Summary
Calcium plays a prominent role during fertilization in many animals. This review focuses on roles of Ca2+ during the events around fertilization in the model organism, Caenorhabditis elegans. Specifically, the role of Ca2+ in sperm, oocytes and the surrounding somatic tissues during fertilization will be discussed, with the focus on sperm activation, meiotic maturation of oocytes, ovulation, sperm-egg interaction and fertilization.
1. Introduction
Calcium plays a ubiquitous role in biological pathways. Fertilization is one such process that exploits Ca2+ mediated signals in several phases and many species across the animal kingdom seem to rely extensively upon Ca2+ signal for this purpose [1].
Caenorhabditis elegans is an attractive model system to study the biology of fertilization [2]. The transparent nature of the body permits the live examination of several key events of fertilization [3]. Especially, monitoring the influx of Ca2+ ions into oocytes enables one to directly correlate the Ca2+ signaling with the hall marks of fertilization in an intact animal.
C. elegans exist as sexually dimorphic males and hermaphrodites. Hermaphrodites are predominantly found in natural population and under normal culture conditions in the lab. Hermaphrodites produce both sperm and oocytes, which enable the animal to self-fertilize to produce self-progeny. Males are found very rarely in nature and produce only sperm. Males can mate with hermaphrodite to sire cross-progeny [4].
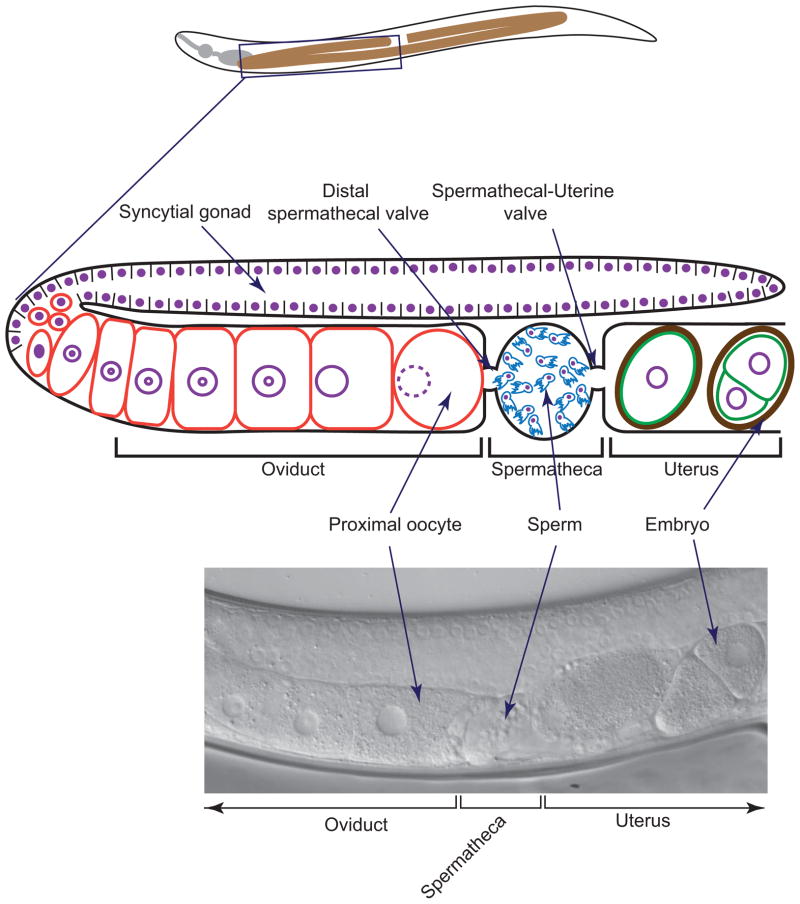
The reproductive system in C. elegans is consists of a simple U shaped tube (figure 1) [5]. At the distal most part of the gonad reside germ cells, which undergo mitotic proliferation, followed by meiotic division to give rise to gametes. During the initial phase of gametogenesis, hermaphrodites produce a limited number of sperm. Thereafter, it continues to produce oocytes only [6].
Figure 1.
Schematic diagram showing the reproductive tract of adult hermaphrodite (top). Oocytes mature and enter one by one into spermatheca, which is the site of sperm storage and fertilization. Fertilized eggs enter into uterus where early embryonic development takes place. Eventually eggs are pushed out through vulva (not shown). DIC micrograph showing part of the adult gonad (bottom).
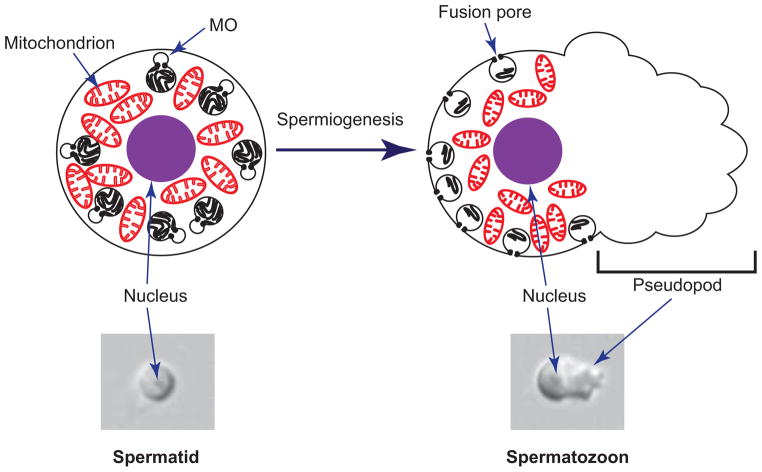
The diploid spermatocytes undergo complete meiotic division to give rise to haploid spermatids through a process called spermatogenesis [7–9]. The spermatids are round in shape, immotile and are incapable of fertilizing oocyte. Spermatids are rapidly activated to form mature spermatozoa through a process called spermiogenesis or sperm activation (figure 2) [8]. During the activation of sperm, pseudopods are formed from one side of the sperm, which enable the sperm to become motile and fertilization-competent. C. elegans sperm contain a specialized Golgi-derived vesicle called membranous organelle (MO). In spermatids, these organelles are sequestered inside the cytosol. During or following sperm activation, MOs fuse with plasma membrane and release their contents outside [9].
