Abstract
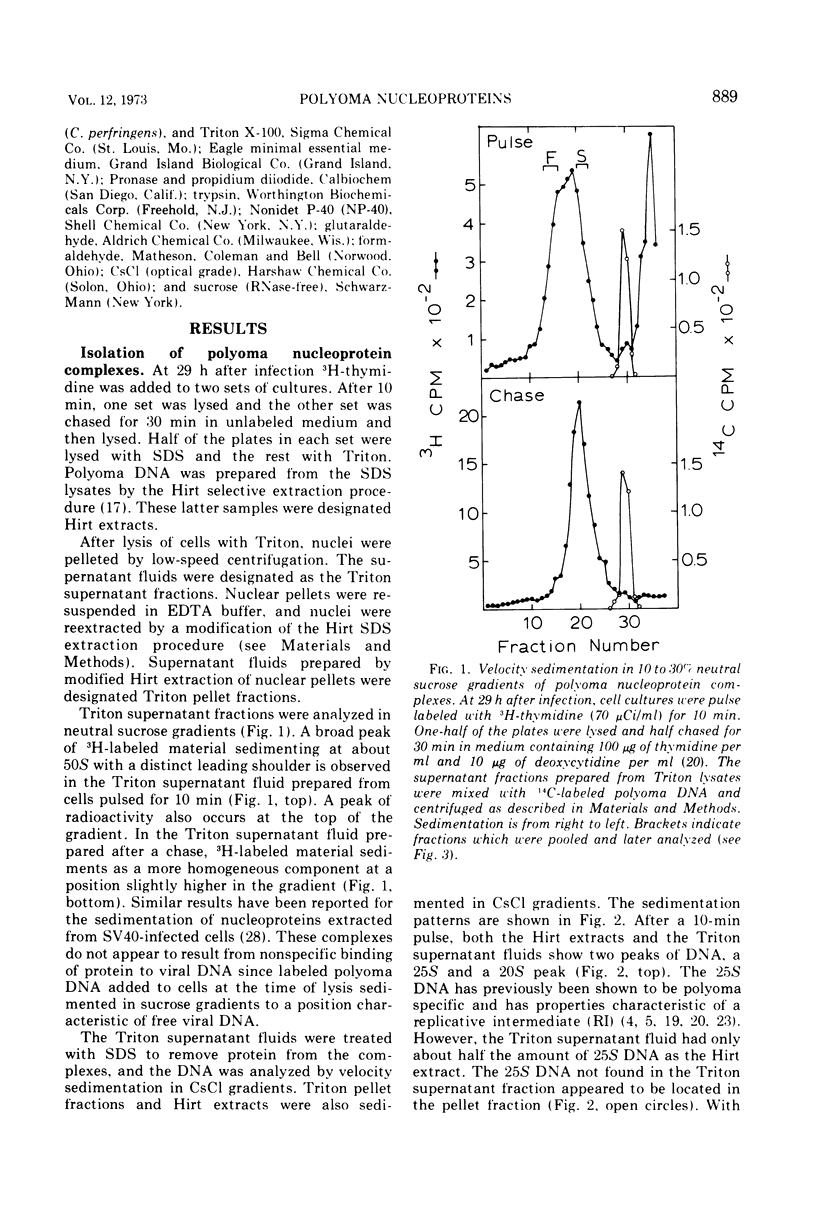
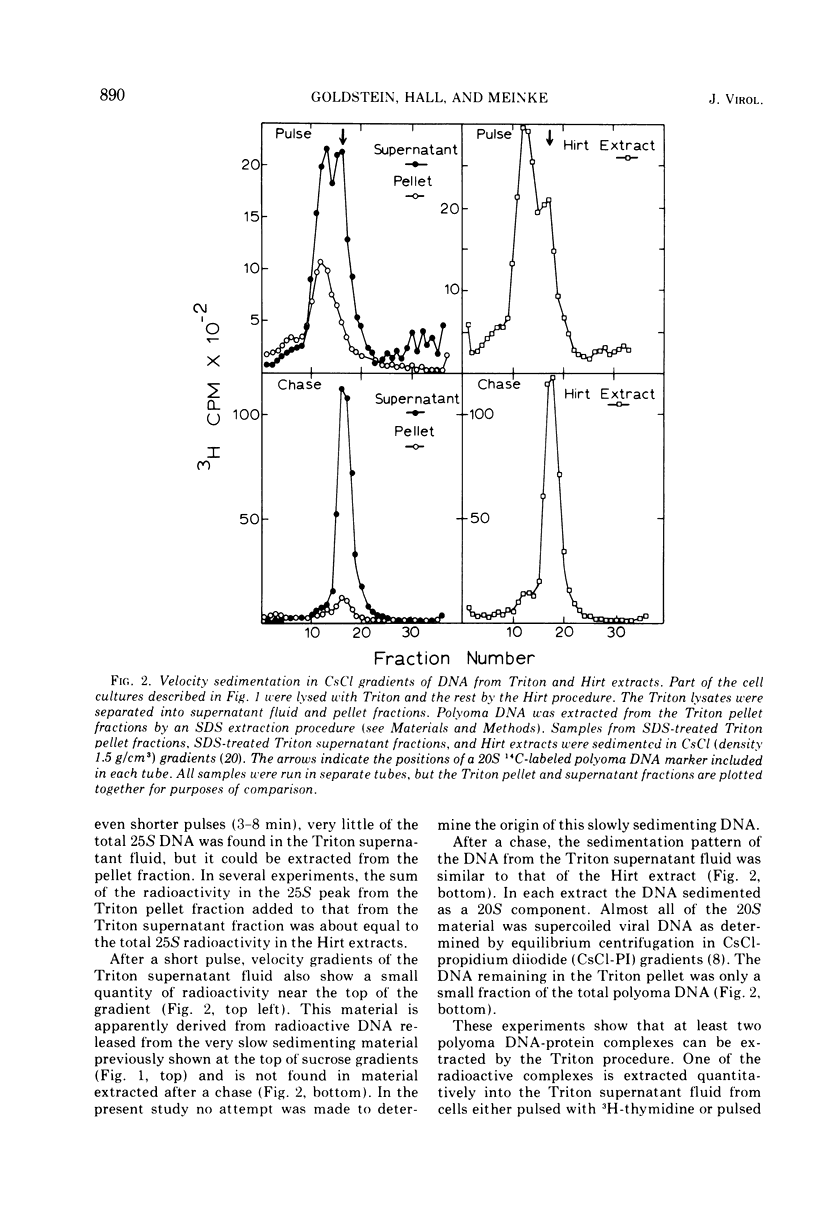
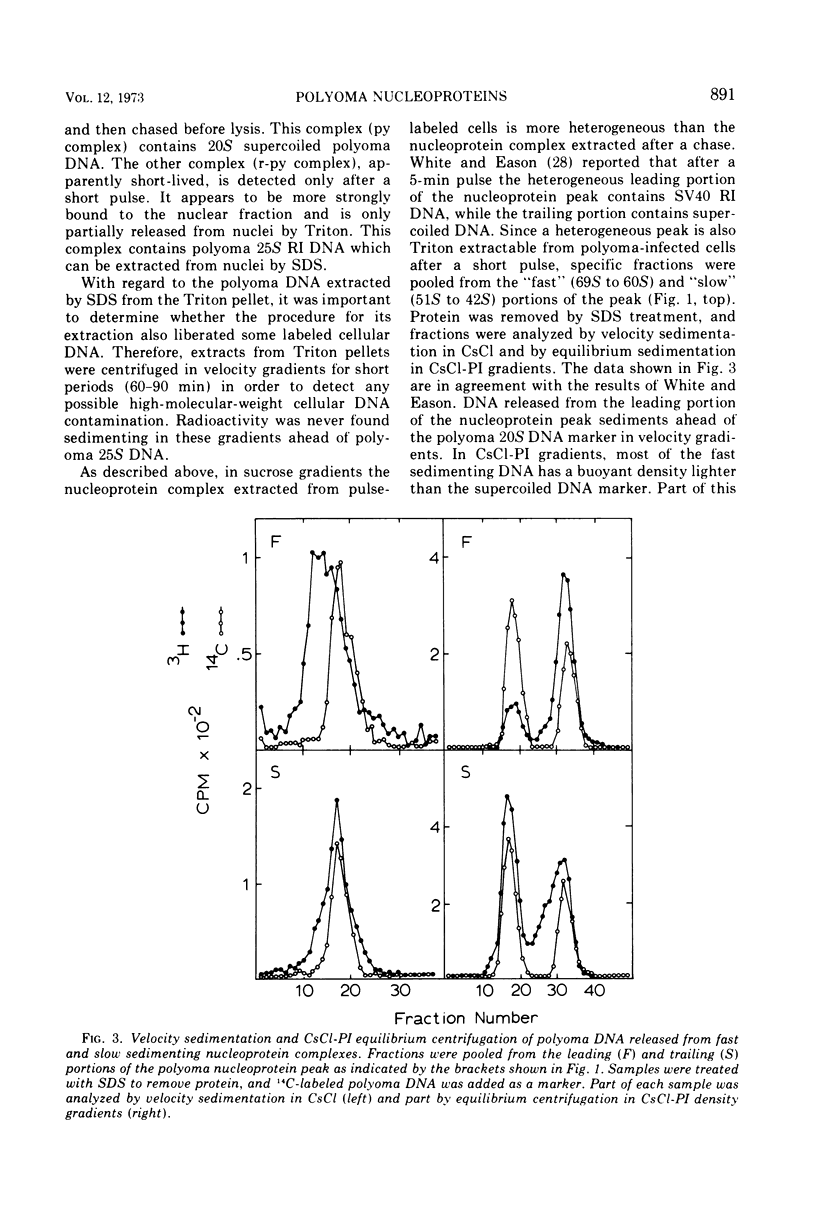
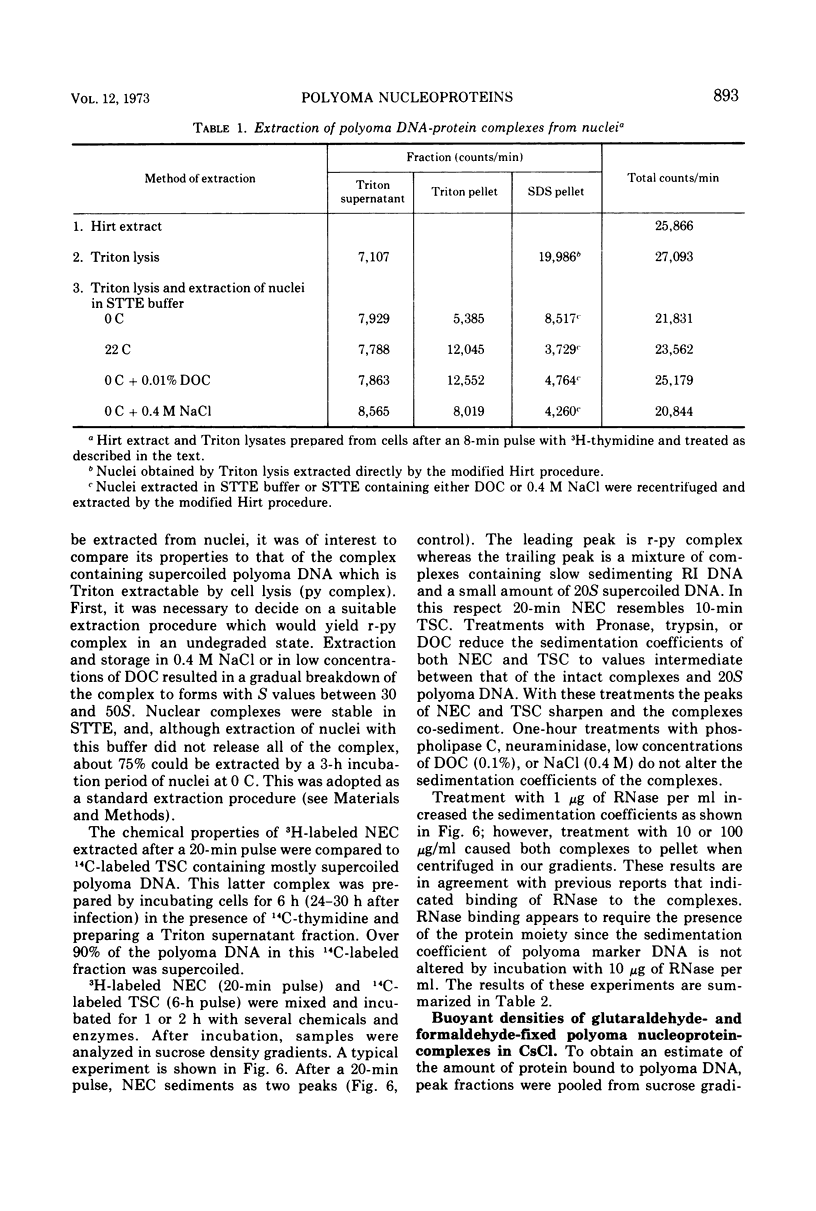
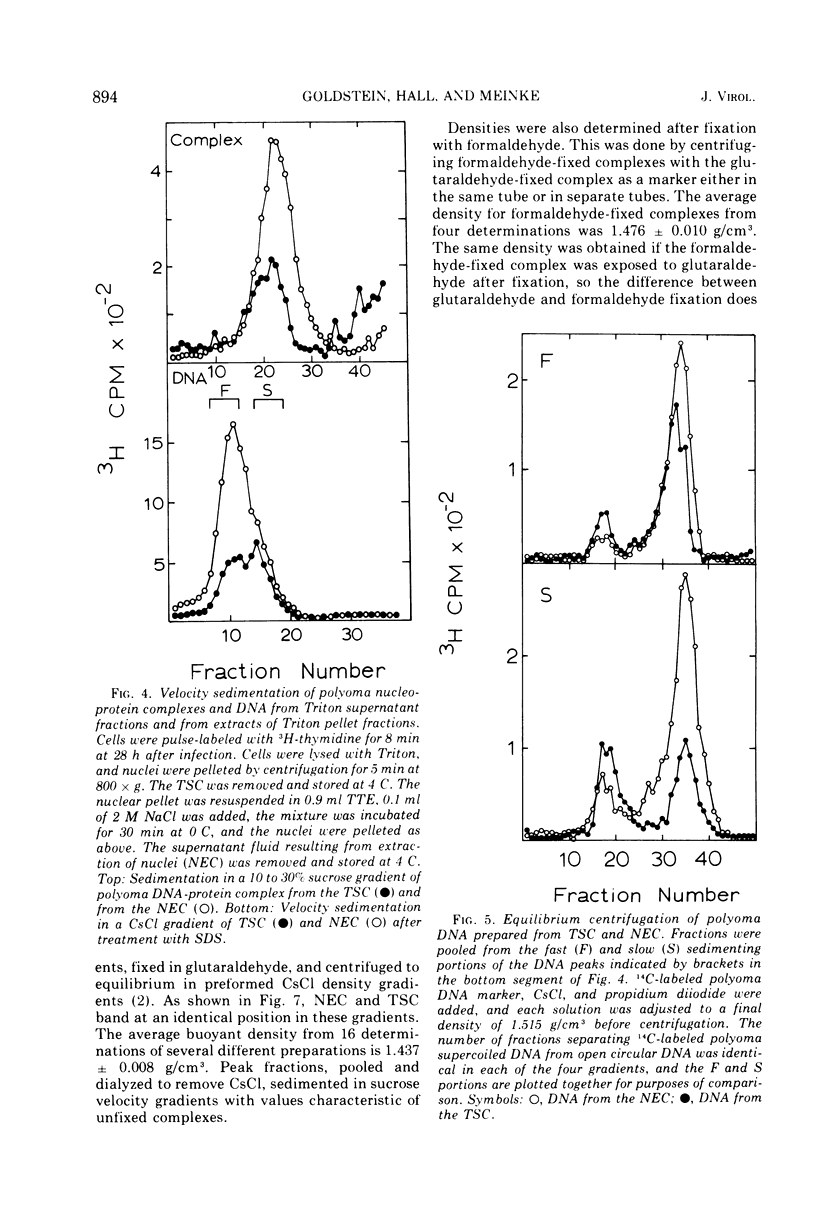
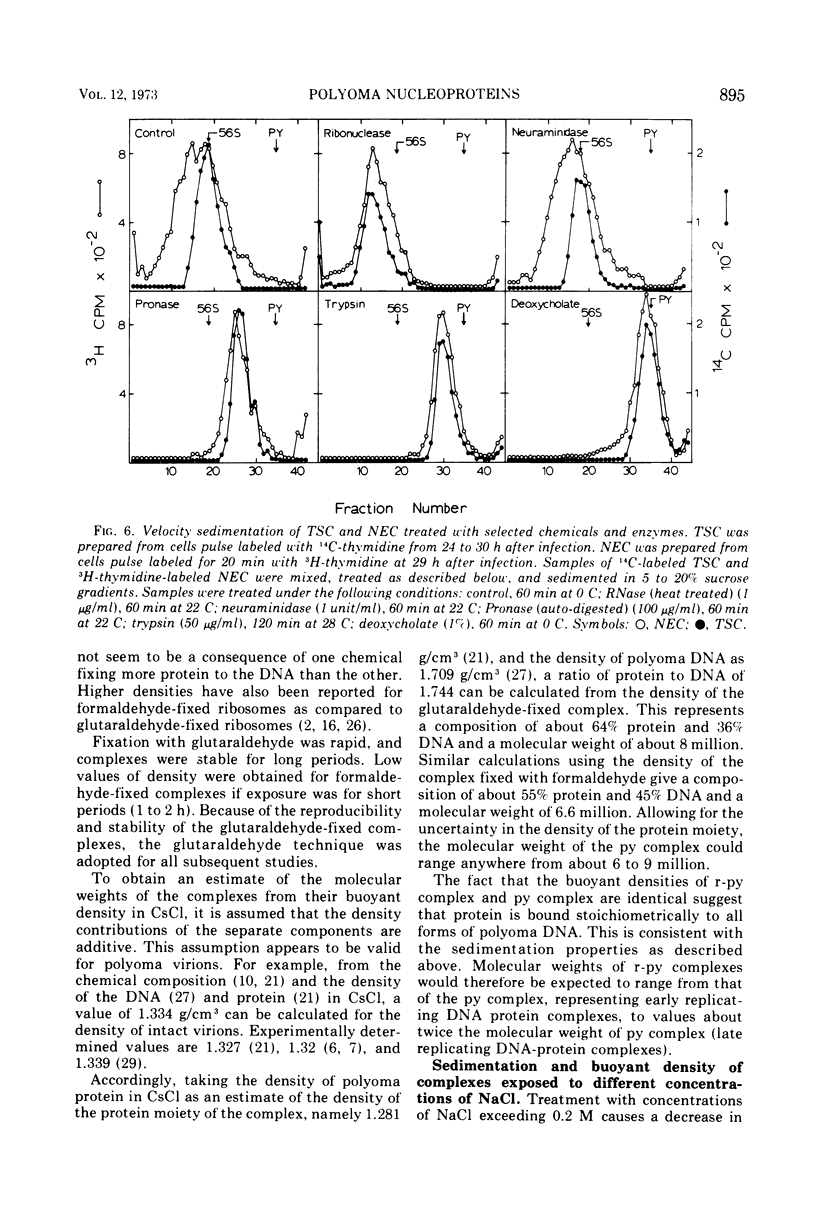
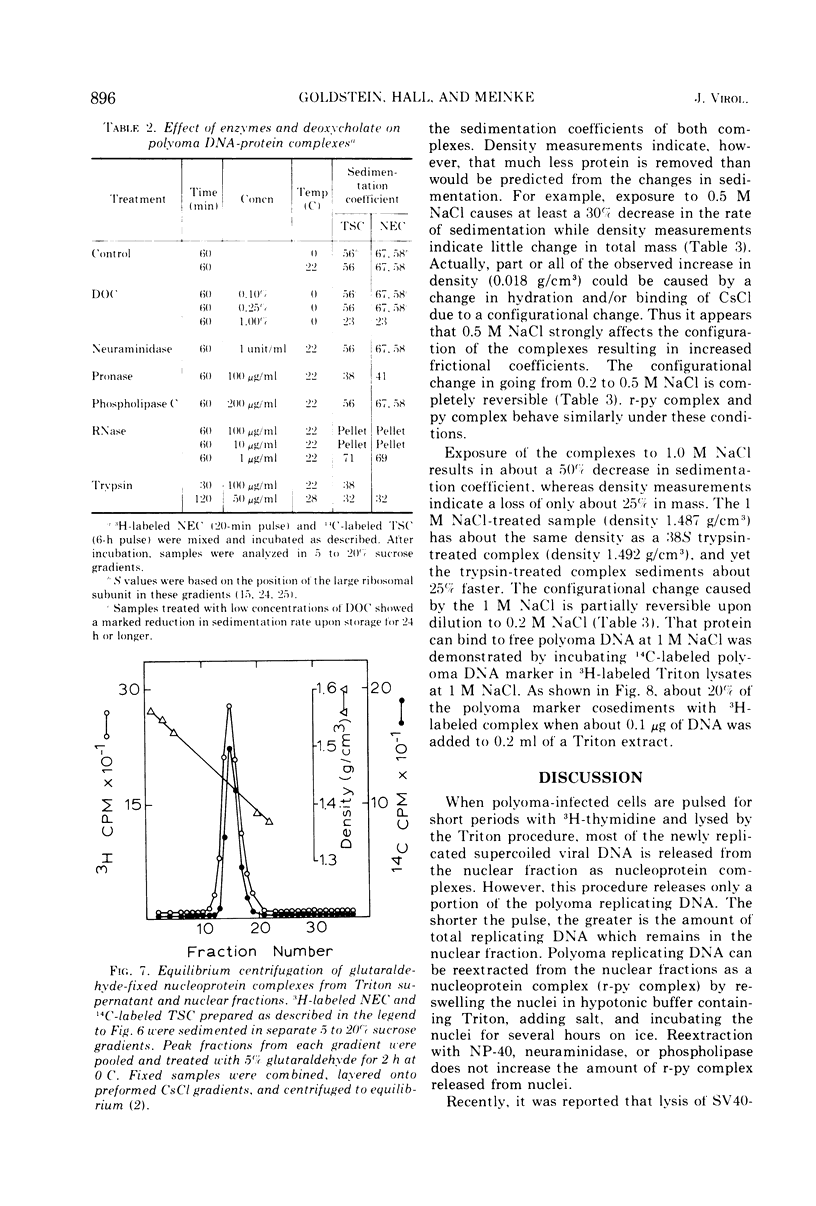
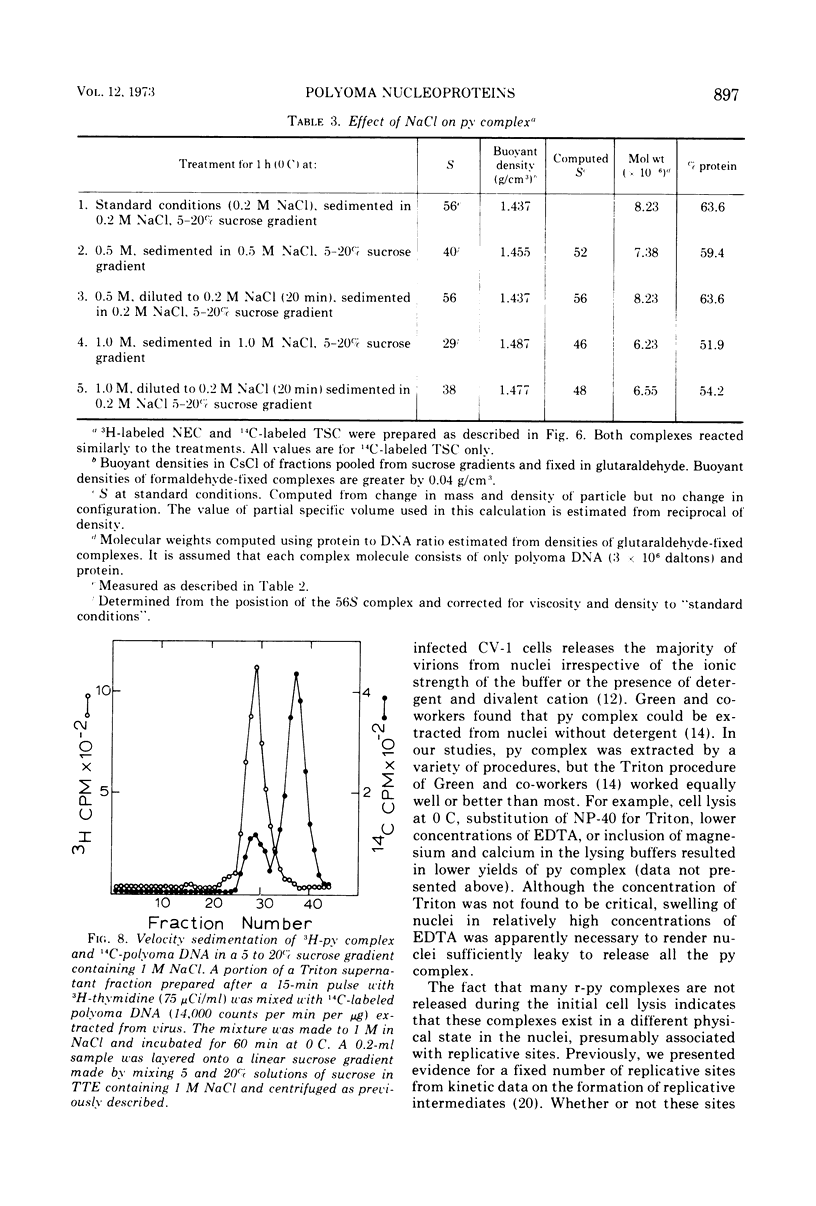
Short-lived nucleoprotein complexes (r-py complex) containing replicating polyoma DNA were isolated from infected cells after lysis with Triton X-100. The Triton lysing procedure of Green, Miller, and Hendler (1971) releases most complexes containing supercoiled viral DNA (py complex) from nuclei, but liberates only a portion of r-py complexes. r-py Complexes are associated more strongly with nuclear sites