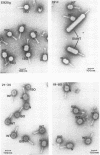
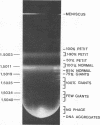
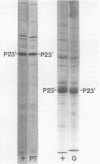
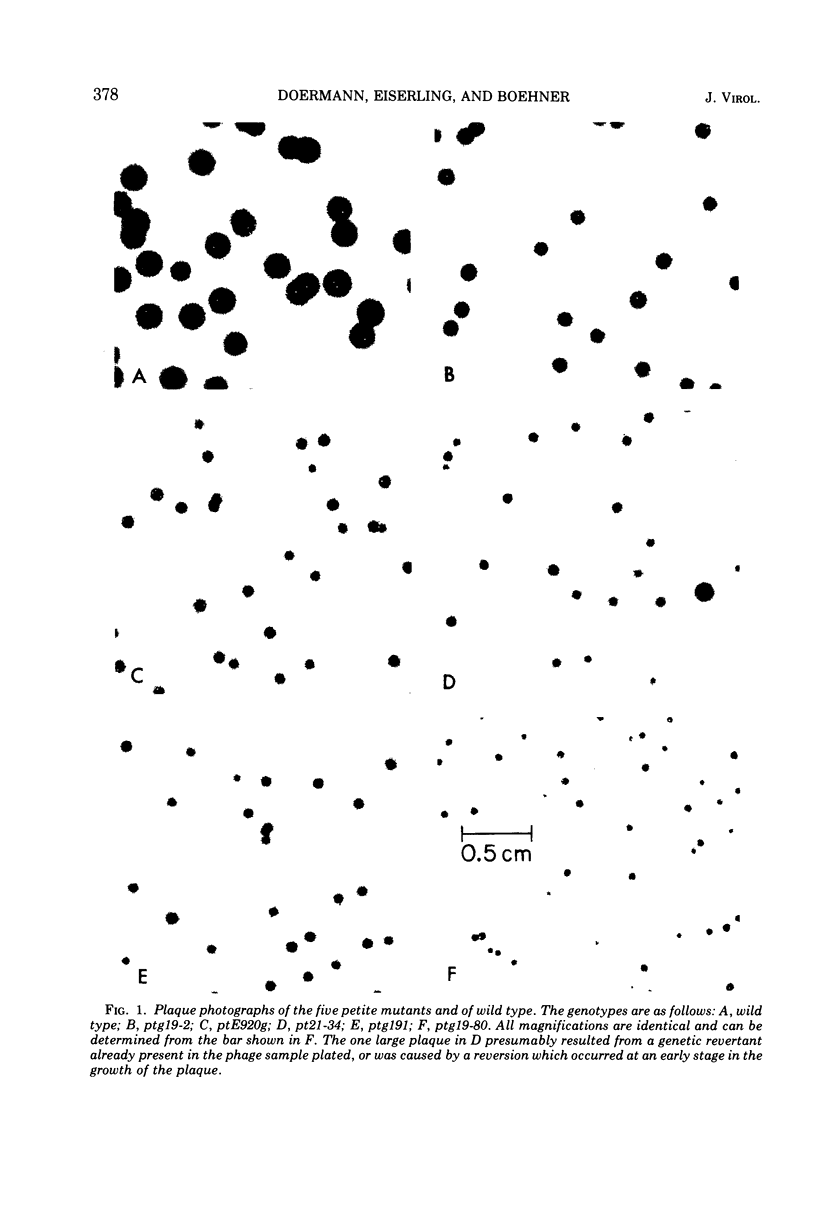
Abstract
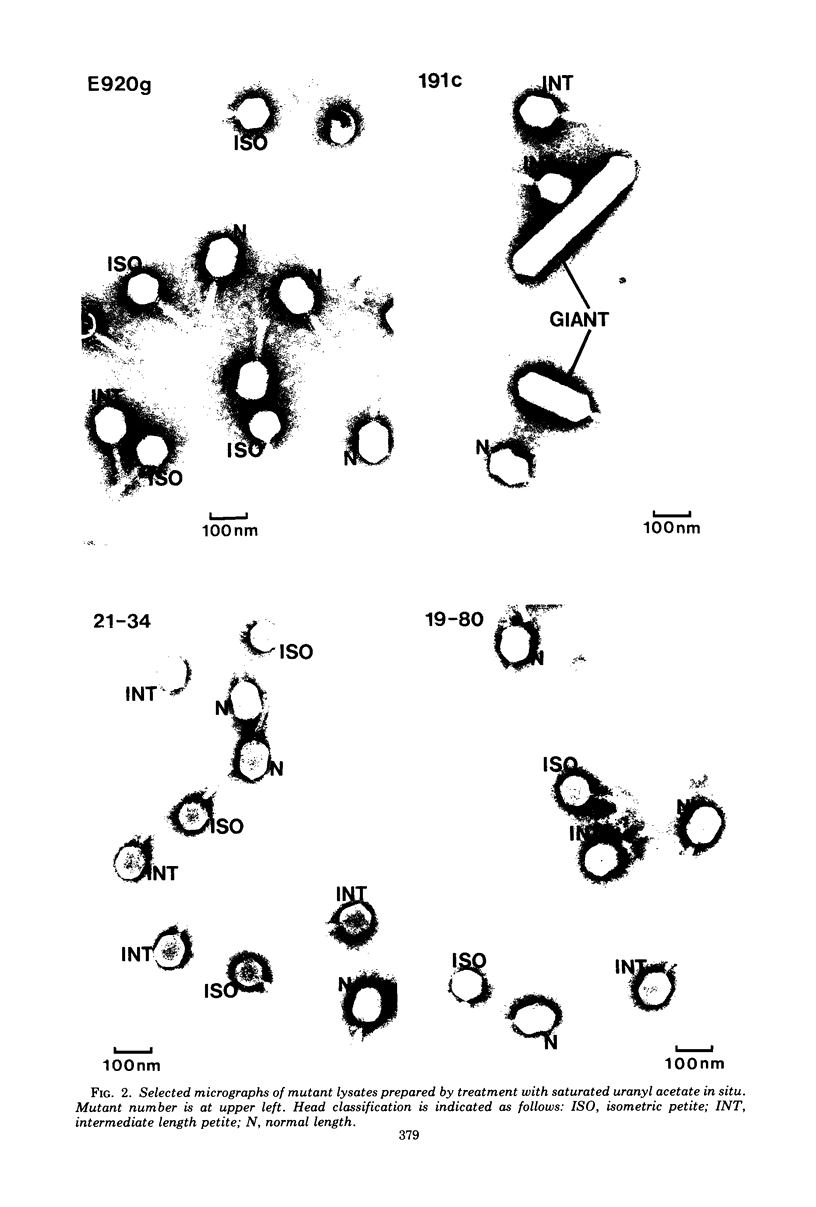
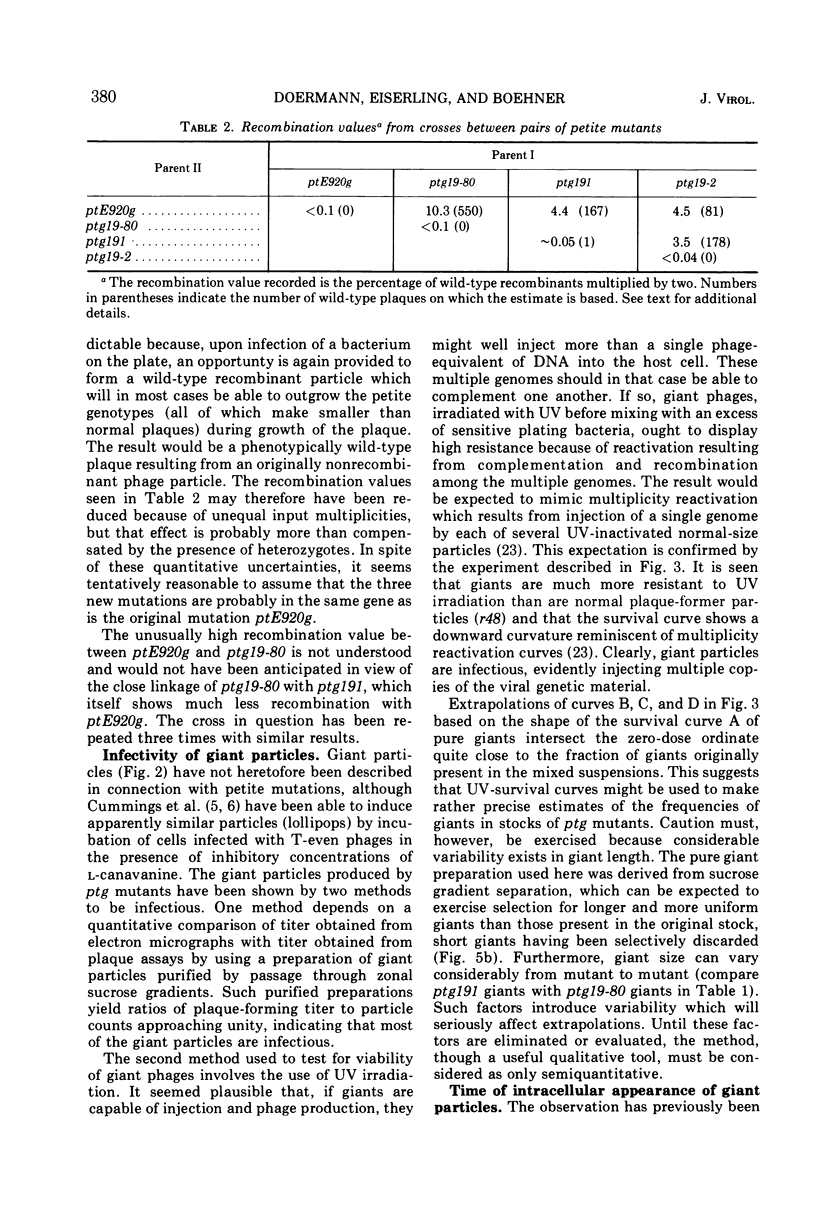
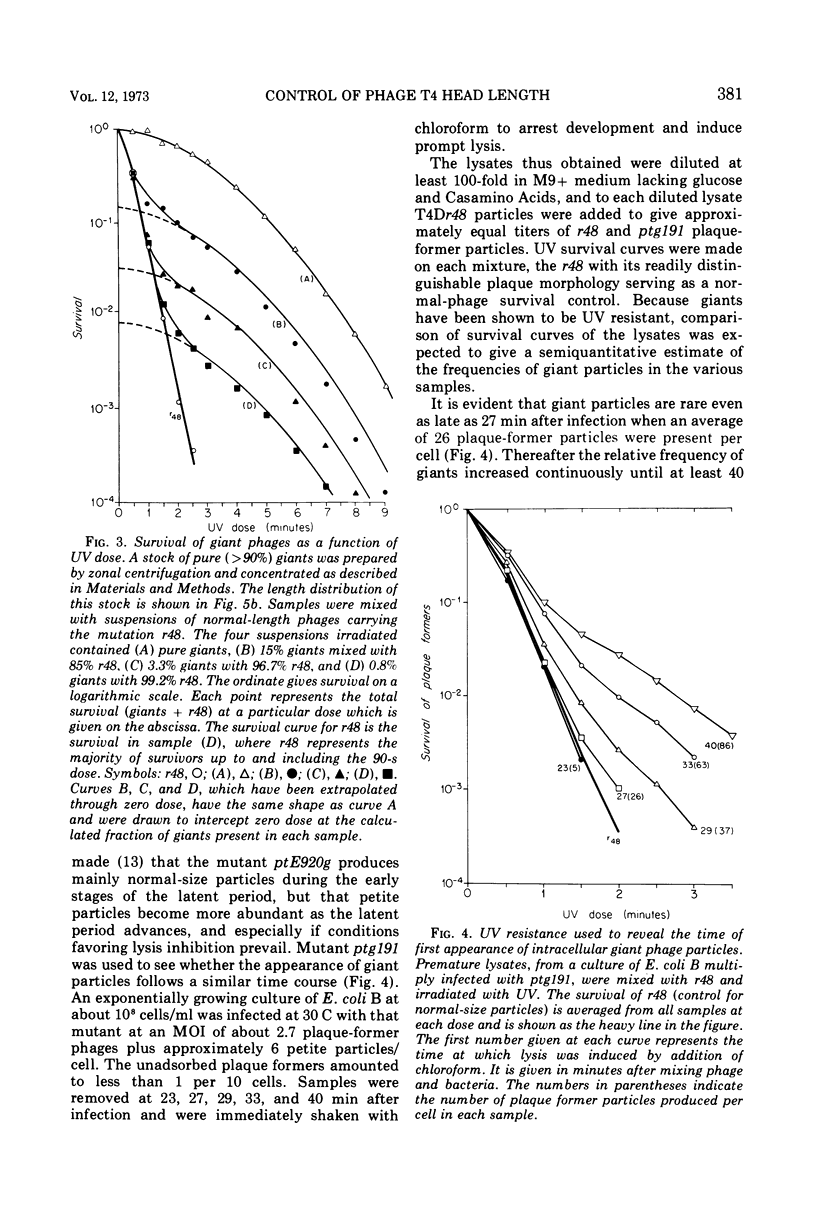
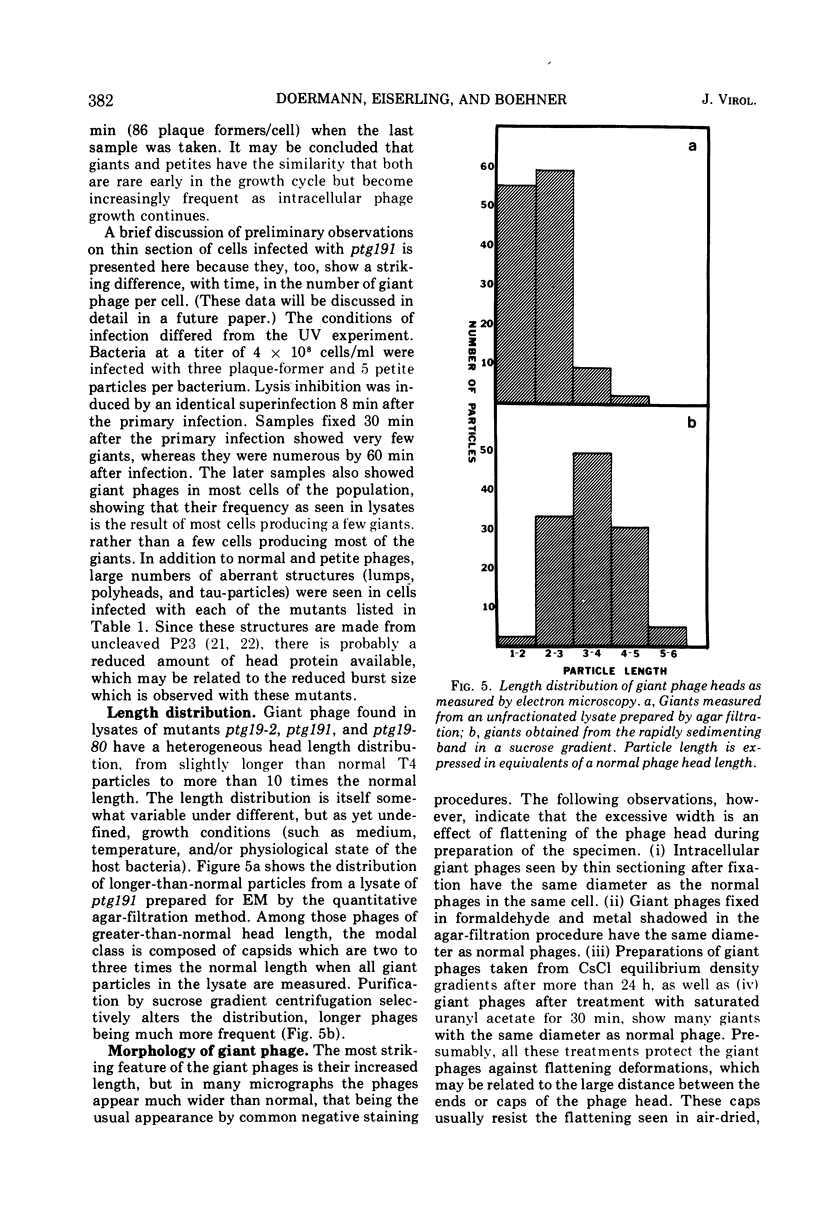
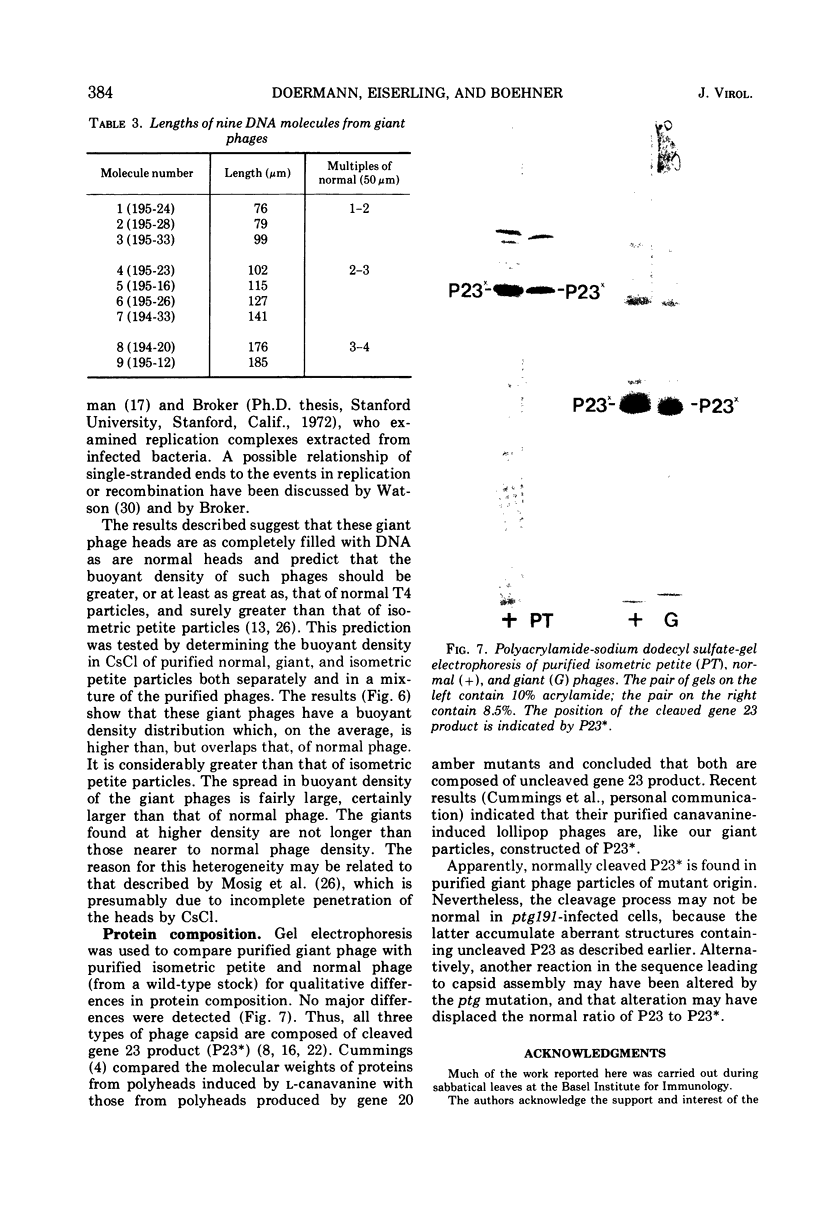
Four new mutants are described whose phenotypic expression affects the length of the head of bacteriophage T4D. All mutants produce some phenotypically normal phage particles. Mutant pt21-34 also produces at least two size classes of phage particle which have heads that are shorter than normal. The other three mutants, ptg19-2, ptg19-80, and ptg191, produce, in addition to phages with normal and with shorter-than-normal heads, giant phages with heads from 1.5 to at least 10 times the normal length. All mutations are clustered near gene 23. Giant phage particles have the following properties: they are infectious and contain and inject multiple genomes as a single