl, sufentanil, or etonitazene were found to stimulate the MOR-mediated production of reactive oxygen molecules, primarily H2O2, via NADH/NADPH oxidase (Koch et al., 2009). The role of ROS and redox homeostasis in regulation of cell growth and survival has been intensively investigated in numerous studies (reviewed by Trachootham et al., 2008). The PLD2-mediated low-level ROS generation by receptor-internalizing or endogenous opioids might be one reason for a lower neurotoxicity compared with noninternalizing opioids under physiologic conditions and this might have implications for analgesic therapy.
VI. Internalization, Phosphatase Activity, and Recovery from Desensitization
The notion that GPCRs remain phosphorylated until endocytosis has occurred has been assumed based on the recycling model of the β-adrenergic receptor (Sibley et al., 1986; Yang et al., 1988). Whether this model applies to MOR is important because some hypotheses propose that tolerance to morphine and other agonists that very weakly induce endocytosis is greater than to high-efficacy agonists such as DAMGO. The idea is that phosphorylated uncoupled MOR accumulates at the cell surface when dephosphorylation and recovery from desensitization requires endocytosis (Schulz et al., 2004). More recent evidence discussed below establishes that a decline in phosphorylation and recovery from desensitization of MOR can occur efficiently at the cell surface regardless of whether strongly or weakly internalizing agonists are examined, so other explanations for the involvement of MOR regulatory mechanisms in tolerance are required.
Schulz et al. (2004) reported that recycling is required to dephosphorylate MOR at Ser375. Phosphorylation of Ser375 persisted long after removal of morphine from cells but was readily reversible using the strongly internalizing agonist DAMGO. More recent studies, including those from the same laboratory (Doll et al., 2011), have established that endocytosis is not necessary for either recovery from desensitization or dephosphorylation of MOR. In addition, Doll et al. (2011) have shown conclusively that dephosphorylation of Ser375 is rapid using both DAMGO and morphine as agonists. This contradicts the earlier study of Schulz et al. (2004) but the explanation may be that morphine did not wash effectively from the cell preparations in the earlier study. Dephosphorylation was enhanced by a brief rinse with low pH in the Doll et al. (2011) study, presumably because low pH facilitates agonist removal from the receptors. More importantly, dephosphorylation of both Ser375 and Thr370 after DAMGO exposure was just as rapid in cells incubated in concanavalin A, which completely blocked endocytosis. Although these findings may not generalize to other phosphorylation sites on MOR, they do establish that sites involved in β-arrestin2 binding dephosphorylate equally efficiently whether or not endocytosis is blocked. Furthermore, Arttamangkul et al. (2006) showed directly that concanavalin A blocks endocytosis of MORs but does not affect recovery from desensitization.
Studies on the recovery from desensitization using locus coeruleus neurons from β-arrestin2 knockout and wild type mice (Dang et al., 2011; Quillinan et al., 2011) are consistent with the study of Doll et al. (2011). If arrestin-dependent endocytosis is required for recovery from desensitization, then recovery for a strongly internalizing agonist should be impaired in β-arrestin2 knockout, but the opposite was found. In wild type mice, MOR recovery was slow after ME-induced desensitization (approximately 60 minutes), similar to results reported earlier for locus coeruleus neurons from rat (Osborne and Williams, 1995; Dang and Williams, 2004) and similar to the rate of MOR recycling reported in cultured cells (Koch et al., 2001; Tanowitz and von Zastrow, 2003). In locus coeruleus neurons from β-arrestin2 knockout mice, MOR recovery was accelerated and was nearly completely recovered within 20 minutes (Dang et al., 2011). Accelerated recovery in the β-arrestin2 knockout was mimicked in wild type locus coeruleus by manipulations that should block arrestin upstream of its association with MOR (an intracellular GRK inhibitor) or endocytosis downstream of arrestin association (an intracellular dynamin inhibitor; Dang et al., 2011). Conversely, recovery from desensitization was slowed by a phosphatase inhibitor under conditions of impaired arrestin association (β-arrestin2 knockout plus GRK inhibitor, Dang et al., 2011). This shows that the recovery from desensitization is rapid even when endocytosis is blocked and the time course is quite consistent with the dephosphorylation rate reported by Doll et al. (2011). The slow recovery from desensitization in wild type locus coeruleus is almost certainly due to the fact that once receptors are endocytosed, relatively slow receptor recycling (Koch et al., 2001; Tanowitz and von Zastrow, 2003) is necessary for recovery of MOR localization and signaling at the surface membrane. However, since β-arrestin likely contributes to both the desensitization (uncoupling) and the receptor recycling events, interpretation of differences in recovery rates after β-arrestin knockout is complicated (see below). It should also be noted that such recovery rates may differ in different neurons because the three most abundant MOR splice variants (MOR1, MOR1A, and MOR1B) recycle at different rates (Koch et al., 1998; Oldfield et al., 2008).
Earlier functional studies using inhibition of cAMP formation as an endpoint showed that monensin (a drug that inhibits endosomal recycling), truncated MOR mutants (Qiu et al., 2003) or MOR splice variants (Koch et al., 2001; Tanowitz and von Zastrow, 2003; Tanowitz et al., 2008) reduced both recycling and recovery of endocytosed MOR. Although these appear to support a requirement for endocytosis and recycling in the recovery of MOR function, assays of MOR function were performed over time scales greatly exceeding acute desensitization of G protein coupling to MOR, β-arrestin2 binding (Oakley et al., 2000), endocytosis (Tanowitz and von Zastrow, 2003; Arttamangkul et al., 2006, 2008), and often recycling (Koch et al., 2001; Tanowitz and von Zastrow, 2003; Arttamangkul et al., 2008; Tanowitz et al., 2008). These studies therefore could not distinguish recovery of functional MOR at the cell surface from the increased MOR surface density (and therefore function) resulting from recycling (Connor et al., 2004), so the findings could also be consistent with and do not refute findings that dephosphorylation is efficient at the cell surface.
The necessity for endocytosis and recycling to dephosphorylate some GPCRs presumably depends on the affinity of arrestins for the agonist occupied receptor (Oakley et al., 1999, 2000). The rapid recovery from desensitization and dephosphorylation of MOR at the cell surface (Dang et al., 2011; Doll et al., 2011; Quillinan et al., 2011) suggests that the affinity of the β-arrestin2 association to MOR is relatively weak (Oakley et al., 2000), so that it can dissociate rapidly prior to endocytosis, thereby exposing the phosphorylated C-terminal residues (Ser375, Thr370, and presumably others) to phosphatases. The very rapid reversal of MOR-β-arrestin2 resonance energy transfer signals upon agonist washout reported by McPherson et al. (2010) for both strongly and weakly internalizing opioids (except etorphine, which has extremely high affinity for MOR) is consistent with this interpretation.
VII. Cellular Adaptations Induced during Morphine Tolerance
Recent promising approaches to limit tolerance have been extensively reviewed and include simultaneous activation of more than one opioid receptor type (e.g., MOR and DOR receptors), selective targeting of hetero-oligomers, or opioids that differentially activate distinct intracellular signaling cascades, possibly involving differential activation of Gα subtypes (Piñeyro and Archer-Lahlou, 2007), and particularly differential G protein activation versus endocytosis (Martini and Whistler, 2007; Christie, 2008; Koch and Höllt, 2008; Berger and Whistler, 2010; von Zastrow, 2010). In keeping with the present focus on MOR regulatory mechanisms, the following sections examine evidence for adaptations in MOR regulation that contribute to tolerance (Fig. 6). As discussed in Biased Agonism and μ-Opioid Receptor Regulation, chronic treatment with morphine and other opioids impairs MOR effector coupling but does not have much effect on MOR binding density. The MOR regulatory mechanisms responsible are still uncertain but multiple adaptive processes are engaged.
Fig. 6.
Summary of adaptations that might contribute to MOR tolerance after chronic exposure to morphine (but perhaps not other agonists). (A) Functional analyses indicate >80% loss of functional MOR is required to account for tolerance after chronic morphine, but this is not accounted for by loss of total MOR binding (see Primary Structure and Structural Diversity of μ-Opioid Receptors). (B) An enhanced rate of MOR desensitization coupled with impaired resensitization should shift the equilibrium toward increased desensitized MOR (Section VII). The dependence of impaired MOR resensitization on GRK and arrestin could explain the loss of morphine tolerance observed β-arrestin2 knockouts (Section VII.B). (C) Although increased endocytosis could contribute to loss of functional MOR, there is evidence (Quillinan et al., 2011) that does not support this idea (Section VII.A and VII.B). (D) Blocking PKC activity reverses tolerance in vivo and in vitro (Section V.G) raising the possibility that persistent PKC phosphorylation of MOR may be required for loss of MOR function. (E) The dependence of impaired MOR resensitization on GRK and arrestin suggests that enhanced interactions may contribute to persistent desensitization. However, there is little direct evidence to support this possibility (Section VII.A, VII.E, VII.F). (F) Reduced recycling of MOR has been observed in locus coeruleus neurons and this should produce accumulation of intracellular MOR. While this occurs in some neurons, it has not been observed in others (see Primary Structure and Structural Diversity of μ-Opioid Receptors).
A. Accelerated Desensitization
Desensitization induced by ME, DAMGO, and morphine (and methadone, Quillinan et al., 2011) are all more pronounced in locus coeruleus (Dang and Williams, 2004, 2005), as well as in periaqueductal gray neurons (Ingram et al., 2008), after withdrawal from chronic exposure to morphine. Enhanced desensitization is one mechanism that would contribute to opioid tolerance. There are many possible adaptations caused by chronic morphine that could be responsible for these observations. One early hypothesis was that chronic morphine treatment enhances endocytosis, but more recent data indicates that the extent of endocytosis is not affected, at least in locus coeruleus (Quillinan et al., 2011). However, reduced recovery from endocytosis after chronic morphine treatment is observed (Quillinan et al., 2011). Other adaptive mechanisms resulting from chronic morphine exposure or withdrawal that may contribute to accelerated desensitization could include those directly involved with MOR phosphorylation, such as increased activation of ERK1/2, GRKs (but GRK2 is decreased; Fan et al., 2002), or arrestins (but β-arrestin2 is decreased in periaqueductal gray; Fan et al., 2003), or upregulation of other proteins such as RGS proteins (Gold et al., 2003), phospholipase D2 (Koch et al., 2006), or spinophilin (Charlton et al., 2008).
