Abstract
The cell culture lines HTG2 and HTG3 were established from a transplantable hamster tumor induced by a murine sarcoma virus (strain Gz-MSV) after 17 and 60 in vivo passages, respectively. The viruses released by these two cell lines markedly differ in morphology, antigenic composition, infectivity, transforming ability, and enzymatic activity. HTG2 virions contained the sarcoma genome but were noninfectious for mouse and hamster cells (S+H-virus). HTG3 virions transformed hamster but not mouse cells. Whereas HTG2 cells and its virus contained murine type C virus gs-1 antigen, all HTG3 clonal lines expressed both murine and hamster type C virus gs-1 antigens. The RNA-dependent DNA polymerase activity of HTG2 virus was very low, whereas that of HTG3 virus was relatively high. HTG2 virions contained electron-lucent centers only. HTG3 virus consisted of the expected mixture of virions with electron-dense and electron-lucent centers. Many broken or incomplete virions were present in both viruses. HTG2 virus is a noninfectious “defective” sarcoma virus without detectable helper virus. Data obtained in these experiments suggest that HTG3 virus is a hamster type C virus pseudotype of Gz-MSV (Gz-MSV [HaLV]). The genome of Gz-MSV is capable of antigenic expression in heterologous cells and in the presence of heterologous viruses. Attempts to chemically activate hamster type C virus (HaLV) from HTG2 cells were unsuccessful. The HTG1 cell culture line, established from another Gz-MSV-induced hamster tumor, initially released a virus indistinguishable from the HTG2 virus. After in vitro passage, spontaneous activation of HaLV occurred in HTG1 cells, and the resultant Gz-MSV (HaLV) had properties similar to those of the HTG3 virus.
Full text
PDF

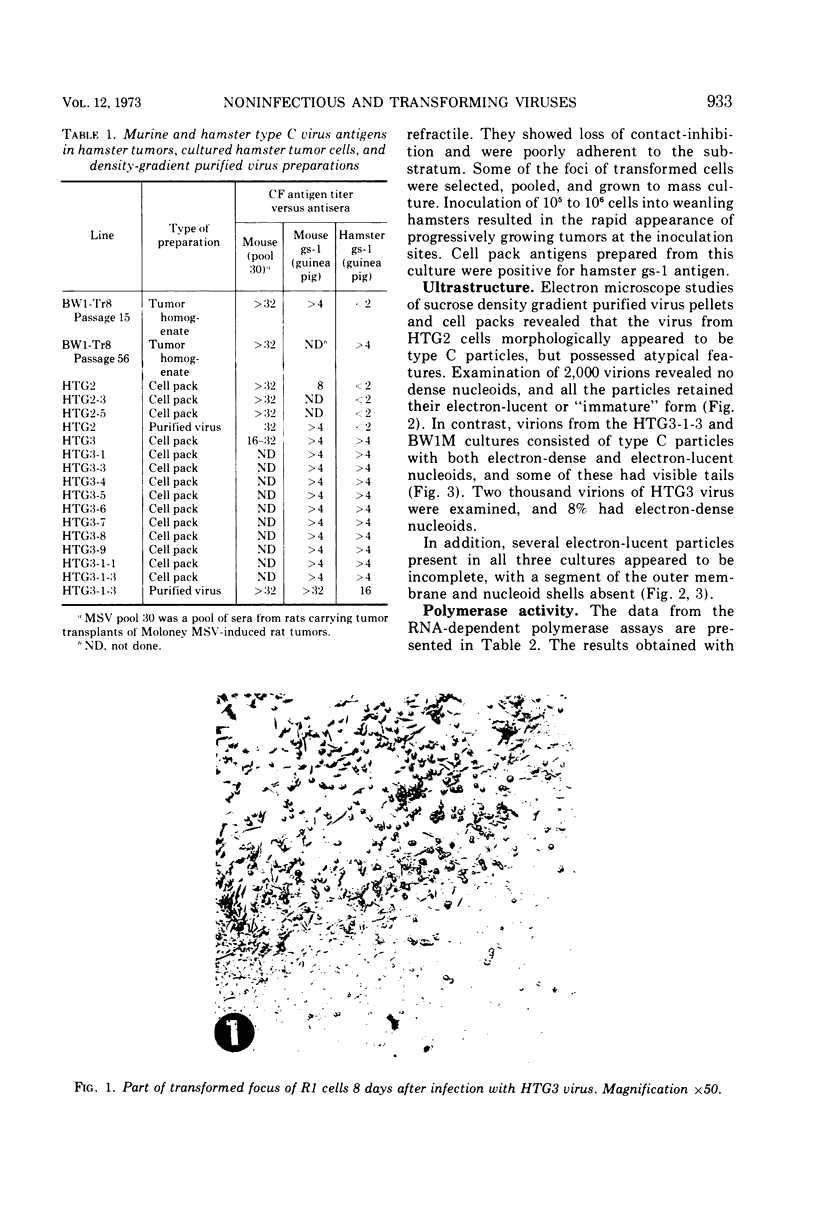



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Chemical induction of focus-forming virus from nonproducer cells transformed by murine sarcoma virus. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3069–3072. doi: 10.1073/pnas.68.12.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. J. RNA tumor viruses. Terminology and ultrastructural aspects of virion morphology and replication. J Natl Cancer Inst. 1972 Aug;49(2):323–327. [PubMed] [Google Scholar]
- Gazdar A. F., Chopra H. C., Sarma P. S. Properties of a murine sarcoma virus isolated from a tumor arising in an NZW-NZB F 1 hybrid mouse. I. Isolation and pathology of tumors induced in rodents. Int J Cancer. 1972 Jan 15;9(1):219–233. [PubMed] [Google Scholar]
