Abstract
Innate immune signaling associated with Toll like receptors (TLRs) is a key pathway involved in the progression of nonalcoholic steatohepatitis (NASH). Here we show that both TLR2 and palmitic acid are required for activation of the inflammasome, IL-1α and IL-1β, resulting in the progression of NASH. Wild type (WT) and TLR2−/− mice were fed a choline deficient amino acid defined (CDAA) diet for 22 weeks to induce NASH. Bone marrow transplanted-TLR2 chimeric mice were generated after the recipient mice were lethally irradiated. Kupffer cells and hepatic stellate cells (HSCs) were isolated from WT mice and stimulated with TLR2 ligand and/or palmitic acid. WT mice on the CDAA diet developed profound steatohepatitis and liver fibrosis. In contrast, TLR2−/− mice had suppressed progression of NASH. While both Kupffer cells and HSCs respond to TLR2 ligand, TLR2 bone marrow chimeric mice demonstrated that Kupffer cells were relatively more important than HSCs in TLR2-mediated progression of NASH. In vitro, palmitic acid alone did not increase TLR2 signaling-target genes including cytokines and inflammasome components in Kupffer cells and HSCs. The TLR2 ligand increased Nod-like receptor protein 3, an inflammasome component, in Kupffer cells, but not in HSCs. In the presence of TLR2 ligand, palmitic acid did induce caspase-1 activation and release of IL-1α and IL-1β in Kupffer cells, however, these effects were not observed in HSCs. In vivo, WT on the CDAA diet showed increased caspase-1 activation in the liver and elevated serum levels of IL-1α and IL-1β levels, which were suppressed in TLR2−/− mice. Conclusion: TLR2 and palmitic acid cooperatively activate inflammasome in Kupffer cells/macrophages in the development of NASH.
Keywords: Kupffer cells, hepatic stellate cells, nalp3, caspase-1, interleukin-1β
Introduction
Nonalcoholic fatty liver disease (NAFLD), the hepatic consequence of the metabolic syndrome, is a significant public health issue in developed countries. A spectrum of NAFLD ranges from simple steatosis to steatosis with progressive liver inflammation and fibrosis, termed nonalcoholic steatohepatitis (NASH), eventually causing liver cirrhosis that may increase the prevalence of hepatocellular carcinoma (1, 2). However, majority of mechanisms involved in the multifactorial events in the development of NASH remain unresolved.
The hepatic innate immune system is activated in the pathogenesis of NASH (3, 4). Toll-like receptors (TLRs) are pattern recognition signal receptors for sensing bacterial and viral derived signature motifs to activate the innate immune system (5). TLR signaling functions in the host defense against invading pathogens through induction of proinflammatory cytokines in immune cells. However, the overactivation of TLR signaling or the breakdown of TLR tolerance produce a large amount of inflammatory cytokines, which ultimately leads to tissue damage (6). Among the 13 TLRs identified in mammals, TLR2, TLR4, and TLR9 are reported to be associated with steatohepatitis (4, 7, 8). TLR4 and TLR9 have been shown to promote hepatic inflammation and fibrosis in experimental NASH (8, 9). However, the role of TLR2 in the pathogenesis of NAFLD is controversial. TLR2 deficiency protected mice from hepatic steatosis induced by high fat diet (HFD) (10, 11). The NASH model induced by methionine and choline deficient (MCD) diet showed that loss of TLR2 increased susceptibility to pathogen-associated steatohepatitis (7, 12). These conflicting data imply the complexity of TLR2-mediated NAFLD.
Inflammasome activation is a pathway required for processing IL-1β and IL-18 from their proforms to active forms through cleavage by caspase-1. Recent reports have demonstrated that loss of inflammasome components protects mice from NAFLD induced by a high fat diet (13, 14). In addition, inactivation of IL-1 receptor signaling attenuates mouse models of NASH (9, 15). These data suggest that the inflammasome contributes to the development of NAFLD. Two-signal hypothesis is accepted in inflammasome activation: the first signal produces pro-IL-1β and inflammasome components, and the second signal assembles inflammasome components to engage in caspase-1 activation. TLRs including TLR2 activate the first signals, and the second signals are activated by danger-associated molecular patterns such as extracellular ATP and urate crystals. Emerging evidence demonstrated that free fatty acids (FFAs) activate the inflammasome (16, 17) as well as TLRs (18–20), which implicate FFAs as promoters of NASH. The elevated plasma FFA concentration in patients with the metabolic syndrome including NAFLD (21) may contribute to the development of NAFLD.
It is often debated which cell types respond to TLR ligands and are responsible for inflammasome activation. Macrophages including Kupffer cells are primary cells to activate the inflammasome and produce TLR-induced cytokines. Hepatic non-immune cells including hepatic stellate cells (HSCs) and hepatocytes also respond to TLR ligands and are the site of inflammasome activation (17, 22).
Here we show that TLR2 is a crucial molecule that promotes liver injury, inflammation and fibrosis in mice fed a choline deficient amino acid defined (CDAA) diet that develop NASH accompanied by obesity and insulin resistance (9, 23). TLR2 signaling increased the expression of proinflammatory cytokines including IL-1β and an inflammasome component, Nod-like receptor protein (NLRP) 3. In cooperation with palmitic acid, the most abundant free fatty acid in the plasma of NAFLD (16, 24), TLR2 signaling activates inflammasome primarily in Kupffer cells, but not in HSCs or hepatocytes.
Materials and Methods
Animals and diet
Wild type (WT) C57BL/6 mice and TLR2−/− mice purchased from Jackson Laboratories (Bar Harbor, ME) were bred in the UCSD vivarium. TLR2−/− mice were backcrossed at least 10 generations onto the C57BL/6 background and displayed a similar hepatic phenotype as WT mice under standard laboratory chow. Male mice were divided into two groups at 8-week-old: choline-supplemented L-amino Acid Defined Diet (CSAA) (Catalog# 518754, Dyets Inc. Bethlehem, PA) and choline-deficient L-amino Acid Defined Diet (Catalog# 518753, Dyets Inc.). Each group included 7 to 10 mice. These diets were continued for 22 weeks without any interruption. The food intake on the CDAA diet was monitored for one month, and similarity in food intake was observed between WT and TLR2−/− mice (Supplementary Figure 1).
To generate chimeric mice, liposomal clodronate was injected intravenously to deplete resident hepatic macrophages one day before the bone marrow transplantation. Bone marrow cells (1×107 cells) obtained from WT or TLR2−/− mice were transplanted through tail veins after the recipient mice were lethally irradiated (10 Gy). CDAA or CSAA diet started eight weeks after bone marrow transplantation. Each group included 5 to 9 mice. The mice received humane care according to US National Institutes of Health recommendations outlined in the “Guide for the Care and Use of Laboratory Animals”. All animal experiments were approved by the University of California San Diego and Akita University Institutional Animal Care and Use Committee.
Histological examination
Hematoxylin and eosin (H-E), Oil Red O, Sirius Red, immunohistochemical staining for αSMA (Dako Cytomation, Kyoto, Japan), F4/80 (eBioScience, San Diego, CA) and Ly6C (Abcam, Cambridge, MA) were performed (9). Sirius red positive area was measured on 10 low power (x40) fields/slide and quantified with the use of NIH imaging software. NAFLD activity score was determined according to the published criteria (25). F4/80-and Ly6C-positive cells were counted on 10 high power (200x) fields per slide.
Quantitative real-time PCR analysis
RNA extracted from livers and cells was converted to cDNA by reverse transcription. Then quantitative real-time PCR were performed using ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster city, CA). Genes were normalized to 18S RNA as an internal control. Sequences of primers were summarized in Supplementary Table 1.
Lipid isolation and measurement
Hepatic lipids were isolated as previously described (9). Triglyceride, total cholesterol and free fatty acid contents were measured using Triglyceride E (Wako, Osaka, Japan), Cholesterol E (Wako), and NEFA C-test (Wako) according to the manufacturer’s instruction.
