Abstract
This report details the efficacy of nitric oxide (NO)-releasing xerogel surfaces composed of N-(6-aminohexyl)aminopropyl trimethoxysilane (AHAP3) and isobutyltrimethoxysilane (BTMOS) against Candida albicans adhesion, viability, and biofilm formation. A parallel plate flow cell assay was used to examine the effect of NO on planktonic fungal cells. Nitric oxide fluxes as low as 14 pmol cm−2 s−1 were sufficient to reduce fungal adhesion by ~49% over controls after 90 min. By utilizing a fluorescence live/dead assay and replicate plating, NO flux was determined to reduce fungal viability in a dose dependent manner. The formation of C. albicans biofilms on NO-releasing xerogel-coated silicon rubber (SiR) coupons was impeded when compared to control (non-NO-releasing) and bare SiR surfaces. Finally, the synergistic efficacy of NO and silver sulfadiazine against C. albicans adhered fungal cells and biofilms is reported with increased killing and biofilm inhibition over NO alone.
Keywords: Nitric oxide, Candida albicans, antimicrobial, fungal adhesion, biofilm
Introduction
Despite sterilization and the development of more hydrophilic coatings, invasive microbial infections remain a significant threat to the success of implanted medical devices, including indwelling catheters (Hetrick and Schoenfisch 2006). Overall, >250,000 catheter-related blood stream infections are reported in the U.S. annually with a mortality rate up to 25% and significant financial burden (O’Grady et al. 2002). Candida albicans, a pathogenic fungus found naturally in the human gastrointestinal system, has been identified as the fourth most common pathogen isolated from catheters (behind coagulase-negative staphylococci, Staphylococcus aureus, and enterococci) (Douglas 2003). Furthermore, C. albicans is characterized by the second highest ratio of colonization to blood stream infection (coagulase-negative staphylococci and staphylococcus aureus), highlighting its virulence (Crump and Collignon 2000; Ramage et al. 2005). Although fungal infections are more common among immunocomprimised patients, the use of broad-spectrum antibiotics in immunocompetent patients often eliminates natural competitive pressure from endogenous bacterial flora, increasing the risk of proliferation of other endogenous microbes and infection (Wey et al. 1989; Cole et al. 1996; Shin et al. 2002; Pfaller and Diekema 2007).
Candida albicans infections typically originate at indwelling medical devices such as central venous catheters (CVCs). Characteristically, pathogenic colonization of medical devices occurs in four stages: adsorption of host proteins, adhesion of single cells to the surface, formation of multiple basal layers, and growth of biofilm structures (e.g., hyphae, extracellular matrix) (Douglas 2003). Fungal biofilms exhibit a greater resistance to antifungal agents (e.g., fluconazole and amphotericin B) than planktonic cells (Hawser and Douglas 1995; Chandra et al. 2001; Douglas 2003). Hawser and Douglas observed that the concentrations of five common antifungal agents used to reduce fungal metabolic activity by 50% were five to eight times higher for C. albicans in biofilms than planktonic cells (Hawser and Douglas 1995). While systemic antifungals may be administered to combat fungal biofilm growth, strains resistant to common antifungals have emerged more recently (Chandra et al. 2001; Douglas 2003).
Both passive and active strategies have been employed to mitigate fungal adhesion to medical implants (Hetrick and Schoenfisch 2006). Passive strategies include surface modifications of polymer brushes (Nejadnik et al. 2008), eugenol derivatives (Rojo et al. 2008), and surface coatings of lauroyl glucose (Dusane et al. 2008). In vivo, the utility of passive strategies remains limited, particularly in their ability to reduce the viability of attached microbes. Active release of antimicrobials (Marini et al. 2007; Malcher et al. 2008) and antibiotics (Rossi et al. 2004) have been shown to effectively reduce both the adhesion and viability of microbes on surfaces, but with increasing concern related to resistant strains (Freitas et al. 1999; Chandra et al. 2001; Silver 2003; Percival et al. 2005; Chow et al. 2007; De Lencastre et al. 2007; Witte et al. 2008), necessitating the development of more effective antimicrobial agents.
Recent work has identified nitric oxide (NO), a highly reactive free radical, as an antimicrobial agent that is produced endogenously (McElhaney-Feser et al. 1998; Weller et al. 2001; Nablo and Schoenfisch 2003; Nablo et al. 2005). Macrophages and other immune cells generate NO via inducible nitric oxide synthase (iNOS) in response to pathogens (MacMicking et al. 1997). Restricting iNOS function and thereby limiting endogenous NO was shown by MacMicking et al. to result in greater infection rates (MacMicking et al. 1995; MacMicking et al. 1997). While NO has been demonstrated as an effective antimicrobial, the potential clinical utility of NO-releasing drugs has been confounded by inadequate delivery. Indeed, sustaining effective NO levels for a given application has proven most challenging (Ghaffari et al. 2006). Our lab has employed sol-gel chemistry to produce materials capable of sustained release of NO via diazeniumdiolate NO donors (Nablo et al. 2001; Nablo and Schoenfisch 2003; Dobmeier and Schoenfisch 2004; Nablo et al. 2005; Nablo et al. 2005; Hetrick and Schoenfisch 2007; Charville et al. 2008). Nitric oxide is stored as two molecule units on secondary amine moieties as a covalently-linked diazeniumdiolate NO donor (Hrabie and Keefer 2002). Such materials have proven beneficial in reducing bacterial adhesion in both in vitro and in vivo models (Nablo et al. 2001; Nablo and Schoenfisch 2003; Dobmeier and Schoenfisch 2004; Nablo et al. 2005; Nablo et al. 2005; Hetrick et al. 2008), and killing surface adhered bacteria (Hetrick et al. 2008). More recently, Hetrick et al. demonstrated that microbial biofilms can be effectively dispersed by NO released from silica particles (Hetrick et al. 2009). While some microbes may decrease their susceptibility to low concentrations of NO and its byproducts by increasing the production of antioxidant enzymes (MacMicking et al. 1997), examples or evidence of bacteria or fungus resistant to high concentrations of NO currently do not exist. Accordingly, interest in the therapeutic applications of NO as an antimicrobial continues to grow.
Although in vitro testing of the efficacy of NO generated by small molecule NO donors against planktonic Candida albicans has proven that NO is a potent antifungal (McElhaney-Feser et al. 1998; Weller et al. 2001), research regarding the effect of NO on the adhesion, viability, and biofilm formation of C. albicans remains incomplete. Herein, we report the effectiveness of surface-generated NO against Candida albicans using model xerogel surfaces with a parallel plate flow cell (fungal adhesion) fluorescence nucleic acid stains and replicate plating on nutrient agar (viability of adherent fungal cells), and a biofilm growth assay.
