Abstract
IFN-γ is critical for immunity against infections with intracellular pathogens, such as Salmonella enterica. However, which of the many cell types capable of producing IFN-γ controls Salmonella infections remains unclear. Using a mouse model of systemic Salmonella infection, we observed that only a lack of all lymphocytes or CD90 (Thy1)+ cells, but not the absence of T cells, Retinoic acid-related orphan receptor (ROR)-γt–dependent lymphocytes, (NK)1.1+ cells, natural killer T (NKT), and/or B cells alone, replicated the highly susceptible phenotype of IFN-γ–deficient mice to Salmonella infection. A combination of antibody depletions and adoptive transfer experiments revealed that early protective IFN-γ was provided by Thy1-expressing natural killer (NK) cells and that these cells improved antibacterial immunity through the provision of IFN-γ. Further analysis of NK cells producing IFN-γ in response to Salmonella indicated that less mature NK cells were more efficient at mediating antibacterial effector function than terminally differentiated NK cells. Inspired by recent reports of Thy1+ NK cells contributing to immune memory, we analyzed their role in secondary protection against otherwise lethal WT Salmonella infections. Notably, we observed that a newly generated Salmonella vaccine strain not only conferred superior protection compared with conventional regimens but that this enhanced efficiency of recall immunity was afforded by incorporating CD4−CD8−Thy1+ cells into the secondary response. Taken together, these findings demonstrate that Thy1-expressing NK cells play an important role in antibacterial immunity.
Keywords: cytokines, infection immunity, intracellular bacteria, vaccination
Intracellular bacteria like Mycobacterium tuberculosis and Salmonella enterica remain serious causes of infections. S. enterica causes gastroenteritis, typhoid fever, and generalized infections in immunocompromised individuals (1, 2). Although S. enterica is typically contracted via oral infection, the critical pathological events that distinguish systemic disease from localized gastrointestinal Salmonellosis occur after its dissemination (3), highlighting the importance of systemic immune responses for the control of invasive S. enterica infections. Reports in humans with genetic defects in the IFN-γ signaling pathway and mouse models of typhoid fever using S. enterica serovar Typhimurium (S. Typhimurium) infections, indicate that IFN-γ is critical for such systemic control of S. enterica infections (4–8). Although it is well established that T cells and natural killer (NK) cells are important sources of IFN-γ, the relative contribution of these different lymphocytes to the IFN-γ–dependent control of S. enterica infections remain poorly characterized (9). The observations that pathogen-specific CD4+ T cells secrete IFN-γ in response to Salmonella (10, 11) and that CD4+ T-cell deficiency impairs clearance of S. Typhimurium (12) led to the extrapolation that CD4+ T cells are the key producers of IFN-γ during Salmonella infections (9, 13). However, as CD4+ T-cell deficiency results in a chronic, nonlethal form of Salmonellosis (12), and mice lacking IFN-γ rapidly succumb to Salmonella infections (4), it appears that other cellular sources of IFN-γ, such as NK cells, could be important in the early response against Salmonella infections.
Although earlier studies have suggested a role for NK cells in Salmonella infection (14–16), the literature on this topic is inconsistent (17–20). For example, whereas IL-15−/− mice, which lack classical NK cells and memory CD8+ T cells (21), had enhanced bacterial dissemination and succumbed to oral infections with WT Salmonella (19), anti-NK1.1 antibody treatment impaired control of Salmonella replication following oral infections with 105 cfu of WT Salmonella but had no effect on infections with higher doses (20). It was even suggested that neutrophils and macrophages, rather than NK, natural killer T (NKT), or T cells, were the dominant sources of IFN-γ during primary infection with Salmonella (22). Thus, given this heterogeneity, the present study was designed to examine the ability of NK cells and T cells to provide IFN-γ in response to S. Typhimurium and to determine their contribution to the control of S. Typhimurium infection in vivo.
Results
Thy1+ CD4−CD8− Cells Are Required for Early Control of Salmonella Infections.
