Abstract
Purpose.
To calculate age-related and per diopter (D) accommodative changes in crystalline lens and ciliary muscle dimensions in vivo in a single cohort of emmetropic human adults ages 30 to 50 years.
Methods.
The right eyes of 26 emmetropic adults were examined using ultrasonography, phakometry, anterior segment optical coherence tomography, and high resolution magnetic resonance imaging. Accommodation was measured both subjectively and objectively.
Results.
In agreement with previous research, older age was linearly correlated with a thicker lens, steeper anterior lens curvature, shallower anterior chamber, and lower lens equivalent refractive index (all P < 0.01). Age was not related to ciliary muscle ring diameter (CMRD) or lens equatorial diameter (LED). With accommodation, lens thickness increased (+0.064 mm/D, P < 0.001), LED decreased (−0.075 mm/D, P < 0.001), CMRD decreased (−0.105 mm/D, P < 0.001), and the ciliary muscle thickened anteriorly (+0.013 to +0.026 mm/D, P < 0.001) and thinned posteriorly (−0.011 to −0.015, P < 0.01). The changes per diopter of accommodation in LED, CMRD, and ciliary muscle thickness were not related to subject age.
Conclusions.
The per diopter ciliary muscle contraction is age independent, even as total accommodative amplitude declines. Quantifying normal biometric dimensions of the accommodative structures and changes with age and accommodative effort will further the development of new IOLs designed to harness ciliary muscle forces.
In this study, emmetropic subjects from 30 to 50 years old were examined to quantitatively analyze accommodative and aging changes in the crystalline lens and ciliary muscle.
Introduction
Presbyopia may be defined, clinically, as a decline in accommodative ability such that the near reading point is receded beyond a comfortable reading distance.1 Patients generally become symptomatic somewhere in the fourth decade of life.2 The massive “Baby Boomer” population, 2.6 million Americans born between 1946 and 1964, is now presbyopic and is retiring later and remaining active in the workforce longer than previous generations.3 Boomers are more willing than their predecessors to spend money on treatments to forestall the effects of aging.4 This population has driven the demand for better treatment options for presbyopia. Reading glasses, monovision, and multifocal corrections have been the mainstay of presbyopic correction for decades, but they are not without compromise and do not provide the same visual utility as the young, naturally accommodating eye.5 The development of an accommodating IOL that could provide clear vision over a full range of distances presents a challenging opportunity for research and development.
Since 2004 the only U.S. Food and Drug Administration (FDA) approved “accommodative IOL” is the Crystalens (Bausch + Lomb, Rochester, NY). Whereas most research on the Crystalens cites subjective improvements in near vision, the few objective studies suggest that only approximately a diopter (D) of objectively measured accommodative change may be realized.6,7 A number of other accommodating IOL designs are in clinical use outside the United States,8 some of which are currently in FDA clinical trials. Whereas preliminary reports suggest that these new designs could provide greater accommodative function, there is still a limited understanding of the ideal dimensions and placement of an accommodating IOL and of how such implants might best be designed to provide maximum accommodation.
If accommodating IOLs are to succeed in providing functional accommodation, a detailed understanding of the young human accommodative system and of age-related dimensional or functional changes is a fundamental starting point. The first significant understanding of the accommodative system comes from nonhuman primate studies.9–14 Edinger-Westphal and pharmacologically stimulated accommodation in rhesus monkeys demonstrate that the lens is the limiting factor in the accommodative response and that the ciliary muscle retains its ability to contract well beyond the onset of presbyopia.9–11,15–17 Only within the last decade have improvements in noninvasive imaging technology allowed for similar studies in humans. To date, no study has undertaken a per diopter analysis of anatomical changes in both the crystalline lens and ciliary muscle in a single cohort of human subjects. Thus, the purpose of this study was to adapt new ocular imaging methods and analysis techniques to quantify changes in the lens and ciliary muscle in vivo in a cohort of human adults and to relate these changes to age and objectively measured accommodative response.
Methods
Recruitment, Enrollment, and Data Collection
Twenty-six volunteers were enrolled, and all subjects completed both study visits. Fifty-four percent of the subjects (n = 14) were female, and 73% were Caucasian (Caucasian: n = 19, Asian: n = 6, African American: n = 1). All subjects gave informed consent prior to enrollment, and the study was approved by The Ohio State University's Biomedical Sciences Institutional Review Board in accordance with the tenets of the Declaration of Helsinki.
Only emmetropic subjects (defined as −0.50 D to +0.50 D spherical equivalent and <1.25 D of astigmatism) were included in the study because refractive error is related to both ocular dimensions and accommodative response.18–21 Eligible patients were 30 to 50 years of age; they were neither pseudophakic nor aphakic, neither strabismic, amblyopic, nor with a history of vision therapy, neither pregnant nor breastfeeding; they had a best-corrected visual acuity of at least 20/25 in each eye, had less than Grade 1 cataract on the Lens Opacity Classification System scale,22 had no systemic disease that would compromise ocular health (e.g., diabetes), and met 7 Tesla (T) magnetic resonance imaging (MRI) safety restrictions (e.g., no metal implants, tattoos, or pacemaker).
Refractive, accommodative, and ocular biometric data were collected on the right eyes for all subjects. Normal room illumination was used for all measurements. Accommodation was measured using a Grand Seiko WV 500 Auto-Refractor (Grand Seiko, Ltd., Hiroshima, Japan) and a Badal lens track using methods described previously.23 The static accommodative response was measured for stimulus demands of 0, 2, 4, and 6 D. The target consisted of four rows of 20/155 Snellen equivalent letters, and subjects were instructed to “pick a letter on the target and carefully focus on it.”24 Five measures were recorded at each stimulus demand, and the spherical equivalent value was averaged.