Experimental
Materials
Isobutyltrimethoxysilane (BTMOS) was purchased from Aldrich (Milwaukee, WI). N-(6-aminohexyl)aminopropyl trimethoxysilane (AHAP3) was purchased from Gelest (Morrisville, PA). Ethanol (absolute), hydrochloric acid, and tetrahydrofuran were purchased from Fisher Scientific (Pittsburgh, PA). Low molecular weight poly(vinyl chloride) (PVC) and silver sulfadiazine (AgSD) were purchased from Sigma-Aldrich (Tullytown, PA). Nitric oxide (99.5%) and argon (Ar) gases were purchased from National Welders Supply (Durham, NC). Class VI medical grade silicon rubber (0.60″ thickness) was purchased from McMaster-Carr (Santa Fe Springs, CA). C. albicans (ATCC# 90028) was obtained from the American Type Culture Collection (Manassas, VA). Yeast peptone dextrose broth was purchased from Becton, Dickinson and Company (Sparks, MD). Nucleic acid stains SYTOc9 and propidium iodide (PI) were purchased from Invitrogen (Carlsbad, CA). Distilled water was purified to 18.2 MΩ·cm with a Millipore Milli-Q Gradient A-10 water purification system (Bedford, MA).
Synthesis of AHAP3/BTMOS xerogel-coated films
Nitric oxide-releasing AHAP3/BTMOS xerogel-coated glass slides were synthesized as described previously (Marxer et al. 2003). Upon mixing ethanol (200 μL), BTMOS (180–120 μL), water (60 μL), and 0.5 M HCl (10 μL) for 1 h, AHAP3 (20–80 μL) was added to the solution and mixed for an additional hour resulting in a sol. The volume percentage of AHAP3 (balance BTMOS) was varied between 10 and 40%. Glass microscope slides were cut to 9 × 24 mm, sonicated in ethanol for 20 min, dried with N2, and UV-cleaned with a BioForce TipCleaner (Ames, IA) for 20 min. The sol (30 μL) was spread onto the clean glass slides, dried for 30 min in a dessicator, and transferred to a 70 °C oven for 3 d. Xerogel-coated slides were then stored in a dessicator at room temperature until use. Xerogels formed in this manner are stable upon immersion in solution (Nablo et al. 2005).
Nitric oxide-releasing 40% AHAP3 (v/v; balance BTMOS) xerogel-coated silicon rubber coupons for use in biofilm studies were synthesized as described previously (Hetrick and Schoenfisch 2007). Following the mixing of ethanol (1.2 mL), water (640 μL), and 0.5 M HCl (110 μL), BTMOS (1.28 mL) was added dropwise, and the sol mixed for 18 h. AHAP3 (860 μL) was then added and the sol mixed for an additional 30 h. Class VI medical-grade silicon rubber (SiR) was cut into 8 × 6 × 2 mm3 coupons, cleaned via sonication in ethanol, sterilized in an autoclave at 120 °C for 20 min, dried for 5 min in a 80 °C oven, and then immobilized sterile syringe needles. A xerogel surface was applied to the SiR coupons via dip-coating into the sol. The xerogel-coated SiR coupons were rotated at 1 rev/sec for 3 d to facilitate even curing, then dried in a 50 °C oven for 1 d. Xerogel-coated SiR squares were then stored in a dessicator at room temperature until use.
Diazeniumdiolate modification of xerogels
Nitric oxide was loaded onto xerogels via exposure of the coated glass slides and SiR coupons to high pressures of NO. The film-coated slides and SiR coupons were placed in a Parr hydrogenation bomb, flushed with Ar at least six times to remove O2, and pressurized with 5 atm NO gas for 3 d. Unreacted NO was then removed from the vessel by flushing with Ar. The NO donor-modified xerogels were stored in a dessicator at −20 °C until use to prevent diazeniumdiolate decomposition.
Evaluation of xerogel stability
The stability of SiR coupons coated with 40% AHAP3-BTMOS was evaluated by measuring the static water contact angle with a KSV Cam200 Contact Angle Goniometer as a function of PBS immersion time. The contact angle of both control and diazeniumdiolate-modified xerogel-coated SiR coupons was measured before and after soaking in PBS at 37 °C for 2 d (the duration of the biofilm experiment).
Growth of standardized C. albicans suspension
Candida albicans was cultured in yeast peptone dextrose (YPD) broth at 37 °C, pelleted by centrifugation, resuspended in 15% glycerol (v/v in PBS) and stored at −80 °C. Cultures for fungal adhesion, viability, and biofilm studies were grown from a −80 °C stock in YPD broth overnight. An aliquot of the overnight culture (1 mL) was inoculated into 100 mL sterile YPD broth, incubated at 37 °C with gentle agitation, and grown to 1 × 106 colony forming units (CFU) per mL as measured by optical density at 650 nm (OD650 ~0.044) and verified by serial 10-fold dilutions in PBS, plating on YPD agar, and enumeration of viable colonies. Cultures for viability and adhesion studies were pelleted by centrifugation (5000 rpm, 15 min) and resuspended in sterile PBS. Cultures for biofilm studies were diluted to 104 CFU/mL in YPD broth.
Determination of fungicidal efficacy of AgSD
The efficacy of AgSD against C. albicans was determined at 24 h using a standard minimum bactericidal concentration (MBC24) assay. The MBC24 is defined as the minimum concentration of AgSD necessary to elicit a 3-log reduction in fungal viability after 24 h in growth conditions. Standardized fungal suspensions were tested against 5 different concentrations of AgSD. Concentrations of AgSD were chosen to bracket the MBC24 with a low and high concentration.
A 104 CFU/mL suspension of C. albicans in YPD broth was diluted to 2 × 104 CFU/mL in YPD broth. A 2x concentration of AgSD in YPD broth was added to an equal volume of fungal suspension, resulting in a 104 CFU/mL fungal concentration and the desired final AgSD concentration. The number of viable fungus was determined via serial dilution and replicate plating on YPD agar at the beginning of the assay, and after 6 and 24 h of incubation at 37 °C on an orbital shaker.
Flow cell-based fungal adhesion studies
A parallel plate flow cell assay was employed to examine the effect of surface NO flux on fungal adhesion to xerogel surfaces (Hetrick and Schoenfisch 2007). Glass slides with control and NO-modified xerogels coated on one side were loaded into a custom-built polycarbonate flow cell device, forming chambers with dimension 2.1 × 0.6 × 0.08 cm3. Two sets of three parallel flow chambers were placed in series so that three control xerogels were placed in front of three NO-releasing xerogels, allowing for 3 replicates to be measured in each experiment. A 106 CFU/mL C. albicans suspension in PBS (25°C) was introduced over the xerogels at 0.2 mL/min using a three-channel peristaltic pump. Fungal coverage to xerogel surfaces was measured by obtaining brightfield micrographs (10x magnification) in real-time at fixed timepoints (5, 20, 40, 60, 90, 120, and 150 min) using a Zeiss Axiovert inverted microscope. Digital images were obtained using a Zeiss Axiocam digital camera (Chester, VA). Fungal adhesion was determined as a function of time using digital thresholding and quantified as percent surface coverage.