Western blot
Protein extracts were electrophoresed and then blotted. Blots were incubated with antibody forα SMA (Sigma, St. Louis, MO) or caspase-1 (Millipore, Temecula, CA).
Measurement for ALT, caspase-1 activity, insulin, IL-1α, IL-1β, and TNFα
A kit for measuring serum alanine transaminase (ALT) levels was obtained from Wako and a capase-1 activity assay kit (catalogue #BF141000) was purchased from R&D (Minneapolis, MN). Enzyme-linked immunoabsorbent assay (ELISA) kits were used for measuring insulin (Shibayagi, Gunma, Japan), IL-1β, IL-1α and TNFα (eBioscience). Insulin resistance was assessed by HOMA-IR [immunoreactive insulin (µU/ml)×FBS (mg/dL)÷405] (26).
Cells and treatment
Kupffer cells, HSCs, and hepatocytes were isolated from mice as previously described (9). In experiments using hepatocytes, 200 µL of liposomal clodronate was injected intravenously one day before isolation to deplete contaminated Kupffer cells. We have confirmed the intact responses of these hepatocytes to TNFα, IL-6 and insulin as assessed by activation of NF-κB, STAT3 and Akt (data not shown). Hepatocytes and Kupffer cells were cultured in serum free media and HSCs were cultured with 1% FBS containing DMEM for 16 hours before stimulation with reagents. Pam3CK4 (5 µg/ml) (EMC Microcollection, Tuebingen, Germany), CpG-ODN (5 µg/ml) (ODN1826: 5’-tccatgacgttcctgacgtt-3’) (invitrogen) and palmitic acid (200 µM) (Sigma) were used to stimulate liver cells.
Statistical analysis
Differences between two groups were compared using Mann Whitney U test. Differences between multiple groups were compared using one-way ANOVA (Dr. SPSS II); p<0.05 was considered significant.
Results
TLR2−/− mice exhibit less inflammation in CDAA diet-induced NASH
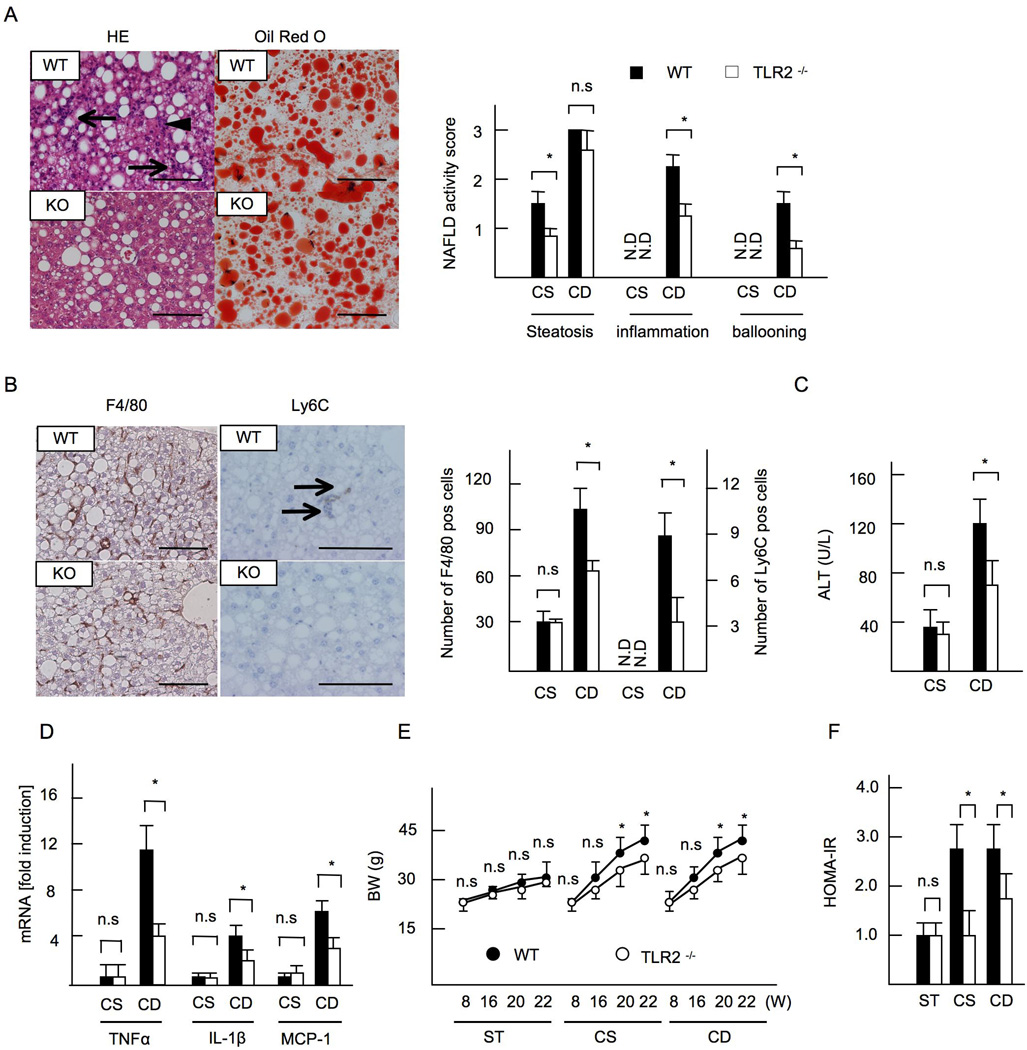
As we reported previously (9), mice on a CDAA diet for 22 weeks resulted in severe steatosis, inflammatory cell infiltration, and hepatocyte ballooning (Figure 1A). TLR2−/− mice on a CDAA diet had similar grades of steatosis (Figure 1A) and hepatic triglyceride content as WT mice (Table 1), whereas inflammatory cell infiltration and hepatocyte ballooning were markedly attenuated in TLR2−/− livers (Figure 1A). Accordingly, the NAFLD activity score in TLR2−/− mice was significantly lower than that in WT mice (Total score, WT versus TLR2−/− mice = 6.5 versus 4.6, p<0.05) (Figure 1A). WT livers on the CDAA diet increased infiltration of inflammatory cells that express F4/80 and Ly6C, while the recruitment of inflammatory cells was decreased in TLR2−/− livers (Figure 1B). Serum ALT levels (Figure 1C) and the expressions of proinflammatory cytokines and chemokines (Figure 1D) were also decreased in TLR2−/− mice in comparison to WT mice. The WT mice fed the CDAA diet also displayed obesity and insulin resistance (Figure 1E, 1F). While similar metabolic phenotypes were shown between WT and TLR2−/− mice fed on standard chow (Table 1), the changes in weight gain and insulin resistance were blunted in TLR2−/− mice on the CDAA diet (Figure 1E, 1F, Table 1). Moderate steatosis was seen in control CSAA diet fed mice, but inflammation was not evident in these animals (Figure 1 A-D).
Figure 1. TLR2−/− mice exhibit less inflammation in CDAA-induced NASH.
WT and TLR2−/− mice were fed CSAA diet (CS) or CDAA diet (CD) for 22 weeks. Closed bars indicate WT mice and open bars represent TLR2−/− mice. (A, left) Hematoxylin and eosin (HE) and oil red O staining. Liver sections on CDAA diet are presented. The grades of steatosis were similar levels in WT and TLR2−/− mice whereas inflammatory cell infiltration (arrows) and hepatocyte ballooning (arrow head) were blunted in TLR2−/− mice. Original magnification,×400 for HE and oil red O staining. Bar 100µm. (A, right) NAFLD activity score. (B) Immunohistochemical staining for F4/80 and Ly6C. Liver sections on CDAA diet are presented. Infiltration of F4/80- and Ly6C-positive cells was suppressed in TLR2−/− mice. Original magnifications,×400 for F4/80,×600 for Ly6C. Bar 100 µm. (B, right) The numbers of F4/80-positive and Ly6C-positve cells. (C) Serum ALT levels. (D) mRNA expression of TNFα, IL-1β, and MCP-1. Genes were normalized to 18S RNA as an internal control. (E, F) Data on mice fed standard chow (ST) were included. (E) Body weight. (F) HOMA-IR. ND; not detected. Data represent mean ±SD, *p<0.05. n.s.; not significant.
