Abstract
Objective
To determine whether behavioral mechanisms explain the association between depressive symptoms and myocardial infarction (MI) or death in individuals with coronary heart disease (CHD).
Background
Depressive symptoms are associated with increased morbidity and mortality in individuals with CHD, but it is unclear how much behavioral mechanisms contribute to this association.
Methods
The study included 4,676 participants with a history of CHD. Elevated depressive symptoms were defined as scores ≥4 on the Center for Epidemiologic Studies Depression 4-item Scale. The primary outcome was definite/probable MI or death from any cause. Incremental proportional hazards models were constructed by adding demographics, comorbidities and medications, then four behavioral mechanisms (alcohol use, smoking, physical inactivity, and medication non-adherence).
Results
At baseline, 638 (13.6%) participants had elevated depressive symptoms. Over a median 3.8 years of follow up, 125 of 638 (19.6%) participants with and 657 of 4038 (16.3%) without elevated depressive symptoms had events. Higher risk of MI or death was observed for elevated depressive symptoms after adjusting for demographics (hazard ratio [HR] 1.41, 95% CI 1.15–1.72), but was no longer significant after adjusting for behavioral mechanisms (HR 1.14, 95% CI 0.93–1.40). The four behavioral mechanisms together significantly attenuated the risk for MI or death conveyed by elevated depressive symptoms (−36.9%, 95% CI −18.9 to −119.1%), with smoking (−17.6%, 95% CI −6.5% to −56.0%) and physical inactivity (−21.0%, 95% CI −9.7% to −61.1%) having the biggest explanatory roles.
Conclusion
Our findings suggest potential roles for behavioral interventions targeting smoking and physical inactivity in patients with CHD and comorbid depression.
Keywords: myocardial infarction, depression, death, physical exercise, smoking
Introduction
In recent years, there has been considerable interest in the negative impact of depression on outcomes among patients with coronary heart disease (CHD) (1). It is estimated that approximately 20% of individuals with CHD meet criteria for major depression, and up to 40% experience some depressive symptoms (2, 3). Furthermore, the presence of depression or elevated depressive symptoms in individuals with CHD has consistently been shown to be associated with a markedly increased risk of adverse events including death and myocardial infarction (MI) (2–7). Because of this, national guidelines have recommended screening and treatment of depression in individuals with CHD (1). However, the evidence for improving cardiac outcomes by treating depression has been limited, and trials of interventions to improve depression thus far have shown equivocal results with regards to improving cardiac risk (8–10).
A better understanding of mechanisms by which depression conveys cardiac risk may suggest alternative approaches for improving outcomes in individuals with concomitant depression and CHD. Recent studies have noted the potential role of behavioral mechanisms that are associated with both depression and cardiac risk. For instance, smoking (2), physical inactivity (2, 11), and medication non-adherence (12) have all been shown to explain part of the increased risk for adverse cardiac outcomes conveyed by depression. However, most studies focused on different individual behavioral mechanisms in selected populations, limiting their generalizability. In the current study, we sought to clarify the collective contribution of behavioral mechanisms to the increased cardiac risk conveyed by elevated depressive symptoms in individuals with CHD. Specifically, we examined the explanatory role played by alcohol use, smoking, physical inactivity, and medication non-adherence in the association between depressive symptoms and MI or death in participants with CHD enrolled in the REason for Geographic and Racial Differences in Stroke (REGARDS) study.
Methods
Details on the REGARDS study have been published previously (13). In brief, the REGARDS study is a population-based cohort study of stroke incidence and cognitive decline with the incidence of CHD being investigated through an ancillary study. The study enrolled adults ≥ 45 years of age from the continental U.S. Potentially eligible participants were identified from commercially available lists of U.S. residents and sent an initial mailing which provided details of the study. This mailing was followed by a telephone call and a subsequent in-home visit, during which time participants were enrolled. Between January 2003 and October 2007, 30,239 African-American and white adults were enrolled. The current analysis was limited to 5,346 participants with a history of CHD (as defined below) at baseline. Of these participants, 26 did not complete depression screening at baseline and 92 were missing follow-up data for outcomes. Additionally, we excluded 520 participants who were missing covariate information. After these exclusions, the analysis included 4,676 participants with complete data. The REGARDS study protocol was approved by the Institutional Review Boards at the participating centers and all participants provided informed consent.
