Abstract
To be successful pathogens, bacteria must often restrict the expression of virulence genes to host environments. This requires a physical or chemical marker of the host environment as well as a cognate bacterial system for sensing the presence of a host to appropriately time the activation of virulence. However, there have been remarkably few such signal–sensor pairs identified, and the molecular mechanisms for host-sensing are virtually unknown. By directly applying a reporter strain of Vibrio cholerae, the causative agent of cholera, to a thin layer chromatography (TLC) plate containing mouse intestinal extracts, we found two host signals that activate virulence gene transcription. One of these was revealed to be the bile salt taurocholate. We then show that a set of bile salts cause dimerization of the transmembrane transcription factor TcpP by inducing intermolecular disulfide bonds between cysteine (C)-207 residues in its periplasmic domain. Various genetic and biochemical analyses led us to propose a model in which the other cysteine in the periplasmic domain, C218, forms an inhibitory intramolecular disulfide bond with C207 that must be isomerized to form the active C207–C207 intermolecular bond. We then found bile salt–dependent effects of these cysteine mutations on survival in vivo, correlating to our in vitro model. Our results are a demonstration of a mechanism for direct activation of the V. cholerae virulence cascade by a host signal molecule. They further provide a paradigm for recognition of the host environment in pathogenic bacteria through periplasmic cysteine oxidation.
The human pathogen Vibrio cholerae is the causative agent of the diarrheal disease cholera. The Vibrio life cycle begins with a free-swimming phase in aquatic environments. Human infection normally starts with the ingestion of food or water contaminated with V. cholerae. As it colonizes small intestines of a host and only when it colonizes, V. cholerae produces an array of virulence factors, including cholera toxin (CT), which causes the diarrhea characteristic of cholera and toxin-coregulated pili (TCP), type IV pili required for intestinal colonization both in animal models and in human volunteers (1, 2).
Bacterial pathogens have evolved highly sophisticated signal transduction systems to coordinately control the expression of virulence determinants to better infect their hosts. Extensive in vitro studies have revealed details of V. cholerae virulence gene regulation (3): AphA and AphB proteins activate transcription of the transmembrane transcription factor TcpP, which in turn activates toxT transcription together with ToxR, which then completes the cascade by activating toxin and TCP production (Fig. 1A). However, all of the experiments to date used artificial in vitro conditions to induce virulence factor production, leaving unanswered which microenvironmental signals in the intestines activate the V. cholerae virulence cascade. It has been reported that certain environmental conditions such as temperature, oxygen concentration, osmolarity, pH, and iron availability (4–6) influence expression of virulence genes in vitro. In addition, bicarbonate and fatty acids, which are abundant in host small intestines, modulate virulence gene expression through ToxT (7–9). Intriguingly, different in vitro conditions are required to activate virulence genes in different biotypes of V. cholerae. In the El Tor biotype responsible for the current cholera pandemic, AKI medium is used (10), in which peptone is substituted for the tryptone found in LB and oxygen concentrations are lowered by incubating cultures without shaking them. For the classical biotype, LB medium at pH 6.5 and 30 °C are required to induce virulence genes. In either case, the mechanism of activation of virulence is not understood, and these conditions obviously differ from the conditions in the small intestine. The relevance of the in vitro signals found in these studies to V. cholerae virulence gene expression within the host is not clear, nor is it clear how various environmental signals are integrated to produce specific gene expression patterns. In this study, we used an ex vivo intestinal model to assess directly the substances in the intestines that lead to virulence activation. We found that at least two host-derived molecules present in mammalian small intestines efficiently induce key V. cholerae virulence regulatory pathways. We identified one of these molecules as the bile salt taurocholate. We further show that bile salts enhance the activity of the transmembrane transcription activator TcpP by inducing intermolecular disulfide bonds between cysteine (C)-207 residues in its periplasmic domain.
Fig. 1.