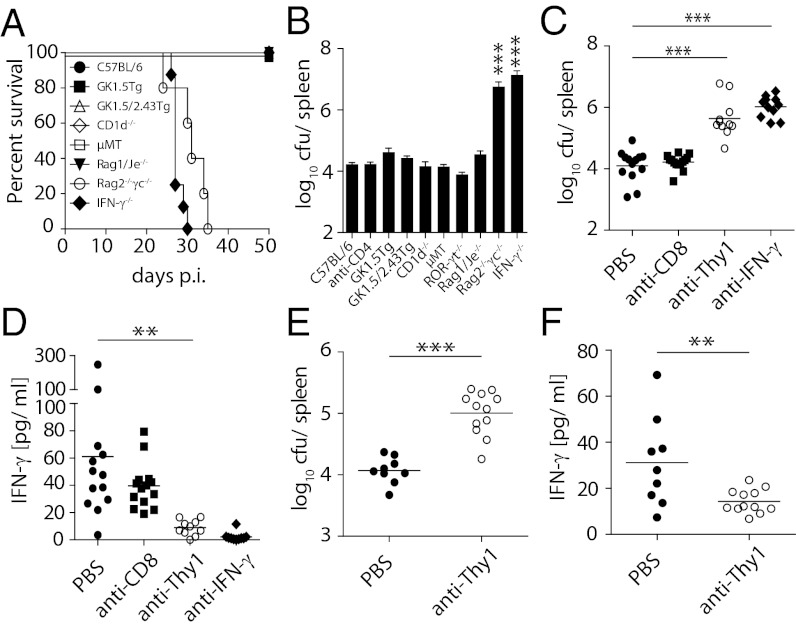
To explore the relative contribution of IFN-γ–producing lymphocytes to early in vivo Salmonella control, we studied the infection in a range of gene-targeted mouse strains with selective deficiencies (Table S1). Given our focus on systemic immune responses, we infected mice i.v. with a low dose of a growth-attenuated ΔaroA ΔaroD S. Typhimurium mutant strain (BRD509) that closely resembles the course of infection in untreated typhoid fever patients (9). Rag2−/−γc−/− mice and IFN-γ−/− mice infected with BRD509 succumbed to the infection within ∼30 d and had greatly elevated bacterial burden (Fig. 1 A and B), confirming that lymphocytes and IFN-γ were critically required for early control of the infection. Consistent with previous reports (23, 24), B cells were not required since µMT mice had comparable bacterial loads as B6 mice (Fig. 1 A and B). While it has been suggested that T cells play the dominant role in mediating early IFN-γ–dependent control of Salmonella infection (9, 13), we observed that mice lacking CD4+ T cells (GK1.5Tg), CD4+ and CD8+ T cells (GK1.5/2.43Tg), all T and B cells (Rag1/Je−/−), or NKT cells (CD1d−/−) controlled Salmonella replication at similar levels to B6 mice (Fig. 1 A and B). Moreover, mice lacking the transcriptional regulator ROR-γt, which is required for the development of IL-17–producing CD4+ T cells and several populations of innate lymphocytes (25), controlled Salmonella replication equally well as B6 mice (Fig. 1B). Although T cells secreted IFN-γ in response to Salmonella (Fig. S1A), these findings indicate that none of these IFN-γ–producing cells were essential for early control of Salmonella replication. Given that NK cells were preserved in all of the mice studied, we surmised that NK cell–derived IFN-γ was sufficient to control early bacterial replication.
Fig. 1.
Thy1-expressing CD3−CD4−CD8− cells are required for early control of S. Typhimurium infection. (A and B) C57BL/6, Rag1/Je−/−, Rag2−/−γc−/−, IFN-γ−/−, CD1d−/−, µMT, ROR-γt−/−, GK1.5Tg, GK1.5/2.43Tg mice were infected i.v. with 200 cfu S. Typhimurium BRD509. Survival was assessed (A) and/or bacterial numbers in the spleen were determined on day 21 after infection (B). (C–F) GK1.5Tg (C and D) and GK1.5/2.43Tg (E and F) mice were injected i.p. with PBS or antibodies against mouse CD8 (2.43), Thy1 (30-H12), or IFN-γ (HB-170-15). Forty-eight hours later mice were infected i.v. with 200 cfu S. Typhimurium BRD509. Injection of depleting antibodies was continued twice weekly for 3 wk. Mice were culled on day 21 after infection, and bacterial numbers (C and E) and serum IFN-γ (D and F) were assessed. Data are representative of at least two pooled independent experiments. Individual data points (C–F) and mean ± SEM of at least six mice per group (B) and at least seven mice per group (A) are shown. Statistical analyses: paired Student t test (E and F) and one-way ANOVA followed by Bonferroni multiple comparison test (B–D). ***P < 0.001, **P < 0.01.
To bypass the potential caveat of unforeseen effects of gene targeting, we confirmed our findings by antibody depletion studies. Depleting CD4+ cells in B6 mice by injecting anti-CD4 antibody (GK1.5) did not affect bacterial counts (Fig. 1B). Similarly, anti-CD8 treatment of GK1.5Tg mice did not alter bacterial load (Fig. 1C), replicating our observations in GK1.5/2.43Tg mice (Fig. 1B). However, treatment of GK1.5Tg and GK1.5/2.43Tg mice with anti-Thy1 antibody significantly impaired Salmonella control, as shown by increased bacterial counts (Fig. 1 C and E) and decreased serum IFN-γ levels (Fig. 1 D and F). Given that Rag1/Je−/− mice controlled the infection as efficiently as B6 mice (Fig. 1 A and B), these results are compatible with Thy1+ non-T and non-B cells providing IFN-γ to control Salmonella infections.
NK Cell Precursors and Immature NK Cells Express Thy1.