Data Collection
The REGARDS study baseline data collection included a computer-assisted telephone interview, an in-home examination, and self-administered questionnaires. Trained research staff conducted telephone interviews to collect data on demographics, education, income, cigarette smoking, physical activity, aspirin and thienopyridine use, and use of antihypertensive, anti-glycemic, and cholesterol lowering medications. During the in-home study visit, trained and certified health professionals conducted a physical examination and collected biological samples. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured two times following a standardized protocol (14). Based on the average of the two blood pressure measurements, hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, and/or self-reported use of antihypertensive medication. Diabetes was defined as a serum glucose ≥ 126 mg/dL for participants who had fasted ≥ 8 hours prior to their in-home study visit, serum glucose ≥ 200 mg/dL for those who had not fasted, or self-report of a prior diagnosis of diabetes with current use of insulin or oral hypoglycemic medications. A history of MI and stroke were identified via self-report. Also, during the in-home visit, participants were asked to provide all medications they had taken in the past two weeks, and medication names were recorded and subsequently coded into drug classes. During the in-home examination, an electrocardiogram was performed. A history of CHD at baseline was defined as a self-reported history of MI or coronary revascularization procedure (percutaneous coronary intervention or coronary artery bypass surgery), or evidence of MI on the study electrocardiogram.
For the current analysis, we focused on four behavioral mechanisms: alcohol use, smoking, physical inactivity, and medication non-adherence. Based on prior literature, light/moderate and heavy alcohol consumption was defined as consuming > 0 to 14 and ≥ 15 alcoholic beverages per week, respectively, for men and > 0 to 7 and ≥ 8 alcoholic beverages per week, respectively, for women (15, 16). Current smoking was determined by affirmative responses to both of the following yes/no questions “Have you smoked at least 100 cigarettes in your lifetime?” and “Do you smoke cigarettes now, even occasionally?” Physical inactivity was assessed through a single question “How many times per week do you engage in intense physical activity, enough to work up a sweat?” with response options of “none”, “1 to 3 times per week” and “4 or more times per week”. Medication non-adherence was assessed using the 4-item Morisky Medication Adherence Scale (MMAS). Each item has a yes/no response option. One point is assigned to each “yes” response, and points are summed (totaling 0–4), with score of 3 or 4 indicating high risk of medication non-adherence (17, 18).
Depressive symptoms
The brief, 4-item Center for Epidemiologic Studies Depression (CES-D) Scale was used to assess the presence of depressive symptoms, by asking how often over the prior week the participant (1) felt depressed, (2) felt lonely, (3) had crying spells and (4) felt sad, with response options of less than 1 day (no points), 1–2 days (1 point), 3–4 days (2 points) and 5–7 days (3 points). Elevated depressive symptoms was defined as having a summed score ≥ 4, which had been reported to have 79.2% sensitivity and 86.4% specificity for meeting previously established threshold for having clinically significant depressive symptoms as assessed by the full 20-item CES-D (19, 20).
Outcomes
The outcomes for the current study included definite/probable MI or death from any cause. Outcome data through December 31, 2008 were used in this analysis. Subsequent to the REGARDS in-home examination, participants were contacted twice yearly by telephone to identify potential events. When a cardiac-related hospitalization was reported, medical records were retrieved and reviewed by a team of trained physicians using a standardized protocol (21). Records were examined for the presence of signs or symptoms suggestive of ischemia, typical rise and fall of cardiac enzymes, and ECG changes consistent with ischemia or MI, guided by the Minnesota code (22, 23). MIs were adjudicated as being definite, probable or possible based on published guidelines (22). For participants who were unable to be contacted for their bi-annual interviews, an interview was conducted with the next of kin listed on study forms and when deaths were reported, the date of death was confirmed through the social security death index, death certificates, or the national death index. Follow-up time was recorded as the number of days from the baseline in-home visit to a participant’s confirmed date of death, occurrence of a definite or probably MI, or their last REGARDS study telephone contact prior to December 31, 2008, whichever occurred first.
Statistical Analyses
Characteristics of REGARDS study participants with a history of CHD were calculated by depressive symptom status and compared via t-tests and chi-square tests, as appropriate. Using the Kaplan-Meier approach, the cumulative incidence was calculated for participants with and without depressive symptoms, separately, for (1) the primary outcome of definite/probable MI or death, (2) definite/probable MI (fatal or non-fatal), and (3) death from any cause. Next, the HR for the primary outcome associated with depressive symptoms was calculated in three nested models. The first model (Model 1) included adjustment for demographics (age, sex, race), education, income and body mass index (BMI). The next model (Model 2) included additional adjustment for co-morbid conditions (hypertension, diabetes mellitus, self-reported history of stroke, and self-reported history of MI) and cardiovascular medication use (aspirin, beta blockers, thienopyridines, ACE-inhibitors/angiotensin receptor blockers, statins, and antidepressants). The final model (Model 3) included additional adjustment for behavioral mechanisms including alcohol use, cigarette smoking, physical inactivity, and medication non-adherence. For physical activity, because those who reported “1 to 3 times per week” and “4 or more times per week” had similar hazard ratios as compared to the reference group of those who reported “none”, the final model considered physical activity as a dichotomous variable where participants who answered “none” were classified as physically inactive. As a sensitivity analysis, we also repeated the above analysis after excluding probable MI (fatal or non-fatal) from the outcome.
