Abstract
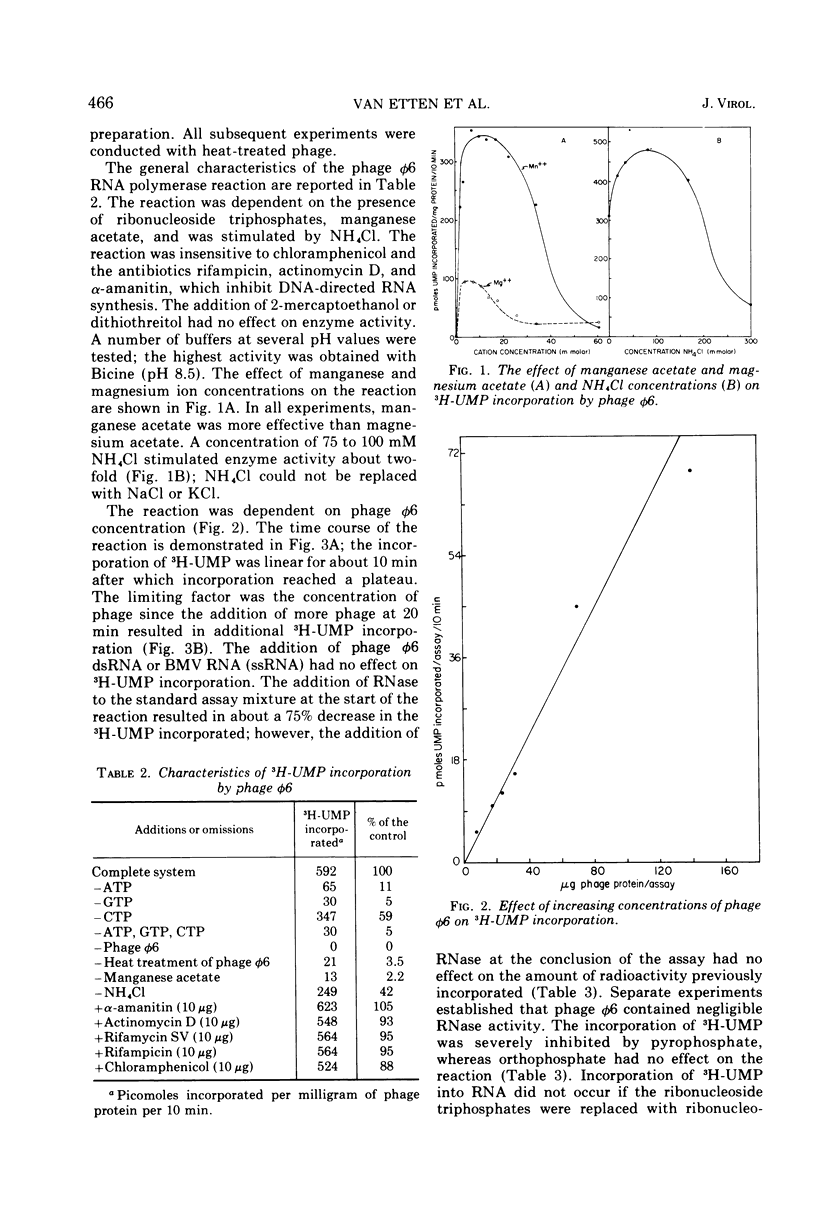
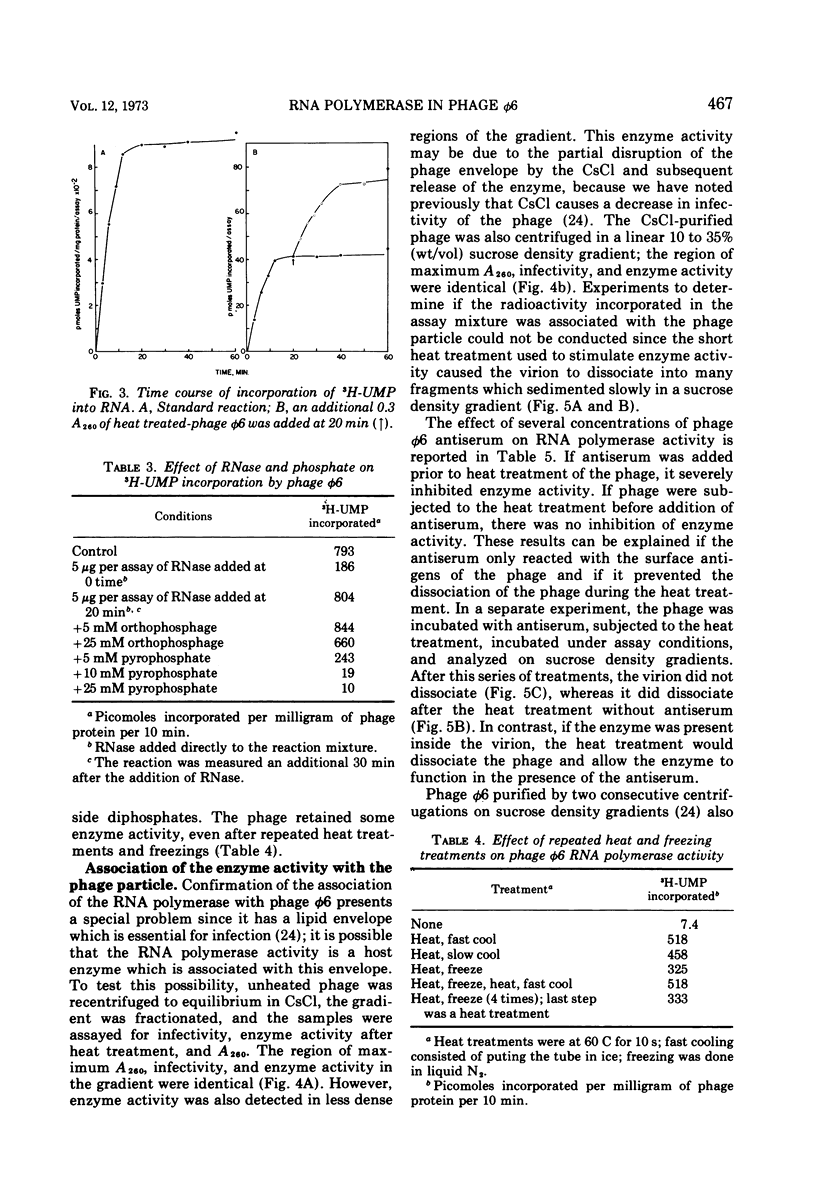
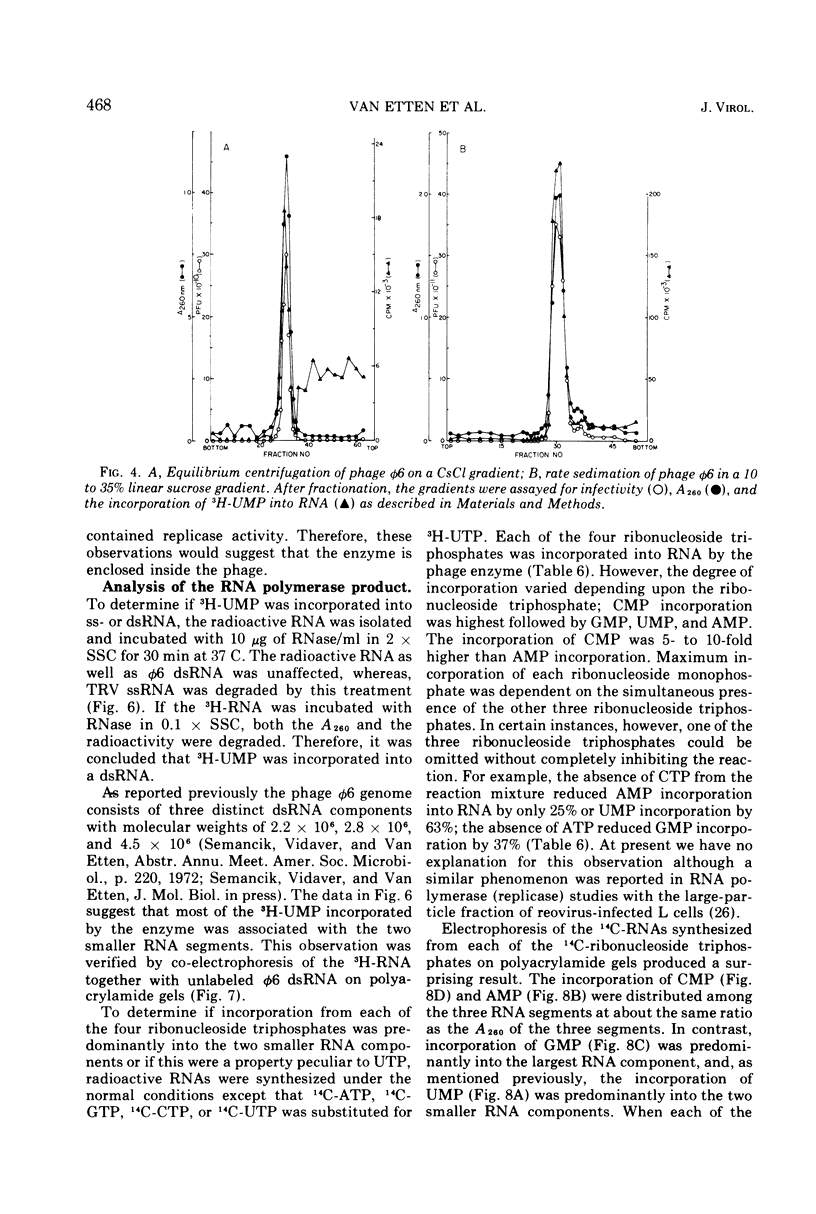
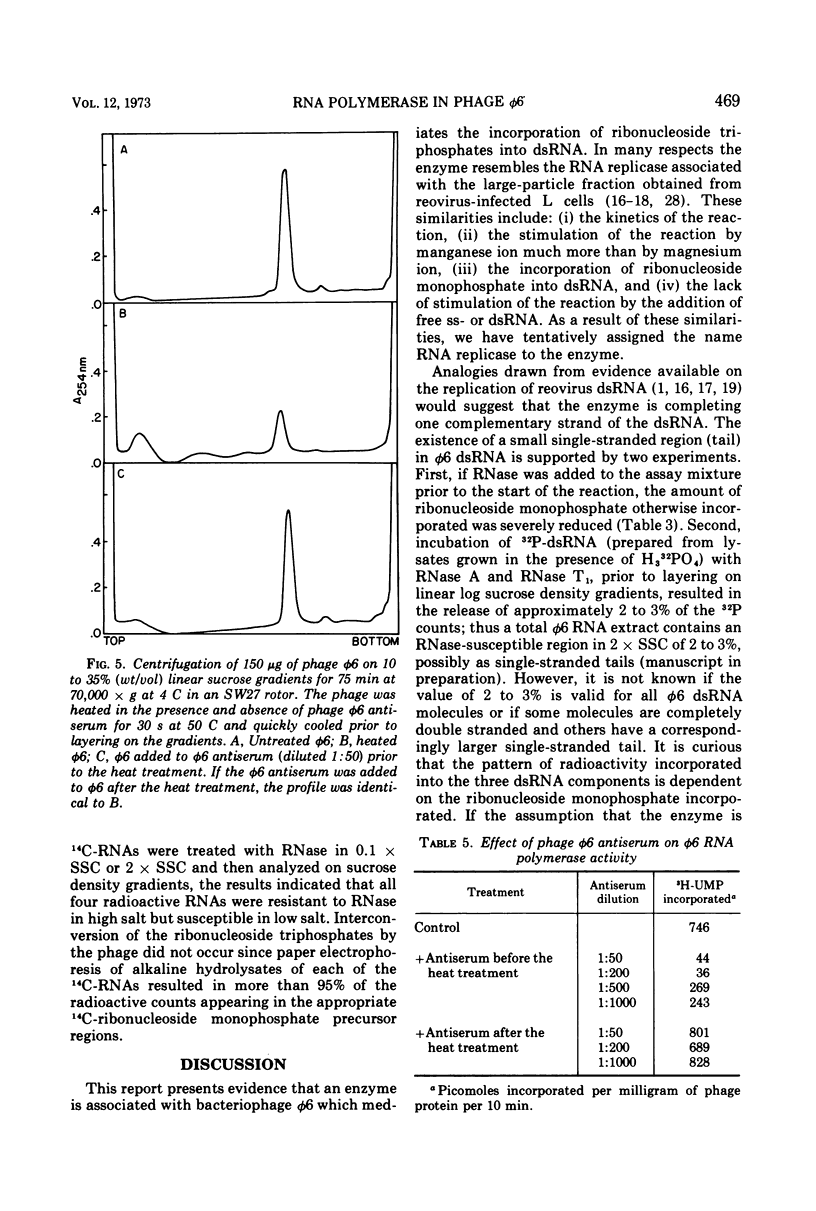
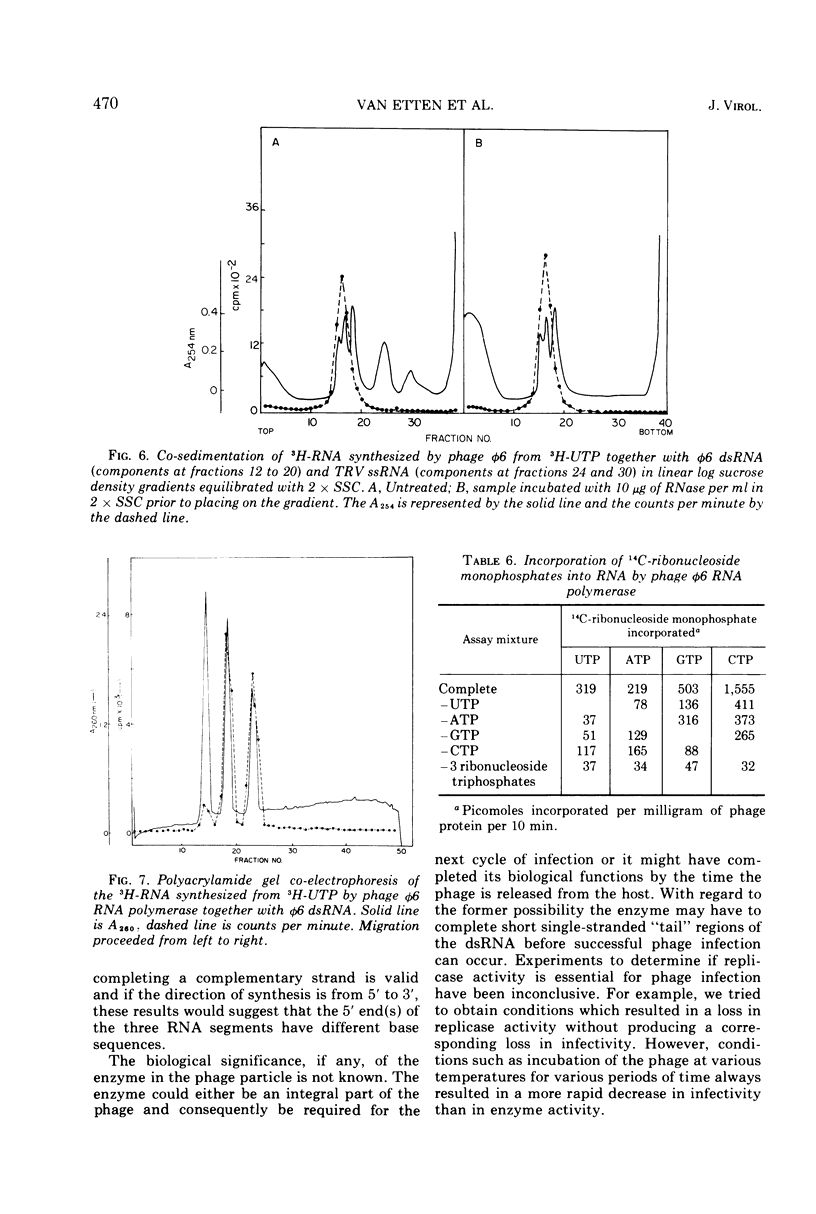
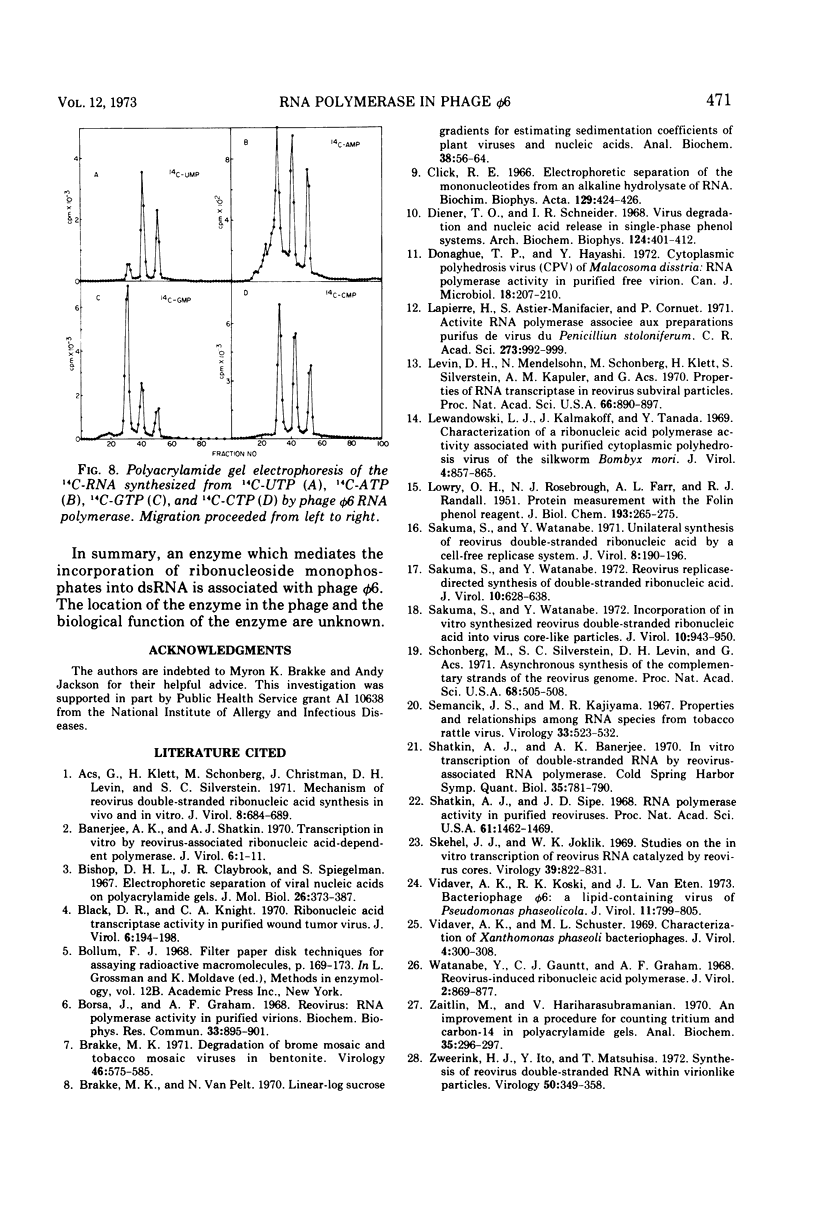
The Pseudomonas phaseolicola bacteriophage φ6 incorporated labeled UTP into an acid-insoluble precipitate. Incorporation was dependent on the presence of manganese acetate, ATP, GTP, CTP, and a short heat treatment of the phage; the reaction was stimulated by NH4Cl. The substitution of 14C-ATP, -CTP or -GTP for UTP, together with the appropriate unlabeled ribonucleoside triphosphates, disclosed that CMP was incorporated to the greatest extent followed by GMP, UMP, and AMP. Radioactive RNAs formed by the reaction were resistant to RNases A and T1 in high salt but susceptible to these nucleases in low salt. The labeled RNA co-sedimented and co-electrophoresed with φ6 double-stranded (ds) RNA. However, the distribution of the radioactivity