Abstract
Significance: The regulation of myocardial function by constitutive nitric oxide synthases (NOS) is important for the maintenance of myocardial Ca2+ homeostasis, relaxation and distensibility, and protection from arrhythmia and abnormal stress stimuli. However, sustained insults such as diabetes, hypertension, hemodynamic overload, and atrial fibrillation lead to dysfunctional NOS activity with superoxide produced instead of NO and worse pathophysiology. Recent Advances: Major strides in understanding the role of normal and abnormal constitutive NOS in the heart have revealed molecular targets by which NO modulates myocyte function and morphology, the role and nature of post-translational modifications of NOS, and factors controlling nitroso-redox balance. Localized and differential signaling from NOS1 (neuronal) versus NOS3 (endothelial) isoforms are being identified, as are methods to restore NOS function in heart disease. Critical Issues: Abnormal NOS signaling plays a key role in many cardiac disorders, while targeted modulation may potentially reverse this pathogenic source of oxidative stress. Future Directions: Improvements in the clinical translation of potent modulators of NOS function/dysfunction may ultimately provide a powerful new treatment for many hearts diseases that are fueled by nitroso-redox imbalance. Antioxid. Redox Signal. 18, 1078–1099.
Introduction: Constitutive NOS and the Heart
Nitric oxide (NO) is generated by enzymes that catalyze the conversion of L-arginine to L-citrulline in a reaction which requires O2 and several cofactors. In mammals, three isoforms of NO synthases (NOS1–3) are encoded by distinct genes (123), two of which (NOS1 or neuronal NOS, and NOS3 or endothelial NOS) are constitutively expressed and synthesize NO in a Ca2+-calmodulin (CaM)-dependent manner (70) (Fig. 1). Both NOS1 and NOS3 are obligate homodimers, each monomer containing an amino-terminal oxygenase domain (N-terminal) and a carboxy-terminal reductase domain (C-terminal). The oxygenase domain contains binding sites for the substrate L-arginine and the cofactor tetrahydrobiopterin (BH4), as well as a cytochrome P-450-type heme active site. The reductase domain binds the flavin cofactors (flavin mononucleotide, flavin adenine dinucleotide) and nicotinamide adenine dinucleotide phosphate (NADPH). On activation of the synthase, the flavins in the reductase domain of NOS serve to transfer electrons from NADPH to the heme iron in the oxygenase domain of the other monomer, allowing the activation of heme-bound molecular oxygen and the synthesis of NO (149).
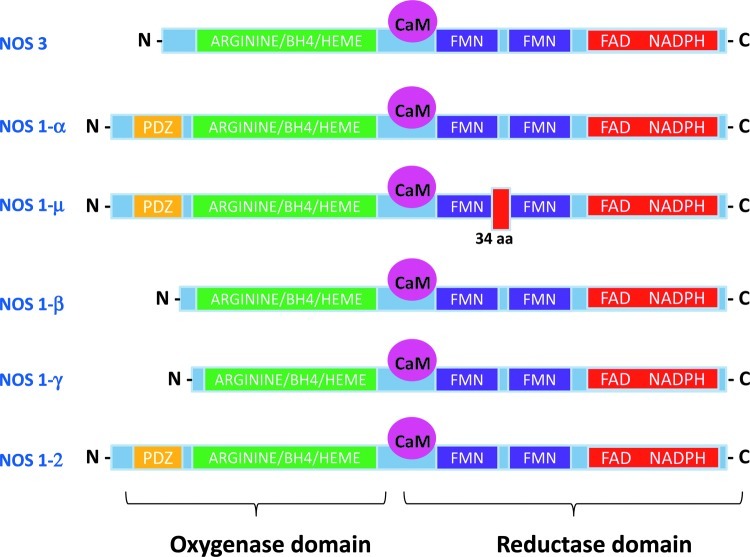
FIG. 1.
Protein structure of nitric oxide synthase 3 (NOS3) and NOS1 splice variants. All NOSs include an oxygenase and a reductase domain and a calmodulin (CaM) binding site. The oxygenase moiety contains the L-arginine, heme, and tetrahydrobiopterin (BH4)-binding domains; whereas the reductase moiety contains the flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide phosphate (NADPH)- binding domains. An additional PDZ domain is found in the N-terminal of the α,μ-NOS1 and NOS1–2 variants and allows the enzyme to bind other proteins with a similar structure. aa, aminoacids. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Both NOS1 and NOS3 are constitutively expressed in the cardiovascular system. NOS3 is mostly found in coronary vascular and endocardial endothelial cells and, to a lesser extent, in cardiac myocytes, where it associates with caveolae (13, 68); whereas NOS1 is predominantly localized to the sarcoplasmic reticulum (SR) (254) and, to a lesser extent, mitochondria (28), the Golgi apparatus (174), and sarcolemmal membrane (167). Although the NOS1 gene can be transcribed into five different mRNA splice forms (Fig. 1), there is limited information about their physiological role or subcellular localization in cardiac myocytes (4). The α and μ-NOS1 contain an N-terminal PSD-95/Discs large/ZO-1 homology domain (PDZ domain), which can tether the enzyme to specific myocardial proteins, such as syntrophin (22). By contrast, the β and γ NOS1 isoforms (which lack the PDZ domain) are thought to reside in the cytoplasm (64). The NOS1–2 variant, although similar to the α isoform, is thought to be enzymatically inactive (23). Alternative splicing in intron13 of the human NOS3 gene has also been reported (145); however, its functional significance in the heart remains to be tested. Both constitutive NOS can translocate to different subcellular domains under certain conditions. In particular, NOS1 appears to shuttle from the SR to the sarcolemmal caveolae in cardiomyocytes from failing hearts (18, 53); by contrast, in endothelial cells, NOS3 has been reported to traffic from the plasmalemmal caveolae to intracellular locations, such as the Golgi apparatus and the endoplasmic reticulum (67).
It is no longer thought that NO synthesized within cardiomyocytes acts as a freely diffusible messenger. Instead, constitutive NO release operates within discrete subcellular domains either by stimulating soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP) or via the S-nitrosylation of specific protein targets. The cGMP-dependent effects of NO are largely mediated by changes in the proteins' phosphorylation state as a result of stimulation of the cGMP-dependent protein kinase (PKG) and/or of changes in the activity of cGMP–stimulated or–inhibited phosphodiesterases (PDE) (72). In the myocardium, constitutive NO production affects the function and phosphorylation state of several proteins that are involved in excitation-contraction coupling (ECC) (e.g., the L-type Ca2+ channel (LTCC) (199, 242), troponin I (135), and phospholamban (PLB) (241, 261), and inhibits both oxygen consumption (143, 253) and β-adrenergic inotropy (82) via a cGMP-dependent mechanism. Furthermore, a direct reaction between NO and thiol groups on cysteine residues causes changes in protein conformation and function that are akin to those induced by phosphorylation (71). Growing evidence supports protein S-nitrosylation as an important mechanism of NO signaling (98), which is implicated in the regulation of the ryanodine receptor Ca2+ release channel (RyR) (65, 85, 244), SR Ca2+ ATPase (SERCA) (17), LTCC (32, 214), and the Kv1.5 channel (166) and the post-translational regulation of β-adrenergic signaling (170, 246). Dysregulated S-nitrosylation of myocardial proteins can result not only from alterations in the expression, compartmentalization, and/or activity of NOS, but also from changes in the activity of denitrosylases such as the S-nitrosoglutathione (GSNO) metabolizing enzyme, GSNO reductase. Indeed, the knockout of GSNO reductase results in enhanced levels of SNO proteins and significantly attenuates experimental asthma and heart failure, while increasing the severity of endotoxic shock in mice (71).
Constitutive NOS Activity and Regulation of Cardiac Function
Paracrine and autocrine actions of NOS3-derived NO
It has long been known that NOS3-derived NO produced in the coronary endothelium modulates the functional characteristics of cardiac myocytes. One of the first demonstrations of this paracrine effect was reported by Paulus et al. in 1995 (172), who stimulated endothelial cell NOS3 by intracoronary infusion of substance P and observed increased left ventricular (LV) diastolic compliance (independent of changes in coronary flow). These effects were later linked to PKG-mediated phosphorylation of troponin I, resulting in a reduction of myofilament Ca2+ sensitivity (135).
The mechanical activation of endothelial cells (shear stress or stretch) and cardiomyocytes (stretch) stimulates the release of NOS3-derived NO (176, 178, 180), and has been proposed to play a role in enhanced stroke volume from a rise in ventricular preload (i.e., the Frank–Starling response). Here, coronary paracrine signaling appears relevant, as denuding coronary endothelium eliminated a preload-stimulated rise in myocardial NO (178). In isolated crystalloid perfused guinea pig hearts, coronary perfusion with L-NG-monomethyl arginine citrate or hemoglobin (NO scavenger) depressed the Frank–Starling reserve (180), though this was not observed in an isolated blood-perfused canine preparation (191). Stretched isolated cardiomyocytes activated NOS3 via Akt-phosphorylation to increase Ca2+ sparks, intracellular Ca2+ transient amplitude, and cell shortening—changes abolished by the genetic deletion of NOS3 or L-Nω-Nitro-L-arginine methyl ester (L-NAME) (176). Lastly, endothelial-derived NO (from bradykinin) can reversibly decrease oxygen consumption by reducing mitochondrial respiration (143, 253), effects that are also attenuated by L-NAME and abolished in NOS3 knockout mice.
Despite these and other studies, in vivo evidence that constitutively expressed NOS3 (in myocyte and endothelial cells) regulates cardiac function under basal conditions remains scant, and evidence from mice genetically lacking NOS3 suggests that any impact is minimal. Basal function is similar between control and NOS3−/− mice, though inotropic and lusitropic responses to isoproterenol (ISO) are enhanced (14, 90). Others have found no differences in rest or ISO stimulated cardiomyocyte function between these models (148, 232). However, stimulation of the β-3 adrenergic receptor (AR) plays an important role in triggering NOS3-derived NO, which, in turn, blunts β1-adrenergic inotropic responses (79) via PKG activation pathways (136); thus, attenuation of β1-adrenergic responses by NO may vary with the intensity of stimulation and the species under investigation.
Actions of NOS1-derived NO
After its detection in SR membrane vesicles of cardiac myocytes in 1999 (254), NOS1 has been shown to act as a major modulator of cardiac function and intracellular Ca2+ fluxes (Fig. 2). In 2002, Ashley et al. reported enhanced contractility and prolonged relaxation in LV myocytes from NOS1−/− mice compared with their wild-type littermates (10). The positive inotropic effect of NOS1 gene deletion on myocardial contraction (both in cardiomyocytes and in vivo) was confirmed by other (25, 55, 199) [but not by all (14, 244)] investigators. Furthermore, Sears et al. reported an increase in Ca2+ current density and larger intracellular Ca2+ transients and Ca2+ stores in the presence of NOS1 inhibition of gene deletion (199). More recently, NO released by NOS1 has been shown to accelerate cardiomyocyte and left ventricular relaxation by increasing PLB phosphorylation of via a cGMP-independent inhibition of serine/threonine protein phosphatases (261).
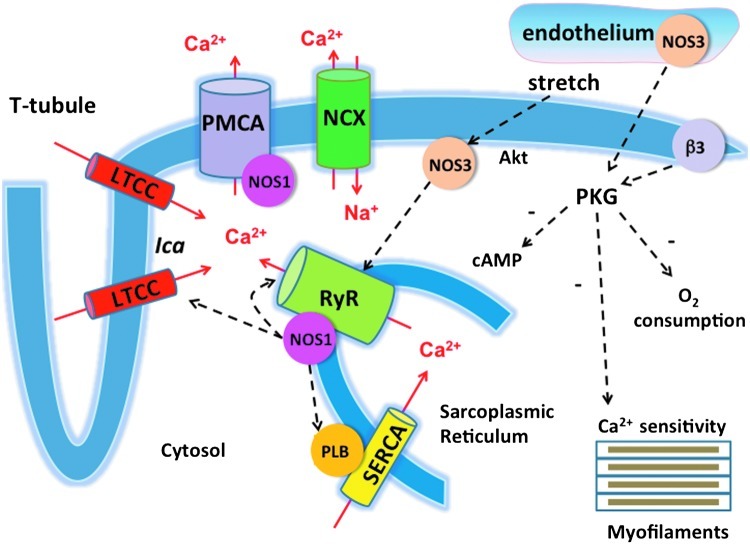
FIG. 2.
Regulation of myocardial Ca2+ fluxes by NOS-derived NO. NOS1-derived NO has been shown to inhibit Ca2+ influx via the L-type Ca2+ channel (LTCC), increase Ca2+ reuptake in the sarcoplasmic reticulum (SR) by increasing phospholamban (PLB) phosphorylation, and regulate the release of Ca2+ from the SR through changes in ryanodine receptor Ca2+ release channel (RyR) S-nitrosylation. NOS1 activity is, in turn, modulated by the Ca2+ flux via the plasmalemma Ca2+ ATPase (plasma membrane calcium/CaM dependent ATPase [PMCA]). Endothelial NOS3-derived NO has been reported to reduce myofilament Ca2+ sensitivity via PKG-mediated phosphorylation, inhibit myocardial oxygen consumption, and regulate intracellular cAMP levels (and thus, β-adrenergic responses) by modulating phosphodiesterase activity. The stimulation of myocardial NOS3 activity by mechanical stretch has been shown to increase the open probability of the RyR Ca2+ release channel. SERCA2a, SR Ca ATPase; NCX, sodium calcium exchanger. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
NOS1-derived NO is also involved in the regulation of RyR function. NOS1 gene deletion has been reported to decrease RyR S-nitrosylation; however, the impact of this finding on RyR open probability remains a matter of debate. Gonzalez et al. reported an increase of diastolic Ca2+ leak from the RyR in NOS1 knockout mice in the absence of differences in channel phosphorylation (85); whereas Wang et al. (244), using the same model, reported a decrease in RyR open probability.
A further mechanism by which NOS1 can modulate ECC is through its interaction with xanthine oxidoreductase (XOR). Khan et al. observed that both enzymes coimmunoprecipitate and colocalize in the SR. In the absence of NOS1, XOR is activated and increases its production of superoxide, which, in turn, reduces myofilament Ca2+ sensitivity (117). Finally, NOS1 has been shown to interact with the sarcolemmal calcium pump (also known as plasma membrane calcium/CaM-dependent ATPase [PMCA]); overexpression of PMCA4b inhibits NOS1 activity and blunts the β-adrenergic contractile response in cardiac myocytes (167).
In addition to its effects in cardiac myocytes, NOS1 acts as a local modulator of the autonomic control of the cardiovascular system. In the heart, NOS1-derived NO increases acetylcholine release from cholinergic neurons via a sGC-cGMP-dependent mechanism. By contrast, NO generated in sympathetic ganglia reduces the synaptic release of noradrenaline [reviewed in Herring and Paterson (97)]. In atrial parasympathetic ganglia, NOS1 is up-regulated by exercise training where it contributes to the enhanced bradycardic response to peripheral vagal stimulation (54). Similarly, an increase in atrial nNOS protein after myocardial infarction has been reported to enhance vagal bradycardia in rats (219). In the central nervous system, NOS1-derived NO exerts an inhibitory role in the regulation of sympathetic outflow, which is blunted in animal models of heart failure (202, 260).
Mechanisms of Constitutive NOS Dysfunction
Since the normal function of constitutive NOS is to generate NO for cell function and adaptation, NOS dysfunction can be considered a state where less NO is generated and/or NO targeting is disrupted. Reduced NO generation by NOS typically coexists with its increased synthesis of O2−, a condition referred to as NOS uncoupling. This and the interaction of NO with oxidants (to form nitroso-oxidant species such as peroxinitrite [ONOO−]) can further impact the fate of any NO generated. To remain functional, NOS requires critical cofactors, an adequate substrate, and a redox environment that facilitates NO targeting. Since each is altered in heart disease, understanding their mechanisms is important.