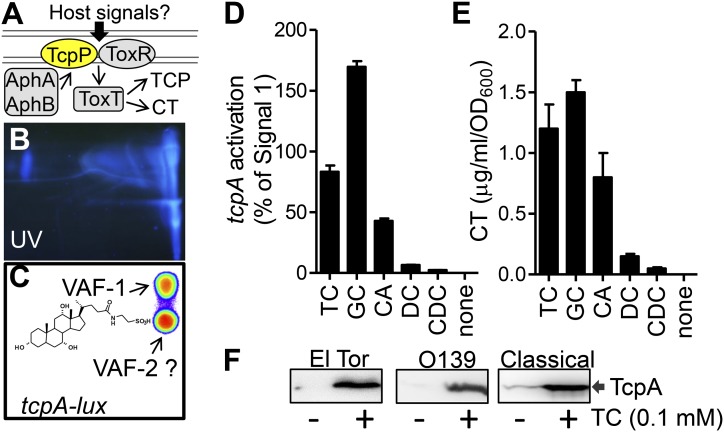
Bile salts activate virulence genes. (A) The V. cholerae virulence regulatory cascade. See text for details. (B and C) Mouse intestinal extracts were chromatographed on cellulose TLC plates and detected by UV (B) or overlaid with LB agar containing V. cholerae PtcpA-lux reporter cells and then incubated anaerobically at 37 °C for 4 h. After incubation, the TLC plate was photographed in the dark using a LAS4010 ImageQuant and analyzed using Living Image 3.2 (C). (Inset) The chemical structure of VAI-1(TC). (D) A total of 100 µM synthetic bile salts (Sigma) was incubated with V. cholerae (PtcpA-lux) anaerobically at 37 °C for 4 h. The luminescence of cells was read using a Bio-Tek Synergy HT spectrophotometer and normalized for growth against optical density at 600 nm and compared with equal amount by mass of purified VAF-1. (E) The corresponding cell-free culture fluids from (D) were assayed in GM1 ganglioside ELISAs for CT (32). (F) TcpA Western blot of El Tor O1 (C6706), El Tor O139 (MO10), and classical O395 grown in LB (El Tor) or LB pH 8.5 [classical, noninducing condition (34)] in the absence or in the presence of 100 µM TC for 5 h. Data are mean and SD of three independent experiments. CA, cholic acid; CDC, chenodeoxycholate; DC, deoxycholate; GC, glycocholate.
Results
Identification of Taurocholate as One of the Host Virulence–Activating Factors.
To determine host factors that V. cholerae identifies as a signal that it is both within a host and is an appropriate time to activate virulence, we developed a tissue model of infection. We incubated V. cholerae containing a PtcpA-lux reporter, which measures the expression of the major virulence gene tcpA (11) by emitting light, with dissected fragments of adult mouse small intestines and placed them in an anaerobic chamber. We found that only under anoxic conditions and in the presence of intestinal fragments did V. cholerae cells activate tcpA transcription. This activation was dependent on all previously characterized major virulence regulators (Fig. S1). These results led us to hypothesize that there exist host virulence-activating factors (VAFs) at the intestinal surface. Further examination found that the VAFs were found in small intestines of infant and adult conventional and germ-free mice, and that they were polar small molecules (Table S1). VAFs were then differentially extracted from adult mouse intestines (Methods) and the concentrated extracts were applied to cellulose TLC plates (Fig. 1B). We overlaid the TLC plate with LB agar containing PtcpA-lux reporter cells and incubated anaerobically at 37 °C for 4 h. Two luminescent spots (induction of tcpA) were observed (Fig. 1C). The top VAF spot was further purified and characterized by high-resolution mass spectrometry, proton NMR and 2D NMR, coupled with our PtcpA-lux–based bioassays (Fig. S2 A–D). We found that this compound was sodium taurocholate (Fig. 1C), one of several bile salts that are synthesized in the liver and secreted into the small intestines as a normal component of digestion (12). V. cholerae is likely to encounter bile salts early upon its entry into the small intestines, its infectious niche, and could thus use bile salts as a molecular signal of this new environment. To confirm that VAF-1 is a bile salt, we tested the virulence-inducing activity of synthetic taurocholate (TC) (Sigma). We found that TC strongly activated tcpA expression at 100 µM in three different pandemic strains of V. cholerae (Fig. 1 D and F). The activity is similar to that of VAF-1 isolated from mouse small intestines. We also tested the activity of other bile salts and found that glycocholate and sodium cholate are active, but that deoxycholate and chenodeoxycholate are not (Fig. 1D, Fig. S2E). Of note, the concentration of bile salts used here is physiologic because the human small intestine has a high bile salt concentration averaging 10 mM during digestion (13). These results suggest that V. cholerae recognizes a set of bile salts as signals to activate virulence genes.
Bile Salts Affect TcpP Activity Through Its Periplasmic Domain.
To elucidate where bile salts act along the virulence regulatory pathway, transcription levels of various components of the virulence cascade were measured in the presence or absence of taurocholate. We found that taurocholate could induce toxT expression, but not the expression of upstream regulators (Fig. 2A). The expression of ToxT is known to be regulated by TcpP and ToxR (3). We measured the expression of a PtoxT-lux reporter in Escherichia coli, which lacks endogenous homologs of either ToxR or TcpP, whereas either V. cholerae ToxR or TcpP was coexpressed from a second plasmid. We found that although taurocholate did not affect ctx promoter activity in the presence of ToxR (Fig. 2B), bile salts strongly induced toxT expression through TcpP (Fig. 2C). TcpP is known to be rapidly degraded through proteolysis by the protease YaeL in the absence of a second protective protein, TcpH (14). To test whether bile salts affect TcpP stability through TcpH, we examined the bile salt effects on TcpP stability (Fig. S3A) and TcpA production in V. cholerae in the presence and in the absence of TcpH and/or YaeL (Fig. S3B). We found that bile salts had no effect on TcpP stability, and that TcpA protein production was dependent on the presence of TcpH only if YaeL was also present, as has been previously reported (14).
