Abstract
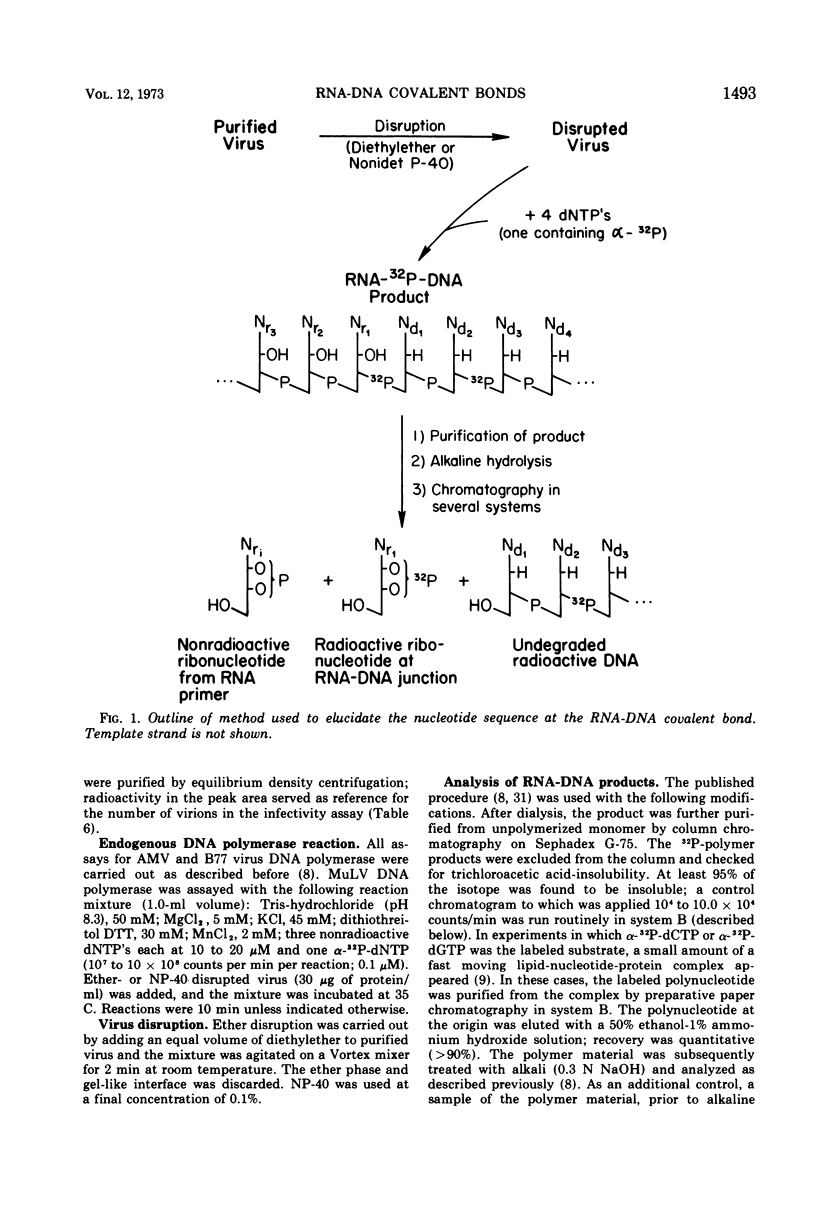
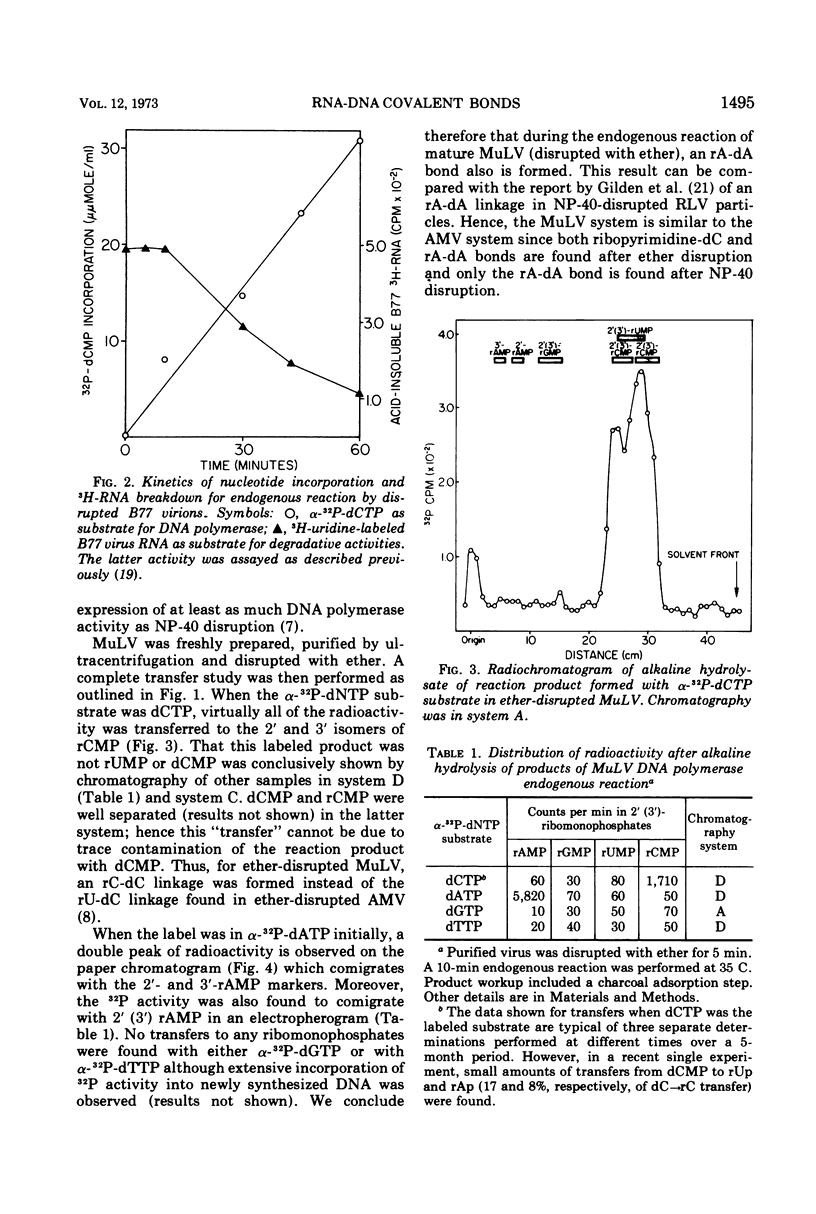
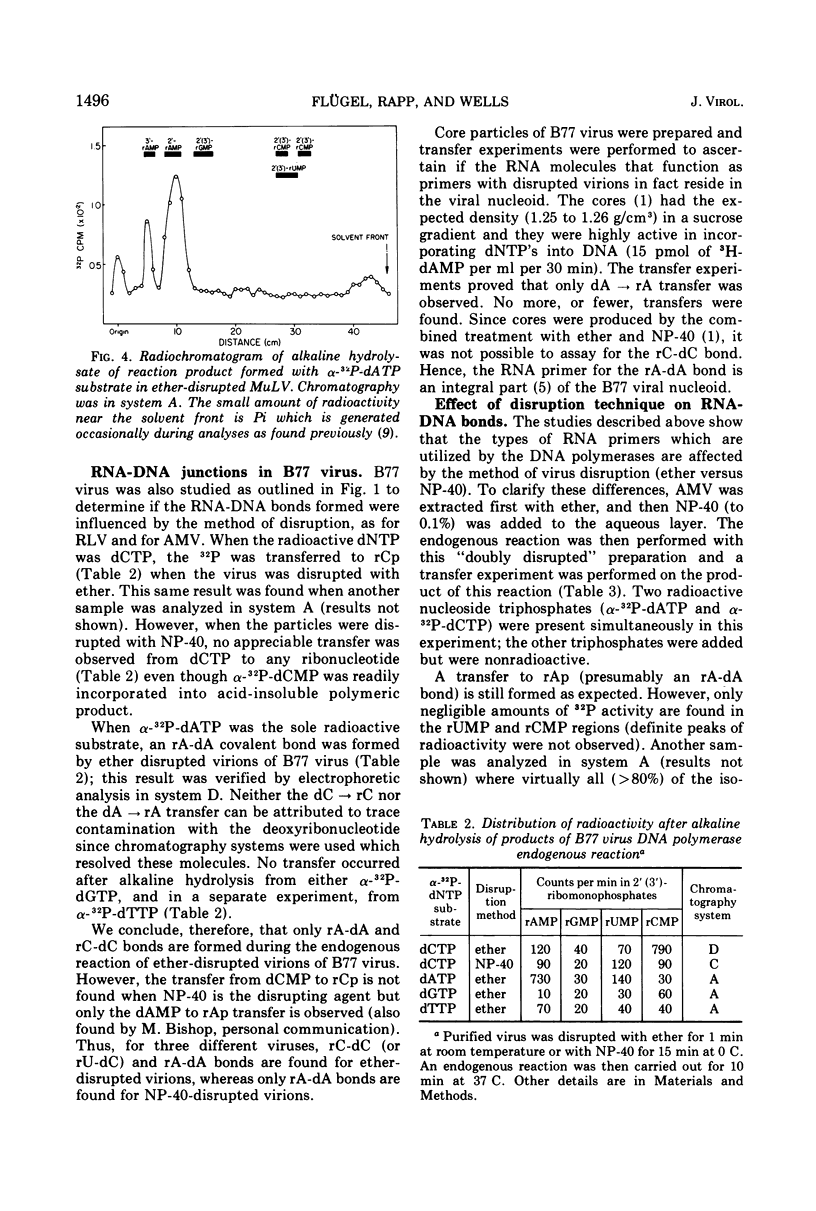
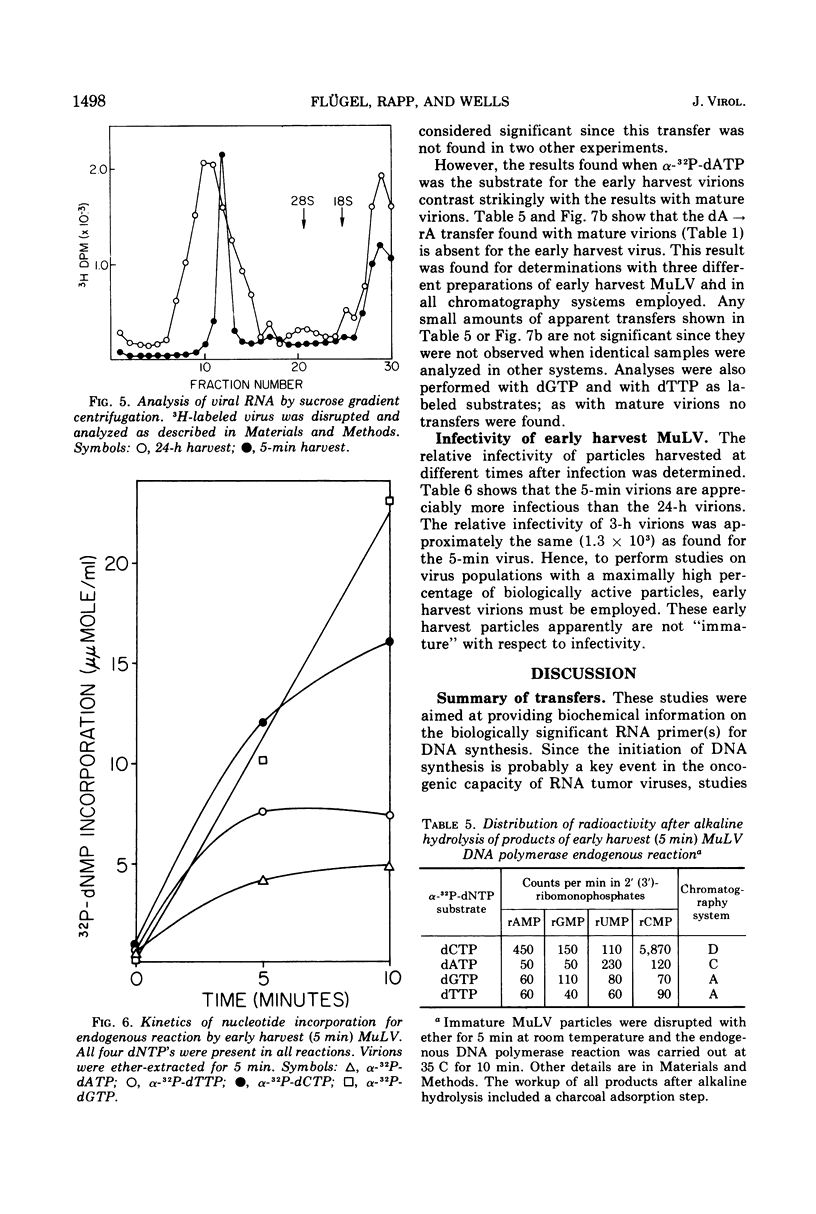
Initiation of DNA synthesis by endogenous RNA primer molecules was studied with three different RNA tumor viruses. The influence of the method of virus disruption on the observed RNA-DNA bonds was ascertained. Ether disrupted virions of both murine leukemia virus (MuLV) and the B77 strain of avian sarcoma virus (B77 virus) have rC-dC and rA-dA covalent linkages between RNA primers and newly synthesized DNA. None of the 14 other possible bonds were formed. Ether-disrupted virions of avian myeloblastosis virus (AMV) have rU-dC and rA-dA linkages. In contrast, work reported herein and from other laboratories shows that Nonidet P-40 (NP-40)-disrupted virions of all three viruses have only the rA-dA junction. Studies with virus particles which were first disrupted with ether and then treated with NP-40 indicated that the detergent treatment disallowed the formation of the ribopyrimidine-dC internucleotide bond. The same transfers are found with AMV in the presence or absence of actinomycin D, where only single-stranded DNA is formed. This finding is