Fluorescence-based qualitative viability studies
The viability of C. albicans adhered to control and NO-releasing xerogel surfaces was assessed qualitatively using a BacLight fluorescent probe nucleic acid stain assay (propidium iodide and SYTOc9). Fungal viability was measured both immediately following microbial adhesion to xerogel-coated glass slides and after incubation of adhered fungal cells at regular time intervals. By incubating xerogels with adhered fungus for extended periods, the effect of surface-based NO flux on fungal viability was assessed. Candida albicans fungi was grown to 106 CFU/mL in YPD broth as described above and resuspended in sterile PBS. Glass slides coated on one side with control and NO-releasing xerogels were incubated in the fungal suspension for 1 h at 37 °C with gentle agitation to evenly adhere cells to the xerogel surface. The substrates were either transferred to a 5 mL solution of fluorescent probes (in PBS) or to 5 mL sterile PBS to maintain NO release. Xerogels incubated in fluorescent probes were removed after 30 min, rinsed in PBS, and dried gently in a N2 stream. Cell viability was assessed via fluorescence microscopy using a Zeiss Axiovert 200 inverted microscope (Chester, VA) equipped with propidium iodide and cyto 9 filters (λ = 530 nm and 630 nm, respectively) from Chroma (Battleboro, VT). Digital images were obtained using a Zeiss Axiocam digital camera (Chester, VA). Brightfield and fluorescence micrographs of the xerogel sides of the glass slides were acquired at 10x magnification. Xerogels incubated in PBS for the time-based studies were fluorescently labeled and imaged as described above.
Quantitative viability studies
The number of viable cells adhered to control and NO-releasing xerogels after long-term incubation in PBS was determined by removing cells and plating on nutrient agar. A standardized suspension of C. albicans (106 CFU/mL) in PBS (25°C) was introduced over the NO-releasing xerogel-coated glass slides at 0.2 mL/min in the parallel plate flow cell. Flow of the fungal suspension was continued until surface coverage of the cells reached 20% as determined by optical microscopy and digital thresholding. Sterile PBS was then exchanged for the fungal suspension without passage of an air-liquid interface at a flow rate of 0.2 mL/min for 5 min to remove non-adhered cells. For AgSD experiments, the fungal suspension was replaced with 160 μg/mL AgSD in sterile PBS. Flow was then stopped and the xerogels were left undisturbed for 15 h at ambient temperature, thus exposing fungal cells to a long-term NO flux. PBS was then removed from the flow cells at a flow rate of 0.2 mL/min, and the slides subsequently removed and imaged at 10x magnification. Each slide was then transferred to 5 mL sterile PBS and sonicated for 15 min to remove adhered cells. Cell viability in the resulting PBS solutions was determined via 10-fold serial dilutions and plating on YPD nutrient agar, followed by enumeration of colony forming units. Complete removal of cells was confirmed by evaluating the substrate surfaces using phase-contrast optical microscopy.
Efficacy of surface-generated NO against fungal biofilms
C. albicans was grown in YPD broth to 1 × 106 CFU/mL (OD650 ~0.044), and serially diluted to 1 × 104 CFU/mL in YPD broth. Control and NO releasing 40% AHAP3-BTMOS SiR coupons immobilized on needles were sterilized under UV light for 20 min. An aliquot (5 mL) of the 1 × 104 CFU/mL C. albicans suspension was added to sterile glass scintillation vials into which control and NO releasing SiR squares were immersed. Vials were incubated at 37 °C with gentle agitation for 2 h to allow fungal adhesion. Substrates were immersed in sterile YPD broth at 37 °C for 48 h to initiate biofilm growth, with a fresh supply of YPD broth introduced at 24 h. For AgSD experiments, substrates were incubated in 160 μg/mL AgSD in YPD broth for 24 h, followed by fresh YPD broth. Biofilm-coated substrates were rinsed by immersion in sterile PBS, followed by sterile water to remove salts. Substrates were immediately affixed to a peltier device set at 11.5 °C in a FEI Quanta 200F scanning electron microscope. Electron micrographs (500 to 1000x magnification) were taken in environmental mode (low vacuum, 5.15 torr) at 50% humidity using a gaseous secondary electron detector (GSED).
Results and Discussion
Nitric oxide release
Previous studies utilizing NO-releasing aminoalkoxysilane xerogels have demonstrated NO’s ability to reduce both bacterial adhesion and viability (Nablo et al. 2001; Nablo and Schoenfisch 2003; Nablo et al. 2005; Hetrick and Schoenfisch 2007). The optical transparency, stability, and NO release tenability (by varying aminosilane composition) of these materials makes them excellent model substrates for evaluating the effect of surface-generated NO on pathogenic fungus. The AHAP3/BTMOS xerogel system was selected for this study due to the obtainable NO release at room and physiological temperatures (>48 h) and material stability (Marxer et al. 2003; Nablo et al. 2005). Furthermore, previous studies established that the surface properties (i.e., water contact angle) of the AHAP3/BTMOS-coated glass slides did not change after exposure to high pressure NO (Marxer et al. 2003). To evaluate the stability of xerogel-coated SiR coupons, static water contact angle measurements were made before and after soaking both control and NO donor-modified substrates in PBS (37 °C) for 2 d. As expected, no significant difference in the measured contact angles was observed during this study (data not shown), indicating that the physical integrity of these substrates are maintained during the bacteria experiments. The NO storage capacity was varied by synthesizing a range of compositions with an AHAP3 content from 10–40% (v/v, balance BTMOS). As expected, the flux, total amount, and NO release kinetics (measured as half-life) varied as a function of aminosilane composition and temperature (Table 1). For all compositions, NO release was characterized as a low but sustained flux at room temperature. At physiological temperatures (i.e., 37°C), larger NO fluxes were measured, but at the sacrifice of release longevity. Indeed, the maximum NO flux from 10–40% (v/v) AHAP3 xerogels at room temperature was an order of magnitude lower than at 37 °C.
Table 1.
Average (from n=3) nitric oxide release at 25 and 37 °C 10–40% AHAP3 xerogels (balance BTMOS, v/v) in PBS (pH 7.4).
| % AHAP3 | Temperature (°C) | [NO]m (pmol cm−2 s−1) | t[NO] (μmol cm−2)* | t1/2 (hr) |
|---|---|---|---|---|
| 10 | 25 | 1.806 ± 0.715 | 0.017 ± 0.005 | 3.872 ± 0.547 |
| 37 | 8.674 ± 1.579 | 0.049 ± 0.004 | 2.450 ± 0.272 | |
| 20 | 25 | 6.117 ±3.035 | 0.078 ± 0.026 | 4.237 ± 1.214 |
| 37 | 33.312 ± 5.590 | 0.324 ± 0.055 | 2.853 ± 0.231 | |
| 30 | 25 | 12.113 ± 4.607 | 0.206 ± 0.091 | 5.552 ± 0.941 |
| 37 | 90.655 ± 26.765 | 0.852 ± .323 | 2.358 ± 0.274 | |
| 40 | 25 | 14.424 ± 7.689 | 0.390 ± 0.158 | 7.615 ± 2.494 |
| 37 | 145.961 ± 38.221 | 2.077 ± 0.656 | 3.9364 ± 0.381 |
Total NO release over 15 h.
Flow cell based fungal adhesion studies
As shown in Figure 1, lower fungal adhesion to the xerogel films was observed with increasing NO release. Absolute fungal adhesion results are provided in Table 2. Percent reduction versus controls, R, was calculated using the following equation:
| (1) |
where ccon and cNO represent the percent bacterial surface coverage on the control and NO-releasing xerogel, respectively. After only 90 min, a 25% reduction in fungal adhesion was observed for NO-releasing 10% AHAP3 xerogels versus controls, indicating that an NO flux as low as ~2 pmol cm−2 s−1 results in a significant reduction in fungal adhesion. Although an increase in the adhesion of C. albicans was observed at both control and NO-releasing xerogels at 150 min, the percent reduction in adhesion of all xerogel compositions versus controls remained unchanged. Furthermore, the differences in fungal adhesion between control xerogels were not statistically significant at any timepoint, regardless of aminosilane concentration. In contrast, the % fungal surface coverage observed for controls and all compositions of NO-releasing xerogels was statistically significant at both 120 and 150 min (p value < 0.05). As shown in Figure 2 for 150 min, a clear trend of decreased fungal adhesion to NO-releasing xerogels with increasing AHAP3 content is apparent.
