Abstract
Significance: Oxidative stress is involved in the pathogenesis of heart failure but clinical antioxidant trials have been unsuccessful. This may be because effects of reactive oxygen species (ROS) depend upon their source, location, and concentration. Nicotinamide adenine dinucleotide phosphate oxidase (Nox) proteins generate ROS in a highly regulated fashion and modulate several components of the heart failure phenotype. Recent Advances: Two Nox isoforms, Nox2 and Nox4, are expressed in the heart. Studies using gene-modified mice deficient in Nox2 activity indicate that Nox2 activation contributes to angiotensin II–induced cardiomyocyte hypertrophy, atrial fibrillation, and the development of interstitial fibrosis but may also positively modulate physiological excitation-contraction coupling. Nox2 contributes to myocyte death under stress situations and plays important roles in postmyocardial infarction remodeling, in part by modulating matrix metalloprotease activity. In contrast to Nox2, Nox4 is constitutively active at a low level and induces protective effects in the heart under chronic stress, for example, by maintaining myocardial capillary density. However, high levels of Nox4 could have detrimental effects. Critical Issues: The effects of Nox proteins during the development of heart failure likely depend upon the isoform, activation level, and cellular distribution, and may include beneficial as well as detrimental effects. More needs to be learnt about the precise regulation of abundance and biochemical activity of these proteins in the heart as well as the downstream signaling pathways that they regulate. Future Directions: The development of specific approaches to target individual Nox isoforms and/or specific cell types may be important for the achievement of therapeutic efficacy in heart failure. Antioxid. Redox Signal. 18, 1024–1041.
Introduction
Oxidative stress is implicated in the pathophysiology of chronic heart failure (CHF) and conditions, such as hypertension and myocardial infarction (MI), that predispose to CHF, as evidenced by a correlation between oxidative stress markers and CHF in human and animal studies (95) and by numerous experimental studies that provide direct molecular evidence for an etiological role of reactive oxygen species (ROS) (8). Clinical trials of antioxidant vitamins, however, have been singularly unsuccessful (35, 176, 192), suggesting that the relationship between oxidative stress and CHF or its antecedent conditions is more complex. Indeed, recent studies suggest that the effects of ROS may be highly dependent upon their source, location, local concentration, and perhaps even nature of the ROS species that is generated (193). In this regard, there has been significant focus on the potential role of ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Noxs) family enzymes in the pathogenesis of CHF, particularly, since these enzymes appear to be especially important in redox signaling (6, 17). Here, we review the regulation of Nox proteins and the evidence supporting their involvement in modulating different components of the phenotype of the failing heart.
NADPH Oxidases
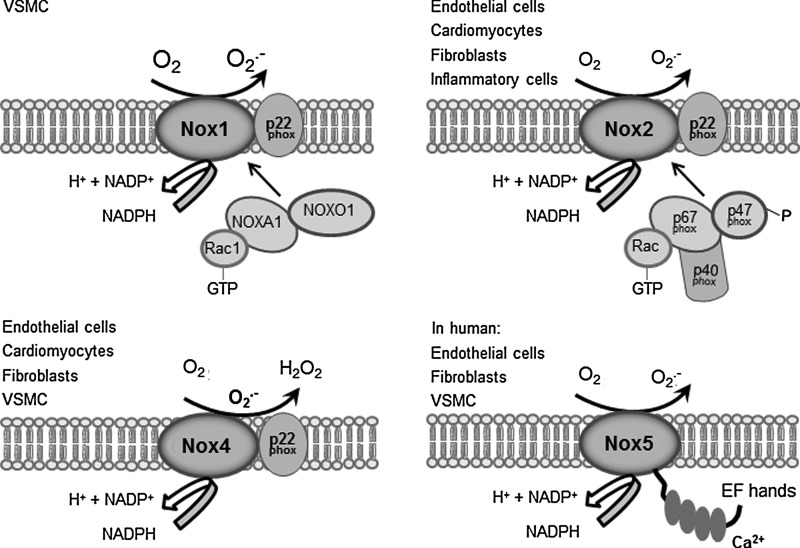
Several potential sources of ROS are present in the cell, including mitochondrial respiratory chain enzymes, xanthine oxidases (XOs), lipoxygenases, myeloperoxidases, uncoupled nitric oxide synthases (NOSs), and Nox proteins (68). Noxs are multi-subunit transmembrane enzymes that utilize NADPH as an electron donor to reduce oxygen to superoxide anion (O2−) and hydrogen peroxide (H2O2). They were initially discovered in phagocytes several decades ago, with the characterization of the Nox2 isoform (also referred to as gp91phox) (117). More recently, 6 other family members each encoded by separate genes have been identified, namely, Nox1, Nox3, Nox4, Nox5, dual oxidase 1 (Duox1), and Duox2 (Fig. 1) (5, 23, 65). These Nox proteins show 21%–59% identity to Nox2, with Nox3 being the most similar and Nox5 the most divergent. Nox5 contains EF-hand calcium-binding motifs not found in Nox1–4 and is absent in rodents. Duox1 and Duox2 show 53% and 47% homology, respectively, to Nox2 within their C-terminal region, but they also contain two N-terminal EF-hand calcium-binding motifs and a peroxidase-like domain (65).
FIG. 1.
Schematic representation of NADPH oxidase 1 (Nox1), Nox2, Nox4, and Nox5 and their accessory subunits. Nox5 is absent in rodents and does not require p22phox, unlike the other isoforms. The cellular distribution of each isoform and the reactive oxygen species (ROS) that are generated are also shown.
Although Nox2 was discovered as a central regulator of microbicidal activity in neutrophils, recent evidence uncovers an important role for Nox2 and other Nox proteins in the cardiovascular system. Noxs regulate a variety of physiological processes, including endothelial cell (EC) migration, angiogenesis, vascular tone, smooth muscle growth, and inflammatory responses, where the tightly regulated generation of ROS is needed to mediate these functions (31). They have also been implicated in pathological processes, such as hypertension, atherosclerosis, restenosis, diabetic vascular disease, cardiac hypertrophy, fibrosis, and heart failure (23, 31, 183). Under these pathological stresses, the quantity of ROS generated by Noxs may increase several fold upon specific stimulation, in turn altering the redox status and the function of macromolecules within the cell.
Cellular distribution of Noxs in the heart
Nox1, Nox2, Nox4, and Nox5 are expressed in the cardiovascular system (with Nox5 only found in higher mammals). These isoforms are found in specific cell types within the heart, including cardiomyocytes, cardiac fibroblasts, vascular smooth muscle cells (VSMCs), ECs, and resident and infiltrating leukocytes.
Nox2 and Nox4 are the predominant isoforms expressed in cardiomyocytes and are involved in regulating several processes as discussed in later sections. The subcellular localization of the two isoforms in cardiomyocytes is distinct, as in other cell types. Activated Nox2 is predominantly expressed at the plasma membrane (88), whereas Nox4 is found intracellularly although the precise location remains controversial. It has been reported to be in a perinuclear location associated with the endoplasmic reticulum (ER) or mitochondria as well as intranuclear (4, 214). The role of Nox4 in cardiomyocytes is still not clear, as discussed later, and it has been suggested that it is only expressed at significant levels in response to cardiac stresses, such as hypoxia, myocardial ischemia, and pressure overload (113, 214).
Freshly cultured cardiac fibroblasts express low levels of Nox4, Nox2, and Nox1, but Nox2 is higher in the adventitial layer of human coronary arteries (184). The latter study showed that Nox5 and Nox4 mRNA are abundantly expressed in cultured human cardiac fibroblasts, whereas Nox1 and Nox2 are barely detectable (39). In general, adventitial fibroblast Nox activity is increased in various vascular diseases, including diabetes mellitus, hypertension, atherosclerosis, and vascular injury (38), but the in vivo contribution of Nox in cardiac fibroblasts to heart function and failure is still unclear.
The predominant Nox isoforms in ECs are also Nox2 and Nox4 (3). Activated Nox2 is again mainly found at the plasma membrane and at perinuclear membranes (126) while Nox4 is localized to the ER (9, 27, 32, 206) and nucleus (114, 156). In contrast, VSMCs express Nox1 and Nox4 (119). While Nox1 is localized to caveolae at the cell surface, Nox4 is found in focal adhesions and inside the nucleus (89). This led to the hypothesis that these two enzymes play different roles within the cell. Nox1 is highly expressed in proliferating VSMCs and its mRNA as well as enzyme activity are further upregulated by agonists, such as angiotensin II (AngII) and platelet-derived growth factor (119). On the contrary, Nox4 is downregulated by the same agonists and has been suggested to be involved in maintaining the quiescent phenotype (118, 119). Another major function of Noxs in VSMCs is the regulation of expression and activity of matrix metalloproteinases (MMPs), thus facilitating vessel remodeling (76).
Leukocytes are the major players of the innate immune response. An essential component of this process is the ability of these cells to generate ROS at a high concentration, crucial for the clearance of invading pathogens. Increased activation of Nox2 in granulocytes has been implicated in a large number of inflammatory pathologies (45). Inflammation is also triggered during the response of the heart to pathological stresses, and Nox2-derived ROS generation within inflammatory cells may be an important contributor to cardiac remodeling (see later sections).
Biochemical regulation of Nox enzymes
The activity of Nox enzyme complexes is regulated through different mechanisms depending on the isoform. Here, we discuss the regulation of Nox2 and Nox4, the main isoforms expressed in cardiomyocytes. Although the regulation of Nox1 is not considered, it is broadly similar to that of Nox2 (23, 65).
