Abstract
The incidence of anxiety, mood, substance abuse disorders and schizophrenia increases during adolescence. Epidemiological evidence confirms that exposure to stress during sensitive periods of development can create vulnerabilities that put genetically predisposed individuals at increased risk for psychiatric disorders. Neuregulin 1 (NRG1) is a frequently identified schizophrenia susceptibility gene that has also been associated with the psychotic features of bipolar disorder. Previously, we established that Type II NRG1 is expressed in the hypothalamic-pituitary-adrenal (HPA) axis neurocircuitry. We also found, using a line of Nrg1 hypomorphic rats (Nrg1Tn), that genetic disruption of Type II NRG1 results in altered HPA axis function and environmental reactivity. The present studies used the Nrg1Tn rats to test whether Type II NRG1 gene disruption and chronic stress exposure during adolescence interact to alter adult anxiety- and fear-related behaviors. Male and female Nrg1Tn and wild type rats were exposed to chronic variable stress (CVS) during mid-adolescence and then tested for anxiety-like behavior, cued fear conditioning and basal corticosterone secretion in adulthood. The disruption of Type II NRG1 alone significantly impacts rat anxiety-related behavior by reversing normal sex-related differences and impairs the ability to acquire cued fear conditioning. Sex-specific interactions between genotype and adolescent stress also were identified such that CVS-treated wild type females exhibited a slight reduction in anxiety-like behavior and basal corticosterone, while CVS-treated Nrg1Tn females exhibited a significant increase in cued fear extinction. These studies confirm the importance of Type II NRG1 in anxiety and fear behaviors and point to adolescence as a time when stressful experiences can shape adult behavior and HPA axis function.
The hypothalamic-pituitary-adrenal (HPA) axis facilitates adaptation to environmental challenges and the adrenal glucocorticoid (GC) hormones (corticosterone in the rat, cortisol in humans) are the principal mediators of this adaptive response. When stress is repeatedly encountered, the chronic elevation of GCs can result in dysregulation of the HPA axis and altered limbic structure and function (McLaughlin et al., 2009). The brain regions most vulnerable to chronic stress are the hippocampus, amygdala and prefrontal cortex (McEwen, 2007). Interestingly, in both humans and rodents, these regions also show significant maturation during adolescence (Giedd, 2004, 2008; Huttenlocher, 1979; Koshibu et al., 2004; Markham et al., 2007; Romeo and Sisk, 2001; Spear, 2000; Zehr et al., 2006)
Adolescence is a critically important period for the development of normal adult behaviors and disturbance of adolescent development contributes to the etiology of several psychiatric disorders (Andersen and Teicher, 2008; Ernst et al., 2009; Paus et al., 2008; Walker et al., 2008; Walker, 2002). Many aspects of normal brain function mature during adolescence including neurotransmitter activity and receptor expression, synapse development and pruning, and myelination (Andersen et al., 2000; Lee et al., 2003; Markham et al., 2007; Teicher et al., 1995). The neural pathways involved in coordinating stress responses also continue development during adolescence (Andersen, 2003; Casey et al., 2008; Spear, 2000) and there may also be modified HPA axis responses to acute and chronic stress during this time (Romeo and Sisk, 2001; Romeo, et al., 2006).
Chronic stress exposure during adolescence can produce long-lasting behavioral and neuroendocrine effects, depending on the timing of exposure, the sex of the animal and the type of stressors used (McCormick et al., 2010; Romeo, 2010). Genetic vulnerabilities may be an additional factor that interacts with adolescent stress exposure to alter adult outcomes relevant to psychiatric disorders. Disruptions of particular genes may create a vulnerability to adolescent stress and ultimately alter the developmental trajectory of the adolescent brain. Since the risk for many psychiatric disorders (i.e. anxiety and mood disorders, schizophrenia and substance abuse) increases during adolescence (Andersen, 2003; Costello et al., 2003; Dahl, 2004; Hankin et al., 1998; Patton and Viner, 2007; Paus et al., 2008; Spear, 2000; Walker, 2002), it is critical to assess interactions between genetic susceptibility and stress exposure during this period of maturation and potential vulnerability.
The specific susceptibility genes that mediate stress interactions within psychiatric populations have not been clearly identified. One candidate may be the Neuregulin 1 (NRG1) gene, which has been repeatedly associated with schizophrenia and bipolar disorder (Harrison and Law, 2006; Prata et al., 2009; Stefansson et al., 2002; Stefansson et al., 2003; Greenwood et al., 2011; Greenwood et al., 2012). In humans and rats, the NRG1 gene is highly complex and can be alternatively spliced into six types (I-VI) of proteins, based on N-terminal structural and functional differences (Falls, 2003a; Esper et al., 2006; Liu et al., 2011). NRG1 signaling via its preferential tyrosine kinase receptor, ErbB4, plays many roles in neural development, including radial neuron migration, axon guidance, myelination, oligodendrocyte development and synapse formation (Falls, 2003a; Mei and Xiong, 2008). In addition, NRG1 is important for adult neural function, including the regulation of the serotonergic system, NMDA, GABAA and α7 nicotinic receptors, modulation of long-term potentiation, transcriptional regulation and hormonal control of puberty (Falls, 2003a; Prevot et al., 2003; Harrison and Law, 2006; Dean et al., 2008).
While gene-environment interactions account for much of the pathology associated with psychiatric disorders (Aguilera et al., 2009; Bayer et al., 1999; Caspi et al., 2003; Howes et al., 2004; Jaaro-Peled et al., 2009; Keri et al., 2009; Mittal et al., 2008; van Os et al., 2008) these interactions have not been extensively investigated during adolescence. However, there are reported gene-environment interactions between NRG1 and stress in adult humans. In patients with schizophrenia, a single nucleotide polymorphism in the 5′ region of NRG1 interacts with psychosocial stress to affect reactivity to expressed emotion (Keri et al., 2009). NRG1 genotype also interacts with job strain to increase risk of heart disease (Hintsanen et al., 2007). Studies in genetically modified animals also demonstrate gene by environment interactions. For example, in heterozygous Nrg1 transmembrane (TM) domain knock out (KO) mice, environmental enrichment during adulthood increases exploratory behavior, while adolescent stress exposure appears to have the opposite effect on adult exploratory behavior (Karl et al., 2007; Desbonnet et al., 2012). These studies suggest that disruption of Nrg1 may interact with chronic adolescent stress on other behaviors relevant to psychiatric disorders.
Previously, we confirmed that a specific isoform of NRG1, Type II, is expressed in the neurocircuitry involved in regulating the HPA axis response to stressors, including the hypothalamic paraventricular nucleus, which integrates and controls the neuroendocrine response to stress (Taylor et al., 2011a). Type II NRG1 is an important isoform to investigate as it is encoded by exons in the 5′ region of the Nrg1 gene that contains many of the risk haplotype associations with schizophrenia and bipolar disorder (Harrison and Law, 2006; Prata et al., 2009; Stefansson et al., 2003; Stefansson et al., 2002; Greenwood et al., 2011; Greenwood et al., 2012). Using a rat model of disrupted Type II NRG1, i.e. the hypomorphic Nrg1Tn rats, we found that male Nrg1Tn rats have significantly higher basal corticosterone (CORT) levels, while female Nrg1Tn rats show enhanced suppression of CORT secretion after recovery from acute restraint stress (Taylor et al., 2011a, 2011b). In addition, sex-specific changes in glucocorticoid (GR) and mineralocorticoid (MR) receptor expression were found, leading to a disrupted MR/GR balance in the hippocampus of males and amygdala of females (Taylor et al., 2011a, 2011b). These findings implicate Type II NRG1 in stress regulation. Behaviorally, we found that male Nrg1Tn rats may be more sensitive to changes in their environment, while female rats are less impacted by mildly stressful aspects in their environment (Taylor et al., 2011a, 2011b).