Figure 2.
Schematic diagram illustrating spermiogenesis in C. elegans (top). Spermiogenesis refers to the process of converting round, immotile spermatids into polarized, motile spermatozoa. Sperm are packed with many mitochondria and Membranous Organelles (MOs). In spermatids, MOs reside inside the cell. During or following activation, MOs fuse with plasma membrane and release their contents outside. The spermatozoon extrudes pseudopod, which is the motility apparatus.
Oocytes undergo several cytological changes in preparation for fertilization and enter into spermatheca serially (see figure 1). The process of entry of oocyte into spermatheca is called ovulation [10]. Contraction of gonadal sheath cells accompanied by the dilation of distal spermatheca valve propels the oocyte into spermatheca [3]. Fertilization takes place in spermatheca, which can house both male derived and hermaphrodite derived sperm. Newly fertilized eggs then enter into the uterus, where early embryonic development takes place. Eventually eggs are pushed out on to the surrounding media through the vulva.
2. Roles of Ca2+ and Ca2+ binding proteins during sperm activation
Depletion of extracellular Ca2+ by treating spermatids with EDTA or EGTA does not compromise sperm activation, which suggests that Ca2+ from extracellular environment may not play a role in sperm activation [11]. In contrast, buffering intacellular Ca2+ by treating the spermatids with BAPTA-AM (a membrane permeable Ca2+ chelator) causes reduction in MO fusion, suggesting that intracellular Ca2+ is necessary for MO fusion and sperm activation [12].
The requirement of intracellular Ca2+ for the complete activation of C. elegans sperm raises an interesting question: is the intracellular pool of Ca2+ regulated in sperm? Most eukaryotic cells sequester Ca2+ in the endoplasmic reticulum (ER) in order to ensure the regulated delivery of Ca2+ into the cytosol [13]. However, most of the organelles, including ER, are discarded during spermatogenesis in C. elegans [14]. Therefore, it is less likely that ER could play a prominent role in regulating Ca2+ homeostasis in C. elegans sperm. Surprisingly, calreticulin, a Ca2+ buffering protein that resides in ER, is detected in C. elegans sperm and plays a role in fertility in C. elegans [15]. Since calreticulin has the propensity to retrotranslocate from ER to cytosol [16], it is likely that the observed CRT-1 staining in C. elegans sperm corresponds to the pool of cytosol-localized CRT-1 [15]. In vitro activated crt-1 mutant spermatozoa are smaller than wild-type sperm, display shorter pseudopods and the nuclei are positioned off center [15]. Since larger sperm outcompete smaller sperm in C. elegans [17], the function of CRT-1 might be critical for the reproductive success of the worm.
In addition to ER, other organelles such as mitochondria, Golgi apparatus, lysosome and nucleus also stores Ca2+ [18]. Since sperm are enriched with mitochondria [19] it would be interesting to investigate the potential role of mitochondria in regulating Ca2+ homeostasis in C. elegans sperm. In addition to mitochondria, sperm are also packed with many MOs. Given that MOs are Golgi-derived organelle and possibly related to lysosome [20], it would be interesting to test the hypothesis that MOs function as a Ca2+ store in C. elegans sperm. Since sperm devoid of nucleus can successfully undergo spermiogenesis and fertilize oocyte [21], the contribution of Ca2+ mobilized from nucleus, if any, should be insignificant for regulating spermiogenesis in C. elegans.
Although intracellular Ca2+is necessary, it’s not sufficient for the sperm activation, since artificial increment of intracellular Ca2+ level by treating spermatids with Ca2+ ionophores does not initiate spermiogenesis [11]. However, it should be noted that the toxicity associated with ionophore treatment could also prevented spermiogenesis.
How is the Ca2+ signal coupled with sperm activation? Calmodulin is one of the Ca2+ binding proteins that are involved in variety of Ca2+ initiated signaling pathways. Surprisingly inhibition, rather than activation, of calmodulin induces spermiogenesis. A variety of calmodulin-inhibitors, such as trifluoperazine (TFP), chlorpromazine (CPZ), naphthalenesulfonamide (W7) induces spermiogenesis in vitro [22]. Although spermiogenesis is initiated by the calmodulin-inhibitor TFP, the resultant sperm do not fully undergo activation, but rather arrest as spermiogenesis intermediates. TFP treated spermatids do not fully form villar projection and are immotile. Upon flushing TFP, the morphology of the sperm become normal spermatozoa and become motile. Conversely, treatment of motile spermatozoa with TFP renders the sperm round and immotile, which suggests the plausible role of calmodulin in sperm-motility.
Formation of pseudopod and acquisition of motility is dependent on the assembly of specialized filaments of Major Sperm Protein (MSP). Given the involvement of kinases and phosphatases in modulating the assembly and disassembly of MSP filaments in nematodes [23–26], it is tempting to speculate that Ca2+/calmodulin kinase – one of the main downstream target proteins of calmodulin - could potentially play a role in regulating the dynamics of MSP filament formation by altering the phosphorylation status of target proteins.