To quantify the amount of association between elevated depressive symptoms and CHD risk that is explained by behavioral risk factors, we used the bootstrap method recommended by Preacher and Hayes (25, 26). Specifically, using the model which included age, sex, race, education, income and body mass index, the percentage of the association between depressive symptoms and the primary outcome explained by co-morbidities, medication use, and behavioral mechanisms was calculated. To do so, we used a 1,000 iteration bootstrap; eachiteration included 4,676 observations chosen at random from the original data set with replacement. For each iteration, we modeled the primary outcome with and without adjustment for the explanatory variables (βafter and βbefore, respectively) and calculated the percent change in the beta coefficient between the two models ([βafter − βbefore]/βbefore). The median difference was used as the percent attenuation with the 2.5th and 97.5th percentiles as empirical 95% confidence intervals. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Of 4,676 REGARDS participants included for analysis, 638 (13.6%) had elevated depressive symptoms. Compared to 4,038 (86.4%) participants without elevated depressive symptoms, those with elevated depressive symptoms were younger, less likely to be male, more likely to be black, less likely to have graduated from high school, more likely to have annual income <$20,000, and have higher body mass index (Table 1). Participants with depressive symptoms were more likely to have hypertension, diabetes mellitus, and a self-reported history of stroke. Participants with depressive symptoms were also more likely to be on thienopyridines and anti-depressants and less likely to be on statins at baseline.
Table 1.
Baseline characteristics of participants with coronary heart disease, by elevated depressive symptoms (Center for Epidemiologic Studies Depression Scale score ≥ 4)
| No Elevated Depressive Symptoms (n=4038) | Elevated Depressive Symptoms (n=638) | P value | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age, Mean (SD), y | 68.9 (8.8) | 65.5 (9.5) | <0.001 |
|
| |||
| Male (%) | 63.1% | 43.3% | <0.001 |
|
| |||
| Black (%) | 32.5% | 49.2% | <0.001 |
|
| |||
| High school graduate, (%) | 85.3% | 71.3% | <0.001 |
|
| |||
| Income < $20,000, (%) | 20.8% | 47.0% | <0.001 |
|
| |||
| Body mass index, Mean (SD) | 29.2 (5.8) | 30.7 (6.8) | <0.001 |
|
| |||
| Comorbidities (%) | |||
|
| |||
| Hypertension | 71.3% | 80.1% | <0.001 |
|
| |||
| Myocardial infarction* | 71.4% | 73.5% | 0.260 |
|
| |||
| Stroke* | 11.2% | 17.1% | <0.001 |
|
| |||
| Diabetes Mellitus | 31.8% | 41.5% | <0.001 |
|
| |||
| Medication use (%) | |||
|
| |||
| Aspirin | 70.2% | 67.1% | 0.110 |
|
| |||
| Beta blocker | 47.1% | 50.9% | 0.073 |
|
| |||
| Thienopyridine | 15.1% | 18.8% | 0.015 |
|
| |||
| Ace-inhibitor/angiotensin receptor blocker | 50.5% | 52.4% | 0.384 |
|
| |||
| Statin | 59.6% | 51.3% | <0.001 |
|
| |||
| Antidepressants | 13.0% | 30.9% | <0.001 |
|
| |||
| Behavioral risk factors (%) | |||
|
| |||
| Alcohol use, | |||
| None | 63.7% | 73.7% | <0.001 |
| Light to moderate | 33.2% | 23.5% | |
| High | 3.1% | 2.8% | |
|
| |||
| Current smoking | 13.8% | 27.6% | <0.001 |
|
| |||
| Physical inactivity | |||
| None | 36.1% | 51.9% | <0.001 |
| 1+ times per week | 63.9% | 48.1% | |
|
| |||
| Morisky scale for medication non-adherence | |||
| 0 (best adherence) | 69.0% | 58.8% | <0.001 |
| 1 | 24.3% | 26.7% | |
| 2 | 4.4% | 8.7% | |
| 3 or 4 (worst adherence) | 2.3% | 5.8% | |
history of myocardial infarction and stroke as identified by self-report
For behavioral mechanisms, participants with elevated depressive symptoms were more likely to report no alcohol use and less likely to report either light-to-moderate or heavy alcohol use (p<0.001 for overall comparison). Those with elevated depressive symptoms were more likely to be current smokers (27.6% vs. 13.8%; p<0.001) and to report physical inactivity (51.9% vs 36.1%; p<0.001). Participants with elevated depressive symptoms reported worse levels of medication adherence (Morisky scale score of 3 or 4, 5.8% vs 2.3%; p<0.001).