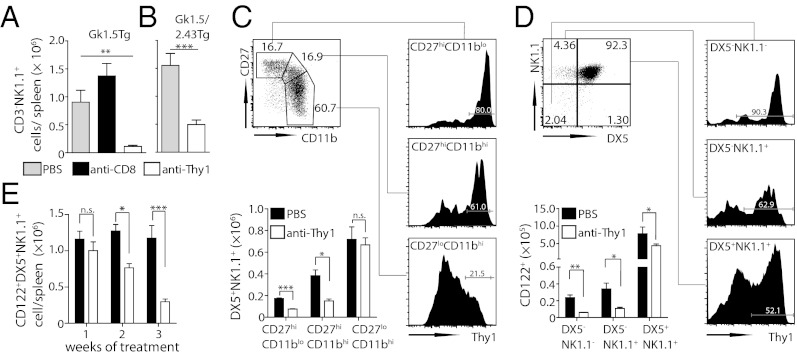
Because Thy1 is expressed on some NK cells (26, 27), it was possible that Thy1-expressing NK cells were required for early control of S. Typhimurium in both the GK1.5Tg and GK1.5/2.43Tg mice. Consistent with this, we found that depletion of Thy1+ cells evoked a significant reduction in NK cell numbers in both mouse strains (Fig. 2 A and B). Because little is known about the expression of Thy1 on NK cells, we examined whether Thy1 expression was associated with distinct developmental stages of NK cells. For this, NK cells were segregated into CD27hi CD11blo NK cells (immature), CD27hi CD11bhi NK cells (mature), and CD27lo CD11bhi NK cells (terminally differentiated) (28, 29). Although all subsets contained Thy1-expressing cells, CD27hi NK cells expressed the highest level of Thy1 (Fig. 2C). Interestingly, this higher expression of Thy1 also rendered CD27hi NK cells preferentially susceptible to Thy1 depletion (Fig. 2C). We excluded contamination with conventional T cells through the use of Rag1/Je−/− mice and T-cell receptor (TCR)/CD3 staining (Fig. S1 C and D). Of note, Thy1 was also highly expressed on committed NK cell precursors, which can be identified among CD3−CD4−CD19−β-TCR− cells by the expression of CD122 (28, 30, 31) and the absence of DX5 and/or NK1.1 expression (28, 32) (Fig. 2D). Consequently, anti-Thy1 treatment of B6 or GK1.5/2.43Tg mice depleted almost all NK cell precursors within 48 h (Fig. 2D). Despite their lower Thy1 expression profile (Fig. 2C), the pool of CD122+DX5+NK1.1+ NK cells was eventually depleted by anti-Thy1 injections. Because this was only observed after 2 wk of antibody treatment, these findings suggest that this was due to impaired replenishment of the terminally differentiated NK cell pool (Fig. 2E). These results provide unique insights into the dynamics of NK cell turnover and suggest that Thy1 expression by NK cells is inversely correlated with their maturation status.
Fig. 2.
NK cell precursors and immature NK cells express Thy1 and are depleted by anti-Thy1 antibodies. (A) GK1.5Tg and (B) GK1.5/2.43Tg mice were injected with antibodies and infected with S. Typhimurium BRD509 as in Fig. 1. Splenic NK cell (CD3−DX5+NK1.1+) numbers were enumerated at day 21 after infection. (C–E) B6 mice were treated i.p. with PBS or anti-Thy1 (30H-12) twice weekly for 3 wk. CD27hi/lo and CD11bhi/lo splenic NK cells (CD3−DX5+NK1.1+) were analyzed for the expression of Thy1 (black histograms) 48 h after the last antibody treatment (C). DX5+/− and NK1.1+/− splenic NK cell precursor subsets (CD19−CD3−CD4−β-TCR−CD122+) were analyzed for the expression of Thy1 (black histograms) and enumerated at 48 h (D) or at different time points over 3 wk (E) after injection of anti-Thy1. Representative FACS plots (C and D) and mean cell numbers ± SEM of at least five mice (A–E) are shown. Data are representative of at least two independent experiments. Cells gated on viable single lymphocytes. Statistical analyses: paired Student t test for individual cell populations (B–D), one-way ANOVA (A), or two-way ANOVA followed by Bonferroni multiple comparison test (E). ***P < 0.001, **P < 0.01, *P < 0.05.
Thy1+ NK Cells Produce IFN-γ and Contribute to Salmonella Control in Vivo.
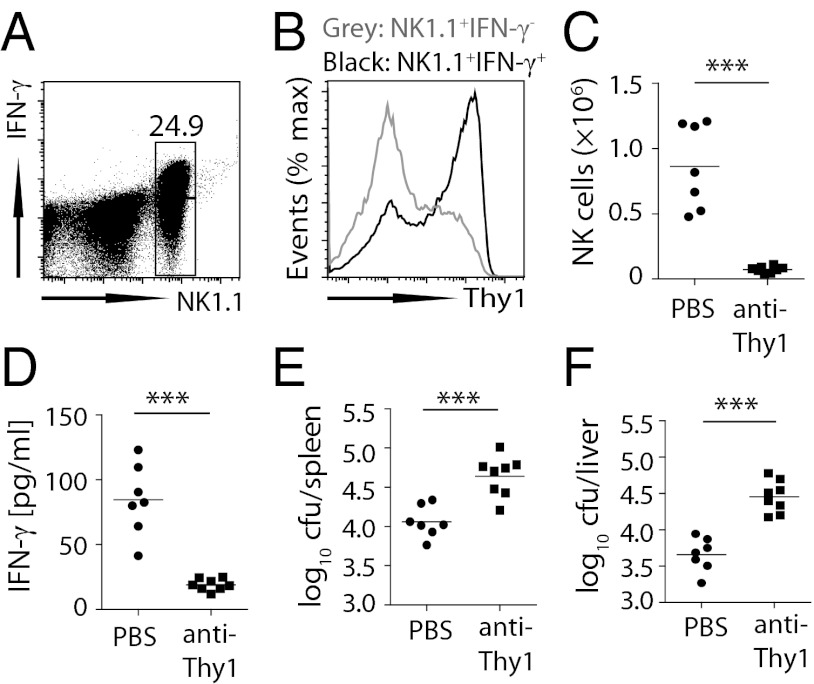
To address whether Thy1-expressing NK cells secreted IFN-γ in response to S. Typhimurium in vivo, we injected heat-killed S. Typhimurium into naïve mice to evoke IFN-γ secretion (33). Approximately two thirds of all splenic NK cells in B6 mice rapidly produced IFN-γ, which again was not due to T-cell contamination, because Rag1/Je−/− mice yielded similar results (Fig. 3 A and B). All IFN-γ–producing NK cells in both B6 and Rag1/Je−/− mice expressed higher levels of Thy1 relative to IFN-γ− NK cells (Fig. 3B; Fig. S1B), indicating that IFN-γ secretion by NK cells in response to Salmonella resided largely with the Thy1+ NK cells. To assess the contribution of Thy1+ NK cells under circumstances where Thy1-mediated T-cell depletion could be excluded, we infected Rag1/Je−/− mice with S. Typhimurium and treated these mice with anti-Thy1 antibody. Thy1 depletion not only reduced total NK cell numbers (Fig. 3C) but, critically, also increased bacterial burden (Fig. 3 E and F) and reduced serum IFN-γ (Fig. 3D). Collectively, these findings support the view that Thy1+ NK cells are highly efficient at mediating IFN-γ–dependent control of S. Typhimurium.