into the three ds-RNA components varied depending on the 14C-ribonucleoside triphosphate used in the reaction. The incorporation of UMP was primarily into the two smaller ds-RNA segments, GMP primarily into the large ds-RNA segment, and CMP and AMP were about equally distributed into all three ds-RNA segments.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. K., Shatkin A. J. Transcription in vitro by reovirus-associated ribonucleic acid-dependent polymerase. J Virol. 1970 Jul;6(1):1–11. doi: 10.1128/jvi.6.1.1-11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Spiegelman S. Electrophoretic separation of viral nucleic acids on polyacrylamide gels. J Mol Biol. 1967 Jun 28;26(3):373–387. doi: 10.1016/0022-2836(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Black D. R., Knight C. A. Ribonucleic acid transcriptase acitvity in purified wound tumor virus. J Virol. 1970 Aug;6(2):194–198. doi: 10.1128/jvi.6.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Graham A. F. Reovirus: RNA polymerase activity in purified virions. Biochem Biophys Res Commun. 1968 Dec 30;33(6):895–901. doi: 10.1016/0006-291x(68)90396-3. [DOI] [PubMed] [Google Scholar]
- Brakke M. K. Degradation of brome mosaic and tobacco mosaic viruses in bentonite. Virology. 1971 Dec;46(3):575–585. doi: 10.1016/0042-6822(71)90061-4. [DOI] [PubMed] [Google Scholar]
- Brakke M. K., Van Pelt N. Linear-log sucrose gradients for estimating sedimentation coefficients of plant viruses and nucleic acids. Anal Biochem. 1970 Nov;38(1):56–64. doi: 10.1016/0003-2697(70)90155-7. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Schneider I. R. Virus degradation and nucleic acid release in single-phase phenol systems. Arch Biochem Biophys. 1968 Mar 20;124(1):401–412. doi: 10.1016/0003-9861(68)90344-5. [DOI] [PubMed] [Google Scholar]
- Donaghue T. P., Hayashi Y. Cytoplasmic polyhedrosis virus (CPV) of Malacosoma disstria: RNA polymerase activity in purified free viroin. Can J Microbiol. 