Abstract
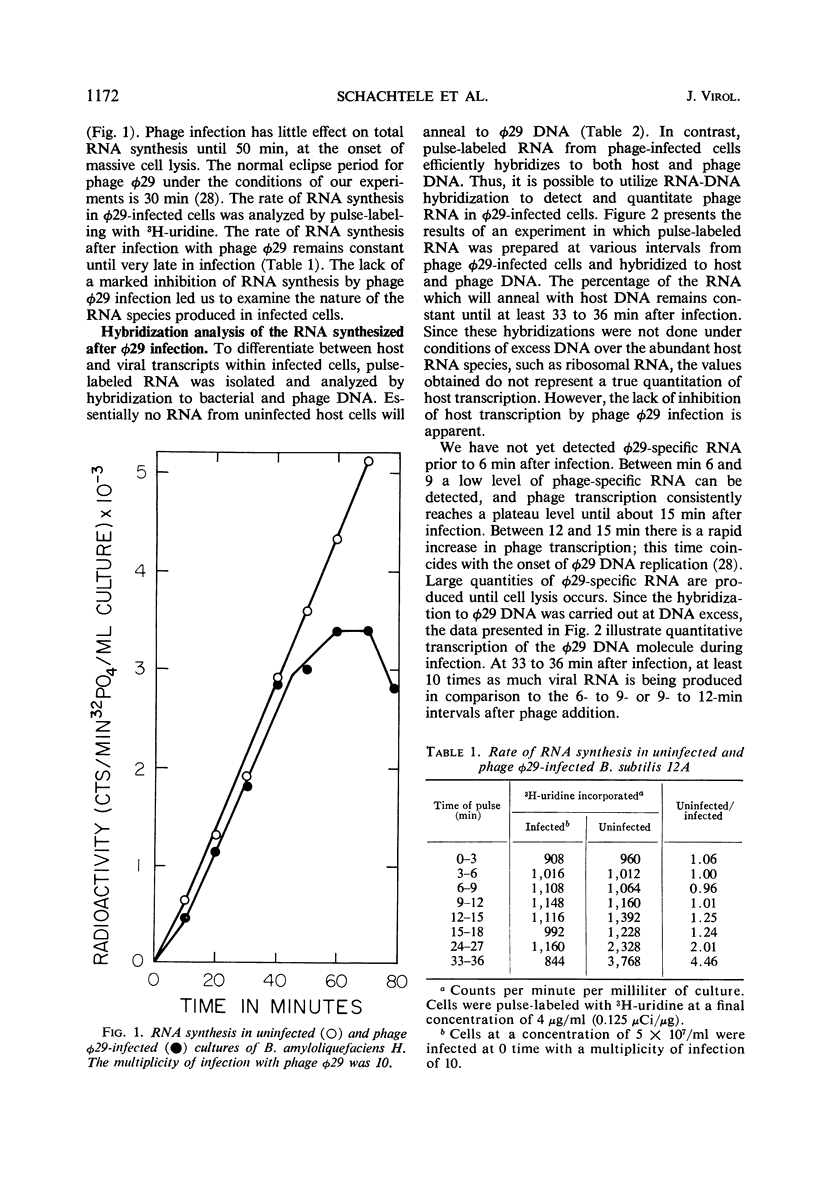
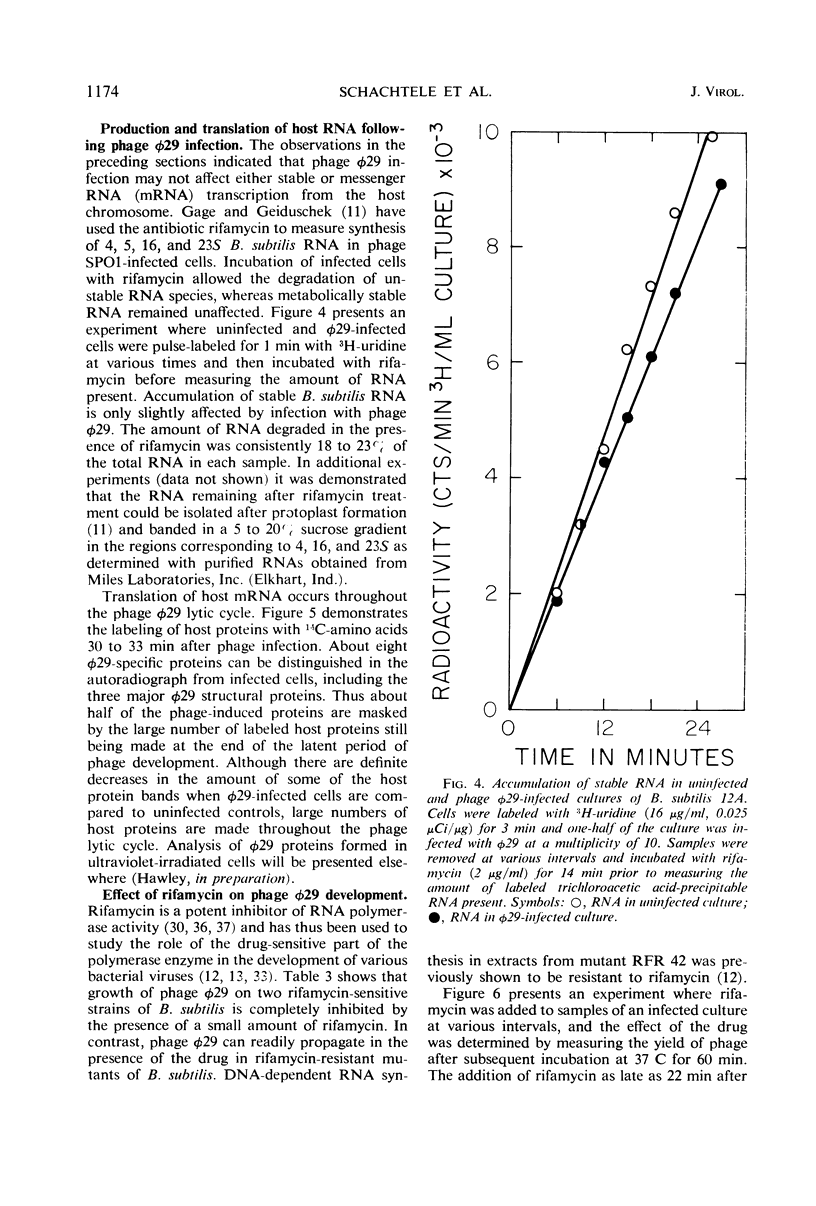
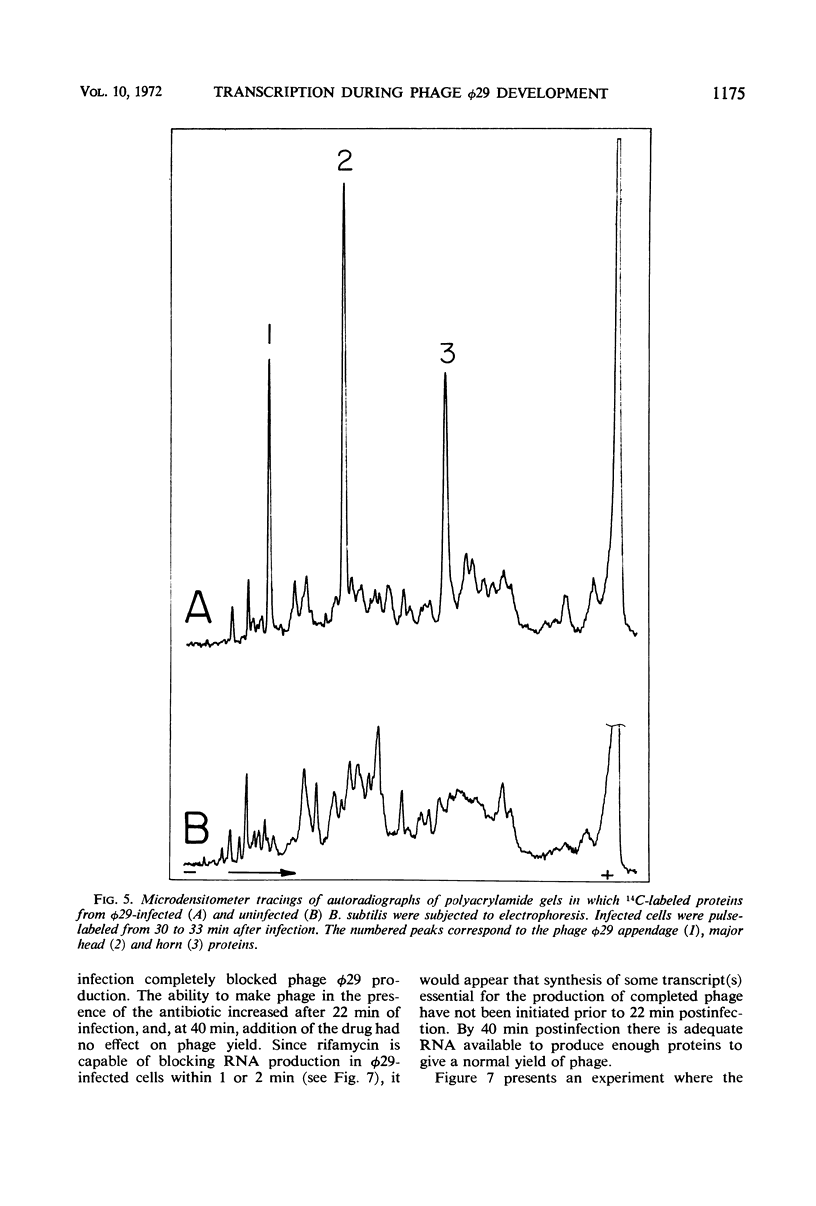
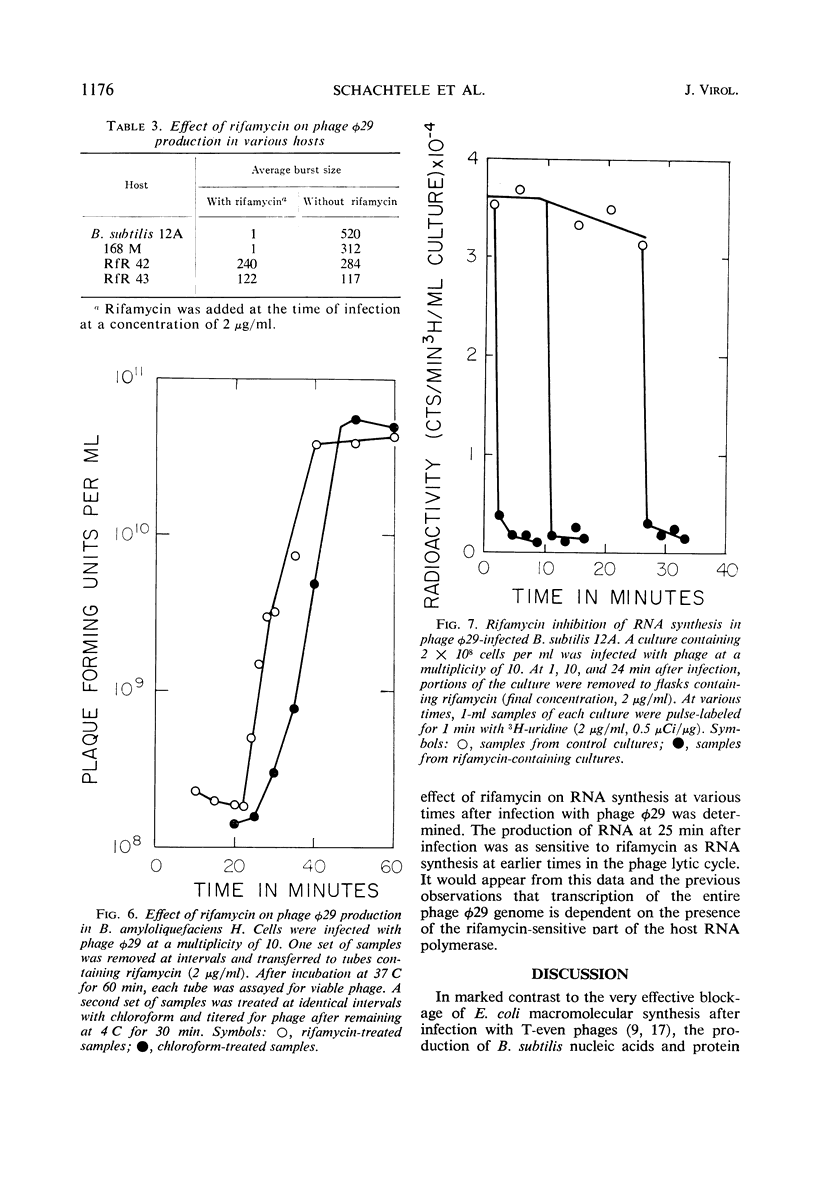
The synthesis of ribonucleic acid (RNA) during development of the virulent Bacillus subtilis bacteriophage φ29 has been analyzed. Transcription of host deoxyribonucleic acid (DNA) continues at the preinfection rate throughout the latent period of viral growth. RNA-DNA hybridization was used to show that host messenger RNA synthesis continues late into the phage lytic cycle. Amino acid-labeling experiments show that this RNA is continuously used to produce protein. Ribosomal RNA production is not inhibited by phage infection. Small quantities of phage-specific RNA first appear between min 6 and 9 after infection. This RNA is made exclusively from one of the φ29 DNA strands. At 12 min postinfection, when phage DNA replication commences, large quantities of viral RNA start to be synthesized. This RNA appears to be transcribed from both strands of φ29 DNA. Studies with rifamycin and rifamycin-resistant host strains showed that the production of all phage φ29-specific RNA requires those components of the host RNA polymerase which are sensitive to this antibiotic. Thus, phage φ29 does not stop transcription of host DNA and may produce only one element for regulation of transcription of its own DNA. These findings may reflect the limited amount of genetic information carried by this phage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez G., Salas E., Pérez N., Celis J. E. Phi29 bacteriophage structural proteins. J Gen Virol. 