Figure 1.
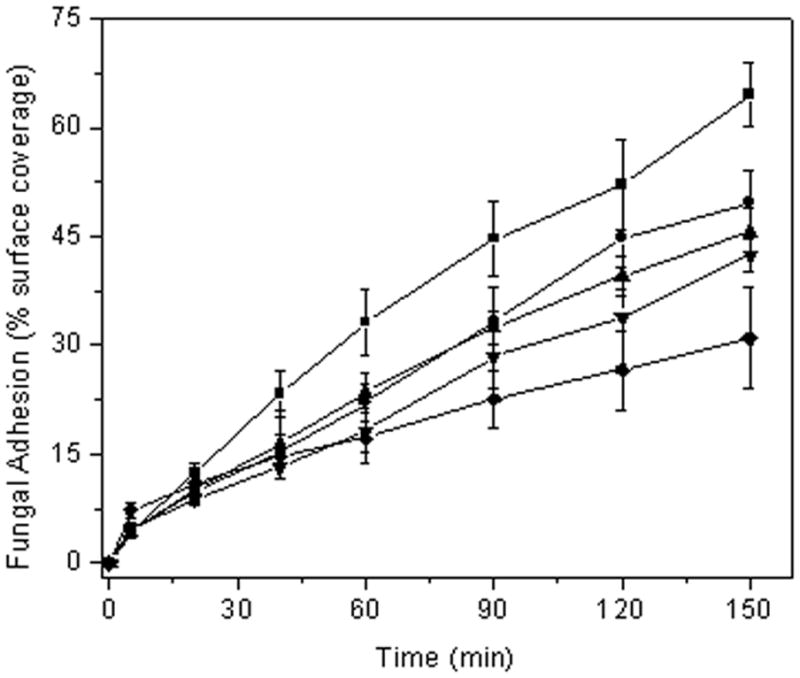
C. albicans adhesion to xerogel-coated glass slides under flowing conditions (0.2 mL/min) at 25 °C. Nitric oxide flux was varied by using 10% (●), 20% (▲), 30% (▼), and 40% (◆) AHAP3 xerogels (balance BTMOS, v/v). Control xerogels of all compositions were averaged (■) as surface coverage values at each timepoint were identical (within error).
Table 2.
C. albicans percent surface coverage to AHAP3 xerogels (balance BTMOS) under flowing conditions (0.2 mL/min) at 25 °C and calculated reduction in surface coverages to 10–40% AHAP3 xerogels over controls (non-NO-releasing).
| % AHAP3 | 90 min
|
150 min
|
||
|---|---|---|---|---|
| % surface coverage | % reductionb | % surface coverage | % reductionb | |
| Controla | 45±5 | N/A | 65±4 | N/A |
| 10% | 33±5 | 27±5 | 50±5 | 23±3 |
| 20% | 32±2 | 29±4 | 46±3 | 29±3 |
| 30% | 28±4 | 38±7 | 43±2 | 34±3 |
| 40% | 23±4 | 49±10 | 31±7 | 52±12 |
Controls are identical to the above xerogel compositions without NO release capabilities. No significant difference was observed between controls of different compositions. Surface coverage values for controls ere the average of all compositions.
Over control.
Figure 2.
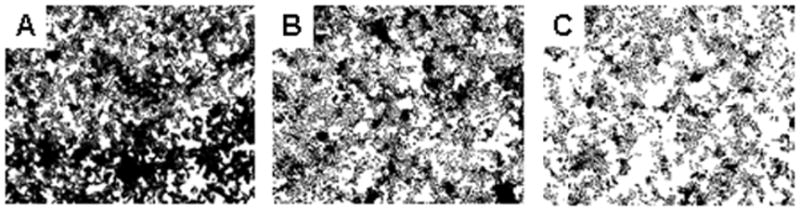
Digitally thresholded phase contrast optical micrographs of C. albicans adhesion to control (A), and NO-releasing 20% (B), and 40% (C) AHAP3 xerogels (balance BTMOS, v/v) following 150 minutes of fungal exposure under flowing conditions (flow rate: 0.2 mL/min) at 25 °C.
To demonstrate that the NO release and not the surface chemistry of the xerogel film resulted in the reduced fungal adhesion, control and NO donor-modified 40% AHAP3/BTMOS xerogels were coated with a thin film of PVC prior to conducting the parallel-plate flow cell assay. As expected, a significant decrease in adhesion was observed for the NO-releasing xerogels versus controls (~28% reduction over controls), confirming that the NO alone leads to decreased fungal adhesion (Figure 3). The overall reduction in adhesion at the PVC-coated 40% AHAP3/BTMOS xerogels was lower than the non-PVC-coated xerogel due to the 20% reduction in NO release upon coating with PVC as previously reported (Nablo and Schoenfisch 2004). The influence of flow rate through the flow cell was also assessed (Figure 4). We expected a decrease in fungal adhesion at higher flow rates due to the increase in shear forces. However, a significant increase in fungal adhesion to control xerogels was observed at the greater flow rate (0.6 mL/min). While counterintuitive based on shear forces, we attribute these results to increased transport of C. albicans to the substrate. As expected, the NO-mediated reduction in adhesion to NO-releasing xerogels versus controls was less pronounced at the larger flow rate (only ~8% reduction in surface coverage over controls at 0.6 mL/min) due to the more rapid clearance of NO from the xerogel surface. This observation provides further evidence of the primary role of NO in reducing fungal adhesion.
Figure 3.
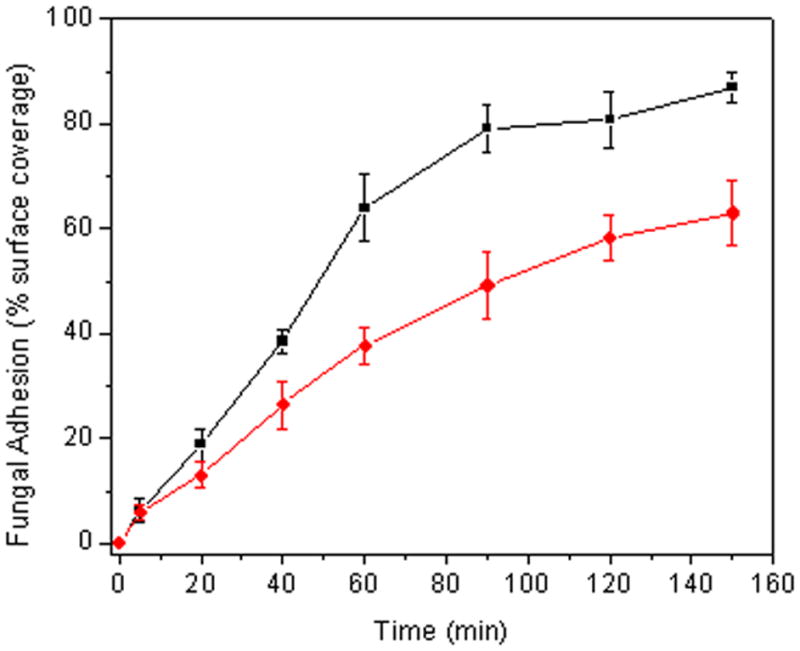
C. albicans adhesion to PVC-coated control (■) and NO-releasing (●) 40% AHAP3 xerogels (balance BTMOS, v/v) under flowing conditions (0.2 mL/min) at 25 °C.