BH4 biosynthesis and regeneration and NOS dysfunction
A critical aspect for NOS generation of NO is the presence of the cofactor BH4. BH4 binds close to the heme active site at the interface between the two monomers, interacting with residues from both. Maintenance and stabilization of NOS dimers is dependent on BH4, and BH4 also plays a direct role in the multistep oxidation of L-arginine through the N-hydroxy-L-arginine intermediate and the subsequent generation of NO (211). Depletion of BH4 and/or its oxidation to BH2 shifts the electron transport process, so that rather than generating NO, the enzyme acts as an oxidase (Fig. 3). This condition, known as NOS uncoupling, now appears to be a major mechanism whereby NOS dysfunction translates to pathological disease. It is, therefore, useful to review the mechanisms controlling BH4 synthesis and regeneration.
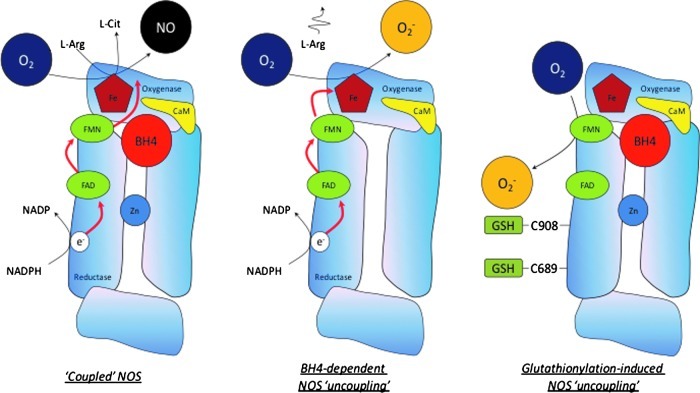
FIG. 3.
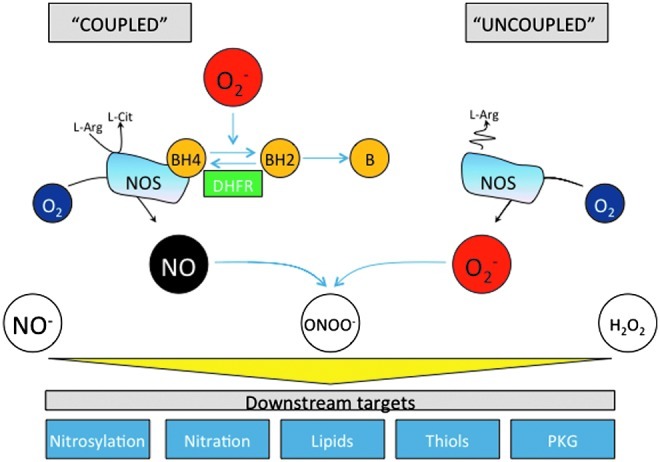
The role of BH4 and its oxidized products in the regulation of NOS activity. In the absence of BH4, or when BH2 binds to the enzyme, NOS becomes “uncoupled” and produces superoxide instead of NO. NOS-derived superoxide, as well as superoxide from other sources such as NADPH oxidase and xanthine oxidoreductase, may, in turn, oxidize BH4 to BH2 and biopterin. Dihydrofolate reductase (DHFR) can recycle BH2 back to BH4. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Intracellular biopterin levels are principally regulated by the activity of the de novo biosynthetic pathway. Guanosine triphosphate cyclohydrolase I (GTPCH) catalyzes the formation of dihydroneopterin 3′ triphosphate (DNTP) from GTP, and BH4 is generated by two further steps through 6-pyruvoyltetrahydropterin synthase and sepiapterin reductase (SPR). GTPCH seems to be the rate-limiting enzyme in BH4 biosynthesis, and the overexpression of GTPCH is sufficient to augment BH4 levels in cultured endothelial cells (31, 49) and in the vascular endothelium and myocardium in vivo (5, 104). Electron paramagnetic resonance spectroscopy studies have shown that BH4 both stabilizes and donates electrons to the ferrous–dioxygen complex in the oxygenase domain, as the initiating step of l-arginine oxidation (86, 187, 235). In this reaction, BH4 forms the protonated trihydrobiopterin cation radical, which is subsequently reduced by electron transfer from NOS flavins (101, 198).
When BH4 availability is limiting, electrons flowing from the reductase domain to the heme are diverted to molecular oxygen instead of to L-arginine, resulting in the formation of superoxide rather than NO. This may lead to the oxidation of BH4 to catalytically incompetent BH2, resulting in a feed-forward cascade of BH4 destruction and further uncoupling of the enzyme (234, 236). Indeed, it has previously been suggested that the relative abundance of NOS3 versus BH4, along with the intracellular BH4:BH2 ratio (instead of the absolute concentration of BH4), may be key determinants of NOS uncoupling (49). This observation is supported by several in vitro and in vivo studies (233, 235) and computational modeling (113) and suggests that mechanisms which regulate the BH4:BH2 ratio, independent of overall biopterin levels, may play a role in controlling NOS coupling that is as important as the well-established role of GTPCH in de novo BH4 biosynthesis. However, others have reported that the absolute level of BH4 and not the biopterin ratio is the principal determinant of NOS activity and uncoupling in endothelial cells and cardiomyocytes (5, 33, 185).
GTPCH protein levels are induced by oxidative stress (204), and bacterial lipopolysaccharide elicits a 2-3-fold increase in GTPCH activity in a variety of rat tissues that constitutively express GTPCH, including cerebellum, liver, spleen, and the adrenal gland. Macrophages, dermal fibroblasts, and tumor cell lines demonstrate a profound increase in GTPCH activity after treatment with IFNγ and tumor necrosis factor alpha (TNFα) (87, 93). BH4 synthesis by GTPCH is subject to feedback inhibition by BH4 and other reduced pterins via a mechanism that requires a regulatory protein known as GTP cyclohydrolase feedback regulatory protein (GFRP) (153). This inhibition of GTPCH by BH4 or GFRP can be reversed by high levels of phenylalanine (153, 259). Given the importance of matching NOS activity with BH4 availability, it is perhaps not surprising to find that stimuli for one also impact the other. For example, aortic GTPCH-1 activity and BH4 levels are enhanced by laminar shear stress by a mechanism shown to involve the phosphorylation of GTPCH-1 at serine 81 (S81) by the α-prime subunit of casein kinase 2 (248). It is proposed that laminar shear, and perhaps S81 phosphorylation of GTPCH-1, disrupts the negative feedback conferred by GFRP in endothelial cells.
In the de novo formation of BH4 from GTP, the conversion of 7,8- DNTP to 6-pyruvoyl tetrahydropterin (PTP) is catalyzed by the zinc-dependent metalloprotein, 6-pyruvoyl tetrahydropterin synthase (PTPS). Although GTPCH is the rate-limiting step to BH4 synthesis in most tissues, PTPS maybe rate limiting in some cells, most notably human hepatocytes, after stimulation with cytokines and other immunological stimuli which induce BH4 synthesis by the up-regulation of GTPCH expression (223). The final steps in the biosynthesis of BH4 are two successive propyl side chain reduction reactions, which are catalyzed by SPR. Studies are only now beginning to elucidate the role of SPR as a site for the regulation of BH4 synthesis; in particular, the genetic knockdown of SPR by RNA interference in endothelial cells is associated with reduction in both intracellular BH4 content and NO production, while in vivo delivery of the SPR gene significantly increases vascular SPR protein expression, activity, BH4 content, NO production, and NO-dependent vasorelaxation (78).
In addition to de novo synthesis, BH4 is modulated by dihydrofolate reductase (DHFR), a key regulator of folate metabolism that also regenerates BH4 from oxidized BH2 (165). Net BH4 bioavailability reflects the balance between de novo synthesis, the oxidation of BH4 to BH2, and the regeneration of BH4 by DHFR; the latter now appears very important for ultimately determining cellular BH4 homeostasis, NO bioavailability, and, ultimately, NOS coupling. Recent studies have investigated the recycling function of DHFR in cultured endothelial cells and mouse models of BH4 overexpression and deficiency (47, 48, 212). Reduced BH4 and NOS uncoupling after angiotensin II exposure are reversed by overexpressing DHFR (34). By contrast, pharmacological inhibition of DHFR activity by methotrexate, or genetic knockdown of DHFR by RNA interference, reduces intracellular BH4 and increases BH2, leading to NOS3 uncoupling in endothelial cells. In cells expressing NOS3 with low biopterin levels, DHFR inhibition or knockdown further decreased the BH4:BH2 ratio and exacerbated NOS3 uncoupling. These data have been corroborated in vivo where NOS uncoupling was induced in the aorta of hph-1 mice after treatment with methotrexate, and prevented by endothelial overexpression of GTPCH (48).
Interestingly, studies have found that DHFR levels and activity decline in experimental models of cardiovascular disease, suggesting that insufficient recycling of BH2–BH4 by DHFR is, at least in part, responsible for the reduced BH4 levels and the accumulation of BH2, leading to NOS uncoupling. For example, DHFR protein levels are significantly decreased in streptozotocin-induced diabetic mice, and diabetes-induced impairment of cardiomyocyte function is exacerbated after DHFR inhibition with methotrexate (186). Furthermore, reduced DHFR activity in adult cardiomyocytes underlies their limited capacity to synthesize BH4 after cytokine stimulation after treatment of rat cardiac allograft recipients with sepiapterin (104). In support of these findings, the up-regulation of BH4 recycling enzymes is sufficient to restore BH4 levels, and effectively “re-couple” NOS3 within the aorta of streptozotocin-induced diabetic mice. Insufficient DHFR activity might also explain impaired vasorelaxation in atherosclerotic vessels from hypercholesterolemic rabbits, despite exposure to sepiapterin as the latter increases intracellular BH2 levels, requiring DHFR to regenerate BH4 (233).
NOS phosphorylation
NOS activity is regulated by a number of protein kinases, and changes in NOS phosphorylation may play an important role in determining and regulating dysfunctional NOS activity in the diseased heart. To date, the majority of evidence stems from vascular studies and the precise role of these post-translational modifications in myocytes remain less clear. In vitro and in vivo experiments have shown that NOS3 activation by phosphorylation at Ser-1177 (human, Ser-1179 in bovine) is mediated by the PI3K/Akt kinase pathway. Transduction of rabbit femoral arteries with constitutively active Akt increased NO-mediated vasodilatation in response to acetylcholine, whereas transduction with dominant-negative Akt attenuates the effect and decreases NO production in endothelial cells (146). Phosphorylation of NOS3 threonine-495 (human) or −497 (bovine) within the CaM binding domain has also been described and appears to be constitutively present in endothelial cells (142). Threonine-495 is a negative regulatory site, and its phosphorylation (probably by protein kinase C [PKC]) is associated with reduced electron flux and NOS3 activity, whereas de-phosphorylation by protein phosphatase-1 enhances the enzyme's activity (152). In cardiac myocytes, application of hydrogen peroxide or angiotensin II transiently increases NOS3 phosphorylation and NO production via an AMPK and Akt-dependent mechanism (196). Pretreatment with PEG-catalase abolished both NOS3 phosphorylation and the positive inotropic effect of angiotensin II, suggesting that myocardial hydrogen peroxide production can modulate both NOS3 activity and inotropy. The angiotensin II-mediated increase in NO was abolished in cardiomyocytes from NOS3−/− mice but unaltered in NOS1−/− myocytes. By contrast, the latter showed a reduction in NO synthesis in response to β-AR stimulation, suggesting that angiotensin II specifically activates myocardial NOS3 activity via a hydrogen peroxide-dependent increase in S1177 and S663 phosphorylation; whereas NOS1 activity is preferentially coupled to β-ARs. Others have reported that the incubation of cardiac myocytes with angiotensin II for 3 h increases NOS1 expression and the speed of relaxation by mechanisms which are dependent on superoxide production by NADPH oxidases (109). Taken together, these findings indicate that myocardial reactive oxygen species (ROS) production may influence both the expression and activity of myocardial constitutive NOSs.
Recent studies have revealed that hearts subjected to various durations of ischemia show a time-dependent reduction in BH4 levels which trigger increased NOS-derived superoxide production. Paradoxically, Akt-mediated phosphorylation, which would usually enhance NOS3-derived NO, further exacerbates the NOS-derived superoxide production, indicating that Ser-1177 phosphorylation is a critical regulator of both coupled and uncoupled NOS3 function (38). However, this may not be relevant with regard to NOS1; although NOS1 contains a potential site for phosphorylation at S1412 at its C-terminal tail that is analogous to the established Akt site found in NOS3 (58, 75, 77), a second phosphorylation site at S847 resides in an α-helix of the autoinhibitory domain of NOS reductase (95, 184). Here, phosphorylation inhibits the binding of CaM and attenuates NOS1 catalytic activity (50, 130).
NOS glutathionylation
Glutathionylation is the specific post-translational modification of protein cysteine residues by the addition of the tripeptide glutathione (GSH), the most abundant and important reducing agent within the cell. Promoted by oxidative stress, glutathionylation can regulate a number of cell processes, including apoptosis, Ca2+ homeostasis, and cellular antioxidant defences (45). Having observed that supplementing dysfunctional NOS with BH4 or L-arginine is often not sufficient to fully restore coupled NOS activity, Chen et al. reported that S-glutathionylation may be a unique mechanism for the redox regulation of NOS3, inducing enzymatic uncoupling and superoxide generation from the reductase domain of the enzyme (40) (Fig. 4). Two highly conserved cysteine residues are critical for NOS3 function, and become glutathionylated under conditions of oxidative stress, promoting NOS3-derived superoxide production. In vitro accumulation of GSSG, induced by exposure of endothelial cells to the GSH reductase inhibitor 1,3-bis(2 chloroethyl)-1-nitrosourea, results in glutathionylation of NOS3; whereas thiol-specific reducing agents reverse both NOS3 glutathionylation and endothelial dysfunction in vessels from spontaneously hypertensive rats (40). There are several proposed mechanisms of S-glutathionylation (1, 21). When the ratio of GSSG:GSH is high, thiol-disulfide exchange with oxidized glutathione (GSSG) may occur. ROS and reactive nitrogen species-derived thiyl radicals can, in turn, react with GSH and protein thiols to allow protein S-glutathionylation. It is believed that the formation of thiol intermediates, such as the thiyl radical, sulfenic acid, or S-nitrosyl, is a more rapid and efficient mechanism for protein S-glutathionylation in vivo and that these mechanisms could play a role in signal transduction. NOS3 thiols can be oxidized by superoxide generated from uncoupled NOS3, exogenous sources, or cellular oxidative stress through the formation of thiyl radicals. Protein thiyl radicals generated from uncoupled NOS3 indeed react with reduced GSH to form NOS3 S-glutathionylation (39).
FIG. 4.
Differential mechanisms of NOS3 uncoupling. When NOS3 is fully coupled, electrons flow from the reductase domain of one subunit into the oxygenase domain of the other, and NO is produced. When NOS3 is uncoupled, L-arginine oxidation becomes uncoupled from electron flow through the enzyme, and superoxide is produced instead of NO. A unique mechanism for the uncoupling of NOS3 has been identified where the modification of C689 and C908 by glutathionylation results in superoxide production from the reductase domain of the enzyme (40). (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Classical uncoupling of NOS3, usually induced by BH4-dependent mechanisms, exhibits superoxide generation from the heme iron within the oxygenase domain, which is inhibited by the L-arginine analogue, N-nitro-L-arginine and its methylester, L-NAME. Here, the nitro-substituted arginine analogue occupies the L-arginine binding site, attenuating the reduction of the heme iron by the reductase domain; the ferric heme does not bind oxygen, rendering it incapable of promoting the production of superoxide. Glutathionylation-induced uncoupling of NOS3, by generating superoxide from flavins within the enzyme's reductase domain, is less sensitive to inhibition by L-NAME. This mechanistic difference between BH4- and glutathionylation induced NOS uncoupling has far-reaching implications; in particular, superoxide generation from the NOS reductase domain, initiated by glutathionylation, might act as a “trigger” for BH4 oxidation and haem-derived superoxide production within the oxygenase domain. Since the importance of NOS coupling in cardiovascular health and disease is now well established, therapeutic strategies targeting the restoration of cardiac and endothelial BH4 levels need to consider these issues.