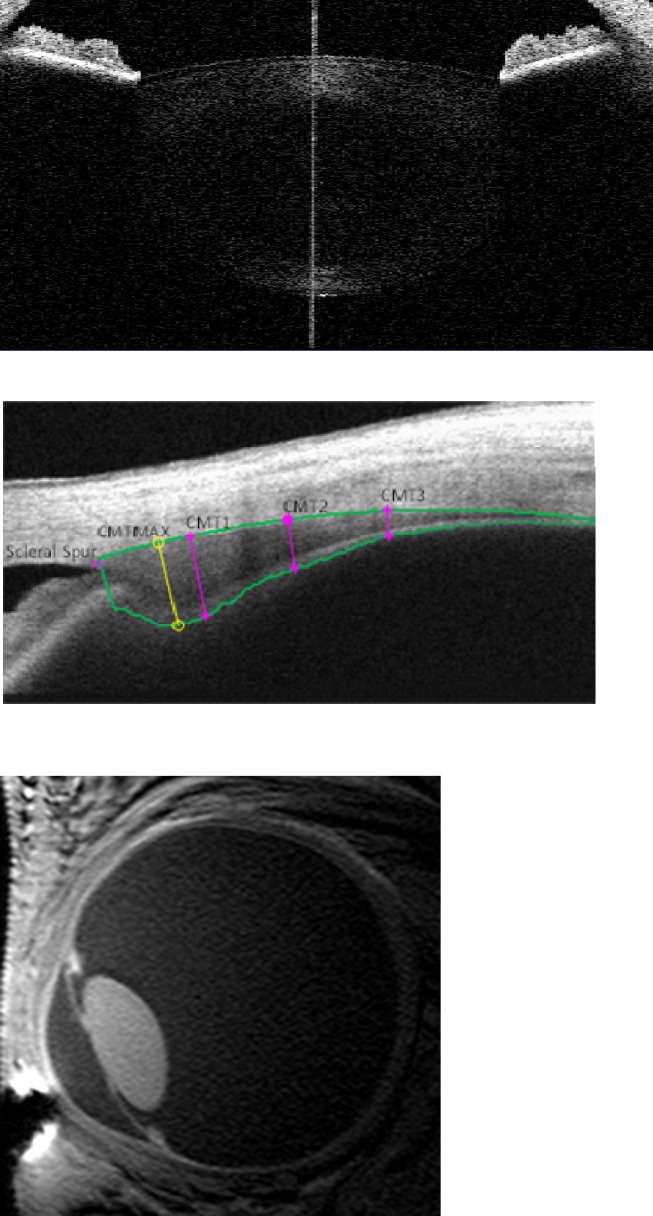
Based on previous work, the “raw image mode” of the Visante anterior-segment optical coherence tomography (OCT; Carl Zeiss Meditec, Dublin, CA) was used to capture images of the crystalline lens, and “enhanced high resolution corneal mode” was used to capture images of the ciliary muscle (Fig. 1).25–27 To image the lens, the subject was seated in front of the OCT with the left eye occluded. The subject was instructed to fixate the Maltese cross target within the instrument, and the examiner aligned the image until the white fixation line originating from a reflection normal to the cornea was visible in the center of the lens.27,28 The internal minus lens system of the OCT was used to increase the accommodative stimulus demand. To image the ciliary muscle, the subject was instructed to look at a similar external Maltese cross target. The distant target was presented on the far wall of the room, and the 2-, 4-, and 6-D targets were presented on an adjustable rod affixed to the instrument. Four lens and ciliary muscle images were recorded at each accommodative stimulus demand and after cycloplegia.
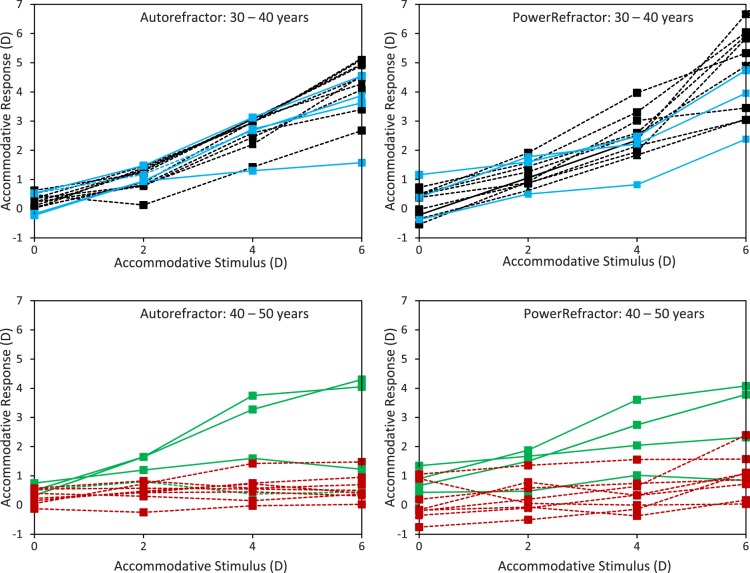
Figure 1. .
OCT images of the crystalline lens (top) and ciliary muscle (middle), and MR imaging of eye (bottom). Ciliary muscle image analysis shows cross-sectional CMT at 1, 2, and 3 mm posterior to the scleral spur as well as maximum thickness.
During OCT imaging, accommodative response was monitored using a PowerRefractor II (PlusOptix Inc., Hillsboro Beach, FL). To allow adequate pupil size during imaging, the pupil was pharmacologically dilated. One drop of 2.5% phenylephrine was instilled in the right eye, and testing resumed 20 minutes after drop instillation. Phenylephrine does not affect static accommodation to a 4-D near target, ciliary muscle dimensions, or the contractile response of the ciliary muscle.29 The PowerRefractor was used in “monocular mode,” a setting which agrees with both autorefraction and subjective refraction in adult subjects.30 The PowerRefractor recorded at 25 Hz, and the data were exported to Microsoft Excel (Microsoft, Redmond, WA) and were filtered for blinks by removing refractive changes >10 D/s.31 The median accommodative response was used for the PowerRefractor data to minimize the effect of potential outliers, as there were more fluctuations when recording continuously than when doing five individual measurements with autorefraction.
To measure the eye under cycloplegia, following accommodative measures, one drop of 1% tropicamide was instilled in the right eye, and testing resumed 20 minutes after drop instillation. Five measures of the subjects' refractive error were recorded with the Grand Seiko WV 500 Auto-Refractor (Grand Seiko, Ltd.).
Purkinje images of the anterior and posterior lens radii of curvature were recorded by video phakometry, and equivalent refractive index was later calculated using data from A-scan ultrasonography and cycloplegic autorefractor measurements.32
A-scan ultrasonography (Model 820; Allergan-Humphrey, San Leandro, CA) was used to measure anterior chamber depth, lens thickness, vitreous chamber depth, and axial length under cycloplegia. As ultrasonography requires corneal contact, a topical anesthetic (0.5% proparacaine) was instilled in the right eye prior to measurement, and five measures were performed and averaged.
Three-dimensional MRIs were acquired on a separate day that was within 1 month of the primary visit. Subjects were scanned in a 7T Philips Achieva (Philips, Cleveland, OH), using a volume head coil (Nova Medical, Inc., Wilmington, MA) for transmission and a custom-built, single loop, 2- by 2.3-cm oval radiofrequency surface coil for reception. Details of the experimental setup have been reported previously.33,34 Briefly, the subject was positioned on the MRI bed in a foam head cushion to limit movement. To decrease motion artifacts from blinks, the imaged (right) eye was taped closed, and the left eye was used to fixate targets. A right-angle mirror was used to allow the subject to fixate the Maltese cross target at the same 0-, 2-, 4-, and 6-D stimulus demands as with the OCT imaging. Inversion-recovery turbo field echo (IR-TFE) sequences were acquired with shot interval and inversion times of 1800 and 900 ms, a repetition time between the TFE read-outs of 6.8 ms, echo time of 2.3 ms, flip angle (α) of 8°, TFE-factor of 260, field of view of 65 × 65 × 8 mm, and a scan time of 34 seconds. Scans were repeated at least eight times for each accommodative demand, depending on subject cooperation and quality of the prior images. Data acquisition for each target took less than 10 minutes. Subjects were given an alarm button to push to pause scanning if they felt a need to move or to relax their accommodation. Images had acquired voxel dimensions of 0.25 × 0.25 × 1.0 mm and were interpolated by the system software (7T Philips Achieva; Philips) to 0.10 × 0.10 × 0.50 mm for analysis (Fig. 1). Due to time and cost considerations, MRI was not performed after cylcoplegia.