Clinical Events, Elevated Depressive Symptoms and Behavioral Mechanisms
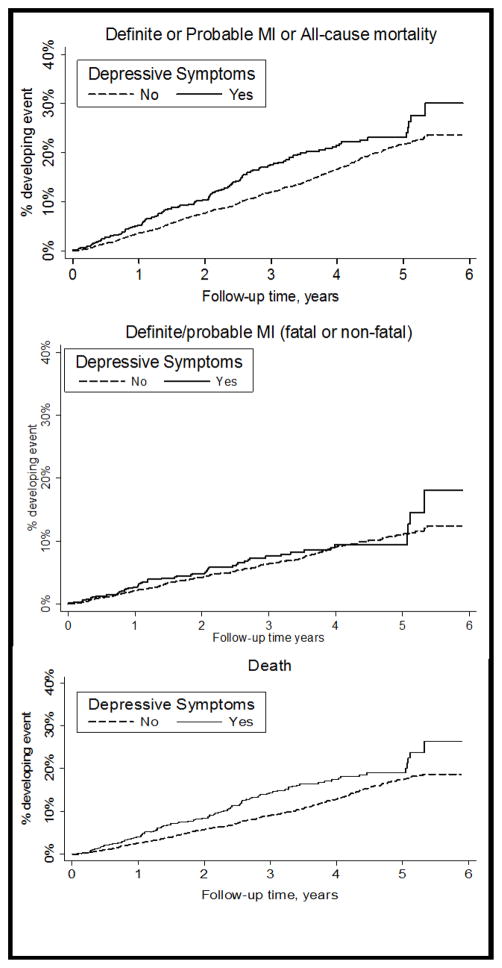
Over a median 3.8 years of follow up, 125 (19.6%) of 638 participants with elevated depressive symptoms experienced the primary outcome of definite/probable MI or death, compared with 657 (16.3%) of 4038 participants without (Figure 1, top panel). After adjusting for demographics, education, income, and BMI (Table 2, Model 1), the HR for MI or death associated with depressive symptoms was 1.41 (95% CI, 1.15–1.72). After further adjustment for medical comorbidities and medications used (Table 2, Model 2), the HR remained statistically significant but decreased to 1.25 (95% CI, 1.02–1.52). Finally, in the full model including all four behavioral mechanisms (Table 2, Model 2), the HR for MI or death associated with elevated depressive symptoms was further attenuated and was no longer statistically significant (HR 1.14, 95% CI 0.93–1.40). For individual behavioral mechanisms, both smoking (HR 2.06, 95% CI 1.71–2.47) and physical inactivity (HR 1.47, 95% CI 1.27–1.71) were associated with a higher risk of MI or death in the fully adjusted model (Table 2, Model 3), whereas levels of alcohol use and medication non-adherence were not.
Figure 1. Kaplan-Meier curves for adverse events.
Cumulative incidence of definite/probable MI or death; definite/probable MI (fatal or non-fatal); or death from any cause, for REGARDS participants with and without elevated depressive symptoms
Table 2.
Association between elevated depressive symptoms (Center for Epidemiologic Studies Depression Scale score of 4 versus <4) and definite/probable MI or death.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
|
| |||
| Elevated Depressive symptoms | 1.41 (1.15 – 1.72) | 1.25 (1.02 – 1.52) | 1.14 (0.93 – 1.40) |
|
| |||
| Age, per 10 years | 1.52 (1.39 – 1.65) | 1.53 (1.40 – 1.67) | 1.64 (1.50 – 1.80) |
|
| |||
| Male | 1.44 (1.22 – 1.69) | 1.46 (1.24 – 1.72) | 1.54 (1.31 – 1.82) |
|
| |||
| Black | 1.11 (0.94 – 1.30) | 1.00 (0.85 – 1.18) | 0.97 (0.82 – 1.14) |
|
| |||
| High school graduate | 0.87 (0.73 – 1.05) | 0.94 (0.78 – 1.13) | 0.97 (0.80 – 1.16) |
|
| |||
| Annual household Income < $20K | 1.53 (1.28 – 1.83) | 1.45 (1.21 – 1.73) | 1.30 (1.09 – 1.56) |
|
| |||
| Body mass index, per 5 kg/m2 | 0.94 (0.88 – 1.01) | 0.89 (0.83 – 0.95) | 0.91 (0.84 – 0.97) |
|
| |||
| Hypertension | * | 1.20 (0.99 – 1.43) | 1.21 (1.01 – 1.44) |
|
| |||
| Myocardial infarction† | * | 1.35 (1.14 – 1.60) | 1.31 (1.11 – 1.55) |
|
| |||
| Stroke† | * | 1.43 (1.19 – 1.72) | 1.34 (1.11 – 1.61) |
|
| |||
| Diabetes Mellitus | * | 1.58 (1.35 – 1.84) | 1.55 (1.33 – 1.80) |
|
| |||
| Aspirin | * | 0.90 (0.77 – 1.05) | 0.89 (0.76 – 1.05) |
|
| |||
| Beta blocker | * | 1.18 (1.02 – 1.37) | 1.18 (1.02 – 1.36) |
|
| |||
| Thienopyridine | * | 1.36 (1.13 – 1.64) | 1.35 (1.12 – 1.63) |
|
| |||
| ACE-inhibitor/angiotensin receptor blocker | * | 1.04 (0.89 – 1.21) | 1.05 (0.90 – 1.22) |
|
| |||
| Statin | * | 0.73 (0.63 – 0.85) | 0.75 (0.64 – 0.87) |
|
| |||
| Antidepressant use | 1.29 (1.07 – 1.56) | 1.20 (0.99 – 1.45) | |
|
| |||
| Alcohol use | * | * | |
| None | 1 (ref) | ||
| Light to moderate | 0.93 (0.79 – 1.09) | ||
| High | 0.69 (0.43 – 1.11) | ||
|
| |||
| Current Smoking | * | * | 2.06 (1.71 – 2.47) |
|
| |||
| Physical activity | * | * | |
| Any | 1 (ref) | ||
| None | 1.47 (1.27 – 1.71) | ||
|
| |||