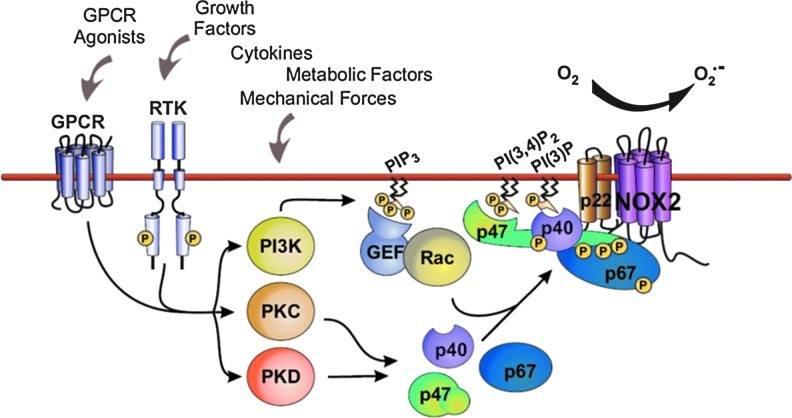
Tight control of Nox2 activation in phagocytes is essential in order to facilitate the killing of ingested pathogens while avoiding the potentially damaging effects of ROS on the host cell. Under resting conditions, Nox2 forms an inactive transmembrane complex with a smaller p22phox subunit (collectively referred to as flavocytochrome b558) that stabilizes both proteins (166). Activation of the enzyme depends upon the binding to the flavocytochrome of four regulatory subunits (p47phox, p67phox, p40phox, and Rac2) that normally reside in the cytoplasm of unstimulated leukocytes (12). Nox2 activation is triggered by different cell surface receptors and their downstream transduction pathways (Fig. 2). Central among these are protein kinase C (PKC)–, phosphoinositide-3-kinase (PI3K)–, protein kinase D (PKD)–, phospholipase D (PLD)–, phospholipase A2 (PLA2)–, and mitogen-activated protein kinase (MAPK)–dependent signaling (23). These kinases phosphorylate p47phox at the C-terminus and relieve an intramolecular Src homology 3 (SH3) domain/C-terminus constraint, allowing the interaction of p47phox with the other cytosolic subunits and with p22phox at the plasma membrane (59, 75). p47phox acts as an “organizer” subunit in this process while p67phox is the “activator” subunit that triggers Nox2 activation upon binding to a specific domain in the protein (196). Homologues of p47phox and p67phox perform similar functions in the activation of Nox1. The recruitment of GTP-bound Rac to the Nox2 complex is a critical step required for full enzyme activation (1, 110). GTP/GDP exchange on Rac promotes a conformational change that regulates its interaction with p67phox and Nox2 (43, 69). The activation of Rac in the vicinity of Nox2 is regulated by the production of 3-phosphorylated phosphoinositides by members of the PI3K enzyme family. Among these lipids, phosphatidylinositol(3, 4, 5)trisphosphate (PIP3) recruits to the plasma membrane several PH-domain containing activators of Rac, such as the guanine-nucleotide-exchange factors (GEFs) P-Rex and Vav1 (105, 201). PI3K products different from PIP3 are also involved in the membrane anchoring of cytosolic subunits, in response to appropriate stimuli. In particular, p40phox specifically binds to PI(3)P through its PX domain (24, 54, 55), while the PX domain of p47phox binds preferentially to PI(3, 4)P2 (102, 104).
FIG. 2.
Mechanism of Nox2 activation. Several signaling pathways, such as protein kinase C (PKC), protein kinase D (PKD), and phosphoinositide-3-kinase (PI3K), are activated downstream of G-protein coupled receptors (GPCRs) or receptor tyrosine kinases (RTKs) and can each lead to post-translational modification of cystosolic regulatory subunits, in particular, p47phox. At the same time, Rac is activated downstream of PI3K. The active Nox2 complex comprises the Nox2-p22phox heterodimer together with p47phox, p67phox, p40phox, and Rac. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Although these mechanisms of Nox2 regulation have been mainly studied in leukocytes, they appear to be generally valid in other cell types, such as ECs and cardiomyocytes (72). However, there may be subtle differences in Nox2 activation between phagocytes and nonphagocytes. For example, the phagocyte Nox2 complex interacts with Rac2 whereas Rac1 is the regulator of endothelial and cardiomyocyte Nox2. There may also be differences in the specific GEFs that are involved. Although definitive comparative data on enzyme kinetics among different cell types are lacking, it appears that nonphagocytic Nox2 enzymes are low-output enzymes that produce substantially less ROS than the neutrophil complex (37, 71). Moreover, in response to cellular stimulation, Nox enzyme activation in VSMCs, ECs, cardiomyocytes, and fibroblasts is slower than in neutrophils (16, 71, 152, 200). The main agonists and stimuli of Nox2 activation in cardiomyocytes and ECs include G-protein coupled receptor agonists (GPCRs), such as AngII and endothelin-1; growth factors; cytokines, such as tumor necrosis factor-α (TNF-α); mechanical forces; and metabolic factors, for example, glucose, insulin, glycated proteins (6, 125), and oxidized low-density lipoprotein (ox-LDL)(137).
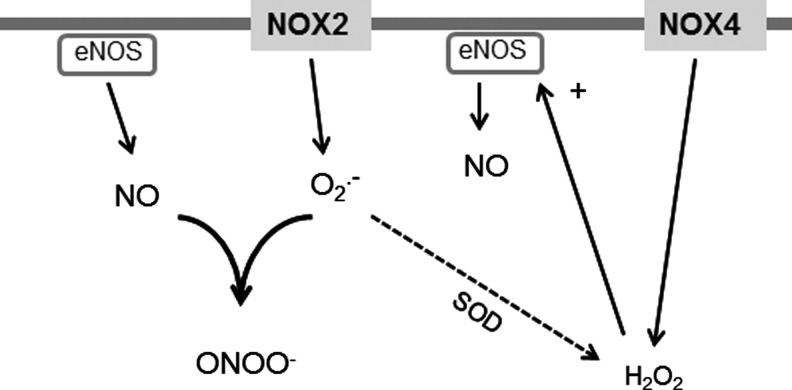
Nox4 also exists in a complex with p22phox that stabilizes both proteins but is otherwise a very differently regulated enzyme compared with Nox2. The activity of Nox4 does not require binding with either Rac or other cytosolic regulatory subunits, and it is in fact a constitutively active enzyme that generates low levels of ROS under basal conditions (34, 53, 141). Another peculiar feature of Nox4 is its ability to generate predominantly H2O2 instead of superoxide (44, 141, 175, 214). The structural basis for this property of Nox4 has recently been suggested to be related to its E-loop (Fig. 3), which differs significantly from that of Nox2 and contains a highly conserved histidine moiety that may hinder superoxide egress and/or provide a source for protons, allowing dismutation to form H2O2 (190). Although H2O2 is more stable and membrane-permeable than O2•− (163), it may still mediate spatially localized signaling because cells have very potent reducing mechanisms, such as peroxiredoxin (164). The propensity of Nox4 to produce predominantly H2O2 rather than O2•− may have important implications for interaction with NO signaling (Fig. 4). Whereas O2•− reacts with NO to generate peroxynitrite and disrupt NO signaling, H2O2 does not undergo this reaction and instead may even enhance NOS activity and signaling (29). Peroxynitrite may have its own distinct signaling effects too. Evidence in support of Nox4-dependent enhancement of NOS signaling is now emerging (36, 173) and is discussed further in later sections. It should be noted, however, that ROS concentration is also an important consideration; very high concentrations of H2O2 may have detrimental effects that outweigh any effect of enhanced NOS signaling. The lack of known regulatory subunits of Nox4 has led to the hypothesis that, in response to several pathological stresses, Nox4 activity is enhanced through an increase in its mRNA and/or protein levels (127, 175, 213). However, recent studies have also suggested that the interaction of Nox4 with two different proteins, Poldip2 and Tks5, could further increase Nox4 activity although the precise mechanisms remain unclear (42, 138). In addition, it has been suggested that increased levels of NADPH and/or FAD could fuel ROS production by Noxs (78, 211), although how such a mechanism may impact on different Nox isoforms remains to be established.
FIG. 3.
Propensity for Nox4 to generate hydrogen peroxide (H2O2) rather than superoxide. (A) Schematic illustration of the Nox4 protein and different loops between transmembrane domains. (B) Nox4 deletion constructs of the E-loop between transmembrane domains 5 and 6 that were studied by Takac et al. (190). FAD binding site (FAD), NADPH binding site (NADPH). (C) Determination of superoxide production by L-012 luminescence in transiently transfected HEK293 cells. (D) Determination of hydrogen peroxide production by luminol+HRP chemiluminescence. Deletion of parts of the E-loop converts Nox4 from a hydrogen peroxide to a superoxide generator. Figure adapted from Ref. (190) with permission. HRP, horseradish peroxidase.
FIG. 4.
Reaction of superoxide versus hydrogen peroxide with nitric oxide (NO). Superoxide inactivates NO to generate peroxynitrite that may have its own effects on signaling. When superoxide and NO are present in similar concentrations, this reaction has faster kinetics than those for superoxide dismutation to hydrogen peroxide. Hydrogen peroxide does not undergo a similar reaction with NO and may enhance nitric oxide synthase (NOS) activity and/or expression levels.
The differences between Nox2 and Nox4 in subcellular localization (previous section) and biochemical regulation seem to be important because both cellular studies and experiments in gene-modified mouse models indicate that changes in Nox2 or Nox4 activity generally result in distinct phenotypes and that one isoform does not usually “compensate” for lack of the other one (9, 34, 119, 161)—although there are exceptions. An important factor contributing to differences between the biological effects of the two isoforms is also likely to be the signaling targets for redox modification, related to the subcellular spatial colocalization of the Nox isoform and its target(s) within the cell (150, 197). For example, Nox2-dependent signaling has been shown to involve colocation of target proteins in the vicinity of the plasma membrane or endosomes (150, 197). In contrast, Nox4 was reported to modulate EGF-induced signaling by oxidizing an ER-resident protein tyrosine phosphatase in ECs (32). It was also reported that Nox4 might physically associate with toll-like receptor after stimulation of ECs with lipopolysaccharide (154). Far fewer such targets have been identified for Nox4 than Nox2 to date.
Role of Nox Enzymes in Cardiac Remodeling and Heart Failure
The failing heart has a complex phenotype that comprises several components, including cardiomyocyte hypertrophy, contractile dysfunction, arrhythmia, myocyte death, fibrosis, and chamber dilatation. Interestingly, many of these components are capable of being independently regulated or even dissociated from each other, suggesting that complex signaling pathways underlie the overall phenotype. A substantial body of evidence generated over the last few years implicates Nox proteins in modulating different components of the heart failure phenotype, generally through alterations in specific redox-regulated signaling pathways (Fig. 5). Nox expression and/or activity have been found to be increased in end-stage human CHF in several independent studies (21, 88, 139), supporting the potential clinical relevance of this pathway.
FIG. 5.
Schematic showing effects of Nox2 on different components of the failing heart phenotype. Signaling pathways modulated by Nox2 are shown.
Cardiac hypertrophy
The role of different Nox isoforms in the development of left ventricular hypertrophy (LVH) has been best assessed in various gene-modified models (183). Increased activation of the renin-angiotensin-aldosterone system is of key importance in the development of heart failure, through multiple effects including the promotion of cardiac hypertrophy. Nox2 was found to be essential for cardiac hypertrophy that develops following short-term (2 weeks) subpressor infusion of AngII, with Nox2 knockout mice displaying less LVH and blunted increases in mRNA levels of molecular markers, such as atrial natriuretic factor (ANF) and β-myosin heavy chain (19). Similar results were found in mice with a cardiomyocyte-specific deletion of Rac1, which had defective Nox2 activation, indicating that it is Nox2 in cardiomyocytes that is stimulated in this setting (172). Consistent with this, AngII-induced hypertrophy of cultured neonatal rat ventricular myocytes (NRVM) involved the activation of Nox2 (149). AngII-induced Nox2-dependent hypertrophy appears to involve the enhanced activation of extracellular-signal-regulated kinase 1/2 (Erk1/2), Akt, and an apoptosis signal-regulating kinase 1 (ASK-1)/nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) pathway (90, 91, 149, 172). In fact, in vivo LVH in response to AngII infusion is attenuated in ASK-1 deficient mice (97). Recently, it was reported that the signaling of AngII-induced cardiomyocyte hypertrophy involved a direct physical interaction of AT1 receptors with Nox2 and the induction of WNT1 inducible signaling pathway protein 1 (WISP1) (178). Cardiomyocyte hypertrophy mediated by other GPCRs, such as noradrenaline and endothelin-1, is also reported to involve Nox2 activation (80, 191, 208). In the case of α1-adrenoreceptor (AR)–stimulated hypertrophy, it was shown that Nox-mediated ROS induced Ras activation through the oxidation of specific thiols, thereby leading to activation of the MEK1/2-ERK1/2 pathway (115). Local AngII release and/or AT1 receptor activation may also contribute to the Nox2-mediated in vivo LVH induced by elevated aldosterone, as in DOCA-salt hypertension models (100, 209).