Calcineurin (a Ser/Thr phosphatase) is one of the downstream target enzymes activated by Ca2+/calmodulin. Interestingly, the pseudopods of calcineurin mutant sperm are smaller in size. Since calcineurin is activated by calmodulin and the full activation of sperm likely depends on calmodulin, it’s possible that Ca2+/calmodulin dependent activation of calcineurin might be essential for the complete execution of spermiogenesis in C. elegans. Epistatic and/or pharmacological analyses using mutants of potential players would give us more details on the signal transduction pathway culminating in spermiogenesis.
All these observations suggest the possible role of calmodulin in blocking the initial onset of spermiogenesis and enabling the sperm to become motility-competent. However, the potential role of calmodulin in regulating sperm activation is unclear. First, calmodulin inhibitors elicit many biological effects that are not dependent on calmodulin [22]. Second, knock down of calmodulin homolog, cmd-1, leads to embryonic lethality [27], meaning that fertilization do occur normally and embryo do form (and then die) following cmd-1 RNAi treatment. Since RNAi treatment does not guarantee complete elimination of transcripts, analysis of cmd-1 null mutant would clear the ambiguity surrounding the in vivo relevance of calmodulin in fertility.
3. Fusion of membranous organelles (MO) with plasma membrane
During the activation of wild-type sperm, membranous organelles (MOs) move toward plasma membrane and fuse with it to form a fusion pore (see figure 2). The fer-1 mutant sperm undergo activation, but the pseudopods are short and stubby [9]. Electron microscopy reveals that although the MOs abut plasma membrane, they fail to fuse with it, suggesting that FER-1 protein is essential for fusion of MOs with plasma membrane.
The FER-1 is a membrane protein that has C2 domains, which commonly mediate Ca2+ assisted events [12, 28]. Three isoforms of FER-1 were recognized by probing the extract of males with polyclonal antibody raised against part of FER-1 protein. The three isoforms are likely arising from proteolytic cleavage [12].
Probing the localization of FER-1 with anti-FER-1 antibody revealed that FER-1 is initially found in the MOs of spermatids. However, in mature spermatozoa, the FER-1 is found all over the plasma membrane, suggesting that after MOs fuse with plasma membrane, FER-1 exit MOs and gets distributed all over the plasma membrane. Quantitative immunoelectron microscopy using anti-FER-1 antibody also shows identical localization dynamics. This localization pattern is similar to one other protein that functions in sperm: TRP-3/SPE-41 [29]. However, it is not clear whether TRP-3/SPE-41 plays any role in the fusion of MOs or providing Ca2+ for FER-1 activation.
The fer-1 mutants are hypersensitive to the depletion of intracellular Ca2+ level. Depleting the intracellular Ca2+ with 1mM of BAPTA-AM (a membrane permeable Ca2+ chelator) doesn’t prevent the wild-type spermatids from fusion of MOs with plasma membrane. However, in specific fer-1 mutant alleles, the same concentration of BAPTA-AM caused the reduction in the MOs fusion, corroborating the idea that Ca2+ might regulate FER-1 function, presumably through C2 domain [12]. FER-1 seems to link the Ca2+ signal with the fusion between MOs and the plasma membrane. The positioning of MOs abutting plasma membrane is independent of FER-1 function.
4. Meiotic maturation of oocytes
In C. elegans, oocytes are arrested in meiotic prophase I before fertilization. Sperm secrete MSP [30], which then bind with MSP receptors (VAB-1 and others) found on the surface of oocyte and initiate signal transduction pathway culminating in the resumption of meiosis or ‘meiotic maturation’ of oocyte [31]. Calcium plays important role during the meiotic maturation of oocytes. At least three proteins linked to the Ca2+ signaling coordinate the meiotic maturation of C. elegans oocytes: inositol 1,4,5-triphosphate receptor (ITR-1), N-methyl D-aspartate type glutamate receptor subunit (NMR-1) and Ca2+/calmodulin dependent protein kinase II (UNC-43) [32]. In response to MSP signaling, these three proteins function in oocytes to regulate meiotic maturation [32].
Endoplasmic reticulum is one of the major warehouses of Ca2+ in most of the eukaryotic cells. Activated phospholipase C generates IP3 from the plasma membrane, which then binds with IP3 receptor found on the ER membrane to mobilize Ca2+ into the cytosol. The gene itr-1 encodes IP3 receptor homolog in C. elegans [33]. ITR-1 represses meiotic maturation in the absence of sperm [32]. The itr-1 reduction-of-function and itr-1 gain-of-function mutant females exhibits increased and decreased rate of oocyte maturation, respectively. Epistatic analyses indicate that itr-1 acts downstream of vab-1 to inhibit meiotic maturation [32].
Similarly, a mutation eliminating Ca2+ channel component NMR-1, results in the increased rate of oocyte maturation in females, which suggest that like ITR-1, NMR-1 also represses meiotic maturation of oocyte in the absence of sperm. Complex genetic analyses suggest that NMR-1 cross-talk with VAB-1 in coupling the MSP signal with meiotic maturation [32].
Increased cytosolic Ca2+ activates calmodulin, which in turn activates many downstream target proteins and UNC-43 is one such Ca2+/calmodulin activated protein kinase. The unc-43 loss-of-function mutant exhibits slower oocyte maturation rates, whereas unc-43 gain-of-function mutant exhibits faster maturation rates, indicating that UNC-43 positively regulates meiotic maturation in C. elegans [32]. Epistatic analysis suggests that in the absence of sperm, NMR-1 negatively regulates UNC-43. Binding of MSP with VAB-1 triggers NMR-1 mediated activation of UNC-43 [32].
