Abstract
The rate of venous thromboembolism (VTE) has been reported to be higher in blacks compared to whites. Non-O blood types have also been associated with a significantly higher VTE risk. Given that a higher proportion of blacks have O blood type, one might have expected that black individuals would have fewer VTE. In this study, we analyzed race, gender, age, ABO/Rh blood type and VTE risk in 60,982 black and white patients admitted over a span of 10 years. The overall occurrence of VTE was 7.6%, higher in males (8.7% males vs. 7.2% females), higher in non-O blood types (8.5% non-O vs. 6.9% O blood type), and increasing with age (5.8% <65yrs, 11.3% ≥65yrs). No difference in VTE rate was noted with Rh antigen positivity. When stratified by age, VTE rate was consistently higher in blacks and non-O blood types. No difference was detected among the various non-O blood types. To assess the potential confounder of comorbidities, we stratified patients according to Charlson comorbidity score. In a subgroup of healthy patients with age-independent Charlson comorbidity scores of 0 (N=28,387), blacks still had an increased VTE risk and this risk was still higher with increasing age and in those with non-O blood types. We conclude that black race and non-O blood types have increased VTE risk when stratified for age and that associated comorbidities do not explain these differences.
Keywords: Blood Type, Venous Thromboembolism, Thrombophilia, Race
Introduction
Venous thromboembolism (VTE) is a complex phenomenon influenced by multiple risk factors, which include cancer, trauma, surgery, prolonged immobility, and certain medical conditions.1,2 VTE risk is generally acknowledged to increase as age advances.1,2 Gender associations are less clear: male gender has been shown to be an independent risk factor for VTE in some studies but not in others.3,4,5,6,7,8 Even less clear is the issue of increased risk due to race. VTE prevalence has been reported to be higher in blacks compared to whites.1,3,4,8,9,10,11,12 However, whether increased morbidity in the different at-risk populations accounts for the perceived racial or ethnic differences has not been studied in detail.
While its exact function remains unknown, ABO blood type has been linked, perhaps via von Willebrand Factor (VWF), to various medical conditions such as VTE,13,14 pepticulcer disease15 and pancreatic cancer.16 Most previous studies have found that individuals with O blood type carry a lower VTE risk compared to other non-O blood types, suggesting that O blood type is protective against VTE.13,17,18,19,20,21 As O blood type is more prevalent in blacks than in whites,22 it is paradoxical to find that VTE prevalence has been observed to be higher in blacks than whites. One caveat shared by the majority of previous ABO blood type studies and VTE risk analyses is the fact that these studies were typically limited to whites and included only a small number of blacks, if at all.10,11,14,17 It is unknown whether the concept of a protective effect of O blood type and its relationship to VWF extends from these studies to the black population, who, despite the increased prevalence of O blood type, have higher baseline Factor VIII and VWF levels than whites.23
There is also little known on whether the Rh blood type, which may function as an membrane transport protein with no known interaction with coagulation factors, has any effect on VTE risk.24 In order to assess how these factors may affect clinical outcome, we conducted a study to investigate the relative importance of blood type, race, age, gender, and comorbidity on VTE risk.
Methods and Materials
Study population
All adult patients (age ≥18yrs) who self-identified as non-Hispanic white or non-Hispanic black and had been admitted to Montefiore Medical Center (MMC) between January 1st, 2000 and December 31st, 2009 were included in the current study. Other demographic groups, i.e. Asian, Hispanic, and those with multiple or unidentified ethnic backgrounds, were excluded.
Data collection
Clinical Looking Glass (CLG) is an interactive software application developed at Montefiore Medical Center that integrates demographic, clinical and administrative datasets and allows them to be reproduced in a programmable format for statistical access. We utilized CLG to screen the study population defined above for information regarding age, gender, race, and ABO/Rh blood types. VTE rate was determined using ICD9 codes either as the primary or secondary diagnosis. We did not differentiate between deep vein thrombosis (DVT) and pulmonary embolism (PE) and they will be referred to collectively as VTE.
Comorbidity information on our population was collected separately using the Charlson comorbidity scoring index.25 The Charlson index considers 22 disease conditions and scores them according to severity, providing a raw Charlson Index. These can be taken into consideration with age-related risk, providing a Combined Charlson Score Index with discrete %10-yr survival estimates. Patients with a Charlson Score of 0 have an estimated 10-year survival of 98.3%, Charlson scores of 1 and 2 have estimated 10-year survivals of 95.9% and 90.2% respectively; Scores of 3-5 have estimated 10-year survivals of 77.5%, 53.4% and 21.4% respectively; Scores of 6 have an estimated 10-year survival of 2.2% while scores ≥7 have a <1 % estimated 10-year survival.