Early studies showed that Nox activity increased in parallel with progression of pressure overload-induced LVH in small animal models (124). However, Nox2 knockout mice developed a similar extent of hypertrophy in response to aortic constriction as wild-type controls (28, 144). This may suggest that the requirement for Nox2 is less important when hypertrophy occurs in response to primarily mechanical stimuli than to agonists such as AngII, in keeping with the knowledge that different signaling pathways are involved in GPCR-agonist-induced versus mechanically induced hypertrophy. Consistent with this notion, Nox2 deletion did not affect hypertrophy when renin-angiotensin system activation was accompanied by concomitant hypertension (and therefore a mechanical load) (100, 144). Nox2 activation nevertheless contributes to the development of contractile dysfunction and interstitial cardiac fibrosis during chronic pressure overload (19, 73), suggesting that these abnormalities involve distinct signaling perturbations than those that drive hypertrophy per se (172).
The lack of inhibition of pressure overload LVH in Nox2 knockout mice could be due to compensatory changes in other Nox isoforms or related signals. Indeed, we found that Nox4 levels were significantly increased by pressure overload (28). To investigate the role of Nox4 in pressure overload LVH, we studied mouse models with a cardiomyocyte-targeted Nox4 overexpression or a knockout of Nox4. These studies unexpectedly demonstrated that Nox4 was actually protective against chronic-load-induced cardiac stress, in that Nox4 knockout mice developed worse LVH, contractile dysfunction, and dilatation whereas Nox4-overexpressing mice showed the opposite phenotype (214). No differences in Nox2 levels upon perturbation of Nox4 were found in these studies. Investigation of underlying mechanisms revealed that an increase in Nox4 levels promoted a better preservation of myocardial capillary density after pressure overload through enhanced activation of hypoxia-inducible factor 1 (Hif1) and the release of vascular endothelial growth factor from cardiomyocytes to exert paracrine angiogenic activity (214). This mechanism is in line with studies in other cellular systems that support an important role of Nox4 in enhancing angiogenesis (41, 198, 207). These findings reveal Nox4 to be a novel inducible regulator of myocardial angiogenesis, a key determinant of cardiac structural and functional compensation to chronic pressure overload (171, 180). In addition to the enhancement of Hif1, cardiac-specific overexpression of Nox4 was found to activate the transcription factor Nrf2 and increase the expression of many genes involved in phase 2 defense (25, 173). Since Nrf2 has been reported to protect against load-induced cardiac hypertrophy (122), this could also contribute to the beneficial effects of Nox4.
The precise mechanism(s) by which Nox4 increases Hif1 activation remains to be fully defined. The major mechanism of regulation of Hif1α levels is via its hydroxylation at specific proline residues by prolyl hydroxylases (PHD) and subsequent targeting for proteosomal degradation (70). In the studies described just now, we found evidence of reduced Hif1α hydroxylation, suggesting that Nox4 may inhibit PHD activity. In fact, a previous study in tumor cells reported that Nox4-derived H2O2 inhibited Hif1 hydroxylation, with evidence that this involved redox modification of the iron center within PHD (66). In the vasculature, however, recent studies showed that Nox4-induced angiogenesis was associated with an increase in eNOS expression and activity (36, 173). NO can augment Hif1α levels through its S-nitrosylation and inhibition of hydroxylation (131), suggesting that an alternative or complementary mechanism for the effects of Nox4 on Hif1 may involve increased eNOS-derived NO (Fig. 6). The contribution of such an NO-dependent mechanism to the protective effects of Nox4 in the heart remains unclear although no changes in eNOS protein levels were found (214). Nevertheless, the potential for Nox4-derived H2O2 to increase eNOS levels and activity is a significant difference from Nox2, the activation of which is generally associated with a decrease in NO actions (26). Interestingly, beneficial effects of endothelial Nox4-derived H2O2 on vasodilatation were recently reported (161), which contrast to the detrimental effects of endothelial Nox2 on vascular function (20, 147).
FIG. 6.
Effects of Nox4 on angiogenesis. Nox4 levels rise in response to pressure overload and hypoxia and trigger an increase in hypoxia-inducible factor-1α (HIF1α) levels. This may be mediated through an increase in eNOS levels and NO production or could involve direct effects of Nox4-derived ROS on prolyl hydroxylases (PHD) enzymes. An increase in HIF1α leads to increased production and release of angiogenic factors, such as vascular endothelial growth factor, from myocytes or other cell types.
In contrast to the work discussed just now, it should be noted that studies from the Sadoshima laboratory using independently generated mouse models of Nox4 overexpression and deletion reported a detrimental role of Nox4 in aortic constriction-induced cardiac dysfunction (113). The reasons for the marked differences between results remain to be elucidated and could possibly be related to methodological differences, such as mouse background strain, strategy of gene targeting, and severity of aortic constriction. Of note, this work found that an important mechanism for cardiac impairment was mitochondrial ROS generation and dysfunction (4, 113). Enhanced generation of mitochondrial ROS primarily from the electron transport chain is considered to be an important pathogenic mechanism in heart failure, especially in its advanced stages, acting via damage to mitochondrial DNA and energetic processes (195). Ultimately, this may result in cell death by causing the mitochondrial permeability transition, a process that results in mitochondrial membrane depolarization and the leak of pro-apoptotic and pro-necrotic proteins into the cytosol (79). Increased mitochondrial ROS result both from an increase in ROS generation and an impairment of mitochondrial antioxidants (10). Importantly, ROS production by other sources (including Noxs and monoamine oxidases) can induce further mitochondrial ROS production, which may act as an amplifying mechanism (48, 101, 108, 220). Future studies need to establish the precise inter-relationship between the generation of ROS by classical mitochondrial enzymes and those derived from different Nox isoforms and other sources in the setting of heart failure, as this data will be germane to the selection of potential therapeutic targets and may also help to decipher the conflicting results referred to previously.
Excitation-contraction coupling and contractile dysfunction
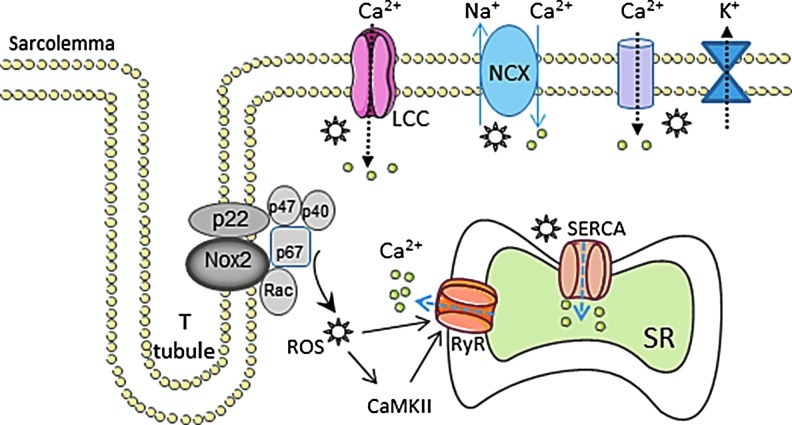
Abnormalities of cardiomyocyte excitation-contraction coupling (ECC) are a fundamental feature of CHF (177). The redox environment influences several components of ECC, typically through oxidative modification of critical reactive cysteine residues in ion channels and transporters (219), but also through modulation of expression levels (194, 218). Increasing evidence supports a role of Nox proteins in these effects but the relevance to heart failure is still poorly explored (Fig. 7).
FIG. 7.
Schematic showing potential effects of Nox2-derived ROS on excitation-contraction coupling. Asterisks denote ROS. Other abbreviations as in the text. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
The open-state probability of L-type calcium channels may be modulated by Nox2 in response to agonists, such as endothelin-1 (212), while AngII reportedly increases expression levels of L-type channel subunits through a Nox-dependent transcriptional pathway (194). Redox regulation of the ryanodine receptor (RyR), which regulates release of calcium from the sarcoplasmic reticulum (SR), has been extensively studied and involves S-glutathionylation and S-nitrosylation (47). An “NADH oxidase” activity associated with the SR has long been recognized (33), and Nox2 was reported to be present in canine cardiac SR (170). Both tachycardia and exercise could enhance Nox activity and increase RyR S-glutathionylation and calcium release rates (169, 170). On the other hand, Nox4 is also reported to localize to the SR (214). Interestingly, Sun et al. recently reported that Nox4 in the SR of skeletal muscle may act as a physiological sensor of O2 tension and generate H2O2 that modulates RyR function (186). Whether Nox4 may play a similar role in the heart is unknown. The SR calcium ATPase pump (SERCA) that mediates re-uptake of calcium into the SR is also susceptible to redox regulation. Increased SERCA oxidation and calcium re-uptake related to increased Nox2 activity have been reported in mouse models of diabetes (128). Contractile dysfunction may also result from abnormalities at the level of the myofilaments and oxidative modification of contractile proteins has been implicated in this process (86). However, whether Nox-derived ROS contribute to this abnormality remains to be addressed.
Recently, Prosser et al. reported a novel role of Nox2 in modulating mechanosensitive stretch-induced calcium release in normal cardiomyocytes (158). Increased calcium release in response to stretch is an important contributor to length-dependent regulation of cardiac function or the Frank–Starling relationship. These authors found that physiological stretch activated Nox2 in the sarcolemma and T-tubules, and that the ROS that were generated sensitized RyR to enhance calcium-induced calcium release—evident as calcium “sparks” on confocal microscopy. This is perhaps the first data to suggest an important physiological role for Nox2 in the cardiac myocyte and addresses a long-standing question as to why these proteins are present even in the normal healthy heart. While this mechanism was beneficial in the physiological setting, Prosser et al. found that excessive activation of Nox2 induced either by AngII stimulation or in a mouse model of muscular dystrophy resulted in abnormal calcium waves instead of sparks (158). Such a mechanism could have relevance to heart failure by contributing to calcium dysregulation, impaired contraction, and arrhythmia. This finding suggests that the level of Nox2 activation and the amount of ROS generated may be a crucial factor in determining the effects on cardiomyocyte function.
Arrhythmia
Atrial fibrillation (AF) is a common arrhythmia complicating CHF, especially in older patients, and increases morbidity. It is associated with electrophysiological and structural remodeling of the atrial myocardium that favors perpetuation of the arrhythmia but the mechanisms underlying these changes remain incompletely understood. Current evidence shows that increased atrial oxidative stress may play an important role in initiating and sustaining AF in animal models and humans (30, 146), and Noxs have emerged as important in this regard (50, 106, 107, 162). Induction of AF with rapid atrial pacing in pigs was associated with increased Nox activation and ROS production in the left atrium (50). Studies from the group of Casadei showed that Nox2 was the main source of ROS in human atrial myocytes and that Nox-derived ROS production was increased in right atrial appendages of patients undergoing cardiac bypass surgery who subsequently developed postoperative AF (106). Further, atrial NADPH-stimulated ROS production was independently associated with an increased risk of postoperative AF (107). Recent work from the same group reported that Nox2 appears to be important mainly in the initiation of AF, either in a goat rapid-pacing model or in humans who develop postoperative AF, whereas uncoupled NOSs and mitochondrial oxidases may be more important ROS sources in the setting of longstanding AF (162). Interestingly, AF develops spontaneously in mice with cardiac-specific overexpression of a constitutively active Rac1, which activates Nox2 (2).