Based on our previous findings that Type II NRG1 plays a sex-specific role in stress regulation and behavioral responses to the environment in adult rats, we sought to test the hypothesis that chronic stress during adolescence interacts with disruption of Type II NRG1 to produce sex-specific behavioral changes in adult anxiety-like behavior, fear conditioning and basal HPA axis activity. In non-stressed animals, we predicted that male Nrg1Tn rats would show increased anxiety-like behavior, enhanced acquisition and impaired extinction of conditioned fear and increases basal CORT, while female Nrg1Tn rats would exhibit decreased anxiety, reduced acquisition and enhanced extinction of fear conditioning and wild type-like basal CORT. The interaction between disrupted Type II NRG1 and adolescent chronic variable stress was predicted to potentiate these genotype specific alterations in the behavioral measures.
2. Experimental Procedures
2.1 Animals
Male and random cycling female Fischer 344 wild-type (WT) and homozygous Nrg1Tn rats were used in the present studies. This model was developed and obtained from the PhysGen Program in Genomic Applications (http://pga.mcw.edu/) at the Medical College of Wisconsin and has been previously described (Lu et al., 2007; Taylor et al., 2011a, 2011b). The Nrg1Tn rats have reduced brain expression of both Type II NRG1 mRNA and protein (Taylor et al., 2011a). All animals were housed in same-sex cages of 2–3 rats in a temperature- and light-controlled (lights on 06.00 h to 20.00 h) facility. Water and chow (Harlan Teklad, Frederick, MD) were available ad libitum. All procedures conform to the guidelines for animal research established by the National Institutes of Health, and were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
2.2 Adolescent Chronic Variable Stress
Adolescent rats were divided into 8 groups: male WT control (CON), female WT CON, male WT chronic variable stress (CVS), female WT CVS, male Nrg1Tn CON, female Nrg1Tn CON, male Nrg1Tn CVS and female Nrg1Tn CVS (n=6–7/group). Animals were exposed to CVS during mid-adolescence (McCormick and Mathews, 2007; Tirelli et al., 2003). Beginning at 37 days of age and continuing through 44 days of age, male and female WT and Nrg1Tn rats in the CVS groups were exposed to a variable stress paradigm (Koenig et al., 2005; Lee et al., 2007; Markham et al., 2010) which precludes HPA axis habituation (Bhatnagar and Dallman, 1998; Girotti et al., 2006; Kinnunen et al., 2003; Solomon et al., 2009). Briefly, the stressors used in this paradigm were: (1) restraint in a well-ventilated cylindrical plexiglas restrainer (Harvard Bioscience, Boston, MA, USA) for 1 h, (2) exposure to a cold environment (4 C) for 6 h, (3) food deprivation for 48 h, (4) housing animals in a cage with water in the bottom for 90 min, (5) 15 min of swim stress, and (6) overcrowded housing conditions overnight. Two to three stressors were administered per day in a randomized order (Table 1; Kinnunen et al., 2003; Koenig et al., 2005; Lee et al., 2007; Markham et al., 2010). Animals in both the CON and CVS groups were weighed at 37 and 44 days of age. Following CVS treatment, animals were left undisturbed in their cages until behavioral testing in adulthood (see experimental timeline - Fig. 1).
Table 1.
Chronic Variable Stress
| Time of Day | |||
|---|---|---|---|
| Age (days) | AM (~8–9) | Mid-day (~12–1) | PM (~4–5) |
| 37 | Swim – 15 min | Restraint – 60 min | Swim – 15 min |
| 38 | Restraint – 60 min | Swim – 15 min | Restraint – 60 min |
| 39 | Cold Exposure – 6 h (9 AM–3 PM) | Food Deprivation – 48 h | |
| 40 | Water in cage – 90 min | Restraint – 60 min | Swim – 15 min |
| 41 | Water in cage – 90 min | Social Stress – overnight (beg. ~3 pm) | |
| 42 | Restraint – 60 min | Swim – 15 min | Restraint – 60 min |
| 43 | Swim – 15 min | Restraint – 60 min | Swim – 15 min |
| 44 | Cold Exposure – 6 h (9 AM–3 PM) | ||
Figure 1. Experimental timeline.
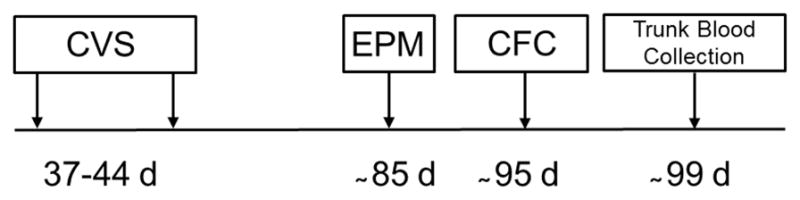
The timeline depicts the age in days (d) of the experimental subjects at each manipulation and behavioral/physiological measurement. CVS: chronic variable stress, EPM: elevated plus maze, CFC: cued fear conditioning.
2.3 Behavioral and Neuroendocrine Measurements
2.3.1 Elevated Plus maze
All groups were tested for anxiety-like behavior in an elevated plus maze (EPM) between 83 and 88 days of age (Fig. 1). Animals were not handled prior to EPM testing. On the morning of testing, all animals were moved to the behavioral testing room and allowed to acclimate for 30 min. Animals of each treatment group and sex were balanced over the testing period to account for time of testing. EPM testing was performed according to standard protocols (Lapiz-Bluhm, et al., 2008; Walf and Frye, 2007). The maze (Stoelting, Inc., Wood Dale, IL) consisted of four arms, each of which was 50 cm × 10 cm. Two opposing arms had 40 cm high walls and the other two were open (without walls). The maze was elevated 50 cm above the floor. To begin the 5 min testing session, each rat was placed onto the center of the maze facing the same open arm. ANY-maze tracking software (Stoelting, Inc) was used to record and analyze the total amount of time spent in the open and closed arms. Time spent in arms was recorded when 75% of the animal’s body was in an arm. An open arm to total arms time ratio (OTR; time in open arms/(time in open + closed arms)) was calculated to account for any differences in locomotor activity and was used for analysis of anxiety-like behavior (Lapiz-Bluhm et al., 2008).
