Abstract
Atherosclerotic plaque rupture is a major cause of myocardial infarction and ischemic stroke. The adhesive strength of the bond between a plaque and the vascular wall, measured as local energy release rate, , is used for quantitative plaque stability estimation. We tested the hypothesis that adhesive strength varies with plaque composition. Matrix metalloproteinase-12 (MMP12) deficiency was previously reported to alter lesion composition. To estimate values, peeling experiments are performed on aortic plaques from apolipoprotein E knockout (apoE KO) and apoE MMP12 double knockout (DKO) male mice after 8 months on high-fat diet. For plaques in apoE KO and apoE MMP12 DKO mice, experimental values for differ significantly (p<0.002) between genotypes, averaging 19.2 Joule/m2 and 12.1 J/m2, respectively. Histology confirms that plaques delaminate along their interface with the underlying internal elastic lamina (IEL) in both genotypes. Quantitative image analysis of stained tissue sections demonstrates a significant positive correlation (p<0.05) between local collagen content of lesions and values in both genotypes, indicating that adhesive strength of plaques depends on local collagen content. Surprisingly, macrophage content of aortic plaques is neither significantly correlated with values nor significantly different between genotypes. The IEL underlying plaques in apoE KO mice is significantly more fragmented (number of breaks and length of breaks) than in apoE MMP12 DKO mice, suggesting that elastin fragmentation also influences adhesion strength of plaques. Overall, our results suggest that plaques adhere more strongly to the underlying IEL in apoE KO mice than in apoE MMP12 DKO mice.
Keywords: atherosclerosis, mouse models, energy release rate, plaque rupture, interfacial delamination, matrix metalloproteinase
1. Introduction
Atherosclerotic plaque rupture is the main cause of myocardial infarction, coronary thrombosis and ischemic stroke. In a previous study, we proposed a new plaque rupture mechanism, plaque separation at the shoulder, and developed a novel quantitative mechanical experiment to measure the adhesive strength between the atherosclerotic plaque and the underlying vascular wall in mouse models using local energy release rate, , as a quantifiable metric for direct comparison of plaque separation strengths (Wang, et al., 2011). Previous studies focused on morphological characteristics of the entire vulnerable plaque, such as fibrous cap thickness and lipid core size, or on local features of the rupture site in human samples, such as presence of micro-calcifications or thinning of the fibrous cap (Vengrenyuk, et al., 2006; Virmani, et al., 2007). Since mouse plaques generally do not rupture spontaneously (Schwartz, et al., 2007), studies of local morphology at the rupture site cannot readily be conducted in mouse models. However, by studying the structure-function relationships between local energy release rate and local plaque composition at non-rupture sites, we can provide quantitative comparisons of relative plaque stability and identify those plaques more likely to fail.
Using transgenic models, we have now explored the correlation between and plaque composition in a mouse model of diet-induced atherosclerosis,. We previously showed that plaques delaminate at the IEL interface in this mouse model (Wang, et al., 2011), suggesting that genetic defects that alter elastin degradation might lead to changes in plaque adhesive strength. MMP-12 (macrophage metalloelastase) is known to be important in elastin degradation (Luttun, et al., 2004), promoting lesion expansion and destabilization through atherosclerotic media destruction and ectasia. Furthermore, plaque composition in MMP-12 deficient mice was reported to be significantly different from that in apoE single knockout controls (Johnson, et al., 2005). Therefore, we expect that the structure of the IEL underlying plaques will differ in apoE KO and apoE MMP-12 DKO mice, due to altered capacity to remodel elastin.
In the current work, we show for the first time that (a) local collagen content of atherosclerotic plaques is positively correlated with local adhesive strength between the plaque and the underlying vascular wall and (b) increased elastin fragmentation in the IEL underlying plaques is positively correlated with increased adhesive strength between the plaque and the underlying vascular wall.