Table 1.
Body/Liver/Fat weight and Lipid Level at 22 weeks after the CSAA and CDAA diet feeding.
| Standard chow | CSAA | CDAA | ||||
|---|---|---|---|---|---|---|
| WT mice (n = 7) |
TLR2−/− mice (n = 7) |
WT mice (n = 10) |
TLR2−/− mice (n = 7) |
WT mice (n = 10) |
TLR2−/− mice (n = 9) |
|
| Body weight (g, start, wk=0) | 22.5 ± 0.95 | 21.4 ± 0.65 | 21.5 ± 1.13 | 22.9 ± 0.93 | 23.0 ± 1.74 | 21.0 ± 0.82 |
| Body weight (g, end, wk=22) | 30.9 ± 1.22 | 29.5 ± 0.73 | 42.0 ± 2.52a | 33.8 ± 1.75 | 42.8 ± 2.02a | 33.5 ± 1.94c |
| Liver weight (g) | 1.22 ± 0.14 | 1.15 ± 0.22 | 1.88 ± 0.21a | 1.37 ± 0.53 | 2.54 ± 0.38a,b | 1.54 ± 0.28c |
| Liver weight (%) | 3.71 ± 0.75 | 3.80 ± 0.66 | 4.44 ± 0.73a | 4.05 ± 0.68 | 5.50 ± 0.64a,b | 5.11 ± 0.58c |
| Epididymal fat (g) | 0.96 ± 0.22 | 0.87 ± 0.36 | 2.24 ± 0.76a | 1.25 ± 0.51 | 2.39 ± 0.52a | 1.01 ± 0.34c |
| Epididymal fat (%) | 2.85 ± 0.34 | 2.95 ± 0.54 | 4.82 ± 0.91a | 4.00 ± 0.77 | 4.70 ± 0.75a | 3.53 ± 0.67c |
| Plasma | ||||||
| Triglyceride (mg/dl) | 36.5 ± 6.25 | 33.8 ± 4.82 | 55.3 ± 7.55 | 48.8 ± 6.12 | 70.5 ± 10.6a | 51.1 ± 8.76 |
| Total cholesterol (mg/dl) | 70.3 ± 6.32 | 66.9 ± 7.12 | 143 ± 12.8a | 99.1 ± 15.8 | 99.6 ± 8.69 | 82.8 ± 7.93 |
| Free fatty acid (mEq/L) | 0.40 ± 0.11 | 0.35 ± 0.08 | 0.47 ± 0.19 | 0.41 ± 0.13 | 0.50 ± 0.21 | 0.36 ± 0.12 |
| Liver | ||||||
| Triglyceride (mg/g liver) | 33.5 ± 3.91 | 30.7 ± 3.67 | 80.4 ± 8.81a | 54.3 ± 6.92 | 155 ± 19.5a,b | 150 ± 20.6 |
| Total cholesterol (mg/g liver) | 11.6 ± 2.56 | 10.6 ± 1.68 | 17.5 ± 2.56 | 12.6 ± 1.05 | 23.7 ± 3.09a | 15.1 ± 2.61 |
| Free fatty acid (mEq/g liver) | 0.60 ± 0.16 | 0.57 ± 0.17 | 2.79 ± 0.85a | 2.55 ± 0.51 | 3.08 ± 0.88a | 2.48 ± 0.62 |
Note: Values are mean ± SD.
Significantly different from WT on Standard chow, p<0.05.
Significantly different from WT on CSAA diet, p<0.05.
Significantly different from WT on CDAA, p<0.05.
Liver fibrosis is suppressed in TLR2−/− mice after CDAA diet feeding
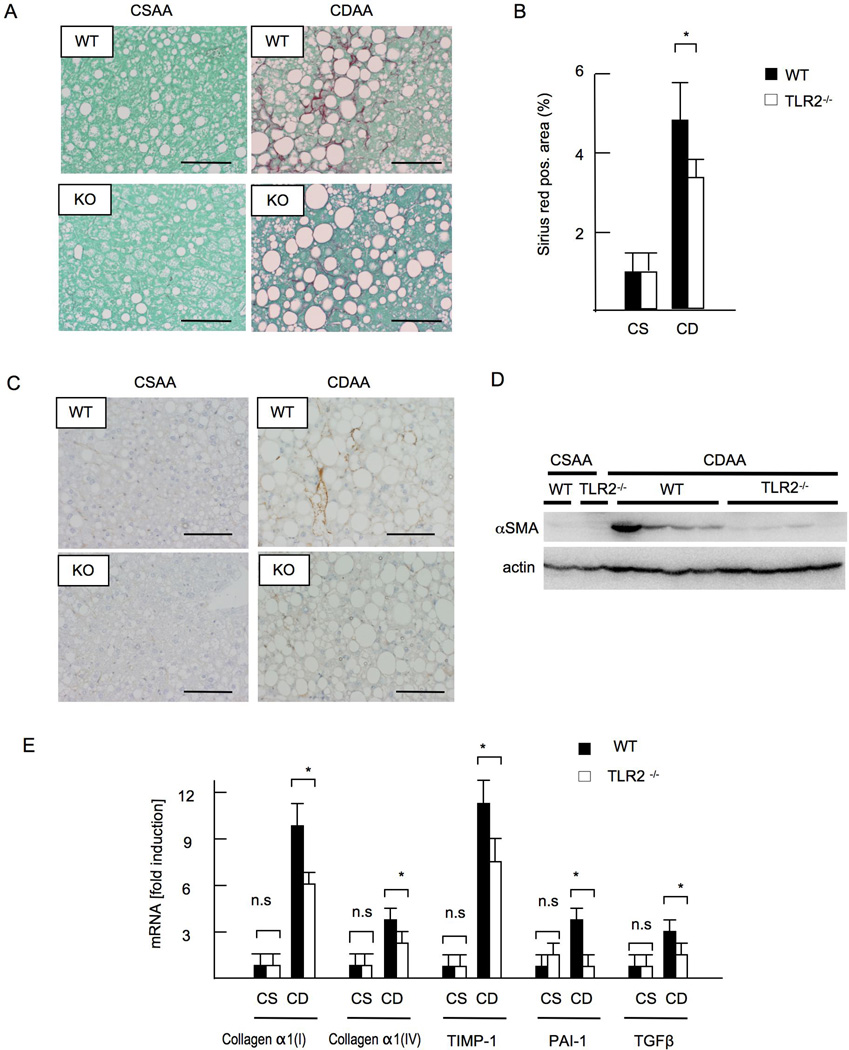
Liver fibrosis is a major manifestation of advanced NASH. The CDAA diet for 22 weeks resulted in perisinusoidal fibrosis in WT mice as assessed by Sirius red staining (Figure 2A, 2B). In contrast, TLR2−/− mice did not show significant liver fibrosis (Figure 2A, 2B). αSMA expression was also attenuated in TLR2−/− mice as examined by immunohistochemistry (Figure 2C) and immunoblotting (Figure 2D). Hepatic mRNA levels of collagen α1(I), collagen α1(IV), transforming growth factor β1 (TGF-β1, tissue inhibitor of metalloproteinase-1 (TIMP-1) and plasminogen activator inhibitor-1 (PAI-1) were decreased in TLR2−/− mice compared with those in WT mice (Figure 2E). Neither WT nor TLR2−/− mice fed a control CSAA diet developed liver fibrosis or HSC activation (Figure 2 A -2E).
Figure 2. TLR2−/− mice develop less liver fibrosis.
WT and TLR2−/− mice were fed CSAA diet (CS) or CDAA diet (CD) for 22 weeks. Closed bars represent WT mice and open bars indicate TLR2−/− mice. (A) Sirius red staining. Original magnification,×400. Bar 100 µm. (B) Sirius red positive area. Liver fibrosis was attenuated in TLR2−/− mice. (C) Immunohistochemical staining for αSMA. Original magnification,×400. Bar 100 µm. (D) Western blotting for αSMA. (E) mRNA expression of fibrogenic genes. Genes were normalized to 18S RNA as an internal control. Data represent mean ±SD, *p<0.05. n.s.; not significant.