2.3.2 Cued Fear Conditioning
Ten days after EPM testing (Fig. 1), cued fear conditioning was conducted according to our published protocol (Markham et al., 2010). An automated fear conditioning system (Coulbourn Instruments, Whitehall, PA, USA) running under the guidance of FreezeFrame software (Coulbourn Instruments) was used to evaluate fear conditioning behavior. Behavioral testing took place in 25.4 cm × 25.4 cm × 19.05 cm chambers contained in sound-attenuated cubicles equipped with a speaker for delivering tones, a ventilation fan for background noise (continuously present throughout the experiment at 60 dB), a house light (continuously present throughout the experiment), and a removable stainless steel grid floor for equally distributed delivery of a mild footshock, controlled via a shocker-scrambler unit automated by the FreezeFrame software (Coulbourn Instruments). The percent time spent freezing was monitored by a videotracking system driven by the FreezeFrame software. Freezing was defined as complete movement cessation, with the exception of respiration. The software automatically determined freezing; however, as indicated by the manufacturer, the software may erroneously score immobility if movement is in the vertical direction. Because animals occasionally jump in response to the footshock, an experimenter (who was blind to the animal’s condition) viewed each video and, if the software had erroneously scored freezing, the operator manually adjusted the threshold to exclude this event. Footshock grids were calibrated before each conditioning session using a digital meter to ensure that the current administered was consistent across animals. Animals received no habituation to the testing chamber prior to behavioral testing; they were moved to the testing room 30 min prior to testing, and were returned to the vivarium at the conclusion of testing each day.
On the morning of the first day, animals underwent fear conditioning, during which time an auditory stimulus was paired with a mild footshock. Following a 60 s acclimation period upon introduction to the chamber, the animal was presented with a tone (90 dB, lasting 10 s) that co-terminated with a footshock (1.0 mA, lasting 1 s). Freezing behavior was measured during the tone presentation. In total, four tone-shock pairings were presented, with each pairing separated by an inter-trial interval that lasted between 60 and 90 s. After the final pairing, the animal remained in the chamber for 60 s and was then returned to its home cage. The entire trial including acclimation lasted 6.5 min. The chamber walls and floor grid were cleaned with 70% ethanol and the drip pan beneath the floor grid was washed with detergent and cleaned with 70% ethanol between each animal.
2.3.3 Cued Fear Extinction
Beginning 24 h after fear conditioning, each animal was returned to the chamber to evaluate fear-related memory and extinction. Multiple contextual features of the chamber were altered (relative to conditioning trials), namely: (1) an opaque plexiglas insert was laid over the grid floor, (2) the chamber walls and floor were cleaned with orange-scented cleaner (instead of ethanol) between animals, (3) the chamber cue light was left on for the entire trial, providing more ambient light in the chamber, (4) the door to the sound-proof chamber was left cracked open, (5) animals remained in an adjoining room (instead of the actual testing room) during periods of group testing and were transported between home cage and testing chamber using an empty holding cage, and (6) the testing chamber was switched on a per-animal basis between conditioning trials and cue extinction trials. Therefore, multiple tactile, olfactory, visual, and other contextual cues were altered between conditioning trials and cue extinction trials. During each 8-min cue extinction trial, no footshocks were delivered and freezing behavior in response to presentation of the tone alone was measured; these trials are an opportunity for the animal to learn that the tone no longer predicts a shock. In total, the tone (90 dB, lasting 10 s) was presented five times per cue extinction trial, separated by an inter-trial interval that lasted between 60 and 90 s. After the final tone presentation, the animal remained in the chamber for 60 s and was then returned to its home cage. There were two sets of extinction trials, occurring 24 h and 96 h (4 days) after the initial conditioning training session.
2.3.4 Footshock Sensitivity
Sensitivity to footshock was assessed in a separate group of adult rats (WT male, n=4; WT female, n=5; Nrg1Tn male, n=5; Nrg1Tn female, n=5). Rats were placed in the fear conditioning chamber, with the house light and fan and presented with uncued footshocks (0.5s) of increasing amplitudes beginning with 0.06 mA. Amperage was manually increased by 0.06 mA until 0.6 mA, after which it was increased by 0.1 mA, and the intervals between shocks ranged from 15 to 25 s. Amperage was increased until a vocalization response was consistently determined. Rat’s received between 12 and 15 shocks. The experimenter recording vocalization responses was blind to the rats’ genotype.
2.3.5 Corticosterone Determination
Four days after cued fear conditioning (Fig. 1), the animals were euthanized between 1000 and 1200 h by rapid decapitation and trunk blood was collected into tubes containing 10% EDTA. Plasma was isolated by centrifugation and was stored at −80°C until use. Plasma CORT concentrations were determined by radioimmunoassay according to protocols provided by the manufacturer (MP Biomedicals, Orangeburg, NY). Samples were run in duplicate. Average intra-assay variation was 12%. Samples that fell below the limits of the standard curve were excluded (i.e. less than 5 ng/ml), resulting in the removal of two female WT CON samples.
2.4 Statistical Methods
Statistical analyses were conducted using SPSS statistical software (version 12.0). Analysis of variance (ANOVA) was used to analyze percent weight gained, behavioral measurements and CORT concentrations. Post hoc tests and planned comparisons, conducted using l-matrix contrast statements in SPSS, were used to follow up any significant main effects or interactions. Statistical outliers were identified with the Grubbs test, resulting in the removal of one WT CON male and one WT CVS female from the EPM analysis.
3. Results
3.1 Percent Weight Gained
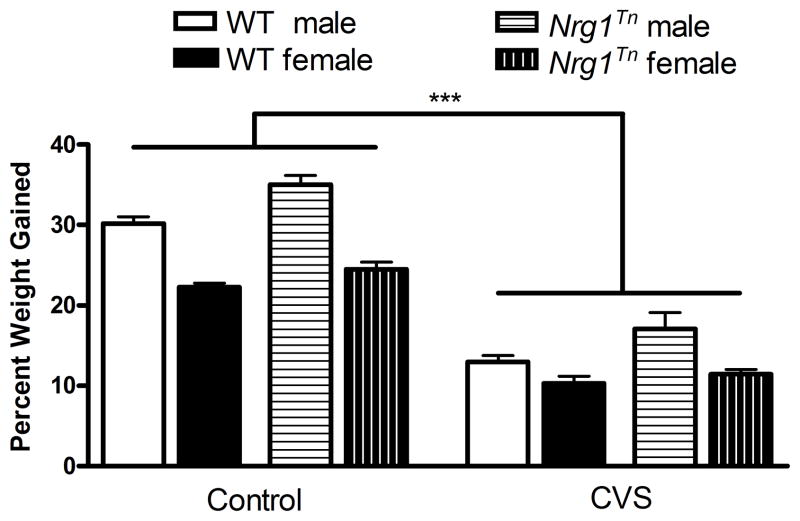
No weight differences were detected in 37-day old rats assigned to the CON and CVS groups (mean starting weights: males: 110.8 ± 1.5 g; females: 97.4 ± 1.4 g). A 2×2×2 ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS)) was conducted on percent weight gained during stress exposure (37–44 days). This analysis confirmed an effect of adolescent CVS exposure (F1,46=390.43, p<0.001; Fig. 2) such that CON animals gained more weight than CVS animals. In addition an effect of genotype was found (F1,46=16.26, p<0.001; Fig. 2) such that Nrg1Tn rats gained more weight than WT rats. Finally, an effect of sex was also found (F1,46=77.51, p<0.001; Fig. 2) indicating that males gained more weight than females
Figure 2. Percent weight gained.
CVS exposure from 37–44 days of age resulted in a significant decrease in percent weight gained over the adolescent stress period (***p<0.001) compared to CON groups. Bars represent mean ± SEM.