Fig. 3.
Thy1+ NK cells produce IFN-γ and contribute to Salmonella control. (A and B) Rag1/Je−/− mice were injected i.v. with 1 × 108 cfu heat-killed S. Typhimurium (HKST), and IFN-γ secretion by CD3−NK1.1+ cells was assessed 2 h later in the spleen (A). IFN-γ+ (black) and IFN-γ− (gray) NK 1.1+ cells were assessed for expression of Thy1 (B). (C–F) Rag1/Je−/− mice were treated i.p. with PBS or anti-Thy1 (30H-12) and infected i.v. with 200 cfu S. Typhimurium BRD509 48 h later. Injection of depleting antibodies was continued twice weekly for 3 wk. Bacterial numbers in spleen (E) and liver (F), serum IFN-γ levels (D), and NK cell numbers (C) were assessed on day 21 after infection. Data are representative of two independent experiments. Representative FACS plot (A) and histogram (B) or individual data points (C–F) are shown. Statistical analysis: paired Student t test. ***P < 0.001.
Transfer of IFN-γ–Competent NK Cells Improves Salmonella Control in Rag2−/−γc−/− Mice.
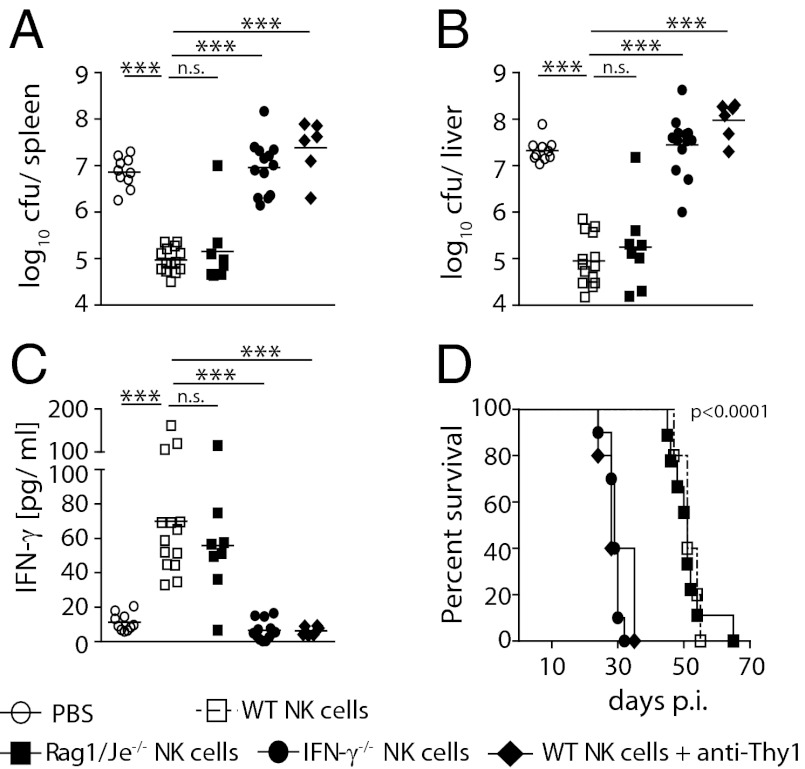
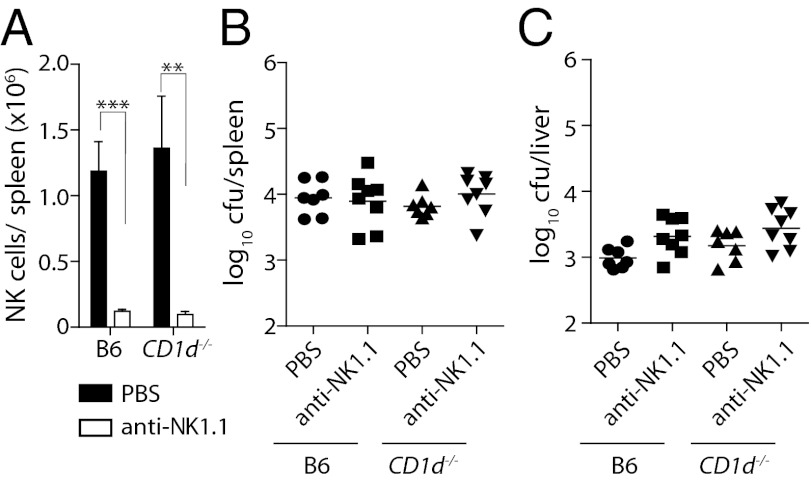
Previous suggestions for a role of NK cells were derived from antibody depletions (16, 17). Considering that some of these treatments also deplete activated T cells (20, 34), it is difficult to gauge to what extent the depletion of non-NK cells contributed to these observations. Similarly, the above experiments cannot exclude a role of other Thy1-expressing cells. We therefore performed transfer studies to conclusively examine whether NK cells alone could mediate Salmonella control. We used a system whereby in vitro activated NK cells were adoptively transferred into Rag2−/−γc−/− mice (35). These in vitro activated NK cells expressed CD122, DX5, and NK1.1 (Fig. S1E) and, upon adoptive transfer, secreted IFN-γ in response to heat killed S. Typhimurium (Fig. S1F). Remarkably, Rag2−/−γc−/− mice receiving NK cells contained ∼100-fold fewer bacteria in the spleen and liver on day 23 after infection (Fig. 4 A and B). The transfer of activated NK cells restored serum IFN-γ levels (Fig. 4C) and significantly prolonged the survival of Rag2−/−γc−/− mice (Fig. 4D). Transfer of IFN-γ–deficient NK cells yielded no such improvement, demonstrating that the protective role of NK cells in S. Typhimurium infections depended on their provision of IFN-γ. Together with the observation that protection could also be achieved by transferring NK cells from Rag1/Je−/− mice, these findings conclusively demonstrate that NK cells improved antibacterial immunity via the provision of IFN-γ. The observation that the transferred NK cells expressed bimodal levels of Thy1 (Fig. S1G) also provided