B. Impaired Recovery from Desensitization
Impairment of the capacity of MOR to recover rapidly from desensitization appears to contribute to morphine tolerance. In addition to enhanced desensitization, the recovery from desensitization is impaired in rat locus coeruleus neurons after withdrawal from chronic morphine (Dang and Williams, 2004). Dang et al. (2011) and Quillinan et al. (2011) recently confirmed this in mouse locus coeruleus and further established that the impairment is arrestin-dependent because recovery from desensitization was normal in β-arrestin2 knockout mice. Impaired recovery after chronic morphine in wild type locus coeruleus neurons was reversed and resembled that in the β-arrestin2 knockout either by disrupting GRK2 function or by inhibition of dynamin function with intracellular inhibitors. These findings link the recovery of MOR desensitization in locus coeruleus to adaptations within the GRK2–β-arrestin2–dynamin–dependent MOR regulation.
Dang et al. (2011) reported that chronic morphine-induced tolerance to the activation of potassium conductance in mouse locus coeruleus neurons was similar to that previously reported in wild type neurons and was abolished in the β-arrestin2 knockout. This is consistent with observations that tolerance to morphine-induced antinociception, as well as tolerance to DAMGO-stimulated GTPγS binding in brainstem and spinal cord membranes, is attenuated in these animals (Bohn et al., 2000, 2002). It was proposed that following chronic morphine, β-arrestin2-dependent regulation of MOR is enhanced, slowing recovery from desensitization, thereby shifting the equilibrium between active and desensitized receptors to an accumulation of desensitized MOR, resulting in MOR tolerance (Dang et al., 2011; Quillinan et al., 2011). As such, ablation of β-arrestin2 in the knockout mice facilitates recovery from desensitization and prevents cellular opioid tolerance in locus coeruleus neurons. Impaired recovery from desensitization may be the cellular mechanism underlying antinociceptive tolerance in vivo if the phenomenon is found to generalize to neurons involved in analgesia.
The mechanisms of enhanced desensitization and β-arrestin2-dependent impairment of recovery during chronic morphine treatment in vivo are still not known. Impaired recovery was observed after very brief exposure to ME and was sensitive to GRK, β-arrestin2, or dynamin inhibition, suggesting a possibly enhanced rate of GRK phosphorylation after chronic morphine that engages β-arrestin2 and clathrin-dynamin-dependent processes (Dang et al., 2011). Quillinan et al. (2011) also reported the impaired recovery was reversed in a transgenic animal in which the activity of an engineered GRK2 could be blocked by a novel agent, 4-amino-1-tert-butyl-3-(1′-naphthyl)pyrazolo[3,4-d]pyrimidine (NaPP1). The dependence of both tolerance and the recovery from desensitization on GRK, β-arrestin2, and dynamin would predict that the explanation for MOR tolerance may be an enhanced rate of endocytosis after chronic morphine. However, Quillinan et al. (2011) found no difference in the extent of ME-induced MOR endocytosis in locus coeruleus neurons from animals chronically treated with morphine, but there was a decrease in the extent of reinsertion of receptors into the plasma membrane. Similarly, the extent of endocytosis induced by DAMGO in spinal cord in vivo was also not reduced by chronic morphine treatment (Trafton and Basbaum, 2004). The latter findings seem at odds with the effects of β-arrestin2 deletion and dynamin inhibition. The mechanism of impaired recovery from desensitization is, therefore, still unclear but a range of adaptations produced by chronic morphine could be responsible. Although untested, it is possible that sites other than Thr370 and Ser375 are more persistently phosphorylated by chronic morphine to enhance other downstream events that are not directly related to increased endocytosis or that postendocytic trafficking and sorting mechanisms are affected by chronic morphine. Although there are important differences between studies that examine desensitization and tolerance, particularly in vivo, it appears that desensitization and perhaps the slowed recovery from desensitization plays a role in tolerance. However, this has only been examined in the locus coeruleus and it is critical to extend these findings to other brain areas, specifically areas associated with modulation of antinociception by opioids.
C. Increased Constitutive Activity of μ-Opioid Receptors
It has been suggested that prolonged exposure to opioid agonists results in an enhanced level of MOR constitutive signaling (i.e., coupling of the receptor to G protein activation in the absence of any drug) (Liu and Prather, 2001; Wang, et al., 2004). This adaptive change could contribute to tolerance by increasing MOR signaling through nonagonist-bound receptors or by enhancing MOR desensitization if constitutively active receptors are susceptible to the same desensitizing and internalizing processes as agonist-bound receptors. However, there is still controversy over whether MORs exhibit constitutive activity and whether it is enhanced by prolonged agonist exposure.
That GPCRs exhibit constitutive activity in the absence of any agonist has been proposed to explain why certain ligands, originally thought to be antagonists, may act to inhibit basal activity of unoccupied receptors (i.e., they are inverse agonists) (Costa and Cotecchia, 2005). The DOR was the first of the opioid receptors where a peptide antagonist, [N,N′-diallyl-Try1.Aib2,3,Leu5]enkephalin (ICI174864), was found to decrease signaling even in the absence of agonist (Costa and Herz, 1989). More recently constitutive activity of expressed MOR was demonstrated using β-chlornaltrexamine (β-CNA) in HEK293 cells (Burford, et al., 2000) and β-funaltrexamine (β-FNA) in GH3 cells (Liu, et al., 2001) and using G protein modulation of calcium channel currents in neurons overexpressing MOR (Mahmoud et al., 2010), although it should be noted that the level of constitutive activity that has been detected is relatively small even when MOR was overexpressed.
Assessments of constitutive activity are confounded by receptor overexpression in transfected cells and possible receptor activation by endogenous opioids during in vivo assays. The significance of constitutive activity of MOR in vivo therefore remains controversial (Connor and Traynor, 2010). Whether reported inverse agonists actually possess negative intrinsic activity is also controversial and may depend on assay methods (usually GTPγS) and conditions (reviewed by Connor and Traynor, 2010), e.g., naloxone has been variously reported to possess positive, neutral, or negative intrinsic activity (see Table 2 of Connor and Traynor 2010). The intrinsic activities of various MOR antagonists will remain controversial until resolved using physiologic conditions even if receptors are overexpressed. Functional studies in dorsal root ganglion neurons cultured from β-arrestin2 knockout but not wild type mice (Walwyn et al., 2007; Lam et al., 2011) detected constitutive activity of MOR using G protein-dependent modulation of calcium channel currents as a reporter of constitutive G protein activation. Constitutive activity was reversed by naloxone and naltrexone (proposed inverse agonists) but not the uniformly accepted neutral antagonist, 6β-naltrexol. As expected of neutral antagonists, 6β-naltrexol (Lam et al., 2011) and d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 (CTAP) (Walwyn et al., 2007) antagonized the inverse agonist actions of naloxone and naltrexone. Walwyn et al., (2007) suggested that β-arrestin is required to target c-Src to constitutively active MOR because c-Src inhibitors mimicked the effect of the β-arrestin knockout.
Constitutive activity of MOR was suggested to be increased after chronic exposure to morphine on the basis of responses to proposed neutral versus inverse agonists in cultured cell lines and animals (Liu and Prather, 2001; Wang, et al., 2004), but this has not been confirmed in other biochemical studies (Divin et al., 2009). Functional studies in neurons and cell lines under physiologic conditions, again utilizing voltage-gated calcium channel modulation by MOR, have also failed to detect constitutive MOR activity after chronic exposure to morphine in locus coeruleus (Connor et al., 1999) and periaqueductal gray neurons (Bagley et al., 2005b) or ShSY5Y cells (Kennedy and Henderson, 1992). In addition to the concerns raised above, it is crucial in such studies to ensure that all agonist has been removed from tissue before evaluating potential inverse agonists following chronic treatment and this may depend somewhat on the physical properties of the agonist used. For example, studies using chronic treatment with strongly lipophilic agonists such as herkinorin (Sally et al., 2010) have reported induction of negative intrinsic activity for a much broader suite of MOR antagonists than all other studies (Connor and Traynor, 2010). The upregulation of adenylyl cyclase activity induced by chronic opioid exposure could also confound attempts to measure changes in constitutive activity. Whether chronic morphine exposure induces constitutive activity of MOR, or whether this is widespread in the nervous system is therefore still uncertain, but if correct, the observation that constitutive MOR activity increases after chronic opioid exposure suggests an active confomational state of MORs that is evident even in the absence of bound ligand.
D. Regulation of G Protein Receptor Kinase in Morphine Tolerance
Among the numerous changes in gene expression regulated by chronic opioid administration (Ammon-Treiber and Hollt, 2005), a relatively early study showed that chronic morphine produced ∼20% increase in GRK2 protein levels in the rat locus coeruleus. This was not observed following acute morphine administration and was specific for the locus coeruleus and for GRK2, as levels of the closely similar kinase GRK3 were not affected (Terwilliger et al., 1994). Increased GRK expression has been shown to potentiate morphine-induced desensitization of MORs (Whistler and von Zastrow, 1998) suggesting that morphine tolerance may be due to increased MOR desensitization. In a more recent investigation, the small increase in GRK2 (or in tyrosine hydroxylase) level in the locus coeruleus following chronic morphine treatment was not reliably found (M. S. Virk, E. K. Lau, J. T. Williams, and M. von Zastrow, unpublished data). No increase in total enzyme level was observed in chronically treated animals until precipitating withdrawal, and then an increase (∼20%) in total GRK2 protein was observed in frontal cortex (Ozaita, et al., 1998). The functional significance of the altered expression/distribution of GRK2 was not determined, nor was it established whether this occurred in neurons expressing MOR. Given the importance of GRK levels to morphine-dependent regulation of receptors in model cell systems, it is conceivable that alterations in GRK2 expression or subcellular distribution following chronic opioid treatment could contribute to significant reprogramming of opioid regulatory responses. It is not presently known if these functional changes in opioid signaling resulted from altered GRK abundance in receptor expressing neurons. It is however a reasonable possibility to consider in future studies.