but can be extracted by prolonged incubation of nuclei in lysing solution. Complexes containing replicating polyoma DNA appear to be precursors to stable complexes containing supercoiled DNA. Sedimentation and buoyant density studies indicate that protein is bound to both r-py complexes and py complexes at a ratio of protein to DNA of about 1 to 2/1. Both types of complexes sediment as if the viral DNA is more compact than free DNA and both undergo major reversible configurational changes with increased salt concentration. Changes resulting from enzymatic and chemical treatment indicate that there may be two or more protein components in both r-py complex and py complex. One component is digested by Pronase and trypsin while another is resistant to the enzymes but released by deoxycholate. The abundance and similarity in chemical and physical properties of protein bound to all forms of polyoma DNA suggest that part of the protein molecules may serve in a structural capacity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Koch M. A., Schlumberger H. D. Structure of simian virus 40. 3. Alkaline degradation of the virus particle. Virology. 1968 Mar;34(3):452–458. doi: 10.1016/0042-6822(68)90065-2. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Huang A. S. Isopycnic separation of subcellular components from poliovirus-infected and normal HeLa cells. Science. 1968 Nov 1;162(3853):572–574. doi: 10.1126/science.162.3853.572. [DOI] [PubMed] [Google Scholar]
- Barban S., Goor R. S. Structural proteins of simian virus 40. J Virol. 1971 Feb;7(2):198–203. doi: 10.1128/jvi.7.2.198-203.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Seiler P. The replication of the ring-shaped DNA of polyoma virus. II. Identification of molecules at various stages of replication. J Mol Biol. 1971 Jul 14;59(1):195–206. doi: 10.1016/0022-2836(71)90421-9. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Unwinding of replicating polyoma virus DNA. J Mol Biol. 1972 Oct 14;70(3):399–413. doi: 10.1016/0022-2836(72)90548-7. [DOI] [PubMed] [Google Scholar]
- Eason R., Vinograd J. Superhelix density heterogeneity of intracellular simian virus 40 deoxyribonucleic acid. J Virol. 