Figure 4.
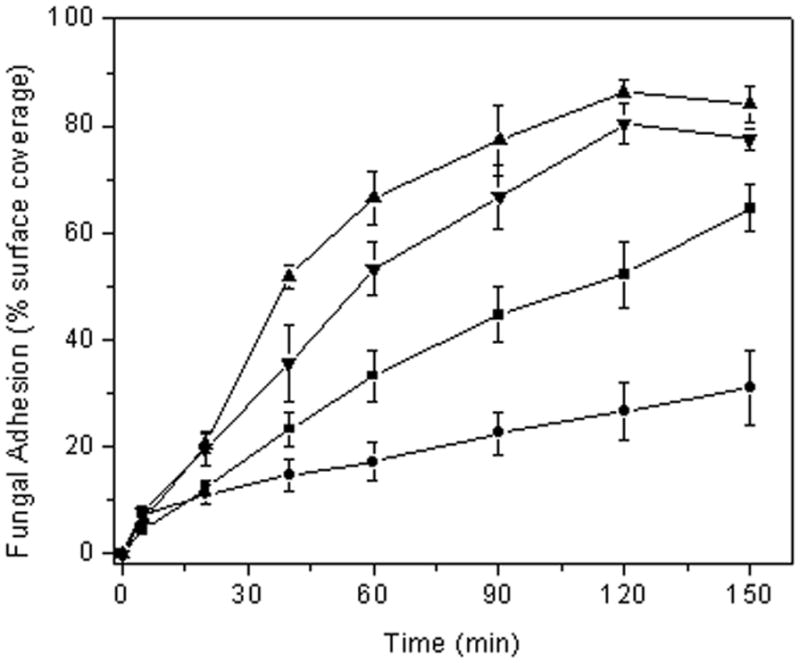
C. albicans adhesion to control (■ and ▲) and NO-releasing (● and ▼) 40% AHAP3 xerogels (balance BTMOS, v/v) under flowing conditions at a flow rate 0.2 mL/min (■ and ●) and 0.6 mL/min (▲ and ▼) at 25 °C.
Fungal viability studies
Critical to the success of indwelling catheters is the ability to both resist fungal adhesion and proliferation of adhered cells (Crump and Collignon 2000; Mermel 2000; Miller and O’Grady 2003). To determine the effect of NO on the viability of adhered fungus in situ, a live/dead assay was conducted using a commercially available kit. While this assay has been used primarily to examine the viability of bacterial cells, Jin and coworkers previously validated its use for similar studies of C. albicans (Jin et al. 2005). We have previously demonstrated that the levels of NO released from xerogel films do not impact the probes (i.e., dyes) used in the assay (Hetrick and Schoenfisch 2007). Candida albicans adhered (by soaking in a fungal suspension) to control and NO-releasing 40% AHAP3/BTMOS xerogels were incubated in PBS for up to 15 h. The fungi were then incubated in a solution containing the two fluorescent probes, SYTO9 and propidium iodide (PI), from a commercially available kit. Since only SYTO9, a green fluorescent dye, enters healthy cells, green fluorescence indicates viable cells. In contrast, red fluorescence suggests significantly compromised cells since PI, a red fluorescent dye, can only penetrate cells with damaged membranes. Example brightfield and fluorescence images of fungi adhered to control and NO-releasing xerogels at 0 and after 11 h incubation are provided in Figures 5 and 6, respectively. Fluorescence micrographs of fungus adhered to control xerogels taken both before (Figure 5B) and after 11 h of incubation in PBS (Figure 5E) exhibited only SYTO9 fluorescence, indicating that cells adhered to controls are initially viable and remain so, even after 11 h in nutrient deficient conditions. In contrast, fungal cells exposed to an NO flux for 11 h had significant red fluorescence due to PI dye penetration (Figure 6D–F), even though such cells were viable at t = 0 h (Figure 5A–C). The emergence of fluorescence due to PI after 11 h of NO release indicated that the cell envelope was compromised. The PI fluorescence was not observed prior to 11 h incubation, suggesting that at earlier periods the level of total NO remained insufficient to cause cell damage (data not shown). In comparison to results published previously for P. aeruginosa, the time required for the appearance of PI was 37% greater (Hetrick and Schoenfisch 2007).
Figure 5.
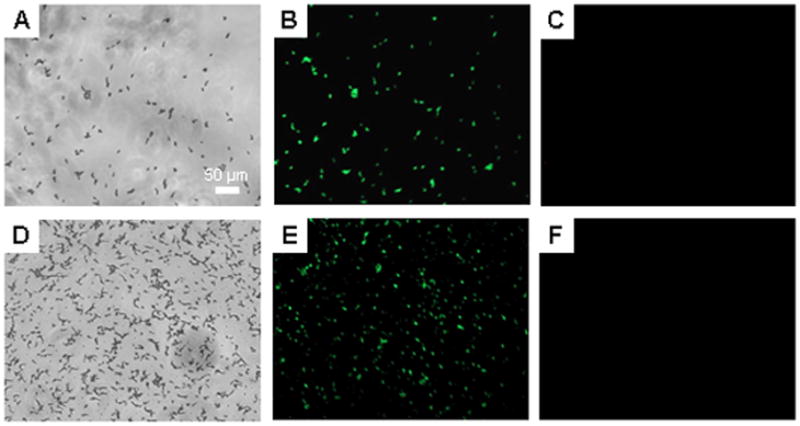
Representative brightfield (A and D), syto 9 fluorescence (B and E), and propidium iodide fluorescence (C and F) optical micrographs (10x magnification) of C. albicans adhered to control 40% AHAP3 xerogels (balance BTMOS, v/v) taken immediately after preparation (A–C), and after 15 h incubation (D–F) in PBS (pH 7.4) at 37 °C.
Figure 6.
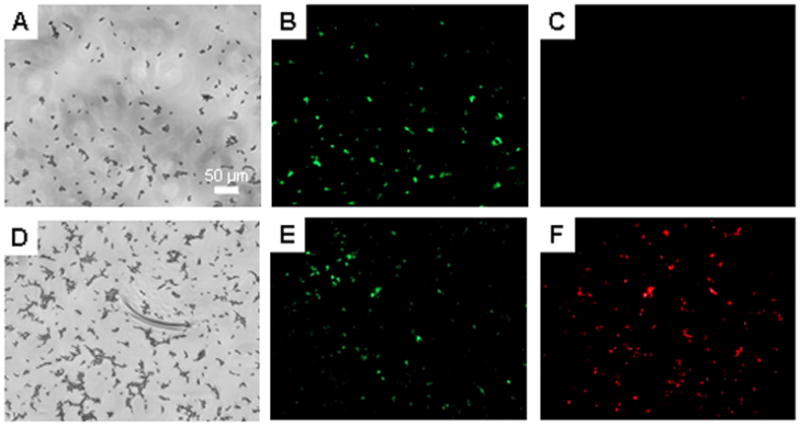
Representative brightfield (A and D), syto 9 fluorescence (B and E), and propidium iodide fluorescence (C and F) optical micrographs (10x magnification) of C. albicans adhered to NO-releasing 40% AHAP3 xerogels (balance BTMOS, v/v) taken immediately after preparation (A–C), and after 15 h incubation (D–F) in PBS (pH 7.4) at 37 °C.