consistent with the notion that virtually all of the significant primers have been recognized. In contrast to mature virions, transfer experiments with ether-disrupted early harvest (5 min) MuLV showed only the rC-dC bond; the rA-dA bond was absent. The short-time harvest contains a significantly higher proportion of infectious virions than 24-h harvests. Also, since the RNA from early harvest virus is appreciably more homogenous than the RNA of mature MuLV, it is concluded that the ribopyrimidine-dC linkage is the more significant initiation event from a biochemical standpoint.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolognesi D. P., Gelderblom H., Bauer H., Mölling K., Hüper G. Polypeptides of avian RNA tumor viruses. V. Analysis of the virus core. Virology. 1972 Mar;47(3):567–578. doi: 10.1016/0042-6822(72)90546-6. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East J. L., Allen P. T., Knesek J. E., Chan J. C., Bowen J. M., Dmochowski L. Structural rearrangement and subunit composition of RNA from released Soehner-Dmochowski murine sarcoma virions. J Virol. 1973 May;11(5):709–720. doi: 10.1128/jvi.11.5.709-720.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Wells R. D. Formation of lipid-nucleotide complex by RNA tumor virus preparations. J Virol. 1973 Dec;12(6):1622–1624. doi: 10.1128/jvi.12.6.1622-1624.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel R. M., Wells R. D. Nucleotides at the RNA-DNA covalent bonds formed in the endogenous reaction by the avian myeloblastosis virus DNA polymerase. Virology. 1972 May;48(2):394–401. doi: 10.1016/0042-6822(72)90050-5. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Grant R. C., Kodama M., Wells R. D. Enzymatic and physical studies on (dI-dC) n -(dI-dC) n and (dG-dC) n -(dG-dC) n . Biochemistry. 1972 Feb 29;11(5):805–815. doi: 10.1021/bi00755a020. [DOI] [PubMed] [Google Scholar]
- Harwood S. J., Wells R. D. Micrococcus luteus deoxyribonucleic acid polymerase. Studies on the initiation of deoxyribonucleic acid synthesis in vitro. J Biol Chem. 1970 Nov 10;245(21):5625–5634. [PubMed] [Google Scholar]
- Hatanaka M., Huebner R. J., Gilden R. V. DNA polymerase activity associated with RNA tumor viruses. Proc Natl Acad Sci U S A. 1970 Sep;67(1):143–147. doi: 10.1073/pnas.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House W., Shearer M., Maroudas N. G. Method for bulk culture of animal cells on plastic film. Exp Cell Res. 1972;71(2):293–296. doi: 