E. Regulation by Arrestin and Endocytosis in Morphine Tolerance
In β-arrestin2 knockout mice there is evidence for attenuated analgesic tolerance to morphine but not fentanyl, methadone, and oxycodone (Bohn et al., 2000, 2002; Raehal and Bohn, 2011). The simplest interpretation of these data is that β-arrestin2-dependent mechanisms contribute to development of tolerance to morphine but not other opioids. Since acutely applied morphine does not efficiently recruit β-arrestin, one would have to propose that chronic morphine treatment results in an increased role of β-arrestin-dependent mechanisms. The findings that cellular tolerance to chronic morphine does not develop for MOR coupling to GIRK channels in locus coeruleus neurons from β-arrestin2 knockout mice (Dang et al., 2011; Quillinan et al., 2011) and for GTPγS binding in brainstem tissue (Bohn et al., 2000) are consistent with this idea, but tolerance to other agonists has not yet been tested. One inconsistency is that in vivo morphine-induced antinociception is increased in the knockout animals but, if anything, MOR coupling to GIRK (Dang et al., 2011; Quillinan et al., 2011) or calcium channels (Walwyn et al., 2007) is less sensitive. It is also important to remember that the antinociceptive responses measured in the analgesia tolerance assays involve a complex circuit response that could be affected by β-arrestin knockout at multiple sites between MOR and the motor response and that deletion of β-arrestin may affect agonist efficacy in this assay in ways that would not be evident by simply recording percent maximal possible effect.
Together, the data speaks to β-arrestin2 dependence for aspects of morphine tolerance and desensitization but not higher efficacy drugs. A possible key to the difference between agonists is the selectivity of agonist-bound receptors for β-arrestin1 or β-arrestin2. Morphine-bound MORs interact only with β-arrestin2, whereas MORs bound to more efficacious agonists (e.g., DAMGO and ME) interact with both β-arrestins 1 and 2 (Groer et al., 2011). In the absence of β-arrestin2, the mechanisms resulting in a desensitized or tolerant state for morphine would be attenuated, whereas for more efficacious agonists, adaptive mechanisms could still recruit β-arrestin1.
There are several roles that arrestin can play in desensitization processes. Multiple proteins (kinases, arrestins, calmodulin, filamin) bind to the intracellular domains of MOR and may act by steric hindrance of key sites for G protein interaction. The efficacy of this mechanism would be dependent on off-and-on rates of the associated proteins, their ability to compete with G protein complexes, and the overall conformational modulation of the receptor-ligand binding site. This simple mechanism is a possible explanation for the morphine phenotype in β-arrestin2 knockout mice—attenuation of in vivo tolerance and cellular desensitization.
Arrestins are also scaffolding proteins involved in clathrin-mediated endocytosis and in orchestrating receptor trafficking toward recycling or lysosomal degradative pathways. Thus arrestins could modulate desensitization by the regulation of active receptors at the cell surface. Internalization may also promote the recovery from desensitization depending on the desensitization process engaged in receptor modification (phosphorylation, protein binding, or ubiqutination). Since the β-arrestins 1 and 2 double knockouts are lethal mutations, the complete absence of arrestins on tolerance mechanisms cannot be assessed in vivo. Although drugs such as morphine induce limited internalization, morphine does induce internalization following overexpression of β-arrestin2. This suggests that the morphine-bound MOR induces a low but functionally effective affinity state for β-arrestin2 binding and clathrin scaffolding that could be significant during chronic receptor stimulation.
Bohn and colleagues reported that development of morphine antinociceptive tolerance (but not withdrawal) is blunted in β-arrestin2 knockout mice. These studies suggest that blocking MOR endocytosis, which is presumably impaired in the β-arrestin2 knockout (but see Arttamangkul et al., 2008; Quillinan et al., 2011), attenuates tolerance. An alternative hypothesis supported by other groups is that induction of MOR endocytosis and recycling limits morphine tolerance, and suppression of endocytosis or recycling enhances it. He et al. (2002) reported that inclusion of an extremely low dose of a strongly internalizing agonist, DAMGO (which had no antinociceptive effect on its own), with constantly infused i.t. morphine limited the development of tolerance and also stimulated MOR endocytosis in spinal cord and cultured cells (but see contrary evidence, Bailey et al., 2003; Koch et al., 2005). This was not observed with either drug alone at the doses used. The authors hypothesized that a very low concentration of DAMGO, which does not induce detectable endocytosis by itself, can stimulate endocytosis of morphine-occupied MOR and thereby reduce tolerance, perhaps via interaction with homomultimers of MOR. Similarly, Kim et al. (2008) studied a transgenic MOR mouse in which part of the C-terminal region of the DOR is substituted into MOR (rMOR). This conferred the ability of morphine to efficiently mediate MOR endocytosis and recycling. The rMOR mice showed similar antinociceptive sensitivity to morphine as wild types but developed less morphine antinociceptive tolerance, as well as less reduction in MOR-activated GTPγS binding in brainstem membranes. Taken together, these studies suggest that MOR endocytosis limits tolerance and, therefore, opioids that do not promote receptor endocytosis should produce greater tolerance than agonists that do promote MOR endocytosis.
The findings described above appear contradictory in terms of the relationship between endocytosis and tolerance. On one hand, blocking β-arrestin2 association with MOR (but not GRK3 or S375A mutants) inhibits morphine tolerance; however, manipulations that enhance MOR endocytosis (and vice versa) impair development of morphine tolerance. Various explanations have been proposed to account for these disparate findings. In the case of manipulations that prevent β-arrestin2 binding, it was proposed that β-arrestin2 association is necessary for, or facilitates MOR desensitization (Bohn et al., 2002, 2004), but this is not the case; see above). It is therefore unclear how β-arrestin2 deletion can account for blunted tolerance in the knockout mice. Two general interpretations (not mutually exclusive) for the inhibition of tolerance were presented (Berger and Whistler, 2010). One interpretation is that strongly internalizing agonists produce less tolerance because the cycles of endocytosis promote dephosphorylation of MOR in endosomes, and active receptors are then recycled to the cell surface. Because morphine poorly stimulates endocytosis of phosphorylated and desensitized MOR (whether MOR is associated with arrestin), the desensitized receptors accumulate at the cell surface causing tolerance. However, evidence discussed in Phosphorylation and μ-Opioid Receptor Regulation strongly suggests that MOR is dephosphorylated (at least at Thr370 and Ser375; Doll et al., 2011) and resensitized (Arttamangkul et al., 2006) when endocytosis is blocked. The other interpretation is that morphine causes persistent signaling that contributes to secondary adaptations involved in tolerance in vivo, whereas endocytosis terminates persistent signaling, limiting downstream counter-adaptations and tolerance. These authors have also provided extensive evidence that such counter-adaptations are more pronounced following chronic morphine stimulation of wild-type MOR compared with the chimeric rMOR that can undergo endocytosis and recycling when stimulated by morphine. The receptor signaling events in the neurons responsible for the analgesic responses have not been directly observed and further study is required.
F. Role of Protein Kinase C in Morphine Tolerance
There is good evidence that PKC plays a significant role in tolerance to the antinociceptive actions of morphine in rodents. First, morphine tolerance can be reduced by administration of PKC inhibitors (Smith et al., 1999, 2003; Inoue and Ueda 2000; Bohn et al., 2002; Hua et al., 2002). Indeed, PKC inhibitors potentiate the antinociceptive effect of a single dose of morphine, indicating that PKC is involved in the tolerance that occurs during the single dose (Watkins et al., 1984; Manning et al., 1996; Bohn et al., 1999). Second, tolerance is decreased when PKC expression is eliminated (Granados-Soto et al., 2000) or reduced by transgenic knockout or by knockdown following small interfering-RNA administration (Zeitz et al., 2001; Hua et al., 2002; Newton et al., 2007). These knockout/knockdown experiments as well as studies using specific inhibitors of individual PKC isoforms (Smith et al., 2007) suggest that PKCα, PKCγ, and, to a lesser extent, PKCε are involved in tolerance to the analgesic effects of morphine. Different PKC isoforms exhibit different anatomical distribution: PKCγ is absent from presynaptic terminals in fully mature rats but can be found in abundance in postsynaptic neurons, whereas in spinal cord, PKCε is only found in presynaptic primary afferent nerve terminals.
It appears that ongoing PKC activity is necessary to maintain morphine antinociceptive tolerance even after it has developed. PKC inhibitors can reverse tolerance to the analgesic actions of morphine when administered 3 days after beginning the morphine treatment in vivo (Smith et al., 1999). In locus coeruleus neurons, cellular tolerance can be observed in brain slices prepared from animals treated with morphine for 3 days (Bailey et al., 2009a) and is reversed by administration of a PKC inhibitor. As with morphine tolerance measured with a nociceptive assay, PKC inhibitors reverse cellular morphine tolerance in locus coeruleus neurons indicating that ongoing PKC activity is required for its maintenance. While PKC is involved in tolerance to morphine, it does not appear to be involved in tolerance to DAMGO. Opposite to the effects of GRK inhibition, a PKC inhibitor blunted short-term analgesic tolerance to morphine but not DAMGO (Hull et al., 2010), and in locus coeruleus neurones, cellular tolerance to DAMGO was not reversed by PKC inhibitors (Bailey et al., 2009a).
Less tolerance develops to the respiratory-depressant actions than the antinociceptive effects of opioids following chronic opioid administration (Ling et al., 1989; Paronis and Woods 1997). This suggests that adaptive processes may differ at both a cellular and circuit level. For example, activation of PKC in pre-Bötzinger nucleus neurons that control respiration increases tolerance to morphine and affords increased protection to death by overdose (Lin et al., 2012).
VIII. Implications and Outstanding Questions
A. Agonist-Selective Tolerance
The distinct agonist-selective mechanisms of MOR regulation of desensitization, endocytosis, and tolerance discussed above (Sections IV to VI) suggest that opioid agonists with improved tolerance liability could be developed. While a truly tolerance-free opioid has not been realized, differences have been noted in the tendency of various opioids to produce tolerance following repeated administration. Morphine, for instance, produced greater opioid tolerance when compared with agonists like DAMGO, sufentanil, or etorphine, when equivalent induction doses and continuous infusions were used to control for pharmacokinetic differences (Stevens and Yaksh 1989; Duttaroy and Yoburn, 1995; Madia et al., 2009). A series of opioid agonists, administered at doses that produce comparable analgesia acutely, further indicated that highly efficacious opioids tend to produce less tolerance following chronic administration than do less efficacious agonists (Stevens and Yaksh 1989; Duttaroy and Yoburn, 1995; Walker and Young, 2001; Madia et al., 2009; Stafford et al., 2001; Dighe et al., 2009). There is also some evidence, such as asymmetric cross-tolerance (Pasternak, 2001) and tolerance-rescue studies (He et al., 2002), to suggest that mechanisms of tolerance may differ for different types of agonist.