1972 Mar;14(3):243–250. doi: 10.1099/0022-1317-14-3-243. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Mosharrafa E. T. Physical and biological properties of phage phi 29 deoxyribonucleic acid. J Virol. 1968 Oct;2(10):1185–1190. doi: 10.1128/jvi.2.10.1185-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Fujita D. J., Ohlsson-Wilhelm B. M., Geiduschek E. P. Transcription during bacteriophage SPO1 development: mutations affecting the program of viral transcription. J Mol Biol. 1971 Apr 28;57(2):301–317. doi: 10.1016/0022-2836(71)90348-2. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Hagen E. W., Zeece V. M., Anderson D. L. A genetic study of temperature-sensitive mutants of the Bacillus subtilis bacteriophage phi 29. Virology. 1971 Mar;43(3):561–568. doi: 10.1016/0042-6822(71)90281-9. [DOI] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R., Brown L. R., Doi R. H. The effect of rifampin on the production of beta22 phage by Bacillus subtilis. Biochem Biophys Res Commun. 1969 Nov 6;37(4):559–566. doi: 10.1016/0006-291x(69)90845-6. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in Bacillus subtilis infected with bacteriophage beta-22. J Virol. 1970 Oct;6(4):381–392. doi: 10.1128/jvi.6.4.381-392.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D. Inhibition of host protein synthesis during infection of Escherichia coli by bacteriophage T4. I. Continued synthesis of host ribonucleic acid. J Virol. 