Therapeutic agents that target Nox2 activation may have some potential in reducing the risk of developing AF. Statins, which downregulate Rac1 activity by suppressing its isoprenylation, are reported to have a favorable effect on risk of developing AF although they may be ineffective in persistent AF (2, 7, 162, 182, 210). Angiotensin-converting enzyme inhibitors and AngII receptor blockers both reduce risk of AF (52, 85), and it is possible that part of the mechanism may involve a reduction in Nox2 activation. Indeed, AngII promotes early afterdepolarizations in rabbit cardiomyocytes through activation of the Nox-ROS-Ca(2+)/calmodulin–dependent protein kinase II (CaMKII) pathway (217). An important role of oxidized CaMKII downstream of Nox2 activation is also supported by work from the Anderson laboratory showing that Nox2-dependent oxidation of CaMKII promotes cardiac sinus node dysfunction in a mouse model of AngII-induced dysrhythmia (189). However, whether any of the above mechanisms are relevant to AF in CHF remains to be established.
Myocyte death
A low level of cardiomyocyte death occurs during chronic cardiac remodeling and contributes to CHF (61). ROS and redox signaling potentially impact on apoptosis at several levels, including upstream signaling pathways that are pro-apoptotic and at the level of the mitochondria (60, 143). The former include pro-apoptotic pathways, such as the activation of ASK-1, JNK, p38MAPK, and CaMKII, as well as anti-apoptotic pathways, such as Akt, Bcl2, and heat shock proteins (143).
There is some evidence to support an involvement of Nox-derived ROS in cardiomyocyte apoptosis. AngII promotes apoptosis of cultured NRVM at least in part via Nox, most likely Nox2 (74, 134, 159). The activation of CaMKII might be a critical convergence point of pro-apoptotic signaling since it is activated both by Ca2+ and by Nox2-derived ROS, downstream of AngII (57). Cardiomyocytes from p47phox−/− mice or from mice expressing an inhibitory peptide against CaMKII were protected against apoptosis induced by chronic AngII infusion (57). The importance of this pathway in other species was further demonstrated by Palomeque et al. (153). Norepinephrine, aldosterone, and doxorubicin are also reported to promote cardiomyocyte apoptosis through the activation of Nox2 (67, 82, 129). Interestingly, apoptosis of rat cardiomyocyte-like H9c2 cells induced by metabolic inhibition was suggested to involve nuclear Nox2 (145). The exposure of rat cardiomyocytes to high glucose upregulates Nox2 activity and increases apoptosis (179), which may be relevant to diabetic heart disease (see the section below entitled Other forms of heart failure).
The possible role of Nox4 in myocyte apoptosis remains controversial. It was reported that adenoviral-mediated overexpression of Nox4 in cardiomyocytes enhanced myocyte apoptosis (4). However, studies in many other cell types reported anti-apoptotic effects of Nox4, for example, through enhancement of Akt activation (14, 41, 51, 120). We did not find any evidence of increased apoptosis in cardiomyocyte-targeted Nox4 transgenic mice up to the age of 12 months (214). It is interesting that Nox4 enhances Akt activation whereas Nox2 was shown to increase CaMKII activation; a likely reason for this could be the different subcellular localization of the two isoforms and presumably different coupling to downstream targets.
Myocardial fibrosis
Myocardial interstitial fibrosis is an important component of the hypertrophied and failing heart and impacts on cardiac passive filling characteristics, tissue hypoxia, and susceptibility to arrhythmia. Increased activation of the renin-angiotensin-aldosterone system, increased mechanical load, and increased cytokine activation are all known to be important in the development of fibrosis. Nox proteins appear to have a major role in the development of fibrosis in different settings. Interstitial fibrosis induced either by subpressor AngII infusion, pressor AngII infusion, or chronic activation of the renin-angiotensin system was inhibited in Nox2 knockout mice (19, 28, 100, 203) or in cardiomyocyte-specific Rac1 knockout mice (172). Nox2 was also implicated in mineralocorticoid receptor agonist aldosterone-induced cardiac fibrosis in mice and rats (100, 188). Moreover, interstitial cardiac fibrosis was abolished in Nox2 knockout mice subjected to aortic constriction or permanent coronary artery ligation (73, 136). Of note, deletion of Nox2 attenuated fibrosis even when the extent of myocyte hypertrophy was unaltered in some of the above studies, indicating a dissociation in the contribution of Nox2 to these phenotypes (100, 188). These data indicate a crucial role of Nox2 in fibrotic remodeling in vivo in response to diverse stimuli. The dissociation between effects of Nox2 on fibrosis and hypertrophy may indicate that the effects on fibrosis involve nonmyocytes or inflammatory cells (148). Some in vitro studies have suggested that Nox4 is involved in the proliferation of cultured human cardiac fibroblasts and their transformation into myofibroblasts in response to transforming growth factor β treatment (39). However, the relevance of this in vivo remains to be established. We did not find a role of Nox4 in promoting fibrosis in gene-modified Nox4 mice subjected to aortic constriction (214).
A number of molecular mechanisms through which Nox2 may promote fibrosis have been suggested. Nox2 may enhance the expression and/or activation of MMPs, which are involved in matrix turnover (11, 168). MMPs not only degrade normal matrix components but also modulate collagen synthesis, for example, by modulating the formation of matrikines and the release of pro-fibrotic growth factors from the extracellular matrix (130). As such, increased MMP activity is commonly associated with increased fibrosis in the failing heart, with the fibrotic tissue comprising altered collagen type, organization, and cross-linking (77, 87, 98). However, matricellular proteins, such as thrombospondin-1, that are activated by MMPs actually inhibit inflammation and fibrosis (63) so that the precise relationship between MMP activation and fibrosis may depend upon context. It was reported that TNF-α-induced induction of MMPs in cardiomyocytes involves Nox and PI3Kγ (11). Nox-mediated MMP activation has also been shown in rabbit hearts in response to combined volume and pressure overload (135), and in age-related cardiac fibrosis in rats (199). Aldosterone-induced in vivo myocardial fibrosis in mice involves the Nox2-dependent activation of NF-κB; upregulation of the profibrotic cytokine connective tissue growth factor; increased expression of procollagen-1, 3, and fibronectin; and increased MMP2 activation (100).
Infiltration by circulating inflammatory cells and the local release of cytokines constitute key pro-fibrotic stimuli within the myocardial tissue in response to pathological stress such as MI and hemodynamic overload (116). Inflammation contributes not only to fibrosis but may also affect cardiomyocyte hypertrophy and viability. Recent studies have highlighted the role of leukocyte PI3Kγ in regulating inflammatory cell recruitment and consequent fibrosis, which may also involve Nox2 activation (40, 92, 155). Mice with catalytically inactive PI3Kγ (PI3Kγ KD) have reduced cardiac fibrosis after transverse aortic constriction, accompanied by attenuated infiltration of inflammatory cells (155). Using a bone marrow chimera approach, it was conclusively demonstrated that the reduction in leukocyte recruitment and fibrosis in the myocardium after aortic constriction was attributable to bone marrow cells (40). Importantly, PI3K has a central role in regulating neutrophil Nox2 activation (81), suggesting that pharmacological targeting of PI3Kγ could impact on Nox2 activation and may at least in part be involved in the effects on fibrotic remodeling.
Post-MI remodeling
Post-MI remodeling includes cell death and inflammation in the early phase of infarct healing, followed by infarct expansion, progressive hypertrophy and fibrosis remote from the infarct site, LV chamber enlargement, and functional deterioration in the late phase. Increased oxidative stress has long been recognized to be involved in this process (109, 181, 187). The specific involvement of Nox proteins was suggested by the finding that Nox2 is upregulated in cardiomyocytes in the infarcted myocardium after acute MI in patients (111) as well as in experimental models (64, 142). Nonspecific inhibitors of Nox2, such as apocynin and statins, were found to have beneficial effects on adverse post-MI remodeling (15, 83, 160). More compelling evidence for the involvement of Nox2 in post-MI remodeling comes from studies in gene-modified mice. Mice deficient in p47phox had no reduction in infarct size after myocardial ischemia/reperfusion or permanent coronary ligation (46, 93). However, these animals were found to have attenuated ventricular dilatation and contractile dysfunction after permanent ligation, as well as higher survival than wild-type controls (46). Likewise, Nox2 knockout mice also displayed less LV dilatation and contractile dysfunction after MI, together with reduced cardiomyocyte hypertrophy, apoptosis, and interstitial fibrosis (136). It has been suggested that adverse effects following myocardial ischemia-reperfusion may involve the ROS-induced induction of lectin-like ox-LDL receptor-1, which in turn mediates the activation of MAPKs and inducible NOS (94).
Increased activation of the sympathetic and renin-angiotensin-aldosterone systems is known to be important in promoting heart failure after MI. It was recently shown that the β1-selective AR antagonist nebivolol exerted beneficial effects on post-MI hypertrophy and contractile dysfunction through the inhibition of Nox (185). However, this agent also has other effects, in particular, an enhancement of NO production (140), raising the question whether the effects on Nox and NO are related. Several studies have shown that increased ROS generation in the central nervous system is implicated in mediating sympathoexcitation post-MI (121, 132). It was reported that Nox4 is upregulated in the paraventricular nucleus after MI and that shRNA-mediated knockdown reduced sympathetic outflow from the brain and improved cardiac function in this setting (96). High plasma levels of aldosterone increase the risk of early death after MI, at least in part by increasing cardiac rupture. Recently, a novel Nox2-mediated mechanism contributing to this lethal complication has been identified (84). He et al. (84) found that Nox2-dependent oxidation of CaMKII mediated these cardiotoxic effects of aldosterone, through an increase in MMP9 expression by cardiomyocytes that promoted cardiac rupture post-MI (Fig. 8). Either the inhibition of CaMKII or a deficiency of Nox2 attenuated the increase in MMP9 activity and protected the heart against rupture after MI. Cardiac-specific deletion of the mineralocorticoid receptor also ameliorates post-MI remodeling, at least in part by reducing Nox2 activation (62).
FIG. 8.