The nature of the tolerance observed depends on the response measured, differences in agonist efficacy, the dosing paradigm selected, the timing of the response measured, and the site of opioid action. Each of these may result in different degrees of tolerance and cross-tolerance (Pawar et al., 2007). Many of these variables are very difficult to control in practice. Yoburn and colleagues have demonstrated that the magnitude of MOR tolerance depends on the method of administration (repeated or continuous) as well as the intrinsic efficacy of the agonist (Pawar et al., 2007; Kumar et al., 2008; Madia et al., 2009). There is good evidence that low intrinsic-efficacy agonists produce greater behavioral tolerance than high efficacy agonists. Low intrinsic-efficacy agonists also usually produce larger rightward shifts in concentration-response curves than high efficacy agonists. This occurs when MOR-effector coupling is impaired either by irreversible antagonists or chronic drug treatment presumably because low intrinsic-efficacy agonists such as morphine must occupy a greater fraction of the total receptor population to produce a given level of effect, due to lesser receptor reserve (Christie et al., 1987; Stevens and Yaksh, 1989; Mjanger and Yaksh, 1991; Connor et al., 1999).
The finding that morphine (and other low-efficacy agonists) produces more behavioral tolerance than high efficacy, strongly internalizing agonists has been widely cited to support the notion that MOR recycling influences tolerance. This is very difficult to test directly in practice because it requires direct comparison of the extent of tolerance produced by morphine with opioids that exhibit comparable intrinsic efficacy for G protein activation but much higher efficacy for endocytosis than morphine, while ensuring equivalent receptor stimulation and duration of action. Furthermore, the receptor desensitization mechanism underlying analgesic tolerance to morphine may not be the same as that responsible for tolerance to more efficacious agonists (Melief et al., 2010). Even at equiactive doses, ligands of different efficacies will be occupying different fractions of receptors and may be initiating different desensitization cascades, further confounding simple interpretation. A recent study used equal analgesic doses of morphine and methadone and found less tolerance was induced with methadone (Enquist et al., 2012), but methadone is also an N-methyl-d-aspartate (NMDA) receptor antagonist (although at very high concentrations, Matsui and Williams 2010). NMDA antagonists have been shown to block opioid receptor tolerance (Trujillo and Akil, 1991; Ebert et al., 1998). The reduction in the development of tolerance induced by methadone was proposed to result from internalization and recycling of MOR but may be a result of NMDA-receptor activity. Endomorphins may be better candidates to test the hypothesis that MOR recycling influences the degree of tolerance induced by an agonist, because their intrinsic efficacies for G protein activation appear similar to morphine and both endomorphin 1 and 2 both efficiently induce MOR endocytosis. Soignier et al. (2004) reported comparable rates of tolerance development and completely symmetrical cross-tolerance during continuous i.c.v. infusion of morphine, endomorphin-1, and endomorphin-2, suggesting tolerance may not be different between strongly and weakly internalizing agonists when intrinsic efficacy is nearly matched. It therefore remains uncertain whether strongly internalizing agonists produce less tolerance than weakly internalizing agonists when matched for intrinsic efficacy. This issue needs to be addressed more thoroughly to establish whether clinical outcomes of analgesic tolerance, as well as side effects such as respiratory depression and constipation, could be altered by directly manipulating bias toward or against agonists with different internalization capacities.
B. Outstanding Issues and Questions in μ-Opioid Receptor Regulation
What is the biophysical basis for agonist-selective regulatory effects of MOR agonists? Do chemically distinct agonists stabilize functionally distinct receptor conformations and how are these conformations translated to produce different effects? Is the basis of such agonist bias the result of activating different G proteins, phosphorylation by different GRKs and/or binding of different arrestins, or phosphorylation by other kinases?
Two major C-terminal groups of phosphorylation sites on MOR (STANT and TSST) are important but the cooperativity between these sites is still not fully understood.
Agonists that do not strongly promote arrestin translocation may produce desensitization/tolerance by as yet unidentified mechanisms involving other potential phosphorylation sites and protein-protein interactions. ERK1/2-, JNK-, and PKC-dependent mechanisms have been implicated, but the mechanisms by which they inactivate MOR are still unknown. It should be noted that these mechanisms have been most thoroughly examined using morphine as the agonist. Whether mechanisms differ for other agonists that do not strongly engage GRK-arrestin regulation, such as oxycodone, is unknown, but there are suggestions that distinct mechanisms are engaged.
How is the dephosphorylation of MOR regulated and what are the functional consequences? There is strong evidence for dephosphorylation and recovery from desensitization at the cell surface, with the steady state of desensitization presumably reflecting the equilibrium of phosphorylation-dephosphorylation reactions.
What role does compartmentalization play in the regulation of MOR signaling? Are there distinct differences between regulation in the soma verses dendrites? What are the principles of presynaptic MOR regulation? Where it has been examined, MOR is regulated differently in nerve terminals, which show little or no rapid desensitization.
The potential involvement of adaptive regulatory mechanisms in constitutive activity of MOR, which remains controversial, is unclear at present.
Ubiquitination of MOR has not been sufficiently explored such that the functional role of this process in MOR signaling is unknown.
How are presently known mechanisms of MOR regulation, as elucidated in studies using in vitro preparations, particularly in genetically modified systems, manifest in the intact human nervous system? Which of these influence clinically relevant processes of drug tolerance? Is there a link between acute desensitization and the development of tolerance?
IX. Concluding Remarks
The large number of distinct regulatory mechanisms implicated suggests that multiple, overlapping and, in some instances, possibly redundant mechanisms contribute to tolerance to opioids. Although there is consensus on a role for some mechanisms (regulation and signaling by the GRK-arrestin system), there are many examples of contradictory mechanisms. This is not surprising because the cellular and molecular adaptations underlying tolerance almost certainly do not have a unitary mechanism, and different cell lines, different neurons and neuronal compartments (i.e., terminal versus somatic compartments) may express different levels of regulatory proteins. In addition, the expression of regulatory proteins is not static and may vary with development, experimental conditions, or metabolic state of the cells. Moreover, as each mechanism in this complex regulatory network is ultimately driven by ligand-mediated MOR activation and regulation, even subtle differences in the effects of individual opioid ligands on receptor function could be physiologically significant. Due to these complexities, MOR regulation in different pathways in vivo may vary, clouding the ability to make direct comparisons between studies. To the extent it has been examined, morphine tolerance in neuronal cell bodies is associated with accelerated MOR desensitization, impaired recovery from desensitization and impaired recycling after endocytosis. The nature of the mechanisms underlying these processes is still unknown. Even less is known of the mechanisms of MOR tolerance in nerve terminals in which tolerance develops in the absence of any rapid desensitization.
Acknowledgments
The authors acknowledge the Vollum Institute for providing support for the initial meeting of authors for a roundtable conference on controversies in MOR regulation that initiated the development of the review. The authors thank Lori Vaskalis of the Vollum Institute for preparation of final figures.
Abbreviations
- CaMKII
calcium calmodulin kinase
- CNS
central nervous system
- DAG
diacylglycerol
- DAMGO
[d-Ala2, N-MePhe4,Gly-ol]enkephalin
- DOR
δ-opioid receptor
- ERK1/2
extracellular signal-regulated kinases
- FRET
fluorescence resonance energy transfer
- GIRK
G protein-gated inwardly rectifying potassium channel
- Gö6976
12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)carbazole
- GPCR
G protein-coupled receptor
- GRK
G protein receptor kinase
- ICI174864
N,N′-diallyl-Try1.Aib2,3,Leu5]enkephalin
- JNK
c-Jun N-terminal kinases
- Kir3
GIRK isoform
- KOR
κ-opioid receptor
- MAPK
mitogen-activated protein kinase
- ME
[Met]5enkephalin
- MOR
μ-opioid receptor
- NaPP1
4-amino-1-tert-butyl-3-(1′-naphthyl)pyrazolo[3,4-d]pyrimidine
- NMDA
N-methyl-d-aspartate
- PKC
protein kinase C
- PLD
phospholipase D
- RAVE
relative activation versus endocytosis
- RGS
regulator of G protein signaling
- rMOR
a transgenic MOR mouse in which part of the C-terminal region of the DOR is substituted into MOR
- ROS
reactive oxygen species
- [35S]GTPγS
35S-labeled guanosine-5′-O-(3-thio)triphosphate
- SL327
[α-[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile
- U0126
1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene
Authorship Contibutions
Wrote or contributed to the writing of the manuscript: Williams, Ingram, Henderson, Chavkin, von Zastrow, Schulz, Koch, Evans, Christie.
Footnotes
This work was supported by grants from the National Institutes of Health National Institute on Drug Abuse [Grants DA01863 (to J.T.W.), DA027625 (to S.I.), DA020836 (to G.H.), K0-5DA20570 (to C.C.), DA012864 (to Mv.Z.), and DA005010 (C.E.)]; the Deutsche Forschungsgemeinschaft [SCH924/11-2, S.S.], the Deutsche Forschungsgemeinschaft [GRK 1167/-1], and the Federal State of Saxony-Anhalt and the “European Regional Development Fund” [Grant ERDF 2007-2013] (to T.K.); and the National Health and Medical Research Council of Australia [Grants 1011979 and 511914] (to M.J.C.).