an opportunity to assess whether Thy1+ and Thy1− NK cells played differential roles in controlling Salmonella infection. Interestingly, Rag2−/−γc−/− mice transferred with NK cells and subsequently treated with anti-Thy1 antibodies twice weekly were no longer able to control the infection (Fig. 4 A and B), indicating that protection from Salmonella infection was critically dependent on Thy1+ NK cells but not Thy1− NK cells. To also address whether NK cells are not only capable (Fig. 4), but also essential, for controlling Salmonella infections in WT mice, we infected B6 mice and CD1d−/− mice that were treated with anti-NK1.1 antibodies with BRD509. Twice weekly administrations of anti-NK1.1 antibodies efficiently depleted NK cells in both mouse strains (Fig. 5A). The depletion of NK1.1+ cells had no effect on the ability of these mice to control Salmonella infections (Fig. 5 B and C), suggesting that, although Thy1+ NK cells can potently control Salmonella replication in vivo, other IFN-γ–producing lymphocytes can compensate for their absence.
Fig. 4.
Transfer of IFN-γ–competent NK cells improves Salmonella control in Rag2−/−γc−/− mice: (A–D) 1 × 106 purified in vitro–activated NK cells or PBS were injected i.v. into naïve Rag2−/−γc−/− mice on day 5 and 6 after in vitro culture. Mice were i.v. infected with 200 cfu BRD509 24 h later. Some mice were injected twice weekly i.p. with antibodies against mouse Thy1 (30-H12). Bacterial numbers in spleen (A) and liver (B) were assessed, and serum samples were analyzed for IFN-γ by cytometric bead array (C) at 23 d after infection. In separate experiments, survival (D) was assessed. Data are representative of at least two independent experiments. Survival plots of 10 mice (D) or individual pooled data points (A–C) are shown. Statistical analyzes: one-way ANOVA followed by Bonferroni multiple comparison test (A–C); log-rank (Mantel-Cox) test (D). ***P < 0.001, **P < 0.01.
Fig. 5.
NK cells are not essential for control of primary infection when other cell types are present. (A) B6 and CD1d−/− mice were injected i.p. with PBS or antibodies against mouse NK1.1 (PK136) and infected i.v. with 200 cfu S. Typhimurium BRD509 48 h later. Injection of depleting antibodies or PBS was continued twice weekly for 3 wk. Splenic NK cell numbers (A) and bacterial counts in spleen (B) and liver (C) were determined on day 21 after infection. Data are representative of two pooled independent experiments with seven to eight mice per group. Statistical analyses: paired Student t test (A). ***P < 0.001, **P < 0.01.
Thy1+ NK Cells Are Required for Optimal Vaccine-Mediated Protection.