Image Analysis
A MATLAB (MathWorks, Natick, MA) program was developed to measure ciliary muscle thickness (CMT) and has been described in detail previously.35 Measurements from the program are reliable, repeatable, and able to detect accommodative changes between and within subjects.25,26,35 Briefly, OCT images were first exported as raw image files. The algorithm segmented the muscle from the relatively brighter ocular tissue and provided cross-sectional CMT measurements at 1, 2, and 3 mm posterior to the scleral spur and at the point of maximum thickness (Fig. 1). A refractive index of 1.41 was used for scleral tissue and 1.38 for ciliary muscle at 1310 nm (the wavelength of the Visante system), and thickness values were converted from pixels to millimeters (128 pixels/mm for high resolution corneal mode).35
OCT crystalline lens images were also analyzed using MATLAB (MathWorks). Lens thickness was measured by manually marking the anterior and posterior lens surfaces parallel to the scanning axis and was corrected for a refractive index of 1.39 at 1310 nm.36 Lens thickness measurements were exported to a Microsoft Excel spreadsheet (Microsoft), and a pixel/mm conversion was applied (64 pixels/mm for raw image mode).35
MR images were analyzed using the Philips DICOM viewer (R2.5 Version 1; provided in the public domain by Philips, http://www.healthcare.philips.com). An examiner masked to subject age and accommodative ability manually scrolled through the three-dimensional dataset and visually identified the eye's central slice. Measurements of the ciliary muscle ring diameter (CMRD), lens thickness, and lens equatorial diameter (LED) were made by the masked reader using straight line calipers. The pixel resolution of the digital calipers was equal to the interpolated voxel size in that plane (0.1 mm). The data were entered into a Microsoft Excel spreadsheet (Microsoft), and measurements from all usable scans were averaged for each biometric measurement (at least six for each parameter for each accommodative stimulus).
Statistical Analysis
Descriptive statistics (mean, median, SD, and range) were calculated for demographic, biometric, and accommodative measures. Linear regression was used to determine which ocular factors were related to age. Ciliary muscle and lens changes with accommodation and age were analyzed using repeated measures regression. In multivariate models, predictors that were not statistically significant were sequentially removed to obtain parsimonious models.
Results
Demographics and Accommodative Function
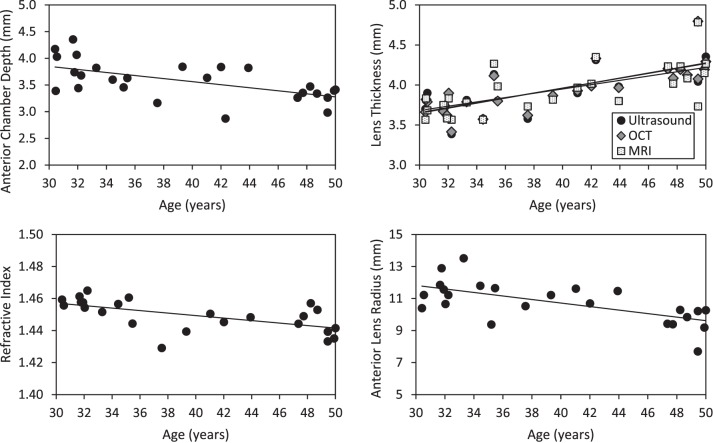
Accommodative responses (Acc Resps) to 0-, 2-, 4-, and 6-D stimulus demands are presented in Figure 2. There was greater variability with the PowerRefractor compared to the Auto-Refractor, but the results between the two instruments were highly correlated (r = 0.89, P < 0.001). Bland-Altman analysis showed no significant bias between the methods (mean difference −0.12 D (Auto-Refractor − PowerRefractor), P = 0.09; 95% limits of agreement −1.56 to 1.30 D), but Auto-Refractor readings were significantly larger (i.e., more negative) for larger accommodative responses (linear regression of difference versus the mean = 0.09 − 0.15 × Acc Resp, P = 0.02). Due to the smaller variability in the Auto-Refractor readings, these were used for comparisons with the biometric measures. In general, accommodative response lagged behind the accommodative demand. For most subjects younger than 40 years, there was a linear increase in accommodative response with increasing demand, whereas subjects older than 40 years showed little change in accommodative response to any target. Linear regression of the maximum accommodative response measured with either the PowerRefractor or Auto-Refractor showed a loss of 0.2 D per year of age (P < 0.001).
Figure 2. .
Accommodative response to 0-, 2-, 4-, and 6-D targets with the Auto-Refractor (left) and PowerRefractor (right). Black dashed lines: 30- < 35-year-old subjects; blue solid lines: 35- < 40-year-old subjects; green solid lines: 40- < 45-year-old subjects; red dashed lines 45- < 50-year-old subjects.
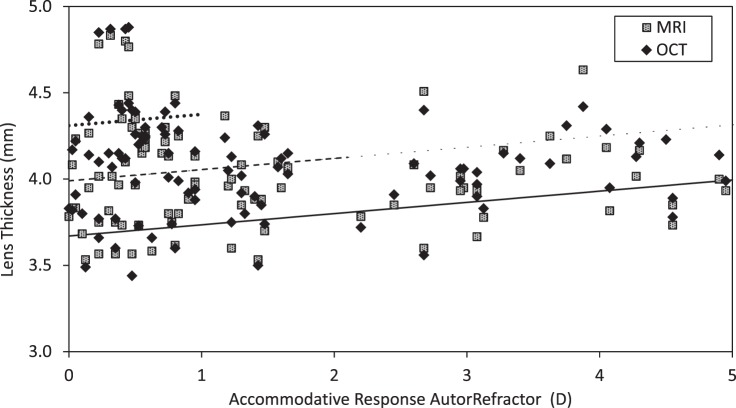
Ocular Dimensions with Age
Baseline biometric data are presented in Table 1. All baseline measurements were conducted under cycloplegia except the MRIs, which are for the distant (0 D) target. The baseline biometric variables were plotted against age to assess potential relationships (i.e., linear, quadratic). Due to the small sample size and lack of obvious nonlinear relationships, linear regression was used. Age was related to thicker lenses, as measured by ultrasonography, OCT, and MRI, steeper anterior lens radius of curvature, shallower anterior chamber depth, and lower equivalent refractive index (Fig. 3). Age was not related to axial length (P = 0.74, 95% confidence intervals [CI]) for slope = −0.044 to 0.032), vitreous chamber depth (P = 0.68, 95% CI = −0.008 to +0.019), posterior lens curvature (P = 0.10, 95% CI = −0.047 to +0.004), CMT at 1, 2, or 3 mm posterior to the scleral spur (CMT1, P = 0.78, 95% CI = −0.007 to +0.005; CMT2, P = 0.71, 95% CI = −0.008 to +0.005; CMT3, P = 0.82, 95% CI = −0.007 to +0.005), maximum CMT (CMTMAX, P = 0.77, 95% CI = −0.007 to +0.005), CMRD (P = 0.74, 95% CI = −0.037 to +0.026), or LED (P = 0.88, 95% CI = −0.014 to +0.012).
Table 1. .