1968 Nov;2(11):1262–1271. doi: 10.1128/jvi.2.11.1262-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Hemphill H. E., Whiteley H. R. Nucleic acid synthesis in bacteriophage SPO2c 1 -infected Bacillus subtilis. J Virol. 1972 May;9(5):776–784. doi: 10.1128/jvi.9.5.776-784.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liljemark W. F., Anderson D. L. Morphology and physiology of the intracellular development of Bacillus subtilis bacteriophage phi25. J Virol. 1970 Jul;6(1):114–124. doi: 10.1128/jvi.6.1.114-124.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharrafa E. T., Schachtele C. F., Reilly B. E., Anderson D. L. Complementary Strands of Bacteriophage phi29 Deoxyribonucleic Acid: Preparative Separation and Transcription Studies. J Virol. 1970 Dec;6(6):855–864. doi: 10.1128/jvi.6.6.855-864.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez E., Ramírez G., Salas M., Viñuela E. Structural proteins of bacteriophage phi 29. Virology. 1971 Sep;45(3):567–576. doi: 10.1016/0042-6822(71)90172-3. [DOI] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Pène J. J. Host macromolecular synthesis in bacteriophage-infected Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):379–386. [PMC free article] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Bolle A., Epstein R. Transcription during bacteriophage T4 development: a demonstration that distinct subclasses of the "early" RNA appear at different times and that some are "turned off" at late times. J Mol Biol. 1970 Apr 28;49(2):271–295. doi: 10.1016/0022-2836(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Hagen E. W., Anderson D. L. Temperature-shift analysis of bacteriophage phi 29 gene expression in Bacillus amyloliquefaciens. J Virol. 