Abstract
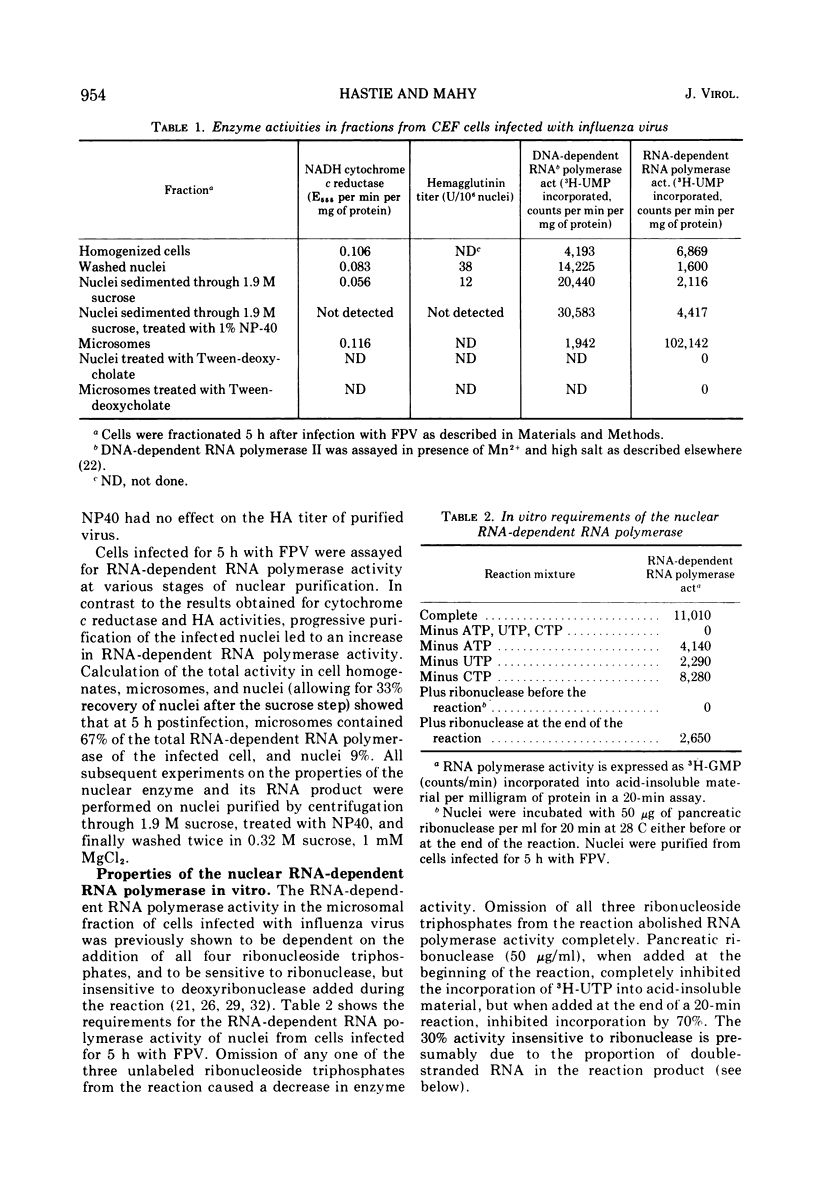
Nuclei purified from chicken embryo fibroblast cells infected with influenza (fowl plague) virus contain an RNA-dependent RNA polymerase. The in vitro activity of this enzyme is insensitive to actinomycin D, and is completely destroyed by preincubation with ribonuclease. Enzyme induction is prevented if cells are treated with actinomycin D or cycloheximide at the time of infection. RNA-dependent RNA polymerase activity increases rapidly in cell nuclei from 1 h postinfection, reaches a maximum at 3 to 4 h, then declines; a similar RNA polymerase activity in the microsomal cell fraction increases from 2 h postinfection and reaches a maximum at 5 to 6 h. The characteristics of the nuclear and microsomal enzymes in vitro are similar with respect to pH and divalent cation requirements. The in vitro products of enzyme activity present in the nuclear and microsomal fractions of cells infected for 3 and 5 h were characterized by sucrose density gradient analysis, and annealing to virion RNA. The microsomal RNA polymerase product contained 67 and 93% RNA complementary to virion RNA at 3 and 5 h, respectively; for the nuclear RNA polymerase product these values were 40% in each case.