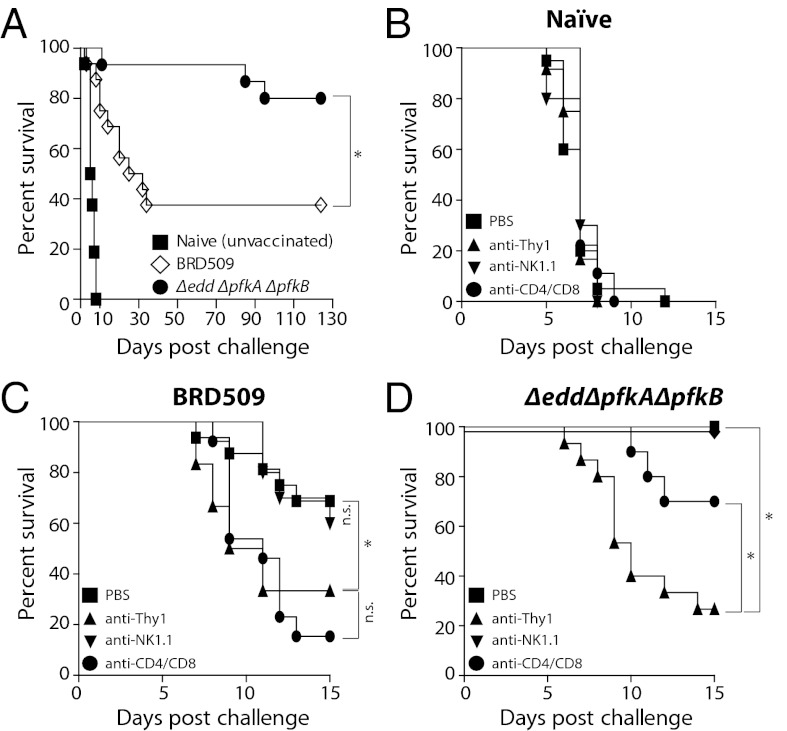
Given the importance of IFN-γ in recall responses against Salmonella (36) and recent reports that Thy1+ NK cells can confer specific immune memory (27), we assessed whether Thy1+ NK cells also contributed to protection against secondary Salmonella infection. Although it is well established that prior infection with growth-attenuated Salmonella strains, such as BRD509, improves subsequent control of otherwise lethal WT S. Typhimurium infections, the degree of such protection is variable (23, 37–39). Indeed, we observed that only ∼50% of BRD509-infected B6 mice survived secondary infections with WT S. Typhimurium beyond 10 to 20 d (Fig. 6A). Considering this limited vaccine efficiency of BRD509 and that of other growth-attenuated Salmonella strains with single mutations in a single metabolic pathway (40), we tested whether manipulations of two or more metabolic pathways might improve the potential of vaccine strains to confer secondary protection. We therefore manipulated several metabolic pathways in Salmonella (Fig. S2) and found that prior infection with Salmonellae carrying mutations in both the Entner-Doudoroff and Embden-Meyerhoff-Parnas metabolic pathways (Δedd ΔpfkA ΔpfkB Salmonella) enabled ∼80–90% of B6 mice to survive secondary WT Salmonella challenges for more than 100 d (P < 0.05; Fig. 6A). To dissect whether this superior protection afforded by Δedd ΔpfkA ΔpfkB Salmonella relative to BRD509 was due to effects on T and/or NK cells, we treated vaccinated and naïve mice with anti-CD4/CD8, anti-NK1.1, or anti-Thy1 antibodies (Fig. 6 B–D). As expected, antibody administration had no effect on naïve mice challenged with WT Salmonella (Fig. 6B). The partial protection after BRD509 vaccination required CD4+ and/or CD8+ T cells, because treatment with anti-CD4/CD8 was similarly efficient as anti-Thy1 antibody in impairing secondary protection (Fig. 6C). Depletion of NK1.1+ cells alone did not affect the ability of vaccinated mice to control the secondary infection (Fig. 6C). In contrast, however, anti-Thy1 antibody treatment had a much more detrimental effect on the survival of Δedd ΔpfkA ΔpfkB Salmonella–vaccinated mice compared with anti-CD4/CD8 antibody–treated mice (P < 0.05; Fig. 6D). Together with our observations that anti-Thy1 treatment impacted NK cell–mediated control of primary Salmonella infections (Figs. 1–3), these results are compatible with the view that the superior capacity of Δedd ΔpfkA ΔpfkB Salmonella to provide secondary protection against virulent Salmonellae was due to its engagement of Thy1+ NK cells in addition to conventional T cells, although potential contributions by other Thy1-expressing cells cannot be excluded.
Fig. 6.
Thy1+ NK cells are required for optimal vaccine-mediated protection. (A) B6 mice were orally infected with 5 × 109 cfu BRD509, Δedd ΔpfkA ΔpfkB Salmonella, or left uninfected. Twelve weeks later, mice were orally infected with 1 × 107 cfu WT Salmonella SL1344. Survival was assessed over 120 d. (B–D) Mice were vaccinated or left uninfected as in A. Two days before challenge with WT Salmonella SL1344, mice were treated i.p. with PBS or antibodies against mouse CD8 (2.43) and CD4 (GK1.5), NK1.1 (PK136), or Thy1 (30-H12). Antibody treatment was maintained twice weekly for the duration of the experiment. Survival was assessed over 15 d. Data are representative of two independent experiments with 9–16 mice per group. Statistical analyses: log-rank (Mantel-Cox) test. *P < 0.05.
Discussion
Our analysis into the functional role of NK cells and other lymphocytes during S. Typhimurium infections was driven by results showing a significant survival advantage of Rag1/Je−/− over Rag2−/−γc−/− mice. Although previous reports provided conflicting results on a potential contribution of NK cells to immunity against Salmonella infections, our adoptive transfer studies provide clear evidence that NK cells can actively control Salmonella replication in vivo. The fact that this protection was dependent on NK cells providing IFN-γ furthermore demonstrates that IFN-γ–independent cytotoxic mechanisms, mediated by granzymes or perforin (41), were not sufficient for NK cell–mediated limitation of bacterial replication. These observations together with the fact that anti-Thy1 antibody treatment efficiently depleted NK cells call into question previous conclusions regarding a protective role of CD4−CD8− T cells during intracellular infections that were derived from Thy1 depletion studies (42–45). It was also intriguing to observe that antibody-mediated depletion of NK1.1+ cells did not impair primary or secondary Salmonella control in WT mice. Bearing in mind the absolute requirement for IFN-γ–producing lymphocytes, and our previous observation that memory CD8+ T cells can also confer protection (33), the most likely explanation as to why Thy1+ NK cells were highly potent, but not irreplaceable in immunity against Salmonella infections, is that one population of IFN-γ–producing lymphocytes can compensate for the absence of another, as previously suggested in models of Listeria monocytogenes infection (46, 47). It is tempting to speculate that this flexibility in the functional contribution of different IFN-γ–producing lymphocytes (NK cells, T cells) serves to provide a sophisticated host response system with inbuilt multilayered safeguards.