Statistical analysis
Only patients who had ABO/Rh blood type information available were included for further analysis. Data were generated by CLG and transferred to Microsoft Excel (Microsoft, Redmond, WA) spreadsheets. Demographic characteristics, blood type analyses, Charlson comoribidy index and VTE rates between groups were compared by Chi-square analysis of proportions. Two tailed t-tests and Chi-square analysis were used to assess the effect of age as appropriate. Odds ratios of VTE for O vs non-O blood types were estimated with logistic regression models within age-race-sex subgroups. In order to limit the impact of comorbidity, analyses were repeated in the subgroup of individuals with Charlson comorbidity score of 0 (no comorbidities). Statistical analyses were performed using SPSS 18 (SPSS, Chicago, IL) and a two-tailed alpha of .05 was used to denote statistical significance.
Results
A total of 60,982 non-Hispanic patients were included in the study; 22,088 of these self-identified as white and 38,894 self-identified as black. The main demographic characteristics of the black and white patient sample are detailed in Table 1. In both racial groups, females constituted the majority, although black females were a much larger proportion of their racial cohort than were white females. The black population was significantly younger than the white. Racial distribution of ABO blood type in our study was similar to what has been previously reported: black have a higher proportion of O and B blood types, and a lower proportion of A and AB blood types, than whites.22 However, our study identified fewer patients with Rh-blood type than have been previously reported.22
Table 1. Characteristics of study population.
| Black | White | p value | |
|---|---|---|---|
|
| |||
| Total | 38,894 | 22,088 | |
|
| |||
| Female (%) | 71.4% | 56.3% | <0.001 |
|
| |||
| Mean age (yrs ±SD) | 49.4±19.4 | 62.9±19.4 | <0.001 |
|
| |||
| ABO blood types (%) | <0.001 | ||
| A | 25.2% | 38.1% | |
| B | 20.4% | 13.8% | |
| AB | 4.2% | 5.1% | |
| O | 50.2% | 43.0% | |
|
| |||
| Rh blood type (%) | <0.001 | ||
| Rh+ | 93.5% | 87.5% | |
| Rh− | 6.5% | 12.5% | |
A total of 4,696 cases of VTE were identified in the study (Table 2), which accounted for 7.7% of total population. Blacks made up 63.8% of the general population and 63.4% of the VTE population. There was no meaningful difference in the overall VTE rate between blacks and whites, and between those patients with Rh+ and those with Rh-blood type. When groups were stratified by age, however, black race was significantly associated with increased VTE. Overall VTE rate was higher in males than females, increased with age, and was higher in those with non-O blood types than those with O blood type for both races. The difference in VTE rate among different non-O blood types was not statistically significant (data not shown) and therefore they will be referred collectively as non-O blood types for the purpose of comparison.
Table 2. VTE rate (% of Study Variable and Hazard Ratio).
| VTE (%) | HR | p-value | |
|---|---|---|---|
| Overall | 7.7% (4,696/60,982) | ||
|
| |||
| Race | 0.61 | ||
| White | 7.80% | 1.01 | |
| Black | 7.70% | 1.00 | |
|
| |||
| Sex | <0.001 | ||
| F | 7.20% | 0.93 | |
| M | 8.70% | 1.13 | |
|
| |||
| Age groups | <0.001 | ||
| 18 – 64 | 5.80% | 0.75 | |
| ≥65 | 11.30% | 1.47 | |
|
| |||
| ABO Blood Type | <0.001 | ||
| Non-O | 8.50% | 1.10 | |
| O | 6.90% | 0.90 | |
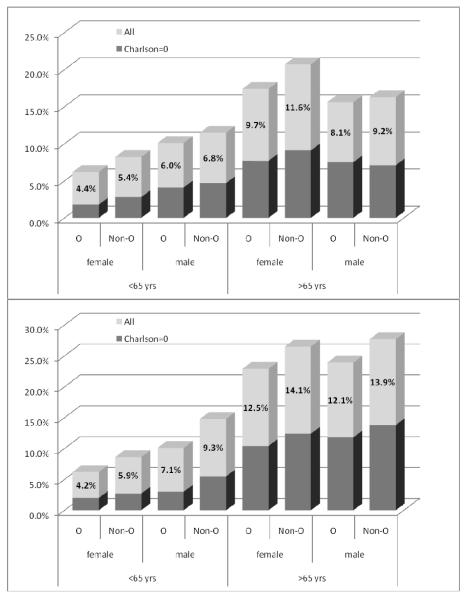
Because of the wide age and gender disparity, we reanalyzed our data stratified for these factors, as depicted in Figure 1. Within each matched age-race-gender group, patients with non-O blood types were significantly associated with a higher VTE rate compared to patients with O blood type (all at p<0.001). When further analyzed for the healthy subgroup of patients (age-independent Charlson index of 0), we found similar trends but statistical significance was achieved only selected populations: male and female blacks younger than 65 (both at p<0.001) and white females younger than 65 (P=0.03). Gender data were less consistent: Male gender was associated with a significantly higher VTE risk only in the younger cohort (Figure 1).