Fig. 2.
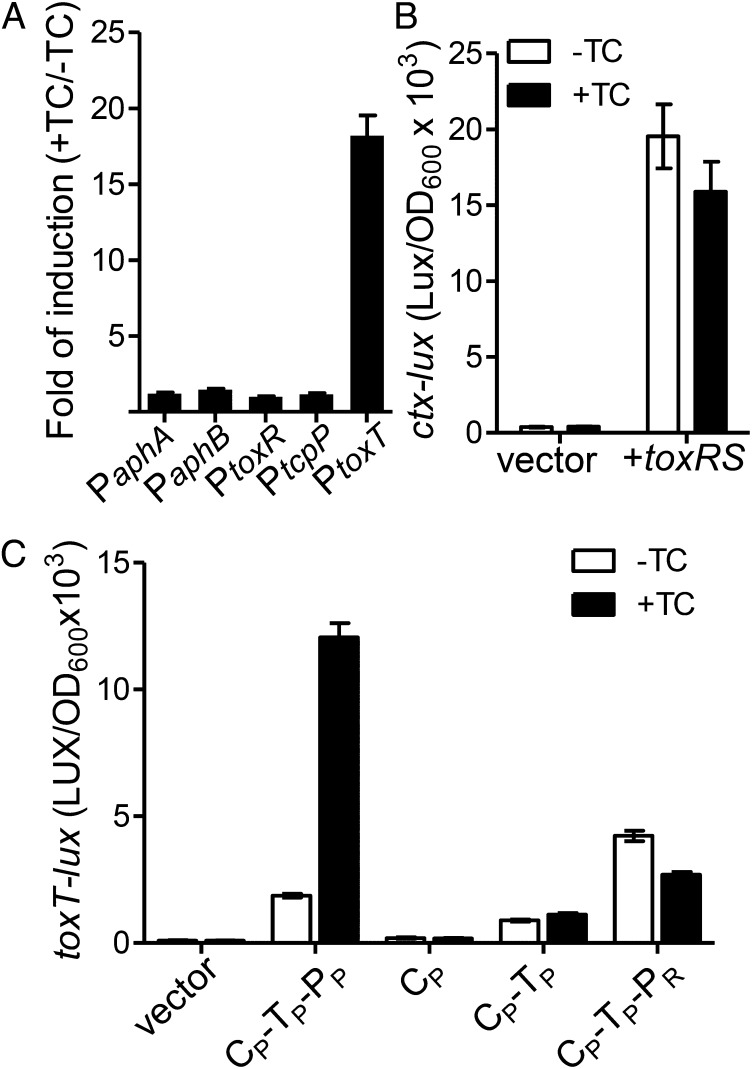
Bile salts regulate TcpP activity through its periplasmic domain. (A) The effect of bile salts on transcription of virulence regulators. V. cholerae containing promoter-lux transcriptional fusion plasmids were grown in LB in the absence or in the presence of 100 µM TC anaerobically at 37 °C until OD600 = 0.2. Luminescence was measured and reported as fold induction (+TC/−TC). (B) E. coli containing Pctx-lux reporter and either vector control or PBAD-toxRS plasmids were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM until OD600 ≈ 0.2. Luminescence was then measured and reported as light units/OD600. (C) E. coli containing PtoxT-lux reporter and either PBAD vector control or indicated PBAD-protein chimeras were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM TC until OD600 = 0.2. Luminescence was then measured and reported as light units/OD600. C, cytoplasmic domain; P, periplasmic domain; P, TcpP; R, ToxR; T, transmembrane domain. Data are mean and SD of three independent experiments.
Similar to ToxR, TcpP is an integral inner membrane protein (15). To examine which TcpP domains sense bile salts, we performed truncation and chimeric protein analysis (Fig. 2C). Deletion of both periplasmic and transmembrane domains, leaving only the cytosolic fragment of TcpP (CP), abolished TcpP activity. However, TcpP with periplasmic domain deletion (CP-TP) demonstrated a basal level of activation of toxT that no longer responded to TC. Interestingly, a chimeric protein containing TcpP cytoplasmic and transmembrane domains fused to the ToxR periplasmic domain (CP-TP-PR) displayed similar activity to CP-TP. Western blot analysis showed that both CP-TP and CP-TP-PR were correctly membrane-associated (Fig. S4). These results suggest that the periplasmic domain of TcpP is critical for sensing bile salts.
Bile Salts Induce TcpP Dimerization Through C207 Disulfide Bond Formation.
It has been suggested that TcpP binds to the toxT promoter as a dimer (16). To test whether bile salts enhance TcpP activity by stimulating dimerization, we used a bacterial adenylate cyclase two-hybrid system (17) in E. coli to examine the bile salt effect on TcpP homodimerization. We found that the full-length TcpP displayed enhanced interaction in the presence of TC (Fig. 3A), and that the transmembrane and periplasmic domains were sufficient for bile salt-induced dimerization. Bile salts failed to stimulate interaction of a TcpP chimeric mutant in which the periplasmic domain was replaced with the ToxR periplasmic domain, again suggesting the importance of the TcpP periplasmic domain in responding to bile salts.