A TLR2 ligand activates both Kupffer cells and HSCs
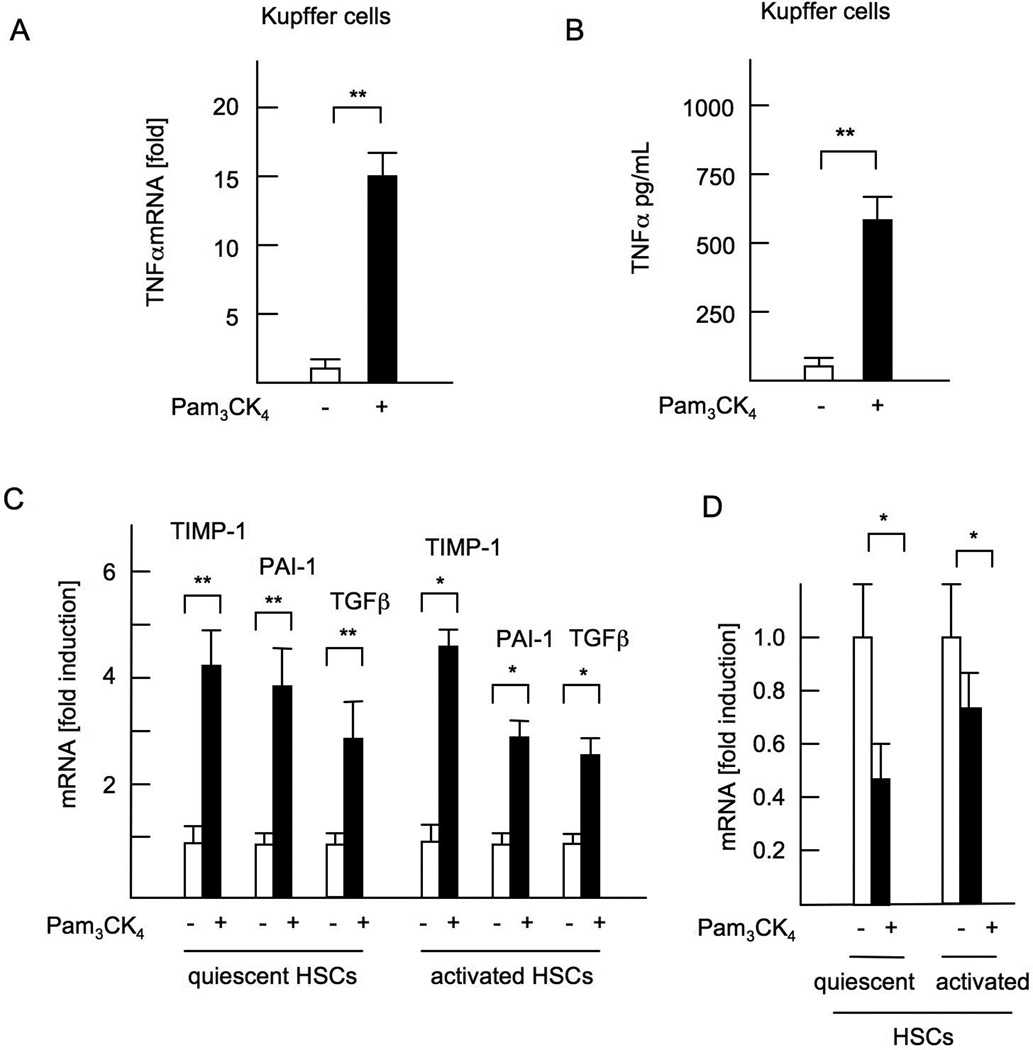
Since Kupffer cells and HSCs are primary cells involved in hepatic inflammation and fibrosis, we examined whether cultured Kupffer cells and HSCs respond to a TLR2 ligand (27). Treatment with Pam3CK4, a synthetic TLR2 ligand, increased TNFα levels in WT-Kupffer cells at the mRNA (Figure 3A) and protein levels (Figure 3B). In both quiescent and culture-activated HSCs, Pam3CK4 treatment increased mRNA expression of fibrogenic genes including TIMP-1, PAI-1, and TGF-β 1 (Figure 3C), and decreased mRNA expression of Bambi, a decoy receptor for TGF-β receptor signaling (Figure 3D), demonstrating that TLR2 ligands induce a profibrogenic phenotype in HSCs.
Figure 3. A synthetic TLR2 ligand activates both Kupffer cells and HSCs.
WT Kupffer cells and HSCs were isolated and cultured in the presence of 5 µg/ml Pam3CK4. (A) mRNA expression of TNFα in Kupffer cells. (B) TNFα concentrations in the supernatant from WT Kupffer cell-culture. (C) mRNA expression of fibrogenic genes in quiescent HSCs and culture-activated HSCs. (D) Bambi mRNA expression in HSCs. Genes were normalized to 18S RNA as an internal control. Data represent mean ±SD, *p<0.05. **p<0.01. n.s.; not significant.
Hematopoietic cells including Kupffer cells contribute to progression of liver inflammation and fibrosis
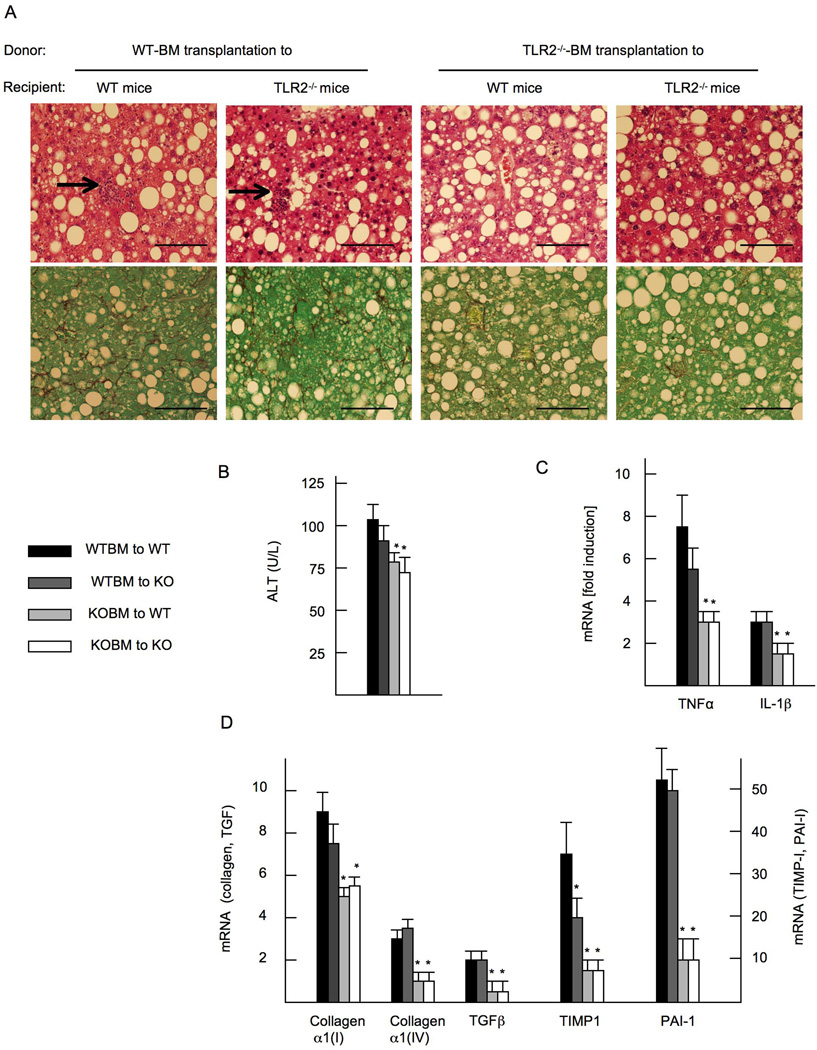
Since both Kupffer cells and HSCs may contribute to TLR2-mediated NASH and fibrosis, we wanted to determine the responsible cells that express functional TLR2 for the development of steatohepatitis. We generated TLR2 bone marrow (BM) chimeric mice, in which hepatic macrophages were reconstituted with transplanted BM cells. As previously reported, more than 90% of Kupffer cells were replaced with transplanted BM cells three months after BM transplantation(23, 28–30). Mice transplanted with WT BM (WT BM cells transplanted to WT mice or TLR2−/− mice) on the CDAA diet feeding showed severe steatosis and inflammatory cell infiltration (Figure 4A). In contrast, mice transplanted with TLR2−/− BM (TLR2−/− BM cells transplanted to WT mice or TLR2−/− mice) had less inflammatory cell infiltration (Figure 4A). Increases in serum ALT and hepatic mRNA levels of inflammatory cytokines were suppressed in mice transplanted with TLR2−/− BM cells (Figure 4B, 4C). These results indicate that hematopoietic cells including Kupffer cells are the primary cell types involved in hepatic inflammation mediated by TLR2. Consistent with the histopathology of liver inflammation, liver fibrosis was attenuated in mice reconstituted with TLR2−/− BM compared to mice with WT BM as assessed by Sirius red staining (Figure 4A). Hepatic mRNA levels of fibrogenic genes were significantly suppressed in mice transplanted with TLR2−/− BM (Figure 4D). Thus, hematopoietic cells including Kupffer cells, but not HSCs, are responsible for TLR2-mediated liver inflammation and fibrosis in NASH.