REFERENCES
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K. (2003) Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev 55:509–550 [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon-Treiber S, Höllt V. (2005) Morphine-induced changes of gene expression in the brain. Addict Biol 10:81–89 [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Alvarez-Maubecin V, Thomas G, Williams JT, Grandy DK. (2000) Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol Pharmacol 58:1570–1580 [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. (2006) Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci 26:4118–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Lau EK, Lu H-W, Williams JT. (2012) Desensitization and trafficking of mu-opioid receptors in locus coeruleus neurons: Modulation by kinases. Mol Pharmacol 81:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Doré AJ, Millecamps M, Stone LS, Pineyro G. (2012) Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists. J Neurosci 32:4827–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G. (2003) Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Chieng BC, Christie MJ, Connor M. (2005b) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. (2003) Mu-opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23:10515–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68 [DOI] [PubMed] [Google Scholar]
- Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson G. (2009a) Role of protein kinase C and mu-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci 29:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009b) Involvement of PKC alpha and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of mu-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauldry SA, Bass DA, Cousart SL, McCall CE. (1991) Tumor necrosis factor alpha priming of phospholipase D in human neutrophils. Correlation between phosphatidic acid production and superoxide generation. J Biol Chem 266:4173–4179 [PubMed] [Google Scholar]
- Bellavite P, Corso F, Dusi S, Grzeskowiak M, Della-Bianca V, Rossi F. (1988) Activation of NADPH-dependent superoxide production in plasma membrane extracts of pig neutrophils by phosphatidic acid. J Biol Chem 263:8210–8214 [PubMed] [Google Scholar]
- Berger AC, Whistler JL. (2010) How to design an opioid drug that causes reduced tolerance and dependence. Ann Neurol 67:559–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schröder H, Schulz S, Höllt V. (2004) Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem 89:553–560 [DOI] [PubMed] [Google Scholar]
- Black JW, Leff P. (1983) Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci 220:141–162 [DOI] [PubMed] [Google Scholar]
- Blanchet C, Lüscher C. (2002) Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector. Proc Natl Acad Sci USA 99:4674–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Sollini M, Lüscher C. (2003) Two distinct forms of desensitization of G-protein coupled inwardly rectifying potassium currents evoked by alkaloid and peptide mu-opioid receptor agonists. Mol Cell Neurosci 24:517–523 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin F-T. (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci 22:10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Caron MG. (2004) G protein-coupled receptor kinase/beta-arrestin systems and drugs of abuse: psychostimulant and opiate studies in knockout mice. Neuromolecular Med 5:41–50 [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, et al. (1998) Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci USA 95:9608–9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser RW, Thompson NT, Randall RW, Garland LG. (1989) Phospholipase D activation is functionally linked to superoxide generation in the human neutrophil. Biochem J 264:617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278:18776–18784 [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 210:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd AL, El-Kouhen R, Erickson LJ, Loh HH, Law PY. (1998) Identification of serine 356 and serine 363 as the amino acids involved in etorphine-induced down-regulation of the mu-opioid receptor. J Biol Chem 273:34488–34495 [DOI] [PubMed] [Google Scholar]
- Burford NT, Wang D, Sadée W. (2000) G-protein coupling of mu-opioid receptors (OP3): elevated basal signalling activity. Biochem J 348:531–537 [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Benovic JL. (1998) G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol 8:335–344 [DOI] [PubMed] [Google Scholar]
- Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell 7:421–432 [DOI] [PubMed] [Google Scholar]
- Celver JP, Lowe J, Kovoor A, Gurevich VV, Chavkin C. (2001) Threonine 180 is required for G-protein-coupled receptor kinase 3- and beta-arrestin 2-mediated desensitization of the mu-opioid receptor in Xenopus oocytes. J Biol Chem 276:4894–4900 [DOI] [PubMed] [Google Scholar]
- Celver J, Xu M, Jin W, Lowe J, Chavkin C. (2004) Distinct domains of the μ-opioid receptor control uncoupling and internalization. Mol Pharmacol 65:528–537 [DOI] [PubMed] [Google Scholar]
- Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. (2008) Multiple actions of spinophilin regulate mu opioid receptor function. Neuron 58:238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. (1984) Opioid receptor reserve in normal and morphine-tolerant guinea pig ileum myenteric plexus. Proc Natl Acad Sci USA 81:7253–7257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Geis C, Sommer C. (2008) Activation of TRPV1 contributes to morphine tolerance: involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci 28:5836–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. (2011) Crystal structure of metarhodopsin II. Nature 471:651–655 [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. (1987) Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol 32:633–638 [PubMed] [Google Scholar]
- Christie MJ. (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154:384–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Loh HH, Law PY. (2008) Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell Signal 20:1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. (2010) Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization. Cell Signal 22:684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KY, Rasmussen SG, Liu T, Li S, DeVree BT, Chae PS, Calinski D, Kobilka BK, Woods VL, Jr, Sunahara RK. (2011) Conformational changes in the G protein Gs induced by the β2 adrenergic receptor. Nature 477:611–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CC, Bruchas MR, Lee ML, Chavkin C. (2010) Phosphorylation of the mu-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol 77:339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Vaughan CW, Chieng B, Christie MJ. (1996) Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol 119:1614–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Borgland SL, Christie MJ. (1999) Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. Br J Pharmacol 128:1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Osborne PB, Christie MJ. (2004) Mu-opioid receptor desensitization: is morphine different? Br J Pharmacol 143:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Traynor J. (2010) Constitutively active μ-opioid receptors. Methods Enzymol 484:445–469 [DOI] [PubMed] [Google Scholar]
- Corbett AD, Henderson G, McKnight AT, Paterson SJ. (2006) 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br J Pharmacol 147 (Suppl 1):S153–S162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Herz A. (1989) Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA 86:7321–7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa T, Cotecchia S. (2005) Historical review: Negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol Sci 26:618–624 [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gomes SD, Stockton SD, Lim MP, Devi LA. (2012) Opioid recepor heteromers in analgesia. Expert Rev Mol Med 14:e9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Ginsburg M, Osman OH. (1968) Acute tolerance to narcotic analgesic drugs in rats. Br Pharmacol Chemother 33:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2004) Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci 24:7699–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2005) Morphine-Induced mu-opioid receptor desensitization. Mol Pharmacol 68:1127–1132 [DOI] [PubMed] [Google Scholar]
- Dang VC, Napier IA, Christie MJ. (2009) Two distinct mechanisms mediate acute mu-opioid receptor desensitization in native neurons. J Neurosci 29:3322–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Chieng B, Azriel Y, Christie MJ. (2011) Cellular morphine tolerance produced by βarrestin-2-dependent impairment of μ-opioid receptor resensitization. J Neurosci 31:7122–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165:1704–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas F, Destainville N, Millot C, Lopez A, Dean D, Salomé L. (2003) Confined diffusion without fences of a g-protein-coupled receptor as revealed by single particle tracking. Biophys J 84:356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebBurman SK, Ptasienski J, Benovic JL, Hosey MM. (1996) G protein-coupled receptor kinase GRK2 is a phospholipid-dependent enzyme that can be conditionally activated by G protein betagamma subunits. J Biol Chem 271:22552–22562 [DOI] [PubMed] [Google Scholar]
- Deng HB, Yu Y, Pak Y, O’Dowd BF, George SR, Surratt CK, Uhl GR, Wang JB. (2000) Role for the C-terminus in agonist-induced mu opioid receptor phosphorylation and desensitization. Biochemistry 39:5492–5499 [DOI] [PubMed] [Google Scholar]
- Dighe SV, Madia PA, Sirohi S, Yoburn BC. (2009) Continuous morphine produces more tolerance than intermittent or acute treatment. Pharmacol Biochem Behav 92:537–542 [DOI] [PubMed] [Google Scholar]
- Divin MF, Bradbury FA, Carroll FI, Traynor JR. (2009) Neutral antagonist activity of naltrexone and 6β-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor. Br J Pharmacol 156:1044–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Aicher SA, Montalmant FL, Milner TA. (2005) Redistribution of mu-opioid receptors in C1 adrenergic neurons following chronic administration of morphine. Exp Neurol 196:365–372 [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. (2011) Agonist-selective patterns of µ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Pöll F, Peuker K, Loktev A, Glück L, Schulz S. (2012) Deciphering mu-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol 167:1259–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG. (2009) Phospholipase D in endocytosis and endosomal recycling pathways. Biochim Biophys Acta 1791:845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82:1226–1236 [DOI] [PubMed] [Google Scholar]
- Ebert B, Thorkildsen C, Andersen S, Christrup LL, Hjeds H. (1998) Opioid analgesics as noncompetitive N-methyl-D-aspartate (NMDA) antagonists. Biochem Pharmacol 56:553–559 [DOI] [PubMed] [Google Scholar]
- Ehlert FJ. (1985) The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol Pharmacol 28:410–421 [PubMed] [Google Scholar]
- Eitan S, Bryant CD, Saliminejad N, Yang YC, Vojdani E, Keith D, Jr, Polakiewicz R, Evans CJ. (2003) Brain region-specific mechanisms for acute morphine-induced mitogen-activated protein kinase modulation and distinct patterns of activation during analgesic tolerance and locomotor sensitization. J Neurosci 23:8360–8369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates mu-opioid receptor internalization. J Biol Chem 276:12774–12780 [DOI] [PubMed] [Google Scholar]
- Erspamer V, Melchiorri P, Broccardo M, Erspamer GF, Falaschi P, Improota G, Negri L, Renda T. (1981) The brain-gut-skin triangle: new peptides. Peptides 2 (Suppl 2):7–16 [DOI] [PubMed] [Google Scholar]
- Evans CJ. (2004) Secrets of the opium poppy revealed. Neuropharmacology 47 (Suppl 1):293–299 [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Zhang X, Yue W, Ma L. (2002) Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology 43:809–816 [DOI] [PubMed] [Google Scholar]
- Fan XL, Zhang JS, Zhang XQ, Yue W, Ma L. (2003) Differential regulation of beta-arrestin 1 and beta-arrestin 2 gene expression in rat brain by morphine. Neuroscience 117:383–389 [DOI] [PubMed] [Google Scholar]