3.2 Elevated Plus Maze
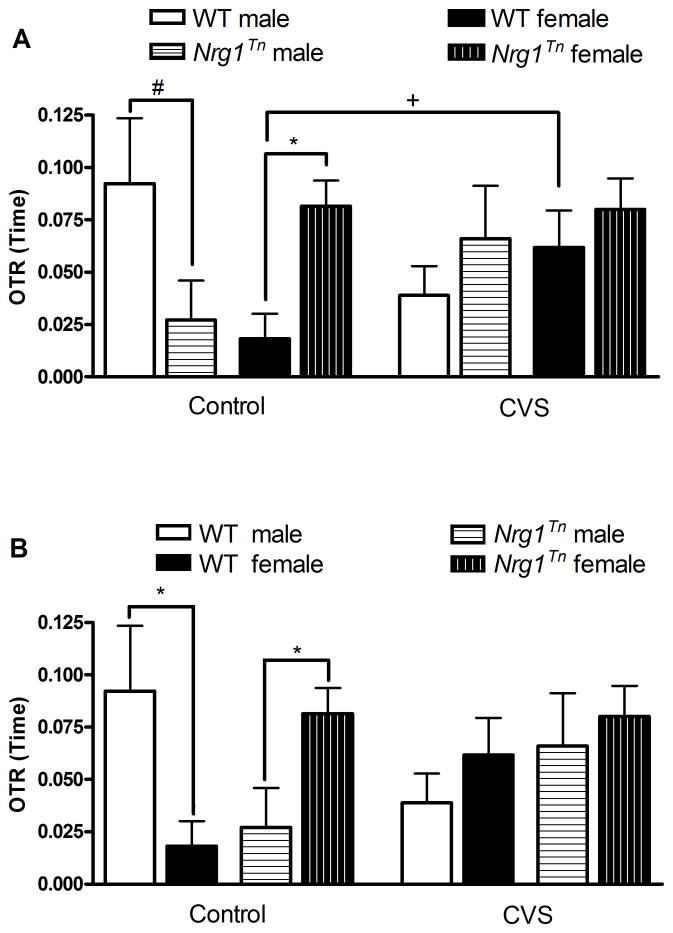
A 2×2×2 ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS)) was conducted on the OTR for time. This analysis revealed a significant three-way interaction between genotype, adolescent stress exposure and sex (F1,44=6.66, p<0.02). Post hoc analysis was used to follow up this three-way interaction (panels A & B of Fig. 3 are the same data presented differently to show group differences more clearly). Genotype differences in same-sex and same-adolescent stress groups were examined first. In CON groups not exposed to adolescent CVS, female Nrg1Tn rats spent significantly more time in the open arms than female WT rats (p<0.01; Fig. 3A), indicating that female Nrg1Tn rats showed less anxiety-like behavior than WT female rats. In contrast, CON male Nrg1Tn rats not exposed to CVS spent slightly less time in the open arms than CON WT rats but the difference was not statistically different (p<0.09; Fig. 3A). Genotype differences found in CON animals were eliminated by adolescent CVS exposure. To examine adolescent stress effects, same-sex and same-genotype groups were analyzed. In females, CVS WT rats spent slightly more time in the open arms than CON WT rats (p<0.08; Fig. 3A), indicating that adolescent CVS exposure resulted in slightly reduced anxiety-like behavior in adult WT females. In males, the opposite pattern appeared, though the effect was not significant, with CVS WT males spending less time in the open arms than CON WT males, indicating that adolescent CVS may have increased anxiety-like behavior in adult WT males. Female Nrg1Tn rats were resistant to adolescent CVS.
Figure 3. Elevated plus maze.
The open to total ratio (OTR) for time (time in open arms/(time in open + closed arms)) is presented, a higher OTR is indicative of decreased anxiety-like behavior. A) In CON groups, female Nrg1Tn rats spent significantly more time in the open arms than female WT rats (**p<0.01) while CON male Nrg1Tn rats tended to spend less time in the open arms than CON WT rats (#p<0.09). A slight effect of adolescent CVS was found such that WT CVS-treated females tended to spend more time in the open arms than WT CON females (+p<0.08). Female Nrg1Tn rats were resistant to adolescent CVS. B) In both WT and Nrg1Tn CON rats, a sex difference was detected. In CON WT animals, males spent more time in the open arms than females (*p<0.04), whereas in CON Nrg1Tn animals, males spent less time in the open arms than females (*p<0.05). CVS exposure eliminated sex differences in both genotypes. Bars represent mean OTR ± SEM.
Finally, sex differences in same-adolescent stress, same-genotype groups were examined. In both WT and Nrg1Tn CON rats, a sex difference was detected. In CON WT animals, males spent more time in the open arms than females (p<0.04; Fig. 3B), whereas in CON Nrg1Tn animals, males spent less time in the open arms than females (p<0.05; Fig. 3B). Adolescent CVS exposure eliminated adult sex differences in both genotypes. No interaction between genotype, sex and adolescent stress was identified in the OTR for entries, time mobile or distance traveled (data not shown).
3.3 Cued Fear Conditioning
To compare overall average freezing to tones across days, a 2×2×2×3 repeated measures ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS) × Day (1–3)) was used with day included as the repeated measure. A main effect of day was found (F2,92 = 41.5, p<0.001) such that average freezing increased from conditioning to the 24 h extinction trials and then decreased again at the 96 h extinction trials (Fig. 4A–C). In addition, between-subjects effects of genotype (F1,46 = 5.4, p<0.03) and sex (F1,46 = 4.9, p<0.04) were also identified, with Nrg1Tn animals exhibiting less overall freezing to tone than WT animals (Fig. 4A–C), and female animals exhibiting less overall freezing to tone than male animals (not shown). No overall effect of adolescent stress exposure was identified.
Figure 4. Cued fear conditioning, memory and extinction across all trials.
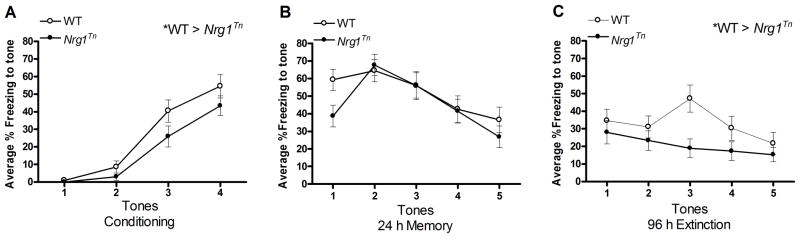
Percent freezing in male and female WT and Nrg1Tn rats are shown in this figure. Freezing to tone increased during the conditioning trials and from conditioning to the 24 h memory trials. Freezing then decreased during the 96 h extinction trials. In addition, Nrg1Tn animals exhibited less overall freezing to tones than WT animals (*p<0.05) during A) conditioning and C) 96 h extinction (*p<0.02). No genotype differences were present at the B) 24h fear memory test. Symbols represent mean percent freezing ± SEM at each individual tone presentation.
3.3.1 Conditioning
A 2×2×2×4 repeated measures ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS) × Tone (1–4)) was used to examine freezing to tone on conditioning trials, with tone included as the repeated measure. This analysis revealed a main effect of tone (F3,138 = 56.65, p<0.001) such that freezing increased with each subsequent tone presentation after the first tone shock pair (Fig. 4A). Note that freezing to the first tone was not expected, as the shock is presented after the tone and therefore the animal would not associate the first tone with an aversive stimulus. Tone did not interact with any other factors. In addition, a between-subjects effect of genotype was detected (F1,46 = 4.12, p<0.05) such that WT animals froze more to tone presentation than Nrg1Tn animals (Fig. 4A).