1971 Jan;7(1):1–7. doi: 10.1128/jvi.7.1.1-7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Stehelin D., Manteuil S., Pages J. Aspects of the encapsidation of simian virus 40 deoxyribonucleic acid. J Virol. 1973 Jan;11(1):107–115. doi: 10.1128/jvi.11.1.107-115.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H. Biosynthetic properties of a polyoma nucleoprotein complex: evidence for replication sites. J Virol. 1972 Jul;10(1):32–41. doi: 10.1128/jvi.10.1.32-41.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Miller H. I., Hendler S. Isolation of a polyoma-nucleoprotein complex from infected mouse-cell cultures. Proc Natl Acad Sci U S A. 1971 May;68(5):1032–1036. doi: 10.1073/pnas.68.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. R., Meinke W., Goldstein D. A. Nucleoprotein complexes containing replicating Simian virus 40 DNA: comparison with polyoma nucleoprotein complexes. J Virol. 1973 Oct;12(4):901–908. doi: 10.1128/jvi.12.4.901-908.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. G., Ruth M. E. The dissociation of rat liver ribosomes by ethylenediaminetetraacetic acid; molecular weights, chemical composition, and buoyant densities of the subunits. Biochemistry. 1969 Mar;8(3):851–856. doi: 10.1021/bi00831a013. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Estes M. K., Pagano J. S. Structure and function of the polypeptides in simian virus 40. I. Existence of subviral deoxynucleoprotein complexes. J Virol. 1972 Jun;9(6):923–929. doi: 10.1128/jvi.9.6.923-929.