Aldosterone-stimulated Nox-dependent oxidation of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) contributes to cardiac rupture following acute myocardial infarction (MI). CaMKII induces the production of matrix metalloproteinase 9 (MMP9) that causes cardiac rupture. Reproduced from Ref. (84) with permission. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Other forms of heart failure
Patients with diabetes may develop a greater extent of cardiac dysfunction and failure that can be attributed simply to myocardial ischemia or changes in cardiac workload (22). While the underlying causes are multifactorial and include prominent metabolic dysregulation, an increase in myocardial ROS production is a contributing factor (22). Nox-derived ROS production may be involved in this process. High glucose increases Nox2 activation in isolated cardiomyocytes and contributes to contractile dysfunction (13, 157) and apoptosis (179). Hyperglycemia-induced Nox2 activation has been shown to contribute to the formation of increased glycated proteins, NF-κB activation, and the upregulation of ANF mRNA in cardiomyocytes (215). Several studies have demonstrated that myocardial Nox activity is increased in models of type 1 and type 2 diabetes (128, 142, 174, 179, 202, 205) and treatment with apocynin was reported to protect against diabetes-induced myocardial fibrosis, hypertrophy, and dysfunction (123, 128, 165). Li et al. used mice with cardiac-specific Rac1 KO to demonstrate that activation of Rac1/Nox2 oxidase is involved in myocardial remodeling during the development of type 1 diabetic cardiomyopathy (123). However, definitive in vivo data to confirm the roles of specific Nox proteins in the development of diabetic heart disease are still lacking.
Anthracycline chemotherapeutic agents, such as doxorubicin, cause dose-dependent cardiotoxicity and heart failure that involves increased ROS generation and oxidative stress (58, 103, 133, 151). Emerging evidence indicates that Nox2-derived ROS are important in the development of doxorubicin-induced cardiotoxicity. Doxorubicin induces rapid Nox-mediated ROS generation in H9c2 cardiomyoblasts (67) and in the mouse heart in vivo (151). Conversely, Nox2 deletion attenuated doxorubicin-stimulated cardiac ROS production and cardiac dysfunction (204). Importantly, the latter group found that genetic polymorphisms of the Nox2 oxidase complex may influence the individual risk of doxorubicin cardiotoxicity in cancer patients (204). Recently, Zhao et al. showed that Nox2-derived ROS contributed to contractile dysfunction, myocardial atrophy, increased cardiomyocyte apoptosis, interstitial fibrosis, and inflammatory cell infiltration in an in vivo model of doxorubicin cardiotoxicity in mice (216).
Therapeutic Interventions Targeting Nox
The studies discussed previously suggest that targeted inhibition of Nox proteins, in particular, Nox2, may be a useful therapeutic strategy in heart failure and its antecedent conditions. The complex regulation and activation of Nox2 and the specific downstream signaling pathways that it modulates offer the potential for multiple complementary approaches to inhibition of detrimental signaling. A diverse range of compounds are potential Nox inhibitors and these have been comprehensively reviewed recently (49, 99). Commonly used agents such as apocynin and diphenylene iodonium are not specific but newer agents in development appear promising (49, 99). Here, we consider a few general issues that may be relevant in developing Nox-targeted therapies for heart failure. Whereas many existing agents or compounds in early development are inhibitors of multiple Nox isoforms, therapies of heart failure may require isoform-specific agents because Nox4 has beneficial effects, such as the preservation of appropriate myocardial vascularization during chronic pressure overload (214). Nox2 may also have some beneficial effects, for example, in ischemic cardiac preconditioning (18) or in ECC (158). Another issue to consider with respect to Nox2 inhibitors is the potential to compromise anti-microbial and/or immune functions of white cells, although it may be feasible to selectively target Nox2 in different cell types due to cell-specific differences in its regulation (99). Further, recent work that severe chronic granulomatous disease is only evident in individuals whose phagocytic ROS production is more than two orders of magnitude lower than healthy controls suggests that safe therapeutic targeting of cardiovascular Nox2 could be feasible (112). Targeting specific molecules upstream or downstream of Nox2 may also be worthwhile. For example, the inhibition of PI3Kγ could be a useful approach to Nox inhibition (167). Likewise, targeting molecules downstream of Nox2 that act as signaling hubs, for example, CaMKII, appears promising (56). Finally, better elucidation of the interactions among Noxs, NOS signaling, and mitochondrial ROS may also offer new targets. In conclusion, the evidence reviewed in this article indicates that Nox-dependent signaling plays important roles in the development of heart failure and may offer novel therapeutic approaches for this condition.
Abbreviations Used
- AF
atrial fibrillation
- Aldo
aldosterone
- ANF
atrial natriuretic factor
- AngII
angiotensin II
- AR
adrenoreceptor
- ASK-1
apoptosis signal-regulating kinase 1
- CaMKII
Ca(2+)/calmodulin-dependent protein kinase II
- CHF
chronic heart failure
- CTGF
connective tissue growth factor
- Duox
dual oxidase
- Duoxa
duox activator
- ECC
excitation-contraction coupling
- ECs
endothelial cells
- ER
endoplasmic reticulum
- Erk
extracellular signal-regulated kinase
- GEFs
guanine-nucleotide-exchange factors
- GPCRs
G-protein coupled receptor agonists
- H2O2
hydrogen peroxide
- HIF1α
hypoxia-inducible factor-1α
- HRP
horseradish peroxidase
- LCC
L-type calcium channels
- LVH
left ventricular hypertrophy
- MAPK
mitogen-activated protein kinase
- MEF2
myocyte enhancer factors 2
- MI
myocardial infarction
- MMPs
matrix metalloproteinases
- MPOs
myeloperoxidases
- MsrA
methionine sulfoxide reductase A
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
sodium-calcium exchange
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NOSs
nitric oxide synthases
- Nox
NADPH oxidase
- Noxa1
Nox activator 1
- Noxo1
Nox organizer 1
- NRVM
neonatal rat ventricular myocytes
- ONOO−
peroxynitrite
- ox-LDL
oxidized low-density lipoprotein
- PDGF
platelet-derived growth factor
- PHD
prolyl hydroxylases
- phox
phagocytic oxidase
- PI3K
phosphoinositide-3-kinase
- PIP3
phosphatidylinositol(3, 4, 5)trisphosphate
- PKC
protein kinase C
- PKD
protein kinase D
- PLA2
phospholipase A2
- PLD
phospholipase D
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- RyR
ryanodine receptor
- SERCA
SR calcium ATPase pump
- SH3
Src homology 3
- SOD
superoxide dismutase
- SR
sarcoplasmic reticulum
- TNF-α
tumor necrosis factor-α
- T-T
T tubule
- VSMCs
vascular smooth muscle cells
- WISP1
WNT1 inducible signaling pathway protein 1
- XOs
xanthine oxidases
Acknowledgments
This work is supported by a Foundation Leducq Transatlantic Network of Excellence Award (E.H. and A.M.S.), the British Heart Foundation (RG/08/011/25922 and RE/08/003 to A.M.S.), and the Department of Health via a National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
Author Disclosure Statement
E.H. is a cofounder of Kither Biotech. None of the other authors have any conflicts to disclose.
References
- 1.Abo A. Pick E. Hall A. Totty N. Teahan CG. Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 2.Adam O. Frost G. Custodis F. Sussman MA. Schäfers HJ. Böhm M. Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 3.Ago T. Kitazono T. Ooboshi H. Iyama T. Han YH. Takada J. Wakisaka M. Ibayashi S. Utsumi H. Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 4.Ago T. Kuroda J. Pain J. Fu C. Li H. Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguirre JS. Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic Biol Med. 2010;49:1342–1353. doi: 10.1016/j.freeradbiomed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akki A. Zhang M. Murdoch C. Brewer A. Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Amar D. Zhang H. Heerdt PM. Park B. Fleisher M. Thaler HT. Statin use is associated with a reduction in atrial fibrillation after noncardiac thoracic surgery independent of C-reactive protein. Chest. 2005;128:3421–3427. doi: 10.1378/chest.128.5.3421. [DOI] [PubMed] [Google Scholar]
- 8.Anilkumar N. Sirker A. Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 9.Anilkumar N. Weber R. Zhang M. Brewer A. Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 10.Aon MA. Cortassa S. O'Rourke B. Redox-optimized ROS balance: A unifying hypothesis. Biochim Biophys Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awad AE. Kandalam V. Chakrabarti S. Wang X. Penninger JM. Davidge ST. Oudit GY. Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-dependent manner. Am J Physiol Cell Physiol. 2010;298:C679–C692. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- 12.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 13.Balteau M. Tajeddine N. de Meester C. Ginion A. Des Rosiers C. Brady NR. Sommereyns C. Horman S. Vanoverschelde JL. Gailly P. Hue L. Bertrand L. Beauloye C. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res. 2011;92:237–246. doi: 10.1093/cvr/cvr230. [DOI] [PubMed] [Google Scholar]
- 14.Basuroy S. Tcheranova D. Bhattacharya S. Leffler CW. Parfenova H. Nox4 NADPH oxidase-derived reactive oxygen species, via endogenous carbon monoxide, promote survival of brain endothelial cells during TNF-α-induced apoptosis. Am J Physiol Cell Physiol. 2011;300:C256–C265. doi: 10.1152/ajpcell.00272.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauersachs J. Galuppo P. Fraccarollo D. Christ M. Ertl G. Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation. 2001;104:982–985. doi: 10.1161/hc3401.095946. [DOI] [PubMed] [Google Scholar]
- 16.Bauldry SA. Nasrallah VN. Bass DA. Activation of NADPH oxidase in human neutrophils permeabilized with Staphylococcus aureus alpha-toxin. A lower Km when the enzyme is activated in situ. J Biol Chem. 1992;267:323–330. [PubMed] [Google Scholar]
- 17.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bell RM. Cave AC. Johar S. Hearse DJ. Shah AM. Shattock MJ. Pivotal role of NOX-2-containing NADPH oxidase in early ischemic preconditioning. FASEB J. 2005;19:2037–2039. doi: 10.1096/fj.04-2774fje. [DOI] [PubMed] [Google Scholar]
- 19.Bendall JK. Cave AC. Heymes C. Gall N. Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 20.Bendall JK. Rinze R. Adlam D. Tatham AL. de Bono J. Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 21.Borchi E. Bargelli V. Stillitano F. Giordano C. Sebastiani M. Nassi PA. d'Amati G. Cerbai E. Nediani C. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta. 2010;1802:331–338. doi: 10.1016/j.bbadis.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Boudina S. Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 23.Brandes RP. Weissmann N. Schröder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 24.Bravo Jn. Karathanassis D. Pacold CM. Pacold ME. Ellson CD. Anderson KE. Butler PJ. Lavenir I. Perisic O. Hawkins PT. Stephens L. Williams RL. The crystal structure of the PX domain from p40phox bound to phosphatidylinositol 3-phosphate. Mol Cell. 2001;8:829–839. doi: 10.1016/s1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- 25.Brewer AC. Murray TVA. Arno M. Zhang M. Anilkumar NP. Mann GE. Shah AM. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med. 2011;51:205–215. doi: 10.1016/j.freeradbiomed.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DI. Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buul JDV. Fernandez-Borja M. Anthony EC. Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal. 2005;7:308–317. doi: 10.1089/ars.2005.7.308. [DOI] [PubMed] [Google Scholar]