3.3.2 Twenty-four Hour Memory
A 2×2×2×5 repeated measures ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS) × Tone (1–5)) was used to examine freezing to tone on 24 h memory trials, with tone included as the repeated measure. This analysis also revealed an effect of tone (F4,184 = 10.85, p<0.001) such that freezing initially increased and then decreased as the tone no longer cued for a subsequent shock, demonstrating within session extinction (Fig. 4B). No effect of any other factor was identified at this time point.
3.3.3 Ninety-six Hour Extinction
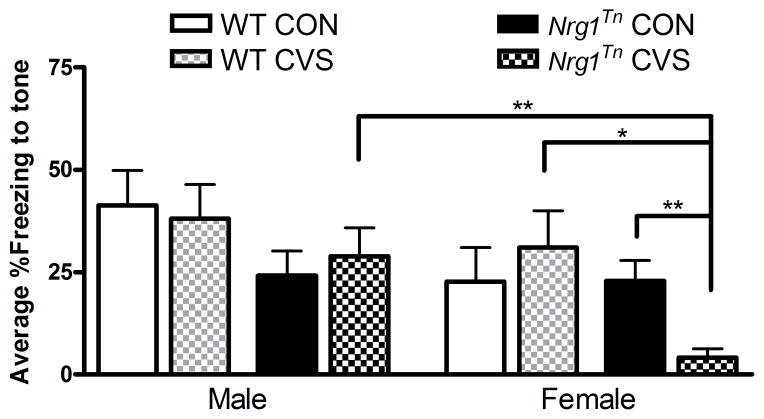
A 2×2×2×5 repeated measures ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS) × Tone (1–5)) was used to examine freezing to tone on 96 h extinction trials, with tone included as the repeated measure. This analysis revealed a less robust effect of tone (F4,184 = 2.44, p<0.05) such that freezing tended to decrease over the session (Fig. 4C). Interestingly, at this time point, between-subjects effects of genotype (F1,46 = 6.8, p<0.02) and sex (F1,46 = 6.4, p<0.02) were identified. These effects mirrored the same effects in the overall analysis, with Nrg1Tn animals exhibiting less overall freezing to tones than WT animals (Fig. 4C)., and female animals exhibiting less overall freezing to tone than male animals (not shown). Furthermore, a trend for genotype and sex to interact with treatment was also identified (F1,46 = 2.96, p≤0.093; Fig. 5). Planned comparisons revealed that exposure to CVS during adolescence resulted in a genotype difference among adult females such that Nrg1Tn CVS females exhibited less freezing than both Nrg1Tn CON females (p<0.01; Fig. 5) and WT CVS females (p<0.03; Fig. 5). In addition, while no sex difference was identified in WT CVS males and females, Nrg1Tn CVS males and females were significantly different, with Nrg1Tn CVS females exhibiting less freezing than Nrg1Tn CVS males (p<0.01; Fig. 5).
Figure 5. Fear extinction during the 96h extinction trials.
Exposure to CVS during adolescence resulted in significant less freezing in the Nrg1Tn females compared to both Nrg1Tn CON females (**p<0.01) and WT CVS females (*p<0.03) during the 96h extinction trials. In addition, while no sex difference was identified in WT CVS males and females, Nrg1Tn CVS males and females were significantly different, with Nrg1Tn CVS females exhibiting less freezing than Nrg1Tn CVS males (**p<0.01). Bars represent mean percent freezing ± SEM.
3.3.4 Footshock Sensitivity
Footshock sensitivity was determined with a 2×2 ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female). This analysis revealed no significant effect of genotype (F1,15 = 0.79, p<0.4), indicating that WT and Nrg1Tn rats have similar pain thresholds for footshock (WT: 0.55 ± 0.05 mA vs. Nrg1Tn: 0.51 ± 0.03 mA). A trend for an effect of sex was also found (F1,15 = 4.06, p<0.062), suggesting that males tended to have a higher threshold than females (data not shown).
3.4 Corticosterone
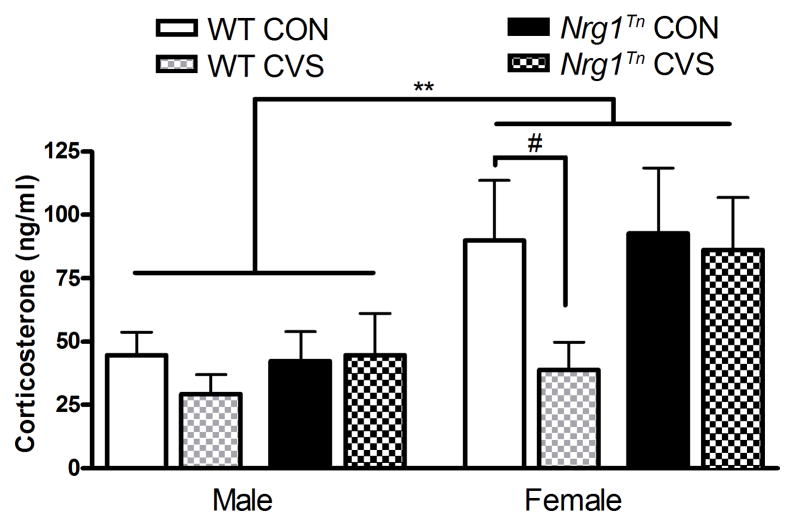
Differences in adult basal CORT secretion were determined with a 2×2×2 ANOVA (Genotype (WT vs. Nrg1Tn) × Sex (male vs. female) × Adolescent Stress (CON vs. CVS)) with time of sacrifice (early, mid, late morning) included as a blocking variable. A significant main effect of sex was found (F1,43= 9.96, p<0.01; Fig. 6) with females exhibiting higher CORT levels than males. Due to the tendency for CVS WT females to show decreased anxiety-like behavior, planned comparisons were used to examine adolescent stress effects in WT female rats. This analysis revealed a trend for CVS WT females to have lower adult basal CORT compared to CON WT females (p<0.06; Fig. 6).
Figure 6. Corticosterone.
Female rats exhibited greater basal corticosterone secretion than male rats (**p<0.01), regardless of genotype or adolescent stress. Adolescent exposure to CVS led to a slight reduction in adult basal corticosterone in WT females (#p<0.06). Bars represent mean corticosterone concentrations (ng/ml) ± SEM.
4. Discussion
In previous work (Taylor et al., 2011a, 2011b) we began to characterize the behavioral and neuroendocrine phenotype of rats with decreased expression of Type II NRG1. In the present report we further describe the role of Type II NRG1 in rat behavior and identify novel environment-induced behavioral changes in these unique animals. The disruption of Type II NRG1 alone significantly impacts rat anxiety-related behavior and reverses normal sex-related differences. In addition, regardless of sex or adolescent CVS exposure, Nrg1Tn rats demonstrated a reduced ability to acquire cued fear conditioning and to respond to the presentation of the tone cue during extinction trials when compared to WT animals without inducing major changes in HPA axis activity. Moreover, adolescent CVS exposure interacts with Nrg1 genotype in a sex-specific way. We predicted that genotype-induced alterations would be potentiated by exposure to adolescent CVS. Surprisingly, we found that adolescent CVS exposure eliminated sex-specific anxiety-related behaviors regardless of genotype, while this developmental insult enhanced cued-fear extinction only in Nrg1Tn female rats, representing a true gene by environment interaction. This is consistent with the reduction in sensitivity to environmental changes and novelty we previously identified (Taylor et al., 2011b). Nrg1Tn males were not significantly affected by adolescent CVS exposure on any measure. Interestingly, only WT females showed a subtle effect of adolescent CVS on anxiety-like behavior, such that open arm time was increased. The present study is also among the first to examine the behavioral effects of chronic adolescent stress in Fischer 344 rats.