Full text
PDF


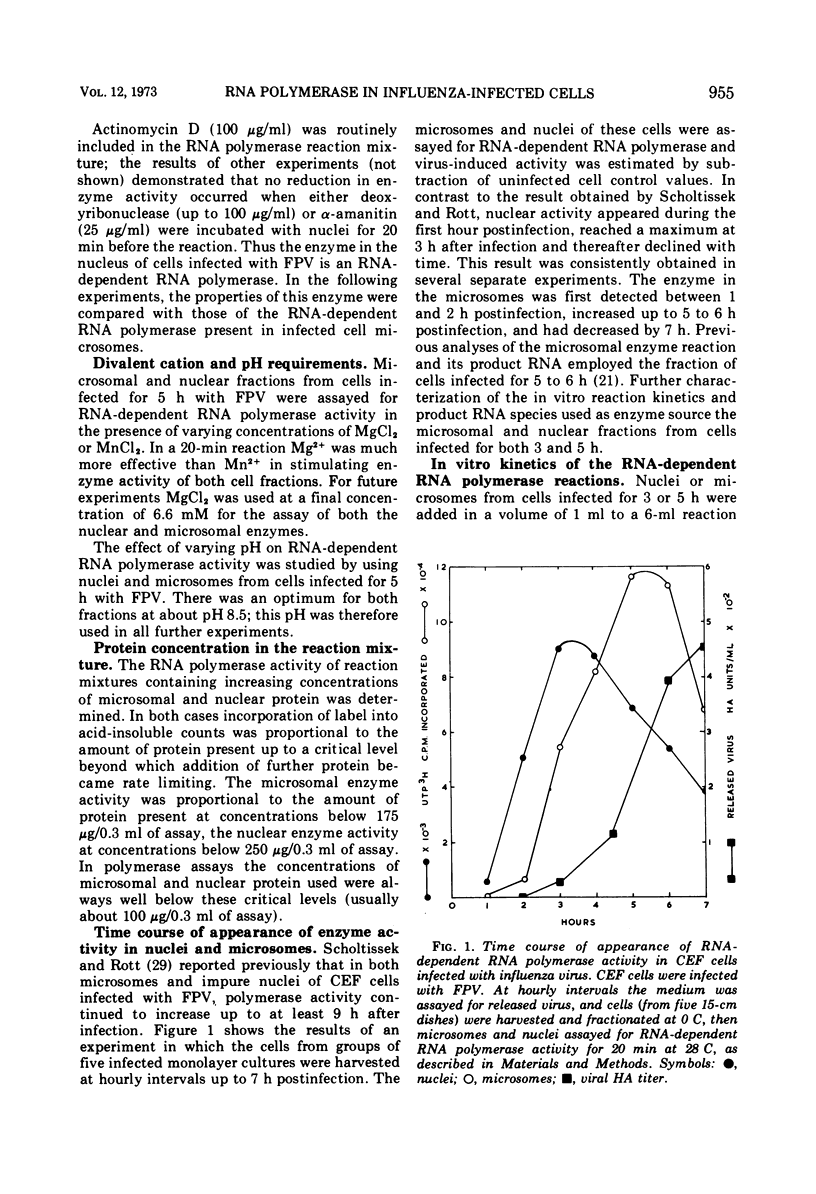






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY R. D. THE EFFECTS OF ACTINOMYCIN D AND ULTRAVIOLET IRRADIATION ON THE PRODUCTION OF FOWL PLAGUE VIRUS. Virology. 1964 Dec;24:563–569. doi: 10.1016/0042-6822(64)90208-9. [DOI] [PubMed] [Google Scholar]
- BREITENFELD P. M., SCHAFER W. The formation of fowl plague virus antigens in infected cells, as studied with fluorescent antibodies. Virology. 1957 Oct;4(2):328–345. doi: 10.1016/0042-6822(57)90067-3. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. D., Duesberg P. H. Myxovirus ribonucleic acids. Annu Rev Microbiol. 1970;24:539–574. doi: 10.1146/annurev.mi.24.100170.002543. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Borland R., Mahy B. W. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in cells infected with influenza virus. J Virol. 1968 Jan;2(1):33–39. doi: 10.1128/jvi.2.1.33-39.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Caliguiri L. A. Isolation and properties of an RNA polymerase from influenza virus-infected cells. J Virol. 1973 Mar;11(3):441–448. doi: 10.1128/jvi.11.3.441-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BREITENFELD P. M. The abortive infection of Earle's L-cells by fowl plague virus. Virology. 1959 Jul;8(3):293–307. doi: 10.1016/0042-6822(59)90031-5. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Mahy B. W. Effects of -amanitin in vivo on RNA polymerase activity of cultured chick embryo fibroblast cell nuclei: resistance of ribosomal RNA synthesis to the drug. FEBS Lett. 1973 May 15;32(1):95–99. doi: 10.1016/0014-5793(73)80746-x. [DOI] [PubMed] [Google Scholar]
- Ho P. P., Walters C. P. Influenza virus-induced ribonucleic acid nucleotidyltransferase and the effect of actinomycin D on its formation. Biochemistry. 1966 Jan;5(1):231–235. doi: 10.1021/bi00865a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson D. E., Wilson P. W., Barker D. C., Kodicek E. Isolation of chick intestinal nuclei. Effect of vitamin D3 on nuclear metabolism. Biochem J. 1969 Nov;115(2):263–268. doi: 10.1042/bj1150263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Maeno K., Kilbourne E. D. Developmental sequence and intracellular sites of synthesis of three structural protein antigens of influenza A2 virus. J Virol. 1970 Feb;5(2):153–164. doi: 10.1128/jvi.5.2.153-164.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Bromley P. A. In vitro product of a ribonucleic acid polymerase induced by influenza virus. J Virol. 