Thy1-mediated NK cell depletion appeared to be largely the consequence of the elimination of Thy1-expressing NK cell precursors and less mature NK cells. Although Thy1loCD27loCD11bhi NK cells were initially not affected by anti-Thy1 treatment, these cells were eventually also depleted, most likely because their replenishment from earlier developmental stages was blocked. As IFN-γ production by NK cells resided mostly with Thy1hiCD27hiCD11blo NK cells, our work suggests that terminally differentiated NK cells do not necessarily confer the most efficient antibacterial activity.
Given that Thy1+ NK cells have been associated with memory responses to viral infections and haptens (27, 48, 49), we also investigated whether targeting these cells through anti-Thy1 antibody affected the outcome of secondary responses to Salmonella. Using different vaccination regimens with growth-attenuated mutants of S. Typhimurium, we made several important observations. First, BRD509-afforded protection from WT infection was not as striking as reported by others (37, 38). In fact, our data are consistent with a study showing that BRD509 infection provided long-term protection to only ∼50% of mice (23). Secondly, the fact that anti-Thy1 antibody depletion impaired BRD509-induced protection as efficiently as a combination of anti-CD4/CD8 antibodies confirmed that this protection was largely dependent on T cells (36). Quite intriguingly however, protection studies with our unique Δedd ΔpfkA ΔpfkB vaccine strain not only revealed its superior capacity to induce secondary protection compared with BRD509, but also showed that Thy1-expressing cells were more important for this type of protection than CD4- or CD8-expressing cells alone. In conjunction with our characterization of the functional role of Thy1+ NK cells in primary responses against Salmonella, these findings are compatible with the view that our unique vaccine strain protects mice with greater efficiency over conventional vaccine strains because the secondary immune response involves both T cells and Thy1+ NK cells, although our data cannot exclude a contribution by other Thy1-expressing cell types. With secondary protection requiring Salmonella-specific Th1 responses (50), it is possible that the greater efficiency of the unique vaccine strain is mediated through Thy1+ NK cells optimizing the ensuing Th1 response, as has been demonstrated in the context of other immune responses (51). Clearly, more studies are required to further delineate the precise nature of the interaction between T cells and other Thy1-expressing cells, such as the NK cells characterized in the present study, during immune responses to Salmonella infection.
Our observations have important implications for future vaccination strategies. Given that IFN-γ secretion in responses to Salmonella was less efficient in terminally differentiated NK cells, attempts at improving antibacterial immunity through potent immune activation might not necessarily lead to the most effective NK cell responses. Such insights might be particularly relevant for the choice of adjuvants in future vaccines. Furthermore, bearing in mind that Salmonellae are among the major causes of fulminant infections in HIV/AIDS patients (2, 52), the potent antibacterial effects of Thy1+ NK cells uncovered here might lead to new therapeutic strategies to improve immunity against Salmonella in situations where T cells are unlikely to contribute.
Materials and Methods
Mice.
C57BL/6, CD45.1, IFN-γ−/−, GK1.5Tg, GK1.5/2.43Tg, µMT, CD1d−/−, Rag1/Je−/−, and Rag2−/−γc−/− mice were bred and maintained at the University of Melbourne animal facility. ROR-γt−/− mice were bred at the Walter and Eliza Hall Institute of Medical Research, Melbourne. All mice were age and sex matched, and experiments were approved by the University of Melbourne Animal Ethics Committee. Accordingly, for survival studies, mice were killed at a weight loss of more than 15%.
Bacterial Strains and Infection.
For primary infections, S. Typhimurium BRD509 was grown statically at 37 °C in Luria-Bertani (LB) broth for 16–18 h and diluted in PBS, and 200 cfu was injected into the tail vein in a volume of 200 μL. For secondary protection studies, mice were pretreated with 10% (wt/vol) sodium bicarbonate, orally infected with 5 × 109 cfu of BRD509 or SL1344 Δedd ΔpfkA ΔpfkB, and challenged orally with 1 × 107 cfu of WT SL1344 12 wk later. The number of replicating bacteria was determined by homogenizing organs from infected mice in 5 mL of sterile PBS. The homogenate was serially diluted and plated onto LB agar plates supplemented with 25 µg/mL streptomycin. Plates were incubated at 37 °C for 24 h.
Antibody-Mediated Depletion of Lymphocyte Subsets and Assessment of Depletion.
Lymphocyte subsets were depleted from B6, CD1d−/−, GK1.5, and GK1.5/2.43 mice by i.p. injections of monoclonal antibodies against CD4, CD8, Thy1, NK1.1, and IFN-γ (HB-170-15). Timing, antibody clones, and doses are listed in Table S2.
Construction of S. Typhimurium Mutants.
Mutants of S. Typhimurium SL1344 were constructed by replacing target genes with an antibiotic resistance cassette as previously described (53). Briefly, ∼0.5-kb regions up and downstream of the chromosomal sequence targeted for deletion were amplified from S. Typhimurium SL1344 genomic DNA and joined to a kan resistance gene (Table S3). Resulting PCR products were flanked by I-SceI sites, ligated into pGEM-T Easy (Promega), and transformed into S. Typhimurium SL1344 carrying the mutagenesis plasmid pACBSR (54). The I-SceI endonuclease and λ Red recombinase genes carried on pACBSR were induced by growth with L-arabinose, promoting homologous recombination of the construct with the targeted chromosomal sequence. All mutations were confirmed by sequencing of chromosomal DNA in the deleted region.