continuous bihelical DNA molecule of greater-than-unit length. Their frequency, relative to the total plaque-former population, increases late in the infectious cycle. They have a normal diameter, variable length, and a buoyant density range in CsCl from equal to slightly greater than that of normal phage. The arrangement of capsomers is visible in the capsids, which are composed of cleaved gene 23 protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Remsen C. C. Bacteriophage T2 as seen with the freeze-etching technique. Virology. 1970 Mar;40(3):703–718. doi: 10.1016/0042-6822(70)90215-1. [DOI] [PubMed] [Google Scholar]
- Boy de la Tour E., Kellenberger E. Aberrant forms of the T-even phage head. Virology. 1965 Oct;27(2):222–225. doi: 10.1016/0042-6822(65)90163-7. [DOI] [PubMed] [Google Scholar]
- Chase M, Doermann A H. High Negative Interference over Short Segments of the Genetic Structure of Bacteriophage T4. Genetics. 1958 May;43(3):332–353. doi: 10.1093/genetics/43.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J. A remeasurement of the molecular weights of T-even bacteriophage substructural proteins. J Virol. 1972 Mar;9(3):547–550. doi: 10.1128/jvi.9.3.547-550.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., Kusy A. R., DeLong S. S. Structural aberrations in T-even bacteriophage. II. Characterization of the proteins contained in aberrant heads. Virology. 1971 May;44(2):425–442. doi: 10.1016/0042-6822(71)90273-x. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Couse N. L., Forrest G. L. Structural defects of T-even bacteriophages. Adv Virus Res. 1970;16:1–41. doi: 10.1016/s0065-3527(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Doermann A. H. Lysis and Lysis Inhibition with Escherichia coli Bacteriophage. J Bacteriol. 1948 Feb;55(2):257–276. doi: 10.1128/jb.55.2.257-276.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H., Parma D. H. Recombination in bacteriophage T4. J Cell Physiol. 1967 Oct;70(2 Suppl):147–164. doi: 10.1002/jcp.1040700411. [DOI] [PubMed] [Google Scholar]