Ocular Dimensions from All Subjects
|
Structure |
Dimension |
Instrument |
Mean ± SD |
Median |
Range |
| Anterior chamber | Depth (mm) | Ultrasound | 3.58 ± 0.36 | 3.54 | 2.87 to 4.35 |
| Vitreous chamber | Depth (mm) | Ultrasound | 16.0 ± 0.7 | 15.9 | 15.1 to 17.6 |
| Axial length | Depth (mm) | Ultrasound | 23.5 ± 0.7 | 23.4 | 22.6 to 24.9 |
| Lens | Thickness (mm) | OCT | 3.95 ± 0.30 | 3.91 | 3.42 to 4.80 |
| MRI | 3.93 ± 0.30 | 3.83 | 3.57 to 4.78 | ||
| Ultrasound | 3.95 ± 0.30 | 3.90 | 3.39 to 4.80 | ||
| Equatorial diameter (mm) | MRI | 9.42 ± 0.24 | 9.39 | 8.97 to 10.03 | |
| Refractive index | Phakometry | 1.45 ± 0.01 | 1.45 | 1.43 to 1.46 | |
| Anterior curvature (mm) | Phakometry | 10.75 ± 1.27 | 10.68 | 7.70 to 13.52 | |
| Posterior curvature (mm) | Phakometry | 7.43 ± 0.46 | 7.44 | 6.82 to 8.46 | |
| Ciliary muscle | Thickness (mm) | ||||
| CMTMAX | OCT | 0.87 ± 0.10 | 0.88 | 0.64 to 1.02 | |
| CMT1 | OCT | 0.80 ± 0.10 | 0.81 | 0.61 to 0.98 | |
| CMT2 | OCT | 0.49 ± 0.11 | 0.51 | 0.19 to 0.72 | |
| CMT3 | OCT | 0.27 ± 0.08 | 0.27 | 0.08 to 0.40 | |
| Ring diameter (mm) | MRI | 11.84 ± 0.55 | 11.85 | 10.71 to 12.90 | |
All measures were conducted under cycloplegia except MRIs, which are reported for the distance target.
Figure 3. .
Statistically significant differences with age. Regression lines fitted to the following equations with age referenced to 30 years and 95% CI for slope in brackets: lens thickness by ultrasound (mm): 3.67 + 0.031 × Age [0.019 to 0.042], P < 0.001; OCT (mm): 3.65 + 0.031 × Age [0.021 to 0.042], P < 0.001; and MRI (mm): 3.68 + 0.027 × Age [0.014 to 0.039], P < 0.001; anterior lens radius of curvature by phakometry (mm): 11.82 − 0.11 × Age [−0.17 to −0.05], P = 0.001; anterior chamber depth by ultrasound (mm): 3.85 − 0.029 × Age [−0.045 to −0.012], P = 0.001; and lens equivalent refractive index (RI) by phakometry: 1.457 − 0.001 × Age [−0.001 to 0.000], P = 0.002.
Ocular Changes with Accommodation
For OCT-measured changes in lens thickness, the interaction between age and accommodative response was not statistically significant (P > 0.27). With the interaction term removed, lens thickness was significantly related to accommodative response and age (Fig. 4). Results were similar for MRI-derived lens thickness. The interaction between age and accommodative response was also not significant (P = 0.30), and the change in lens thickness was significantly related to both age and accommodative response (both P < 0.001) with similar coefficients to those from OCT (Fig. 4).
Figure 4. .
Lens thickness (LT) with accommodation. Black lines fitted to the regression equation for OCT data with age fixed at 30 years (solid line), 40 years (dashed line), and 50 years (dotted line) and 95% CI in brackets: LT by OCT (mm) = 3.67 + 0.065 × Acc Resp + 0.032 × (Age − 30) [Acc Resp = 0.057 to 0.072, Age − 0.020 to 0.043]; PAccResp < 0.001, PAge < 0.001. MRI regression equation is similar and not plotted: LT by MRI (mm) = 3.68 + 0.055 × Acc Resp + 0.028 × (Age − 30) [Acc Resp = 0.039 to 0.071, Age = 0.016 to 0.041], PAccResp < 0.001, PAge < 0.001.
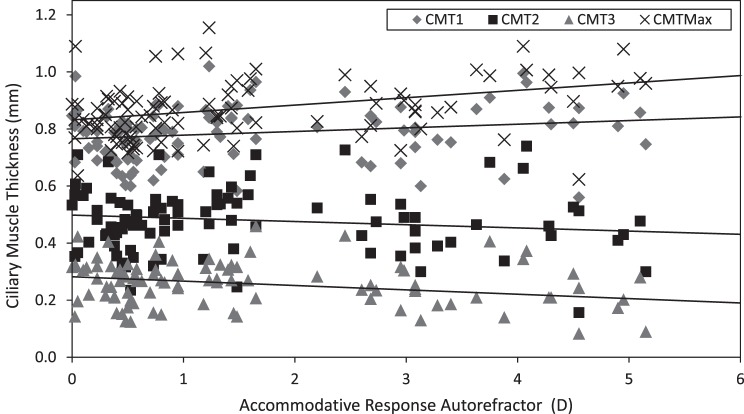
For CMT, neither the interaction between age and accommodation nor age as a main effect were statistically significant for CMTMAX, CMT1, CMT2, or CMT3 (P > 0.42), and age terms were removed from the models. The ciliary muscle thickened anteriorly (CMTMAX and CMT1) and thinned posteriorly (CMT2 and CMT3) with accommodation (Fig. 5).
Figure 5. .
Ciliary muscle thickness with accommodation at points 1, 2, and 3 mm posterior to the scleral spur (CMT1, CMT2, CMT3) and the site of maximum width (CMTMAX). Lines fitted to regression equations with 95% CI in brackets: CMTMAX (mm) = 0.832 + 0.026 × Acc Resp [0.013 to 0.039], P < 0.001; CMT1 (mm) = 0.765 + 0.013 × Acc Resp [0.004 to 0.022], P = 0.005; CMT2 (mm) = 0.498 − 0.011 × Acc Resp [−0.003 to −0.019], P = 0.005; CMT3 (mm) = 0.282 − 0.015 × Acc Resp [−0.009 to −0.022], P < 0.001.