1970 Sep;6(3):259–268. doi: 10.1128/jvi.6.3.259-268.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Bromley P. A. Synthesis of ribonuclease-resistant ribonucleic acid by fowl-plague-virus-induced ribonucleic acid polymerase. Biochem J. 1969 Oct;114(4):64P–64P. doi: 10.1042/bj1140064pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy B. W., Hastie N. D., Armstrong S. J. Inhibition of influenza virus replication by -amanitin: mode of action. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1421–1424. doi: 10.1073/pnas.69.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D. P., Baluda M. A. Ribonucleic acid synthesis in cells infected with influenza virus. J Virol. 1968 Feb;2(2):99–109. doi: 10.1128/jvi.2.2.99-109.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E., Miller H., Doyle M., Blatti S. RNA-dependent RNA polymerase activity in influenza virions. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1369–1371. doi: 10.1073/pnas.68.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Ruck B. J., Brammer K. W., Page M. G., Coombes J. D. The detection and characterization of an induced RNA polymerase in the chorioallantoic membranes of embryonated eggs infected with influenza A2 virus. Virology. 1969 Sep;39(1):31–41. doi: 10.1016/0042-6822(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R. Ribonucleic acid nucleotidyl transferase induced in chick fibroblasts after infection with an influenza virus. J Gen Virol. 1969 Jan;4(1):125–137. doi: 10.1099/0022-1317-4-1-125. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Synthesis in vitro of RNA complementary to parental viral RNA by RNA polymerase induced by influenza virus. Biochim Biophys Acta. 1969 Apr 22;179(2):389–397. doi: 10.1016/0005-2787(69)90047-1. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Burke D. C. Ribonucleic acid synthesis in chick embryo cells infected with fowl-plague virus. J Virol. 1969 Apr;3(4):429–438. doi: 10.1128/jvi.3.4.429-438.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. RNA-dependent RNA polymerase activity of the influenza virus. Virology. 1971 Sep;45(3):793–796. doi: 10.1016/0042-6822(71)90197-8. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Hampson A. W., Layton J. E., White D. O. The polypeptides of influenza virus. IV. An analysis of nuclear accumulation. Virology. 1970 Nov;42(3):744–752. doi: 10.1016/0042-6822(70)90320-x. [DOI] [PubMed] [Google Scholar]