To gain a more thorough understanding about the relationship between NO flux and fungal viability, we evaluated the viability of adhered fungal cells using a range of NO-releasing xerogels (10, 20, 30 and 40% AHAP3-BTMOS). Viable cells were counted as colony forming units on nutrient agar. Candida albicans adhesion was first standardized at 20% coverage on the NO-releasing xerogels in a parallel plate flow cell (as described above) to allow direct comparison of fungal viability between each xerogel composition. After incubation for 15 h in PBS, adherent cells were removed from the xerogel surfaces in an ultrasonic bath, spread on nutrient agar and counted. Previous studies have shown this method of cell removal to be safe removing C. albicans from surfaces (Sherertz et al. 1990; Lewis et al. 2002). Removal of cells was confirmed after sonication by evaluating the surfaces using optical microscopy. Of note, sonication of control xerogels resulted in incomplete removal of fungus cells, and therefore comparison to NO-releasing xerogels was not possible. Replicate plating experiments confirmed that cells were not killed during this period of sonication (data not shown). As expected, the number of viable cells removed from the xerogel surfaces was inversely proportional to the xerogel’s aminosilane content, and therefore the NO flux and total NO released from (Figure 7). Optical micrographs taken of each substrate after the 15 hr incubation in PBS but before removal of cells via sonication showed that the majority of cells are in their oval yeast form, rather than growing as hyphae (data not shown). While the total NO release from 10 and 40% AHAP3-BTMOS xerogels was 0.049 and 2.077 μmol cm−2, respectively, the number of viable cells removed from 40% AHAP3 xerogels was ~42% less that that for 10% AHAP3 xerogels. The incomplete removal of fungus from controls further demonstrates the detrimental effect of NO on fungal adhesion. When compared to P. aeruginosa (96% decrease in viability between bacteria adhered to 10% and 40% AHAP3 xerogels) (Hetrick and Schoenfisch 2007), the observed reduction is attenuated.
Figure 7.
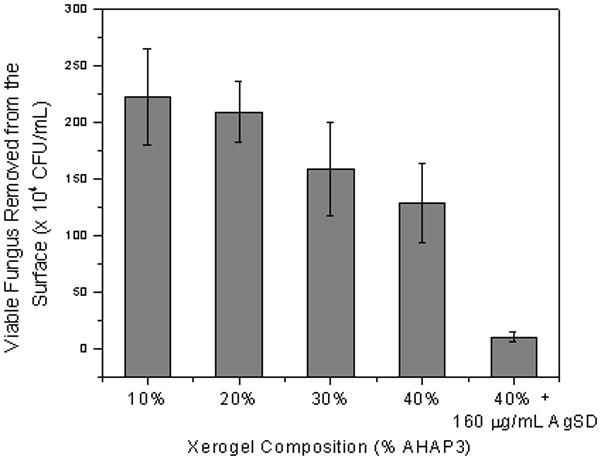
Viable C. albicans removed from 10 – 40% AHAP3 xerogels (balance BTMOS, v,v) and 40% AHAP3 xerogels in the presence of a sub-fungicidal concentration of AgSD after 15 h of exposure of adhered fungus in PBS at 37 °C. Initial fungal surface coverage was identical at the start of the assay (20% coverage).
While the data suggests that NO-releasing materials may be effective at reducing fungal adhesion and proliferation, NO alone does not appear to be as potent against C. albicans as it is against other biofilm-forming bacteria. Decreased efficacy of NO against C. albicans may be attributed to cell size. Indeed, C. albicans are up to an order of magnitude larger in diameter than P. aeruginosa (Matias et al. 2003; Sudbery et al. 2004). As a result, much of the yeast cell would be located further from the source of NO generation, minimizing exposure. Additionally, C. albicans are characterized by thick cellular envelopes (200–300 nm) (Chaffin et al. 1998) while P. aeruginosa, for example, is <50 nm (Chaffin et al. 1998; Matias et al. 2003). The thicker cell envelope may therefore afford C. albicans improved protection against NO and its reactive byproducts. Furthermore, a marked difference in the efficacy of broad spectrum antimicrobials such as chlorhexidine and silver sulfadiazine against C. albicans and P. aeruginosa has been reported by Schierholz and coworkers (Schierholz et al. 2000), suggesting that C. albicans may be less susceptible to broad spectrum antimicrobials in general.
To compensate for the decreased NO efficacy, the combination of NO and silver suldaziazine (AgSD) was investigated to further reduce colonization of implanted surfaces by C. albicans. McElhaney-Feser et al. previously reported that a combination of NO and azole-based antifungal agents resulted in synergistic killing (e.g., improved efficacy in combination than the sum of the efficacy of each individual agent) (McElhaney-Feser et al. 1998). Following a previous report (McElhaney-Feser et al. 1998), the efficacy of AgSD as an antifungal was determined by exposing C. albicans to a range of concentrations of AgSD in nutrient broth and measuring viability after 6 and 24 h (data not shown). Exposing C. albicans to a AgSD concentration of 800 μg/mL for 24 h resulted in complete killing (no viable cells). Although some growth inhibition was observed at 200 and 400 μg/mL AgSD, the cells remained completely viable (no inhibition) at a concentration of 100 μg/mL. Of note, no killing was observed at 6 h incubation at AgSD concentrations up to 1.6 mg/mL. To test the surface-localized anti-fungal efficacy of AgSD in combination with NO, fungal cells adhered to an NO-releasing 40% AHAP3-BTMOS xerogel were incubated in a PBS solution containing AgSD at a sub-fungicidal concentration of 160 μg/mL. As shown in Figure 7, the combination of NO and AgSD resulted in a ~95% reduction of viable cells compared to the NO release from 10% AHAP3-BTMOS xerogels alone.