- Feng B, Li Z, Wang JB. (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol Pharmacol 79:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32:829–839 [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. (1996) Opioid desensitization: interactions with G-protein-coupled receptors in the locus coeruleus. J Neurosci 16:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölich N, Dees C, Paetz C, Ren X, Lohse MJ, Nikolaev VO, Zenk MH. (2011) Distinct pharmacological properties of morphine metabolites at G(i)-protein and β-arrestin signaling pathways activated by the human μ-opioid receptor. Biochem Pharmacol 81:1248–1254 [DOI] [PubMed] [Google Scholar]
- Fyfe LW, Cleary DR, Macey TA, Morgan MM, Ingram SL. (2010) Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther 335:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabra BH, Bailey CP, Kelly E, Smith FL, Henderson G, Dewey WL. (2008) Pre-treatment with a PKC or PKA inhibitor prevents the development of morphine tolerance but not physical dependence in mice. Brain Res 1217:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaibelet G, Millot C, Lebrun C, Ravault S, Sauliere A, Andre A, Lagane B, Lopez A (2008) Cholesterol content drives distinct pharmacological behaviours of micro-opioid receptor in different microdomains of the CHO plasma membrane. Mol Membr Biol 25:423–435. [DOI] [PubMed]
- Galandrin S, Oligny-Longpré G, Bouvier M. (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28:423–430 [DOI] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. (2004) Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol 66:97–105 [DOI] [PubMed] [Google Scholar]
- Gold SJ, Han MH, Herman AE, Ni YG, Pudiak CM, Aghajanian GK, Liu RJ, Potts BW, Mumby SM, Nestler EJ. (2003) Regulation of RGS proteins by chronic morphine in rat locus coeruleus. Eur J Neurosci 17:971–980 [DOI] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. (1998) Role of arrestins in G-protein-coupled receptor endocytosis. Adv Pharmacol 42:429–433 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Moore S, Bell RM, Dush M. (2003) Functional analysis of a phosphatidic acid binding domain in human Raf-1 kinase: mutations in the phosphatidate binding domain lead to tail and trunk abnormalities in developing zebrafish embryos. J Biol Chem 278:45690–45696 [DOI] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485:400–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL. (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85:395–404 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Bartzsch K, Widera A, Becker A, Höllt V, Kocj T. (2006) Development of tolerance and sensitization to different opioid agoinsts in rats. Psychopharmacology 186:177–184 [DOI] [PubMed] [Google Scholar]
- Grecksch G, Just S, Pierstorff C, Imhof A-K, Gluck L, Doll C, Lupp A, Becker A, Koch T, Stumm R, et al. (2011) Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A μ-opioid receptor knock-in mice. J Neurosci 31:13890–13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer CE, Schmid CL, Jaeger AM, Bohn LM. (2011) Agonist-directed interactions with specific beta-arrestins determine mu-opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem 286:31731–31741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. (2003) Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci 23:4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstock-Debic H, Kim K-A, Yu YJ, von Zastrow M. (2005) Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci 25:7847–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Vaughan CW, Christie MJ. (2003) Modulation of GABA release during morphine withdrawal in midbrain neurons in vitro. Neuropharmacology 45:575–584 [DOI] [PubMed] [Google Scholar]
- Harris GC, Williams JT. (1991) Transient homologous mu-opioid receptor desensitization in rat locus coeruleus neurons. J Neurosci 11:2574–2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. (2002) Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 108:271–282 [DOI] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, Molloy JE, Birdsall NJ. (2010) Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci USA 107:2693–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Moore A, Malkmus S, Murray SF, Dean N, Yaksh TL, Butler M. (2002) Inhibition of spinal protein kinase Calpha expression by an antisense oligonucleotide attenuates morphine infusion-induced tolerance. Neuroscience 113:99–107 [DOI] [PubMed] [Google Scholar]
- Huang P, Xu W, Yoon SI, Chen C, Chong PL, Unterwald EM, Liu-Chen LY. (2007) Agonist treatment did not affect association of mu opioid receptors with lipid rafts and cholesterol reduction had opposite effects on the receptor-mediated signaling in rat brain and CHO cells. Brain Res 1184:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. (2010) The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther 332:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. (1998) Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci 18:10269–10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Macey TA, Fossum EN, Morgan MM. (2008) Tolerance to repeated morphine administration is associated with increase potency of opioid agonists. Neuropsychopharmacology 33:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Ueda H. (2000) Protein kinase C-mediated acute tolerance to peripheral mu-opioid analgesia in the bradykinin-nociception test in mice. J Pharmacol Exp Ther 293:662–669 [PubMed] [Google Scholar]
- Jenkins GM, Frohman MA. (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70:676–685 [DOI] [PubMed] [Google Scholar]
- Johnson EE, Christie MJ, Connor M. (2005) The role of opioid receptor phosphorylation and trafficking in adaptations to persistent opioid treatment. Neurosignals 14:290–302 [DOI] [PubMed] [Google Scholar]
- Kasai RS, Suzuki KG, Prossnitz ER, Koyama-Honda I, Nakada C, Fujiwara TK, Kusumi A. (2011) Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol 192:463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. (1996) Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem 271:19021–19024 [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. (1998) mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol 53:377–384 [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G. (2007) Agonist-selective mechanisms of GPCR desensitization. Br J Pharmacol 153 (Suppl 1):S379–S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (1997) Pharmacologic Analysis of Drug Receptor Interaction, Ed. 3rd Lippincott-Raven, Philadelphia [Google Scholar]
- Kenakin T. (2011) Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 336:296–302 [DOI] [PubMed] [Google Scholar]
- Kennedy C, Henderson G. (1992) Chronic exposure to morphine does not induce dependence at the level of the calcium channel current in human SH-SY5Y cells. Neuroscience 49:937–944 [DOI] [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, et al. (2008) Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol 18:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Mayer P, Raulf E, Höllt V. (1997) Site mutation in the rat mu-opioid receptor demonstrates the involvement of calcium/calmodulin-dependent protein kinase II in agonist-mediated desensitization. J Neurochem 69:1767–1770 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Schröder H, Wolf R, Raulf E, Höllt V. (1998) Carboxyl-terminal splicing of the rat mu opioid receptor modulates agonist-mediated internalization and receptor resensitization. J Biol Chem 273:13652–13657 [DOI] [PubMed] [Google Scholar]
- Koch T, Kroslak T, Averbeck M, Mayer P, Schröder H, Raulf E, Höllt V. (2000) Allelic variation S268P of the human mu-opioid receptor affects both desensitization and G protein coupling. Mol Pharmacol 58:328–334 [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schröder H, Kahl E, Höllt V. (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276:31408–31414 [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Schulz S, Liang Y, Klein J, Hollt V. (2003) ADP-ribosylation factor-dependent phospholipase D2 activation is required for agonist-induced mu-opioid receptor endocytosis. J Biol Chem 278:9979–9985 [DOI] [PubMed] [Google Scholar]
- Koch T, Brandenburg LO, Liang Y, Schulz S, Beyer A, Schröder H, Höllt V. (2004) Phospholipase D2 modulates agonist-induced mu-opioid receptor desensitization and resensitization. J Neurochem 88:680–688 [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Höllt V. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol 67:280–287 [DOI] [PubMed] [Google Scholar]
- Koch T, Wu DF, Yang LQ, Brandenburg LO, Höllt V. (2006) Role of phospholipase D2 in the agonist-induced and constitutive endocytosis of G-protein coupled receptors. J Neurochem 97:365–372 [DOI] [PubMed] [Google Scholar]
- Koch T, Höllt V. (2008) Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther 117:199–206 [DOI] [PubMed] [Google Scholar]
- Koch T, Seifert A, Wu DF, Rankovic M, Kraus J, Börner C, Brandenburg LO, Schröder H, Höllt V. (2009) mu-opioid receptor-stimulated synthesis of reactive oxygen species is mediated via phospholipase D2. J Neurochem 110:1288–1296 [DOI] [PubMed] [Google Scholar]
- Kovoor A, Nappey V, Kieffer BL, Chavkin C. (1997) Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in xenopus oocytes. J Biol Chem 272:27605–27611 [DOI] [PubMed] [Google Scholar]
- Kovoor A, Celver JP, Wu A, Chavkin C. (1998) Agonist induced homologous desensitization of mu-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol Pharmacol 54:704–711 [PubMed] [Google Scholar]
- Kroslak T, Laforge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. (2007) The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem 103:77–87 [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. (1998) The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol 38:289–319 [DOI] [PubMed] [Google Scholar]
- Kuszak AJ, Pitchiaya S, Anand JP, Mosberg HI, Walter NG, Sunahara RK. (2009) Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 284:26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sunkaraneni S, Sirohi S, Dighe SV, Walker EA, Yoburn BC. (2008) Hydromorphone efficacy and treatment protocol impact on tolerance and mu-opioid receptor regulation. Eur J Pharmacol 597:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagane B, Gaibelet G, Meilhoc E, Masson JM, Cézanne L, Lopez A. (2000) Role of sterols in modulating the human mu-opioid receptor function in Saccharomyces cerevisiae. J Biol Chem 275:33197–33200 [DOI] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. (2011) Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking β-arrestin 2. Mol Pain 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal 4:ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. (2000) Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol 40:389–430 [DOI] [PubMed] [Google Scholar]
- Lazar DF, Medzihradsky F. (1992) Altered microviscosity at brain membrane surface induces distinct and reversible inhibition of opioid receptor binding. J Neurochem 59:1233–1240 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Stadel JM, Caron MG. (1983) Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem 52:159–186 [DOI] [PubMed] [Google Scholar]
- Lemberg KK, Kontinen VK, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA. (2006) Antinociception by spinal and systemic oxycodone: why does the route make a difference? In vitro and in vivo studies in rats. Anesthesiology 105:801–812 [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. (2009) Reward processing by the opioid system in the brain. Physiol Rev 89:1379–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt ES, Clark MJ, Jenkins PM, Martens JR, Traynor JR. (2009) Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J Biol Chem 284:22108–22122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AH, Wang HL. (2001) G protein-coupled receptor kinase 2 mediates mu-opioid receptor desensitization in GABAergic neurons of the nucleus raphe magnus. J Neurochem 77:435–444 [DOI] [PubMed] [Google Scholar]
- Lin HY, Law PY, Loh HH. (2012) Activation of protein kinase C (PKC)α or PKCε as an approach to increase morphine tolerance in respiratory depression and lethal overdose. J Pharmacol Exp Ther 341:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling GS, Paul D, Simantov R, Pasternak GW. (1989) Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci 45:1627–1636 [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Lavie Y, Tang X. (1999) Localization and possible functions of phospholipase D isozymes. Biochim Biophys Acta 1439:245–263 [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X. (2000) Phospholipase D: molecular and cell biology of a novel gene family. Biochem J 345:401–415 [PMC free article] [PubMed] [Google Scholar]