1972 Feb;18(2):207–210. doi: 10.1139/m72-032. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapierre H., Astier-Manifacier S., Cornuet P. Activité RNA polymérase associée aux préparations purifiées de virus du Penicillium stoloniferum. C R Acad Sci Hebd Seances Acad Sci D. 1971 Sep 13;273(11):992–994. [PubMed] [Google Scholar]
- Levin D. H., Mendelsohn N., Schonberg M., Klett H., Silverstein S., Kapuler A. M., Acs G. Properties of RNA transcriptase in reovirus subviral particles. Proc Natl Acad Sci U S A. 1970 Jul;66(3):890–897. doi: 10.1073/pnas.66.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski L. J., Kalmakoff J., Tanada Y. Characterization of a Ribonucleic Acid Polymerase Activity Associated with Purified Cytoplasmic Polyhedrosis Virus of the Silkworm Bombyx mori. J Virol. 1969 Dec;4(6):857–865. doi: 10.1128/jvi.4.6.857-865.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Incorporation of in vitro synthesized reovirus double-stranded ribonucleic acid into virus corelike particles. J Virol. 1972 Nov;10(5):943–950. doi: 10.1128/jvi.10.5.943-950.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Reovirus replicase-directed synthesis of double-stranded ribonucleic acid. J Virol. 1972 Oct;10(4):628–638. doi: 10.1128/jvi.10.4.628-638.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma S., Watanabe Y. Unilateral synthesis of reovirus double-stranded ribonucleic acid by a cell-free replicase system. J Virol. 1971 Aug;8(2):190–196. doi: 10.1128/jvi.8.2.190-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg M., Silverstein S. C., Levin D. H., Acs G. Asynchronous synthesis of the complementary strands of the reovirus genome. Proc Natl Acad Sci U S A. 1971 Feb;68(2):505–508. doi: 10.1073/pnas.68.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Kajiyama M. R. Properties and relationships among RNA species from tobacco rattle virus. Virology. 1967 Nov;33(3):523–532. doi: 10.1016/0042-6822(67)90129-8. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Joklik W. K. Studies on the in vitro transcription of reovirus RNA catalyzed by reovirus cores. Virology. 1969 Dec;39(4):822–831. doi: 10.1016/0042-6822(69)90019-1. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Schuster M. L. Characterization of Xanthomonas phaseoli Bacteriophages. J Virol. 1969 Sep;4(3):300–308. doi: 10.1128/jvi.4.3.300-308.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Gauntt C. J., Graham A. F. Reovirus-induced ribonucleic acid polymerase. J Virol. 1968 Sep;2(9):869–877. doi: 10.1128/jvi.2.9.869-877.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. An improvement in a procedure for counting tritium and carbon-14 in polyacrylamide gels. Anal Biochem. 1970 May;35(1):296–297. doi: 10.1016/0003-2697(70)90038-2. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Ito Y., Matsuhisa T. Synthesis of reovirus double-stranded RNA within virionlike particles. Virology. 1972 Nov;50(2):349–358. doi: 10.1016/0042-6822(72)90386-8. [DOI] [PubMed] [Google Scholar]