Figure 1.
VTE rates in O and non-O blood types according to age, sex and Charlson score. Top: Whites, Bottom: Blacks. All numbered comparisons: p<0.001
As expected, VTE rate increased with increasing Charlson scores, regardless of race, gender or ABO blood type (Table 3). Within each comorbidity group, VTE rate was still higher for those with non-O blood types.
Table 3. VTE rate according to age-dependent Charlson score.
| VTE Rate (%) |
|||||
|---|---|---|---|---|---|
| Overall | Charlson ≤2 | Charlson 3 – 5 | Charlson ≥6 | ||
|
| |||||
| Race | White | 7.7 | 4 | 9.4 | 11.4 |
| Black | 7.8 | 4.4 | 11.5 | 14.9 | |
|
| |||||
| Gender | Female | 7.2 | 3.7 | 11.2 | 13.6 |
| Male | 8.7 | 5.8 | 9.5 | 12.9 | |
|
| |||||
|
Blood Type |
O | 6.9 | 3.6 | 9.6 | 12.8 |
| Non-O | 8.5 | 4.9 | 11.3 | 13.7 | |
Shade denotes statistical significance (p<0.01)
We performed logistic regression analyses to evaluate the relative contributions of each risk factor. We found that in patients younger than 65 years, male sex appeared to be the most important risk factor, followed by non-O blood type, while black race appeared to be the least important factor. In contrast, in patients with age 65 years or older, black race appeared to be the most important risk factor, followed by non-O blood type, while male gender appeared to be protective (Table 4).
Table 4. Logistic Regression Analysis for VTE Risk.
| Age groups | Risk factors | OR | 95% CI |
|---|---|---|---|
|
| |||
| < 65 yrs | Male gender | 1.57 | [1.44, 1.72] |
| Non-O blood type | 1.34 | [1.23, 1.46] | |
| Black race | 1.16 | [1.05, 1.28] | |
|
| |||
| ≥65 yrs | Black race | 1.39 | [1.27, 1.51] |
| Non-O blood type | 1.18 | [1.08, 1.28] | |
| Male gender | 0.88 | [0.80, 0.96] | |
Discussion
The primary goal of the current study was to investigate whether O blood type is protective against VTE in the black population in a role similar to what has been demonstrated for the white population. While the relationship between ABO blood types and VTE risk has been investigated quite extensively, the role of race remains elusive, as most previous studies have primarily focused on the white population13,17,18,19,20,21. Our very large patient population demonstrated that our white and black populations had very different demographics. It is necessary to stratify these groups to ensure that the VTE differences are not secondary to confounding variables such as age, gender and comorbidities. With the advantage of a huge database, we have been able to demonstrate that significant differences in VTE rate for O and non-O blood type hold for the black population and that this is an independent risk factor in blacks.
While there is no conclusive explanation on how ABO blood type influences VTE, there is evidence that the effect may be mediated by ABO blood group antigenic determinants expressed on both the N- and O-linked carbohydrate structures of VWF which appear to influence clearance rate of VWF.26,27 As the half-life of VWF is longer in those with non-O blood types, circulating levels of VWF and Factor VIII, which are known risk factors for VTE, tend to be higher in those with non-O blood types than those with O blood type.28 On the other hand, race also plays a role in determining the level of FVIII: despite a higher proportion of O blood type22, blacks generally have a higher level of Factor VIII and VWF than whites.29 Since the association of lower Factor VIII and VWF levels with O blood type may be the mechanism whereby O blood type plays a protective role, we were uncertain whether the protective effect of O blood type would still be present.