1971 Sep;8(3):352–354. doi: 10.1128/jvi.8.3.352-354.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Oman R. W., Anderson D. L. Effect of elevated temperature on deoxyribonucleic acid synthesis in bacteriophage phi-29-infected Bacillus amyloliquefaciens. J Virol. 1970 Oct;6(4):430–437. doi: 10.1128/jvi.6.4.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Rogers P. Canavanine death in Escherichia coli. J Mol Biol. 1965 Dec;14(2):474–489. doi: 10.1016/s0022-2836(65)80197-8. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Oyama Y., Nakajima K., Yura T. Role of host RNA polymerase for lambda phage development. Biochem Biophys Res Commun. 1969 Aug 15;36(4):533–538. doi: 10.1016/0006-291x(69)90337-4. [DOI] [PubMed] [Google Scholar]
- Talavera A., Jimenez F., Salas M., Viñuela E. Temperature-sensitive mutants of bacteriophage phi-29. Virology. 1971 Dec;46(3):586–595. doi: 10.1016/0042-6822(71)90062-6. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Nüesch J., Knüsel F., Staehelin M. Action of rifamycins on RNA polymerase. Biochim Biophys Acta. 1968 Mar 18;157(1):215–217. doi: 10.1016/0005-2787(68)90285-2. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. B., Ennis H. L. Potassium requirement for synthesis of macromolecules in Bacillus subtilis infected with bacteriophage 2C. J Virol. 1969 Jan;3(1):1–7. doi: 10.1128/jvi.3.1.1-7.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