Efficacy of surface-generated NO against fungal biofilms
Due to the ability of biofilms to protect microbes from therapeutics (Douglas 2003), we examined the effect of surface generated NO on the viability of preformed biofilms. Control and NO-releasing (40% AHAP3-BTMOS) xerogel-coated medical grade silicon rubber coupons were exposed to a fungal suspension for 2 h and then transferred to sterile nutrient broth for 2 d (transferring to fresh broth after 1 d) to facilitate biofilm formation. As expected, environmental scanning electron micrographs of each substrate revealed a substantial qualitative difference in the morphology of the biofilm between the control and NO-releasing xerogels (Figure 8). Densely packed communities of fungus were found over large areas on control substrates (Figure 8A), while only small clusters of cells and hyphae were observed on the NO-releasing substrates (Figure 8C). These results suggest that surface-generated NO release alters biofilm growth. Although the density of the biofilm appears to be reduced as a result of NO exposure alone, the formation of hyphae may signal the presence of chlamydospores, and potentially increased C. albicans pathogenesis (Cassone et al. 1973). The emergence of hyphae on the NO-releasing substrates may be attributed to a localized anaerobic environment near the NO-releasing inteface. Previous studies have reported an increase in chlamydospore formation on solid surfaces in anaerobic media (Montazeri and Hedrick 1984). Of note, the formation of hyphae was not observed during the flow-cell experiments (Figures 2, 5, and 6). In this manner, slight convection may minimize this effect. Nevertheless, we sought to evaluate the effectiveness of NO in combination with another biocide (AgSD). Biofilms were grown on control and NO-releasing xerogels in the presence of 160 g/mL AgSD. Controls with and without AgSD treatment were indistinguishable, confirming that the AgSD alone had little effect on biofilm growth at the chosen concentration (Figure 8B). When AgSD treatment was combined with NO release, only sporadic clusters of cells were present with no hyphal growth (Figure 8D). The absence of hyphae suggests that the combination of AgSD and NO release may have arrested the growth and morphogenesis of planktonic cells that managed to adhere.
Figure 8.
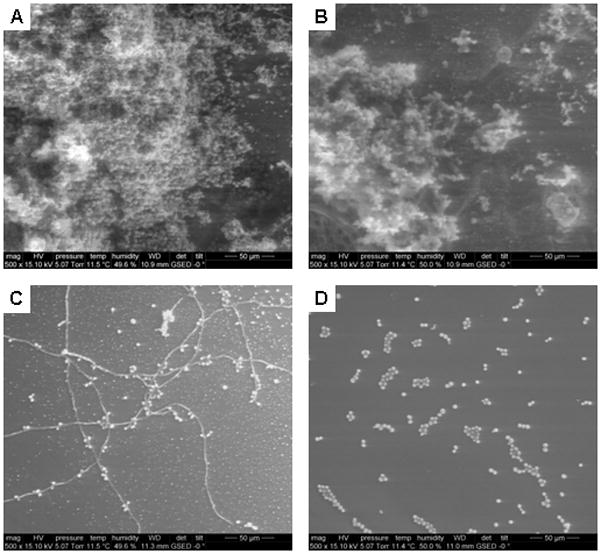
Representative environmental scanning electron micrographs of C. albicans biofilms attached to control (A and B) and NO-releasing (C and D) 40% AHAP3-BTMOS xerogels exposed to sterile YPD broth alone (A and C) and 160 μg/mL AgSD in nutrient broth (B and D) at 37 °C.
Conclusions
The results described herein demonstrate that surface generated NO is an effective inhibitor of C. albicans adhesion at concentrations as low as ~2 pmol cm−2 s−1. The majority of adhered cells were killed, with viability dependent on the quantity of NO released. Biofilm formation was reduced when compared to formation on control and blank (bare SiR) substrates. Due to a reduced efficacy against C. albicans relative to pathogenic bacteria at similar NO concentrations and the emergence of hyphae after NO exposure, future studies should examine both the antifungal and cytotoxic properties of greater NO fluxes. Nevertheless, the synergy study suggests that the combination of surface-generated NO and sub-MBC concentrations of AgSD greatly increases killing over NO alone. Further studies will examine the antimicrobial efficacy effect of broad-spectrum leachable antimicrobials doped into NO-releasing xerogels against C. albicans and other biofilm-forming fungus including C. parapsilosis and C. tropicalis.
Acknowledgments
This research was supported by the National Institutes of Health (EB000708). The authors would like to thank Wallace Ambrose at the Chapel Hill Analytical and Nanofabrication Laboratory for assistance with ESEM.
References
- Cassone A, Simonett N, Strippol V. Ultrastructural Changes in Wall During Germ-Tube Formation from Blastospores of Candida-Albicans. Journal of General Microbiology. 1973;77:417–426. doi: 10.1099/00221287-77-2-417. [DOI] [PubMed] [Google Scholar]
- Chaffin WL, Lopez-Ribot JL, Casanova M, Gozalbo D, Martinez JP. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiology and Molecular Biology Reviews. 1998;62:130–180. doi: 10.1128/mmbr.62.1.130-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–908. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- Charville GW, Hetrick EM, Geer CB, Schoenfisch MH. Reduced bacterial adhesion to fibrinogen-coated substrates via nitric oxide release. Biomaterials. 2008;29:4039–4044. doi: 10.1016/j.biomaterials.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow VCY, Hawkey PM, Chan EWC, Chin ML, Au TK, Fung DKC, Chan RCY. High-level gentamicin resistance mediated by a Tn4001-like transposon in seven nonclonal hospital isolates of Streptococcus pasteurianus. Antimicrobial Agents and Chemotherapy. 2007;51:2508–2513. doi: 10.1128/AAC.00603-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GT, Halawa AA, Anaissie EJ. The role of the gastrointestinal tract in hematogenous candidiasis: From the laboratory to the bedside. Clinical Infectious Diseases. 1996;22:S73–S88. doi: 10.1093/clinids/22.supplement_2.s73. [DOI] [PubMed] [Google Scholar]
- Crump JA, Collignon PJ. Intravascular catheter-associated infections. European Journal of Clinical Microbiology & Infectious Diseases. 2000;19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- De Lencastre H, Oliveira D, Tomasz A. Antibiotic resistant Staphylococcus aureus: a paradigm of adaptive power. Current Opinion in Microbiology. 2007;10:428–435. doi: 10.1016/j.mib.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobmeier KP, Schoenfisch MH. Antibacterial properties of nitric oxide-releasing sol-gel microarrays. Biomacromolecules. 2004;5:2493–2495. doi: 10.1021/bm049632u. [DOI] [PubMed] [Google Scholar]
- Douglas LJ. Candida biofilms and thier role in infection. Trends in Microbiology. 2003;11:30–36. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Dusane DH, Rajput JK, Kumar AR, Nancharaiah YV, Venugopalan VP, Zinjarde SS. Disruption of fungal and bacterial biofilms by lauroyl glucose. Letters in Applied Microbiology. 2008;47:374–379. doi: 10.1111/j.1472-765X.2008.02440.x. [DOI] [PubMed] [Google Scholar]
- Freitas FIS, Guedes-Stehling E, Siqueira-Junior JP. Resistance to gentamicin and related aminoglycosides in Staphylococcus aureus isolated in Brazil. Letters in Applied Microbiology. 1999;29:197–201. doi: 10.1046/j.1365-2672.1999.00617.x. [DOI] [PubMed] [Google Scholar]