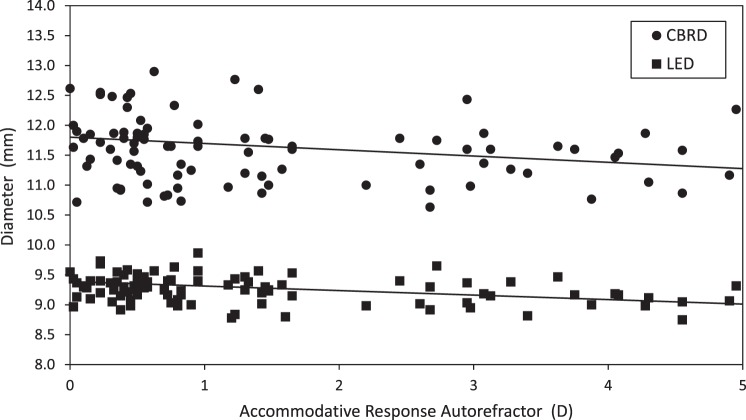
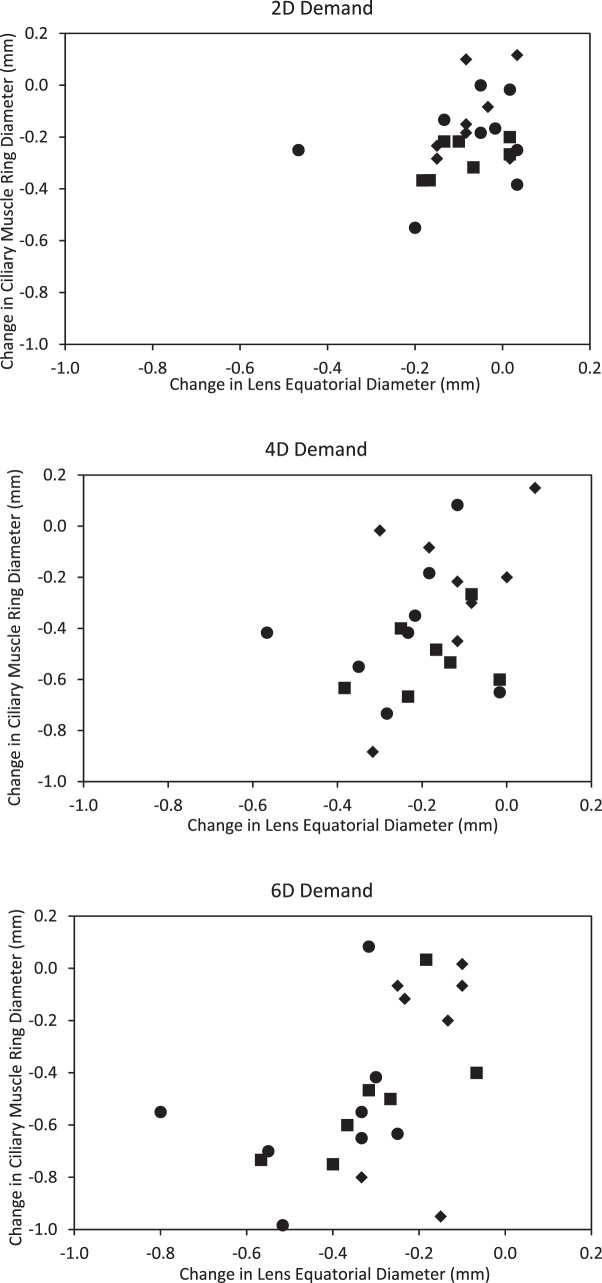
For MRI-measured LED, neither the interaction between accommodation and age, nor age as a main effect, was statistically significant (P > 0.41). Likewise, for CMRD, neither the interaction term nor age as a main effect (P > 0.20) was statistically significant. With accommodative effort, CMRD decreased approximately 100 μm/D and LED decreased 75 μm/D (Fig. 6). To further explore this relationship, change in CMRD versus change in LED was plotted in age groups for each of the three accommodative demands (Fig. 7). As expected, most subjects younger than age 45 years demonstrated commensurate changes in both the lens and ciliary muscle with increasing accommodative demands. At the 6-D demand, two subjects older than 45 years of age showed ciliary muscle change with little lens change, suggestive of a stiffened, presbyopic lens. The remaining subjects older than 45 years showed little to no change in either the lens or ciliary muscle for the 6-D stimulus. This could be because the subjects did not try to focus on an accommodative target they knew they could not clear, or because the ciliary muscle did not contract despite their effort.
Figure 6. .
LED and CBRD with accommodation. Lines fitted to regression equations with 95% CI in brackets: LED (mm) = 9.39 − 0.075 × Acc Resp [−0.099 to −0.053], P < 0.001; CBRD (mm) = 11.80 − 0.105 × Acc Resp [−0.149 to −0.062], P < 0.001.
Figure 7. .
Change in LED with CMRD contraction for 2-D (top), 4-D (middle), and 6-D (bottom) stimuli. Circles: 30- to 35-year-old subjects; squares: 35- to 45-year-old subjects; diamonds: 45- < 50-year-old subjects.
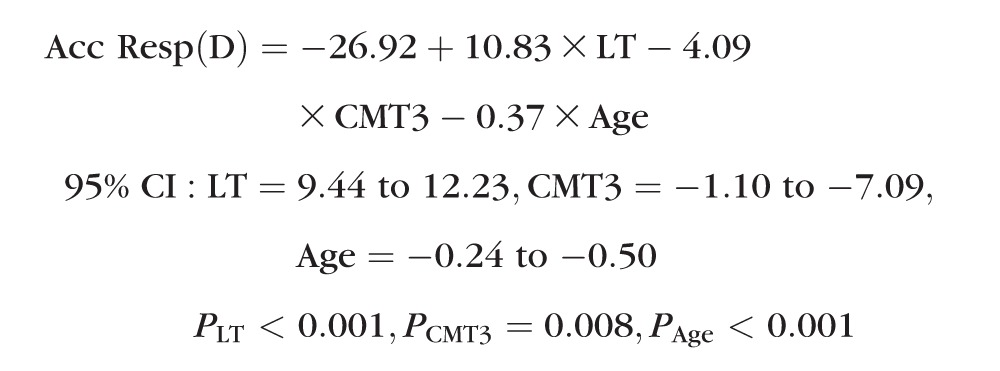
Finally, a multivariate model was used to examine which of the measured factors were most closely related to accommodative response. Using a backwards removal procedure, age, lens thickness (LT), and CMT at 3 mm were retained in the final model:
 |
Discussion
Accommodative Ability
In agreement with previous reports, subjects tended to overaccommodate to a distant target and underaccommodate to near targets (2, 4, and 6 D).37 Anderson and colleagues predicted a maximum monocular accommodative response between 5.66 and 0.46 D for subjects aged 30 to 50 years.38 This range broadly agrees with our maximum amplitudes of 0.03 to 5.15 D, measured with the Grand Seiko WV 500 Auto-Refractor (Grand Seiko, Ltd.), and 0.17 to 6.67 D with the PowerRefractor.
Ocular Dimensions with Age
Table 2 provides an overview of changes in ocular dimensions with age. This study found that vitreous chamber depth and axial length do not change with age and that anterior chamber depth decreases with age, in accordance with prior studies.39,40 Lenticular changes with age have been well established by multiple independent studies using various imaging techniques. Despite differences in resolution and assumptions of refractive index and speed of sound, ultrasonography, OCT, and MRI measurements were in good agreement with each other and with previous findings in both animal models and humans.15,27,40–43 Approximately 90% of the decrease in anterior chamber depth is accounted for by lens growth, suggesting that the posterior lens surface remains relatively stable in position with age. Significant anterior lens steepening was found with age, consistent with previous reports using phakometry, MRI, and Scheimpflug photography.40,44,45 Whereas some early studies suggest an increase in posterior lens curvature with age, more recent studies agree that the posterior lens is unaltered with age.40,44,46,47 There was a significant decrease in equivalent refractive index with age. The change reported here was smaller than that reported previously, likely due to the narrow age range of this study sample.40,45
Table 2. .
