Abstract
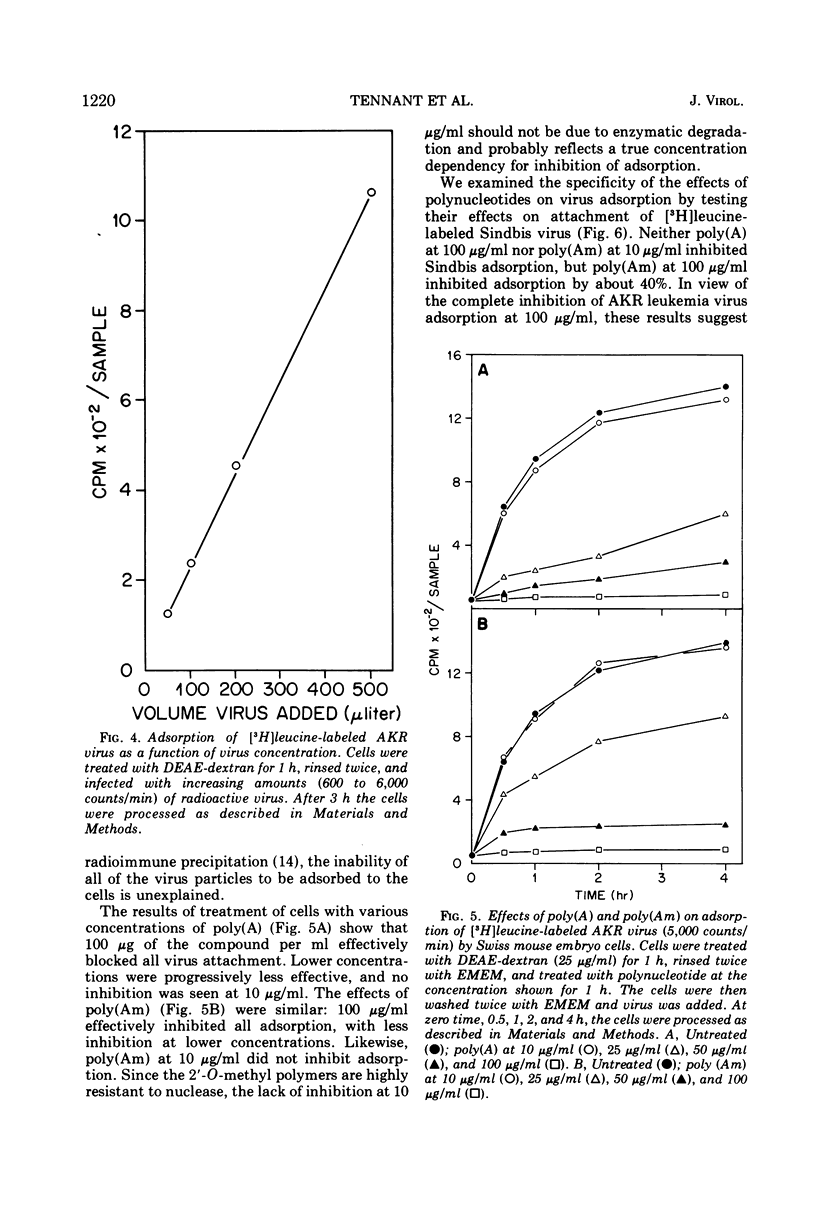
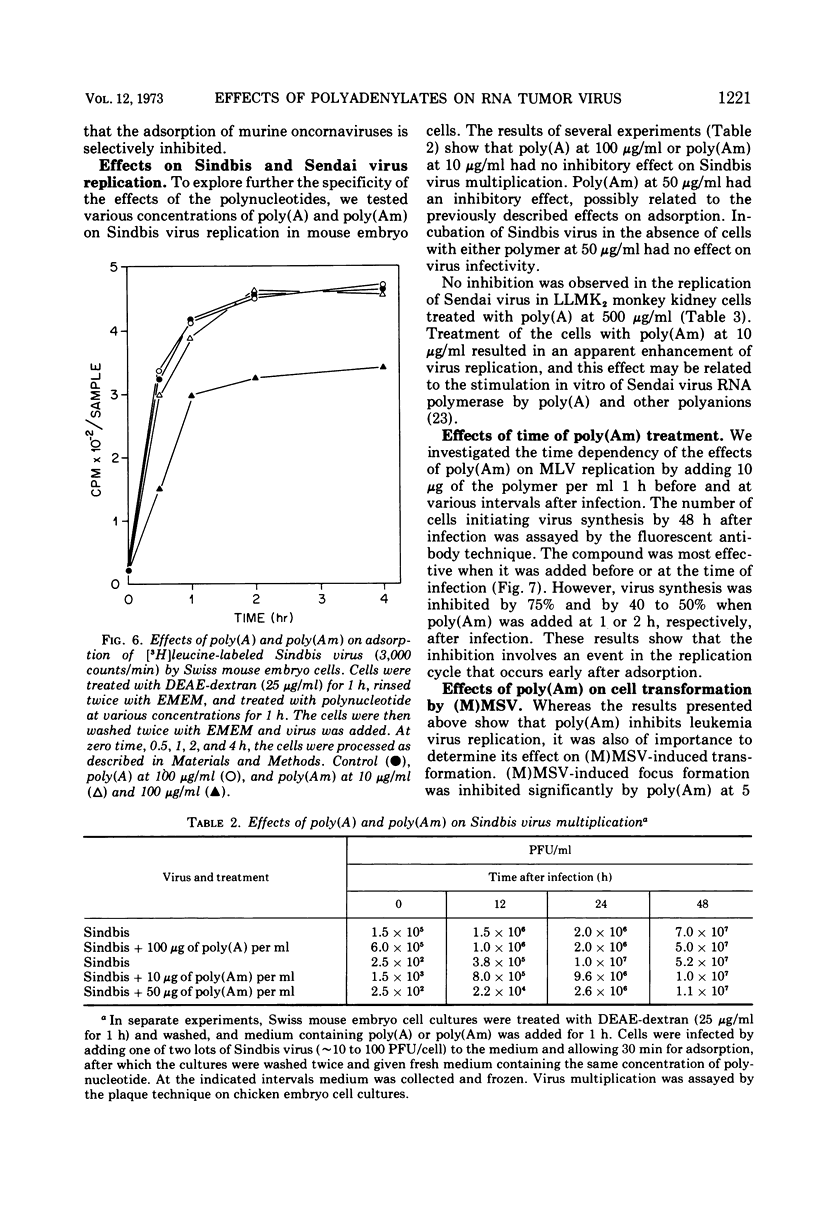
Single-stranded polyribonucleotides, which competitively inhibit murine RNA tumor virus reverse transcriptase in vitro, were tested as inhibitors of various virus functions in cell culture. The compounds had two concentration-dependent effects. At high concentrations (100 μg/ml), both poly(adenylic acid) [poly(A)] and poly(2′-O-methyladenylic acid) [poly(Am)] inhibited the uptake of radioactively labeled leukemia virus by Swiss mouse embryo cells, but neither had a similar effect on Sindbis virus adsorption. At low concentrations (10 μg/ml), poly(Am) did not inhibit the uptake of leukemia virus but did inhibit virus replication by 85%; in contrast, the replication of Sendai virus and Sindbis virus was not inhibited significantly at this concentration. Both compounds were effective only when added prior to or early during virus infection. Poly(Am) was a much more effective inhibitor than poly(A), probably due to the nuclease resistance of the former compound. Poly(Am) at 5 μg/ml also inhibited transformation of 3T3 cells by Moloney sarcoma virus. However, neither poly(A) nor poly(Am) at high concentrations inhibited the activation of endogenous leukemia virus by iododeoxyuridine in AKR mouse embryo cells. These results suggest that virus reverse transcriptase plays an essential role in both the replication of exogenous murine leukemia viruses and the transformation of cells by murine sarcoma viruses but probably has no role in the activation of endogenous leukemia virus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Abrell J. W., Smith R. G., Robert M. S., Gallo B. C. DNA polymerases from RNA tumor viruses and human cells: inhibition by polyuridylic acid. Science. 1972 Sep 22;177(4054):1111–1114. doi: 10.1126/science.177.4054.1111. [DOI] [PubMed] [Google Scholar]
- Balduzzi P., Morgan H. R. Mechanism of oncogenic transformation by Rous sarcoma virus. I. Intracellular inactivation of cell-transforming ability of Rous sarcoma virus by 5-bromodeoxyuridine and light. J Virol. 1970 Apr;5(4):470–477. doi: 10.1128/jvi.5.4.470-477.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Temin H. M. Light inactivation of focus formation by chicken embryo fibroblasts infected with avian sarcoma virus in the presence of 5-bromodeoxyuridine. Nature. 1970 Nov 14;228(5272):622–624. doi: 10.1038/228622a0. [DOI] [PubMed] [Google Scholar]