2. Materials and Methods
2.1. Animal Model, Specimen Preparation and Mechanical Experiments
ApoE KO and apoE MMP-12 DKO male mice are used in this study. Six-week-old mice are fed a high-fat Western diet for 8 months to develop atherosclerosis throughout the aorta (Nakashima, et al., 1994). Mice are sacrificed and dissected to expose the aorta. The descending aorta is cut open longitudinally to expose each plaque. A small initial flaw is introduced at the proximal end of the plaque to initiate delamination. Animals used in this study were euthanized by humane methods in accordance with PHS guidelines and as approved by the university Institutional Animal Care and Use Committee.
Cyclic peeling experiments are performed to obtain local values. Briefly, energy used for delamination in one peeling cycle is averaged by the area newly exposed by plaque delamination in that cycle. Details of the experimental setup and data processing can be found in Wang et al. (Wang, et al., 2011). Each delamination experiment generated a series of local values as delamination proceeded stepwise from the proximal edge of the plaque.
2.2. Histology
After the peeling experiment, delaminated plaques are immersion-fixed in 10% neutral-buffered formalin for at least 24 hours. The small size of plaque specimens makes it difficult to directly embed them in paraffin in an appropriate orientation. Therefore, specimens are first embedded in 10% agarose gel. The agarose blocks are cut into cubes and trimmed to ensure the correct orientation for each plaque. Specimens are oriented to obtain transverse sections starting at the proximal end of the plaque. Agarose blocks are dehydrated in graded alcohols and processed using conventional methods for paraffin embedding.
The protocol for correlating experimental delamination data (hereafter referred to as experimental mechanics data) with histological data is shown schematically in Figure 1. Embedded plaque specimens are sectioned at 5 μm along their entire length. For every 10 sections, we collected the first 5 sections as a set. Since specimens shrink during dehydration and embedding (Lowder, et al., 2007), we record the total number of sets to calculate the lengths of plaques after embedding (Lf = section thickness × number of sets × sections/set), enabling us to obtain a more accurate correlation with experimental mechanics data. The first section of each set is stained with Masson's Trichrome for general morphology, and the remaining sections are stained to identify specific plaque components, as described below. Images are taken under 100× magnification with a Zeiss Axioskop 2 microscope and analyzed with image processing software ImagePro Plus (Media Cybernetics). During delamination experiments, black tissue marking dye is applied to the luminal surface of the plaque to distinguish newly exposed area under the plaque. Tissue marking dye is applied after creation of a small flaw at the proximal end of each plaque and before initiation of peeling cycles. Thus, in serial sections of delaminated plaques, tissue marking dye coating both surfaces of the “peel arm” appears in the first few images, then decreases on the abluminal surface corresponding to newly exposed area. The transition from sections with abluminal dye to those without is identified as the mechanical experiment starting point on the delaminated specimens. As shown schematically in Figure 1, this starting point corresponds to axial position x=0 during the first peeling cycle. Along the remaining plaque length, the mechanical experiment data point for each cycle is correlated with plaque composition of a representative set of cross-sections taken from the plaque segment delaminated during that cycle. The length of the plaque segment corresponding to each data point can be determined from the displacement length recorded by the Bose actuator. The sum of all such displacements is the total plaque length represented by mechanical experiment data points (Lo). The total length of each delaminated plaque after fixation and embedding (Lf) can be calculated as described above from the number of sections and section thickness. The position of each histological section in the processed plaque which corresponds to an experimental mechanics data point is estimated by assuming uniform strain and multiplying the experimental displacement value by a proportionality constant, Lf/Lo, defined as total fixed length of the plaque (Lf) divided by total fresh length (Lo) (see Figure 1).
Figure 1. Schematic of strategy for correlating experimental biomechanics data with histological sections.
Experimental biomechanics data and histology are correlated by calculating the lengths of plaques before and after fixation and embedding, and aligning the data sets. Each experimental data point (diamonds in top figure) corresponds to a force-displacement curve (inset) acquired during delamination of a specific length of plaque. Total unfixed plaque length is calculated by summing individual delamination event lengths (displacements) measured by the Bose mechanical test system. Plaques shrink after fixation and embedding (schematic, third panel). After sectioning (bottom panel), embedded plaque length is calculated as total number of sections × section thickness. Each experimental biomechanics data point corresponds to several groups of sections (enclosed in gray brackets), representing a length of delaminated plaque in the fixed specimen. Both unfixed and fixed plaque lengths are corrected for the initial peel arm length (black segment at left of plaque) and aligned.