Arginine metabolism: asymmetric dimethyl arginine and arginase
NOS requires L-arginine to function and generate NO, and abnormal arginine metabolism also plays a role in NOS dysfunction. Two pathways have been recently highlighted (237): One is the conversion of L-arginine to asymmetric dimethyl arginine (ADMA) by protein arginine methyltransferase, and the second is its catabolism to urea or ornithine by arginase I and/or II. Both reduce the available supply of L-arginine, and in case of ADMA, further inhibit NOS by competing for L-arginine binding. Enhanced levels of ADMA are accompanied by NOS uncoupling, which is associated with vascular and cardiac dysfunction (7). In vivo levels of ADMA are increased by gene deletion of dimethylarginine dimethylaminohydrolase-1—an enzyme that metabolizes ADMA—leading to increased ADMA, NOS dysfunction, and vascular disease (138).
Increasing the level or activity of arginases also compromises NOS function by limiting available substrate. Arginase is a manganese metalloenzyme which is central to the hepatic urea metabolism, and exists in two distinct isoforms that are widely distributed. Arginase I is the primary form in the liver, while arginase II is more widely expressed. Both are expressed in endothelial cells and vascular smooth muscle that vary with vessel type and species. While first thought to be primarily involved with disposal of amino acid and nucleotide nitrogen, arginase has been recently revealed to play a key role in cardiovascular biology by its regulation of NOS3 and NOS1 (19). Arginase is activated by oxidative stress via a Rho/Rho-kinase pathway in bovine aortic endothelial cells (35, 210), and its up-regulation with aging, ischemic heart disease, hypertension, and heart failure supports its role in NOS dysfunction (194).
The fate of NO: role of redox chemistry
In addition to NOS dysregulation, the bio-availability of NO for targeting sGC or other protein thiols for S-nitrosylation reactions depends on local redox chemistry. As noted, oxidative stress shifts BH4 to BH2, and reactive species such as ONOO− (15) (which are formed from NO and superoxide) can directly impede NOS activity by modification of the central zinc (263). NOS uncoupling provides the ideal microenvironment for this chemistry, as both oxidants and NO are generated by the same enzyme, and this is thought to be an important factor limiting both cGMP-dependent and independent signaling linked to NO (116). ONOO− targets remains far from being well understood, though several proteins have been identified [e.g., SERCA (124)] and [e.g., PKCɛ (12)].
Consequences of NOS Dysfunction in Cardiovascular Disease
NOS, endothelial function, and vascular disease
Given the central role of NO signaling in endothelial function, smooth muscle proliferation, thrombosis, and inflammation, it comes as no surprise that NOS dysfunction is a potent contributor to vascular pathophysiology (96). The importance of NOS3 to vascular homeostasis has been demonstrated by genetic gain and loss of function studies, with deletion worsening neointimal medial thickening and vascular remodeling after injury (160, 258); whereas endothelial-targeted NOS3 overexpression suppresses the same (114) and reduces atherosclerosis in the ApoE-null mouse (231). Significantly, the deletion of NOS3 removed both NO-dependent vasorelaxation and oxidant-coupled hyperpolarization (150). While uncoupled NOS3 generates less NO, its synthesis of O2−, subsequently converted to H2O2 by Cu,Zn-superoxide dismutase, also results in vasodilation (159, 205). The latter was recently coupled to oxidation of C46 residues in PKG1α to form an internal disulfide bond and activate the kinase in a cGMP-independent manner (27). Mice with a knock-in mutated PKG1α (C46S) cannot undergo this oxidation, and develop hypertension(181). Along with previous NOS3 deletion studies, these results suggest a novel linkage between NOS3-derived oxidants and vasomotor tone. Depressed NOS1 activity also likely plays a role in vascular disease. Interestingly, NOS1 regulates peripheral and coronary flow in humans (200, 201), and its expression rises in early- and advanced-stage atheroma (249) and in the neointima, endothelial cells, and macrophages in vascular lesions. This is likely to play a counter-responsive role, as NOS1 gene deletion models also develop worse vascular pathobiology after vessel injury (131). Although NOS1 nor NOS3 gene deletion alone is sufficient to induce significant vascular disease in the absence of a pathological stress or concomitant disease, combining deletions displays a striking phenotype. Deletion of all three NOS isoforms in mice results in spontaneous coronary artery disease, myocardial infarction, and sudden cardiac death (163, 228), which are marked by intimal inflammation and coronary vasospasm. Thus, defects in one isoform alone may be compensated by others, but combined defects are likely to be markedly pathogenic.
NOS3 also contributes to mechano-signaling in the coronary vasculature, which is important to matching coronary reserve with ventricular demands with exertional stress. The failing and hypertrophied heart has depressed coronary dilator reserve, and NOS dysfunction has long been known to play an important role. Increases in pulsatile flow involve both a rise in phasic shear stress and cyclic wall stretch, both of which are involved with NOS activation via post-translational modifications, notably Akt phosphorylation, that not only involve overlapping (e.g., tyrosine kinase cSrc) but also unique (e.g., VEGFR2 dependent for shear, or independent for pulse-stretch) signaling (140). Reduced vessel compliance—a common feature of aging and many cardiovascular diseases—would suppress a stretch mechano-signal; this may blunt Akt and, thus, NOS3 activation, and dampen cytoprotection against oxidant stress (140). Through this mechanism, dysfunctional NOS activity may be linked to structural changes in the vessel wall, independent of endothelial biology per se.
In addition to modulating vascular tone and proliferation, endothelial-derived NO impacts inflammation. Early studies focused on the role of inducible NOS2 (208) (222); however, NOS3 and NOS1 now appear to be major participants as well (61, 238), providing a more tonic level of NO release. For example, both NOS1−/− and NOS3−/− mice display 2- or 6-fold increases, respectively, in leukocytes rolling at baseline (134, 137), and 9-10-fold increases after inflammatory stimulation.
NOS dysfunction and myocardial ischemia/reperfusion injury and infarction
The cell-specific role of NOS1 and NOS3 in the ischemic and infarcted ventricle is somewhat uncertain, as targeted gene-deletion models are lacking; however, both isoforms are thought to play a role. Mice globally lacking either NOS1 or NOS3 display worse LV function and remodeling after coronary ligation-infarction (55, 195). Both endothelial-and myocyte-restricted overexpression of NOS3 reduces the severity of postinfarction pathophysiology (107, 110). Somewhat analogous findings were reported for NOS1, where myocyte-specific conditional transgenic overexpression of NOS1 led to reduced infarct size, improved function, and reduced oxidative stress after coronary ligation (28). The same authors reported the up-regulation and translocation of NOS1 to mitochondria in response to ischemia/reperfusion; similarly, transgenically overexpressed NOS1 localized to mitochondria coupled to HSP90 translocation (28). Unlike NOS3 for which expression changes in hypertrophy and heart failure are often not observed, NOS1 expression rises in human HF (53) and after myocardial infarction in rats, where translocation to the plasma membrane and association with caveolin-3 (Cav-3) is observed (18, 52). Although this may reduce the capacity of NOS1 to impact oxidative stress, it also leads to retargeting, with increased cGMP/PKG and S-NO mediated inhibition of the LTCC (214) and β-AR signaling (18, 55, 214) that may protect the heart while down-regulating function.
The mechanisms underpinning NO-derived cardiac protection to ischemia-reperfusion injury involves mitochondrial KATP channels via PKG phosphorylation (255), S-nitrosylation of sarcoplasmic and mitochondrial calcium uptake ATPases and of the hypoxia inducible factor 1α (141), and ONOO-induced tyrosine-nitration of PKCɛ (12). The relative role of NOS3 and NOS1 to these mechanisms may depend on redox conditions that impact NOS coupling, sGC-NO responsiveness (226), and potentially PKG activity (27). S-nitrosylation of a variety of target proteins during ischemic preconditioning is thought to act as a cysteine shield, protecting residues from subsequent oxidation and functional damage on reperfusion (126).
NOS dysfunction and cardiac hypertrophy and failure
Changes in NOS3 and NOS2 in experimental and human cardiac failure and pathological hypertrophy bear many similarities with results obtained in the postischemic/infarcted heart. NOS3 expression has been observed to decline in some studies (60, 177), while others find no change, and NOS1 expression typically increases (18, 52). Gain-and-loss-of-function models have targeted pressure overload, and here, the results are somewhat mixed, likely highlighting the importance of redox environment and status of NOS (i.e., uncoupled or not) in determining the response. For example, chronic NOS3 deletion induces concentric hypertrophy (14), and has been found to worsen remodeling after pressure overload (197). Myocyte-targeted up-regulation protected against this loading model (29). However, others employing abdominal aortic banding found increased hypertrophy but less chamber dilation in NOS3−/−(189). With a more severe ascending constriction model, Takimoto et al. (218) found that NOS3−/− mice were paradoxically protected, displaying neither dilation nor progressive LV systolic dysfunction as compared with controls, and linked this to the lack of oxidative stress in mice lacking NOS3. Thus, while NOS3 may usually be protective against maladaptive stress, once uncoupled, it potently contributes to the pathophysiology, itself becoming a major ROS generator. In this condition, its absence results in improved biology.
In vitro studies have demonstrated the uncoupling of NOS1 activity in response to ROS via both reversible oxidation of BH4 (induced predominantly by superoxide) and irreversible heme degradation (induced by peroxynitrite) (213). Less is known about the mechanisms and functional consequences of NOS1 uncoupling in vivo, though it may in part account for left ventricular diastolic dysfunction. In deoxycorticosterone acetate-salt mice, treatment with the partial selective NOS1 inhibitor 7-nitroindazole reduced superoxide in left ventricular tissue, while oral BH4 supplementation maintained or restored PLB phosphorylation. NOS1 inhibition or gene deletion also impairs myocardial relaxation by reducing the PLB phosphorylated fraction (241, 261).
The role of NOS2 (inducible NOS) to pathological LV remodeling in heart failure is still being defined. Targeted overexpression in myocytes is protective against pressure overload (245) and as previously noted, this may relate to antioxidant effects (169). However, whether NOS2 provides a source of ROS due to uncoupling under stress is less clear, as the deletion of NOS3 appears sufficient to suppress ROS activity (218). Furthermore, since much of NOS2 signaling appears less dependent on cGMP and more on S-NO modifications, the impact of up- or down-regulation is likely to depend on the local redox environment, not only affecting the enzyme itself, but also the proteins whose cysteines would be targeted for S-nitrosylation.
The scaffolding protein Cav-3 plays an important role in NOS function in the cardiac myocyte, and evidence supports the role of Cav-3 dysregulation in heart disease. In a study conducted on mouse models of dilated cardiomyopathy (adenosine A1-receptor or TNFα overexpression), Cav-3 expression declined and correlated with functional deficits as well as reduced Akt activation and SR-ATPase gene expression (66). In this study, human-failing myocardium Cav-3 trended to a decline, though some studies of experimental heart failure have reported increased Cav-3 expression (15). Mice genetically lacking Cav-3 develop hypertrophic cardiomyopathy that is accompanied by activation of the p42/44 mitogen activated protein kinase (MAPK) pathway (251), while cardiomyocyte-targeted over-expression of Cav-3 (100) may attenuate cardiac hypertrophy, increasing cGMP levels, a-type and b-type natriuretic peptide (ANP/BNP) expression, and enhancing nuclear targeting of Akt, itself shown to be anti-hypertrophic (227). Cav-3-NOS modulation likely contributes to these effects. For example, in the pressure-overloaded heart, NO-dependent activation of sGC is markedly depressed, in part due to oxidation coupled to a decline in its expression within Cav-3 microdomains (226). In hearts genetically lacking Cav-3, sGC activation by NO or by direct small-molecule activators is also lost (226—indicating a critical role of the protein in normal interactions between NOS-NO generation and sGC-cGMP production. Since caveolae also serve as a major nexus for a broad range of cell signaling, coordinating receptors, kinases, ion channels, and the dystrophin-glycoprotein complex (80), more than NOS3 dysregulation is involved when Cav-3 is abnormal. Loss-of-function mutations in Cav-3 have been linked to several forms of muscular dystrophy, including limb girdle, hyper-creatine kinase, and rippling muscle disease (80). Several case reports that suggest Cav-3 mutations (e.g., T77M) (225) can result in hypertrophic cardiomyopathy, though this remains rare, and whether the disease involves NOS dysregulation is unclear. NOS1 colocalizes with Cav-3 in skeletal muscle but not in normal cardiac myocytes. However, some have observed plasma-membrane translocation of NOS1 in heart failure syndromes, where interaction with Cav-3 may well play a role in altered signaling (16, 53).
NOS and cardiac transplantation
Despite general success with human heart transplantation, graft rejection continues to be a primary cause for postoperative mortality and morbidity (46). Most research has focused on inducible NOS2, which may have positive and negative effects, the latter being coupled to mitochondrial (220), metabolic (139), and apoptotic (215) modulation. NOS3 is also likely involved. While depressed NOS3 is observed and thought to contribute to graft/host vasculopathy (57, 175), data regarding the benefits of gene therapy that enhance NOS3 are mixed (106, 108, 257). This could reflect redox imbalance with a graft rejection; so, NOS3 becomes more likely to be uncoupled if up-regulated.
NOS and angiogenesis
There is evidence that NOS-derived NO controls the process of angiogenesis during heart development (262). This regulation becomes even more important during pathological conditions where the formation of new vessels plays a key role in the remodeling of the heart. Recent findings suggest that NOS3 regulates the migration of endothelial-derived progenitor cells (EPC) which are produced by the bone marrow and mobilize on stimulation to induce neovascularization (173). NOS3−/− mice have a reduced recruitment of EPC and impaired angiogenesis (3). The absence of NOS3 prevents the up-regulation and mobilization of EPC after aortic constriction, resulting in enhanced cardiac hypertrophy and fibrosis, and reduced capillary formation (115). These negative effects are reversed when wild-type bone marrow is transplanted in NOS3−/− mice. EPC are also recruited to the myocardium during ischemia preconditioning (103), although whether this is causing a beneficial effect on cardiac remodeling has not yet been elucidated. In myocardial infarction models, EPC mobilization and angiogenesis can be increased by the administration of estradiol or atorvastatin (105, 132); in both cases, the increase in EPC recruitment is mediated by NOS.
NOS and arrhythmia
Both NOS1 and NOS3 play key roles in electrical activation of the heart, and their dysfunction contributes to arrhythmia. Mice lacking NOS3 display a slower heart rate, and an increase in the transient inward current that has been coupled to aminoglycoside-induced ventricular ectopy and tachycardia (182). While NOS3−/− myocytes have normal resting action potential duration (APD) and LTCC current under basal condition, their response to ISO is altered, with longer APD and augmented ICa associated with increases in early and delayed afterdepolarizations (243). The latter may be related to a loss-of-negative cGMP/PKG effects on the β-subunit of the LTCC (256). NOS1 plays a key role in Ca2+ cycling, and its absence results in an increase in peak and diastolic calcium and ventricular ectopy (25, 199). Mice lacking NOS1 develop worse arrhythmia and SCD after myocardial infarction (25).
A novel mechanism linking NOS and arrhythmia has been revealed by genetic studies of mutations in the sodium channel Nav1.5. The channel protein forms a complex with NOS1, PMCA4b, and a-syntrophin, and the late Na+ current is enhanced by S-nitrosylation (9, 36, 112, 230). A mutation in SNTA1 enhances S-NO modifications, and is a cause for long QT syndrome (LQT3). Furthermore, genome wide association studies showed a genetic variant in the gene encoding the NOS1 adaptor protein, CAPON, to be associated with prolongation of the QT interval (9) and sudden cardiac death (112). The SNP is in a noncoding region, and mechanisms by which it confers changes in NO-dependent signaling remain underway.