Figure 4. Hematopoietic cells including Kupffer cells are crucial for the development of liver inflammation and fibrosis in NASH.
TLR2 bone marrow (BM) chimeric mice, in which hepatic macrophages were reconstituted with transplanted BM cells, were generated. Mice on CDAA diet are presented. (A) HE staining (upper) and Sirius red staining (lower). WT BM-transplanted mice show inflammatory cell infiltration (arrows), which are blunted in TLR2−/− BM transplanted mice. Liver fibrosis is attenuated in TLR2−/− BM transplanted mice. Original magnification, × 400. Bar 100 µm. (B) Serum ALT levels. (C) mRNA expression of TNFα and IL-1β. (D) mRNA expression of fibrogenic genes. Genes were normalized to 18S RNA as an internal control. Data represent mean ±SD, *p<0.05.
TLR2 ligand and palmitic acid cooperatively activate the inflammasome in Kupffer cells
We sought to elucidate the mechanism by which Kupffer cells are the primary cell types in TLR2-mediated NASH. Since palmitic acid has been reported to be a TLR2 ligand(19, 20), we tested whether palmitic acid can produce TLR2-mediated cytokines that are induced by Pam3CK4 (Figure 3). In contrast to previous studies (18, 19), WT Kupffer cells did not increase TNFα and CD68 in response to palmitic acid, ranging from 10 to 500 µM (Supplementary Figure 2A, 2B). Furthermore, palmitic acid did not increase mRNA expression of fibrogenic factors in HSCs (Supplementary Figure 2C). These data indicate that palmitic acid alone would not function as a TLR ligand in Kupffer cells or HSCs.
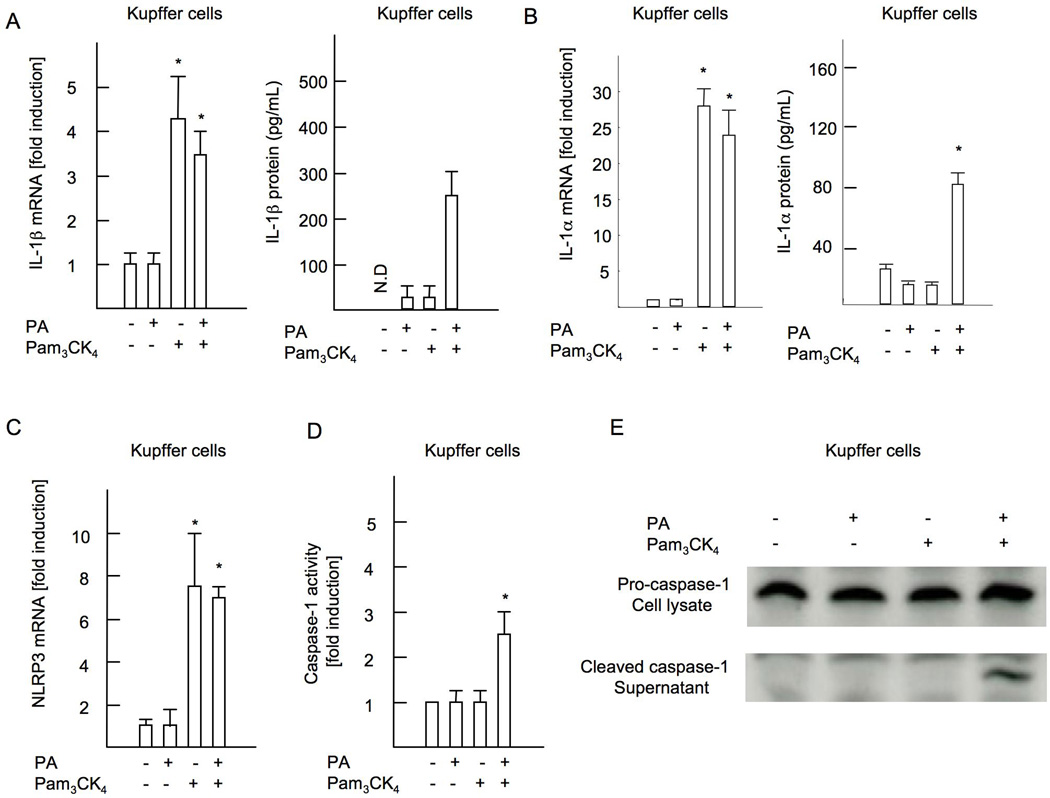
Next, we tested whether palmitic acid contributes to inflammasome activation. Pam3CK4 increased mRNA expression of IL-1β and IL-1α in WT Kupffer cells, but palmitic acid did not (Figure 5A, B). Neither Pam3CK4 nor palmitic acid increased IL-1β mRNA in HSCs (Supplementary Figure 3A). We then measured the active form of IL-1β and IL-1α in supernatant. In the presence of Pam3CK4, palmitic acid induced secretion of active form of IL-1β and IL-1α from Kupffer cells (Figure 5B, D). In contrast, HSCs did not secrete IL-1β by the same treatment (Supplementary Figure 3B), and palmitic acid or Pam3CK4 alone did not induce IL-1β secretion in either Kupffer cells or HSCs, suggesting that two-step stimulation with TLR2 ligand and palmitic acid is required for the inflammasome activation. To assess inflammasome activation by TLR2 ligands and palmitic acid, we measured the expression of inflammasome components including NLRP3, ASC, NLRP1 and AIM2. Among them, Pam3CK4 induced NLRP3 expression in Kupffer cells, but not in HSCs (Figure 5C, Supplementary Figure 3C), and NLRP3 was not induced by palmitic acid treatment alone (Figure 5C). In agreement with the findings shown above, Kupffer cells primed with Pam3CK4 increased caspase-1 activity in response to palmitic acid (Figure 5D, E). As expected, palmitic acid did not activate caspase-1 in HSCs primed with Pam3CK4 (Supplementary Figure 3D). We further examined hepatocytes to respond to Pam3CK4 and palmitic acid. Primary hepatocytes isolated from mice pretreated with liposomal clodronate to deplete Kupffer cells in vivo did not increase levels of IL-1β mRNA and protein, NLRP3 mRNA and caspase-1 activity by Pam3CK4 and palmitic acid treatment (Supplementary Figure 4A-D). TNFα production was also not seen in these hepatocytes in response to Pam3CK4 (Supplementary Figure 4E, F). These results indicate that hepatocytes are not the responsible cell types for TLR2-mediated inflammasome activation.