The most important error in correlating experimental mechanics data to histological data is likely to be underestimation of the fixed plaque length due to loss of sections during paraffin sectioning; based on estimates of how many sections are typically lost, this would be no more than 5% error in Lf. During mechanical experimentation, axial position measurements (displacements) output by the Bose actuator are very accurate, ±30 μm, or the equivalent of 6 paraffin sections (i.e., positions are accurately known to within a single group of sections).
Sets of sections corresponding to locations of mechanical experiment data points are selected for further examination. The second section from each set is stained with Picrosirius Red (PSR) to determine fibrillar collagen content. The third section from each set is stained for macrophages. using a Mac-3 immunohistochemistry method. Five micron-thick paraffin-embedded sections are deparaffinized in Histoclear and rehydrated. Antigen retrieval is performed in boiling citrate buffer. Sections are stained for macrophages using rat anti-mouse Mac-3 monoclonal antibody (1:500, Pharmingen), followed by biotinylated donkey anti-rat IgG and alkaline phosphatase–streptavidin. Vector Red Substrate kit is used for color development. Positive and negative controls are used for immunohistochemical staining to define background staining levels (Lessner, et al., 2004). Positive controls consisted of plaques from mouse aortic arch, while negative controls omitted the primary antibody. Our studies indicate that morphological features of serial sections within each set are very similar. Therefore, we used the result from one section in each set as a representative of plaque composition for the entire set.
2.3. Image Analysis
For consistency in illumination conditions and image acquisition, all images for each type of staining are taken on the same day and quantified using the same parameter settings. PSR-stained sections are imaged in brightfield for total area measurement and under cross-polarized transmitted light for collagen area measurement. Mac-3 stained sections are imaged in brightfield. Color images are segmented in ImagePro to identify Mac-3-positive area (pink-stained regions). Collagen content and macrophage content are expressed as percentages of total plaque area.
2.4 Histological Analysis of IEL Fragmentation
To detect potential differences in IEL fragmentation between mouse strains, separate specimens of intact, undelaminated plaques were prepared from perfusion-fixed aortas of apoE KO and apoE MMP-12 DKO mice after eight months on Western diet. After embedding, plaque specimens from the descending aorta were sectioned as described above and stained with Masson's Trichrome to identify the region of maximum plaque base length. Sections from each plaque at its maximum were stained with Verhoeff's stain for visualization of elastic fibers. Images acquired with an AxioImager microscope were analyzed using ImagePro software to quantify IEL length under the plaque, number of breaks in the IEL, and total length of breaks. IEL continuity under each plaque was quantified by dividing the number of breaks in the IEL by the total length of the IEL, and by calculating the fractional length occupied by breaks in the IEL (sum of all break lengths divided by total length of the IEL).
2.5. Statistical Analysis
Values are expressed as mean value ± experimentally estimated standard deviation. For comparison of group means of values, a two-tailed Wilcoxon test is performed. The group mean for both local collagen content and macrophage content are analyzed with the two-tailed Mann-Whitney test. In all cases, statistical significance is assumed when the two-tailed probability is <0.05.
3. Results
3.1. Local Energy Release Rate during Plaque Delamination Varies with Mouse Genotype
Figures 2A and 2B present a summary in histogram form of the measurements for 52 (55) data points obtained from 9 (11) atherosclerotic plaque delamination experiments performed using aorta specimens from 8 apoE KO (7 apoE MMP-12 DKO) mice, respectively. Statistical parameters describing the distributions of all measured data are given in Table 1. For plaques in apoE KO mice, experimental values for averaged 19.2±15.6 Joule/m2 (95% confidence interval of the mean, ±4.3 J/m2). For plaques in apoE MMP12 DKO mice, values averaged 12.1±13.0 J/m2 (95% confidence interval, ±3.5 J/m2). The positive skewness values of 1.46 and 2.20 differ significantly from zero, indicating that both distributions are non-normal. A two-tailed Mann-Whitney test showed a significant effect of genotype on average values (p<0.002).