Dysfunctional NOS activity has also been linked to atrial fibrillation (AF). Short-term AF leads to reduced atrial NOS activity and NO bioavailability (30), coupled with an increase in Rac1 activity and superoxide production from NOX2 oxidases. In contrast, long-standing AF and atrial structural remodeling are associated with increased mitochondrial oxidase activity and NOS uncoupling secondary to a reduction in atrial BH4 content and an increase in arginase activity (121, 185). In human atrial myocytes, NO donors prolong APD by decreasing IKv4.3 and human Ito1 (84); whereas endogenous NO increases the inwardly rectifying K+ current, IK1 and shorten the APD by S-nitrosylating Kir2.1 channels (83). These findings suggest that the decreased S-nitrosylation of Kir2.1 channels observed in human samples from patients with AF may represent a compensatory mechanism which attenuates the shortening of APD and atrial refractory period and, thus, atrial electrical remodeling in AF. Although recent data support the use of statins to counter NOX2-dependent atrial superoxide production as a strategy which prevents the new onset of AF after cardiac surgery (6), other findings indicate that this approach is unlikely to be successful in long-standing AF (185).
Therapeutic Approaches for Fixing NOS-opathy
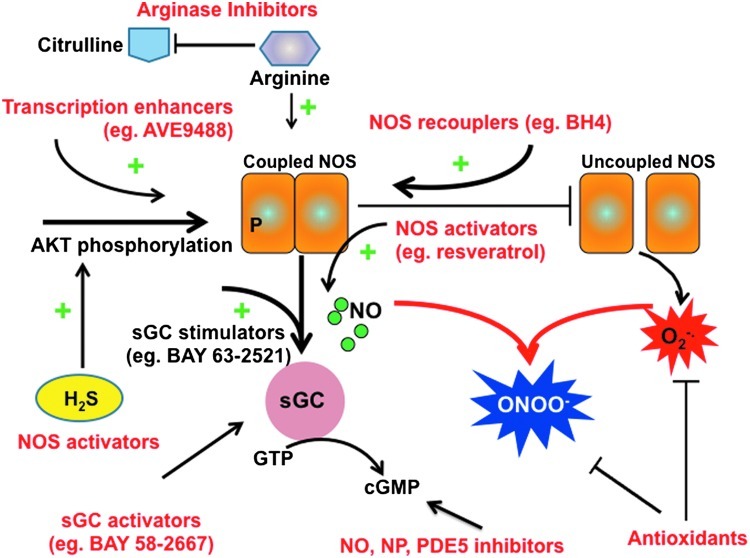
The approaches that are used for ameliorating NOS dysfunction in cardiovascular disease have targeted each of the features we have just reviewed. They fall into six categories: organic nitrates, NOS activators, NOS recouplers, arginase inhibitors, enhancers of cGMP/PKG signaling, and modulators of NOS-ROS interaction (Fig. 5). Although these appear as promising targets, clinical translational remains limited, though several approaches are being actively pursued in patients with heart disease.
FIG. 5.
Therapeutic approaches for treating “NOS-opathy.” Schematic showing various components of NOS-related dysfunction and where and how different therapeutic approaches are attempting to ameliorate this pathophysiology. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars).
Organic nitrates
Organic nitrates are used in the treatments of ischemic heart disease and heart failure by increasing blood NO concentration via their denitration. Nitroglycerin (glyceryl trinitrite [GTN]) is the most common form, and it rapidly improves cardiac hemodynamics by vasodilation, augmenting cGMP in vascular smooth muscle (224). GTN denitration has been previously attributed to reactions with GSH S-transferase, cytochrome P450, and XOR (76); however, recent data indicate (41) that mitochondrial aldehyde dehydrogenase 2 (mtALDH2) is the major enzyme (128, 129). GTN bioactivation may also result in the formation of other NO-related species that activate sGC in a haem-independent manner. While among the initial agents used to reduce cardiac loading in heart failure, organo-nitrates lost favor due to the clinical superiority of other vasodilators (e.g., angiogensin converting enzyme inhibition) and tolerance. The latter is now thought to be due to the chronic suppression of mtALDH2 after long-term exposure (216). Nonetheless, recent evidence of the benefits of this drug class combined with hydralazline in the African-American sub-population of heart failure patients has revived interest (221).
NOS activators
Akt activation of NOS3 has been recently mimicked by administration of the gas, hydrogen sulfide (H2S) (179). H2S stimulates a two-fold increase in NO production coupled to Ser-1177 phosphorylation, and the inhibition of Akt prevents these effects. Current H2S “donors” are short-lived, and will have to be modified to generate a meaningful pharmaceutical approach, but this strategy is evolving (247).
Several methods have been recently described as enhancing NOS3 expression. Two small-molecule NOS3 transcription enhancers, 4-fluoro-N-indan-2-yl-benzamide (AVE9488) and 2,2-difluoro-benzo[1,3]dioxole-5-carboxylic acid indan-2-ylamide (AVE3085) were identified by high throughput screening. Both AVE9488 and AVE3085 stimulate eNOS transcription in vivo and in vitro (250). They reduce atherosclerotic plaque formation in apoE knockout mice but not in the apoE/NOS3 double knockout mice (250), supporting specificity for NOS3. AVE30085 improves endothelial dysfunction in diabetic mice through increased NO production and reduced oxidative stress in the vascular wall (37). These compounds may prove useful by enhancing NOS3 expression, though the status of NOS—that is, redox modulation of coupling or uncoupling—could impact their net benefit, and more studies are required.
Another approach that increases NOS3 expression is the polyphenol antioxidant trans-resveratrol (240), which augments NO production (122). Trans-resveratrol also prevents NOS3 uncoupling and lowers oxidative stress while preserving endothelial function (20), and it can up-regulate GTPCH-1 (252), suggesting multiple mechanisms whereby it may prove useful for treating NOS-opathy. Lastly, triterpenoids, such as betulinic acid isolated from birch tree bark, have been shown to improve endothelium-dependent relaxation in rats with L-NAME-induced hypertension by their ability to reduce oxidative stress (74). Betulinic acid may also up-regulate NOS3 expression and reduce NADPH oxidase expression in human endothelial cells through a PKC-dependent mechanism (209).
Lastly, the racemic mixture d- and l-nibivolol, a third-generation selective β1-adrenoceptor blocker with NO-mediated vasodilating and antioxidant (102) properties, is another intriguing method used. It reduces superoxide in endothelial cells to boost NO bioavailability (43), and can inhibit NADPH oxidase activity in various models of hypertension (168). This results in a net enhancement of NO production (63, 151, 207) with the increased activity of NOS3 playing a role (171). Such activity is observed in both endothelium and heart (147), although NOS3 activation has not been observed in end-stage human heart failure (24). Cardiac activity of nibivolol in the nonfailing heart has been coupled to β3-AR stimulation to enhance NO synthesis and promote angiogenesis (188), suggesting potential use in ischemia-reperfusion injury (8). To date, there have been only a few controlled clinical trials in heart failure. The SENIORS trial (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure) showed Nebivolol induced a modest, borderline significant decline in all causes of mortality or cardiovascular hospital admission (69). A trial of HFpEF, a disorder that is commonly associated with hypertension, was recently reported, where the drug potentially failed to benefit patients by suppression chronotropic reserve (44).
NOS recouplers
BH4 is a critical regulator of NOS coupling and function, suggesting that it may be a beneficial therapeutic agent in vascular disease. Pharmacological supplementation of BH4 improves endothelium-dependent relaxation and NOS coupling (155, 157). BH4 aids electron transfer from the NOS reductase to the oxidase domains, assisting in the production of NO and L-citrulline from L-arginine. It stabilizes NOS in its active homodimeric form (183). Moens et al. (157) found that the administration of BH4 to mice with well-established left ventricular pathological remodeling, fibrosis, and dysfunction induced by pressure overload led to the amelioration of these abnormalities and the reversal of maladaptive remodeling. This occurred in association with “re-coupling” of NOS, which accounted for most of the ROS generation in the control (placebo treated) group. Intriguingly, reversal of the pathology/pathophysiology by BH4 was not coupled with enhanced PKG activation. Rather, the data suggest retargeting of NO due to an improvement in myocardial NO/ROS imbalance. More recently, BH4 was shown to improve left ventricular diastolic dysfunction in a mouse model of hypertension via an increase in coupled NOS1 activity (206). BH4 has also been used to treat ischemia/reperfusion (62), and recent data have coupled this beneficial effect with angiogenesis (203).
However, despite initial enthusiasm based on experimental studies, clinical trials of BH4 to treat disorders linked to uncoupled NOS activity (i.e., hypertension, sickle cell disease, cardio-renal syndrome, pulmonary hypertension, and coronary artery endothelial dysfunction) have all been disappointing [reviewed in (156)]. Part of the problem may be differences in the tissue uptake of exogenous BH4, which appears to be species dependent. Another factor is that orally consumed BH4 is rapidly oxidized, and should be reconverted by DHFR to be effective. Even in mice, where exogenous BH4 is effective for treating heart disease, increasing the dose led to a paradox reversal of benefit, re-appearance of ROS, and this was coupled with a decline in the BH4/BH2 ratio toward baseline (155). Variability in both uptake and redox modification of exogenous BH4 depending on the patient and disease would greatly complicate its clinical pharmacology.
There are several alternatives to BH4 itself. Statins can increase BH4 bioavailability in endothelial cells by up-regulating GCH1 gene expression (94), and could assist in NOS uncoupling. Folic acid has been studied, and it can improve the binding affinity of BH4 to eNOS, enhancing the reduction of BH2 to produce BH4, and chemically stabilizing BH4 (158). However, the doses required are unclear, and initial efforts have not been encouraging (59).
Arginase inhibitors
The importance of arginase in disease conditions has been based in large part on the use of small-molecule inhibitors, such as N(omega)-hydroxy-nor-L-arginine, which reverses hypertension in the spontaneously hypertensive rat (11). Other inhibitors [e.g., S-(2-boronoethyl)-l-cysteine] have been used to demonstrate the impact of arginase up-regulation on suppressing NOS signaling in aged vessels (19), and to ameliorate vascular aging (42, 111, 120). Commercial development of clinical arginase inhibitors has begun (Arginetix, Inc., now Immune Control), and assessment of the efficacy of this approach may follow.
cGMP-enhancing approaches
Another approach for tackling NOS-opathy is to concentrate on the cGMP-dependent component of the pathway. While recognizing that this represents a component of NO signaling that may or may not be central depending on the condition, nonetheless, studies have revealed the benefit of enhancing cGMP and thereby PKG activation in the hypertrophied and failing heart. There are three primary means for achieving this: exogenous administration of NO donors or stimulators of an alternative natriuretic-peptide-coupled cGMP synthetic pathway, the direct activation/stimulation of sGC itself, or the inhibition of cGMP hydrolysis by selective PDE. All three have been clinically utilized and/or investigated. The use of NO donors and NPs has the longest history. The former remains limited due to tolerance that has multiple causes, including oxidative stress and the associated down-regulation of aldehyde dehydrogenase-II (216), PDE up-regulation (119), arginase activation (118), and NOS uncoupling. NP administration has not only been primarily used to reduce ventricular load, but may also have benefits on the heart, though its use has been limited to date due to requirements for intravenous administration. This may be changing, however, in the near future (239).
Activators and stimulators of sGC represent a fairly new class of drugs, and several are now in advanced stages of clinical trials. Some such as cinaciguat (BAY 58-2667) function through an NO-independent mechanism, whereas others (stimulators, riociguat, BAY 63-2521) improve the binding of sGC to NO. Low-dose intravenous Cinaciguat decreased diastolic blood pressure and heart rate without lowering systolic blood pressure, whereas at higher doses, mean arterial pressure declined as plasma cGMP increased (73). In a Phase II study, patients with pulmonary hypertension treated with riociguat displayed increased exercise capacity and reduced pulmonary vascular resistance (81). Other trials are ongoing to examine their role in heart failure.
Lastly, studies have also revealed how the inhibition of cGMP-selective PDE5a with drugs such as sildenafil or tadalafil (both widely used to treat erectile dysfunction and pulmonary hypertension) can improve cardiac hypertrophy/failure pathophysiology and remodeling. Sildenafil suppresses as well as reverses existing hypertrophy/dysfunction in mice subjected to pressure overload (162, 217). This and similar agents also protect against I/R injury (192, 193), doxorubicin toxicity (127), and precondition stem cells to improve postimplant survival and function (99). They are also being studied in muscular dystrophy, where both skeletal and cardiac failure have been linked to the loss of dystrophin and NOS1 activity (2, 125). There have been several clinical trials, showing the improvement in heart failure symptoms, cardiac remodeling and function (89), and peripheral vascular reserve and endothelial function (88). A multicenter clinical trial (RELAX) that studies the effects of sildenafil in patients with heart failure and a preserved ejection fraction is near completion; results are due in late 2012.
ROS/NO interactions
The recognition that nitroso-redox imbalance can adversely impact the heart has led to several therapeutic approaches. Broad antioxidant strategies, such as with Vitamin E and C supplementation, have been disappointing. Some found HF risk may even increase, as in the HOPE-TOO trial conducted in patients with vascular disease or diabetes-mellitus-prescribed vitamin E (144). Other trials using folic acid, B-vitamins, and other agents have so far not improved cardiovascular risk.
A few other strategies have been proposed, though clinical data remain controversial. For example, XOR is proposed to negatively impact the nitroso-redox balance in the cardiovascular system (229), and XOR inhibitors such as allopurinol or oxypurinol can improve cardiac function and/or remodeling in the experimental failing (190) and infarcted (154) heart. This may be related to targeting of the NOS1-XO interactome (117) and amelioration of vascular oxidative stress to improve endothelial function (133). However, to date, human HF trials of XOR inhibition have not shown clinical benefit (92, 164). Another approach was the combined use of hydralazine and isosorbide dinitrate. A retrospective analysis of earlier trials led to the hypothesis that, while not particularly effective in the Caucasian patients with HF, the hydralazine-isosorbide dinitrate combination may benefits to African Americans. The clinical trial, reported in 2004 (221), showed a surprising efficacy on mortality and led to the first racial-targeted FDA approval of a therapy. One hypothesis was that hydralazine acted as an anti-oxidant and suppressor of nitrate tolerance, and combined with isosorbide dinitrate, could favorably impact the nitroso-redox imbalance (51, 91, 161). Why this should work only in African Americans—if indeed this is the relevant mechanism—remains unclear, as one would imagine that NOS/ROS imbalance is not unique to this population. The greater incidence of hypertension in African Americans and, thus, potential loading impact remains an alternative explanation.
Conclusions
In summary, NO is a critical regulator of cardiovascular homeostasis, and abnormalities in the performance of its primary constitutive synthases play an important role in heart disease. Growing evidence points to post-translational modifications underlying many of the NOS-opathies, resulting in an imbalance of nitrosative and oxidative environments. This impacts both ongoing NO generation and its chemistry as well as the subsequent capacity to stimulate cGMP and PKG activation or modify targeted0 protein residues. Several new avenues to ameliorate NOS activity or its downstream pathways have shown promising results, but more trials are obviously needed.