- 28.Byrne JA. Grieve DJ. Bendall JK. Li JM. Gove C. Lambeth JD. Cave AC. Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 29.Cai H. Davis ME. Drummond GR. Harrison DG. Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol. 2001;21:1571–1576. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 30.Carnes CA. Chung MK. Nakayama T. Nakayama H. Baliga RS. Piao S. Kanderian A. Pavia S. Hamlin RL. McCarthy PM. Bauer JA. Van Wagoner DR. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89:e32–e38. doi: 10.1161/hh1801.097644. [DOI] [PubMed] [Google Scholar]
- 31.Cave AC. Brewer AC. Narayanapanicker A. Ray R. Grieve DJ. Walker S. Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 32.Chen K. Kirber MT. Xiao H. Yang Y. Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherednichenko G. Zima AV. Feng W. Schaefer S. Blatter LA. Pessah IN. NADH oxidase activity of rat cardiac sarcoplasmic reticulum regulates calcium-induced calcium release. Circ Res. 2004;94:478–486. doi: 10.1161/01.RES.0000115554.65513.7C. [DOI] [PubMed] [Google Scholar]
- 34.Clempus RE. Sorescu D. Dikalova AE. Pounkova L. Jo P. Sorescu GP. Lassegue B. Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook NR. Albert CM. Gaziano JM. Zaharris E. MacFadyen J. Danielson E. Buring JE. Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craige SM. Chen K. Pei Y. Li C. Huang X. Chen C. Shibata R. Sato K. Walsh K. Keaney JF. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation/clinical perspective. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross AR. Parkinson JF. Jones OT. The superoxide-generating oxidase of leucocytes. NADPH-dependent reduction of flavin and cytochrome b in solubilized preparations. Biochem J. 1984;223:337–344. doi: 10.1042/bj2230337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csányi G. Taylor WR. Pagano PJ. NOX and inflammation in the vascular adventitia. Free Radic Biol Med. 2009;47:1254–1266. doi: 10.1016/j.freeradbiomed.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cucoranu I. Clempus R. Dikalova A. Phelan PJ. Ariyan S. Dikalov S. Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-{beta}1-Induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 40.Damilano F. Franco I. Perrino C. Schaefer K. Azzolino O. Carnevale D. Cifelli G. Carullo P. Ragona R. Ghigo A. Perino A. Lembo G. Hirsch E. Distinct effects of leukocyte and cardiac phosphoinositide 3-kinase γ activity in pressure overload-induced cardiac failure/clinical perspective. Circulation. 2011;123:391–399. doi: 10.1161/CIRCULATIONAHA.110.950543. [DOI] [PubMed] [Google Scholar]
- 41.Datla SR. Peshavariya H. Dusting GJ. Mahadev K. Goldstein BJ. Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 42.Diaz B. Shani G. Pass I. Anderson D. Quintavalle M. Courtneidge SA. Tks5-dependent, Nox-mediated generation of reactive oxygen species is necessary for invadopodia formation. Sci Signal. 2009;2:ra53. doi: 10.1126/scisignal.2000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diekmann D. Abo A. Johnston C. Segal AW. Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 44.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HH. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinauer MC. Orkin SH. Chronic granulomatous disease. Annu Rev Med. 1992;43:117–124. doi: 10.1146/annurev.me.43.020192.001001. [DOI] [PubMed] [Google Scholar]
- 46.Doerries C. Grote K. Hilfiker-Kleiner D. Luchtefeld M. Schaefer A. Holland SM. Sorrentino S. Manes C. Schieffer B. Drexler H. Landmesser U. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 47.Donoso P. Sanchez G. Bull R. Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci. 2011;16:553–567. doi: 10.2741/3705. [DOI] [PubMed] [Google Scholar]
- 48.Doughan AK. Harrison DG. Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 49.Drummond GR. Selemidis S. Griendling KK. Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudley SC. Hoch NE. McCann LA. Honeycutt C. Diamandopoulos L. Fukai T. Harrison DG. Dikalov SI. Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage. Circulation. 2005;112:1266–1273. doi: 10.1161/CIRCULATIONAHA.105.538108. [DOI] [PubMed] [Google Scholar]
- 51.Edderkaoui M. Nitsche C. Zheng L. Pandol SJ. Gukovsky I. Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J Biol Chem. 2011;286:7779–7787. doi: 10.1074/jbc.M110.200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehrlich JR. Hohnloser SH. Nattel S. Role of angiotensin system and effects of its inhibition in atrial fibrillation: clinical and experimental evidence. Eur Heart J. 2006;27:512–518. doi: 10.1093/eurheartj/ehi668. [DOI] [PubMed] [Google Scholar]
- 53.Ellmark SHM. Dusting GJ. Ng Tang Fui M. Guzzo-Pernell N. Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 54.Ellson C. Davidson K. Anderson K. Stephens LR. Hawkins PT. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellson CD. Gobert-Gosse S. Anderson KE. Davidson K. Erdjument-Bromage H. Tempst P. Thuring JW. Cooper MA. Lim ZY. Holmes AB. Gaffney PRJ. Coadwell J. Chilvers ER. Hawkins PT. Stephens LR. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat Cell Biol. 2001;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 56.Erickson JR. He BJ. Grumbach IM. Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Erickson JR. Joiner Ml. Guan X. Kutschke W. Yang J. Oddis CV. Bartlett RK. Lowe JS. O'Donnell SE. Aykin-Burns N. Zimmerman MC. Zimmerman K. Ham AJ. Weiss RM. Spitz DR. Shea MA. Colbran RJ. Mohler PJ. Anderson ME. A Dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ewer MS. Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 59.Faust LR. el Benna J. Babior BM. Chanock SJ. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J Clin Invest. 1995;96:1499–1505. doi: 10.1172/JCI118187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Filomeni G. Ciriolo MR. Redox control of apoptosis: an update. Antioxid Redox Signal. 2006;8:2187–2192. doi: 10.1089/ars.2006.8.2187. [DOI] [PubMed] [Google Scholar]
- 61.Foo RS. Mani K. Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fraccarollo D. Berger S. Galuppo P. Kneitz S. Hein L. Schütz G. Frantz S. Ertl G. Bauersachs J. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction/clinical perspective. Circulation. 2011;123:400–408. doi: 10.1161/CIRCULATIONAHA.110.983023. [DOI] [PubMed] [Google Scholar]
- 63.Frangogiannis NG. Ren G. Dewald O. Zymek P. Haudek S. Koerting A. Winkelmann K. Michael LH. Lawler J. Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 64.Fukui T. Yoshiyama M. Hanatani A. Omura T. Yoshikawa J. Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun. 2001;281:1200–1206. doi: 10.1006/bbrc.2001.4493. [DOI] [PubMed] [Google Scholar]
- 65.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Gerald D. Berra E. Frapart YM. Chan DA. Giaccia AJ. Mansuy D. Pouysségur J. Yaniv M. Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 67.Gilleron Mn. Marechal X. Montaigne D. Franczak J. Neviere R. Lancel S. NADPH oxidases participate to doxorubicin-induced cardiac myocyte apoptosis. Biochem and Biophys Res Commun. 2009;388:727–731. doi: 10.1016/j.bbrc.2009.08.085. [DOI] [PubMed] [Google Scholar]
- 68.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gorzalczany Y. Sigal N. Itan M. Lotan O. Pick E. Targeting of Rac1 to the phagocyte membrane is sufficient for the induction of NADPH oxidase assembly. J Biol Chem. 2000;275:40073–40081. doi: 10.1074/jbc.M006013200. [DOI] [PubMed] [Google Scholar]
- 70.Greer SN. Metcalf JL. Wang Y. Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 72.Griendling KK. Sorescu D. Ushio-Fukai M. NAD(P)H Oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 73.Grieve DJ. Byrne JA. Siva A. Layland J. Johar S. Cave AC. Shah AM. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol. 2006;47:817–826. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 74.Grishko V. Pastukh V. Solodushko V. Gillespie M. Azuma J. Schaffer S. Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am J Physiol Heart Circ Physiol. 2003;285:H2364–H2372. doi: 10.1152/ajpheart.00408.2003. [DOI] [PubMed] [Google Scholar]
- 75.Groemping Y. Lapouge K. Smerdon SJ. Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 76.Grote K. Flach I. Luchtefeld M. Akin E. Holland SM. Drexler H. Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 77.Gunja-Smith Z. Morales AR. Romanelli R. Woessner JF., Jr. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol. 1996;148:1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 78.Gupte SA. Levine RJ. Gupte RS. Young ME. Lionetti V. Labinskyy V. Floyd BC. Ojaimi C. Bellomo M. Wolin MS. Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 80.Häuselmann SpP. Rosc-Schlüter BI. Lorenz V. Plaisance I. Brink M. Pfister O. Kuster GM. β1-Integrin is up-regulated via Rac1-dependent reactive oxygen species as part of the hypertrophic cardiomyocyte response. Free Radic Biol Med. 2011;51:609–618. doi: 10.1016/j.freeradbiomed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 81.Hawkins PT. Davidson K. Stephens LR. The role of PI3Ks in the regulation of the neutrophil NADPH oxidase. Biochem Soc Symp. 2007;74:59–67. doi: 10.1042/BSS0740059. [DOI] [PubMed] [Google Scholar]
- 82.Hayashi H. Kobara M. Abe M. Tanaka N. Gouda E. Toba H. Yamada H. Tatsumi T. Nakata T. Matsubara H. Aldosterone nongenomically produces NADPH oxidase-dependent reactive oxygen species and induces myocyte apoptosis. Hypertens Res. 2008;31:363–375. doi: 10.1291/hypres.31.363. [DOI] [PubMed] [Google Scholar]
- 83.Hayashidani S. Tsutsui H. Shiomi T. Suematsu N. Kinugawa S. Ide T. Wen J. Takeshita A. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–873. doi: 10.1161/hc0702.104164. [DOI] [PubMed] [Google Scholar]