The medical and cultural definitions of race are unclear and changing. We chose to use the current recommended definition of self-identification rather than ‘ancestral’ markers since it is uncertain at this juncture whether it is genes,30,31,32 phenotype, or associated cultures which underlie putative associations. We found no meaningful difference in the initial crude VTE rate between blacks and whites, but on closer scrutiny, this appears to be due to confounding effects from the different age and gender composition between the white and black populations. The Bronx, as is true of many inner city areas, retains an elderly white population while becoming home to a newer and mostly younger black population. There were also a higher proportion of females, in both groups but especially in the black cohort. This may reflect the greater utilization of medical care by women.33 We also stratified for Charlson score since we were concerned that differences in obesity, diabetes and various comorbidities between blacks and whites might confound any observed relationship of race with VTE. By examining VTE rate in a subgroup of patients whose age-independent Charlson score was 0 and was presumed to be otherwise healthy, we tried to control for this to some extent. In this smaller group of patients, we found a trend in VTE rate differences similar to that of the general patient population, suggesting that these differences cannot be explained by comorbidity alone.
Our study finds non-O blood types are associated with higher VTE risk independent of comorbidity. When examined within black race, we found, similar to what has been reported for whites, that the VTE rate in blacks was consistently higher in those with non-O blood types than in those with O blood type, suggesting that O blood type may also be protective against VTE in blacks. When further stratified by age and gender, associations with O blood type remained statistically significant, suggesting that O blood type may be a protective factor against VTE independent of age and gender.
As a retrospective study, the current study is subject to the usual limitations. Our study focused on an inpatient population and relied on ICD-9 coding diagnoses and we did not differentiate provoked vs idiopathic VTE. Additionally, since this study examined overall rate, we did not include any recurrent VTE after the initial captured VTE. However, these caveats were true for all cohorts analyzed. We did not have access to VWF or Factor VIII data so we could not examine whether the levels of these factors could account for some of our observations. We provide evidence of associations, not causality. VTE is a complex process influenced and impacted by multiple hereditary and acquired risk factors. Our current study provides some insight into how these factors may interact to determine individual risk profile.
Acknowledgements
We would like to acknowledge Eran Bellin MD PhD for his development of Clinical Looking Glass and to his staff for their support in data retrieval.
This publication was supported in part by the CTSA Grant UL1 RR025750, KL2 RR025749 and TL1 RR025748 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessary represent the official view of the NCRR or NIH.
Footnotes
Disclosure of Confliict of Interests
The authors state that they have no confliict of interest.
Explanation of Author Contributions:
HHB designed the research project; CF performed the research; CF, HHB and HWC analyzed the data. CF and HHB wrote the paper with helpful additions of HWC.
The authors declare no conflict of interest or financial involvement in the research, analysis or preparation of this manuscript.
References
- 1.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Jr., Spencer FA. Risk Factors for Venous Thromboembolism. Circulation. 2003;107:9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 3.Keenan CR, White RH. The effects of race/ethnicity and sex on the risk of venous thromboembolism. Curr Opin Pulm Med. 2007;13:377–83. doi: 10.1097/MCP.0b013e3281eb8ef0. [DOI] [PubMed] [Google Scholar]
- 4.White RH, Dager WE, Zhou H, Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96:267–73. doi: 10.1160/TH06-07-0365. [DOI] [PubMed] [Google Scholar]
- 5.Holst AG, Jensen G, Prescott E. Risk Factors for Venous Thromboembolism: Results From the Copenhagen City Heart Study. Circulation. 2010;121:1896–903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoodi BK, Gansevoort RT, Veeger NJGM, Matthews AG, Navis G, Hillege HL, van der Meer J. Microalbuminuria and Risk of Venous Thromboembolism. JAMA. 2009;301:1790–7. doi: 10.1001/jama.2009.565. [DOI] [PubMed] [Google Scholar]
