Abstract
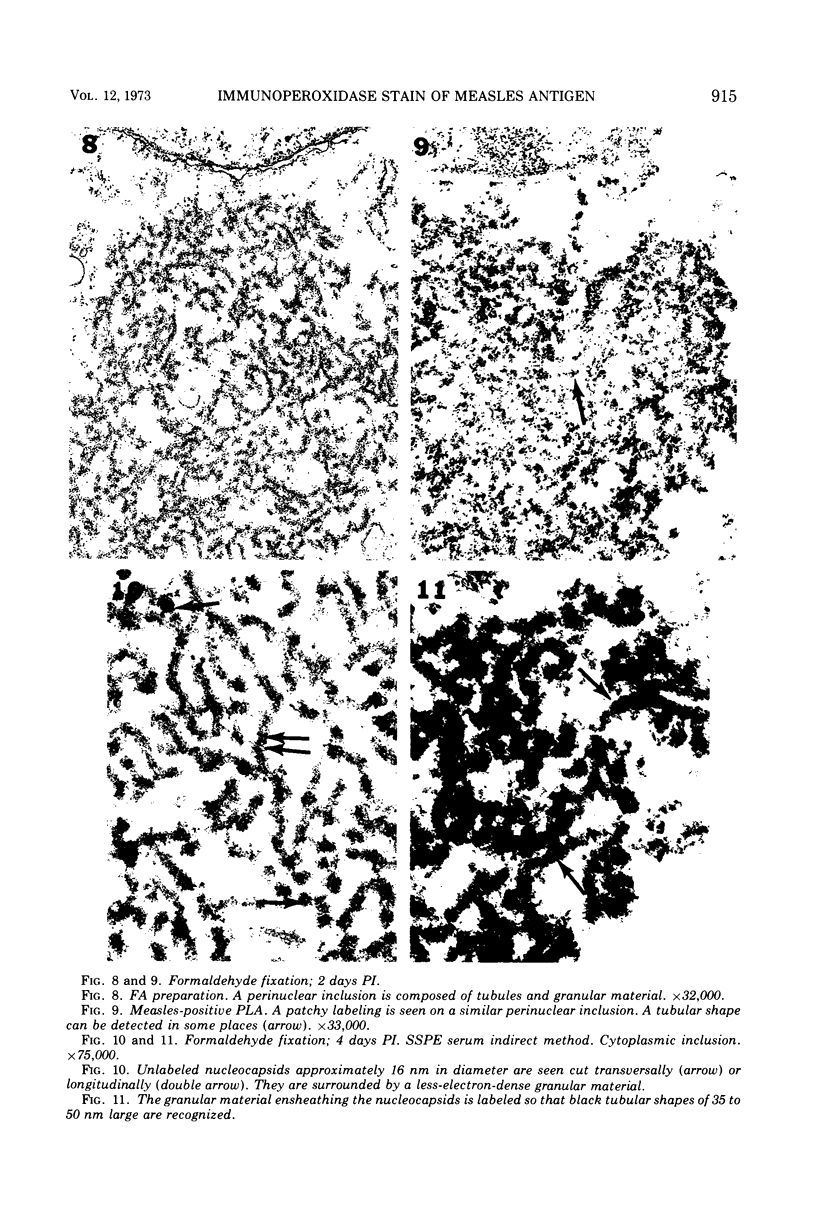
A specific electron microscopy staining technique for measles antigen has been developed by using Vero cells infected with a subacute sclerosing panencephalitis (SSPE) measles virus strain and fixed in glutaraldehyde or formaldehyde. Peroxidase-labeled antibody was prepared according to the method of Avrameas (4). Sera from SSPE patients with high measles antibody titer as well as normal human sera with and without measles antibody were used. With both fixatives, specific labeling was obtained on the surface of infected cells, on the budding site, and on complete viral particles. The cell membrane staining sometimes had a patchy distribution in that the reaction was most intense on the surface projections in front of each nucleocapsid. This suggests modification of the cell membrane in association with the nucleocapsids. In contrast, no label was detected on the membranes of the cells during the latent period from penetration through maturation of the virus. In formaldehyde-fixed cultures, cytoplasmic inclusions were stained, and this label was located on the “fuzzy” material around the nucleocapsids. The smooth type of nucleocapsids, mainly seen in the nucleus, were never labeled. These findings suggest that the antigenic nature of the “fuzzy” nucleocapsids in the cytoplasm may be different from that of the “smooth” nucleocapsids. The immunoperoxidase method gives good resolution of viral antigenic sites at high magnifications under electron microscopy and may be of value in studies on the immunopathogenesis of SSPE and other chronic viral infections.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMEIDA J. D., HOWATSON A. F. A negative staining method for cell-associated virus. J Cell Biol. 1963 Mar;16:616–620. doi: 10.1083/jcb.16.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson H. T., Smith G. H., Hoffman H. A., Rowe W. P. Use of enzyme-labeled antibody for electron microscope localization of lymphocytic choriomeningitis virus antigens in infected cell cultures. J Natl Cancer Inst. 1969 Mar;42(3):497–515. [PubMed] [Google Scholar]
- Apostolov K., Almeida J. D. Interaction of Sendai (HVJ) virus with human erythrocytes: a morphological study of haemolysis cell fusion. J Gen Virol. 1972 Jun;15(3):227–234. doi: 10.1099/0022-1317-15-3-227. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. Peroxidase labelled antibody and Fab conjugates with enhanced intracellular penetration. Immunochemistry. 1971 Dec;8(12):1175–1179. doi: 10.1016/0019-2791(71)90395-8. [DOI] [PubMed] [Google Scholar]
- Bretton R., Ternynck T., Avrameas S. Comparison of peroxidase and ferritin labelling of cell surface antigens. Exp Cell Res. 1972 Mar;71(1):145–155. doi: 10.1016/0014-4827(72)90272-8. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Reese T. S. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969 Mar;40(3):648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape C. A., Martinez A. J., Robertson J. T., Hamilton R., Jabbour J. T. Adult onset of subacute sclerosing panencephalitis. Arch Neurol. 1973 Feb;28(2):124–127. doi: 10.1001/archneur.1973.00490200072010. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Duc-Nguyen H., Rosenblum E. N. Immuno-electron microscopy of the morphogenesis of mumps virus. J Virol. 1967 Apr;1(2):415–429. doi: 10.1128/jvi.1.2.415-429.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- Howe C., Morgan C., de Vaux St Cyr C., Hsu K. C., Rose H. M. Morphogenesis of type 2 parainfluenza virus examined by light and electron microscopy. J Virol. 1967 Feb;1(1):215–237. doi: 10.1128/jvi.1.1.215-237.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurstak E., Belloncik S., Onji P. A., Montplaisir S., Martineau B. Localisation par l'immunoperoxydase des antigènes du virus cytomégalique en culture cellulaire de fibroblastes humains. Microscopie photonique et électronique. Arch Gesamte Virusforsch. 1972;38(1):67–76. [PubMed] [Google Scholar]
