Abstract
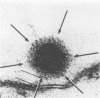
Highly purified human adenovirus type 2 directly penetrated the plasma membranes of KB cells. The process of membrane penetration resulted in the appearance of large numbers of adenovirions free in the cytoplasm of the infected cells. The virions underwent a morphological change as they penetrated the cell surface. Penetration of the plasma membranes and the accompanying alteration in virion morphology was dependent on a function associated with the intact cells, because neither event occurred when purified virions were added to isolated cell membranes. Inactivation of the adenovirions with heat or antibodies before inoculation of the cells reduced the infectivity of the virus population and prevented the appearance of virions free in the cytoplasm. The inactivation of the virions did not significantly reduce the number of virus particles which were found in cell vacuoles and pinocytotic vesicles.
Full text
PDF






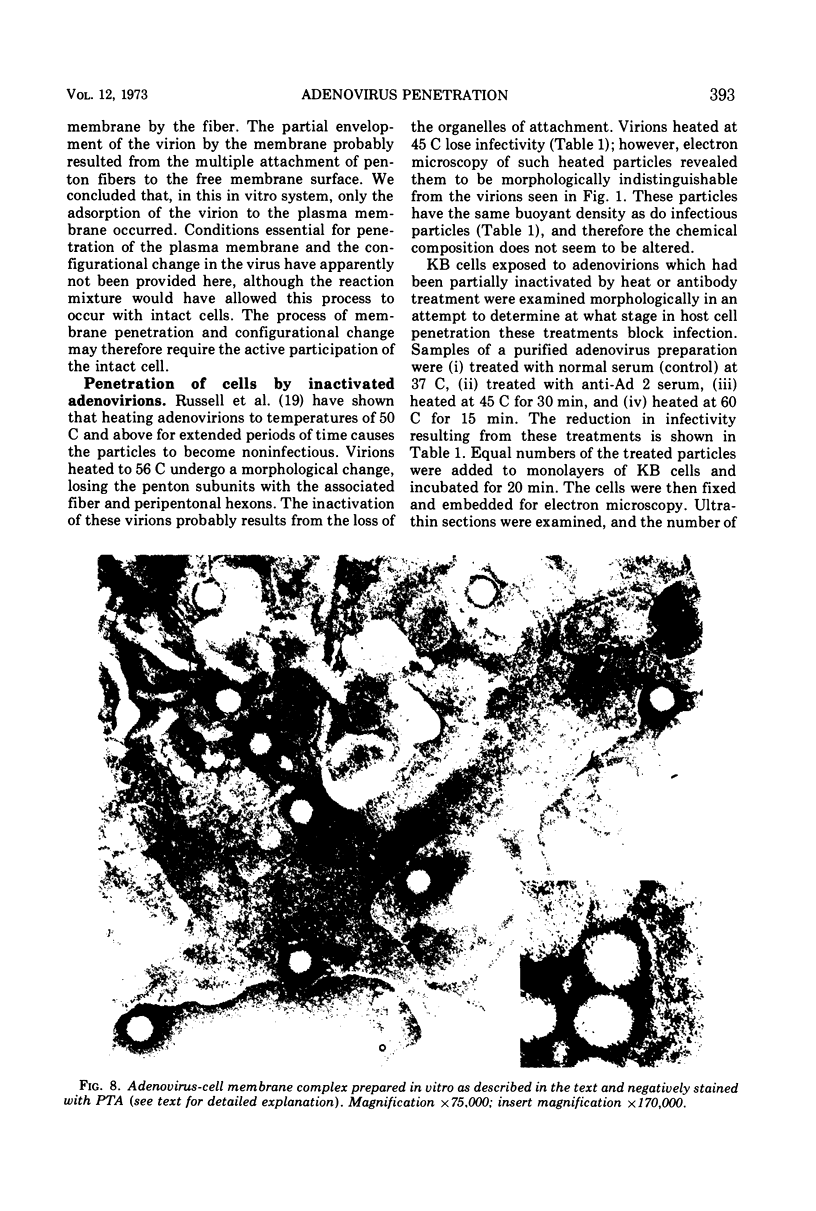
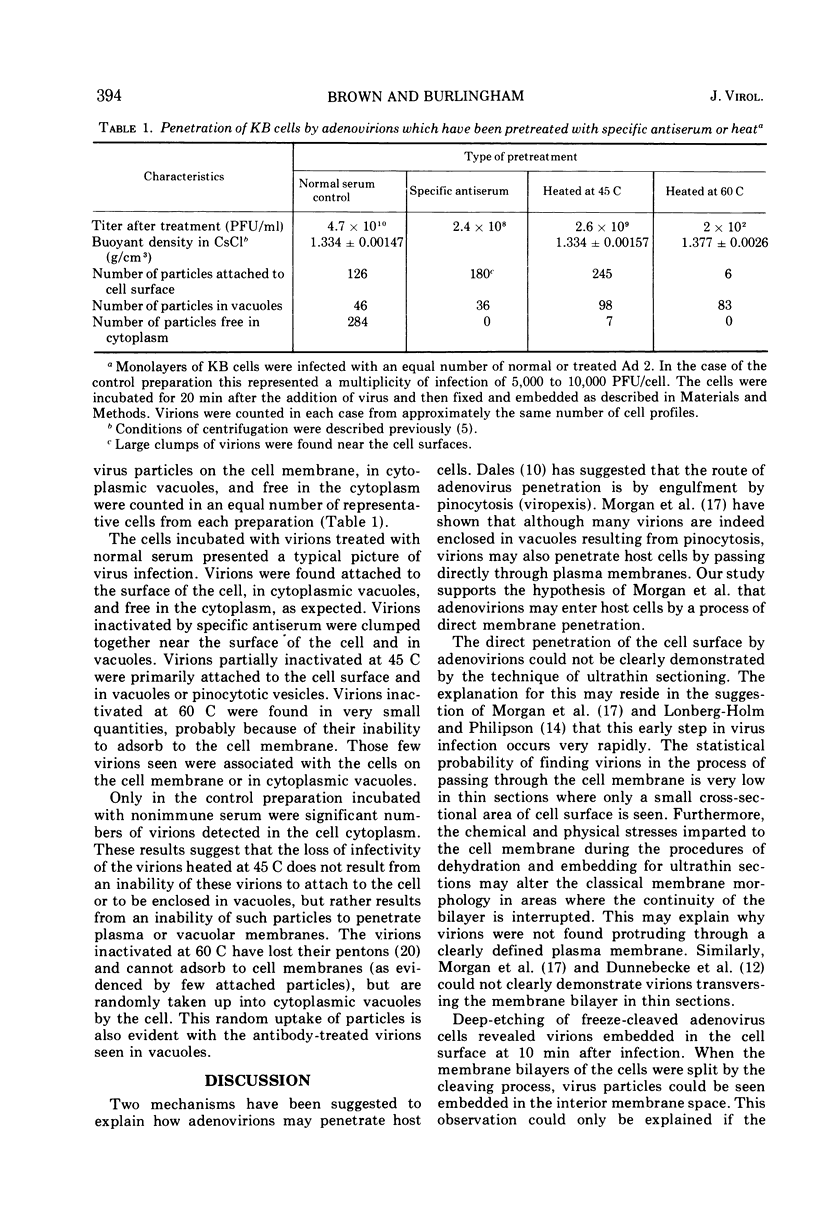


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. An endonuclease in cells infected with adenovirus and associated with adenovirions. Virology. 1972 Apr;48(1):1–13. doi: 10.1016/0042-6822(72)90108-0. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. II. Comparative observations on the penetration of types 1, 5, 7, and 12. Virology. 1970 Mar;40(3):478–485. doi: 10.1016/0042-6822(70)90190-x. [DOI] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnebacke T. H., Levinthal J. D., Williams R. C. Entry and release of poliovirus as observed by electron microscopy of cultured cells. J Virol. 1969 Oct;4(4):505–513. doi: 10.1128/jvi.4.4.505-513.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Valentine R. C., Pereira H. G. The effect of heat on the anatomy of the adenovirus. J Gen Virol. 1967 Oct;1(4):509–522. doi: 10.1099/0022-1317-1-4-509. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]