| Morisky scale for | * | * | |
| medication adherence | |||
| 0 (best adherence) | 1 (ref) | ||
| 1 | 1.15 (0.97 – 1.35) | ||
| 2 | 1.23 (0.90 – 1.67) | ||
| 3 or 4 (worst adherence) | 0.80 (0.51 – 1.25) | ||
Models also included adjustment for geographic region of residence.
not included in regression model
history of myocardial infarction and stroke as identified by self-report
Only 14.0% (95% CI, −2.5% to −44.3%) of the excess risk for MI or death associated with elevated depressive symptoms was due to medical comorbidities including hypertension, history of MI, history of stroke, and diabetes mellitus (Table 3). Medication use did not significantly attenuate the HR for elevated depressive symptoms (−27.4%, 95% CI, −12.7% to 86.1%). In contrast, inclusion of all four behavioral mechanisms into the model attenuated more than one third of the risk for MI or death associated with elevated depressive symptoms (−36.9%, 95% CI −18.9% to −119.1%), with smoking and physical inactivity having the biggest impact (Table 3).
Table 3.
Attenuation in the association between elevated depressive symptoms (Center for Epidemiologic Studies Depression Scale of ≥ 4 versus < 4) and definite/probable MI or death, after addition of clinical and behavioral variables.*
| % Attenuation | |
|---|---|
| Hypertension/MI/stroke/diabetes mellitus | −14.0% (−2.5%, −44.3%) |
| Medications (aspirin, beta blockers, ACE-I/ARBs, thienopyridine, statins, antidepressants) | −27.4% (−12.7%, 86.1%) |
| Alcohol use | 0% (4.8%, 3.0%) |
| Current smoking | −17.6% (−6.5%, −56.0%) |
| Physical inactivity | −21.0% (−9.7%, −61.1%) |
| Medication non-adherence | −3.6% (2.5%, −16.1%) |
| All behavioral mechanisms | −36.9% (−18.9% − 119.1%) |
Baseline model is Model 1 in Table 3 and includes adjustment for age, race, sex, geographic region of residence, income, education, and body mass index.
Individual Outcomes and Sensitivity Analyses
As a secondary analysis, we further explored the role of behavioral mechanisms in explaining the association between elevated depressive symptoms and the individual components of the primary outcome (definite/probable MI and all-cause mortality, separately), using the same incremental proportional hazards models specified above. During the follow-up period, 101 (15.8%) of 638 participants with elevated depressive symptoms died, compared to 504 (12.5%) of 4,038 participants without elevated depressive symptoms; 50 (7.8%) of 638 participants with elevated depressive symptoms and 314 of (7.8%) 4038 participants without experienced a definite/probable MI (Figure 1, middle and bottom panels). The results of proportional hazards models for death were consistent with the findings from the primary analyses: the HR for elevated depressive symptoms was 1.48 (95% CI 1.18–1.85) in the model containing demographic covariates and BMI only, but was no longer statistically significant (HR 1.20, 95% CI 0.96–1.50) in the fully adjusted model containing behavioral mechanisms. For definite/probable MI, a similar trend was observed, though none of the HRs met statistical significance (Figure 2). In sensitivity analysis excluding probable MI events, the findings were similar.
Figure 2. Hazard ratios for elevated depressive symptoms in incremental proportional hazard models, with the outcomes of death from any cause; definite/probable MI (fatal or non-fatal); or the composite of both endpoints.