4.1 Adult Anxiety
Exposure to CVS during mid-adolescence resulted in a significant attenuation of percent weight gained in all groups. This is consistent with the well-documented effects of exposure to CVS to reduce body weight in both adolescent and adult rodents (Isgor et al., 2004; Jankord et al., 2010; Solomon et al., 2009). In adulthood, all experimental groups underwent behavioral testing on the EPM. In control groups, disruption of Nrg1 resulted in a trend toward increased anxiety-like behavior in males and significantly decreased anxiety-like behavior in females compared to WT animals. These genotype differences resulted in opposite sex differences, with CON WT females exhibiting greater anxiety-like behavior than CON WT males, which is consistent with the findings of others in different assays of anxiety-like behavior in Fischer 344 rats (Skripuletz et al., 2010). On the other hand, in CON Nrg1Tn animals, males demonstrated significantly greater anxiety-like behavior than females. The sex-specific effect of genotype in Nrg1Tn rats is interesting in that it reverses the sex-differences in anxiety-like behavior found in the WT rats, potentially inducing female rats to be more resilient to what would normally be an anxiety-provoking environment. These findings in the EPM confirm and extend our earlier findings in Nrg1Tn rats using simple habituation behavior as an indication of anxiety (Taylor et al., 2011a, 2011b).
Studies in mouse models of disrupted Nrg1 have also examined anxiety-like behavior with conflicting results. One study with heterozygous Nrg1 TM domain KO mice found males to have increased anxiety-like behavior on open arm time in the EPM (Boucher et al., 2007) while others reported no effect of deleting the TM domain on the EPM in either male or female mice (O’Tuathaigh et al., 2008). In heterozygous Nrg1 EGF domain KO mice, no difference was detected between WT and KO mice on the EPM (Duffy et al., 2008), while female mice over-expressing Type I NRG1 tended to show a mild increase in anxiety-like behavior on the EPM (Deakin et al., 2009). Thus anxiety-related phenotypes appear to be both varied and specific to the type and location of genetic disruption in the Nrg1 gene, as well as the sex of the animal.
In WT female rats, adolescent CVS resulted in a slight reduction in anxiety-like behavior, while no effect was found in the WT males. Others have found that chronic stress during mid-adolescence leads to either increased (McCormick, et al., 2008) or decreased anxiety-like behavior in adulthood (Wilken et al., 2012). These discrepant findings may be due to differences in chronic stress paradigms as social stress lead to increased anxiety-like behavior, while intermittent physical stressors lead to decreased anxiety-like behavior on the EPM (McCormick et al., 2008; Wilken et al., 2012). Anxiety-like behavior in female Nrg1Tn rats was completely unchanged by adolescent CVS. Consistent with our previous findings, disruption of Nrg1 in female rats may make them less susceptible to the apparent reprogramming of anxiety-like behavior by chronic stress during adolescence due to decreased environmental reactivity (Taylor et al., 2011b). On the other hand, male Nrg1Tn rats exhibited a slight but non-significant decrease in anxiety-like behavior following adolescent CVS. This was an unanticipated pattern given our previous findings that male Nrg1Tn rats demonstrate enhanced reactivity to environmental changes. However, Desbonnet and colleagues (2012) recently investigated the effect of adolescent social defeat stress on adult behaviors in male Nrg1 TM domain KO mice and observed a small but significant anxiolytic effect of adolescent social defeat on novelty-induced exploration in these KO mice. Together these data indicate a subtle gene by environment interaction between Nrg1 and adolescent stress.
4.2 Adult Cued Fear Conditioning
In cued fear conditioning, rodents learn to associate a neutral stimulus (tone) with an aversive event (shock). The resulting behavior (freezing) to the tone is measured and used as an indication that the rodent associates the tone with the shock. Nrg1Tn rats, regardless of sex or adolescent stress exposure, displayed a reduced ability to learn the association between the tone and shock. The development of conditioned fear is known to be dependent on normal amygdala (AMG) function (LeDoux, 2003). Interestingly, we previously found that female, but not male, Nrg1Tn rats have a subtle disruption to the MR/GR ratio in the AMG (Taylor et al., 2011a, 2011b). Importantly, the possibility that Nrg1Tn rats have an altered pain threshold to footshock was ruled out, and we previously confirmed normal auditory function in the Nrg1Tn rats (Taylor et al., 2011b). In heterozygous Nrg1 EGF and TM domain KO mice, impaired short-term contextual fear memory was found, though cued-conditioning was either not examined or not impaired (Ehrlichman et al., 2009; Duffy et al., 2010). Of potential relevance to the impaired association learning demonstrated here, we have found that male Nrg1Tn rats do not exhibit impairments on the Morris water maze (a hippocampal dependent spatial memory task) but do show working and reference memory impairments in the Can Test (a more prefrontal cortex and dorsal striatum dependent discrimination memory task) suggesting that the neurocircuitry underlying learning and memory is disrupted by the Nrg1 mutation (A.R. Taylor, unpublished observations).
All experimental groups regardless of genotype or stress exposure, demonstrated a memory for the tone-shock association, indicated by increased freezing at the 24 h extinction session relative to the conditioning session. During the 96 h extinction session, female rats demonstrated less freezing than males, and Nrg1Tn rats demonstrated less freezing behavior than WT rats. Interestingly, at this time point, CVS-treated female Nrg1Tn rats displayed significantly less freezing to the tone than female Nrg1Tn CON rats, female WT CVS rats and male Nrg1Tn CVS rats, suggesting significantly greater extinction of the cue-induced fear response. Extinction of cue-conditioned fear memory is PFC-mediated (Morgan and LeDoux, 1995; Quirk et al., 2000; Milad and Quirk, 2002). Whether the increased extinction in female Nrg1Tn CVS rats is due to enhanced PFC-mediated extinction or to a disruption of AMG-mediated acquisition of fear conditioning remains to be determined. To date, only one other study has examined the effects of adolescent stress on adult fear conditioning. In this study, no effect of exposure to predator odor during early adolescence was found on fear conditioning or retrained fear conditioning, though males did demonstrate impaired extinction (Toledo-Rodriguez and Sandi, 2007). Interestingly, open arm time in the EPM and enhanced fear extinction may be suggestive of a generalized reduction in anxiety which has also been reported in animals with deficiencies in the NRG1 receptor, i.e. ErbB4 (Shamir et al., 2012). Together these studies point to the importance of NRG1/ErbB4 in anxiety-related behavioral phenotypes (Shamir et al., 2012).