5. Propelling oocyte into spermatheca
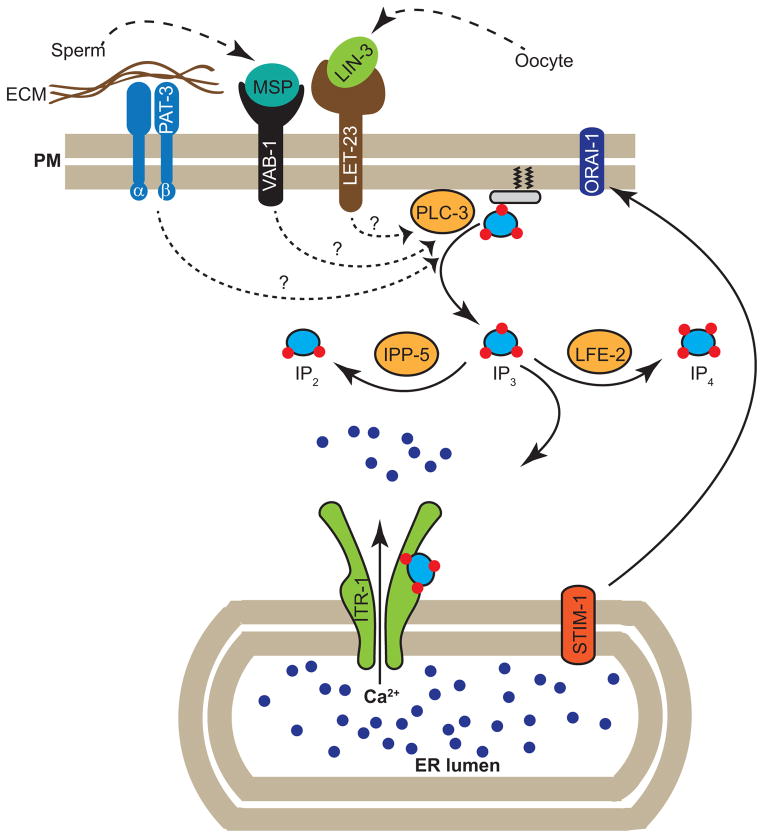
Contraction of gonadal sheath cells, accompanied by dilation of distal spermathecal valve, push the oocyte into spermatheca. IP3 signaling plays an important role in both contraction of sheath cells and dilation of spermatheca valve (see figure 3). Sheath cell contraction requires extracellular Ca2+, since removal of extracellular Ca2+ inhibits contractility of isolated gonads [34]. Oocytes secrete LIN-3/EGF ligand which binds with LET-23/EGF receptor found on the gonadal sheath cells to activate PLC-3/phospholipase C γ. PLC-3 converts PIP2 into IP3 from membrane which in turn binds with IP3 receptor (ITR-1) on the ER membrane to mobilize Ca2+. Several lines of evidence support this model: LET-23, PLC-3 and ITR-1 are expressed in sheath cells [34, 35]. Loss-of-function mutants and/or knock-down of let-23, plc-3 and itr-1 causes reduced sheath contractility and ovulation [34].
Figure 3.
Cartoon showing the molecular components involved in modulating Ca2+si gnal for regulating gonadal sheath cell contraction. Oocytes secrete EGF ligand (LIN-3), which binds with EGF receptor (LET-23). Sperm secrete MSP which bind with VAB-1/Epherin receptor. Extracellular Matrix components surrounding sheath cell bind with integrin (PAT-3). Ligands-recptors interactions trigger signal transduction pathway that likely activates PLC-γ that results in the generation of Inositol triphosphate (IP3). LFE-2 phosphorylates IP3 to form IP4. IPP-5 dephosphorylates IP3 to form IP2. IP3 binds with IP3 receptor (ITR-1) found on the endoplasmic reticulum to mobilize Ca2+ from ER. Depletion of Ca2+ from ER is sensed by ER Ca2+ sensor, STIM-1, which then activates Ca2+ release-activated Ca2+ channel (CRAC), ORAI-1 present on the plasma membrane to replenish the internal Ca2+ store.
The dilation of distal spermatheca valve also depends on LIN-3/LET-23 mediated generation of IP3 [36]. The transcription factors FOS-1 and JUN-1 promote the expression of phospholipase C (plc-1) in spermatheca [37]. PLC-1 generates IP3 which in turn binds with ITR-1 to mobilize Ca2+ from ER. The level of IP3 is regulated by altering its phosphorylation status. The enzymes IP3 kinase (LFE-2) and type I 5 phosphatase (IPP-5) converts IP3 into IP4 and IP2, respectively [36, 38]. Tight regulation of IP3 may be critical in order to ensure that only single oocyte will enter spermatheca per cycle. For example, the spermatheca of ipp-5 mutant engulfs two oocytes per cycle, presumably due to the presence of excessive IP3 [38].
LET-413 (Drosophila Scribble homolog) and DLG-1 (Drosophila Discs large homolog) maintain polarity in spermatheca and positively regulate ovulation through regulating the IP3 pathway. Depletion of let-413 or dlg-1 causes ovulation-failure and sterility [39]. The ipp-5 loss-of-function mutation and itr-1 gain-of-function mutation can partially suppress the sterility observed in let-413 or dlg-1 knock-down animals, suggesting that the LET-413 and DLG-1 positively regulates the IP3 pathway [39]. The extracellular matrix (ECM) surrounding the spermatheca also contributes to ovulatory signal. The ECM components EPI-1 (laminin-α) and EMB-9 (Collagen IV, α1) likely activate integrins (PAT-3) to generate IP3 [40] Knock down of epi-1, emb-9 orpat-3 causes sterility, which can be rescued by ipp-5 loss-of-function mutation or itr-1 gain-of-function mutation, implicating the link between ECM components and the IP3 pathway [40].