- Liu JG, Ruckle MB, Prather PL. (2001) Constitutively active mu-opioid receptors inhibit adenylyl cyclase activity in intact cells and activate G-proteins differently than the agonist [D-Ala2,N-MePhe4,Gly-ol5]enkephalin. J Biol Chem 276:37779–37786 [DOI] [PubMed] [Google Scholar]
- Liu JG, Anand KJ. (2001) Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev 38:1–19 [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. (2001) Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol 60:53–62 [DOI] [PubMed] [Google Scholar]
- Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, Li QF, Jeffry J, Kim JY, Loh HH, et al. (2011) Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 147:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. (2001) Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci 14:1091–1104 [DOI] [PubMed] [Google Scholar]
- Macé G, Miaczynska M, Zerial M, Nebreda AR. (2005) Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J 24:3235–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. (2009) Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther 331:412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Lowe JD, Chavkin C. (2006) Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem 281:34515–34524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. (2009) Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 207:413–422 [DOI] [PubMed] [Google Scholar]
- Mahmoud S, Margas W, Trapella C, Caló G, Ruiz-Velasco V. (2010) Modulation of silent and constitutively active nociceptin/orphanin FQ receptors by potent receptor antagonists and Na+ ions in rat sympathetic neurons. Mol Pharmacol 77:804–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BH, Mao J, Frenk H, Price DD, Mayer DJ. (1996) Continuous co-administration of dextromethorphan or MK-801 with morphine: attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain 67:79–88 [DOI] [PubMed] [Google Scholar]
- Martini L, Whistler JL. (2007) The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol 17:556–564 [DOI] [PubMed] [Google Scholar]
- Matsui A, Williams JT. (2010) Activation of μ-opioid receptors and block of Kir3 potassium channels and NMDA receptor conductance by L- and D-methadone in rat locus coeruleus. Br J Pharmacol 161:1403–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383:819–823 [DOI] [PubMed] [Google Scholar]
- McCulloch DA, Lutz EM, Johnson MS, Robertson DN, MacKenzie CJ, Holland PJ, Mitchell R. (2001) ADP-ribosylation factor-dependent phospholipase D activation by VPAC receptors and a PAC(1) receptor splice variant. Mol Pharmacol 59:1523–1532 [DOI] [PubMed] [Google Scholar]
- McDermott M, Wakelam MJ, Morris AJ. (2004) Phospholipase D. Biochem Cell Biol 82:225–253 [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Chavkin C. (2001) Tyrosine phosphorylation of the mu-opioid receptor regulates agonist intrinsic efficacy. Mol Pharmacol 59:1360–1368 [DOI] [PubMed] [Google Scholar]
- McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. (2006) Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol 176:7039–7050 [DOI] [PubMed] [Google Scholar]
- McPhail LC, Qualliotine-Mann D, Waite KA. (1995) Cell-free activation of neutrophil NADPH oxidase by a phosphatidic acid-regulated protein kinase. Proc Natl Acad Sci USA 92:7931–7935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J, Rivero G, Baptist M, Llorente J, Al-Sabah S, Krasel C, Dewey WL, Bailey CP, Rosethorne EM, Charlton SJ, et al. (2010) μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 78:756–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. (1995) The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci 15:2396–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CJ. (2009) Inhibition of EGF-induced ERK/MAP kinase-mediated astrocyte proliferation by mu opioids: integration of G protein and beta-arrestin 2-dependent pathways. J Neurochem 110:662–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjanger E, Yaksh TL. (1991) Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther 258:544–550 [PubMed] [Google Scholar]
- Molinari P, Vezzi V, Sbraccia M, Grò C, Riitano D, Ambrosio C, Casella I, Costa T. (2010) Morphine-like opiates selectively antagonize receptor-arrestin interactions. J Biol Chem 285:12522–12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Christie MJ. (2011) Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol 164:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulédous L, Díaz MF, Gutstein HB. (2007) Extracellular signal-regulated kinase (ERK) inhibition does not prevent the development or expression of tolerance to and dependence on morphine in the mouse. Pharmacol Biochem Behav 88:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller DL, Unterwald EM. (2004) In vivo regulation of extracellular signal-regulated protein kinase (ERK) and protein kinase B (Akt) phosphorylation by acute and chronic morphine. J Pharmacol Exp Ther 310:774–782 [DOI] [PubMed] [Google Scholar]
- Narita M, Ioka M, Suzuki M, Narita M, Suzuki T. (2002) Effect of repeated administration of morphine on the activity of extracellular signal regulated kinase in the mouse brain. Neurosci Lett 324:97–100 [DOI] [PubMed] [Google Scholar]
- Newton PM, Kim JA, McGeehan AJ, Paredes JP, Chu K, Wallace MJ, Roberts AJ, Hodge CW, Messing RO. (2007) Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav 6:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Vitek LV. (1980) The effect of chronic morphine treatment of excitatory junction potentials in the mouse vas deferens. Br J Pharmacol 68:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. (1999) Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 274:32248–32257 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275:17201–17210 [DOI] [PubMed] [Google Scholar]
- Ogier-Denis E, Pattingre S, El Benna J, Codogno P. (2000) Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275:39090–39095 [DOI] [PubMed] [Google Scholar]
- Oldfield S, Braksator E, Rodriguez-Martin I, Bailey CP, Donaldson LF, Henderson G, Kelly E. (2008) C-terminal splice variants of the mu-opioid receptor: existence, distribution and functional characteristics. J Neurochem 104:937–945 [DOI] [PubMed] [Google Scholar]
- Osborne PB, Williams JT. (1995) Characterization of acute homologous desensitization of mu-opioid receptor-induced currents in locus coeruleus neurones. Br J Pharmacol 115:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. (1995) Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci 15:1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita A, Escribá PV, Ventayol P, Murga C, Mayor F, Jr, García-Sevilla JA. (1998) Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem 70:1249–1257 [DOI] [PubMed] [Google Scholar]
- Pak Y, O’Dowd BF, George SR. (1997) Agonist-induced desensitization of the mu opioid receptor is determined by threonine 394 preceded by acidic amino acids in the COOH-terminal tail. J Biol Chem 272:24961–24965 [DOI] [PubMed] [Google Scholar]
- Paronis CA, Woods JH. (1997) Ventilation in morphine-maintained rhesus monkeys. II: Tolerance to the antinociceptive but not the ventilatory effects of morphine. J Pharmacol Exp Ther 282:355–362 [PubMed] [Google Scholar]
- Pasternak GW. (2001) Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci 22:67–70 [DOI] [PubMed] [Google Scholar]
- Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. (2007) Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol 563:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock RL, Hentges ST. (2011) Differential expression and sensitivity of presynaptic and postsynaptic opioid receptors regulating hypothalamic proopiomelanocortin neurons. J Neurosci 31:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppel K, Boekhoff I, McDonald P, Breer H, Caron MG, Lefkowitz RJ. (1997) G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J Biol Chem 272:25425–25428 [DOI] [PubMed] [Google Scholar]
- Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, Kress HG, Langford R, Likar R, Raffa RB, et al. (2008) Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 8:287–313 [DOI] [PubMed] [Google Scholar]
- Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, Lowe J, Low MJ, Pintar JE, Chavkin C. (2007) The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 146:1795–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeyro G, Archer-Lahlou E. (2007) Ligand-specific receptor states: implications for opiate receptor signalling and regulation. Cell Signal 19:8–19 [DOI] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Dorner LF, Kansra V, Comb MJ. (1998) A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J Biol Chem 273:12402–12406 [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. (2007) Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol 69:511–534 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Law PY, Loh HH. (2003) Mu-opioid receptor desensitization: role of receptor phosphorylation, internalization, and representation. J Biol Chem 278:36733–36739 [DOI] [PubMed] [Google Scholar]
- Qiu Y, Wang Y, Law PY, Chen HZ, Loh HH. (2011) Cholesterol regulates micro-opioid receptor-induced beta-arrestin 2 translocation to membrane lipid rafts. Mol Pharmacol 80:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. (2011) Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci 31:4434–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Schmid CL, Groer CE, Bohn LM. (2011) Functional selectivity at the μ-opioid receptor: implications for understanding opioid analgesia and tolerance. Pharmacol Rev 63:1001–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. (2011) The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, Violin JD, Lefkowitz RJ. (2011) Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol 80:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic M, Jacob L, Rankovic V, Brandenburg LO, Schröder H, Höllt V, Koch T. (2009) ADP-ribosylation factor 6 regulates mu-opioid receptor trafficking and signaling via activation of phospholipase D2. Cell Signal 21:1784–1793 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, DeVree BT, Zou Y, et al. (2011) Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh A, Cooper A, Guy-David L, Reuveny E. (2010) Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell 143:750–760 [DOI] [PubMed] [Google Scholar]
- Ravindranathan A, Joslyn G, Robertson M, Schuckit MA, Whistler JL, White RL. (2009) Functional characterization of human variants of the mu-opioid receptor gene. Proc Natl Acad Sci USA 106:10811–10816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero G, Llorente J, McPherson J, Cooke A, Mundell SJ, McArdle CA, Rosethorne EM, Charlton SJ, Krasel C, Bailey CP, Henderson G, Kelly E (2012) Endomorphin-2: A Biased Agonist at the μ Opioid Receptor. Mol Pharmacol. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Rodriguez-Martin I, Braksator E, Bailey CP, Goodchild S, Marrion NV, Kelly E, Henderson G. (2008) Methadone: does it really have low efficacy at micro-opioid receptors? Neuroreport 19:589–593 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, et al. (2011) Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469:236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FB, Smith MT. (1997) The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain 73:151–157 [DOI] [PubMed] [Google Scholar]
- Rossi F, Grzeskowiak M, Della Bianca V, Calzetti F, Gandini G. (1990) Phosphatidic acid and not diacylglycerol generated by phospholipase D is functionally linked to the activation of the NADPH oxidase by FMLP in human neutrophils. Biochem Biophys Res Commun 168:320–327 [DOI] [PubMed] [Google Scholar]
- Sally EJ, Xu H, Dersch CM, Hsin LW, Chang LT, Prisinzano TE, Simpson DS, Giuvelis D, Rice KC, Jacobson AE, et al. (2010) Identification of a novel “almost neutral” micro-opioid receptor antagonist in CHO cells expressing the cloned human mu-opioid receptor. Synapse 64:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samways DS, Henderson G. (2006) Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal 18:151–161 [DOI] [PubMed] [Google Scholar]