Figure 2. Distribution of local energy release rate () in apoE KO (A) and apoE MMP-12 DKO (B) mice.
The distributions of values from both groups are positively skewed. Data were acquired from 9 apoE KO plaques (eight mice) and 11 apoE MMP-12 DKO plaques (seven mice).
Table 1.
Statistical parameters for distributions of experimental energy release rate measurements
| ApoE KO | ApoEMMP-12DKO | |
|---|---|---|
| Mean | 19.2 | 12.1* |
| Median | 16.0 | 8.0 |
| SD | 15.6 | 13.0 |
| Kurtosis | 2.0 | 4.6 |
| Skewness | 1.5 | 2.2 |
| Minimum | 1.7 | 1.6 |
| Maximum | 70.3 | 57.9 |
| Count | 52 | 55 |
P<0.002
In a few cases, we observed alternate failure mechanisms, such as delamination within the media (1 plaque, 4% of experimental total), fibrous cap tearing (1 plaque, 4% of experimental total), or delamination within branch arteries where the plaque had extended during development (most common alternate mechanism; 2 plaques, 8% of experimental total). The maximum observed values of for delamination of any plaque segment were 71.5 J/m2 (delamination with plaque extending into the intercostal artery) and 93.6 J/m2 (“trouser tear” through distal plaque segment). Experiments in which an alternative failure mechanism is clearly evident in the video record of the delamination experiment are excluded from the following analysis of plaque composition/adhesive strength correlations.
3.2. Histology
Location of Delamination Plane
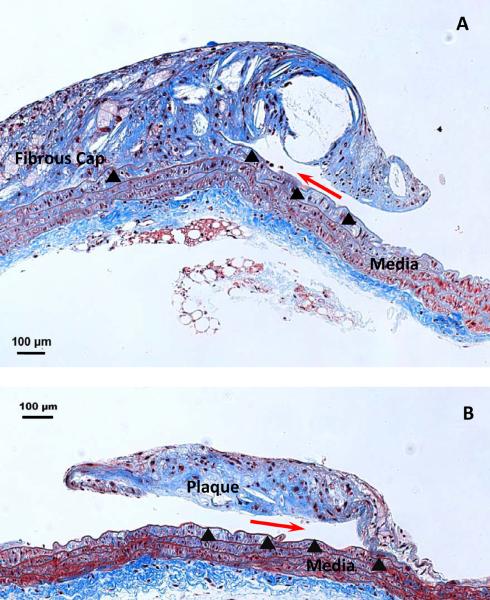
Histological studies at 200× magnification indicate that delamination events propagated along the interface of plaques and the underlying IEL in both mouse strains (Figure 3). Examination at the light microscopic level revealed an intact IEL after the delamination process. In some cases, gaps in the IEL are noted prior to delamination, as shown in Figure 3A to the left of the delamination front. Since the location of the delamination plane is identical in both strains of mice, we sought to identify differences in local plaque composition that might contribute to differences in measured local values.
Figure 3. Histological demonstration of delamination surface location.
A. Masson's Trichrome staining of a longitudinal section of a partially delaminated plaque from an apoE KO mouse (200×, scale bar=100 μm), leaving part of it attached to the underlying IEL (arrowheads). The crack front (red arrow) is located between the atherosclerotic plaque cap and its underlying IEL; B. A partially delaminated plaque from an apoE MMP-12 DKO mouse (200×, scale bar=100 Pm). Plaque separation occurred at the same interface. Collagen is stained blue, nuclei purple-black, and smooth muscle cells red.
Plaque Collagen Content
Representative images from the same set of sections showing Trichrome staining under brightfield and PSR staining under cross-polarized transmitted light are shown in Figures 4A and 4B, respectively. For apoE KO mice, the local collagen content averaged 9.1%±12.1%. For apoE MMP-12 DKO mice, the collagen content averaged 6.6%±7.1%.1
Figure 4. Representative images of histological study.