Depletion of Ca2+ in the ER is sensed by a Ca2+ sensor which then activates a Ca2+ release - activated Ca2+ (CRAC) channel present on the plasma membrane to replenish the internal Ca2+ store [41]. C. elegans proteome has an ER Ca2+sensor, STIM-1 and CRAC channel ORAI-1. Both STIM-1 and ORAI-1 are found in the gonadal sheath cells and spermatheca [42–44]. Orai1 first maintains the sustained response and at the termination of the stimulus it functions to replenish the stores. Maintaining the sustained response is an important function of the Orai1-mediated Ca2+ influx. Knock down of stim-1 or orai-1 causes identical phenotypes: sheath cells do not contract well and distal spermatheca fail to open during ovulation, which indicates that CRAC channel activity positively regulates ovulation in C. elegans (see figure 3) [42–44].
6. Cell surface localization of TRP-3/SPE-41 channel by SPE-38
TRP-3/SPE-41 is a transient receptor potential Ca2+ permeable channel that plays a role in the terminal phase of fertilization [29]. trp-3/spe-41 mutants are nearly sterile. Several lines of evidences suggest that SPE-41/TRP-3 functions in the sperm. First, TRP-3/SPE-41 is expressed in sperm, not in oocyte. Second, supplying wild-type sperm to trp-3/spe-41 mutant hermaphrodites (by crossing with wild-type males) rescues the sterile phenotype. Third, trp-3/spe-41 mutant males do not sire progeny. Like the rest of the members of a similar class of mutants [19, 45], all aspects of sperm development and differentiation occur normally in trp-3/spe-41 mutants [29]. The trp-3/spe-41 spermatozoa are morphologically indistinguishable from the wild-type spermatozoa; the sperm comes in direct contact with oocyte but fails to fertilize it [29].
Xu and Sternberg developed a protocol to measure changes in intracellular Ca2+ level in C. elegans sperm [29]. Two types of Ca2+ channels are found in C. elegans sperm: constitutively active Ca2+ -permeable channel (CAC) and store-operated channel (SOC). As the name suggests, CAC is constitutively active, therefore, Ca2+ will immediately enter through this class of channel upon increasing the concentration of extracellular Ca2+. On the other hand, the SOC is activated in response to the status of intracellular Ca2+ store. Intracellular Ca2+ store can be depleted by the application of ionomycin and thapsigargin. When the sperm is subsequently exposed to Ca2+, a larger influx of Ca2+ occurs through SOC. This phenomenon is called Store Operated Calcium Entry (SOCE).
The C. elegans spermatids exhibit very little CAC and SOCE activities. The mature spermatozoa, however, exhibit prominent SOCE activity. The SOCE activity is dramatically reduced in trp-3/spe-41 mutant spermatozoa, suggesting that TRP-3/SPE-41 channel contributes to the SOCE in C. elegans spermatozoa [29].
TRP-3/SPE-41 is initially localized to the MOs in spermatids. In mature spermatozoa, TRP-3/SPE-41 exit MOs and gets distributed all over the plasma membrane. Since the TRP-3/SPE-41 channels are sequestered in MOs in spermatids - and therefore unexposed to extracellular milieu - barely any SOCE is detected in spermatids. The fusion of MOs with the plasma membrane redistributes TRP-3/SPE-41 channels on to the plasma membrane, which now are directly exposed to extracellular milieu, thereby contributing SOCE activity.
The localization of TRP-3/SPE-41 is dependent on another protein, SPE-38, which is a novel four-pass transmembrane protein that functions in fertilization [46]. Like trp-3/spe-41 mutant, spe-38 mutants undergo normal spermatogenesis and spermiogenesis, come in direct contact with the oocyte, but fail to fertilize them. SPE-38 is initially localized to MOs in spermatids. In mature spermatozoa, SPE-38 segregates to pseudopod.
In spe-38 mutant spermatozoa, TRP-3/SPE-41 is trapped within the MOs, indicating that SPE-38 regulates the localization of TRP-3/SPE-41 (see figure 4) [47]. Considering the fact that fusion of MOs with plasma membrane occurs normally in spe-38 spermatozoa [46], the inability of TRP-3/SPE-41 to exit MOs suggests that distribution of proteins from fused MOs to plasma membrane is regulated. SPE-38 and TRP-3/SPE-41 physically interact with each other and this interaction is regulated by their cytoplasmic regions [47].
Figure 4.
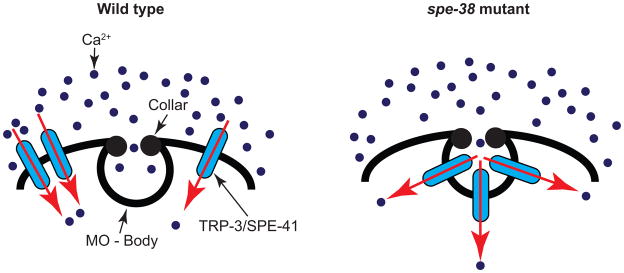
Ca2+ influx in wild-type and spe-38 spermatozoa. In wild-type spermatozoa, TRP-3/SPE-41 channels are found all over the plasma membrane. In spe-38 spermatozoa, TRP-3/SPE-41 channels are trapped within the fused MOs. However, TRP-3/SPE-41 channels are directly exposed to extracellular milieu and hence can function from fused MOs.
Following the fusion of MOs with plasma membrane, MO resident proteins seem to be selectively permitted past fused MOs. An epitope recognized by the monoclonal antibody 1CB4 continues to reside in MOs even after the fusion of MOs with plasma membrane [12, 48]. Similarly, TRP-3/SPE-41 resides in the fused MOs of spe-38 mutant spermatozoa, suggesting that diffusion of proteins from the fused MOs to plasma membrane is regulated by SPE-38.