Fig. 3.
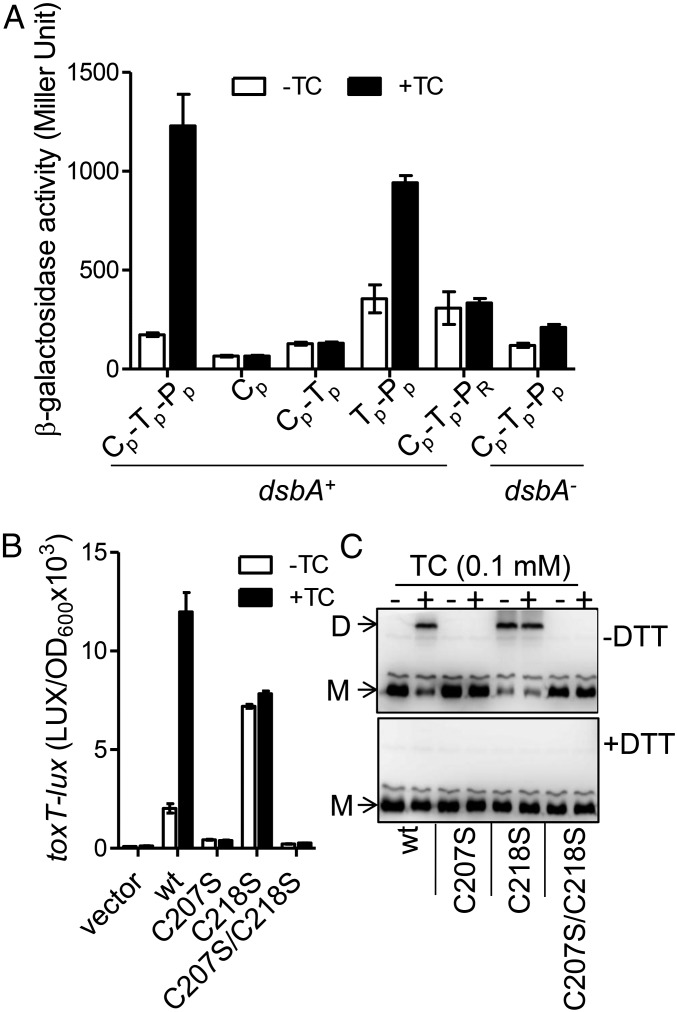
Bile salts mediate TcpP dimerization through cysteine-207. (A) Bile salts promote TcpP–TcpP interaction. Full-length TcpP and truncated/chimeric TcpP were fused with the T25 and T18 domains of adenylate cyclase (CyaA) from Bordetella pertussis, respectively, and the T25, T18 fusion pairs were introduced into E. coli cyaA mutants (17) or cyaA/dsbA double mutants (35). Cultures were grown at 30 °C for 8 h and β-galactosidase activity was measured and reported as Miller Units (36). The annotation is the same as in Fig. 2C. (B) V. cholerae ΔtcpPH (pBAD-tcpPH) containing PtoxT-lux reporter and WT or cysteine mutant tcpP under the control of the PBAD promoter on plasmids were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM TC until OD600 ≈ 0.2. Luminescence was then measured and reported as light units/OD600. Data are mean and SD of three independent experiments. (C) V. cholerae tcpP deletion mutants containing PBAD-controlled plasmids harboring TcpP and its cysteine mutant derivatives fused with C-terminal FLAG tags were grown in LB containing 0.01% arabinose in the absence or in the presence of 100 µM TC. Then, 1-mg cell lysates were applied to a nonreducing SDS/PAGE (without DTT, Upper) or to a reducing SDS/PAGE (with 10 mM DTT in the loading buffer, Lower), and subjected to the Western blot using anti-FLAG antibody. D, dimer; M, monomer.
A possible mechanism of dimerization of transmembrane proteins is the formation of disulfide bonds between periplasmic domains, because the periplasm is a more oxidizing environment than the cytoplasm. To test if disulfide bonds might form in the TcpP periplasmic domain to cause dimerization, we first tested the dimerization of TcpP in E. coli lacking dsbA, a strain that is deficient in the formation of disulfide bonds in periplasmic proteins. We found that bile salts failed to stimulate TcpP interaction (Fig. 3A, last two columns) in this strain, indicating the likely involvement of disulfide bond formation in TcpP activity. We confirmed that TcpP disulfide bond formation is affected by V. cholerae DsbA using a differential thiol trapping method (18) (Fig. S5). As expected, we found that oxidized cysteine residues were present in TcpP in dsbA+ cells but not in dsbA− cells.