Serial sections of delaminated plaques at each mechanical experiment data point are stained with Masson's Trichrome (A), Picrosirius Red (B, cross-polarized transmitted light image), and Mac-3 (C, pink areas), to determine general morphology, fibrillar collagen content and macrophage content, respectively. The fractional areas of collagen and macrophages are quantified with ImagePro image analysis software.
Plaque Macrophage Content
Figure 4C shows a representative picture of Mac-3 staining from the same set of sections. The pink areas are Mac-3 positive areas. Comparison of Mac-3 staining in plaques of apoE KO and apoE MMP12 DKO mice showed no notable differences in macrophage distribution. Both strains of mice had strongest Mac-3 staining both near the lumen and in the shoulder regions of plaques. For apoE KO mice, the local macrophage content in plaques averaged 2.6%±4.8%. For apoE MMP-12 DKO mice, local macrophage content averaged 1.7%±3.0%. Statistical analysis indicates that the mean values of macrophage content in plaques from these two mouse strains are not significantly different (p>0.05). We note that, in general, macrophage content in these advanced aortic plaques is very low compared to that in aortic arch plaques from apoE KO mice (used as a positive control for Mac-3 staining, data not shown).
IEL Fragmentation
Table 2 compares the continuity of the IEL in the two strains of mice. Both the number of breaks per unit length of IEL under the plaque and the fractional length of breaks were significantly greater in apoE KO mice than in apoE MMP-12 DKO mice. Representative images of IEL fragmentation in each mouse strain are shown in Supplemental Data Figure S1.
Table 2.
IEL Fragmentation in apoE KO vs. apoE MMP12 DKO mice
| ApoE KO | ApoEMMP-12DKO | |
|---|---|---|
| IEL breaks per plaque base length (×104), μm−1 | 9.1 ± 8.0 | ‡3.5 ± 4.2 |
| Total length of breaks/plaque base length | 0.080 ± 0.067 | *0.015 ± 0.041 |
| n | 14 | 15 |
P<0.05, 2-tailed t-test
P<0.01, 2-tailed t-test
3.3. Correlation between Plaque Composition and Adhesive Strength
Correlations between lesion composition and values from the two strains of mice are shown in Figure 5 (collagen) and Figure 6 (macrophages). Importantly, positive linear statistical correlations are observed between collagen content and values in both groups. The values are correlated with local fibrillar collagen content in plaques from apoE KO mice, as demonstrated by a slope which is significantly greater than zero (p<0.05). A similar correlation is observed in apoE MMP-12 DKO mice (p<0.001 for non-zero slope). The slope for apoE MMP-12 DKO mice is significantly steeper than that for apoE KO mice, indicating a stronger relationship between collagen content and adhesive strength in mice lacking MMP-12. Somewhat surprisingly, the slopes of the correlation plots of values vs. fractional macrophage content for both groups are not significantly different from zero (p>0.05). This indicates that there are no significant correlations between macrophage content and values for either group, implying that the measured values are independent of the macrophage content for the two mouse strains.
Figure 5. Correlation between G values and local collagen content of delaminated plaques.
Image analysis of Picrosirius Red-stained tissue sections demonstrated a positive linear correlation between local collagen content in lesions and values in both apoE KO mice (A) and apoE MMP12 DKO mice (B).
Figure 6. Correlation between G values and local macrophage content of delaminated plaques.
Macrophage content of aortic plaques is neither significantly correlated with values nor significantly different between apoE KO mice and apoE MMP12 DKO mice.
4. Discussion
Plaque rupture in human arteries has been observed both within the fibrous cap and at the shoulder region, where the plaque adjoins the adjacent normal wall. Tearing in the shoulder region could readily evolve into delamination (adhesive failure) of the plaque from the underlying vessel. In this regard, recent computational studies have shown that the maximum von Mises stress can occur either on the fibrous cap away from the vessel-plaque interface or at the upstream edge of the plaque where it emerges from the vessel wall, depending on the plaque geometry (Belzacq, et al., 2012). These results are consistent with clinical observations of plaque failure on the upstream side of the plaque (Groen, et al., 2007). Additional support for a delamination-type mechanism comes from clinical imaging of “mobile floating plaques” (Cho, et al., 2002; Ferrero, et al., 2009), which appear in vivo as plaque segments that are fully separated from the vessel wall. Since shoulder region rupture is likely to be a combination of local processes, one approach for quantifying rupture resistance is to determine the adhesive strength of the bond between the plaque and the vascular wall, which is the approach we have taken in this study.