- Calvin M., Joss U. R., Hackett A. J., Owens R. B. Effect of rifampicin and two of its derivatives on cells infected with Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1441–1443. doi: 10.1073/pnas.68.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. A., Brockman W. W., Borden E. C. Streptovaricins inhibit focus formation by MSV (MLV) complex. Nat New Biol. 1971 Aug;232(33):212–214. doi: 10.1038/newbio232212a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Merigan T. C. Requirement of a stable secondary structure for the antiviral activity of polynucleotides. Nature. 1969 Jun 21;222(5199):1148–1152. doi: 10.1038/2221148a0. [DOI] [PubMed] [Google Scholar]
- Diggelmann H., Weissmann C. Rifampicin inhibits focus formation in chick fibroblasts infected with Rous sarcoma virus. Nature. 1969 Dec 27;224(5226):1277–1279. doi: 10.1038/2241277a0. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., O'Connor T. E. Replication of defective and competent forms of murine sarcoma virus in mouse cell cultures. Virology. 1970 Jun;41(2):233–243. doi: 10.1016/0042-6822(70)90075-9. [DOI] [PubMed] [Google Scholar]
- Gallo R. C. Reverse transcriptase, the DNA polymerase of oncogenic RNA viruses. Nature. 1971 Nov 26;234(5326):194–198. doi: 10.1038/234194a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitha P. M., Teich N. M., Lowy D. R., Pitha J. Inhibition of murine leukemia virus replication by poly(vinyluracil) and poly(vinyladenine). Proc Natl Acad Sci U S A. 1973 Apr;70(4):1204–1208. doi: 10.1073/pnas.70.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. J., Balduzzi P. Mechanism of oncogenic transformation by Rous sarcoma virus. II. Effect of rifampin on Rous sarcoma virus infection. J Virol. 1971 Jul;8(1):62–65. doi: 10.1128/jvi.8.1.62-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Robinson W. S. Inhibition of growth of uninfected and rous sarcoma virus-infected chick-embryo fibroblasts by rifampicin. J Natl Cancer Inst. 1971 Apr;46(4):785–788. [PubMed] [Google Scholar]