Age-Related Change in Biometric Values for the Current Study and Previous In Vivo Studies in Human Adults
|
Structure |
Difference per Year of Life |
||
|
Current Study Findings and Method |
Previous Studies Findings and Method |
||
| Axial length (mm) | No change (US) | No change (US)39,40 | |
| Vitreous chamber depth (mm) | No change (US) | No change (US)39,40 | |
| Anterior chamber depth (mm) | −0.031 (US) | −0.011 to −0.029 (US or MRI)39,40,44 | |
| Crystalline lens | |||
| Thickness (mm) | +0.031 (US) | +0.018 to +0.029 (US, OCT, or MRI)27,39–41,44,47 | |
| +0.031 (OCT) | |||
| +0.027 (MRI) | |||
| Equatorial diameter (mm) | No change (MRI) | +0.006 to +0.007 (MRI)40,65, No change (MRI)41,51 | |
| Anterior curvature (mm) | −0.11 (ph) | −0.04 to −0.08 (ph)40,44 | |
| Posterior curvature (mm) | No change (ph) | No change (ph or MRI)39,40 | |
| Equivalent refractive index | −0.001 (ph) | −0.004 (ph or MRI)40,45 | |
| Ciliary muscle | |||
| Thickness (mm) | No change (OCT) | No change to −0.003 (OCT)54 | |
| Ring diameter (mm) | No change (MRI) | −0.015 to −0.025 (MRI)47,51 | |
US, ultrasound. ph indicates phakometry, Scheimpflug photography, or similar optical method. “No change” indicates no statistically significant difference across ages (all cross-sectional studies).
We did not find a statistically significant change in LED with age. Removal of the young lens from the eye will cause it to assume its most accommodated shape and decrease the equatorial diameter; thus, early in vitro studies that reported an increase in diameter with age were not necessarily showing an age-related increase.48–50 An in vivo study of rhesus monkeys showed no change in LED with age, but the few in vivo human studies were equivocal.15,41,47,51 The original MRI studies by Strenk and coworkers reported no change, while a more recent study comparing 18- to 29-year-old subjects to 60- to 70-year-old subjects found a slight increase of approximately 0.01 mm/y.40,41,47,51 The 95% CI estimated with our sample suggested that an increase of approximately 0.01 mm/y is plausible.
There are relatively few reports that quantify ciliary muscle changes with age. In monkeys, prior studies show a decrease in the circumlental space (as measured by the distance between the lens edge and tip of the ciliary muscle) with age.11 Strenk and colleagues were the first to use MRI to study the CMRD in humans in vivo and found an age-related decrease of approximately 0.025 mm/y in a population of 48 subjects of unknown refractive error, aged 22 to 91 years.51,52 Kasthurirangan et al. confirmed these findings in two groups of 15 young (age range 19–29 years) and 15 old (age range 60–70 years) subjects but suggested that the difference may be only half as large (0.015 mm/y).47 The current study found no statistically significant change in CMRD, perhaps due to the relatively small sample size and limited age range; however, our 95% CI does include the possibility of a decrease as large as 0.037 mm/y.
The current study also found no significant difference in ciliary muscle cross-sectional thickness with age. Sheppard and Davies used a similar technique to acquire images but estimated CMT using calipers and applied a refractive index post hoc and oblique to the scan acquisition line. Potential inaccuracies introduced by these methods have been described.53 Nevertheless, in subsets of their full dataset (due to technical failures with some data), they reported a small but statistically significant decrease in cross-sectional thickness with age.54 In 45 emmetropic subjects, they found a decrease of 0.002 mm/y at a point 2 mm posterior to the scleral spur; however, this finding was not evident in 34 myopic subjects.54 They also found a decrease of 0.003 mm/y in maximum ciliary muscle width in a subset of 37 subjects. In vitro studies show an alteration in the relative proportion of muscle fibers, an increase in connective tissue beginning in the third decade, and atrophy of the muscle beyond the sixth decade.55–57 Based on both our parameter estimates (−0.008 to +0.005 mm/y) and those of Sheppard and Davies,58 it seems that any potential age-related changes in CMT are likely small and of little clinical significance when compared to the overall thickness (0.3 to 0.8 mm) and changes in thickness with accommodative effort (0.01 to 0.03 mm/D).
The Lens and Ciliary Muscle with Accommodation
Table 3 compares the accommodative changes from the current study to those of previous human studies. The change in lens thickness with accommodation has been well documented by multiple independent studies, and the parameters reported in the current study fall well within previously reported ranges.27,41,59–62 Nonhuman primate studies have demonstrated an accommodative decrease in LED of approximately 0.055 mm/D.15,63 Only in the last decade have human studies reported in vivo changes in LED in enough people to accurately quantify this parameter in humans.41,47,51,60 Neither the Kasthurirangan and Jones studies of the same dataset41,47 nor Strenk's study51 measure accommodative response. They assumed subjects accommodated accurately to stimulus demands as high as 7 to 8 D. Assuming accurate accommodation and ignoring accommodative lag would underestimate the true coefficient. The present study correlated an objectively measured accommodative response to biometric changes to determine a decrease in equatorial diameter of 0.075 mm/D.
Table 3. .
Accommodative Change in Biometric Values for the Current Study Compared to Other In Vivo Studies in Human Adults
|
Structure |
Change per Diopter of Accommodation |
||
|
Current Study Findings and Method |
Previous Studies Findings and Method* |
||
| Crystalline lens | Thickness (mm) | +0.064 (OCT) | +0.042 to +0.080 (OCT, US, ph, or MRI)27,41,47,59–61,65–67 |
| +0.065 (MRI) | |||
| Equatorial diameter (mm) | −0.075 (MRI) | −0.046 to −0.090 (MRI)41,47,51,60 | |
| Ciliary muscle | Thickness (mm) | +0.026 CMTMAX (OCT) | |
| +0.013 CMT1 (OCT) | +0.071 CM25 (OCT)58 | ||
| −0.011 CMT2 (OCT) | 0 to −0.007 CM2 (OCT)58 | ||
| −0.015 CMT3 (OCT) | |||
| Ring diameter (mm) | −0.105 (MRI) | −0.063 to −0.088 (MRI)47,52 | |
ph indicates phakometry, Scheimpflug photography, or similar optical method. CM25 is located at a point 25% of the estimated muscle length and roughly corresponds to the CMT1 position. CM2 is equivalent to CMT2.
References 41, 47, 51, 52, and 64 measured accommodative change with stimulus demand but did not measure accommodative response. The values provided in the table are calculated as responses per diopter of accommodative stimulus demand. The values from the current study are calculated from accommodative response.
To date, only two groups have measured ciliary muscle ring contraction with accommodation in the human eye. Strenk et al. reported an average decrease of 0.64 mm to an 8-D accommodative stimulus in 40 subjects aged 22 to 91 years.52 Kasthurirangan et al. measured CMRD in 15 subjects 19 to 29 years old and found a mean decrease of 0.44 mm with maximum accommodation (which ranged from a 4.8- to 6.9-D stimulus).47 Assuming that subjects made an effort to focus accurately on the target, regardless of age, these studies suggest a range of approximately 0.063 to 0.088 mm/D. These values are smaller than the 0.105 mm/D found in the current study; but, as above, it is well known that subjects generally underaccommodate to near demands, so previous calculations assuming a full accommodative response underestimate the true dioptric change.64
Quantification of cross-sectional ciliary muscle accommodative changes in humans in vivo was first reported by Sheppard and Davies.58 Some of their measurement locations were based on an estimated muscle length; so, while the results may not be quantitatively comparable, they are qualitatively similar. In this study, an accommodative change per diopter was quantified and showed thickening in two regions of the anterior muscle (CMT1 and CMTMAX) and thinning in two regions of the posterior muscle (CMT2 and CMT3).