- 84.He BJ. Joiner Ml. Singh MV. Luczak ED. Swaminathan PD. Koval OM. Kutschke W. Allamargot C. Yang J. Guan X. Zimmerman K. Grumbach IM. Weiss RM. Spitz DR. Sigmund CD. Blankesteijn WM. Heymans S. Mohler PJ. Anderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Healey JS. Baranchuk A. Crystal E. Morillo CA. Garfinkle M. Yusuf S. Connolly SJ. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 86.Heusch P. Canton M. Aker S. van de Sand A. Konietzka I. Rassaf T. Menazza S. Brodde OE. Di Lisa F. Heusch G. Schulz R. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol. 2010;160:1408–1416. doi: 10.1111/j.1476-5381.2010.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heymans S. Luttun A. Nuyens D. Theilmeier G. Creemers E. Moons L. Dyspersin GD. Cleutjens JP. Shipley M. Angellilo A. Levi M. Nube O. Baker A. Keshet E. Lupu F. Herbert JM. Smits JF. Shapiro SD. Baes M. Borgers M. Collen D. Daemen MJ. Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 88.Heymes C. Bendall JK. Ratajczak P. Cave AC. Samuel JL. Hasenfuss G. Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 89.Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 90.Hingtgen SD. Tian X. Yang J. Dunlay SM. Peek AS. Wu Y. Sharma RV. Engelhardt JF. Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 91.Hirotani S. Otsu K. Nishida K. Higuchi Y. Morita T. Nakayama H. Yamaguchi O. Mano T. Matsumura Y. Ueno H. Tada M. Hori M. Involvement of nuclear factor-{kappa}B and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 92.Hirsch E. Katanaev VL. Garlanda C. Azzolino O. Pirola L. Silengo L. Sozzani S. Mantovani A. Altruda F. Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmeyer MR. Jones SP. Ross CR. Sharp B. Grisham MB. Laroux FS. Stalker TJ. Scalia R. Lefer DJ. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 94.Hu C. Chen J. Dandapat A. Fujita Y. Inoue N. Kawase Y. Jishage K. Suzuki H. Li D. Hermonat PL. Sawamura T. Mehta JL. LOX-1 abrogation reduces myocardial ischemia-reperfusion injury in mice. J Mol Cell Cardiol. 2008;44:76–83. doi: 10.1016/j.yjmcc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Ide T. Tsutsui H. Kinugawa S. Suematsu N. Hayashidani S. Ichikawa K. Utsumi H. Machida Y. Egashira K. Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- 96.Infanger DW. Cao X. Butler SD. Burmeister MA. Zhou Y. Stupinski JA. Sharma RV. Davisson RL. Silencing Nox4 in the paraventricular nucleus Improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res. 2010;106:1763–1774. doi: 10.1161/CIRCRESAHA.109.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Izumiya Y. Kim S. Izumi Y. Yoshida K. Yoshiyama M. Matsuzawa A. Ichijo H. Iwao H. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 98.Janicki JS. Brower GL. Gardner JD. Chancey AL. Stewart JA. The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev. 2004;9:33–42. doi: 10.1023/B:HREV.0000011392.03037.7e. [DOI] [PubMed] [Google Scholar]
- 99.Jaquet V. Scapozza L. Clark RA. Krause KH. Lambeth JD. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 2009;11:2535–2552. doi: 10.1089/ars.2009.2585. [DOI] [PubMed] [Google Scholar]
- 100.Johar S. Cave AC. Narayanapanicker A. Grieve DJ. Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 101.Kaludercic N. Carpi A. Menabò R. Di Lisa F. Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kanai F. Liu H. Field SJ. Akbary H. Matsuo T. Brown GE. Cantley LC. Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 103.Kang YJ. Chen Y. Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 104.Karathanassis D. Stahelin RV. Bravo J. Perisic O. Pacold CM. Cho W. Williams RL. Binding of the PX domain of p47(phox) to phosphatidylinositol 3, 4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim C. Marchal CC. Penninger J. Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 106.Kim YM. Guzik TJ. Zhang YH. Zhang MH. Kattach H. Ratnatunga C. Pillai R. Channon KM. Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 107.Kim YM. Kattach H. Ratnatunga C. Pillai R. Channon KM. Casadei B. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2001;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 108.Kimura S. Zhang GX. Nishiyama A. Shokoji T. Yao L. Fan YY. Rahman M. Suzuki T. Maeta H. Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- 109.Kinugawa S. Tsutsui H. Hayashidani S. Ide T. Suematsu N. Satoh S. Utsumi H. Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- 110.Knaus UG. Heyworth PG. Evans T. Curnutte JT. Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 111.Krijnen PAJ. Meischl C. Hack CE. Meijer CJLM. Visser CA. Roos D. Niessen HWM. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol. 2003;56:194–199. doi: 10.1136/jcp.56.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhns DB. Alvord WG. Heller T. Feld JJ. Pike KM. Marciano BE. Uzel G. DeRavin SS. Priel DAL. Soule BP. Zarember KA. Malech HL. Holland SM. Gallin JI. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuroda J. Ago T. Matsushima S. Zhai P. Schneider MD. Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. PNAS. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuroda J. Nakagawa K. Yamasaki T. Nakamura Ki. Takeya R. Kuribayashi F. Imajoh-Ohmi S. Igarashi K. Shibata Y. Sueishi K. Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 115.Kuster GM. Pimentel DR. Adachi T. Ido Y. Brenner DA. Cohen RA. Liao R. Siwik DA. Colucci WS. {alpha}-Adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. [DOI] [PubMed] [Google Scholar]
- 116.Kuwahara F. Kai H. Tokuda K. Takeya M. Takeshita A. Egashira K. Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction. Hypertension. 2004;43:739–745. doi: 10.1161/01.HYP.0000118584.33350.7d. [DOI] [PubMed] [Google Scholar]
- 117.Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 118.Lassègue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 119.Lassègue B. Griendling KK. NADPH Oxidases: Functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee JK. Edderkaoui M. Truong P. Ohno I. Jang KT. Berti A. Pandol SJ. Gukovskaya AS. NADPH oxidase promotes pancreatic cancer cell survival via inhibiting JAK2 dephosphorylation by tyrosine phosphatases. Gastroenterology. 2007;133:1637–1648. doi: 10.1053/j.gastro.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Leenen FHH. Brain mechanisms contributing to sympathetic hyperactivity and heart failure. Circ Res. 2007;101:221–223. doi: 10.1161/CIRCRESAHA.107.158261. [DOI] [PubMed] [Google Scholar]
- 122.Li J. Ichikawa T. Villacorta L. Janicki JS. Brower GL. Yamamoto M. Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 123.Li J. Zhu H. Shen E. Wan L. Arnold JM. Peng T. Deficiency of Rac1 Blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li JM. Gall NP. Grieve DJ. Chen M. Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 125.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 126.Li JM. Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 127.Li S. Tabar SS. Malec V. Eul BG. Klepetko W. Weissmann N. Grimminger F. Seeger W. Rose F. Hänze J. NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxid Redox Signal. 2008;10:1687–1698. doi: 10.1089/ars.2008.2035. [DOI] [PubMed] [Google Scholar]
- 128.Li SY. Yang X. Ceylan-Isik A. Du M. Sreejayan N. Ren J. Cardiac contractile dysfunction in Lep Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 129.Li Y. Arnold JM. Pampillo M. Babwah AV. Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med. 2009;46:51–61. doi: 10.1016/j.freeradbiomed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 130.Li YY. McTiernan CF. Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 131.Lima B. Lam GKW. Xie L. Diesen DL. Villamizar N. Nienaber J. Messina E. Bowles D. Kontos CD. Hare JM. Stamler JS. Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. PNAS. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lindley TE. Doobay MF. Sharma RV. Davisson RL. Superoxide is involved in the central nervous system activation and sympathoexcitation of myocardial infarction-induced heart failure. Circ Res. 2004;94:402–409. doi: 10.1161/01.RES.0000112964.40701.93. [DOI] [PubMed] [Google Scholar]
- 133.Lipshultz SE. Rifai N. Dalton VM. Levy DE. Silverman LB. Lipsitz SR. Colan SD. Asselin BL. Barr RD. Clavell LA. Hurwitz CA. Moghrabi A. Samson Y. Schorin MA. Gelber RD. Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145–153. doi: 10.1056/NEJMoa035153. [DOI] [PubMed] [Google Scholar]
- 134.Liu JJ. Li DL. Zhou J. Sun L. Zhao M. Kong SS. Wang YH. Yu XJ. Zhou J. Zang WJ. Acetylcholine prevents angiotensin II-induced oxidative stress and apoptosis in H9c2 cells. Apoptosis. 2011;16:94–103. doi: 10.1007/s10495-010-0549-x. [DOI] [PubMed] [Google Scholar]
- 135.Liu Y. Huang H. Xia W. Tang Y. Li H. Huang C. NADPH oxidase inhibition ameliorates cardiac dysfunction in rabbits with heart failure. Mol Cell Biochem. 2010;343:143–153. doi: 10.1007/s11010-010-0508-4. [DOI] [PubMed] [Google Scholar]
- 136.Looi YH. Grieve DJ. Siva A. Walker SJ. Anilkumar N. Cave AC. Marber M. Monaghan MJ. Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 137.Lu J. Mitra S. Wang X. Khaidakov M. Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 138.Lyle AN. Deshpande NN. Taniyama Y. Seidel-Rogol B. Pounkova L. Du P. Papaharalambus C. Lassègue B. Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maack C. Kartes T. Kilter H. Schafers HJ. Nickenig G. Bohm M. Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of Rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 140.Maffei A. Di Pardo A. Carangi R. Carullo P. Poulet R. Gentile MT. Vecchione C. Lembo G. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 141.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 142.Matsushima S. Kinugawa S. Yokota T. Inoue N. Ohta Y. Hamaguchi S. Tsutsui H. Increased myocardial NAD(P)H oxidase-derived superoxide causes the exacerbation of postinfarct heart failure in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H409–H416. doi: 10.1152/ajpheart.01332.2008. [DOI] [PubMed] [Google Scholar]
- 143.Matsuzawa A. Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- 144.Maytin M. Siwik DA. Ito M. Xiao L. Sawyer DB. Liao R. Colucci WS. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. [DOI] [PubMed] [Google Scholar]
- 145.Meischl C. Krijnen P. Sipkens J. Cillessen S. Muńoz I. Okroj M. Ramska M. Muller A. Visser C. Musters R. Simonides W. Hack C. Roos D. Niessen H. Ischemia induces nuclear NOX2 expression in cardiomyocytes and subsequently activates apoptosis. Apoptosis. 2006;11:913–921. doi: 10.1007/s10495-006-6304-7. [DOI] [PubMed] [Google Scholar]
- 146.Mihm MJ. Yu F. Carnes CA. Reiser PJ. McCarthy PM. Van Wagoner DR. Bauer JA. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104:174–180. doi: 10.1161/01.cir.104.2.174. [DOI] [PubMed] [Google Scholar]
- 147.Murdoch C. Alom-Ruiz S. Wang M. Zhang M. Walker S. Yu B. Brewer A. Shah A. Role of endothelial Nox2 NADPH oxidase in angiotensin II-induced hypertension and vasomotor dysfunction. Basic Res Cardiol. 2011;106:527–538. doi: 10.1007/s00395-011-0179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Murdoch CE. Brewer A. Zhang M. Vanhoutte D. Heymans S. Shah AM. Endothelial-specific overexpression of Nox2 enhances angiotensin II-induced cardiac dysfunction and fibrosis. Circulation. 2008;118:S314. [Google Scholar]
- 149.Nakagami H. Takemoto M. Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 150.Oakley FD. Abbott D. Li Q. Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pacher P. Liaudet L. Bai P. Mabley JG. Kaminski PM. Virág L. Deb A. Szabò E. Ungvári Zn. Wolin MS. Groves JT. Szabò C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 152.Pagano PJ. Chanock SJ. Siwik DA. Colucci WS. Clark JK. Angiotensin II Induces p67phox mRNA expression and NADPH oxidase superoxide generation in rabbit aortic adventitial fibroblasts. Hypertension. 1998;32:331–337. doi: 10.1161/01.hyp.32.2.331. [DOI] [PubMed] [Google Scholar]