- Leduc E. H., Wicker R., Avrameas S., Bernhard W. Ultrastructural localization of SV40T antigen with enzyme-labelled antibody. J Gen Virol. 1969 Jun;4(4):609–614. doi: 10.1099/0022-1317-4-4-609. [DOI] [PubMed] [Google Scholar]
- McFerran J. B., Nelson R., McCracken J. M., Ross J. G. Viruses isolated from sheep. Nature. 1969 Jan 11;221(5176):194–195. doi: 10.1038/221194a0. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Compans R. W., Choppin P. W. Proteins of vesicular stomatitis virus and of phenotypically mixed vesicular stomatitis virus-simian virus 5 virions. J Virol. 1971 Nov;8(5):722–729. doi: 10.1128/jvi.8.5.722-729.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M., Imagawa D. T. Electron microscopy of measles virus replication. J Virol. 1969 Feb;3(2):187–197. doi: 10.1128/jvi.3.2.187-197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai T., Shand F. L., Howatson A. F. Development of measles virus in vitro. Virology. 1969 May;38(1):50–67. doi: 10.1016/0042-6822(69)90127-5. [DOI] [PubMed] [Google Scholar]
- Norrby E. C., Magnusson P. Some morphological characteristics of the internal component of measles virus. Arch Gesamte Virusforsch. 1965;17(3):443–447. doi: 10.1007/BF01241199. [DOI] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Katz M., Koprowski H. Comparison of subacute sclerosing panencephalitis and measles viruses: an electron microscope study. J Virol. 1971 Jan;7(1):176–187. doi: 10.1128/jvi.7.1.176-187.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyanagi S., ter Meulen V., Müller D., Katz M., Koprowski H. Electron microscopic observations in subacute sclerosing panencephalitis brain cell cultures: their correlation with cytochemical and immunocytological findings. J Virol. 1970 Sep;6(3):370–379. doi: 10.1128/jvi.6.3.370-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Périer O., Vanderhaeghen J. J., Pelc S. Subacute sclerosing leuco-encephalitis. Electron microscopic finding in two cases with inclusion bodies. Acta Neuropathol. 1967 Jul 5;8(4):362–380. doi: 10.1007/BF00696673. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Feldman L. A., Sheppard R. D., Bornstein M. B. Ultrastructural study of long-term measles infection in cultures of hamster dorsal-root ganglion. J Virol. 1971 Sep;8(3):318–329. doi: 10.1128/jvi.8.3.318-329.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T. S., Karnovsky M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967 Jul;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi A. A., Norrby E., Panelius M. Identification of different measles virus-specific antibodies in the serum and cerebrospinal fluid from patients with subacute sclerosing pancencephalitis and multiple sclerosis. Infect Immun. 1972 Sep;6(3):248–254. doi: 10.1128/iai.6.3.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabo A. L., Petricciani J. C., Kirschstein R. L. Immunoperoxidase localization of herpes zoster virus and simian virus 40 in cell culture. Appl Microbiol. 1972 May;23(5):1001–1009. doi: 10.1128/am.23.5.1001-1009.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubertini T., Wilkie B. N., Noronha F. Use of horseradish peroxidase-labeled antibody for light and electron microscope localization of reovirus antigen. Appl Microbiol. 1971 Mar;21(3):534–538. doi: 10.1128/am.21.3.534-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J., Iwasaki Y. Isolation and characterization of subacute sclerosing panencephalitis virus nucleocapsids. J Virol. 1972 Dec;10(6):1220–1227. doi: 10.1128/jvi.10.6.1220-1227.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Muelen V., Enders-Ruckle G., Müller D., Joppich G. Immunhistological, microscopical and neurochemical studies on encephalitides. 3. Subacute progressive panencephalitis. Virological and immunhistological studies. Acta Neuropathol. 1969;12(3):244–259. doi: 10.1007/BF00687648. [DOI] [PubMed] [Google Scholar]