Interestingly, transmission electron microscopy of mature spermatozoa shows electron dense ‘collar’ at the site of fusion pore [9]. This observation raises the possibility that the collar might act as a barrier for the free diffusion of proteins from MOs to plasma membrane and selective trafficking of proteins across the collar region is facilitated by proteins such as SPE-38 (refer to figure 4). At present, the identities of the individual constituents of the electron dense collar region are unknown. Isolating mutants defective in the MO collar would help us better understand the protein(s) that make or maintain the MO collar and illustrate the nature of the barrier between fused MOs and plasma membrane. Also, this would shine light on how SPE-38 assists in trafficking TRP-3/SPE-41 past the collar region.
Even though TRP-3/SPE-41 channels are stuck in MOs in the absence of SPE-38, the MOs are in direct contact with the extracellular milieu. As a result, TRP-3/SPE-41 is able to function from the fused MOs, as evidenced by the normal SOC activity in spe-38 spermatozoa (figure 4) [47]. The observation that the TRP-3/SPE-41 mediated Ca2+ transients occur normally in spe-38 spermatozoa, yet fail to fertilize oocyte, raises two possibilities. First, it is possible that the channel activity of TRP-3/SPE-41 is separable from its ability to serve as a sperm-egg recognition molecule. Second, unlike wild-type sperm, where TRP-3/SPE-41 channels are scattered all over the plasma membrane, the TRP-3/SPE-41 mediated Ca2+ influx is restricted only from the regions where MOs fuse with plasma membrane in spe-38 mutants. Therefore, the spatial pattern of Ca2+ influx, and not the overall quantity of Ca2+ influx, might be decisive in enabling the sperm fertilization-competent.
What triggers TRP-3/SPE-41 channel activity? Following the co-expression of TRP-3/SPE-41 channel with a histamine receptor (H1R) in a mammalian cell line, treatment of histamine exhibited SOCE activity [29]. Similarly, STIM1 can bind with and activate TRPC in mammals [49]. Identifying endogenous protein(s) that trigger the activation of TRP-3/SPE-41 in C. elegans will be useful in delineating the mechanistic details of coupling the channel activity with fertilization.
7. Generation and propagation of Ca2+ wave following fertilization
Samuel et al. monitored the dynamics of intracellular Ca2+ during fertilization in C. elegans oocytes [50]. The distal region of the gonad is syncytial in nature, which permits the flow of an injected Ca2+ indicator dye (Calcium Green-1 dextran) into developing oocytes. When fully developed, the mature oocytes incorporate Ca2+ indicator dye in their cytoplasm, permitting the visualization of changes in intracellular Ca2+ levels [50]. The resting concentration of Ca2+ is estimated to be around 50–100nM in oocyte.
Fertilization triggers elevation of intracellular Ca2+ level to around 250nM [50]. The onset of Ca2+ elevation occurs at the leading edge of the oocyte – the edge of the oocyte that initially enters into the spermatheca [50]. Since the spermatheca is loaded with sperm, it means that the leading edge of the oocyte is the one that comes in direct contact with sperm at first [51]. Therefore, sperm usually enters at the leading edge of the oocyte. The point of sperm entry determines anterior-posterior axis in C. elegans [51]. Coincident with the sperm entry, the onset of Ca2+ elevation occurs at the leading edge of oocyte, which raises an interesting possibility that Ca2+ signaling might help specify anterior-posterior axis in C. elegans [50].
Recently, Jun Takayama and Shuichi Onami probed the fertilization induced Ca2+ transient in C. elegans oocyte using a more sensitive microscope (personal communication). They found that fertilization triggers biphasic Ca2+ waves in oocytes. First, a fast local wave appeared at the site of sperm-entry and disappeared shortly thereafter. Second, a slow global wave propagated from the site of ‘local wave’ to the opposite pole. The second response is reminiscent of the Ca2+ induced Ca2+ release activity (CICR) (Jun Takayama and Shuichi Onami, personal communication).
Two lines of evidence suggest that sperm triggers biphasic Ca2+ wave upon fertilization in C. elegans. First, mutant sperm that fails to enter oocyte (spe-9 sperm) does not initiate Ca2+ wave in oocyte. Second, a mutant sperm that does enter oocyte, but fails to activate it (spe-11 sperm), exhibits normal biphasic Ca2+ wave (Jun Takayama and Shuichi Onami, personal communication). TRP-3/SPE-41 channel dictates the onset of local wave. The trp-3 mutants are nearly sterile and produce few progeny [29]. The initial fast ‘local wave’ was completely missing in those rarely fertilized trp-3 mutant oocytes, indicating that TRP-3/SPE-41 channel is essential for the onset of fast ‘local wave’. Although a nearly normal ‘global wave’ was generated in fertilized trp-3 oocyte, there had been a significant delay in its onset, suggesting that TRP-3/SPE-41 is essential for regulating the temporal pattern of ‘global wave’ generation (Jun Takayama and Shuichi Onami, personal communication).
It would be interesting to investigate what channel(s) contributes towards the propagation of ‘global wave’ of Ca2+ in oocyte following fertilization. Many Ca2+ channels are enriched in oocytes [52]. Unlike sperm, oocytes are amenable to RNAi. Therefore, systematic knock-down of Ca2+ channels enriched in oocyte can be conducted. Any deviation from the wild type pattern of ‘global wave’, following the knock-down of any of these genes might reveal the Ca2+ channels required for ‘global wave’ formation after fertilization. Since many of these channels might also function in somatic tissue, performing such screen in a genetic background [rrf-1(pk1417)] that would permit knockdown of genes selectively in germ line and spare the somatic tissue [53] would offer practical advantage.