Abbreviations Used
- β-AR
β-adrenergic receptor
- ACE
angiogensin converting enzyme
- ADMA
asymmetric dimethyl arginine
- ANP/BNP
a-type and b-type natriuretic peptide
- APD
action potential duration
- BH4
tetrahydrobiopterin
- CaM
calmodulin
- CAPON
carboxy-terminal PDZ ligand of nNOS
- Cav-3
caveolin 3
- cGMP
cyclic guanosine monophosphate
- DHFR
dihyrdofolate reductase
- DNTP
dihydroneopterin 3′ triphosphate
- ECC
excitation-contraction coupling
- EPC
endothelial-derived progenitor cells
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- GFRP
GTP cyclohydrolase feedback regulatory protein
- GSH
glutathione
- GSNO
S-nitrosoglutathione
- GTN
glyceryl trinitrite
- GTPCH
guanosine triphosphate cyclohydrolase I
- H2S
hydrogen sulfide
- ISO
isoproterenol
- L-NAME
L-Nω-nitro-L-arginine methyl ester
- L-NMMA
L-NG-monomethyl arginine citrate
- LTCC
L-type Ca2+ channel
- MAPK
mitogen activated protein kinase
- mtALDH2
mitochondrial aldehyde dehydrogenase 2
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
sodium calcium exchanger
- NO
nitric oxide
- NOS
nitric oxide synthase
- ONOO−
peroxinitrite
- PDE5
phosphodiesterase type 5
- PKC
protein kinase C
- PKG
protein kinase G
- PLB
phospholamban
- PMCA
plasma membrane calcium/CaM dependent ATPase
- PTP
6-pyruvoyl tetrahydropterin
- PTPS
6-pyruvoyl tetrahydropterin synthase
- ROS
reactive oxygen species
- RyR
ryanodine receptor Ca2+ release channel
- SERCA
sarcoplasmic reticulum Ca2+ ATPase
- sGC
soluble guanylate cyclase
- SPR
sepiapterin reductase
- SR
sarcoplasmic reticulum
- TNFα
tumor necrosis factor alpha
- XOR
xanthine oxidoreductase
Acknowledgments
This study was supported by Fondation Leducq, National Institutes of Health, and the British Heart Foundation.
References
- 1.Adachi T. Modulation of vascular sarco/endoplasmic reticulum calcium ATPase in cardiovascular pathophysiology. Adv Pharmacol. 2010;59:165–195. doi: 10.1016/S1054-3589(10)59006-9. [DOI] [PubMed] [Google Scholar]
- 2.Adamo CM. Dai DF. Percival JM. Minami E. Willis MS. Patrucco E. Froehner SC. Beavo JA. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aicher A. Heeschen C. Mildner-Rihm C. Urbich C. Ihling C. Technau-Ihling K. Zeiher AM. Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 4.Alderton WK. Cooper CE. Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alp NJ. Mussa S. Khoo J. Guzik TJ. Cai S. Jefferson A. Rockett KA. Channon KM. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniades C. Demosthenous M. Reilly S. Margaritis M. Zhang MH. Antonopoulos A. Marinou K. Nahar K. Jayaram R. Tousoulis D. Bakogiannis C. Sayeed R. Triantafyllou C. Koumallos N. Psarros C. Miliou A. Stefanadis C. Channon KM. Casadei B. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59:60–70. doi: 10.1016/j.jacc.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 7.Antoniades C. Shirodaria C. Leeson P. Antonopoulos A. Warrick N. Van-Assche T. Cunnington C. Tousoulis D. Pillai R. Ratnatunga C. Stefanadis C. Channon KM. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur Heart J. 2009;30:1142–1150. doi: 10.1093/eurheartj/ehp061. [DOI] [PubMed] [Google Scholar]
- 8.Aragon JP. Condit ME. Bhushan S. Predmore BL. Patel SS. Grinsfelder DB. Gundewar S. Jha S. Calvert JW. Barouch LA. Lavu M. Wright HM. Lefer DJ. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arking DE. Pfeufer A. Post W. Kao WH. Newton-Cheh C. Ikeda M. West K. Kashuk C. Akyol M. Perz S. Jalilzadeh S. Illig T. Gieger C. Guo CY. Larson MG. Wichmann HE. Marban E. O'Donnell CJ. Hirschhorn JN. Kaab S. Spooner PM. Meitinger T. Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 10.Ashley EA. Sears CE. Bryant SM. Watkins HC. Casadei B. Cardiac nitric oxide synthase 1 regulates basal and beta-adrenergic contractility in murine ventricular myocytes. Circulation. 2002;105:3011–3016. doi: 10.1161/01.cir.0000019516.31040.2d. [DOI] [PubMed] [Google Scholar]
- 11.Bagnost T. Ma L. da Silva RF. Rezakhaniha R. Houdayer C. Stergiopulos N. Andre C. Guillaume Y. Berthelot A. Demougeot C. Cardiovascular effects of arginase inhibition in spontaneously hypertensive rats with fully developed hypertension. Cardiovasc Res. 2010;87:569–577. doi: 10.1093/cvr/cvq081. [DOI] [PubMed] [Google Scholar]
- 12.Balafanova Z. Bolli R. Zhang J. Zheng Y. Pass JM. Bhatnagar A. Tang XL. Wang O. Cardwell E. Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- 13.Balligand JL. Kobzik L. Han X. Kaye DM. Belhassen L. O'Hara DS. Kelly RA. Smith TW. Michel T. Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. J Biol Chem. 1995;270:14582–14586. doi: 10.1074/jbc.270.24.14582. [DOI] [PubMed] [Google Scholar]
- 14.Barouch LA. Harrison RW. Skaf MW. Rosas GO. Cappola TP. Kobeissi ZA. Hobai IA. Lemmon CA. Burnett AL. O'Rourke B. Rodriguez ER. Huang PL. Lima JA. Berkowitz DE. Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Beigi F. Oskouei BN. Zheng M. Cooke CA. Lamirault G. Hare JM. Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide. 2009;21:226–233. doi: 10.1016/j.niox.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bencsik P. Kupai K. Giricz Z. Gorbe A. Huliak I. Furst S. Dux L. Csont T. Jancso G. Ferdinandy P. Cardiac capsaicin-sensitive sensory nerves regulate myocardial relaxation via S-nitrosylation of SERCA: role of peroxynitrite. Br J Pharmacol. 2008;153:488–496. doi: 10.1038/sj.bjp.0707599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendall JK. Damy T. Ratajczak P. Loyer X. Monceau V. Marty I. Milliez P. Robidel E. Marotte F. Samuel J-L. Heymes C. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in {beta}-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- 19.Berkowitz DE. White R. Li D. Minhas KM. Cernetich A. Kim S. Burke S. Shoukas AA. Nyhan D. Champion HC. Hare JM. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt SR. Lokhandwala MF. Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258–264. doi: 10.1016/j.ejphar.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Biswas S. Chida AS. Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Brenman JE. Chao DS. Gee SH. Mcgee AW. Craven SE. Santillano DR. Wu ZQ. Huang F. Xia HH. Peters MF. Froehner SC. Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha-1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 23.Brenman JE. Xia H. Chao DS. Black SM. Bredt DS. Regulation of neuronal nitric oxide synthase through alternative transcripts. Dev Neurosci. 1997;19:224–231. doi: 10.1159/000111211. [DOI] [PubMed] [Google Scholar]
- 24.Brixius K. Song Q. Malick A. Boelck B. Addicks K. Bloch W. Mehlhorn U. Schwinger RH. ENOS is not activated by nebivolol in human failing myocardium. Life Sci. 2006;79:1234–1241. doi: 10.1016/j.lfs.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Burger DE. Lu X. Lei M. Xiang F-L. Hammoud L. Jiang M. Wang H. Jones DL. Sims SM. Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- 26. This reference has been deleted.
- 27.Burgoyne JR. Madhani M. Cuello F. Charles RL. Brennan JP. Schroder E. Browning DD. Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317:1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 28.Burkard N. Williams T. Czolbe M. Blomer N. Panther F. Link M. Fraccarollo D. Widder JD. Hu K. Han H. Hofmann U. Frantz S. Nordbeck P. Bulla J. Schuh K. Ritter O. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010;122:1588–1603. doi: 10.1161/CIRCULATIONAHA.109.933630. [DOI] [PubMed] [Google Scholar]
- 29.Buys ES. Raher MJ. Blake SL. Neilan TG. Graveline AR. Passeri JJ. Llano M. Perez-Sanz TM. Ichinose F. Janssens S. Zapol WM. Picard MH. Bloch KD. Scherrer-Crosbie M. Cardiomyocyte-restricted restoration of nitric oxide synthase 3 attenuates left ventricular remodeling after chronic pressure overload. Am J Physiol Heart Circ Physiol. 2007;293:H620–H627. doi: 10.1152/ajpheart.01236.2006. [DOI] [PubMed] [Google Scholar]
- 30.Cai H. Li Z. Goette A. Mera F. Honeycutt C. Feterik K. Wilcox JN. Dudley SC., Jr. Harrison DG. Langberg JJ. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: potential mechanisms for atrial thrombosis and stroke. Circulation. 2002;106:2854–2858. doi: 10.1161/01.cir.0000039327.11661.16. [DOI] [PubMed] [Google Scholar]
- 31.Cai S. Khoo J. Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res. 2005;65:823–831. doi: 10.1016/j.cardiores.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 32.Campbell DL. Stamler JS. Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carnicer R. Hale AB. Liu X. Bendall JK. Lim GBS. Alp NJ. Channon KM. Casadei B. Cardiomyocyte-targeted overexpression of GTP cyclohydrolase-1 increases nNOS activity and hastens myocardial relaxation. Eur Heart J. 2011;32:154. [Google Scholar]
- 34.Chalupsky K. Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandra S. Romero MJ. Shatanawi A. Alkilany AM. Caldwell RB. Caldwell RW. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–519. doi: 10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang KC. Barth AS. Sasano T. Kizana E. Kashiwakura Y. Zhang Y. Foster DB. Marban E. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci U S A. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheang WS. Wong WT. Tian XY. Yang Q. Lee HK. He GW. Yao X. Huang Y. Endothelial nitric oxide synthase enhancer reduces oxidative stress and restores endothelial function in db/db mice. Cardiovasc Res. 2011;92:267–275. doi: 10.1093/cvr/cvr233. [DOI] [PubMed] [Google Scholar]
- 38.Chen CA. Druhan LJ. Varadharaj S. Chen YR. Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen CA. Lin CH. Druhan LJ. Wang TY. Chen YR. Zweier JL. Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem. 2011;286:29098–29107. doi: 10.1074/jbc.M111.240127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CA. Wang TY. Varadharaj S. Reyes LA. Hemann C. Talukder MA. Chen YR. Druhan LJ. Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z. Zhang J. Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chicoine LG. Paffett ML. Young TL. Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 43.Cominacini L. Fratta Pasini A. Garbin U. Nava C. Davoli A. Criscuoli M. Crea A. Sawamura T. Lo Cascio V. Nebivolol and its 4-keto derivative increase nitric oxide in endothelial cells by reducing its oxidative inactivation. J Am Coll Cardiol. 2003;42:1838–1844. doi: 10.1016/j.jacc.2003.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Conraads VM. Metra M. Kamp O. De Keulenaer GW. Pieske B. Zamorano J. Vardas PE. Bohm M. Dei Cas L. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–225. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 45.Cooper AJ. Pinto JT. Callery PS. Reversible and irreversible protein glutathionylation: biological and clinical aspects. Expert Opin Drug Metab Toxicol. 2011;7:891–910. doi: 10.1517/17425255.2011.577738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cotts WG. Johnson MR. The challenge of rejection and cardiac allograft vasculopathy. Heart Failure Rev. 2001;6:227–240. doi: 10.1023/a:1011414307636. [DOI] [PubMed] [Google Scholar]
- 47.Crabtree MJ. Channon KM. Dihydrofolate reductase and biopterin recycling in cardiovascular disease. J Mol Cell Cardiol. 2009;47:749–751. doi: 10.1016/j.yjmcc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Crabtree MJ. Hale AB. Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50:1639–1646. doi: 10.1016/j.freeradbiomed.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crabtree MJ. Tatham AL. Al-Wakeel Y. Warrick N. Hale AB. Cai S. Channon KM. Alp NJ. Quantitative regulation of intracellular endothelial nitric oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 50.Craig DH. Chapman SK. Daff S. Calmodulin activates electron transfer through neuronal nitric-oxide synthase reductase domain by releasing an NADPH-dependent conformational lock. J Biol Chem. 2002;277:33987–33994. doi: 10.1074/jbc.M203118200. [DOI] [PubMed] [Google Scholar]
- 51.Daiber A. Oelze M. Coldewey M. Kaiser K. Huth C. Schildknecht S. Bachschmid M. Nazirisadeh Y. Ullrich V. Mulsch A. Munzel T. Tsilimingas N. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem Biophys Res Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 52.Damy T. Ratajczak P. Robidel E. Bendall JK. Oliviero P. Boczkowski J. Ebrahimian T. Marotte F. Samuel JL. Heymes C. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;17:1934–1936. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- 53.Damy T. Ratajczak P. Shah AM. Camors E. Marty I. Hasenfuss G. Marotte F. Samuel JL. Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 54.Danson EJ. Paterson DJ. Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise-trained mice. J Physiol. 2003;546:225–232. doi: 10.1113/jphysiol.2002.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson D. Lygate CA. Zhang M-H. Hulbert K. Neubauer S. Casadei B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation. 2005;112:3729–3737. doi: 10.1161/CIRCULATIONAHA.105.539437. [DOI] [PubMed] [Google Scholar]
- 56. This reference has been deleted.