It should also be noted that plaque failure in the human abdominal aorta has been reported in the literature (van Dijk, et al., 2010). The major difference between plaque failure in the abdominal aorta and in the carotids and coronaries is that total occlusion of the vessel typically will not occur in the former case, making these events clinically silent.
Based on the existing evidence, we expect that the prevailing mechanism of plaque failure will depend on both the distribution of maximum stresses on the plaque and on the material properties at the locations of highest stress, which would include the delamination strength in the plaque-vessel interface region, quantified here as local energy release rate, .
The distributions of values from apoE KO and apoE MMP-12 DKO mice are both non-normal, as confirmed by calculated values of skewness and kurtosis. To compare the two distributions, the authors performed a two-tailed Mann-Whitney test. The results clearly show that the mean values of the two mouse strains are significantly different (p<0.002), indicating that genotype has an effect on measured average values. This observed difference in average value could not be explained by separation at different planes within the vessel wall, as histology showed that delamination occurred at the interface of plaque and IEL in both mouse strains.
Measured values in this study are consistent with those previously reported for plaque delamination in apoE KO mice (Wang, et al., 2011). Furthermore, values reported herein fall within the same order of magnitude as dissection energies measured during delamination of human carotid artery specimens at or near the intima-media boundary (52 ± 31 J/m2 in the longitudinal direction and 36 ± 7 J/m2 in the circumferential direction, respectively) (Tong, et al., 2011). In Tong's work, delamination typically occurred within the media near the intima-media interface, which may account for the higher energies required to delaminate these specimens. In particular, it is important to note that longitudinal delamination of human carotid artery specimens at the intima-media interface resulted in a much rougher fracture interface (Tong, et al., 2011) than that observed in our work.
Quantitative image analysis of PSR-stained tissue sections demonstrated a positive linear correlation between local fibrillar collagen content of lesions and values in both strains of mice (p<0.05). Our results indicate that local fibrillar collagen content is positively correlated with plaque stability to delamination; that is, collagen content is positively correlated with local adhesive strength between a plaque and the vascular wall in both strains of mice.
It is noted that a previous study reported that collagen content of plaques and surrounding aortic walls at 25 weeks of diet is unaltered by MMP-12 deficiency in apoE KO mice (Luttun, et al., 2004). Our results for lesional collagen content agree qualitatively with this work, where fractional collagen area in both strains of mice is reported to be comparable (37.4% versus 39.6%).
Surprisingly, immunohistochemistry showed that macrophage content of aortic plaques is neither significantly correlated with values nor significantly different between the two strains of mice examined in this work. Our results are in contrast to a previous study that reported plaque macrophage content in apoE MMP-12 DKO mice is lower than in apoE KO mice (Johnson, et al., 2005). The apparent discrepancy may be explained by differences in plaque location and age between our work and that of Johnson and colleagues. Johnson's group examined plaques in mouse brachiocephalic arteries at the 8-week time point, while we used plaques from mouse descending aortas after 8 months of Western diet. The brachiocephalic trunk is one of the earliest sites of atherosclerotic plaque development in mouse models (Nakashima, et al., 1994). In Johnson, et al., macrophage content is quantified either in both the plaque and the adjacent arterial wall or solely in the adjacent arterial wall. In contrast, we measured macrophage content only in delaminated plaques. In the advanced plaques analyzed in the current work, a majority of macrophage foam cells might already have undergone necrosis, producing cell debris which would not be detected by immunohistochemistry. Our results agree more closely with those of Luttun and coworkers, which indicated that macrophage content in plaques and surrounding aortic walls in the descending aortas after 25 weeks of diet are similar in apoE KO and apoE MMP12 DKO mice (Luttun, et al., 2004). In advanced plaques, macrophage content decreases compared with that at earlier stages due to apoptosis or necrosis. We observed very little Mac-3-positive staining as a percentage of total area of delaminated plaques (2.6%±4.8% in apoE KO vs. 1.7%±3.0% in apoE MMP12 DKO). The paucity of macrophages in these advanced plaques may explain why local macrophage content is not significantly correlated with values.