8. Conclusions
Calcium plays important roles in orchestrating various events that prepares and facilitates gamete maturation, interaction and ultimately, fertilization, in C. elegans. Full activation of sperm requires intracellular Ca2+. Oocytes are arrested in meiotic prophase I and sperm-derived hormone, MSP relieves the block by modulating the activity of at least three proteins that function in Ca2+ signaling. Propelling maturing oocyte into spermatheca requires sheath cell contraction and spermatheca valve dilation, for which IP3 mediated mobilization of Ca2+ from ER plays an important role. Finally, fertilization requires TRP-3/SPE-41 Ca2+ permeable channel, whose localization is governed by SPE-38. Calcium waves occur immediately following fertilization in oocytes, which requires TRP-3/SPE-41 channels.
Acknowledgments
We thank Lokesh Lahoti for providing the DIC image of C. elegans gonad; Sina Rahimi for providing sperm images. We thank Singson lab members for providing useful suggestions. We thank Lokesh Lahoti and Sunny Dharia for providing feedback on the usage of English. This work was supported by a grant from NIH (R01 HD054681) to Andrew Singson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 2.Singson A, Hang JS, Parry JM. Genes required for the common miracle of fertilization in Caenorhabditis elegans. Int J Dev Biol. 2008;52:647–656. doi: 10.1387/ijdb.072512as. [DOI] [PubMed] [Google Scholar]
- 3.McCarter J, Bartlett B, Dang T, Schedl T. On the control of oocyte meiotic maturation and ovulation in Caenorhabditis elegans. Dev Biol. 1999;205:111–128. doi: 10.1006/dbio.1998.9109. [DOI] [PubMed] [Google Scholar]
- 4.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbard EJ, Greenstein D. The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- 7.L’Hernault SW. Spermatogenesis. WormBook; 2006. pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu DS, Shakes DC. Spermatogenesis. Adv Exp Med Biol. 2013;757:171–203. doi: 10.1007/978-1-4614-4015-4_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward S, Argon Y, Nelson GA. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J Cell Biol. 1981;91:26–44. doi: 10.1083/jcb.91.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenstein D. Control of oocyte meiotic maturation and fertilization. WormBook; 2005. pp. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson GA, Ward S. Vesicle fusion, pseudopod extension and amoeboid motility are induced in nematode spermatids by the ionophore monensin. Cell. 1980;19:457–464. doi: 10.1016/0092-8674(80)90520-6. [DOI] [PubMed] [Google Scholar]
- 12.Washington NL, Ward S. FER-1 regulates Ca2+ -mediated membrane fusion during C. elegans spermatogenesis. J Cell Sci. 2006;119:2552–2562. doi: 10.1242/jcs.02980. [DOI] [PubMed] [Google Scholar]
- 13.Rizzuto R, Duchen MR, Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 14.Muhlrad PJ, Ward S. Spermiogenesis initiation in Caenorhabditis elegans involves a casein kinase 1 encoded by the spe-6 gene. Genetics. 2002;161:143–155. doi: 10.1093/genetics/161.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park BJ, Lee DG, Yu JR, Jung SK, Choi K, Lee J, Kim YS, Lee JI, Kwon JY, Singson A, Song WK, Eom SH, Park CS, Kim DH, Bandyopadhyay J, Ahnn J. Calreticulin, a calcium-binding molecular chaperone, is required for stress response and fertility in Caenorhabditis elegans. Mol Biol Cell. 2001;12:2835–2845. doi: 10.1091/mbc.12.9.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afshar N, Black BE, Paschal BM. Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol. 2005;25:8844–8853. doi: 10.1128/MCB.25.20.8844-8853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaMunyon CW, Ward S. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc Biol Sci. 1998;265:1997–2002. doi: 10.1098/rspb.1998.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Curr Opin Cell Biol. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Singson A. Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev Biol. 2001;230:101–109. doi: 10.1006/dbio.2000.0118. [DOI] [PubMed] [Google Scholar]
- 20.Gleason EJ, Hartley PD, Henderson M, Hill-Harfe KL, Price PW, Weimer RM, Kroft TL, Zhu GD, Cordovado S, L’Hernault SW. Developmental genetics of secretory vesicle acidification during Caenorhabditis elegans spermatogenesis. Genetics. 191:477–491. doi: 10.1534/genetics.112.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadler PL, Shakes DC. Anucleate Caenorhabditis elegans sperm can crawl, fertilize oocytes and direct anterior-posterior polarization of the 1-cell embryo. Development. 2000;127:355–366. doi: 10.1242/dev.127.2.355. [DOI] [PubMed] [Google Scholar]
- 22.Shakes DC, Ward S. Initiation of spermiogenesis in C. elegans: a pharmacological and genetic analysis. Dev Biol. 1989;134:189–200. doi: 10.1016/0012-1606(89)90088-2. [DOI] [PubMed] [Google Scholar]
- 23.Singaravelu G, Singson A. New insights into the mechanism of fertilization in nematodes. Int Rev Cell Mol Biol. 2011;289:211–238. doi: 10.1016/B978-0-12-386039-2.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeClaire LL, 3rd, Stewart M, Roberts TM. A 48 kDa integral membrane phosphoprotein orchestrates the cytoskeletal dynamics that generate amoeboid cell motility in Ascaris sperm. J Cell Sci. 2003;116:2655–2663. doi: 10.1242/jcs.00469. [DOI] [PubMed] [Google Scholar]
- 25.Yi K, Buttery SM, Stewart M, Roberts TM. A Ser/Thr kinase required for membrane-associated assembly of the major sperm protein motility apparatus in the amoeboid sperm of Ascaris. Mol Biol Cell. 2007;18:1816–1825. doi: 10.1091/mbc.E06-08-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi K, Wang X, Emmett MR, Marshall AG, Stewart M, Roberts TM. Dephosphorylation of major sperm protein (MSP) fiber protein 3 by protein phosphatase 2A during cell body retraction in the MSP-based amoeboid motility of Ascaris sperm. Mol Biol Cell. 2009;20:3200–3208. doi: 10.1091/mbc.E09-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karabinos A, Bussing I, Schulze E, Wang J, Weber K, Schnabel R. Functional analysis of the single calmodulin gene in the nematode Caenorhabditis elegans by RNA interference and 4-D microscopy. Eur J Cell Biol. 2003;82:557–563. doi: 10.1078/0171-9335-00347. [DOI] [PubMed] [Google Scholar]