- Ghaffari A, Miller CC, McMullin B, Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide. 2006;14:21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hawser SP, Douglas LJ. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrobial Agents and Chemotherapy. 1995;39:2128–2131. doi: 10.1128/aac.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chemical Society Reviews. 2006;35:780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- Hetrick EM, Schoenfisch MH. Antibacterial nitric oxide-releasing xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials. 2007;28:1948–1956. doi: 10.1016/j.biomaterials.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009;30:2782–2789. doi: 10.1016/j.biomaterials.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. Acs Nano. 2008;2:235–246. doi: 10.1021/nn700191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chemical Reviews. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- Jin Y, Zhang T, Samaranayake Y, Fang H, Yip H, Samaranayake L. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia. 2005;159:353–360. doi: 10.1007/s11046-004-6987-7. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Kontoyiannis DP, Darouiche RO, Raad, Prince RA. Antifungal activity of amphotericin B, fluconazole, and voriconazole in an in vitro model of Candida catheter-related bloodstream infection. Antimicrobial Agents and Chemotherapy. 2002;46:3499–3505. doi: 10.1128/AAC.46.11.3499-3505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annual Review of Immunology. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, Chen H, Mudgett JS. Altered Responses to Bacterial-Infection and Endotoxic-Shock in Mice Lacking Inducible Nitric-Oxide Synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Malcher M, Volodkin D, Heurtault B, Andre P, Schaaf P, Mohwald H, Voegel JC, Sokolowski A, Ball V, Boulmedais F, Frisch B. Embedded silver ions-containing liposomes in polyelectrolyte multilayers: Cargos films for antibacterial agents. Langmuir. 2008;24:10209–10215. doi: 10.1021/la8014755. [DOI] [PubMed] [Google Scholar]
- Marini M, De Niederhausern S, Iseppi R, Bondi M, Sabia C, Toselli M, Pilati F. Antibacterial activity of plastics coated with silver-doped organic-inorganic hybrid coatings prepared by sol-gel processes. Biomacromolecules. 2007;8:1246–1254. doi: 10.1021/bm060721b. [DOI] [PubMed] [Google Scholar]
- Marxer SM, Rothrock AR, Nablo BJ, Robbins ME, Schoenfisch MH. Preparation of nitric oxide (NO)-releasing sol-gels for biomaterial applications. Chemistry of Materials. 2003;15:4193–4199. [Google Scholar]
- Matias VRF, Al-Amoudi A, Dubochet J, Beveridge TJ. Cryo-transmission electron Microscopy of frozen-hydrated sections of Escherichia coli and Pseudomonas aeruginosa. Journal of Bacteriology. 2003;185:6112–6118. doi: 10.1128/JB.185.20.6112-6118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney-Feser GE, Raulli RE, Cihlar RL. Synergy of Nitric Oxide and Azoles against Candida Species In Vitro. Antimicrobial Agents and Chemotherapy. 1998;42:2342–2346. doi: 10.1128/aac.42.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermel LA. Prevention of Intravascular Catheter-Related Infections. Ann Intern Med. 2000;132:391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- Miller DL, O’Grady NP. Guidelines for the prevention of intravascular catheter-related infections: Recommendations relevant to interventional radiology. Journal of Vascular and Interventional Radiology. 2003;14:133–136. doi: 10.1016/s1051-0443(07)60120-1. [DOI] [PubMed] [Google Scholar]
- Montazeri M, Hedrick HG. Factors Affecting Spore Formation of a Candida-Albicans Strain. Applied and Environmental Microbiology. 1984;47:1341–1342. doi: 10.1128/aem.47.6.1341-1342.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nablo BJ, Schoenfisch MH. Antibacterial properties of nitric oxide-releasing sol-gels. Journal of Biomedical Materials Research Part A. 2003;67A:1276–1283. doi: 10.1002/jbm.a.20030. [DOI] [PubMed] [Google Scholar]
- Nablo BJ, Schoenfisch MH. Poly(vinyl chloride)-coated sol-gels for studying the effects of nitric oxide release on bacterial adhesion. Biomacromolecules. 2004;5:2034–2041. doi: 10.1021/bm049727w. [DOI] [PubMed] [Google Scholar]
- Nablo BJ, Chen TY, Schoenfisch MH. Sol-gel derived nitric-oxide releasing materials that reduce bacterial adhesion. Journal of the American Chemical Society. 2001;123:9712–9713. doi: 10.1021/ja0165077. [DOI] [PubMed] [Google Scholar]
- Nablo BJ, Rothrock AR, Schoenfisch MH. Nitric oxide-releasing sol-gels as antibacterial coatings for orthopedic implants. Biomaterials. 2005;26:917–924. doi: 10.1016/j.biomaterials.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Nablo BJ, Prichard HL, Butler RD, Klitzman B, Schoenfisch MH. Inhibition of implant-associated infections via nitric oxide. Biomaterials. 2005;26:6984–6990. doi: 10.1016/j.biomaterials.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Nejadnik MR, van der Mei HC, Norde W, Busscher HJ. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials. 2008;29:4117–4121. doi: 10.1016/j.biomaterials.2008.07.014. [DOI] [PubMed] [Google Scholar]
- O’Grady NP, Alexander M, Dellinger P, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. Guidelines for the prevention of intravascular catheter-related infections. Pediatrics. 2002;110 doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. Journal of Hospital Infection. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clinical Microbiology Reviews. 2007;20:133. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, Saville SP, Thomas DP, Lopez-Ribot JL. Candida biofilms: an update. Eukaryotic Cell. 2005;4:633–638. doi: 10.1128/EC.4.4.633-638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo L, Barcenilla JM, Vazquez B, Gonzalez R, Roman JS. Intrinsically antibacterial materials based on polymeric derivatives of eugenol for biomedical applications. Biomacromolecules. 2008;9:2530–2535. doi: 10.1021/bm800570u. [DOI] [PubMed] [Google Scholar]
- Rossi S, Azghani AO, Omri A. Antimicrobial efficacy of a new antibiotic-loaded poly(hydroxybutyric-co-hydroxyvaleric acid) controlled release system. Journal of Antimicrobial Chemotherapy. 2004;54:1013–1018. doi: 10.1093/jac/dkh477. [DOI] [PubMed] [Google Scholar]
- Schierholz JM, Fleck C, Beuth J, Pulverer G. The antimicrobial efficacy of a new central venous catheter with long-term broad-spectrum activity. J Antimicrob Chemother. 2000;46:45–50. doi: 10.1093/jac/46.1.45. [DOI] [PubMed] [Google Scholar]
- Sherertz RJ, Raad II, Belani A, Koo LC, Rand KH, Pickett DL, Straub SA, Fauerbach LL. 3-Year Experience with Sonicated Vascular Catheter Cultures in a Clinical Microbiology Laboratory. Journal of Clinical Microbiology. 1990;28:76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. Journal of Clinical Microbiology. 2002;40:1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiology Reviews. 2003;27:341–353. doi: 10.1016/S0168-6445(03)00047-0. [DOI] [PubMed] [Google Scholar]
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends in Microbiology. 2004;12:317–324. doi: 10.1016/j.tim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C. Antimicrobial effect of acidified nitrite on dermatophyte fungi, Candida and bacterial skin pathogens. Journal of Applied Microbiology. 2001;90:648–652. doi: 10.1046/j.1365-2672.2001.01291.x. [DOI] [PubMed] [Google Scholar]
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Risk Factors for Hospital-Acquired Candidemia: A Matched Case-Control Study. Arch Intern Med. 1989;149:2349–2353. [PubMed] [Google Scholar]
- Witte W, Cuny C, Klare I, Nuebel U, Strommenger B, Werner G. Emergence and spread of antibiotic-resistant Gram-positive bacterial pathogens. International Journal of Medical Microbiology. 2008;298:365–377. doi: 10.1016/j.ijmm.2007.10.005. [DOI] [PubMed] [Google Scholar]