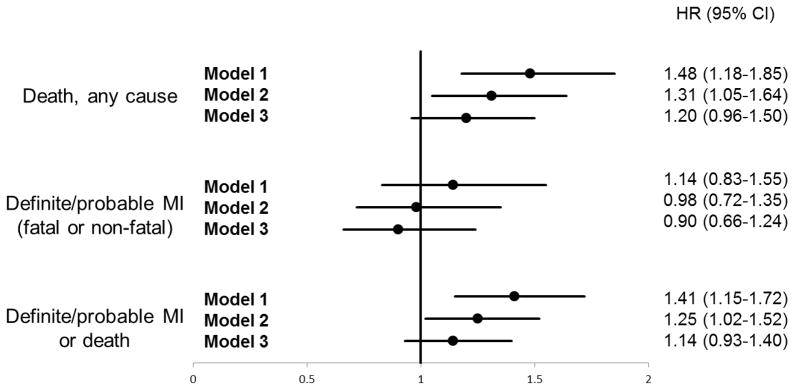
Model 1: adjusted for demographics, education, income and BMI.
Model 2: adjusted for all covariates in model 1, with additional adjustment for comorbidities and medication use.
Model 3: adjusted for all covariates in model 2, with additional adjustment for behavioral mechanisms (alcohol use, current smoking, physical inactivity, and medication non-adherence).
Discussion
In this analysis of a large cohort of participants from across the continental US enrolled in the REGARDS study, we found that self-reported behavioral mechanisms explained a substantial proportion of the excess risk of MI or death associated with elevated depressive symptoms in individuals with CHD. Of the four behavioral mechanisms that were assessed, smoking and physical inactivity were the most substantial contributors, each accounting for around one fifth of the relationship between elevated depressive symptoms and cardiac risk. We also confirmed that elevated depressive symptoms are associated with an increased risk of MI or death in individuals with CHD, a relationship that was mainly driven by the increased risk of death.
Our results are consistent with and extend the findings of prior studies in this field (2, 11). In an analysis of 5,888 participants enrolled in the Cardiovascular Health Study, Win et al. reported that physical inactivity accounted for 25% of the increased risk for cardiovascular mortality associated with depression (11). We observed an effect size of a similar magnitude for physical inactivity in our cohort, but also considered other behavioral mechanisms such as smoking, alcohol use, or medication non-adherence, which were not accounted for in the Cardiovascular Health Study. Our results are also similar to that from the Heart and Soul Study, which found that self-reported physical inactivity explained ~30% and smoking ~10% of the association between depressive symptoms and increased cardiovascular events, while the contribution of medication non-adherence was similarly of borderline significance as observed in our cohort (2). The prevalence of elevated depressive symptoms was slightly lower in our cohort (13.6%) compared to these prior studies, which may be due to our study sample being population based as well as our use of a different measure and threshold for defining elevated depressive symptoms. However, our larger sample size and the robust bootstrapping method we used go further to support the role of smoking and physical inactivity as key behavioral mechanisms that explain the association between elevated depressive symptoms and the risk of MI or death.
In our study, alcohol use and medication non-adherence did not attenuate the relationship between elevated depressive symptoms and MI or death. There are several potential explanations. The precise impact of alcohol consumption on cardiovascular risk remains under debate (16, 27), and it has been proposed that physical activity may be a confounder for epidemiological observations that link light-to-moderate alcohol use with decreased number of cardiac events (28). Supporting this, alcohol consumption was not associated with MI or death in the full model that included physical activity in our study, and thus would not be expected to explain the association between elevated depressive symptoms and cardiac outcomes. For medication non-adherence, although we used the 4-item Morisky scale rather than a single-item assessment as was done in the Heart and Soul Study, underreporting of medication non-adherence is a known challenge for assessments based on self-report (29). It is possible that more objective measurement tools would demonstrate a more important role for medication non-adherence. Consistent with this, a study of 168 patients with acute coronary syndrome has previously shown that aspirin non-adherence measured using electronic pill-bottles accounted for a substantial amount of the risk for increased death or major adverse cardiovascular events associated with elevated depressive symptoms (12).
In our analysis, we found that the association of elevated depressive symptoms to death was significant, whereas that between elevated depressive symptoms and MI was not. A similarly non-significant association between elevated depressive symptoms and MI was also seen in the Heart and Soul Study, while a relationship between elevated depressive symptoms and other cardiovascular events such as stroke and heart failure was more pronounced (2). A meta-analysis by van Melle et al. noted a stronger link between depression and death in post-MI patients than that between depression and cardiovascular events including recurrent MI (30). Therefore, there is a possibility that in patients with CHD, depression increases the risk for death through causes other than recurrent myocardial infarction, potentially via mechanisms for which smoking and physical inactivity are contributors (3, 31).