- 57.Demers P. Elkouri S. Sirois MG. Cartier R. Coronary artery endothelial dysfunction after ischemia-reperfusion and acute untreated rejection in a canine heterotopic heart transplantation model. Transplantation. 2001;71:26–32. doi: 10.1097/00007890-200101150-00005. [DOI] [PubMed] [Google Scholar]
- 58.Dimmeler S. Fleming I. Fisslthaler B. Hermann C. Busse R. Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt- dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 59.Doshi SN. Naka KK. Payne N. Jones CJ. Ashton M. Lewis MJ. Goodfellow J. Flow-mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci (Lond) 2001;101:629–635. [PubMed] [Google Scholar]
- 60.Drexler H. Kastner S. Strobel A. Studer R. Brodde OE. Hasenfuss G. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol. 1998;32:955–963. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 61.Duma D. Fernandes D. Bonini MG. Stadler K. Mason RP. Assreuy J. NOS-1 derived NO is an essential triggering signal for the development of systemic inflammatory responses. Eur J Pharmacol. 2011;668:285–292. doi: 10.1016/j.ejphar.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 62.Dumitrescu C. Biondi R. Xia Y. Cardounel AJ. Druhan LJ. Ambrosio G. Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proc Natl Acad Sci U S A. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edes I. Gasior Z. Wita K. Effects of nebivolol on left ventricular function in elderly patients with chronic heart failure: results of the ENECA study. Eur J Heart Failure. 2005;7:631–639. doi: 10.1016/j.ejheart.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Eliasson MJ. Blackshaw S. Schell MJ. Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci U S A. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eu JP. Xu L. Stamler JS. Meissner G. Regulation of ryanodine receptors by reactive nitrogen species. Biochem Pharmacol. 1999;57:1079–1084. doi: 10.1016/s0006-2952(98)00360-8. [DOI] [PubMed] [Google Scholar]
- 66.Feiner EC. Chung P. Jasmin JF. Zhang J. Whitaker-Menezes D. Myers V. Song J. Feldman EW. Funakoshi H. Degeorge BR., Jr. Yelamarty RV. Koch WJ. Lisanti MP. McTiernan CF. Cheung JY. Bristow MR. Chan TO. Feldman AM. Left ventricular dysfunction in murine models of heart failure and in failing human heart is associated with a selective decrease in the expression of caveolin-3. J Card Fail. 2011;17:253–263. doi: 10.1016/j.cardfail.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng Y. Venema VJ. Venema RC. Tsai N. Caldwell RB. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem Biophys Res Commun. 1999;256:192–197. doi: 10.1006/bbrc.1998.9790. [DOI] [PubMed] [Google Scholar]
- 68.Feron O. Belhassen L. Kobzik L. Smith TW. Kelly RA. Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 69.Flather MD. Shibata MC. Coats AJ. Van Veldhuisen DJ. Parkhomenko A. Borbola J. Cohen-Solal A. Dumitrascu D. Ferrari R. Lechat P. Soler-Soler J. Tavazzi L. Spinarova L. Toman J. Bohm M. Anker SD. Thompson SG. Poole-Wilson PA. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 70.Fleming I. Busse R. Signal transduction of eNOS activation. Cardiovasc Res. 1999;43:532–541. doi: 10.1016/s0008-6363(99)00094-2. [DOI] [PubMed] [Google Scholar]
- 71.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Francis SH. Busch JL. Corbin JD. Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frey R. Muck W. Unger S. Artmeier-Brandt U. Weimann G. Wensing G. Pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase activator cinaciguat (BAY 58–2667) in healthy male volunteers. J Clin Pharmacol. 2008;48:1400–1410. doi: 10.1177/0091270008322906. [DOI] [PubMed] [Google Scholar]
- 74.Fu JY. Qian LB. Zhu LG. Liang HT. Tan YN. Lu HT. Lu JF. Wang HP. Xia Q. Betulinic acid ameliorates endothelium-dependent relaxation in L-NAME-induced hypertensive rats by reducing oxidative stress. Eur J Pharm Sci. 2011;44:385–391. doi: 10.1016/j.ejps.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 75.Fulton D. Gratton JP. McCabe TJ. Fontana J. Fujio Y. Walsh K. Franke TF. Papapetropoulos A. Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fung HL. Bauer JA. Mechanisms of nitrate tolerance. Cardiovasc Drugs Ther. 1994;8:489–499. doi: 10.1007/BF00877927. [DOI] [PubMed] [Google Scholar]
- 77.Gallis B. Corthals GL. Goodlett DR. Ueba H. Kim F. Presnell SR. Figeys D. Harrison DG. Berk BC. Aebersold R. Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 78.Gao L. Pung YF. Zhang J. Chen P. Wang T. Li M. Meza M. Toro L. Cai H. Sepiapterin reductase regulation of endothelial tetrahydrobiopterin and nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2009;297:H331–H339. doi: 10.1152/ajpheart.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gauthier C. Leblais V. Kobzik L. Trochu JN. Khandoudi N. Bril A. Balligand JL. Le Marec H. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest. 1998;102:1377–1384. doi: 10.1172/JCI2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gazzerro E. Sotgia F. Bruno C. Lisanti MP. Minetti C. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur J Hum Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghofrani HA. Hoeper MM. Halank M. Meyer FJ. Staehler G. Behr J. Ewert R. Weimann G. Grimminger F. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J. 2010;36:792–799. doi: 10.1183/09031936.00182909. [DOI] [PubMed] [Google Scholar]
- 82.Godecke A. Heinicke T. Kamkin A. Kiseleva I. Strasser RH. Decking UK. Stumpe T. Isenberg G. Schrader J. Inotropic response to beta-adrenergic receptor stimulation and anti-adrenergic effect of ACh in endothelial NO synthase-deficient mouse hearts. J Physiol. 2001;532:195–204. doi: 10.1111/j.1469-7793.2001.0195g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gómez R. Caballero R. Barana A. Amorós I. Calvo E. López JA. Klein H. Vaquero M. Osuna L. Atienza F. Almendral J. Pinto Á. Tamargo J. Delpón E. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res. 2009;105:383–392. doi: 10.1161/CIRCRESAHA.109.197558. [DOI] [PubMed] [Google Scholar]
- 84.Gómez R. Núñez L. Vaquero M. Amorós I. Barana A. de Prada T. Macaya C. Maroto L. Rodríguez E. Caballero R. López-Farré A. Tamargo J. Delpón E. Nitric oxide inhibits Kv4.3 and human cardiac transient outward potassium current (Ito1) Cardiovasc Res. 2008;80:375–384. doi: 10.1093/cvr/cvn205. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez DR. Beigi F. Treuer AV. Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorren AC. List BM. Schrammel A. Pitters E. Hemmens B. Werner ER. Schmidt K. Mayer B. Tetrahydrobiopterin-free neuronal nitric oxide synthase: evidence for two identical highly anticooperative pteridine binding sites. Biochemistry. 1996;35:16735–16745. doi: 10.1021/bi961931j. [DOI] [PubMed] [Google Scholar]
- 87.Gross SS. Levi R. Madera A. Park KH. Vane J. Hattori Y. Tetrahydrobiopterin synthesis is induced by LPS in vascular smooth muscle and is rate-limiting for nitric oxide production. Adv Exp Med Biol. 1993;338:295–300. doi: 10.1007/978-1-4615-2960-6_61. [DOI] [PubMed] [Google Scholar]
- 88.Guazzi M. Casali M. Berti F. Rossoni G. Colonna VD. Guazzi MD. Endothelium-mediated modulation of ergoreflex and improvement in exercise ventilation by acute sildenafil in heart failure patients. Clin Pharmacol Ther. 2008;83:336–341. doi: 10.1038/sj.clpt.6100306. [DOI] [PubMed] [Google Scholar]
- 89.Guazzi M. Vicenzi M. Arena R. Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 90.Gyurko R. Kuhlencordt P. Fishman MC. Huang PL. Modulation of mouse cardiac function in vivo by eNOS and ANP. Am J Physiol Heart Circ Physiol. 2000;278:H971–H981. doi: 10.1152/ajpheart.2000.278.3.H971. [DOI] [PubMed] [Google Scholar]
- 91.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 92.Hare JM. Mangal B. Brown J. Fisher C., Jr. Freudenberger R. Colucci WS. Mann DL. Liu P. Givertz MM. Schwarz RP. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 93.Hattori Y. Gross SS. GTP cyclohydrolase I mRNA is induced by LPS in vascular smooth muscle: characterization, sequence and relationship to nitric oxide synthase. Biochem Biophys Res Comm. 1993;195:435–441. doi: 10.1006/bbrc.1993.2062. [DOI] [PubMed] [Google Scholar]
- 94.Hattori Y. Nakanishi N. Akimoto K. Yoshida M. Kasai K. HMG-CoA reductase inhibitor increases GTP cyclohydrolase I mRNA and tetrahydrobiopterin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:176–182. doi: 10.1161/01.atv.0000054659.72231.a1. [DOI] [PubMed] [Google Scholar]
- 95.Hayashi Y. Nishio M. Naito Y. Yokokura H. Nimura Y. Hidaka H. Watanabe Y. Regulation of neuronal nitric-oxide synthase by calmodulin kinases. J Biol Chem. 1999;274:20597–20602. doi: 10.1074/jbc.274.29.20597. [DOI] [PubMed] [Google Scholar]
- 96.Heitzer T. Schlinzig T. Krohn K. Meinertz T. Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 97.Herring N. Paterson DJ. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp Physiol. 2009;94:46–53. doi: 10.1113/expphysiol.2008.044776. [DOI] [PubMed] [Google Scholar]
- 98.Hess DT. Stamler JS. Regulation by S-nitrosylation of protein posttranslational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoke NN. Salloum FN. Kass DA. Das A. Kukreja RC. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells. 2012;30:326–335. doi: 10.1002/stem.789. [DOI] [PubMed] [Google Scholar]
- 100.Horikawa YT. Panneerselvam M. Kawaraguchi Y. Tsutsumi YM. Ali SS. Balijepalli RC. Murray F. Head BP. Niesman IR. Rieg T. Vallon V. Insel PA. Patel HH. Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol. 2011;57:2273–2283. doi: 10.1016/j.jacc.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hurshman AR. Krebs C. Edmondson DE. Huynh BH. Marletta MA. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- 102.Ignarro LJ. Different pharmacological properties of two enantiomers in a unique beta-blocker, nebivolol. Cardiovasc Ther. 2008;26:115–134. doi: 10.1111/j.1527-3466.2008.00044.x. [DOI] [PubMed] [Google Scholar]
- 103.Ii M. Nishimura H. Iwakura A. Wecker A. Eaton E. Asahara T. Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 104.Ionova IA. Vasquez-Vivar J. Whitsett J. Herrnreiter A. Medhora M. Cooley BC. Pieper GM. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am J Physiol Heart Circ Physiol. 2008;295:H2178–H2187. doi: 10.1152/ajpheart.00748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iwakura A. Shastry S. Luedemann C. Hamada H. Kawamoto A. Kishore R. Zhu Y. Qin G. Silver M. Thorne T. Eaton L. Masuda H. Asahara T. Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 106.Iwata A. Sai S. Nitta Y. Chen M. de Fries-Hallstrand R. Dalesandro J. Thomas R. Allen MD. Liposome-mediated gene transfection of endothelial nitric oxide synthase reduces endothelial activation and leukocyte infiltration in transplanted hearts. Circulation. 2001;103:2753–2759. doi: 10.1161/01.cir.103.22.2753. [DOI] [PubMed] [Google Scholar]
- 107.Janssens S. Pokreisz P. Schoonjans L. Pellens M. Vermeersch P. Tjwa M. Jans P. Scherrer-Crosbie M. Picard MH. Szelid Z. Gillijns H. Van de Werf F. Collen D. Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circ Res. 2004;94:1256–1262. doi: 10.1161/01.RES.0000126497.38281.23. [DOI] [PubMed] [Google Scholar]
- 108.Jeppsson A. Pellegrini C. O'Brien T. Miller VM. Tazelaar HD. Taner CB. McGregor CG. Gene transfer of endothelial nitric oxide synthase to pulmonary allografts: impact on acute rejection. Transpl Int. 2000;13(Suppl 1):S591–S596. doi: 10.1007/s001470050409. [DOI] [PubMed] [Google Scholar]
- 109.Jin CZ. Jang JH. Wang Y. Kim JG. Bae YM. Shi J. Che CR. Kim SJ. Zhang YH. Neuronal nitric oxide synthase is up-regulated by angiotensin II and attenuates NADPH oxidase activity and facilitates relaxation in murine left ventricular myocytes. J Mol Cell Cardiol. 2012;52:1274–1281. doi: 10.1016/j.yjmcc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 110.Jones SP. Greer JJ. van Haperen R. Duncker DJ. de Crom R. Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci U S A. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jung C. Gonon AT. Sjoquist PO. Lundberg JO. Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res. 2010;85:147–154. doi: 10.1093/cvr/cvp303. [DOI] [PubMed] [Google Scholar]
- 112.Kao WH. Arking DE. Post W. Rea TD. Sotoodehnia N. Prineas RJ. Bishe B. Doan BQ. Boerwinkle E. Psaty BM. Tomaselli GF. Coresh J. Siscovick DS. Marban E. Spooner PM. Burke GL. Chakravarti A. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kar S. Kavdia M. Modeling of biopterin-dependent pathways of eNOS for nitric oxide and superoxide production. Free Radic Biol Med. 2011;51:1411–1427. doi: 10.1016/j.freeradbiomed.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawashima S. Yamashita T. Ozaki M. Ohashi Y. Azumi H. Inoue N. Hirata K-I. Hayashi Y. Itoh H. Yokoyama M. Endothelial NO synthase overexpression inhibits lesion formation in mouse model of vascular remodeling. Arterioscler Thromb Vasc Biol. 2001;21:201–207. doi: 10.1161/01.atv.21.2.201. [DOI] [PubMed] [Google Scholar]
- 115.Kazakov A. Muller P. Jagoda P. Semenov A. Bohm M. Laufs U. Endothelial nitric oxide synthase of the bone marrow regulates myocardial hypertrophy, fibrosis, and angiogenesis. Cardiovasc Res. 2012;93:397–405. doi: 10.1093/cvr/cvr305. [DOI] [PubMed] [Google Scholar]
- 116.Kelm M. Dahmann R. Wink D. Feelisch M. The nitric oxide/superoxide assay. Insights into the biological chemistry of the NO/O-2. interaction. J Biol Chem. 1997;272:9922–9932. doi: 10.1074/jbc.272.15.9922. [DOI] [PubMed] [Google Scholar]
- 117.Khan SA. Lee K. Minhas KM. Gonzalez DR. Raju SV. Tejani AD. Li D. Berkowitz DE. Hare JM. Neuronal nitric oxide synthase negatively regulates xanthine oxidoreductase inhibition of cardiac excitation-contraction coupling. Proc Natl Acad Sci U S A. 2004;101:15944–15948. doi: 10.1073/pnas.0404136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khong S. Andrews K. Huynh N. Venardos K. Aprico A. Michell D. Zarei M. Moe K. Dusting G. Kaye D. Chin-Dusting J. Arginase II inhibition prevents nitrate tolerance. Br J Pharmacol. 2012;166:2015–2023. doi: 10.1111/j.1476-5381.2012.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim D. Rybalkin SD. Pi X. Wang Y. Zhang C. Munzel T. Beavo JA. Berk BC. Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 120.Kim JH. Bugaj LJ. Oh YJ. Bivalacqua TJ. Ryoo S. Soucy KG. Santhanam L. Webb A. Camara A. Sikka G. Nyhan D. Shoukas AA. Ilies M. Christianson DW. Champion HC. Berkowitz DE. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim YM. Guzik TJ. Zhang YH. Zhang MH. Kattach H. Ratnatunga C. Pillai R. Channon KM. Casadei B. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 122.Klinge CM. Wickramasinghe NS. Ivanova MM. Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 123.Knowles RG. Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Knyushko T. Sharov VS. Williams TD. Shoneich C. Bigelow DJ. 3-nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44:13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 125.Kobayashi YM. Rader EP. Crawford RW. Iyengar NK. Thedens DR. Faulkner JA. Parikh SV. Weiss RM. Chamberlain JS. Moore SA. Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kohr MJ. Sun J. Aponte A. Wang G. Gucek M. Murphy E. Steenbergen C. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koka S. Kukreja RC. Attenuation of doxorubicin-induced cardiotoxicity by tadalafil: a long acting phosphodiesterase-5 inhibitor. Mol Cell Pharmacol. 2010;2:173–178. [PMC free article] [PubMed] [Google Scholar]