Because disulfide bonds form between cysteine residues, we performed mutagenesis of the two periplasmic cysteine residues of TcpP—C207 and C218—to serine, which replaces the sulfur atom of cysteine with a far less reactive oxygen atom while minimizing steric effects on protein folding. Interestingly, a C207S mutation abolished TcpP bile salt activation (Fig. 3B), suggesting involvement of at least this residue in the activity of the protein. Likewise, the TcpPC207S/C218S double mutant did not activate toxT transcription either. Remarkably, the C218S mutation rendered the activation of toxT constitutive and bile salt–independent (Fig. 3B), but not in the dsbA mutant (Fig. S6). This suggested to us that C218 might be a regulatory residue that controls the response to bile salts through a mechanism involving disulfide bonds. We tested this hypothesis by examining the multimerization of mutant TcpP by nonreducing SDS/PAGE. We found, as expected, that bile salts stimulate wild type (WT) TcpP to form dimers (Fig. 3C), whereas few dimers were detected in strains containing TcpPC207S and TcpPC207S/C218S, whether or not bile salts were present. However, TcpP dimer formation was found to be constitutive in TcpPC218S mutants and independent of bile salts, again suggesting that the C218 residue regulates the bile salt–dependent dimerization of the protein. Importantly, all dimers were abolished in the presence of dithiothreitol (DTT) (Fig. 3C), suggesting that the dimers resulted from reversible disulfide bond formation.
These results led us to propose a model (Fig. 4A) in which TcpP forms inhibitory intramolecular disulfide bonds between C207 and C218 in the absence of stimuli. In the host intestines, bile salts stimulate TcpP intermolecular C207–C207 disulfide bond formation, which results in activation of virulence gene expression.
Fig. 4.
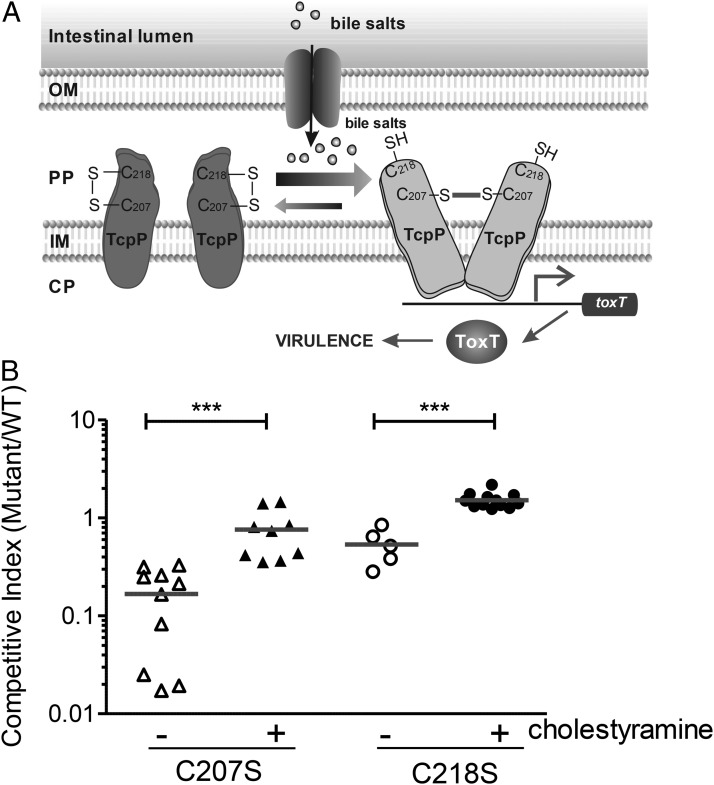
Sensing bile salts in vivo. (A) A working model: an intermolecular disulfide bond at C207 is critical for TcpP activity. In the absence of bile salts, an intramolecular disulfide bond forms between C207 and C218. Upon encountering bile salts, isomerization takes place to cause an intermolecular disulfide bond between two subunits of TcpP at C207. (B) In vivo competition assay using an infant mouse model. Six-day-old CD-1 infant mice were inoculated with the mixture of TcpP cysteine mutant and WT at 1:1 ratio with or without cholestyramine. After a 6-h period of colonization, intestinal homogenates were collected, and the ratio of mutant-to-WT bacteria was determined and normalized against input ratios. ***P < 0.001 (Student t test).
Bile Salt Sensing Is Important for V. cholerae Pathogenesis in Vivo.
To assess the importance of bile salt effects on V. cholerae pathogenesis, we examined the intestinal colonization ability of tcpP cysteine mutants (Fig. 4B). The C207S mutant exhibited a competitive disadvantage in colonizing the small intestines of infant mice, suggesting that the inability to sense bile salts through TcpP is disadvantageous to survival in the intestines. The C218S mutant displayed a slight defect in colonization, possibly because of the overall lower virulence induction compared with that of WT (Fig. 3B). No growth defect was detected when C207S and C218 mutants were grown in vitro (Fig. S7A). More importantly, coadministration of the bile salt–sequestering resin cholestyramine abolished the competitive disadvantage for both mutant strains, implying an important role for bile salt–dependent virulence gene activation in vivo. This suggests that inducible virulence expression is optimal, but that in the absence of a signal, constitutive expression of virulence confers an advantage. The colonization defect of C207S mutants or WT in the presence of cholestyramine was not as dramatic as that of the tcpP null mutant, which displayed ∼1,000-fold colonization disadvantages over WT (Fig. S7B). Because one additional virulence-activating factor was detected from mouse intestines (Fig. 1A), it is likely that V. cholerae responds to multiple host signals to activate virulence genes.