4.3 Adult Basal Corticosterone
Previously, in naïve animals, we reported that male, but not female, Nrg1Tn rats exhibit elevated basal CORT levels. However, we did not find a similar change in basal CORT levels in this study. This difference is likely due to the repeated testing that all animals experienced in the studies reported herein. In the present studies, regardless of genotype or adolescent stress exposure, female rats exhibited increased basal CORT secretion compared to male rats. This sex difference has been reported in a variety of rat strains, including Fischer 344 rats (Chisari et al., 1995). In addition, in WT females, adolescent CVS exposure resulted in a trend toward lower basal CORT secretion compared to CON females. No effect of adolescent CVS was identified in Nrg1Tn rats. The evidence for long lasting effects of adolescent stress on adult HPA axis function is mixed. One study showed a decrease in afternoon CORT concentrations in adult mice (Sterlemann et al., 2008). However, most report no effect on basal or stress-induced CORT and ACTH secretion, though some studies find increased basal and stress-induced CORT secretion in adulthood (Isgor et al., 2004; McCormick et al., 2008; McCormick et al., 2010; Toledo-Rodriguez and Sandi, 2007). Importantly, most of these studies were conducted in only male rats (McCormick et al., 2010).
5. General Comments and Conclusions
There are several potential limitations with our study. The first potential limitation is related to the timing of the stress exposure administration. It was recently reported that stressors administered in early adolescence (e.g. days 22–33) lead to a broad range of outcomes in adulthood (Wilken et al., 2012). The rather small impact of adolescent stress reported in this study maybe due to the later adolescent period during which we applied our stress manipulation. An additional limitation regarding the Nrg1Tn rat preparation is the use of the Fischer 344 rats as the background strain. Fischer 344 rats are known to have enhanced HPA axis responses to stressors compared to several other rat strains (Dhabhar et al., 1993; Dhabhar et al., 1995; Glowa et al., 1992). It is possible that the enhanced response to stressors in Fischer 344 rats may mask or preclude some effects of the disruption of Type II NRG1. Use of a different strain background could be more informative regarding the effects of an interaction between disrupted Type II NRG1 and chronic adolescent stress on anxiety-like behavior, fear extinction and basal HPA axis activity in adulthood. Finally, it should be noted that the present study used random cycling female rats. It is possible that the fluctuating levels of estradiol and progesterone during the estrous cycle could have impacted the behavioral and physiological outcomes presented here. Estrous cyclicity was not monitored because of potential confounding effects on some behavioral outcomes, particularly the EPM (S.B. Taylor, unpublished observations). Interestingly, we previously reported that disruption of Type II NRG1 did not alter estrous cyclicity, the time of pubertal onset or other measures of reproductive function (Taylor et al., 2011b).
In this study, we show that disruption of Type II NRG1 resulted in the reversal of sex differences in anxiety-like behavior and the emergence of adolescent CVS-induced sex differences in the extinction of conditioned fear memory. Our earlier studies revealed that disruption of Type II NRG1 leads to sex-specific changes in response to an acute stressor and sex-specific alterations in GR and MR receptor expression in males and females (Taylor et al., 2011a, 2011b). Together, these findings indicate that there may be a complex interaction between gonadal and adrenal hormones and brain NRG1 function. Unfortunately, little is known about the sex-specific expression and function of NRG1 in the brain. However, it is known that various isoforms of NRG1, including Type II, show increased expression in the anterior pituitary at the beginning of estrus in female rats (Zhao and Ren, 2011a). In addition, NRG1 expression in spinal cord astrocytes is significantly increased only in the presence of circulating progesterone in female rats following spinal cord injury (Lacroix-Fralish, 2008). Finally, evidence suggests that expression of the NRG1 receptors, including ErbB4, is up-regulated by the synthetic glucocorticoid, dexamethasone, in epithelial cells (Dammann et al., 2006). Together these studies provide evidence to support NRG1-gonadal/adrenal hormone interactions in the brain, but further studies are needed to systematically examine how steroid hormones interact with NRG1 in the brain.
In conclusion, the present studies confirm the importance of Type II NRG1 in sex-specific rat anxiety-like behavior and cued fear acquisition. Furthermore, we present evidence to suggest that adolescent stress exposure has rather minor effects and does not appear to potentiate the effects of disrupted Type II NRG1. If anything, disruption of Type II NRG1 seems to confer a resistance or resiliency in the face of adolescent stress. The present studies also suggest that the effects of chronic adolescent stress are more prominent in female rats than males in the Fischer 344 strain. Subtle indications of anxiolytic effects of adolescent CVS were identified in WT females, while we observed a more significant gene by environment interaction in Nrg1Tn females in the extinction of fear-related memories.
Research Highlights.
Normal sex differences in anxiety behavior are reversed in Nrg1Tn rats.
Adolescent stress exposure ameliorates sex differences in anxiety behavior.
Disruption of Type II NRG1 disrupts fear learning and extinction.
A gene by stress by sex interaction was revealed in extinction of fear memory.
Acknowledgments
This work was supported by grants and contracts from NIDA (N01DA59909) and NIMH (R01MH73826, P50MH82999).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibanez MI, Ruiperez MA, Ortet G, Fananas L. Early adversity and 5-htt/bdnf genes: New evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009;39(9):1425–32. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37(2):167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: The basis of the “two hit hypothesis”. J Psychiatr Res. 1999;33(6):543–8. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–39. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Boucher AA, Arnold JC, Duffy L, Schofield PR, Micheau J, Karl T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of delta9-tetrahydrocannabinol. Psychopharmacology (Berl) 2007;192(3):325–36. doi: 10.1007/s00213-007-0721-3. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-htt gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chisari A, Carino M, Perone M, Gaillard RC, Spinedi E. Sex and strain variability in the rat hypothalamo-pituitary-adrenal (hpa) axis function. J Endocrinol Invest. 1995;18(1):25–33. doi: 10.1007/BF03349692. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–44. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dammann CE, Nassimi N, Liu W, Nielsen HC. ErbB receptor regulation by dexamethasone in mouse type II epithelial cells. Eur Respir J. 2006;28(6):1117–1123. doi: 10.1183/09031936.06.00132305. [DOI] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type i overexpressing transgenic mice. Neuroreport. 2009;20(17):1523–8. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B, Karl T, Pavey G, Boer S, Duffy L, Scarr E. Increased levels of serotonin 2A receptors and serotonin transporter in the CNS of neuregulin 1 hypomorphic/mutant mice. Schizophr Res. 2008;99(1–3):341–349. doi: 10.1016/j.schres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, O’Tuathaigh C, Clarke G, O’Leary C, Petit E, Clarke N, Tighe O, Lai D, Harbey R, Cryan JF, Dinan TG, Waddington JL. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: A putative model of gene × environment interaction. Brain, Behavior, and Immunity. 2012;26(4):660–71. doi: 10.1016/j.bbi.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels--a comparison between sprague-dawley, fischer 344 and lewis rats. Brain Res. 1993;616(1–2):89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Differential activation of adrenal steroid receptors in neural and immune tissues of sprague dawley, fischer 344, and lewis rats. J Neuroimmunol. 1995;56(1):77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Lai D, Boucher AA, Karl T. Cognition in transmembrane domain neuregulin 1 in mutant mice. Neuroscience. 2010;170(3):800–807. doi: 10.1016/j.neuroscience.2010.07.042. [DOI] [PubMed] [Google Scholar]