Though we have shown local fibrillar collagen content is positively correlated with values, this observation does not fully account for the observed difference in plaque adhesive strength between apoE KO and apoE MMP12 DKO mice. Plaques in our model typically delaminate along the IEL-plaque interface; therefore, the authors performed detailed studies of the microstructure of extracellular matrix (ECM) at this interface, since IEL integrity is likely to be an important factor in determining adhesive strength. Specifically, the authors suggest that the difference in values between these two strains of mice may reflect the important role of MMP-12 in elastin degradation. MMP-12 deficiency leads to markedly diminished capacity of macrophages to degrade ECM, elastin in particular, and to penetrate reconstituted basement membranes (Fan, et al., 2004; Shipley, et al., 1996; Wang, et al., 2004). Luttun's group found that in apoE KO mice, the loss of MMP-12 decreases elastin degradation by 5- to 8-fold, length of breaks in the IEL per plaque base length more than 4-fold, and incidence of ectasia by 16-fold, respectively (Luttun, et al., 2004). As noted in Section 3.2, we confirmed that the IEL underlying plaques in the descending aorta in our model shows fewer, shorter breaks in the apoE MMP-12 DKO mice than in the apoE KO mice at the 8-month time point (Table 2). Gaps in the IEL potentially allow cell migration between intima and media, which may be associated with deposition of matrix fibers bridging the two layers. In composite materials, fiber bridging increases delamination resistance (Spearing and Evans, 1992); thus, development of fiber bridging between the plaque and the IEL is expected to reinforce plaque stability to delamination. The observed decrease in number of gaps and total gap length in the IEL of apoE MMP-12 DKO mice might be associated with a reduction in fiber bridging in these mice. We speculate that differences in fiber bridging could account for observed differences in average energy release rate between apoE KO and apoE MMP-12 DKO mice, but further characterization of the microstructure at the plaque-IEL interface is needed to support this hypothesis.
Overall, our results demonstrate that plaques adhere more strongly to the underlying IEL in apoE KO mice than in apoE MMP-12 DKO mice (consistent with the observation that elastin fragmentation is higher in the apoE KO strain, allowing for increased fiber bridging), while plaque adhesive strength correlates with local fibrillar collagen content in both mouse genotypes. These results suggest that clinical interventions which increase collagen content may stabilize plaques against failure by a delamination mechanism.
Supplementary Material
Acknowledgements
This work is funded by NSF CMMI-0926301 and CMMI-1200358 (S.M.L., M.A.S), NIH/NCRR P20 RR021949 (subcontract, S.M.L.), and NSF EPS-0903795. The study sponsors have played no role in the study design, analysis, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors have no conflicts of interest to report.
Though statistical analysis indicates that mean values of local collagen content in plaques from apoE KO and apoE MMP-12 DKO mice are not significantly different (p>0.05), the observed differences in collagen content in the two mouse strains will be shown to have a significant influence on energy release rate.