Discussion
Bile salts are the components of bile, a mixture including many distinct molecules. Bile salts have previously been implicated in virulence in Vibrio parahaemolyticus (19), but the mechanism of induction is unclear. Interestingly, it has been shown that in a classical strain of V. cholerae (20), bile salts also may affect ToxR activity to enhance ctx production independent of ToxT. Although the induction in this case was rather minimal compared with the maximal ctx expression, it does suggest that bile salts may play additional roles in regulating virulence. In addition, it is possible that other host signal molecules, such as bicarbonate (7) and the VAF-2 in the intestinal extract identified in this study (Fig. 1B), participate in activating virulence gene expression. Further studies are required to fully understand how V. cholerae integrates these host signals. Intriguingly, fatty acids present in bile negatively regulate virulence gene expression (9), indicative of fine-tuned negative feedback of virulence gene regulation, further illuminating the complexity of the host–pathogen interaction.
Few examples exist of host-derived signal molecules that activate a bacterial pathogen’s virulence gene expression. In enterohemorrhagic E. coli, host catecholamines activate production of virulence factors through a two-component system (21). In the plant pathogen Agrobacterium tumefaciens, virulence genes are induced by plant phenolic compounds (22). Although many studies have already shown a crucial role for periplasmic disulfide bond formation in the virulence of multiple bacterial pathogens (23), the disulfide bonds were generally important for accessory functions, such as assembly of extracellular adhesins, rather than for directly sensing the host environment. Here we found that alterations in disulfide bonds in V. cholerae TcpP form in response to a host factor to dimerize and directly stimulate virulence gene expression. In this way, V. cholerae monitors normal host digestive processes to up-regulate its virulence cascade. We speculate that other enteric bacterial pathogens, the virulence-activating signals for which are largely unknown, may also depend on disulfide bond formation as a signal for virulence gene up-regulation in response to host signal molecules.
Materials and Methods
Strains, Plasmids, and Culture Conditions.
All V. cholerae strains used in this study were derived from E1 Tor C6706 (24) unless otherwise noted, and were propagated in LB media containing appropriate antibiotics at 37 °C. Cultures were grown microaerophilically (stationary growth) or anaerobically (vinyl anaerobic chambers, Coy Laboratory Products) unless otherwise noted.
Transcriptional lux reporters of promoter regions of aphA, aphB, tcpP, toxR, toxT, and tcpA in the pBBR-lux vector (25) have been described previously (6). Plasmids for overexpressing virulence regulators were either described previously (26) or constructed by cloning the PCR-amplified coding regions into pBAD24 (27) or pACYC117 (28). In-frame deletion strains used in this study were either described in previous publications (6) or constructed as described previously (29). TcpP truncation and chimeric mutants as well as cysteine mutations were constructed by overlap extension PCR (30). Chromosomal replacement of WT tcpP with cysteine mutants was constructed by cloning PtcpP-tcpP (C207S, C218S, or C207S/C218S) into a suicide vector containing a V. cholerae intergenic fragment between VCA0104 and VCA0105. The resulting plasmids were introduced into V. cholerae a tcpP partial deletion mutant (TcpPΔ67–89), in which tcpH is still intact, by selecting for double-crossover recombination events.
VAF Identification Using an ex Vivo Model and Chemical Analysis.
All animal studies were carried out in accordance with the animal protocols that were approved by the Institutional Animal Care and Use Committee of University of Pennsylvania. Fragments of small intestines from 5-wk-old CD-1 mice were cut open and mounted in dishes covered with LB medium. V. cholerae cells containing a PtcpA-lux reporter were then loaded on the top of opened intestinal tissues and dishes were placed at 37 °C in an anaerobic chamber (O2−) or ambient incubator (O2+). After 4 h, dishes were photographed in the dark using a LAS4010 ImageQuant (GE Healthcare Life Sciences) and analyzed using Living Imageо 3.2 software (PerkinElmer).