- 28.Achanzar WE, Ward S. A nematode gene required for sperm vesicle fusion. J Cell Sci. 1997;110(Pt 9):1073–1081. doi: 10.1242/jcs.110.9.1073. [DOI] [PubMed] [Google Scholar]
- 29.Xu XZ, Sternberg PWA. C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114:285–297. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 30.Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- 31.Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17:187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrigan C, Subramanian R, Miller MA. Eph and NMDA receptors control Ca2+/calmodulin-dependent protein kinase II activation during C. elegans oocyte meiotic maturation. Development. 2005;132:5225–5237. doi: 10.1242/dev.02083. [DOI] [PubMed] [Google Scholar]
- 33.Baylis HA, Furuichi T, Yoshikawa F, Mikoshiba K, Sattelle DB. Inositol 1,4,5-trisphosphate receptors are strongly expressed in the nervous system, pharynx, intestine, gonad and excretory cell of Caenorhabditis elegans and are encoded by a single gene (itr-1) J Mol Biol. 1999;294:467–476. doi: 10.1006/jmbi.1999.3229. [DOI] [PubMed] [Google Scholar]
- 34.Yin X, Gower NJ, Baylis HA, Strange K. Inositol 1,4,5-trisphosphate signaling regulates rhythmic contractile activity of myoepithelial sheath cells in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3938–3949. doi: 10.1091/mbc.E04-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dal Santo P, Logan MA, Chisholm AD, Jorgensen EM. The inositol trisphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- 36.Clandinin TR, DeModena JA, Sternberg PW. Inositol trisphosphate mediates a RAS-independent response to LET-23 receptor tyrosine kinase activation in C. elegans. Cell. 1998;92:523–533. doi: 10.1016/s0092-8674(00)80945-9. [DOI] [PubMed] [Google Scholar]
- 37.Hiatt SM, Duren HM, Shyu YJ, Ellis RE, Hisamoto N, Matsumoto K, Kariya K, Kerppola TK, Hu CD. Caenorhabditis elegans FOS-1 and JUN-1 regulate plc-1 expression in the spermatheca to control ovulation. Mol Biol Cell. 2009;20:3888–3895. doi: 10.1091/mbc.E08-08-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bui YK, Sternberg PW. Caenorhabditis elegans inositol 5-phosphatase homolog negatively regulates inositol 1,4,5-triphosphate signaling in ovulation. Mol Biol Cell. 2002;13:1641–1651. doi: 10.1091/mbc.02-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pilipiuk J, Lefebvre C, Wiesenfahrt T, Legouis R, Bossinger O. Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev Biol. 2009;327:34–47. doi: 10.1016/j.ydbio.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 40.Xu X, Lee D, Shih HY, Seo S, Ahn J, Lee M. Linking integrin to IP3 signaling is important for ovulation in Caenorhabditis elegans. FEBS Lett. 2005;579:549–553. doi: 10.1016/j.febslet.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 41.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446:284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 42.Strange K, Yan X, Lorin-Nebel C, Xing J. Physiological roles of STIM1 and Orai1 homologs and CRAC channels in the genetic model organism Caenorhabditis elegans. Cell Calcium. 2007;42:193–203. doi: 10.1016/j.ceca.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorin-Nebel C, Xing J, Yan X, Strange K. CRAC channel activity in C. elegans is mediated by Orai1 and STIM1 homologues and is essential for ovulation and fertility. J Physiol. 2007;580:67–85. doi: 10.1113/jphysiol.2006.124883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan X, Xing J, Lorin-Nebel C, Estevez AY, Nehrke K, Lamitina T, Strange K. Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J Gen Physiol. 2006;128:443–459. doi: 10.1085/jgp.200609611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcello MR, Singaravelu G, Singson A. Fertilization. Adv Exp Med Biol. 2013;757:321–350. doi: 10.1007/978-1-4614-4015-4_11. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee I, Richmond A, Putiri E, Shakes DC, Singson A. The Caenorhabditis elegans spe-38 gene encodes a novel four-pass integral membrane protein required for sperm function at fertilization. Development. 2005;132:2795–2808. doi: 10.1242/dev.01868. [DOI] [PubMed] [Google Scholar]
- 47.Singaravelu G, Chatterjee I, Rahimi S, Druzhinina MK, Kang L, Xu XZ, Singson A. The sperm surface localization of the TRP-3/SPE-41 Ca2+ -permeable channel depends on SPE-38 function in Caenorhabditis elegans. Dev Biol. 2012;365:376–383. doi: 10.1016/j.ydbio.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okamoto H, Thomson JN. Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous tissue of the nematode Caenorhabditis elegans. J Neurosci. 1985;5:643–653. doi: 10.1523/JNEUROSCI.05-03-00643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, Malik AB, Tiruppathi C. The Ca2+ sensor stromal interaction molecule 1 (STIM1) is necessary and sufficient for the store-operated Ca2+ entry function of transient receptor potential canonical (TRPC) 1 and 4 channels in endothelial cells. Mol Pharmacol. 81:510–526. doi: 10.1124/mol.111.074658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuel AD, Murthy VN, Hengartner MO. Calcium dynamics during fertilization in C. elegans. BMC Dev Biol. 2001;1:8. doi: 10.1186/1471-213X-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- 52.Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 53.Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RH, Fire A. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]