In addition to these mechanistic insights, our study also has important implications for future research and potential interventions for patients with CHD and elevated depressive symptoms. Given that prior studies of pharmacologic and counseling approaches of depression treatment demonstrated only limited efficacy in reducing cardiac risk in that patient population (8–10), our results suggest that there may be a role for intermediary interventions that explicitly target health behaviors. Despite clear guidelines, many individuals with CHD, and especially those with depressive symptoms, still do not meet current recommendations for smoking cessation and exercise (32). The recently published UPBEAT trial demonstrated that a 4-months exercise intervention was able to reduce depressive symptoms in patients with CHD, but the trial was not powered to find differences in cardiac outcomes (33). Given our findings that behavioral risk factors explain a substantial part of the association between elevated depressive symptoms and the risk of MI or death, future trials of interventions targeting these particular behaviors to improve cardiac outcomes in patients with CHD and elevated depressive symptoms are urgently needed.
There are several strengths to our study. The REGARDS study includes a large number of women and African-Americans and recruited adults from across the continental U.S., supporting the generalizability of our findings. Further, we used the statistical approach of bootstrapping to quantify the amount of attenuation of the relationship between elevated depressive symptoms and the risk of MI or death after behavioral mechanisms were considered, which allowed us greater confidence in the robustness of our results. Finally, although we used brief, self-reported items to measure behavioral mechanisms and depressive symptoms at a single point in time, their ease-of-use and positive associations with outcomes suggests a potential role for broader application in clinical and research settings.
There are several possible limitations to our study. As stated above, the measures of behavioral risk factors including smoking, physical inactivity, alcohol use, and medication non-adherence in our study were self-reported, and their exact prevalence may be underestimated. The REGARDS study did not assess other potential biological factors, such as endothelial dysfunction and heart rate variability, which have been proposed to explain the association between depressive symptoms and cardiac risk (1, 34). Prior studies, however, suggest the role played by these biological mechanisms may be modest (2, 7, 35, 36). It is also possible that there is a survivor bias, as those individuals with elevated depressive symptoms and who are at the highest risk for death would not have survived to be enrolled in our study. However, this would tend to bias our results towards the null, and thus would only underestimate the associations that we have identified between elevated depressive symptoms, clinical events, and behavioral mechanisms. Finally, causality cannot be ascertained in our observational study. For instance, it is possible that physical inactivity and depressive symptoms are both markers of disease severity, rather than causal determinants of cardiac risk. Future studies, possibly using a cross-lagged path design (37), may help to clarify the complex relationships between depressive symptoms, cardiac risk, and behavioral mechanisms such as physical inactivity and smoking.
In summary, we found that in a large cohort of participants from across the continental U.S. enrolled in the REGARDS study, elevated depressive symptoms are associated with increased risk of MI or death in individuals with a history of CHD. Furthermore, a substantial proportion of the relationship between elevated depressive symptoms and the risk for MI or death was explained by behavioral mechanisms, the most important of which were smoking and physical inactivity. These findings add to our understanding of the mechanisms by which elevated depressive symptoms may convey increased risk for adverse cardiovascular outcomes for individuals with CHD, and suggest that behavioral interventions to target smoking cessation and increase physical activity could reduce the excessive cardiac risk associated with depression in CHD patients.
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.2 The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Dr. Ye is supported by an ACC/Merck Research Fellowship award, and by National Institutional of Health Grant T32HL007854-16. Dr. Safford receives salary support from Amgen, Inc and Pfizer, Inc for research studies.