To purify VAFs, small intestines were removed from the mice and flushed with double-distilled (dd) H2O (50 mL/mouse). The intestinal flush was then autoclaved and extracted with phenol-chloroform and subsequently ethyl acetate. The aqueous phase was then precipitated with 70% (vol/vol) ethanol. The supernatant was dried using a rotary evaporator and resuspended with ddH2O, then dialyzed against ddH2O using a 1-kDa-cutoff membrane. The contents outside of the dialysis bag were then dried again and resuspended in ddH2O. The samples were then applied on cellulose TLC plates (Sorbent Technologies) and 1-butanol:2-propanol:H2O (1:4:4) was used for the first- and chloroform:methanol (9:1) as the second-dimension solvent. After chromatography, the plates were dried and photographed under UV light. The TLC plates were overlaid with LB agar (0.75%) containing V. cholerae (pPtcpA-lux) reporter cells (∼5 × 107/mL) and appropriate antibiotics and incubated at 37 °C anaerobically for 4 h. Photographs were taken in the dark using the ImageQuant LAS4000. The corresponding active spots were recovered from TLC plates, and VAF-1 was further purified by applying it to an anion-exchange Q-Sepharose column and eluted with gradients of NaCl (0–200 mM). The fractions containing VAF activity were dried and dissolved in DMSO and purified on a C8 (2) Luna Phenomenex semiprep column (5 µm, 250 × 10 mm) with UV detection (254 nm) using a 3 min‒1 CH3CN/water gradient (30–100% CH3CN, 0.2% TFA) to give pure VAF-1. 1H and 2D NMR data (Bruker, 600 MHz, DMSO-d6) were consistent with that reported for sodium taurocholate (31) [high-resolution MS (electrospray ionization-time of flight) m/z (MH+) calculated for C26H46NO7S 516.2995, found 516.2987].
Measurement of Virulence Gene Expression and Virulence Factor Production.
Overnight cultures of E. coli or V. cholerae strains containing virulence promoter luxCDABE transcriptional fusions were subcultured at a dilution of 1:100 in LB with or without bile salts indicated and grown anaerobically until OD600 ∼0.2. Luminescence was measured using a Bio-Tek Synergy HT spectrophotometer and normalized for growth against OD600. Luminescence expression is reported as light units/OD600. TCP production was measured by Western blot analysis using an anti-TcpA polyclonal antibody (26) and CT production was measured by a GM1 ganglioside ELISA CT assay (32).
Bacterial Two-Hybrid System to Determine Bile Salt Effects on TcpP-TcpP Interaction.
Full-length or truncated tcpP fragments, as well as tcpP-toxR chimerics were PCR amplified and cloned into the inducible expression vectors pUT18C and pKT25, respectively (17). To analyze the dimerization of each construct, β-galactosidase measurements are performed as described previously (17). Briefly, overnight cultures of E. coli BTH101 or BTH101ΔdsbA mutants containing both pUT-18C-fusion and pKT25-fusion constructs were subcultured at a dilution of 1:100 in LB medium containing 0.5 mM isopropyl β-D-1-thiogalactopyranoside with or without 100 μM taurocholate and incubated without shaking at 30 °C for 8 h. Cultures were then assayed for β-galactosidase activity.
Nonreducing SDS/PAGE to Examine Bile Salt Effects on TcpP Disulfide Bond Formation.
PBAD promoter-controlled FLAG-tagged TcpP WT and cysteine mutants were introduced into V. cholerae tcpPH (pBAD-tcpH). Overnight cultures were refreshed at a dilution of 1:100 in 5 mL of LB medium containing appropriated antibiotics and grown at 37 °C stationary until midlog phase. A total of 100 μM taurocholate and 0.02% arabinose were then added to the cultures and continued to incubate for 2 h. Cell pellets were then resuspended in SDS/PAGE sampling buffer without DTT and proteins were separated on SDS/PAGE. TcpP was detected by a Western blot using HRP-conjugated monoclonal anti-FLAG tag antibody (Sigma). Where indicated, DTT (50 mM) was added in the sampling buffers to reduce protein disulfide bonds.
In Vivo Competition.
The infant mouse colonization assay was performed as described previously (33) with modifications. Briefly, V. cholerae tcpP mutant strains (lacZ+) were mixed one-to-one with the WT strain (lacZ−). Approximately 105 cells were inoculated into 6-d-old CD-1 sucking mice by oral gavage with 100 µL PBS or PBS containing 1% (wt/vol) cholestyramine. Additional cholestyramine was inoculated twice within 4 h by oral gavage. After a 6-h period of colonization, mice were killed, small intestines were collected and homogenized, and the ratio of mutant to WT bacteria determined by plating on LB agar containing X-Gal.
Supplementary Material
Acknowledgments
We thank Mark Goulian for E. coli dsbA mutant strain and helpful discussions. This study is supported by National Institutes of Health and National Institute of Allergy and Infectious Diseases Grant R01 AI080654 (to J.Z.), Natural Sciences Foundation of China Key Project 30830008 (to B.K.), and the Natural Sciences Foundation of China Young Scientist Award 30900036 (to H.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218039110/-/DCSupplemental.