- Saulière-Nzeh Ndong A, Millot C, Corbani M, Mazères S, Lopez A, Salomé L. (2010) Agonist-selective dynamic compartmentalization of human Mu opioid receptor as revealed by resolutive FRAP analysis. J Biol Chem 285:14514–14520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Schulz S, Klutzny M, Koch T, Händel M, Höllt V. (2000) Involvement of mitogen-activated protein kinase in agonist-induced phosphorylation of the mu-opioid receptor in HEK 293 cells. J Neurochem 74:414–422 [DOI] [PubMed] [Google Scholar]
- Schulz R, Wüster M, Krenss H, Herz A. (1980) Lack of cross-tolerance on multiple opiate receptors in the mouse vas deferens. Mol Pharmacol 18:395–401 [PubMed] [Google Scholar]
- Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. (2004) Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J 23:3282–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Strasser RH, Benovic JL, Daniel K, Lefkowitz RJ. (1986) Phosphorylation/dephosphorylation of the β-adrenergic receptor regulates its functional coupling to adenylate cyclase and subcellular distribution. Proc Natl Acad Sci USA 83:9408–9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. (2000) Chronic heroin self-administration desensitizes mu opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci 20:4555–4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Lohmann AB, Dewey WL. (1999) Involvement of phospholipid signal transduction pathways in morphine tolerance in mice. Br J Pharmacol 128:220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Elzey MJ, Dewey WL. (2003) The expression of a high level of morphine antinociceptive tolerance in mice involves both PKC and PKA. Brain Res 985:78–88 [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127:129–139 [DOI] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. (2010) Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev 62:701–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soignier RD, Vaccarino AL, Fanti KA, Wilson AM, Zadina JE. (2004) Analgesic tolerance and cross-tolerance to i.c.v. endomorphin-1, endomorphin-2, and morphine in mice. Neurosci Lett 366:211–214 [DOI] [PubMed] [Google Scholar]
- Sosnowski M, Yaksh TL. (1990) Differential cross-tolerance between intrathecal morphine and sufentanil in the rat. Anesthesiology 73:1141–1147 [DOI] [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. (2001) mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacol Biochem Behav 69:233–237 [DOI] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, Evans C, Brecha NC. (1996) Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci USA 93:9241–9246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyaert J, Kobilka BK. (2011) Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol 21:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Yaksh TL. (1989) Time course characteristics of tolerance development to continuously infused antinociceptive agents in rat spinal cord. J Pharmacol Exp Ther 251:216–223 [PubMed] [Google Scholar]
- Stockton SD, Jr, Devi LA. (2012) Functional relevance of μ-δ opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend 121:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ritchie K, Kajikawa E, Fujiwara T, Kusumi A. (2005) Rapid hop diffusion of a G-protein-coupled receptor in the plasma membrane as revealed by single-molecule techniques. Biophys J 88:3659–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminath G, Deupi X, Lee TW, Zhu W, Thian FS, Kobilka TS, Kobilka B. (2005) Probing the beta2 adrenoceptor binding site with catechol reveals differences in binding and activation by agonists and partial agonists. J Biol Chem 280:22165–22171 [DOI] [PubMed] [Google Scholar]
- Tan M, Groszer M, Tan AM, Pandya A, Liu X, Xie CW. (2003) Phosphoinositide 3-kinase cascade facilitates mu-opioid desensitization in sensory neurons by altering G-protein-effector interactions. J Neurosci 23:10292–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Walwyn WM, Evans CJ, Xie CW. (2009) p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A-adrenergic receptors in neurons. J Biol Chem 284:6270–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz M, von Zastrow M. (2003) A novel endocytic recycling signal that distinguished the membrane trafficking of naturally occurring opioid receptors. J Biol Chem 278:45978–45986 [DOI] [PubMed] [Google Scholar]
- Tanowitz M, Hislop JN, von Zastrow M. (2008) Alternative splicing determines the post-endocytic sorting fate of G-protein-coupled receptfet alors. J Biol Chem 283:35614–35621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Jin W, Cheong YP, Lowe J, Caron MG, Lefkowitz RJ, Chavkin C. (2004) G-protein receptor kinase 3 (GRK3) influences opioid analgesic tolerance but not opioid withdrawal. Br J Pharmacol 141:55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Ortiz J, Guitart X, Nestler EJ. (1994) Chronic morphine administration increases beta-adrenergic receptor kinase (beta ARK) levels in the rat locus coeruleus. J Neurochem 63:1983–1986 [DOI] [PubMed] [Google Scholar]
- Thompson AA, Liu W, Chun E, Katritch V, Wu H, Vardy E, Huang XP, Trapella C, Guerrini R, Calo G, et al. (2012) Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. (1999) Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension 34:976–982 [DOI] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Basbaum AI. (2004) [d-Ala2,N-MePhe4,Gly-ol5]enkephalin-induced internalization of the μ-opioid receptor in the spinal cord of morphine tolerant rats. Neuroscience 125:541–543 [DOI] [PubMed] [Google Scholar]
- Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. (2005) Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry 44:6133–6143 [DOI] [PubMed] [Google Scholar]
- Trujillo KA and Akil H (1991) Inhibition of morphine tolerance and dependence by the NMDA receptor antagonists MK-801 Science 251:85-87. [DOI] [PubMed]
- Tsao PI, von Zastrow M. (2000) Downregulation of G protein-coupled receptors. Curr Opin Neurobiol 10:365–369 [DOI] [PubMed] [Google Scholar]
- Umans JG, Inturrisi CE. (1981) Pharmacodynamics of subcutaneously administered diacetylmorphine, 6-acetylmorphine and morphine in mice. J Pharmacol Exp Ther 218:409–415 [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. (2001) Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience 108:467–477 [DOI] [PubMed] [Google Scholar]
- Vergarajauregui S, San Miguel A, Puertollano R. (2006) Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization. Traffic 7:686–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk MS, Williams JT. (2008) Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol 73:1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M. (2010) Regulation of opioid receptors by endocytic membrane traffic: mechanisms and translational implications. Drug Alcohol Depend 108:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. (2003) Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol 13:348–353 [DOI] [PubMed] [Google Scholar]
- Waite KA, Wallin R, Qualliotine-Mann D, McPhail LC. (1997) Phosphatidic acid-mediated phosphorylation of the NADPH oxidase component p47-phox. Evidence that phosphatidic acid may activate a novel protein kinase. J Biol Chem 272:15569–15578 [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. (2004) Opioid receptors. Annu Rev Biochem 73:953–990 [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. (2001) Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 154:131–142 [DOI] [PubMed] [Google Scholar]
- Walwyn WM, Wei W, Xie CW, Chiu K, Kieffer BL, Evans CJ, Maidment NT. (2006) Mu opioid receptor-effector coupling and trafficking in dorsal root ganglia neurons. Neuroscience 142:493–503 [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. (2007) Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci 27:5092–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadée W. (2004) Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther 308:512–520 [DOI] [PubMed] [Google Scholar]
- Wang HL. (2000) A cluster of Ser/Thr residues at the C-terminus of mu-opioid receptor is required for G protein-coupled receptor kinase 2-mediated desensitization. Neuropharmacology 39:353–363 [DOI] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Hsu CY, Huang PC, Chow YW, Li AH. (2002) Identification of two C-terminal amino acids, Ser(355) and Thr(357), required for short-term homologous desensitization of mu-opioid receptors. Biochem Pharmacol 64:257–266 [DOI] [PubMed] [Google Scholar]
- Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. (2012) Reduced expression of the mu opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience 205:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ma W, Chabot JG, Quirion R. (2009) Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J 23:2576–2586 [DOI] [PubMed] [Google Scholar]
- Wang Z, Chabot JG, Quirion R. (2011) On the possible role of ERK, p38 and CaMKII in the regulation of CGRP expression in morphine-tolerant rats. Mol Pain 7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Kinscheck IB, Mayer DJ. (1984) Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide. Science 224:395–396 [DOI] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. (2008) Structural insights into G-protein-coupled receptor activation. Curr Opin Struct Biol 18:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfield GH, Rasmussen SG, Su M, Dutta S, DeVree BT, Chung KY, Calinski D, Velez-Ruiz G, Oleskie AN, Pardon E, et al. (2011) Structural flexibility of the G alpha s alpha-helical domain in the beta2-adrenoceptor Gs complex. Proc Natl Acad Sci USA 108:16086–16091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. (1998) Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci USA 95:9914–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. (1999) Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron 23:737–746 [DOI] [PubMed] [Google Scholar]
- Williams JT, Christie MJ, Manzoni O. (2001) Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev 81:299–343 [DOI] [PubMed] [Google Scholar]
- Wolf R, Koch T, Schulz S, Klutzny M, Schröder H, Raulf E, Bühling F, Höllt V. (1999) Replacement of threonine 394 by alanine facilitates internalization and resensitization of the rat mu opioid receptor. Mol Pharmacol 55:263–268 [DOI] [PubMed] [Google Scholar]
- Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, et al. (2012) Structure of the human κ-opioid receptor in complex with JDTic. Nature 485:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LQ, Seifert A, Wu DF, Wang X, Rankovic V, Schröder H, Brandenburg LO, Höllt V, Koch T. (2010) Role of phospholipase D2/phosphatidic acid signal transduction in micro- and delta-opioid receptor endocytosis. Mol Pharmacol 78:105–113 [DOI] [PubMed] [Google Scholar]
- Yang S-D, Fong Y-L, Benovic JL, Sibley DR, Caron MG, Lefkowitz RJ. (1988) Dephosphorylation of the β 2-adrenergic receptor and rhodopsin by latent phosphatase 2. J Biol Chem 263:8856–8858 [PubMed] [Google Scholar]
- Yu Y, Zhang L, Yin X, Sun H, Uhl GR, Wang JB. (1997) Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem 272:28869–28874 [DOI] [PubMed] [Google Scholar]
- Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. (2009) Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J Neurosci 29:222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Dhavan R, Chevalier MW, Yudowski GA, von Zastrow M. (2010) Rapid delivery of internalized signaling receptors to the somatodendritic surface by sequence-specific local insertion. J Neurosci 30:11703–11714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, Basbaum AI. (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94:245–253 [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. (1998) Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA 95:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhao H, Qiu Y, Loh HH, Law PY. (2009) Src phosphorylation of micro-receptor is responsible for the receptor switching from an inhibitory to a stimulatory signal. J Biol Chem 284:1990–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Chu J, Qiu Y, Loh HH, Law PY. (2008a) Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci USA 105:9421–9426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. (2008b) Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol 73:178–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouwail S, Pettitt TR, Dove SK, Chibalina MV, Powner DJ, Haynes L, Wakelam MJ, Insall RH. (2005) Phospholipase D activity is essential for actin localization and actin-based motility in Dictyostelium. Biochem J 389:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]