Accommodative changes in both CMRD and cross-sectional thickness were linear per diopter of accommodative response and did not vary with age. This finding supports the Hess-Gullstrand theory of presbyopia, which suggests that the amount of ciliary muscle contraction per diopter of accommodative response is constant throughout life and that there is an increasing latent amount of ciliary muscle force with age that does not result in accommodative output due to the nonmalleable presbyopic lens.
Conclusions
The results of this study, in which a variety of ocular imaging methods were employed in conjunction with objective measurements of accommodation, supported previous human and animal studies and demonstrated consistent age-related changes in crystalline lens size and shape. More importantly, this study provided quantitative, per diopter of accommodation measurements of lens and ciliary muscle changes in humans in vivo. This and future research in myopes and hyperopes can help create a database of normative ocular changes that may prove useful for the development of new accommodating IOL designs.
Footnotes
Supported by National Institutes of Health National Eye Institute K23-EY019097 (KR), U10-EY08893 (KZ), Alfred P. Sloan Fellowship (CYK), National Science Foundation grant DMS1216742 (C-YK), and American Academy of Optometry Bausch + Lomb and Presidents Circle Ezell Fellowships (KR).
Disclosure: K. Richdale, Bausch & Lomb (C); L.T. Sinnott, None; M.A. Bullimore, Carl Zeiss Meditec (C), Alcon (C), Elenza (C); P.A. Wassenaar, None; P. Schmalbrock, None; C.-Y. Kao, None; S. Patz, Becton Dickinson & Company (I), Qiagen NV (I); D.O. Mutti, None; A. Glasser, None; K. Zadnik, None
References
- 1. Atchison DA. Accommodation and presbyopia. Ophthalmic Physiol Opt. 1995; 15: 255–272 [PubMed] [Google Scholar]
- 2. Murphy SL, Xu JQ, Kochanek KD. Deaths: preliminary data for 2010. Natl Vital Stat Rep. 2012; 60: 30 [Google Scholar]
- 3. National Institute on Aging Growing Older in America: The Health and Retirement Study. National Institutes of Health, U.S. Department of Health and Human Services. 2007. Available at: http://www.nia.nih.gov/health/publication/growing-older-america-health-and-retirement-study. Accessed January 17, 2013 [Google Scholar]
- 4. Moschis G, Lee E, Mathur A, Strautman J. The Maturing Marketplace: Buying Habits of Baby Boomers and Their Parents. Westport, CT: Quorum Books; 2000. [Google Scholar]
- 5. Richdale K, Mitchell GL, Zadnik K. Comparison of multifocal and monovision soft contact lens corrections in patients with low-astigmatic presbyopia. Optom Vis Sci. 2006; 83: 266–273 [DOI] [PubMed] [Google Scholar]
- 6. Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction: past, present, and future. Curr Opin Ophthalmol. 2012; 23: 40–46 [DOI] [PubMed] [Google Scholar]
- 7. Sheppard AL, Bashir A, Wolffsohn JS, Davies LN. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clin Exp Optom. 2010; 93: 441–452 [DOI] [PubMed] [Google Scholar]
- 8. Bohorquez V, Alarcon R. Long-term reading performance in patients with bilateral dual-optic accommodating intraocular lenses. J Cataract Refract Surg. 2010; 36: 1880–1886 [DOI] [PubMed] [Google Scholar]
- 9. Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999; 106: 863–872 [DOI] [PubMed] [Google Scholar]
- 10. Croft MA, Glasser A, Heatley G, et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006; 47: 1076–1086 [DOI] [PubMed] [Google Scholar]
- 11. Croft MA, Glasser A, Heatley G, et al. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006; 47: 1087–1095 [DOI] [PubMed] [Google Scholar]
- 12. Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998; 275: R1885–R1897 [DOI] [PubMed] [Google Scholar]
- 13. Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982; 23: 23–31 [PubMed] [Google Scholar]
- 14. Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular ageing. Eye. 1987; 1 (Pt 2); 222–230 [DOI] [PubMed] [Google Scholar]
- 15. Wendt M, Croft MA, McDonald J, Kaufman PL, Glasser A. Lens diameter and thickness as a function of age and pharmacologically stimulated accommodation in rhesus monkeys. Exp Eye Res. 2008; 86: 746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croft MA, McDonald JP, Nadkarni NV, Lin TL, Kaufman PL. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exper Eye Res. 2009; 89: 824–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ostrin LA, Glasser A. Edinger-Westphal and pharmacologically stimulated accommodative refractive changes and lens and ciliary process movements in rhesus monkeys. Exper Eye Res. 2007; 84: 302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zadnik K, Mutti DO, Fusaro RE, Adams AJ. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci. 1995; 36: 1581–1587 [PubMed] [Google Scholar]
- 19. Oliveira C, Tello C, Liebmann JM, Ritch R. Ciliary body thickness increases with increasing axial myopia. Am J Ophthalmol. 2005; 140: 324–325 [DOI] [PubMed] [Google Scholar]
- 20. Garner LF, Stewart AW, Owens H, Kinnear RF, Frith MJ. The Nepal Longitudinal Study: biometric characteristics of developing eyes. Optom Vis Sci. 2006; 83: 274–280 [DOI] [PubMed] [Google Scholar]
- 21. Bailey MD, Sinnott LT, Mutti DO. Ciliary body thickness and refractive error in children. Invest Ophthalmol Vis Sci. 2008; 49: 4353–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chylack LT Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. Arch Ophthalmol. 1993; 111: 831–836 [DOI] [PubMed] [Google Scholar]
- 23. Mutti DO, Mitchell GL, Hayes JR, et al. Accommodative lag before and after the onset of myopia. Invest Ophthal Vis Sci. 2006; 47: 837–846 [DOI] [PubMed] [Google Scholar]
- 24. Stark LR, Atchison DA. Subject instructions and methods of target presentation in accommodation research. Invest Ophthalmol Vis Sci. 1994; 35: 528–537 [PubMed] [Google Scholar]
- 25. Lossing LA, Richdale K, Sinnott LT, Bailey MD. Changes in ciliary body thickness with accommodation [ E-abstract 95815] Optom Vis Sci. 2009; 86 [Google Scholar]
- 26. Lossing LA, Sinnott LT, Kao C-Y, Richdale K, Bailey MD. Measuring changes in ciliary muscle thickness with accommodation in young adults. Optom Vis Sci. 2012; 89: 719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richdale K, Bullimore MA, Zadnik K. Lens thickness with age and accommodation by optical coherence tomography. Ophthalmic Physiol Opt. 2008; 28: 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang D, Li Y, Radhakrishnan S. Optical coherence tomography of the anterior segment of the eye. Ophthalmol Clin North Am. 2004; 17: 1–6 [DOI] [PubMed] [Google Scholar]