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Mass M., Fine R., Murakami W. T. Protein composition of polyoma virus. J Mol Biol. 1968 Aug 28;36(1):167–177. doi: 10.1016/0022-2836(68)90227-1. [DOI] [PubMed] [Google Scholar]
- Meinke W., Goldstein D. A. Studies on the structure and formation of polyoma DNA replicative intermediates. J Mol Biol. 1971 Nov 14;61(3):543–563. doi: 10.1016/0022-2836(71)90064-7. [DOI] [PubMed] [Google Scholar]
- Murakami W. T., Fine R., Harrington M. R., Sassan Z. B. Properties and amino acid composition of polyoma virus purified by zonal ultracentrifugation. J Mol Biol. 1968 Aug 28;36(1):153–166. doi: 10.1016/0022-2836(68)90226-x. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TASHIRO Y., SIEKEVITZ P. LOCALIZATION ON HEPATIC RIBOSOMES OF PROTEIN NEWLY SYNTHESIZED IN VIVO. J Mol Biol. 1965 Feb;11:166–173. doi: 10.1016/s0022-2836(65)80048-1. [DOI] [PubMed] [Google Scholar]
- TASHIRO Y., SIEKEVITZ P. ULTRACENTRIFUGAL STUDIES ON THE DISSOCIATION OF HEPATIC RIBOSOMES. J Mol Biol. 1965 Feb;11:149–165. doi: 10.1016/s0022-2836(65)80047-x. [DOI] [PubMed] [Google Scholar]
- WEIL R. The denaturation and the renaturation of the DNA of polyoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:480–487. doi: 10.1073/pnas.49.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- White M., Eason R. Nucleoprotein complexes in simian virus 40-infected cells. J Virol. 1971 Oct;8(4):363–371. doi: 10.1128/jvi.8.4.363-371.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