- Rottman F., Heinlein K. Polynucleotides containing 2'-O-methyladenosine. I. Synthesis by polynucleotide phosphorylase. Biochemistry. 1968 Jul;7(7):2635–2641. doi: 10.1021/bi00847a028. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W., Lander M. R., Pugh W. E., Teich N. Noninfectious AKR mouse embryo cell lines in which each cell has the capacity to be activated to produce infectious murine leukemia virus. Virology. 1971 Dec;46(3):866–876. doi: 10.1016/0042-6822(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Somers K., Kit S. Clonal isolation of murine sarcoma virus (MSV): characterization of virus produced from transformed cells. Virology. 1971 Dec;46(3):774–785. doi: 10.1016/0042-6822(71)90079-1. [DOI] [PubMed] [Google Scholar]
- Stone H. O., Kingsbury D. W. Stimulation of Sendai virion transcriptase by polyanions. J Virol. 1973 Feb;11(2):243–249. doi: 10.1128/jvi.11.2.243-249.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tennant R. W., Richter C. B. Murine leukemia virus: restriction in fused permissive and nonpermissive cells. Science. 1972 Nov 3;178(4060):516–518. doi: 10.1126/science.178.4060.516. [DOI] [PubMed] [Google Scholar]
- Ting R. C., Yang S. S., Gallo R. C. Reverse transcriptase, RNA tumour virus transformation and derivatives of rifamycin SV. Nat New Biol. 1972 Apr 12;236(67):163–166. doi: 10.1038/newbio236163a0. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Kenney F. T. Inhibition of the DNA polymerase of Rauscher leukemia virus by single-stranded polyribonucleotides. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2198–2202. doi: 10.1073/pnas.68.9.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Hanafusa H. Effect of rifampicin and a derivative on cells transformed by Rous sarcoma virus. Cancer Res. 1971 Dec;31(12):2032–2036. [PubMed] [Google Scholar]
- Wu A. M., Ting R. C., Paran M., Gallo R. C. Cordycepin inhibits induction of murine leukovirus production by 5-iodo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3820–3824. doi: 10.1073/pnas.69.12.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]