- Duffy L, Cappas E, Scimone A, Schofield PR, Karl T. Behavioral profile of a heterozygous mutant mouse model for egf-like domain neuregulin 1. Behav Neurosci. 2008;122(4):748–59. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Luminais SN, White SL, Rudnick ND, Ma N, Dow HC, Kreibich AS, Abel T, Brodkin ES, Hahn CG, Siegel SJ. Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res. 1294:116–127. doi: 10.1016/j.brainres.2009.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacol Biochem Behav. 2009;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Esper RM, Pankonin MS, Loeb JA. Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res Rev. 2006;51(2):161–175. doi: 10.1016/j.brainresrev.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284(1):14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: Insights from neuroimaging. J Adolesc Health. 2008;42(4):335–43. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–81. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Geyer MA, Gold PW, Sternberg EM. Differential startle amplitude and corticosterone response in rats. Neuroendocrinology. 1992;56(5):719–23. doi: 10.1159/000126298. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leaonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuany DW, Tsuang MT, Teretsky BI, Freedman R, Breff DL. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am J Psychiatry. 2011;168(9):930–46. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 1998;107(1):128–40. doi: 10.1037//0021-843x.107.1.128. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60(2):132–40. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hintsanen M, Elovainio M, Puttonen S, Kivimaki M, Raitakari OT, Lehtimaki T, Rontu R, Juonala M, Kahonen M, Viikari J, Keltikangas-Jarvinen L. Neuregulin-1 genotype moderates the association between job strain and early atherosclerosis in young men. Ann Behav Med. 2007;33(2):148–55. doi: 10.1007/BF02879896. [DOI] [PubMed] [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, Murray RM. Pathways to schizophrenia: The impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(Suppl 1):S7–S13. doi: 10.1017/S1461145704004122. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: Understanding disturbed postnatal brain maturation through neuregulin-1-erbb4 and disc1. Trends Neurosci. 2009;32(9):485–95. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Solomon MB, Albertz J, Flak JN, Zhang R, Herman JP. Stress vulnerability during adolescent development in rats. Endocrinology. 2010;152(2):629–38. doi: 10.1210/en.2010-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: Implications for understanding schizophrenia. Genes Brain Behav. 2007;6(7):677–87. doi: 10.1111/j.1601-183X.2006.00298.x. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Seres I, Kelemen O. A polymorphism of the neuregulin 1 gene (snp8nrg243177/rs6994992) affects reactivity to expressed emotion in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(3):418–20. doi: 10.1002/ajmg.b.30812. [DOI] [PubMed] [Google Scholar]
- Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86(3):736–48. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: Potential relevance to schizophrenia. Behav Brain Res. 2005;156(2):251–61. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koshibu K, Levitt P, Ahrens ET. Sex-specific, postpuberty changes in mouse brain structures revealed by three-dimensional magnetic resonance microscopy. Neuroimage. 2004;22(4):1636–45. doi: 10.1016/j.neuroimage.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Lacroix-Fralish ML. Sex-specific pain modulation: the growth factor, neuregulin-1, as a pro-nociceptive cytokine. Neurosci Lett. 2008;437(3):184–187. doi: 10.1016/j.neulet.2008.02.074. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bedard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20(10):1115–37. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Corticosterone alters n-methyl-d-aspartate receptor subunit mrna expression before puberty. Brain Res Mol Brain Res. 2003;115(1):55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–67. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bates R, Yim DM, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang JZ, Xiong WC, Mei L. Specific Regulation of NRG1 Isoform Expression by Neuronal Activity. J Neurosci. 2011;31(23):8491–8501. doi: 10.1523/JNEUROSCI.5317-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged sleeping beauty transposon system. Mamm Genome. 2007;18(5):338–46. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2010;4:173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ. Hpa function in adolescence: Role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav. 2007;86(2):220–33. doi: 10.1016/j.pbb.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P. Investigations of hpa function and the enduring consequences of stressors in adolescence in animal models. Brain Cogn. 2010;72(1):73–85. doi: 10.1016/j.bandc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40(2):166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9(6):437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: The role of obstetric complications. Schizophr Bull. 2008;34(6):1083–94. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cotex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- O’Tuathaigh CM, O’Connor AM, O’Sullivan GJ, Lai D, Harvey R, Croke DT, Waddington JL. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‘knockout’ of the schizophrenia risk gene neuregulin-1. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(2):462–6. doi: 10.1016/j.pnpbp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–9. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata DP, Breen G, Osborne S, Munro J, St Clair D, Collier DA. An association study of the neuregulin 1 gene, bipolar affective disorder and psychosis. Psychiatr Genet. 2009;19(3):113–6. doi: 10.1097/YPG.0b013e32832a4f69. [DOI] [PubMed] [Google Scholar]
- Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G. Normal female sexual development requires neuregulin-erbB receptor signaling in hypothalamic astrocytes. J Neurosci. 2003;23(1):230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20(16):6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889(1–2):71–7. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147(4):1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31(2):232–40. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, Buonanno A. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Kruschinski C, Pabst R, von Horsten S, Stephan M. Postnatal experiences influence the behavior in adult male and female fischer and lewis rats. Int J Dev Neurosci. 2010;28(7):561–71. doi: 10.1016/j.ijdevneu.2010.07.235. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2009;22(1):13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71(4):877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, Gunnarsdottir S, Walker N, Petursson H, Crombie C, Ingason A, Gulcher JR, Stefansson K, St Clair D. Association of neuregulin 1 with schizophrenia confirmed in a scottish population. Am J Hum Genet. 2003;72(1):83–7. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Muller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: Implications for stress-related disorders. Horm Behav. 2008;53(2):386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Taylor AR, Taylor SB, Koenig JI. Reduction of Type II Neuregulin-1 in rats causes working and reference memory deficits. Neuroscience letters. In press. http://dx.doi.org/10.1016/j.neulet.2012.10.018.
- Taylor SB, Taylor AR, Markham JA, Geurts AM, Kanaskie BZ, Koenig JI. Disruption of the neuregulin 1 gene in the rat alters hpa axis activity and behavioral responses to environmental stimuli. Physiol Behav. 2011a;104(2):205–14. doi: 10.1016/j.physbeh.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SB, Markham JA, Taylor AR, Kanaskie BZ, Koenig JI. Sex-specific neuroendocrine and behavioral phenotypes in hypomorphic type II neuregulin 1 rats. Behav Brain Res. 2011b;224(2):223–232. doi: 10.1016/j.bbr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89(2):167–72. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27(1–2):163–78. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plast. 2007;2007:71203. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: Review of epidemiological findings and future directions. Schizophr Bull. 2008;34(6):1066–82. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF. Adolescent neurodevelopment and psychopathology. Curr Dir Psychol Sci. 2002;11(1):24–28. [Google Scholar]
- Wilkin MM, Waters P, McCormick CM, Menard JL. Intermittent physical stress during early- and mid-adolescence differentially alter rats’ anxiety- and depression-like behaviors in adulthood. Behavioral Neuroscience. 2012;126(2):344–60. doi: 10.1037/a0027258. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male syrian hamster. J Neurobiol. 2006;66(6):578–90. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ren SG. Neuregulin-1 (Nrg1) is mainly expressed in rat pituitary gonadotroph cells and possibly regulates prolactin (PRL) secretion in a juxtacrine manner. J Neuroendocrinol. 2011;23(12):1252–1262. doi: 10.1111/j.1365-2826.2011.02223.x. [DOI] [PubMed] [Google Scholar]
- Zhao WJ, Ren SG. Endogenous neuregulin-1 expression in the anterior pituitary of female Wistar-Furth rats during the estrous cycle. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(6):921–927. [PubMed] [Google Scholar]