References
- Belzacq T, Avril S, Leriche E, Delache A. Mechanical action of the blood onto atheromatous plaques: influence of the stenosis shape and morphology. Comp. Methods Biomech. Biomed. Eng. 2012:1–12. doi: 10.1080/10255842.2012.697898. on line ahead of print. [DOI] [PubMed] [Google Scholar]
- Cho YP, Kwon TW, Kim GE. Sonographic appearance of a free-floating atheromatous plaque in a patient with acute stroke. J Clin Ultrasound. 2002;30(5):317–21. doi: 10.1002/jcu.10064. [DOI] [PubMed] [Google Scholar]
- Fan J, Wang X, Wu L, Matsumoto SI, Liang J, Koike T, Ichikawa T, Sun H, Shikama H, Sasaguri Y, Watanabe T. Macrophage-specific overexpression of human matrix metalloproteinase-12 in transgenic rabbits. Transgenic Research. 2004;13(3):261–9. doi: 10.1023/b:trag.0000034717.70729.61. [DOI] [PubMed] [Google Scholar]
- Ferrero E, Gaggiano A, Ferri M, Nessi F. Mobile floating carotid plaque post-trauma. Diagnosis and treatment. Interact Cardiovasc Thorac Surg. 2009;8(4):496–7. doi: 10.1510/icvts.2008.198754. [DOI] [PubMed] [Google Scholar]
- Groen HC, Gijsen FJ, van der Lugt A, Ferguson MS, Hatsukami TS, van der Steen AF, Yuan C, Wentzel JJ. Plaque rupture in the carotid artery is localized at the high shear stress region: a case report. Stroke. 2007;38(8):2379–81. doi: 10.1161/STROKEAHA.107.484766. [DOI] [PubMed] [Google Scholar]
- Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinase-3, metalloproteinase-7, metalloproteinase-9, and metalloproteinase-12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proceedings of the National Academy of Sciences USA. 2005;102(43):15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessner SM, Martinson DE, Galis ZS. Compensatory vascular remodeling during atherosclerotic lesion growth depends on matrix metalloproteinase-9 activity. Arteriosclerosis Thrombosis and Vascular Biology. 2004;24(11):2123–9. doi: 10.1161/01.ATV.0000141840.27300.fd. [DOI] [PubMed] [Google Scholar]
- Lowder ML, Li S, Carnell PH, Vito RP. Correction of distortion of histological sections of arteries. Journal of Biomechanics. 2007;40:445–450. doi: 10.1016/j.jbiomech.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Luttun A, Lutgens E, Manderveld A, Maris K, Collen D, Carmeliet P, Moons L. Loss of matrix metalloproteinase-9 or matrix metalloproteinase-12 protects apolipoprotein E-deficient mice against atherosclerotic media destruction but differentially affects plaque growth. Circulation. 2004;109(11):1408–14. doi: 10.1161/01.CIR.0000121728.14930.DE. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133–40. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27(4):705–13. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- Shipley JM, Wesselschmidt RL, Kobayashi DK, Ley TJ, Shapiro SD. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc Natl Acad Sci U S A. 1996;93(9):3942–6. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearing SM, Evans AG. The role of fiber bridging in the delamination resistance of fber-reinforced composites. Acta Metallurgica et Materialia. 1992;40(9):2191–2199. [Google Scholar]
- Tong J, Sommer G, Regitnig P, Holzapfel GA. Dissection properties and mechanical strength of tissue components in human carotid bifurcations. Ann Biomed Eng. 2011;39(6):1703–19. doi: 10.1007/s10439-011-0264-y. [DOI] [PubMed] [Google Scholar]
- van Dijk RA, Virmani R, von der Thusen JH, Schaapherder AF, Lindeman JH. The natural history of aortic atherosclerosis: a systematic histopathological evaluation of the peri-renal region. Atherosclerosis. 2010;210(1):100–6. doi: 10.1016/j.atherosclerosis.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103(40):14678–83. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virmani R, Burke AP, Farb A, Gold HK, Finn AV, Kolodgie FD. Plaque rupture. In: Virmani R, Narula J, Leon MB, Willerson JT, editors. The Vulnerable Atherosclerotic Plaque: Strategies for Diagnosis and Management. Blackwell Futura; Malden, MA: 2007. pp. 37–59. [Google Scholar]
- Wang X, Liang J, Koike T, Sun H, Ichikawa T, Kitajima S, Morimoto M, Shikama H, Watanabe T, Sasaguri Y, Fan J. Overexpression of human matrix metalloproteinase-12 enhances the development of inflammatory arthritis in transgenic rabbits. American Journal of Pathology. 2004;165(4):1375–83. doi: 10.1016/S0002-9440(10)63395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ning J, Johnson JA, Sutton MA, Lessner SM. Development of a quantitative mechanical test of atherosclerotic plaque stability. Journal of Biomechanics. 2011;44(13):2439–45. doi: 10.1016/j.jbiomech.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.