- EPSTEIN R. H. A study of multiplicity-reactivation in the bacteriophage T4. I. Genetic and functional analysis of T4D-K12(lambda) complexes. Virology. 1958 Oct;6(2):382–404. doi: 10.1016/0042-6822(58)90090-4. [DOI] [PubMed] [Google Scholar]
- Edgar R S. Phenotypic Properties of Heterozygotes in the Bacteriophage T4. Genetics. 1958 Mar;43(2):235–248. doi: 10.1093/genetics/43.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Dickson R. C. Assembly of viruses. Annu Rev Biochem. 1972;41:467–502. doi: 10.1146/annurev.bi.41.070172.002343. [DOI] [PubMed] [Google Scholar]
- Eiserling F. A., Geiduschek E. P., Epstein R. H., Metter E. J. Capsid size and deoxyribonucleic acid length: the petite variant of bacteriophage T4. J Virol. 1970 Dec;6(6):865–876. doi: 10.1128/jvi.6.6.865-876.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREIFELDER D. A NOVEL METHOD FOR THE RELEASE OF BACTERIOPHAGE DNA. Biochem Biophys Res Commun. 1965 Jan 4;18:141–144. doi: 10.1016/0006-291x(65)90897-1. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., ARBER W. Electron microscopical studies of phage multiplication. I. A method for quantitative analysis of particle suspensions. Virology. 