Abbreviation list
- CHD
coronary heart disease
- BMI
body mass index
- HR
hazard ratio
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lichtman JH, Bigger JT, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, et al. Depression and Coronary Heart Dis ease. Circulation. 2008;118(17):1768–75. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 2.Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–88. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 Suppl 2):S20–7. doi: 10.1016/j.amjmed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Carney RM, Rich MW, Tevelde A, Saini J, Clark K, Jaffe AS. Major depressive disorder in coronary artery disease. Am J Cardiol. 1987;60(16):1273–5. doi: 10.1016/0002-9149(87)90607-2. [DOI] [PubMed] [Google Scholar]
- 5.Zellweger MJ, Osterwalder RH, Langewitz W, Pfisterer ME. Coronary artery disease and depression. Eur Heart J. 2004;25(1):3–9. doi: 10.1016/j.ehj.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Lesperance F, Frasure-Smith N, Talajic M, Bourassa MG. Five-year risk of cardiac mortality in relation to initial severity and one-year changes in depression symptoms after myocardial infarction. Circulation. 2002;105(9):1049–53. doi: 10.1161/hc0902.104707. [DOI] [PubMed] [Google Scholar]
- 7.Ye S, Denton E-g, Wasson L, Davidson K. Epidemiology and Management of Depression Following Coronary Heart Disease Diagnosis in Women. Current Cardiovascular Risk Reports. :1–9. doi: 10.1007/s12170-012-0234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA. 2003;289(23):3106–16. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- 9.Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev. 2011;9:CD008012. doi: 10.1002/14651858.CD008012.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, et al. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med. 2010;170(7):600–8. doi: 10.1001/archinternmed.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Win S, Parakh K, Eze-Nliam CM, Gottdiener JS, Kop WJ, Ziegelstein RC. Depressive symptoms, physical inactivity and risk of cardiovascular mortality in older adults: the Cardiovascular Health Study. Heart. 2011;97(6):500–5. doi: 10.1136/hrt.2010.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieckmann N, Burg MM, Kronish IM, Chaplin WF, Schwartz JE, Davidson KW. Aspirin Adherence, Depression and One-Year Prognosis after Acute Coronary Syndrome. Psychother Psychosom. 2011;80(5):316–8. doi: 10.1159/000323168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Woolson RF, Egan BM, Nicholas JS, Adams RJ, Howard G, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4(1):32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services, National Institutes of Health, National Institue on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician’s guide. Bethesda, Md: National Institue on Alcohol Abuse and Alcoholism; 2007. [Accessed February 27, 2009]. NIH publication no. 07–3769. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. [Google Scholar]
- 16.O’Keefe JH, Bybee KA, Lavie CJ. Alcohol and Cardiovascular Health: The Razor-Sharp Double-Edged Sword. J Am Coll Cardiol. 2007;50(11):1009–14. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 17.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Shalansky SJ, Levy AR, Ignaszewski AP. Self-reported Morisky score for identifying nonadherence with cardiovascular medications. Ann Pharmacother. 2004;38(9):1363–8. doi: 10.1345/aph.1E071. [DOI] [PubMed] [Google Scholar]
- 19.Melchior LA, Huba GJ, Brown VB, Reback CJ. A Short Depression Index for Women. Educational and Psychological Measurement. 1993;53(4):1117–25. [Google Scholar]
- 20.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–27. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 23.Prineas RJ, Crow RS, Zhang Z-M. Minnesota Code Manual of Electrocardiographic Findings. 2. London: Springer-Verlag; 2010. [Google Scholar]
- 24.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158(6):848–56. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 25.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 26.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Communication Monographs. 2009;76(4):408–20. [Google Scholar]
- 27.Mukamal KJ, Chen CM, Rao SR, Breslow RA. Alcohol Consumption and Cardiovascular Mortality Among U.S. Adults, 1987 to 2002. J Am Coll Cardiol. 2010;55(13):1328–35. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrett DH, Anda RF, Croft JB, Serdula MK, Lane MJ. The association between alcohol use and health behaviors related to the risk of cardiovascular disease: the South Carolina Cardiovascular Disease Prevention Project. J Stud Alcohol. 1995;56(1):9–15. doi: 10.15288/jsa.1995.56.9. [DOI] [PubMed] [Google Scholar]
- 29.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 30.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 31.Whang W, Kubzansky LD, Kawachi I, Rexrode KM, Kroenke CH, Glynn RJ, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol. 2009;53(11):950–8. doi: 10.1016/j.jacc.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 33.Blumenthal JA, Sherwood A, Babyak MA, Watkins LL, Smith PJ, Hoffman BM, et al. Exercise and Pharmacological Treatment of Depressive Symptoms in Patients With Coronary Heart Disease: Results From the UPBEAT (Understanding the Prognostic Benefits of Exercise and Antidepressant Therapy) Study. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pozuelo L, Tesar G, Zhang J, Penn M, Franco K, Jiang W. Depression and heart disease: What do we know, and where are we headed? Cleveland Clinic Journal of Medicine. 2009;76(1):59–70. doi: 10.3949/ccjm.75a.08011. [DOI] [PubMed] [Google Scholar]
- 35.Cooper DC, Tomfohr LM, Milic MS, Natarajan L, Bardwell WA, Ziegler MG, et al. Depressed Mood and Flow-Mediated Dilation: A Systematic Review and Meta-Analysis. Psychosomatic Medicine. 2011;73(5):360–9. doi: 10.1097/PSY.0b013e31821db79a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, et al. Relation of inflammation to depression and incident coronary heart disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study) Am J Cardiol. 2009;103(6):755–61. doi: 10.1016/j.amjcard.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, et al. Course of Depressive Symptoms and Medication Adherence After Acute Coronary Syndromes: An Electronic Medication Monitoring Study. J Am Coll Cardiol. 2006;48(11):2218–22. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]