Figure 5. Palmitic acid activates inflammasome in cooperation with TLR2 ligand in Kupffer cells.
Kupffer cells were isolated from WT mice and cultured in the presence of 5 µg/ml Pam3CK4 and/or 200 µM palmitic acid. (A) mRNA expression of IL-1β and active IL-1β in the supernatant. (B) mRNA expression of IL-1α and active IL-1α in the supernatant. (C) mRNA expression in NLRP3. Genes were normalized to 18S RNA as an internal control. (D) Caspase-1 activity. (E) Immunobotting for the active form of caspase-1 is shown. N.D; not detected. Data represent mean ±SD, *p<0.05.
Diminished inflammasome activation in TLR2−/− mice
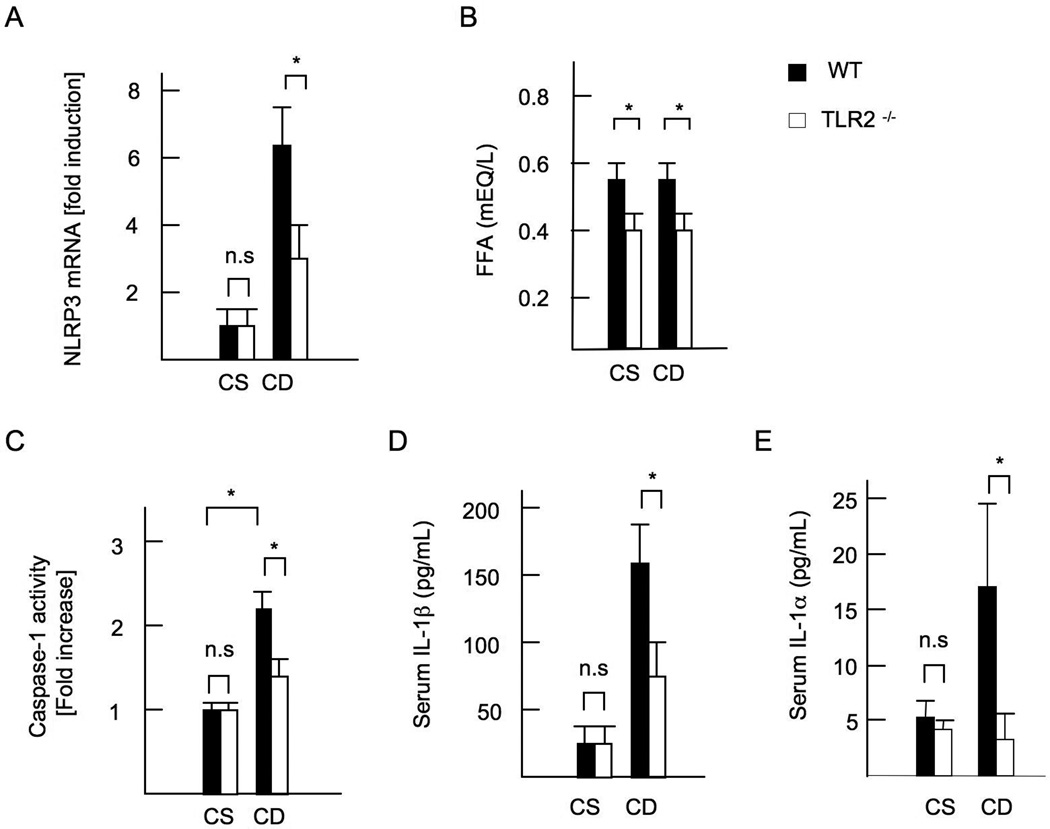
Finally, we investigated inflammasome activation in vivo. WT mice on the CDAA diet increased expressions of IL-1β mRNA (Figure 1F) and the inflammasome component NLRP3 in the liver (Figure 6A). In contrast, this induction was diminished in TLR2−/− mice liver (Figure 1F, 6A). Moreover, portal vein FFA levels were lower in TLR2−/− mice than in WT mice (Figure 6B). In addition, WT mice on the CDAA diet exhibited increased hepatic caspase-1 activity and serum levels of IL-1β and IL-1α (Figure 6C-E) whereas TLR2−/− mice had lower activity of caspase-1 in the liver and decreased IL-1β and IL-1α levels in sera (Figure 6C-E). These results demonstrate that TLR2 is required for activation of the inflammasome in mice fed the CDAA diet.
Figure 6. Inflammasome activation is abolished in TLR2−/− mice.
WT and TLR2−/− mice were fed control CSAA (CS) diet, and CDAA (CD) diet for 22 weeks. Closed bars represent WT mice and open bars indicate TLR2−/− mice. (A) Hepatic mRNA expression of NLRP3. Genes were normalized to 18S RNA as an internal control. (B) FFA concentrations in the portal vein. (C) Caspase-1 activation in the liver. (D) Serum IL-1β concentrations. (E) Serum IL-1α concentrations. N.D; not detected. Data represent mean ±SD, *p<0.05. n.s.; not significant.
The collaborative effect of TLR2 and TLR9 ligands on cytokine production in Kupffer cells
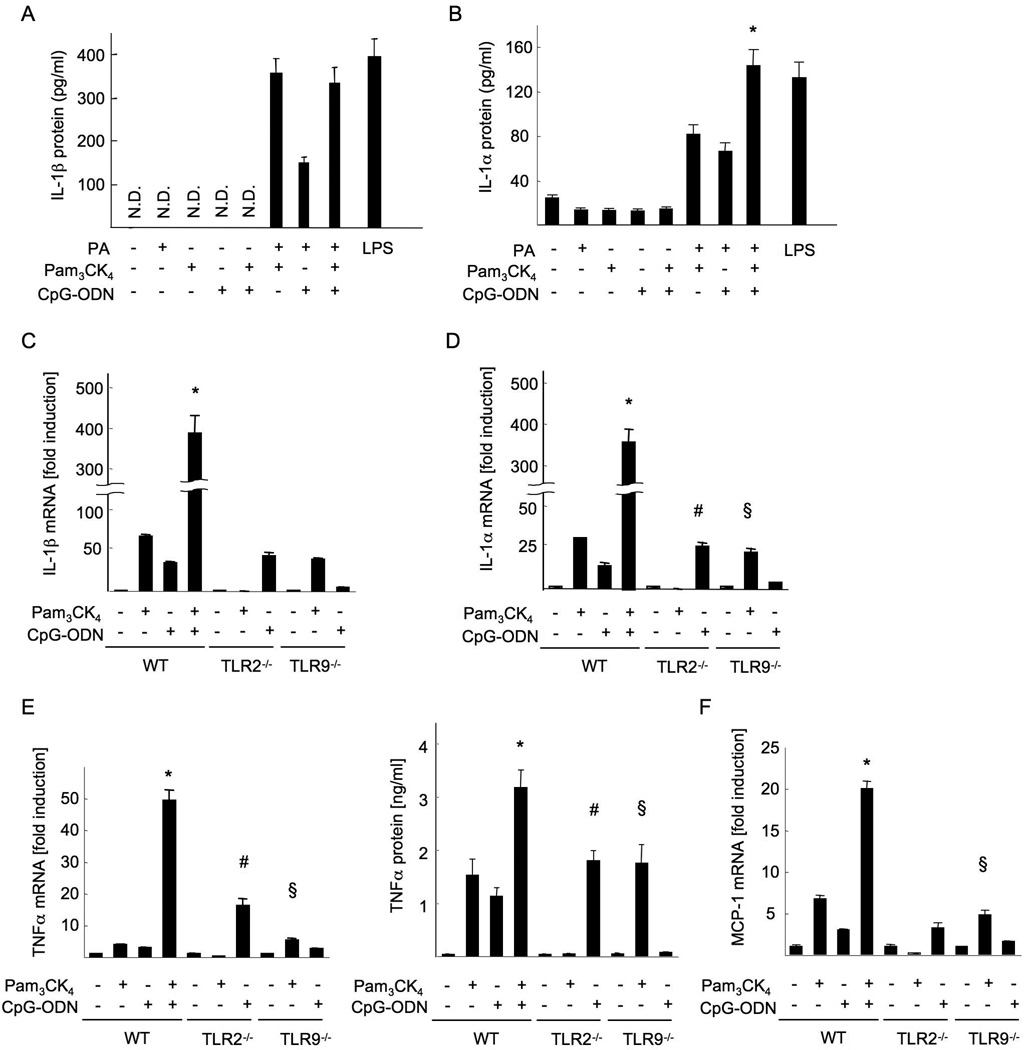
Since TLR9 signaling promotes NASH progression through IL-1β(9), we investigated whether palmitic acids participate in TLR9-mediated IL-1 production. In combination with CpG-ODN, a synthetic TLR9 ligand, palmitic acids functioned to secrete IL-1α and IL-1β in Kupffer cells (Figure 7A, B). Then, we examined the collaborative effect of TLR2 and TLR9 ligands in Kupffer cells. TLR2 and TLR9 ligands synergistically upregulated mRNA levels of proinflammtory cytokines including IL-1β, IL-1α, TNFα and MCP-1 (Figure 7C-F). The synergistic effects of TLR2 and TLR9 ligands were also observed in the secretion of IL-1α and TNFα proteins (Figure 7A, B, E). These results suggest that TLR2 and TLR9 signaling collaboratively induce cytokine production in Kupffer cells that promote NASH.