- 7.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular Risk Factors and Venous Thromboembolism Incidence: The Longitudinal Investigation of Thromboembolism Etiology. Arch Intern Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 8.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 9.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128:737–40. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, Becker RC, Goldberg RJ. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–7. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd. Trends in the Incidence of Deep Vein Thrombosis and Pulmonary Embolism: A 25-Year Population-Based Study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 12.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–7. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 13.Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, Rosamond WD, Folsom AR. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology. J Thromb Haemost. 2007;5:1455–61. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- 14.Morelli VM, De Visser MC, Vos HL, Bertina RM, Rosendaal FR. ABO blood group genotypes and the risk of venous thrombosis: effect of factor V Leiden. J Thromb Haemost. 2005;3:183–5. doi: 10.1111/j.1538-7836.2004.01071.x. [DOI] [PubMed] [Google Scholar]
- 15.Edgren G, Hjalgrim H, Rostgaard K, Norda R, Wikman A, Melbye M, Nyrén O. Risk of Gastric Cancer and Peptic Ulcers in Relation to ABO Blood Type: A Cohort Study. Amer J Epidemiol. 2010;172:1280–5. doi: 10.1093/aje/kwq299. [DOI] [PubMed] [Google Scholar]
- 16.Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, Giovannucci EL, Fuchs CS. ABO Blood Group and the Risk of Pancreatic Cancer. J Natl Cancer Inst. 2009;101:424–31. doi: 10.1093/jnci/djp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trégouët D-A, Heath S, Saut N, Biron-Andreani C, Schved J-F, Pernod G, Galan P, Drouet L, Zelenika D, Juhan-Vague I, Alessi MC, Tiret L, Lathrop M, Emmerich J, Morange PE. Common susceptibility alleles are unlikely to contribute as strongly as the FV and ABO loci to VTE risk: results from a GWAS approach. Blood. 2009;113:5298–303. doi: 10.1182/blood-2008-11-190389. [DOI] [PubMed] [Google Scholar]
- 18.Clark P, Walker ID, Govan L, Wu O, Greer IA. The GOAL study: a prospective examination of the impact of factor V Leiden and ABO(H) blood groups on haemorrhagic and thrombotic pregnancy outcomes. Br J Haematol. 2008;140:236–40. doi: 10.1111/j.1365-2141.2007.06902.x. [DOI] [PubMed] [Google Scholar]
- 19.Larsen TB, Johnsen SP, Gislum M, Moller CA, Larsen H, Sorensen HT. ABO blood groups and risk of venous thromboembolism during pregnancy and the puerperium. A population-based, nested case-control study. J Thromb Haemost. 2005;3:300–4. doi: 10.1111/j.1538-7836.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:62–9. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 21.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–23. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 22.Garratty G, Glynn SA, McEntire R. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–6. doi: 10.1111/j.1537-2995.2004.03338.x. [DOI] [PubMed] [Google Scholar]
- 23.Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, Ouyang P, Folsom AR. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups:the MESA study. J Thromb Haemost. 2006;4:2629–35. doi: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- 24.Westhoff CM. The structure and function of the Rh antigen complex. Semin Hematol. 2007;44:42–50. doi: 10.1053/j.seminhematol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Gallinaro L, Cattini MG, Sztukowska M, Padrini R, Sartorello F, Pontara E, Bertomoro A, Daidone V, Pagnan A, Casonato A. A shorter von Willebrand factor survival in O blood group subjects explains how ABO determinants influence plasma von Willebrand factor. Blood. 2008;111:3540–45. doi: 10.1182/blood-2007-11-122945. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H Antigen Expressed on Circulating von Willebrand Factor Is Modified by ABO Blood Group Genotype and Is a Major Determinant of Plasma von Willebrand Factor Antigen Levels. Arterioscler Thromb Vasc Biol. 2002;22:335–41. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 28.Tsai AW CM, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113:636–42. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Cushman M, Tsai MY, Aleksic N, Heckbert SR, Boland LL, Tsai AW, Yanez ND, Rosamond WD. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002;99:2720–5. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Narváez EA, Rosenberg L, Wise LA, Reich D, Palmer JR. Validation of a Small Set of Ancestral Informative Markers for Control of Population Admixture in African Americans. Am J Epidemiol. 2011;173:587–92. doi: 10.1093/aje/kwq401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tandon A, Patterson N, Reich D. Ancestry informative marker panels for African Americans based on subsets of commercially available SNP arrays. Genet Epidemiol. 2011;35:80–3. doi: 10.1002/gepi.20550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G SD, Zhou J, Doumatey A, Huang H, Gerry NP, Herbert A, Christman MF, Chen Y, Dunston GM, Faruque MU, Rotimi CN, Adeyemo A. Development of admixture mapping panels for African Americans from commercial high-density SNP arrays. BMC Genomics. 2010;11:417. doi: 10.1186/1471-2164-11-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinkhasov RM, Wong J, Kashanian J, Lee M, Samadi DB, Pinkhasov MM, Shabsigh R. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract. 2010;64:475–87. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]