- 153.Palomeque J. Rueda OV. Sapia L. Valverde CA. Salas M. Petroff MV. Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 154.Park HS. Chun JN. Jung HY. Choi C. Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 155.Patrucco E. Notte A. Barberis L. Selvetella G. Maffei A. Brancaccio M. Marengo S. Russo G. Azzolino O. Rybalkin SD. Silengo L. Altruda F. Wetzker R. Wymann MP. Lembo G. Hirsch E. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 156.Pendyala S. Gorshkova IA. Usatyuk PV. He D. Pennathur A. Lambeth JD. Thannickal VJ. Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2008;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Privratsky JR. Wold LE. Sowers JR. Quinn MT. Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 158.Prosser BL. Ward CW. Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 159.Qin F. Patel R. Yan C. Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med. 2006;40:236–246. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 160.Qin F. Simeone M. Patel R. Inhibition of NADPH oxidase reduces myocardial oxidative stress and apoptosis and improves cardiac function in heart failure after myocardial infarction. Free Radic Biol Med. 2007;43:271–281. doi: 10.1016/j.freeradbiomed.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 161.Ray R. Murdoch CE. Wang M. Santos CX. Zhang M. Alom-Ruiz S. Anilkumar N. Ouattara A. Cave AC. Walker SJ. Grieve DJ. Charles RL. Eaton P. Brewer AC. Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 162.Reilly SN. Jayaram R. Nahar K. Antoniades C. Verheule S. Channon KM. Alp NJ. Schotten U. Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation/clinical perspective. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 163.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 164.Rhee SG. Woo HA. Kil IS. Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287:4403–4410. doi: 10.1074/jbc.R111.283432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roe ND. Thomas DP. Ren J. Inhibition of NADPH oxidase alleviates experimental diabetes-induced myocardial contractile dysfunction. Diabetes Obes Metab. 2011;13:465–473. doi: 10.1111/j.1463-1326.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 166.Rotrosen D. Yeung CL. Leto TL. Malech HL. Kwong CH. Cytochrome b558: the flavin-binding component of the phagocyte NADPH oxidase. Science. 1992;256:1459–1462. doi: 10.1126/science.1318579. [DOI] [PubMed] [Google Scholar]
- 167.Ruckle T. Schwarz MK. Rommel C. PI3K[gamma] inhibition: towards an “aspirin of the 21st century”? Nat Rev Drug Discov. 2006;5:903–918. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 168.Rude MK. Duhaney TA. Kuster GM. Judge S. Heo J. Colucci WS. Siwik DA. Sam F. Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension. 2005;46:555–561. doi: 10.1161/01.HYP.0000176236.55322.18. [DOI] [PubMed] [Google Scholar]
- 169.Sanchez G. Escobar Ma. Pedrozo Z. Macho P. Domenech Rl. Härtel S. Hidalgo C. Donoso P. Exercise and tachycardia increase NADPH oxidase and ryanodine receptor-2 activity: possible role in cardioprotection. Cardiovasc Res. 2008;77:380–386. doi: 10.1093/cvr/cvm011. [DOI] [PubMed] [Google Scholar]
- 170.Sanchez G. Pedrozo Z. Domenech RLJ. Hidalgo C. Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol. 2005;39:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 171.Sano M. Minamino T. Toko H. Miyauchi H. Orimo M. Qin Y. Akazawa H. Tateno K. Kayama Y. Harada M. Shimizu I. Asahara T. Hamada H. Tomita S. Molkentin JD. Zou Y. Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 172.Satoh M. Ogita H. Takeshita K. Mukai Y. Kwiatkowski DJ. Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. PNAS. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schröder K. Zhang M. Benkhoff S. Mieth A. Pliquett R. Kosowski J. Kruse C. Luedike P. Michaelis UR. Weissmann N. Dimmeler S. Shah AM. Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase/novelty and significance. Circ Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 174.Serpillon S. Floyd BC. Gupte RS. George S. Kozicky M. Neito V. Recchia F. Stanley W. Wolin MS. Gupte SA. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Serrander L. Cartier L. Bedard K. Banfi B. Lardy B. Plastre O. Sienkiewicz A. Forro L. Schlegel W. Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sesso HD. Buring JE. Christen WG. Kurth T. Belanger C. MacFadyen J. Bubes V. Manson JE. Glynn RJ. Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shah AM. Mann DL. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shanmugam P. Valente AJ. Prabhu SD. Venkatesan B. Yoshida T. Delafontaine P. Chandrasekar B. Angiotensin-II type 1 receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:928–938. doi: 10.1016/j.yjmcc.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shen E. Li Y. Li Y. Shan L. Zhu H. Feng Q. Arnold JM. Peng T. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58:2386–2395. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shiojima I. Sato K. Izumiya Y. Schiekofer S. Ito M. Liao R. Colucci WS. Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shiomi T. Tsutsui H. Matsusaka H. Murakami K. Hayashidani S. Ikeuchi M. Wen J. Kubota T. Utsumi H. Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- 182.Shiroshita-Takeshita A. Schram G. Lavoie J. Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation. 2004;110:2313–2319. doi: 10.1161/01.CIR.0000145163.56529.D1. [DOI] [PubMed] [Google Scholar]
- 183.Sirker A. Zhang M. Shah A. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sorescu D. Weiss D. Lassègue B. Clempus RE. Szocs K. Sorescu GP. Valppu L. Quinn MT. Lambeth JD. Vega JD. Taylor WR. Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 185.Sorrentino SA. Doerries C. Manes C. Speer T. Dessy C. Lobysheva I. Mohmand W. Akbar R. Bahlmann F. Besler C. Schaefer A. Hilfiker-Kleiner D. Lüscher TF. Balligand JL. Drexler H. Landmesser U. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J Am Coll Cardiol. 2011;57:601–611. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 186.Sun QA. Hess DT. Nogueira L. Yong S. Bowles DE. Eu J. Laurita KR. Meissner G. Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. PNAS. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun Y. Oxidative stress and cardiac repair/remodeling following infarction. Am J Med Sci. 2007;334:197–205. doi: 10.1097/MAJ.0b013e318157388f. [DOI] [PubMed] [Google Scholar]
- 188.Sun Y. Zhang J. Lu L. Chen SS. Quinn MT. Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Swaminathan PD. Purohit A. Soni S. Voigt N. Singh MV. Glukhov AV. Gao Z. He BJ. Luczak ED. Joiner ML. Kutschke W. Yang J. Donahue JK. Weiss RM. Grumbach IM. Ogawa M. Chen PS. Efimov I. Dobrev D. Mohler PJ. Hund TJ. Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Takac I. Schröder K. Zhang L. Lardy B. Anilkumar N. Lambeth JD. Shah AM. Morel F. Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tanaka K. Honda M. Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;37:676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 192.The Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 193.Toren F. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 194.Tsai CT. Wang DL. Chen WP. Hwang JJ. Hsieh CS. Hsu KL. Tseng CD. Lai LP. Tseng YZ. Chiang FT. Lin JL. Angiotensin II increases expression of α1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–1485. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 195.Tsutsui H. Kinugawa S. Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 196.Uhlinger DJ. Taylor KL. Lambeth JD. p67-phox enhances the binding of p47-phox to the human neutrophil respiratory burst oxidase complex. J Biol Chem. 1994;269:22095–22098. [PubMed] [Google Scholar]
- 197.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vallet P. Charnay Y. Steger K. Ogier-Denis E. Kovari E. Herrmann F. Michel JP. Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 199.Wang M. Zhang J. Walker SJ. Dworakowski R. Lakatta EG. Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Watson F. Robinson J. Edwards SW. Protein kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J Biol Chem. 1991;266:7432–7439. [PubMed] [Google Scholar]
- 201.Welch HCE. Coadwell WJ. Ellson CD. Ferguson GJ. Andrews SR. Erdjument-Bromage H. Tempst P. Hawkins PT. Stephens LR. P-Rex1, a PtdIns(3, 4, 5)P3- and Gβ-regulated guanine-nucleotide exchange factor for Rac. Cell. 2002;108:809–821. doi: 10.1016/s0092-8674(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 202.Wendt MC. Daiber A. Kleschyov AL. Mülsch A. Sydow K. Schulz E. Chen K. Keaney J. Lassègue B. Walter U. Griendling KK. Münzel T. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic Biol Med. 2005;39:381–391. doi: 10.1016/j.freeradbiomed.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 203.Whaley-Connell A. Govindarajan G. Habibi J. Hayden MR. Cooper SA. Wei Y. Ma L. Qazi M. Link D. Karuparthi PR. Stump C. Ferrario C. Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab. 2007;293:E355–E363. doi: 10.1152/ajpendo.00632.2006. [DOI] [PubMed] [Google Scholar]
- 204.Wojnowski L. Kulle B. Schirmer M. Schlüter G. Schmidt A. Rosenberger A. Vonhof S. Bickeböller H. Toliat MR. Suk EK. Tzvetkov M. Kruger A. Seifert S. Kloess M. Hahn H. Loeffler M. Nürnberg P. Pfreundschuh M. Trümper L. Brockmöller J. Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 205.Wold LE. Ceylan-Isik AF. Fang CX. Yang X. Li SY. Sreejayan N. Privratsky JR. Ren J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–1429. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 206.Wu RF. Ma Z. Liu Z. Terada LS. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local ras activation. Mol Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xia C. Meng Q. Liu LZ. Rojanasakul Y. Wang XR. Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 208.Xiao L. Pimentel DR. Wang J. Singh K. Colucci WS. Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha 1-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 209.Xu J. Carretero OA. Liao TD. Peng H. Shesely EG. Xu J. Liu TS. Yang JJ. Reudelhuber TL. Yang XP. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1328–H1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yagi S. Akaike M. Aihara Ki. Ishikawa K. Iwase T. Ikeda Y. Soeki T. Yoshida S. Sumitomo-Ueda Y. Matsumoto T. Sata M. Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension. 2010;55:918–923. doi: 10.1161/HYPERTENSIONAHA.109.146076. [DOI] [PubMed] [Google Scholar]
- 211.Yazdanpanah B. Wiegmann K. Tchikov V. Krut O. Pongratz C. Schramm M. Kleinridders A. Wunderlich T. Kashkar H. Utermohlen O. Bruning JC. Schutze S. Kronke M. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 212.Zeng Q. Zhou Q. Yao F. O'Rourke ST. Sun C. Endothelin-1 regulates cardiac L-type calcium channels via NAD(P)H oxidase-derived superoxide. J Pharmacol Exp Ther. 2008;326:732–738. doi: 10.1124/jpet.108.140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang L. Sheppard OR. Shah AM. Brewer AC. Positive regulation of the NADPH oxidase NOX4 promoter in vascular smooth muscle cells by E2F. Free Radic Biol Med. 2008;45:679–685. doi: 10.1016/j.freeradbiomed.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 214.Zhang M. Brewer AC. Schröder K. Santos CXC. Grieve DJ. Wang M. Anilkumar N. Yu B. Dong X. Walker SJ. Brandes RP. Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. PNAS. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang M. Kho AL. Anilkumar N. Chibber R. Pagano PJ. Shah AM. Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 216.Zhao Y. McLaughlin D. Robinson E. Harvey AP. Hookham MB. Shah AM. McDermott BJ. Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhao Z. Fefelova N. Shanmugam M. Bishara P. Babu GJ. Xie LH. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2011;50:128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhou C. Ziegler C. Birder LA. Stewart AFR. Levitan ES. Angiotensin II and stretch activate NADPH oxidase to destabilize cardiac Kv4.3 channel mRNA. Circ Res. 2006;98:1040–1047. doi: 10.1161/01.RES.0000218989.52072.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zima AV. Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 220.Zorov DB. Filburn CR. Klotz LO. Zweier JL. Sollott SJ. Reactive oxygen species (Ros-induced) Ros release. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]