Figure 7. TLR2 and TLR9 ligands synergistically produce inflammatory cytokines in Kupffer cells.
Kupffer cells were isolated from WT, TLR2−/− and TLR9−/− mice, and cultured in the presence of 5 µg/ml Pam3CK4, 5 µg/ml CpG-ODN and/or 200 µM palmitic acid. (A,B,E) IL-1β (A), IL-1α (B) and TNFα (E, right) proteins in the supernatant. (C-F) mRNA expression of IL-1β (C), IL-1α (D), TNFα (E, left) and MCP-1 (F). Genes were normalized to 18S RNA as an internal control. N.D; not detected. Data represent mean ±SD, *; significant differences to WT KCs treated with Pam3CK4 or CpG-ODN, #; significant differences to WT KCs treated with CpG-DNA, §; no significant difference with WT KCs treated with Pam3CK4.
Discussion
We have previously demonstrated that activation of TLR4 signaling is crucial for the development of liver fibrosis through amplification of TGFβ signaling in HSCs (28). In murine NASH models, TLR4 ligand LPS and TLR9 ligand bacterial DNA were elevated in the plasma (9, 23), and the signaling of TLR4 and TLR9 promoted NASH development. MyD88, an adaptor molecule for TLR2, TLR4 and TLR9, is crucial for NASH progression (9). The present study demonstrates that TLR2 signaling acts as a promoter of liver inflammation and fibrosis in CDAA diet-induced NASH (Figure 1 and 2). Interestingly, TLR2−/− mice displayed less liver inflammation and fibrosis but similar levels of hepatic steatosis as WT mice, indicating that TLR2 signaling contributes to liver inflammation and fibrosis, but that hepatic lipid accumulation is independent of TLR2. Although a TLR2 ligand can activate both Kupffer cells and HSCs (Figure 3), our in vivo study using TLR2 BM chimeric mice demonstrated that Kupffer cells are responsible for TLR2-mediated liver inflammation and fibrosis (Figure 4). The activation of the inflammasomes, and the production of IL-1α and IL-1β in WT mice on the CDAA diet were suppressed in TLR2-deficient mice (Figure 6). In Kupffer cells, the cooperative actions mediated by TLR2 ligand and palmitic acids are required for activation of inflammasomes and production of IL-1α and IL-1β (Figure 5). We also found that TLR2 and TLR9 signaling synergistically induce production of inflammatory cytokines that can promote NASH (Figure 7).
A previous study reported that TLR2−/− deficiency aggravates NASH developed on an MCD diet (12). When mice fed a MCD diet, TLR2−/− mice are more susceptible to LPS-induced inflammatory and fibrogenic responses than WT mice (7, 12). The present study used the CDAA diet that produces weight gain, insulin resistance and liver fibrosis, in contrast to MCD diet that causes weight loss, improvement of insulin signaling, and only very mild liver fibrosis. Our data showed that the portal vein FFA levels were elevated in mice fed the CDAA diet, but not the MCD diet (Supplementary Figure 5). Because the majority of NASH patients develop obesity, insulin resistance and liver fibrosis with elevation of FFA levels (21), we propose that the CDAA diet model is more relevant NASH model to human NASH than the MCD diet model. The murine HFD model induces obesity, insulin resistance and hepatic steatosis, but liver inflammation is very mild and fibrosis does not develop. TLR2−/− mice and MyD88−/− mice had less steatosis than WT mice on a HFD(10, 11). Similarly, TLR2−/− mice exhibited less hepatic inflammation and fibrosis on the CDAA diet, which influences obesity and systemic insulin resistance. Notably, TLR2−/− mice on the CDAA diet developed similar degree of hepatic steatosis, but insulin resistance was suppressed. Alternatively, CSAA diet caused much less hepatic steatosis than CDAA diet, but induced similar degree of insulin resistance caused by CDAA diet (Figure 1)(9, 31). These findings suggest that systemic insulin resistance, simple steatosis and NASH substantially affect one another, but could be caused by different etiologies, and steatosis is not a preliminary hepatic condition for NASH (32).
The inflammasome is a multi-protein platform containing NLRP1, NLRP3, NLRC4 and AIM2 that activates caspase-1 through the adaptor protein ASC. Activated caspase-1 proteolytically cleaves pro-IL-1β and pro-IL-18 to generate active forms of IL-1β and IL-18(33). A recent report demonstrated that IL-1α secretion also requires inflammasome activation (34). Inflammasome activation has been reported to be important for the development of obesity, hepatic steatosis and insulin resistance (13, 14, 16). In these reports, NLRP3−/−, ASC−/− and caspase-1−/− mice on a HFD had less hepatic steatosis and obesity and improved insulin sensitivity (13, 14, 16). The function of the inflammasome in a NASH model induced by MCD diet is controversial. Two reports showed that activation of inflammasome components including caspase-1 promotes liver inflammation and fibrogenesis in NASH induced by MCD diet (17, 35). However, a recent study demonstrated that mice deficient in caspase-1, ASC or NLRP3 developed more steatosis by MCD diet (36). The results from our present and previous studies are in agreement with concept that inflammasome activation promotes NASH and insulin resistance. In particular, TLR2−/− mice fed a CDAA diet exhibited decreased expression of inflammasome components including NLRP3, casapae-1, IL-1α and IL-1β, and TLR2−/− mice and IL-1R−/− mice showed reduced body weight gain and suppression of insulin resistance, liver injury, inflammation and fibrosis compared with WT mice (9). TLR ligands alone are sufficient for induction of NLRP3 through NF-κB, but are insufficient to activate the inflammasomes(37, 38). Additional factors such as particular bacterial toxins, extracellular ATP or certain danger signals are required for assembly of inflammasome components and activation (33, 39, 40). Dietary factors, such as uric acids, cholesterol crystal and palmitic acid were also reported as a trigger to assemble the inflammasome components in macrophages (16, 41, 42). Wen et al demonstrated that LPS activates inflammasome in cooperation with palmitic acid, one of the most abundant saturated fatty acids in plasma of NAFLD, in BM-derived macrophages (16, 42). Similarly, our data demonstrated that the TLR2 or TLR9 ligand alone failed to activate inflammasomes, while palmitic acid in concert with a TLR2 or TLR9 ligand induces inflammasome activation in Kupffer cells (Figure 5, 7) (9). Importantly, in hepatic macrophages/ Kupffer cells, LPS alone can activate inflammasomes to produce IL-1β, IL-1α and IL-18 through the MyD88-independent TRIF-dependent pathway (Figure 7A, B) (43, 44). Although palmitic acid has been characterized as a TLR2 and TLR4 ligand to induce inflammatory cytokines (18–20), data from recent research and ours did not support this effect of palmitic acid in both Kupffer cells and HSCs (45).