- 128.Kollau A. Beretta M. Russwurm M. Koesling D. Keung WM. Schmidt K. Mayer B. Mitochondrial nitrite reduction coupled to soluble guanylate cyclase activation: lack of evidence for a role in the bioactivation of nitroglycerin. Nitric Oxide. 2009;20:53–60. doi: 10.1016/j.niox.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 129.Kollau A. Hofer A. Russwurm M. Koesling D. Keung WM. Schmidt K. Brunner F. Mayer B. Contribution of aldehyde dehydrogenase to mitochondrial bioactivation of nitroglycerin: evidence for the activation of purified soluble guanylate cyclase through direct formation of nitric oxide. Biochem J. 2005;385:769–777. doi: 10.1042/BJ20041354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Komeima K. Hayashi Y. Naito Y. Watanabe Y. Inhibition of neuronal nitric-oxide synthase by calcium/calmodulin-dependent protein kinase IIalpha through Ser847 phosphorylation in NG108-15 neuronal cells. J Biol Chem. 2000;275:28139–28143. doi: 10.1074/jbc.M003198200. [DOI] [PubMed] [Google Scholar]
- 131.Kuhlencordt PJ. Hotten S. Schodel J. Rutzel S. Hu K. Widder J. Marx A. Huang PL. Ertl G. Atheroprotective effects of neuronal nitric oxide synthase in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:1539–1544. doi: 10.1161/01.ATV.0000223143.88128.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Landmesser U. Engberding N. Bahlmann FH. Schaefer A. Wiencke A. Heineke A. Spiekermann S. Hilfiker-Kleiner D. Templin C. Kotlarz D. Mueller M. Fuchs M. Hornig B. Haller H. Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 133.Landmesser U. Spiekermann S. Dikalov S. Tatge H. Wilke R. Kohler C. Harrison DG. Hornig B. Drexler H. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–3078. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 134.Laroux FS. Lefer DJ. Kawachi S. Scalia R. Cockrell AS. Gray L. Van der Heyde H. Hoffman JM. Grisham MB. Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid Redox Signal. 2000;2:391–396. doi: 10.1089/15230860050192161. [DOI] [PubMed] [Google Scholar]
- 135.Layland J. Li JM. Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee DI. Vahebi S. Tocchetti CG. Barouch LA. Solaro RJ. Takimoto E. Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lefer DJ. Jones SP. Girod WG. Baines A. Grisham MB. Cockrell AS. Huang PL. Scalia R. Leukocyte-endothelial cell interactions in nitric oxide synthase-deficient mice. Am J Physiol. 1999;276:H1943–H1950. doi: 10.1152/ajpheart.1999.276.6.H1943. [DOI] [PubMed] [Google Scholar]
- 138.Leiper J. Nandi M. Torondel B. Murray-Rust J. Malaki M. O'Hara B. Rossiter S. Anthony S. Madhani M. Selwood D. Smith C. Wojciak-Stothard B. Rudiger A. Stidwill R. McDonald NQ. Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13:198–203. doi: 10.1038/nm1543. [DOI] [PubMed] [Google Scholar]
- 139.Lewis NP. Tsao PS. Rickenbacher PR. Xue C. Johns RA. Haywood GA. von der Leyen H. Trindade PT. Cooke JP. Hunt SA. Billingham ME. Valantine HA. Fowler MB. Induction of nitric oxide synthase in the human cardiac allograft is associated with contractile dysfunction of the left ventricle. Circulation. 1996;93:720–729. doi: 10.1161/01.cir.93.4.720. [DOI] [PubMed] [Google Scholar]
- 140.Li M. Chiou KR. Bugayenko A. Irani K. Kass DA. Reduced wall compliance suppresses Akt-dependent apoptosis protection stimulated by pulse perfusion. Circ Res. 2005;97:587–595. doi: 10.1161/01.RES.0000181432.73920.b1. [DOI] [PubMed] [Google Scholar]
- 141.Lima B. Lam GK. Xie L. Diesen DL. Villamizar N. Nienaber J. Messina E. Bowles D. Kontos CD. Hare JM. Stamler JS. Rockman HA. Endogenous S-nitrosothiols protect against myocardial injury. Proc Natl Acad Sci U S A. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin MI. Fulton D. Babbitt R. Fleming I. Busse R. Pritchard KA., Jr. Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 143.Loke KE. McConnell PI. Tuzman JM. Shesely EG. Smith CJ. Stackpole CJ. Thompson CI. Kaley G. Wolin MS. Hintze TH. Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res. 1999;84:840–845. doi: 10.1161/01.res.84.7.840. [DOI] [PubMed] [Google Scholar]
- 144.Lonn E. Bosch J. Yusuf S. Sheridan P. Pogue J. Arnold JM. Ross C. Arnold A. Sleight P. Probstfield J. Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 145.Lorenz M. Hewing B. Hui J. Zepp A. Baumann G. Bindereif A. Stangl V. Stangl K. Alternative splicing in intron 13 of the human eNOS gene: a potential mechanism for regulating eNOS activity. Faseb J. 2007;21:1556–1564. doi: 10.1096/fj.06-7434com. [DOI] [PubMed] [Google Scholar]
- 146.Luo Z. Fujio Y. Kureishi Y. Rudic RD. Daumerie G. Fulton D. Sessa WC. Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide- dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maffei A. Di Pardo A. Carangi R. Carullo P. Poulet R. Gentile MT. Vecchione C. Lembo G. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension. 2007;50:652–656. doi: 10.1161/HYPERTENSIONAHA.107.094458. [DOI] [PubMed] [Google Scholar]
- 148.Martin SR. Emanuel K. Sears CE. Zhang YH. Casadei B. Are myocardial eNOS and nNOS involved in the beta-adrenergic and muscarinic regulation of inotropy? a systematic investigation. Cardiovasc Res. 2006;70:97–106. doi: 10.1016/j.cardiores.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 149.Masters BS. McMillan K. Sheta EA. Nishimura JS. Roman LJ. Martasek P. Neuronal nitric oxide synthase, a modular enzyme formed by convergent evolution: structure studies of a cysteine thiolate-liganded heme protein that hydroxylates L-arginine to produce NO. as a cellular signal [published erratum appears in FASEB J 10(9): 1107, 1996] FASEB J. 1996;10:552–558. doi: 10.1096/fasebj.10.5.8621055. [DOI] [PubMed] [Google Scholar]
- 150.Matoba T. Shimokawa H. Nakashima M. Hirakawa Y. Mukai Y. Hirano K. Kanaide H. Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McEniery CM. Schmitt M. Qasem A. Webb DJ. Avolio AP. Wilkinson IB. Cockcroft JR. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44:305–310. doi: 10.1161/01.HYP.0000137983.45556.6e. [DOI] [PubMed] [Google Scholar]
- 152.Michell BJ. Chen Z. Tiganis T. Stapleton D. Katsis F. Power DA. Sim AT. Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 153.Milstien S. Jaffe H. Kowlessur D. Bonner TI. Purification and cloning of the GTP cyclohydrolase I feedback regulatory protein, GFRP. J Biol Chem. 1996;271:19743–19751. doi: 10.1074/jbc.271.33.19743. [DOI] [PubMed] [Google Scholar]
- 154.Minhas KM. Saraiva RM. Schuleri KH. Lehrke S. Zheng M. Saliaris AP. Berry CE. Barouch LA. Vandegaer KM. Li D. Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 155.Moens AL. Ketner EA. Takimoto E. Schmidt TS. O'Neill CA. Wolin MS. Alp NJ. Channon KM. Kass DA. Bi-modal dose-dependent cardiac response to tetrahydrobiopterin in pressure-overload induced hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:564–569. doi: 10.1016/j.yjmcc.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moens AL. Kietadisorn R. Lin JY. Kass D. Targeting endothelial and myocardial dysfunction with tetrahydrobiopterin. J Mol Cell Cardiol. 2011;51:559–563. doi: 10.1016/j.yjmcc.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 157.Moens AL. Takimoto E. Tocchetti CG. Chakir K. Bedja D. Cormaci G. Ketner EA. Majmudar M. Gabrielson K. Halushka MK. Mitchell JB. Biswal S. Channon KM. Wolin MS. Alp NJ. Paolocci N. Champion HC. Kass DA. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moens AL. Vrints CJ. Claeys MJ. Timmermans JP. Champion HC. Kass DA. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2008;294:H1971–H1977. doi: 10.1152/ajpheart.91503.2007. [DOI] [PubMed] [Google Scholar]
- 159.Morikawa K. Shimokawa H. Matoba T. Kubota H. Akaike T. Talukder MA. Hatanaka M. Fujiki T. Maeda H. Takahashi S. Takeshita A. Pivotal role of Cu, Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moroi M. Zhang L. Yasuda T. Virmani R. Gold HK. Fishman MC. Huang PL. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Munzel T. Kurz S. Rajagopalan S. Thoenes M. Berrington WR. Thompson JA. Freeman BA. Harrison DG. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J Clin Invest. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nagayama T. Hsu S. Zhang M. Koitabashi N. Bedja D. Gabrielson KL. Takimoto E. Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nakata S. Tsutsui M. Shimokawa H. Suda O. Morishita T. Shibata K. Yatera Y. Sabanai K. Tanimoto A. Nagasaki M. Tasaki H. Sasaguri Y. Nakashima Y. Otsuji Y. Yanagihara N. Spontaneous myocardial infarction in mice lacking all nitric oxide synthase isoforms. Circulation. 2008;117:2211–2223. doi: 10.1161/CIRCULATIONAHA.107.742692. [DOI] [PubMed] [Google Scholar]
- 164.Nasr G. Maurice C. Allopurinol and global left myocardial function in heart failure patients. J Cardiovasc Dis Res. 2010;1:191–195. doi: 10.4103/0975-3583.74262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nichol CA. Lee CL. Edelstein MP. Chao JY. Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proc Natl Acad Sci U S A. 1983;80:1546–1550. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nunez L. Vaquero M. Gomez R. Caballero R. Mateos-Caceres P. Macaya C. Iriepa I. Galvez E. Lopez-Farre A. Tamargo J. Delpon E. Nitric oxide blocks hKv1.5 channels by S-nitrosylation and by a cyclic GMP-dependent mechanism. Cardiovasc Res. 2006;72:80–89. doi: 10.1016/j.cardiores.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 167.Oceandy D. Cartwright EJ. Emerson M. Prehar S. Baudoin FM. Zi M. Alatwi N. Venetucci L. Schuh K. Williams JC. Armesilla AL. Neyses L. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115:483–492. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 168.Oelze M. Daiber A. Brandes RP. Hortmann M. Wenzel P. Hink U. Schulz E. Mollnau H. von Sandersleben A. Kleschyov AL. Mulsch A. Li H. Forstermann U. Munzel T. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 169.Otani H. The role of nitric oxide in myocardial repair and remodeling. Antioxid Redox Signal. 2009;11:1913–1928. doi: 10.1089/ars.2009.2453. [DOI] [PubMed] [Google Scholar]
- 170.Ozawa K. Whalen EJ. Nelson CD. Mu Y. Hess DT. Lefkowitz RJ. Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parenti A. Filippi S. Amerini S. Granger HJ. Fazzini A. Ledda F. Inositol phosphate metabolism and nitric-oxide synthase activity in endothelial cells are involved in the vasorelaxant activity of nebivolol. J Pharmacol Exp Ther. 2000;292:698–703. [PubMed] [Google Scholar]
- 172.Paulus WJ. Vantrimpont PJ. Shah AM. Paracrine coronary endothelial control of left ventricular function in humans. Circulation. 1995;92:2119–2126. doi: 10.1161/01.cir.92.8.2119. [DOI] [PubMed] [Google Scholar]
- 173.Pearson JD. Endothelial progenitor cells—an evolving story. Microvasc Res. 2010;79:162–168. doi: 10.1016/j.mvr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 174.Percival JM. Anderson KN. Huang P. Adams ME. Froehner SC. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Perrault LP. Bidouard JP. Janiak P. Villeneuve N. Bruneval P. Vilaine JP. Vanhoutte PM. Time course of coronary endothelial dysfunction in acute untreated rejection after heterotopic heart transplantation. J Heart Lung Transplant. 1997;16:643–657. [PubMed] [Google Scholar]
- 176.Petroff MG. Kim SH. Pepe S. Dessy C. Marban E. Balligand JL. Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- 177.Piech A. Massart PE. Dessy C. Feron O. Havaux X. Morel N. Vanoverschelde JL. Donckier J. Balligand JL. Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2002;282:H219–H231. doi: 10.1152/ajpheart.2002.282.1.H219. [DOI] [PubMed] [Google Scholar]
- 178.Pinsky DJ. Patton S. Mesaros S. Brovkovych V. Kubaszewski E. Grunfeld S. Malinski T. Mechanical transduction of nitric oxide synthesis in the beating heart. Circ Res. 1997;81:372–379. doi: 10.1161/01.res.81.3.372. [DOI] [PubMed] [Google Scholar]
- 179.Predmore BL. Julian D. Cardounel AJ. Hydrogen sulfide increases nitric oxide production from endothelial cells by an akt-dependent mechanism. Front Physiol. 2011;2:104. doi: 10.3389/fphys.2011.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Prendergast BD. Sagach VF. Shah AM. Basal release of nitric oxide augments the Frank-Starling response in the isolated heart. Circulation. 1997;96:1320–1329. doi: 10.1161/01.cir.96.4.1320. [DOI] [PubMed] [Google Scholar]
- 181.Prysyazhna O. Rudyk O. Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012;18:286–290. doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rakhit A. Maguire CT. Wakimoto H. Gehrmann J. Li GK. Kelly RA. Michel T. Berul CI. In vivo electrophysiologic studies in endothelial nitric oxide synthase (eNOS)-deficient mice. J Cardiovasc Electrophysiol. 2001;12:1295–1301. doi: 10.1046/j.1540-8167.2001.01295.x. [DOI] [PubMed] [Google Scholar]
- 183.Raman CS. Li H. Martasek P. Kral V. Masters BS. Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- 184.Rameau GA. Chiu LY. Ziff EB. Bidirectional regulation of neuronal nitric-oxide synthase phosphorylation at serine 847 by the N-methyl-D-aspartate receptor. J Biol Chem. 2004;279:14307–14314. doi: 10.1074/jbc.M311103200. [DOI] [PubMed] [Google Scholar]
- 185.Reilly SN. Jayaram R. Nahar K. Antoniades C. Verheule S. Channon KM. Alp NJ. Schotten U. Casadei B. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124:1107–1117. doi: 10.1161/CIRCULATIONAHA.111.029223. [DOI] [PubMed] [Google Scholar]
- 186.Ren J. Duan J. Thomas DP. Yang X. Sreejayan N. Sowers JR. Leri A. Kajstura J. Gao F. Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 187.Rodriguez-Crespo I. Gerber NC. Ortiz de Montellano PR. Endothelial nitric-oxide synthase. Expression in Escherichia coli, spectroscopic characterization, and role of tetrahydrobiopterin in dimer formation. J Biol Chem. 1996;271:11462–11467. doi: 10.1074/jbc.271.19.11462. [DOI] [PubMed] [Google Scholar]
- 188.Rozec B. Gauthier C. Beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–673. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 189.Ruetten H. Dimmeler S. Gehring D. Ihling C. Zeiher AM. Concentric left ventricular remodeling in endothelial nitric oxide synthase knockout mice by chronic pressure overload. Cardiovasc Res. 2005;66:444–453. doi: 10.1016/j.cardiores.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 190.Saavedra WF. Paolocci N. St John ME. Skaf MW. Stewart GC. Xie JS. Harrison RW. Zeichner J. Mudrick D. Marban E. Kass DA. Hare JM. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res. 2002;90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 191.Saeki A. Recchia FA. Senzaki H. Kass DA. Minimal role of nitric oxide in basal coronary flow regulation and cardiac energetics of blood-perfused isolated canine heart. J Physiol. 1996;491(Pt 2):455–463. doi: 10.1113/jphysiol.1996.sp021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Salloum F. Yin C. Xi L. Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–597. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 193.Salloum FN. Abbate A. Das A. Houser JE. Mudrick CA. Qureshi IZ. Hoke NN. Roy SK. Brown WR. Prabhakar S. Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–H1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 194.Santhanam L. Christianson DW. Nyhan D. Berkowitz DE. Arginase and vascular aging. J Appl Physiol. 2008;105:1632–1642. doi: 10.1152/japplphysiol.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saraiva RM. Minhas KM. Raju SV. Barouch LA. Pitz E. Schuleri KH. Vandegaer K. Li D. Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 196.Sartoretto JL. Kalwa H. Pluth MD. Lippard SJ. Michel T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc Natl Acad Sci U S A. 2011;108:15792–15797. doi: 10.1073/pnas.1111331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Scherrer-Crosbie M. Ullrich R. Bloch KD. Nakajima H. Nasseri B. Aretz HT. Lindsey ML. Vancon AC. Huang PL. Lee RT. Zapol WM. Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- 198.Schmidt PP. Lange R. Gorren AC. Werner ER. Mayer B. Andersson KK. Formation of a protonated trihydrobiopterin radical cation in the first reaction cycle of neuronal and endothelial nitric oxide synthase detected by electron paramagnetic resonance spectroscopy. J Biol Inorg Chem. 2001;6:151–158. doi: 10.1007/s007750000185. [DOI] [PubMed] [Google Scholar]