10.1016/0014-4827(72)90296-0. [DOI] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. Isolation and characterization of a protein that stimulates DNA synthesis from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2331–2335. doi: 10.1073/pnas.69.8.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Wells R. D. Properties of the exonucleolytic activities of the Micrococcus luteus deoxyribonucleic acid polymerase. J Biol Chem. 1972 May 10;247(9):2667–2674. [PubMed] [Google Scholar]
- Mizutani S., Temin H. M., Kodama M., Wells R. T. DNA ligase and exonuclease activities in virions of rous sarcoma virus. Nat New Biol. 1971 Apr 21;230(16):232–235. doi: 10.1038/newbio230232a0. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Vogt P. K. Effects of genetic cellular resistance on cell transformation and virus replication in chicken hematopoietic cell cultures infected with avian myeloblastosis virus (BAI-A). Virology. 1968 Aug;35(4):487–497. doi: 10.1016/0042-6822(68)90278-x. [DOI] [PubMed] [Google Scholar]
- Okabe H., Lovinger G. G., Gilden R. V., Hatanaka M. The nucleotides at the RNA-DNA joint formed by the DNA polymerase of Rauscher leukemia virus. Virology. 1972 Dec;50(3):935–938. doi: 10.1016/0042-6822(72)90451-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruprecht R. M., Goodman N. C., Spiegelman S. Conditions for the selective synthesis of DNA complementary to template RNA. Biochim Biophys Acta. 1973 Jan 19;294(2):192–203. [PubMed] [Google Scholar]
- Sugino A., Okazaki R. RNA-linked DNA fragments in vitro. Proc Natl Acad Sci U S A. 1973 Jan;70(1):88–92. doi: 10.1073/pnas.70.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Faras A. J., Varmus H. E., Goodman H. M., Levinson W. E., Bishop J. M. Transcription of ribonucleic acid by the ribonucleic acid directed deoxyribonucleic acid polymerase of Rous sarcoma virus and deoxyribonucleic acid polymerase I of Escherichia coli. Biochemistry. 1973 Jan 30;12(3):460–467. doi: 10.1021/bi00727a016. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Flügel R. M., Larson J. E., Schendel P. F., Sweet R. W. Comparison of some reactions catalyzed by deoxyribonucleic acid polymerase from avian myeloblastosis virus, Escherichia coli, and Micrococcus luteus. Biochemistry. 1972 Feb 15;11(4):621–629. doi: 10.1021/bi00754a025. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J Mol Biol. 1970 Apr 28;49(2):319–342. doi: 10.1016/0022-2836(70)90248-2. [DOI] [PubMed] [Google Scholar]