- Gazdar A. F., Phillips L. A., Sarma P. S., Peebles P. T., Chopra H. C. Presence of sarcoma genome in a "non-infectious" mammalian virus. Nat New Biol. 1971 Nov 17;234(46):69–72. doi: 10.1038/newbio234069a0. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Russell E., Bassin R. H. In vivo enhancement of a murine sarcoma virus by diethylaminoethyl-dextran. Proc Soc Exp Biol Med. 1971 May;137(1):310–314. doi: 10.3181/00379727-137-35567. [DOI] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., TURNER H. C., LANE W. T. SPECIFIC ADENOVIRUS COMPLEMENT-FIXING ANTIGENS IN VIRUS-FREE HAMSTER AND RAT TUMORS. Proc Natl Acad Sci U S A. 1963 Aug;50:379–389. doi: 10.1073/pnas.50.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Chang N. H., Lee Y. K., Gilden R. V. Envelope antigen relationships among three hamster-specific sarcoma viruses and a hamster-specific helper virus. J Gen Virol. 1970 Oct;9(1):19–33. doi: 10.1099/0022-1317-9-1-19. [DOI] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Lee Y. K., Toni R., Gilden R. Hamster-tropic sarcomagenic and nonsarcomagenic viruses derived from hamster tumors induced by the Gross pseudotype of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1970 Feb;65(2):310–317. doi: 10.1073/pnas.65.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff G., Huebner R. J., Oroszlan S., Toni R., Gilden R. V. Immunological identity of the group-specific antigen of hamster-specific C-type viruses and an indigenous hamster virus. J Gen Virol. 1970 Oct;9(1):27–33. doi: 10.1099/0022-1317-9-1-27. [DOI] [PubMed] [Google Scholar]
- Klement V., Hartley J. W., Rowe W. P., Huebner R. J. Recovery of a hamster-specific, focus-forming, and sarcomagenic virus from a "noninfectious" hamster tumor induced by the Kirsten Mouse sarcoma virus. J Natl Cancer Inst. 1969 Oct;43(4):925–934. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oroszlan S., Fisher C. L., Stanley T. B., Gilden R. V. Proteins of the murine C-type RNA tumour viruses: isolation of a group-specific antigen by isoelectric focusing. J Gen Virol. 1970 Jul;8(1):1–10. doi: 10.1099/0022-1317-8-1-1. [DOI] [PubMed] [Google Scholar]
- Peebles P. T., Haapala D. K., Gazdar A. F. Deficiency of viral ribonucleic acid-dependent deoxyribonucleic acid polymerase in noninfectious virus-like particles released from murine sarcoma virus-transformed hamster cells. J Virol. 1972 Mar;9(3):488–493. doi: 10.1128/jvi.9.3.488-493.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M. S., Smith R. G., Gallo R. C., Sarin P. S., Abrell J. W. Viral and cellular DNA polymerase: comparison of activities with synthetic and natural RNA templates. Science. 1972 May 19;176(4036):798–800. doi: 10.1126/science.176.4036.798. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Sarma P. S., Gazdar A. F., Turner H. C., Kunchorn P. D. Gazdar strain of murine sarcoma virus. Biologic and antigenic interactions with the heterologous hamster host. Proc Soc Exp Biol Med. 1972 Jul;140(3):928–933. doi: 10.3181/00379727-140-36582. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Sarngadharan M. G., Sarin P. S., Reitz M. S., Gallo R. C. Reverse transcriptase activity of human acute leukaemic cells: purification of the enzyme, response to AMV 70S RNA, and characterization of the DNA product. Nat New Biol. 1972 Nov 15;240(98):67–72. doi: 10.1038/newbio240067a0. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]