- 199.Sears CE. Bryant SM. Ashley EA. Lygate CA. Rakovic S. Wallis HL. Neubauer S. Terrar DA. Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- 200.Seddon M. Melikian N. Dworakowski R. Shabeeh H. Jiang B. Byrne J. Casadei B. Chowienczyk P. Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 201.Seddon MD. Chowienczyk PJ. Brett SE. Casadei B. Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- 202.Sharma NM. Zheng H. Mehta PP. Li Y-F. Patel KP. Decreased nNOS in the PVN leads to increased sympathoexcitation in chronic heart failure: role for CAPON and Ang II. Cardiovasc Res. 2011;92:348–357. doi: 10.1093/cvr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shimazu T. Otani H. Yoshioka K. Fujita M. Okazaki T. Iwasaka T. Sepiapterin enhances angiogenesis and functional recovery in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H2061–H2072. doi: 10.1152/ajpheart.00525.2011. [DOI] [PubMed] [Google Scholar]
- 204.Shimizu S. Shiota K. Yamamoto S. Miyasaka Y. Ishii M. Watabe T. Nishida M. Mori Y. Yamamoto T. Kiuchi Y. Hydrogen peroxide stimulates tetrahydrobiopterin synthesis through the induction of GTP-cyclohydrolase I and increases nitric oxide synthase activity in vascular endothelial cells. Free Radic Biol Med. 2003;34:1343–1352. doi: 10.1016/s0891-5849(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 205.Shimokawa H. Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 206.Silberman GA. Fan TH. Liu H. Jiao Z. Xiao HD. Lovelock JD. Boulden BM. Widder J. Fredd S. Bernstein KE. Wolska BM. Dikalov S. Harrison DG. Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sorrentino SA. Doerries C. Manes C. Speer T. Dessy C. Lobysheva I. Mohmand W. Akbar R. Bahlmann F. Besler C. Schaefer A. Hilfiker-Kleiner D. Luscher TF. Balligand JL. Drexler H. Landmesser U. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1-blockade. J Am Coll Cardiol. 2011;57:601–611. doi: 10.1016/j.jacc.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 208.Stadler K. Bonini MG. Dallas S. Duma D. Mason RP. Kadiiska MB. Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis. Am J Physiol Endocrinol Metab. 2008;295:E456–E462. doi: 10.1152/ajpendo.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Steinkamp-Fenske K. Bollinger L. Xu H. Yao Y. Horke S. Forstermann U. Li H. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. J Pharmacol Exp Ther. 2007;322:836–842. doi: 10.1124/jpet.107.123356. [DOI] [PubMed] [Google Scholar]
- 210.Steppan J. Ryoo S. Schuleri KH. Gregg C. Hasan RK. White AR. Bugaj LJ. Khan M. Santhanam L. Nyhan D. Shoukas AA. Hare JM. Berkowitz DE. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- 212.Sugiyama T. Levy BD. Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J Biol Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sun J. Druhan LJ. Zweier JL. Dose dependent effects of reactive oxygen and nitrogen species on the function of neuronal nitric oxide synthase. Arch Biochem Biophys. 2008;471:126–133. doi: 10.1016/j.abb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sun J. Picht E. Ginsburg KS. Bers DM. Steenbergen C. Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel alpha1 subunit and reduced ischemia/reperfusion injury. Circ Res. 2006;98:403–411. doi: 10.1161/01.RES.0000202707.79018.0a. [DOI] [PubMed] [Google Scholar]
- 215.Szabolcs MJ. Ma N. Athan E. Zhong J. Ming M. Sciacca RR. Husemann J. Albala A. Cannon PJ. Acute cardiac allograft rejection in nitric oxide synthase-2(-/-) and nitric oxide synthase-2(+/+) mice: effects of cellular chimeras on myocardial inflammation and cardiomyocyte damage and apoptosis. Circulation. 2001;103:2514–2520. doi: 10.1161/01.cir.103.20.2514. [DOI] [PubMed] [Google Scholar]
- 216.Szocs K. Lassegue B. Wenzel P. Wendt M. Daiber A. Oelze M. Meinertz T. Munzel T. Baldus S. Increased superoxide production in nitrate tolerance is associated with NAD(P)H oxidase and aldehyde dehydrogenase 2 downregulation. J Mol Cell Cardiol. 2007;42:1111–1118. doi: 10.1016/j.yjmcc.2007.03.904. [DOI] [PubMed] [Google Scholar]
- 217.Takimoto E. Champion HC. Li M. Belardi D. Ren S. Rodriguez ER. Bedja D. Gabrielson KL. Wang Y. Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 218.Takimoto E. Champion HC. Li M. Ren S. Rodriguez ER. Tavazzi B. Lazzarino G. Paolocci N. Gabrielson KL. Wang Y. Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Takimoto Y. Aoyama T. Tanaka K. Keyamura R. Yui Y. Sasayama S. Augmented expression of neuronal nitric oxide synthase in the atria parasympathetically decreases heart rate during acute myocardial infarction in rats. Circulation. 2002;105:490–496. doi: 10.1161/hc0402.102662. [DOI] [PubMed] [Google Scholar]
- 220.Tatsumi T. Matoba S. Kawahara A. Keira N. Shiraishi J. Akashi K. Kobara M. Tanaka T. Katamura M. Nakagawa C. Ohta B. Shirayama T. Takeda K. Asayama J. Fliss H. Nakagawa M. Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J Am Coll Cardiol. 2000;35:1338–1346. doi: 10.1016/s0735-1097(00)00526-x. [DOI] [PubMed] [Google Scholar]
- 221.Taylor AL. Ziesche S. Yancy C. Carson P. D'Agostino R., Jr. Ferdinand K. Taylor M. Adams K. Sabolinski M. Worcel M. Cohn JN. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 222.Thoenes M. Forstermann U. Tracey WR. Bleese NM. Nussler AK. Scholz H. Stein B. Expression of inducible nitric oxide synthase in failing and non-failing human heart. J Mol Cell Cardiol. 1996;28:165–169. doi: 10.1006/jmcc.1996.0016. [DOI] [PubMed] [Google Scholar]
- 223.Thony B. Auerbach G. Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 224.Torfgard KE. Ahlner J. Mechanisms of action of nitrates. Cardiovasc Drugs Ther. 1994;8:701–717. doi: 10.1007/BF00877117. [DOI] [PubMed] [Google Scholar]
- 225.Traverso M. Gazzerro E. Assereto S. Sotgia F. Biancheri R. Stringara S. Giberti L. Pedemonte M. Wang X. Scapolan S. Pasquini E. Donati MA. Zara F. Lisanti MP. Bruno C. Minetti C. Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro. Lab Invest J Tech Methods Pathol. 2008;88:275–283. doi: 10.1038/labinvest.3700713. [DOI] [PubMed] [Google Scholar]
- 226.Tsai EJ. Liu Y. Koitabashi N. Bedja D. Danner T. Jasmin JF. Lisanti MP. Friebe A. Takimoto E. Kass DA. Pressure-overload-induced subcellular relocalization/oxidation of soluble guanylyl cyclase in the heart modulates enzyme stimulation. Circ Res. 2012;110:295–303. doi: 10.1161/CIRCRESAHA.111.259242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tsujita Y. Muraski J. Shiraishi I. Kato T. Kajstura J. Anversa P. Sussman MA. Nuclear targeting of Akt antagonizes aspects of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2006;103:11946–11951. doi: 10.1073/pnas.0510138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tsutsui M. Nakata S. Shimokawa H. Otsuji Y. Yanagihara N. Spontaneous myocardial infarction and nitric oxide synthase. Trends Cardiovasc Med. 2008;18:275–279. doi: 10.1016/j.tcm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 229.Tziomalos K. Hare JM. Role of xanthine oxidoreductase in cardiac nitroso-redox imbalance. Front Biosci J Virtual Libr. 2009;14:237–262. doi: 10.2741/3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ueda K. Valdivia C. Medeiros-Domingo A. Tester DJ. Vatta M. Farrugia G. Ackerman MJ. Makielski JC. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.van Haperen R. de Waard M. van Deel E. Mees B. Kutryk M. van Aken T. Hamming J. Grosveld F. Duncker DJ. de Crom R. Reduction of blood pressure, plasma cholesterol, and atherosclerosis by elevated endothelial nitric oxide. J Biol Chem. 2002;277:48803–48807. doi: 10.1074/jbc.M209477200. [DOI] [PubMed] [Google Scholar]
- 232.Vandecasteele G. Eschenhagen T. Scholz H. Stein B. Verde I. Fischmeister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med. 1999;5:331–334. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- 233.Vasquez-Vivar J. Duquaine D. Whitsett J. Kalyanaraman B. Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol. 2002;22:1655–1661. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- 234.Vasquez-Vivar J. Hogg N. Martasek P. Karoui H. Pritchard KA., Jr. Kalyanaraman B. Tetrahydrobiopterin-dependent inhibition of superoxide generation from neuronal nitric oxide synthase. J Biol Chem. 1999;274:26736–26742. doi: 10.1074/jbc.274.38.26736. [DOI] [PubMed] [Google Scholar]
- 235.Vasquez-Vivar J. Kalyanaraman B. Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 236.Vasquez-Vivar J. Martasek P. Whitsett J. Joseph J. Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Visser M. Paulus WJ. Vermeulen MA. Richir MC. Davids M. Wisselink W. de Mol BA. van Leeuwen PA. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12:1274–1281. doi: 10.1093/eurjhf/hfq158. [DOI] [PubMed] [Google Scholar]
- 238.Vo PA. Lad B. Tomlinson JA. Francis S. Ahluwalia A. Autoregulatory role of endothelium-derived nitric oxide (NO) on lipopolysaccharide-induced vascular inducible NO synthase expression and function. J Biol Chem. 2005;280:7236–7243. doi: 10.1074/jbc.M411317200. [DOI] [PubMed] [Google Scholar]
- 239.Vogel MW. Chen HH. Novel natriuretic peptides: new compounds and new approaches. Curr Heart Fail Rep. 2011;8:22–27. doi: 10.1007/s11897-010-0038-0. [DOI] [PubMed] [Google Scholar]
- 240.Wallerath T. Deckert G. Ternes T. Anderson H. Li H. Witte K. Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 241.Wang H. Kohr MJ. Traynham CJ. Wheeler DG. Janssen PM. Ziolo MT. Neuronal nitric oxide synthase signaling within cardiac myocytes targets phospholamban. Am J Physiol Cell Physiol. 2008;294:C1566–C1575. doi: 10.1152/ajpcell.00367.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang H. Kohr MJ. Traynham CJ. Ziolo MT. Phosphodiesterase 5 restricts NOS3/soluble guanylate cyclase signaling to L-type Ca2+ current in cardiac myocytes. J Mol Cell Cardiol. 2009;47:304–314. doi: 10.1016/j.yjmcc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang H. Kohr MJ. Wheeler DG. Ziolo MT. Endothelial nitric oxide synthase decreases beta-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am J Physiol Heart Circ Physiol. 2008;294:H1473–H1480. doi: 10.1152/ajpheart.01249.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang H. Viatchenko-Karpinski S. Sun J. Gyorke I. Benkusky NA. Kohr MJ. Valdivia HH. Murphy E. Gyorke S. Ziolo MT. Regulation of myocyte contraction via neuronal nitric oxide synthase: role of ryanodine receptor S-nitrosylation. J Physiol. 2010;588:2905–2917. doi: 10.1113/jphysiol.2010.192617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.West MB. Rokosh G. Obal D. Velayutham M. Xuan YT. Hill BG. Keith RJ. Schrader J. Guo Y. Conklin DJ. Prabhu SD. Zweier JL. Bolli R. Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation. 2008;118:1970–1978. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Whalen EJ. Foster MW. Matsumoto A. Ozawa K. Violin JD. Que LG. Nelson CD. Benhar M. Keys JR. Rockman HA. Koch WJ. Daaka Y. Lefkowitz RJ. Stamler JS. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 247.Whiteman M. Le Trionnaire S. Chopra M. Fox B. Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 248.Widder JD. Chen W. Li L. Dikalov S. Thony B. Hatakeyama K. Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res. 2007;101:830–838. doi: 10.1161/CIRCRESAHA.107.153809. [DOI] [PubMed] [Google Scholar]
- 249.Wilcox JN. Subramanian RR. Sundell CL. Tracey WR. Pollock JS. Harrison DG. Marsden PA. Expression of multiple isoforms of nitric oxide synthase in normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:2479–2488. doi: 10.1161/01.atv.17.11.2479. [DOI] [PubMed] [Google Scholar]
- 250.Wolfhart P. Xu H. Endlich A. Habermeier A. Closs EI. Hubschle T. Mang C. Strobel H. Suzuki T. Kleinert H. Förstermann U. Ruetten H. Li H. Antiatherosclerotic effects of small-molecular-weight compounds enhancing endothelial nitric-oxide synthase (eNOS) expression and preventing eNOS uncoupling. J Pharmocol Exp Ther. 2008;325:370–379. doi: 10.1124/jpet.107.128009. [DOI] [PubMed] [Google Scholar]
- 251.Woodman SE. Park DS. Cohen AW. Cheung MW. Chandra M. Shirani J. Tang B. Jelicks LA. Kitsis RN. Christ GJ. Factor SM. Tanowitz HB. Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 252.Xia N. Daiber A. Habermeier A. Closs EI. Thum T. Spanier G. Lu Q. Oelze M. Torzewski M. Lackner KJ. Munzel T. Forstermann U. Li H. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 253.Xie YW. Wolin MS. Role of nitric oxide and its interaction with superoxide in the suppression of cardiac muscle mitochondrial respiration. Involvement in response to hypoxia/reoxygenation. Circulation. 1996;94:2580–2586. doi: 10.1161/01.cir.94.10.2580. [DOI] [PubMed] [Google Scholar]
- 254.Xu KY. Huso DL. Dawson TM. Bredt DS. Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Xu Z. Ji X. Boysen PG. Exogenous nitric oxide generates ROS and induces cardioprotection: involvement of PKG, mitochondrial KATP channels, and ERK. Am J Physiol Heart Circ Physiol. 2004;286:H1433–H1440. doi: 10.1152/ajpheart.00882.2003. [DOI] [PubMed] [Google Scholar]
- 256.Yang L. Liu G. Zakharov SI. Bellinger AM. Mongillo M. Marx SO. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res. 2007;101:465–474. doi: 10.1161/CIRCRESAHA.107.156976. [DOI] [PubMed] [Google Scholar]
- 257.Yap J. O'Brien T. Pellegrini C. Barber DA. Tazelaar HD. Severson SR. Miller VM. McGregor CG. Distribution and function of recombinant endothelial nitric oxide synthase in transplanted hearts. Cardiovasc Res. 1999;42:720–727. doi: 10.1016/s0008-6363(99)00010-3. [DOI] [PubMed] [Google Scholar]
- 258.Yogo K. Shimokawa H. Funakoshi H. Kandabashi T. Miyata K. Okamoto S. Egashira K. Huang P. Akaike T. Takeshita A. Different vasculoprotective roles of NO synthase isoforms in vascular lesion formation in mice. Arterioscler Thromb Vasc Biol. 2000;20:E96–E100. doi: 10.1161/01.atv.20.11.e96. [DOI] [PubMed] [Google Scholar]
- 259.Yoneyama T. Brewer JM. Hatakeyama K. GTP cyclohydrolase I feedback regulatory protein is a pentamer of identical subunits. Purification, cDNA cloning, and bacterial expression. J Biol Chem. 1997;272:9690–9696. doi: 10.1074/jbc.272.15.9690. [DOI] [PubMed] [Google Scholar]
- 260.Zhang K. Mayhan WG. Patel KP. Nitric oxide within the paraventricular nucleus mediates changes in renal sympathetic nerve activity. Am J Physiol. 1997;273:R864–R872. doi: 10.1152/ajpregu.1997.273.3.R864. [DOI] [PubMed] [Google Scholar]
- 261.Zhang YH. Zhang MH. Sears CE. Emanuel K. Redwood C. El-Armouche A. Kranias EG. Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]
- 262.Zhao X. Lu X. Feng Q. Deficiency in endothelial nitric oxide synthase impairs myocardial angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H2371–H2378. doi: 10.1152/ajpheart.00383.2002. [DOI] [PubMed] [Google Scholar]
- 263.Zou MH. Shi C. Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]