1957 Apr;3(2):245–255. doi: 10.1016/0042-6822(57)90091-0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Der Kamp C. K.-V. On a modification of the gene product P23 according to its use as subunit of either normal capsids of phage T4 or of polyheads. FEBS Lett. 1970 Jun 1;8(3):140–144. doi: 10.1016/0014-5793(70)80247-2. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Dulbecco R. Genetic Recombinations Leading to Production of Active Bacteriophage from Ultraviolet Inactivated Bacteriophage Particles. Genetics. 1949 Mar;34(2):93–125. doi: 10.1093/genetics/34.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. F. The shape of the T-even bacteriophage head. Virology. 1965 Aug;26(4):567–576. doi: 10.1016/0042-6822(65)90319-3. [DOI] [PubMed] [Google Scholar]
- Mosig G., Carnighan J. R., Bibring J. B., Cole R., Bock H. G., Bock S. Coordinate variation in lengths of deoxyribonucleic acid molecules and head lengths in morphological variants of bacteriophage T4. J Virol. 1972 May;9(5):857–871. doi: 10.1128/jvi.9.5.857-871.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma D. H. The structure of genomes of individual petit particles of the bacteriophage T4D mutant E920/96/41. Genetics. 1969 Oct;63(2):247–261. doi: 10.1093/genetics/63.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STREISINGER G. Phenotypic mixing of host range and serological specificities in bacteriophages T2 and T4. Virology. 1956 Jun;2(3):388–398. doi: 10.1016/0042-6822(56)90033-2. [DOI] [PubMed] [Google Scholar]
- Walker D. H., Jr, Mosig G., Bayer M. E. Bacteriophage T4 head models based on icosahedral symmetry. J Virol. 1972 May;9(5):872–875. doi: 10.1128/jvi.9.5.872-875.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Yanagida M., DeRosier D. J., Klug A. Structure of the tubular variants of the head of bacteriophage T4 (polyheads). II. Structural transition from a hexamer to a 6+1 morphological unit. J Mol Biol. 1972 Apr 14;65(3):489–499. doi: 10.1016/0022-2836(72)90203-3. [DOI] [PubMed] [Google Scholar]