- 29. Richdale K, Bailley MD, Sinnott LT, Kao CY, Zadnik K, Bullimore M. The effect of phenylephrine on the ciliary muscle and accommodation. Optom Vis Sci. 2012; 89: 1507–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen PM, Radhakrishnan H, O'Leary DJ. Repeatability and validity of the PowerRefractor and the Nidek AR600-A in an adult population with healthy eyes. Optom Vis Sci. 2003; 80: 245–251 [DOI] [PubMed] [Google Scholar]
- 31. Harb E, Thorn F, Troilo D. Characteristics of accommodative behavior during sustained reading in emmetropes and myopes. Vision Res. 2006; 46: 2581–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mutti DO, Zadnik K, Adams AJ. A video technique for phakometry of the human crystalline lens. Invest Ophthalmol Vis Sci. 1992; 33: 1771–1782 [PubMed] [Google Scholar]
- 33. Richdale K, Schmalbrock P, Wassenaar P, Frederick E, Patz S, Bullimore M. Ocular imaging with ultra-high field MRI [E-abstract 80039]. Optom Vis Sci. 2008; 82 [Google Scholar]
- 34. Richdale K, Wassenaar P, Teal Bluestein K, et al. 7 Tesla MR imaging of the human eye in vivo. J Magn Reson Imaging. 2009; 30: 924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kao CY, Richdale K, Sinnott LT, Grillott LE, Bailey MD. Semiautomatic extraction algorithm for images of the ciliary muscle. Optom Vis Sci. 2011; 88: 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atchison DA, Smith G. Chromatic dispersions of the ocular media of human eyes. J Opt Soc Am A Opt Image Sci Vis. 2005; 22: 29–37 [DOI] [PubMed] [Google Scholar]
- 37. Charman WN. The eye in focus: accommodation and presbyopia. Clin Exp Optom. 2008; 91: 207–225 [DOI] [PubMed] [Google Scholar]
- 38. Anderson HA, Hentz G, Glasser A, Stuebing KK, Manny RE. Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: a study of objectively measured accommodative amplitudes from age 3. Invest Ophthalmol Vis Sci. 2008; 49: 2919–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989; 29: 1685–1692 [DOI] [PubMed] [Google Scholar]
- 40. Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann PG. Age-related changes in optical and biometric characteristics of emmetropic eyes. J Vis. 2008; 8: 29.1–29.20 [DOI] [PubMed] [Google Scholar]
- 41. Jones CE, Atchison DA, Pope JM. Changes in lens dimensions and refractive index with age and accommodation. Optom Vis Sci. 2007; 84: 990–995 [DOI] [PubMed] [Google Scholar]
- 42. Dubbelman M, van der Heijde GL, Weeber HA. The thickness of the aging human lens obtained from corrected Scheimpflug images. Optom Vis Sci. 2001; 78: 411–416 [DOI] [PubMed] [Google Scholar]
- 43. Bullimore M, Mitchell GL, Jones L, Reuter KS. Factors affecting the accommodative response in an adult myopic population [ E-abstract 070082] Optom Vis Sci. 2007; 84 [Google Scholar]
- 44. Koretz JE, Strenk SA, Strenk LM, Semmlow JL. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment: a comparative study. J Opt Soc Am A Opt Image Sci Vis. 2004; 21: 346–354 [DOI] [PubMed] [Google Scholar]
- 45. Dubbelman M, Van der Heijde GL. The shape of the aging human lens: curvature, equivalent refractive index and the lens paradox. Vision Res. 2001; 41: 1867–1877 [DOI] [PubMed] [Google Scholar]
- 46. Kirschkamp T, Dunne M, Barry JC. Phakometric measurement of ocular surface radii of curvature, axial separations and alignment in relaxed and accommodated human eyes. Ophthalmic Physiol Opt. 2004; 24: 65–73 [DOI] [PubMed] [Google Scholar]
- 47. Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. MRI study of the changes in crystalline lens shape with accommodation and aging in humans [ published online ahead of print March 25, 2011]. J Vis. doi:10.1167/11.3.19 [DOI] [PubMed] [Google Scholar]
- 48. Rosen AM, Denham DB, Fernandez V, et al. In vitro dimensions and curvatures of human lenses. Vision Res. 2006; 46: 1002–1009 [DOI] [PubMed] [Google Scholar]
- 49. Glasser A, Campbell MC. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999; 39: 1991–2015 [DOI] [PubMed] [Google Scholar]
- 50. Smith P. Diseases of the crystalline lens and capsule: on the growth of the crystalline lens. Trans Ophthalmol Soc UK. 1883; 3: 79–99 [Google Scholar]
- 51. Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci. 1999; 40: 1162–1169 [PubMed] [Google Scholar]
- 52. Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg. 2006; 32: 1792–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bailey MD. How should we measure the ciliary muscle? Invest Ophthalmol Vis Sci. 2011; 52: 1817–1818 [DOI] [PubMed] [Google Scholar]
- 54. Sheppard AL, Davies LN. The effect of ageing on in vivo human ciliary muscle morphology and contractility. Invest Ophthalmol Vis Sci. 2011; 52: 1809–1816 [DOI] [PubMed] [Google Scholar]
- 55. Tamm E, Croft MA, Jungkunz W, Lutjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol. 1992; 110: 871–876 [DOI] [PubMed] [Google Scholar]
- 56. Tamm ER, Lutjen-Drecoll E. Ciliary body. Microsc Res Tech. 1996; 33: 390–439 [DOI] [PubMed] [Google Scholar]
- 57. Pardue MT, Sivak JG. Age-related changes in human ciliary muscle. Optom Vis Sci. 2000; 77: 204–210 [DOI] [PubMed] [Google Scholar]
- 58. Sheppard AL, Davies LN. In vivo analysis of ciliary muscle morphologic changes with accommodation and axial ametropia. Invest Ophthalmol Vis Sci. 2010; 51: 6882–6889 [DOI] [PubMed] [Google Scholar]
- 59. Ostrin L, Kasthurirangan S, Win-Hall D, Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci. 2006; 83: 657–665 [DOI] [PubMed] [Google Scholar]
- 60. Sheppard AL, Evans CJ, Singh KD, Wolffsohn JS, Dunne MCM, Davies LN. Three-dimensional magnetic resonance imaging of the phakic crystalline lens during accommodation. Invest Ophthalmol Vis Sci. 2011; 52: 3689–3697 [DOI] [PubMed] [Google Scholar]
- 61. Bolz M, Prinz A, Drexler W, Findl O. Linear relationship of refractive and biometric lenticular changes during accommodation in emmetropic and myopic eyes. Br J Ophthalmol. 2007; 91: 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dubbelman M, Van der Heijde GL, Weeber HA. Change in shape of the aging human crystalline lens with accommodation. Vision Res. 2005; 45: 117–132 [DOI] [PubMed] [Google Scholar]
- 63. Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest Ophthalmol Vis Sci. 2006; 47: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Anderson HA, Glasser A, Manny RE, Stuebing KK. Age-related changes in accommodative dynamics from preschool to adulthood. Invest Ophthalmol Vis Sci. 51: 614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci. 2008; 49: 2531–2540 [DOI] [PubMed] [Google Scholar]
- 66. Dubbelman M, Van der Heijde GL, Weeber HA, Vrensen GF. Changes in the internal structure of the human crystalline lens with age and accommodation. Vision Res. 2003; 43: 2363–2375 [DOI] [PubMed] [Google Scholar]
- 67. Garner LF, Yap MK. Changes in ocular dimensions and refraction with accommodation. Ophthalmic Physiol Opt. 1997; 17: 12–17 [PubMed] [Google Scholar]