References
- 1.Miller VL, Taylor RK, Mekalanos JJ. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell. 1987;48(2):271–279. doi: 10.1016/0092-8674(87)90430-2. [DOI] [PubMed] [Google Scholar]
- 2.Herrington DA, et al. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007;75(12):5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krukonis ES, DiRita VJ. From motility to virulence: Sensing and responding to environmental signals in Vibrio cholerae. Curr Opin Microbiol. 2003;6(2):186–190. doi: 10.1016/s1369-5274(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 5.Skorupski K, Taylor RK. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol Microbiol. 1997;25(6):1003–1009. doi: 10.1046/j.1365-2958.1997.5481909.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, et al. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc Natl Acad Sci USA. 2011;108(2):810–815. doi: 10.1073/pnas.1014640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abuaita BH, Withey JH. Bicarbonate Induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun. 2009;77(9):4111–4120. doi: 10.1128/IAI.00409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowden MJ, et al. Structure of Vibrio cholerae ToxT reveals a mechanism for fatty acid regulation of virulence genes. Proc Natl Acad Sci USA. 2010;107(7):2860–2865. doi: 10.1073/pnas.0915021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee A, Dutta PK, Chowdhury R. Effect of fatty acids and cholesterol present in bile on expression of virulence factors and motility of Vibrio cholerae. Infect Immun. 2007;75(4):1946–1953. doi: 10.1128/IAI.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwanaga M, et al. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol Immunol. 1986;30(11):1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, et al. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci USA. 2008;105(28):9769–9774. doi: 10.1073/pnas.0802241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmann AF. Bile acids: The good, the bad, and the ugly. News Physiol Sci. 1999;14:24–29. doi: 10.1152/physiologyonline.1999.14.1.24. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci USA. 2006;103(12):4333–4334. doi: 10.1073/pnas.0600780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matson JS, DiRita VJ. Degradation of the membrane-localized virulence activator TcpP by the YaeL protease in Vibrio cholerae. Proc Natl Acad Sci USA. 2005;102(45):16403–16408. doi: 10.1073/pnas.0505818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krukonis ES, DiRita VJ. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol Cell. 2003;12(1):157–165. doi: 10.1016/s1097-2765(03)00222-3. [DOI] [PubMed] [Google Scholar]
- 16.Goss TJ, Seaborn CP, Gray MD, Krukonis ES. Identification of the TcpP-binding site in the toxT promoter of Vibrio cholerae and the role of ToxR in TcpP-mediated activation. Infect Immun. 2010;78(10):4122–4133. doi: 10.1128/IAI.00566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci USA. 1998;95(10):5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leichert LI, Jakob U. Protein thiol modifications visualized in vivo. PLoS Biol. 2004;2(11):e333. doi: 10.1371/journal.pbio.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotoh K, et al. Bile acid-induced virulence gene expression of Vibrio parahaemolyticus reveals a novel therapeutic potential for bile acid sequestrants. PLoS ONE. 2010;5(10):e13365. doi: 10.1371/journal.pone.0013365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung DT, Mekalanos JJ. Bile acids induce cholera toxin expression in Vibrio cholerae in a ToxT-independent manner. Proc Natl Acad Sci USA. 2005;102(8):3028–3033. doi: 10.1073/pnas.0409559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc Natl Acad Sci USA. 2006;103(27):10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YW, Jin S, Sim WS, Nester EW. Genetic evidence for direct sensing of phenolic compounds by the VirA protein of Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1995;92(26):12245–12249. doi: 10.1073/pnas.92.26.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heras B, et al. DSB proteins and bacterial pathogenicity. Nat Rev Microbiol. 2009;7(3):215–225. doi: 10.1038/nrmicro2087. [DOI] [PubMed] [Google Scholar]
- 24.Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect Immun. 2006;74(2):1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer BK, Bassler BL. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc Natl Acad Sci USA. 2007;104(27):11145–11149. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA. 2002;99(5):3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang AC, Cohen SN. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Stern AM, Liu Z, Kan B, Zhu J. Virulence regulator AphB enhances toxR transcription in Vibrio cholerae. BMC Microbiol. 2010;10:3. doi: 10.1186/1471-2180-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 1988;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Farrant RD, Lindon JC, Nicholson JK. Two-dimensional 1H-1H and 13C-1H maximum-quantum correlation NMR spectroscopy with application to the assignment of the NMR spectra of the bile salt sodium taurocholate. Magn Reson Chem. 1995;33(3):212–219. [Google Scholar]
- 32.Gardel CL, Mekalanos JJ. Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol. 1994;235:517–526. doi: 10.1016/0076-6879(94)35167-8. [DOI] [PubMed] [Google Scholar]
- 33.Gardel CL, Mekalanos JJ. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect Immun. 1996;64(6):2246–2255. doi: 10.1128/iai.64.6.2246-2255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakrabarti S, Sengupta N, Chowdhury R. Role of DnaK in in vitro and in vivo expression of virulence factors of Vibrio cholerae. Infect Immun. 1999;67(3):1025–1033. doi: 10.1128/iai.67.3.1025-1033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippa AM, Goulian M. Perturbation of the oxidizing environment of the periplasm stimulates the PhoQ/PhoP system in Escherichia coli. J Bacteriol. 2012;194(6):1457–1463. doi: 10.1128/JB.06055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.