HMGB-1 released from damaged hepatocytes may activate TLR2 in addition to TLR4 as an endogenous ligand in NASH. Intestinal microflora can also be the source of TLR2 ligand because of contradictory phenotypes in metabolic disease in TLR2−/− mice between SPF and non-SPF conditions that affect the composition of gut flora(10, 11, 46). Oral bacteria contribute to the progression of metabolic disease including atherosclerosis (47, 48). Thus, endogenous ligands, such as HMGB-1, and the components derived from intestinal microflora or oral bacteria can be a ligand for TLR2 in NASH.
The present study demonstrates that TLR2−/− mice exhibited reduced NASH compared to WT mice, but seemed more NASH pathology than in TLR9−/− mice (9). Our data suggest that TLR2−/− Kupffer cells are more sensitive to produce inflammatory cytokines than WT Kupffer cells in response to TLR9 ligand whereas TLR9−/− Kupffer cells have similar response to TLR2 ligand in comparison to WT Kupffer cells (Figure 7). It is conceivable that TLR9 signaling may compensates the expression of inflammatory cytokines in TLR2−/− Kupffer cells, which may explain the mechanisms underlying suppressed NASH pathology in TLR9−/− mice compared to TLR2−/− mice. However, liver inflammation in TLR2−/− mice was still less than WT mice because NASH progression requires the synergy of TLR2 and TLR9 signaling. This synergy induces the production of inflammatory cytokines in Kupffer cells that promote NASH. In particular, TLR2 and TLR9-mediated IL-1 induces fibrogenic responses including the upregulation of TIMP-1 and PAI-1, and the downregulation of Bambi, an endogenouse inhibitor of the TGFβ; thus the combination of these responses promotes liver fibrosis in HSCs (9).
Supplementary Material
Acknowledgement
We thank Rie Seki, Karin Diggle, Jingyi Isabelle Song (Department of Medicine, UCSD, La Jolla, CA) and Yukie Komatsu (Akita University Graduate School of Medicine) for excellent technical assistance.
Financial support
This study is supported by NIH grant R01AA02172 (ES), R01DK085252 (ES), R24DK090962 (DAB), R01GM041804 (DAB), and the pilot grant from the UCSD Digestive Diseases Research Development Center (DK080506) (ES), Takeda Science Foundation (KM), The Mochida Memorial Foundation for Medical and Pharmaceutical Research (KM), Mishima Kaiun Memorial Foundation (KM), and by JSPS [Grant-in-Aid for Scientific Research (C)] (KM).
Nonstandard abbreviations used
- ALT
alanine transaminase
- BM
bone marrow
- CCR
CC chemokine receptor
- CDAA
choline-deficient amino-acid defined
- CSAA
choline-supplemented amino acid defined
- ELISA
enzyme-linked immunoabsorbent assay
- HE
hematoxylin and eosin
- HFD
high fat diet
- HOMA-IR
homeostasis model assessment of insulin resistance
- HSC
hepatic stellate cell
- IL-1β
interleukin-1β
- MCD
methionine and choline deficient
- MCP
monocyte chemoattractant protein
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NLRP
Nod-like receptor protein
- PAI-1
plasminogen activator inhibitor-1
- TGF-β1
transforming growth factor β1
- TIMP-1
tissue inhibitor of metalloproteinase-1
- TLR
Toll-like receptor
- TNF α
tumor necrosis factor α
- WT
wild type
Footnotes
Disclosures
No conflict of interest
References
- 1.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 2.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maher JJ, Leon P, Ryan JC. Beyond insulin resistance: Innate immunity in nonalcoholic steatohepatitis. Hepatology. 2008;48:670–678. doi: 10.1002/hep.22399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miura K, Seki E, Ohnishi H, Brenner DA. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic Fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847. doi: 10.1155/2010/362847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 7.Szabo G, Velayudham A, Romics L, Jr, Mandrekar P. Modulation of non-alcoholic steatohepatitis by pattern recognition receptors in mice: the role of toll-like receptors 2 and 4. Alcohol Clin Exp Res. 2005;29:140S–145S. doi: 10.1097/01.alc.0000189287.83544.33. [DOI] [PubMed] [Google Scholar]
- 8.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334. e327. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, et al. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–1806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 11.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera CA, Gaskin L, Allman M, Pang J, Brady K, Adegboyega P, Pruitt K. Toll-like receptor-2 deficiency enhances non-alcoholic steatohepatitis. BMC Gastroenterol. 2010;10:52. doi: 10.1186/1471-230X-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, Neale GA, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. 2011;108:15324–15329. doi: 10.1073/pnas.1100255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isoda K, Sawada S, Ayaori M, Matsuki T, Horai R, Kagata Y, Miyazaki K, et al. Deficiency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. J Biol Chem. 2005;280:7002–7009. doi: 10.1074/jbc.M412220200. [DOI] [PubMed] [Google Scholar]
- 16.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–144. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 20.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 21.de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, Mahmood S, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1248–G1257. doi: 10.1152/ajpgi.90223.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, Osterreicher CH, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137:1467–1477. e1465. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002;5:545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Brun P, Castagliuolo I, Pinzani M, Palu G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 28.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 29.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes non-alcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00365.2011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsutsui H, Imamura M, Fujimoto J, Nakanishi K. The TLR4/TRIF-Mediated Activation of NLRP3 Inflammasome Underlies Endotoxin-Induced Liver Injury in Mice. Gastroenterol Res Pract. 2010;2010:641865. doi: 10.1155/2010/641865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, et al. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Dixon LJ, Berk M, Thapaliya S, Papouchado BG, Feldstein AE. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab Invest. 2012 doi: 10.1038/labinvest.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Connor W, Jr, Harton JA, Zhu X, Linhoff MW, Ting JP. Cutting edge: CIAS1/cryopyrin/PYPAF1/NALP3/CATERPILLER 1.1 is an inducible inflammatory mediator with NF-kappa B suppressive properties. J Immunol. 2003;171:6329–6333. doi: 10.4049/jimmunol.171.12.6329. [DOI] [PubMed] [Google Scholar]
- 38.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto M, Yaginuma K, Tsutsui H, Sagara J, Guan X, Seki E, Yasuda K, et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells. 2004;9:1055–1067. doi: 10.1111/j.1365-2443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 42.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seki E, Tsutsui H, Nakano H, Tsuji N, Hoshino K, Adachi O, Adachi K, et al. Lipopolysaccharide-induced IL-18 secretion from murine Kupffer cells independently of myeloid differentiation factor 88 that is critically involved in induction of production of IL-12 and IL-1beta. J Immunol. 2001;166:2651–2657. doi: 10.4049/jimmunol.166.4.2651. [DOI] [PubMed] [Google Scholar]
- 44.Imamura M, Tsutsui H, Yasuda K, Uchiyama R, Yumikura-Futatsugi S, Mitani K, Hayashi S, et al. Contribution of TIR domain-containing adapter inducing IFN-beta-mediated IL-18 release to LPS-induced liver injury in mice. J Hepatol. 2009;51:333–341. doi: 10.1016/j.jhep.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Abergel A, Sapin V, Dif N, Chassard C, Darcha C, Marcand-Sauvant J, Gaillard-Martinie B, et al. Growth arrest and decrease of alpha-SMA and type I collagen expression by palmitic acid in the rat hepatic stellate cell line PAV-1. Dig Dis Sci. 2006;51:986–995. doi: 10.1007/s10620-005-9031-y. [DOI] [PubMed] [Google Scholar]
- 46.Caricilli AM, Picardi PK, de Abreu LL, Ueno M, Prada PO, Ropelle ER, Hirabara SM, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212. doi: 10.1371/journal.pbio.1001212. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, Lu Y, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1405–1411. doi: 10.1161/01.ATV.0000082462.26258.FE. [DOI] [PubMed] [Google Scholar]
- 48.Erridge C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis. 2011;216:1–6. doi: 10.1016/j.atherosclerosis.2011.02.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.