Assessment of ex Vivo IFN-γ Secretion and Serum IFN-γ.
Ex vivo IFN-γ secretion by distinct lymphocyte subsets was assessed as previously described (33). Briefly, mice were injected i.v. with 1 × 108 cfu of heat-inactivated Salmonella. Two hours later, 1 × 106 splenocytes were stained with the Mouse IFN-γ secretion assay detection kit (Miltenyi Biotec) and analyzed by flow cytometry. Serum concentrations of IFN-γ were analyzed using the BD Cytometric Bead Array (BD Biosciences) according to the manufacturer’s instructions.
Flow Cytometry.
To assess depletion of distinct lymphocyte subsets and expression of surface antigens, splenocytes were stained with monoclonal antibodies (BD Pharmingen) against CD4 (GK1.5), CD8α (53-6.7), CD3 (145-2C11), CD19 (ID3), CD11b (M1/70), CD27 (LG-3A10), CD122 (TM-β1), β-TCR (H57-597), CD49b (DX5), NK1.1 (PK136), Thy1.2 (53-2.1), and CD122 (TM-β1) as described elsewhere. Samples were analyzed using a FACSCantoII or LSRII (BD Biosciences). Propidium iodide (2 μg/mL) was added to exclude dead cells.
Isolation, Enrichment, and In Vitro Activation of NK Cells.
Spleen and lymph nodes from donor mice were harvested. Non-NK cells were depleted using a NK cell enrichment kit and MACS technology (Miltenyi Biotec). Isolated and purified NK cells were cultured in RPMI-1640 medium supplemented with 10% (vol/vol) FCS, 2 mM L-glutamine, 5 × 10−5 mM β-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM Hepes, and 1,000 IU/mL recombinant human IL-2 (Peprotech) for 5 and 6 d at a density of ∼5 × 105 cells/mL. NK cells (1 × 106) were adoptively transferred i.v. into naïve Rag2−/−γc−/− mice on day 5 and 6 after culture.
Data Analysis.
Flow cytometry data were analyzed using FlowJo software (Treestar), and statistical analysis was performed using GraphPad Prism version 5.0.
Supplementary Material
Acknowledgments
We thank Drs. J. Tschopp, W. Chen, O. L. Wijburg, and D. I. Godfrey for reagents, bacteria, mice, and helpful discussions. This research is supported by the National Health and Medical Research Council of Australia (Program grant: Fighting infection, exploiting host-pathogen interactions). S.B. and D.M.A. were recipients of NH&MRC Career Development awards. M.J.S. was supported by a NH&MRC Australia fellowship. G.T.B. was supported by a Sylvia and Charles Viertel fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222047110/-/DCSupplemental.
References
- 1.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. 2010;50(2):241–246. doi: 10.1086/649541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet. 2012;379(9835):2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53–66. doi: 10.1038/nrmicro1788. [DOI] [PubMed] [Google Scholar]
- 4.VanCott JL, et al. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4(11):1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 5.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280(5368):1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 6.Jouanguy E, et al. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11(3):346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 7.Fang FC. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 8.Henry SC, et al. Balance of Irgm protein activities determines IFN-gamma-induced host defense. J Leukoc Biol. 2009;85(5):877–885. doi: 10.1189/jlb.1008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougan G, John V, Palmer S, Mastroeni P. Immunity to salmonellosis. Immunol Rev. 2011;240(1):196–210. doi: 10.1111/j.1600-065X.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 10.Mittrücker HW, Köhler A, Kaufmann SHE. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70(1):199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alaniz RC, Cummings LA, Bergman MA, Rassoulian-Barrett SL, Cookson BT. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J Immunol. 2006;177(6):3983–3993. doi: 10.4049/jimmunol.177.6.3983. [DOI] [PubMed] [Google Scholar]
- 12.Hess J, Ladel C, Miko D, Kaufmann SHE. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: Major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156(9):3321–3326. [PubMed] [Google Scholar]
- 13.Mastroeni P, Ménager N. Development of acquired immunity to Salmonella. J Med Microbiol. 2003;52(Pt 6):453–459. doi: 10.1099/jmm.0.05173-0. [DOI] [PubMed] [Google Scholar]
- 14.Maskell DJ, Hormaeche CE, Harrington KA, Joysey HS, Liew FY. The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microb Pathog. 1987;2(4):295–305. doi: 10.1016/0882-4010(87)90127-6. [DOI] [PubMed] [Google Scholar]
- 15.Hormaeche CE, Mastroeni P, Arena A, Uddin J, Joysey HS. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990;70(2):247–250. [PMC free article] [PubMed] [Google Scholar]
- 16.Ramarathinam L, Niesel DW, Klimpel GR. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150(9):3973–3981. [PubMed] [Google Scholar]
- 17.Schafer R, Eisenstein TK. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992;60(3):791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrington L, Srikanth CV, Antony R, Shi HN, Cherayil BJ. A role for natural killer cells in intestinal inflammation caused by infection with Salmonella enterica serovar Typhimurium. FEMS Immunol Med Microbiol. 2007;51(2):372–380. doi: 10.1111/j.1574-695X.2007.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashkar AA, Reid S, Verdu EF, Zhang K, Coombes BK. Interleukin-15 and NK1.1+ cells provide innate protection against acute Salmonella enterica serovar Typhimurium infection in the gut and in systemic tissues. Infect Immun. 2009;77(1):214–222. doi: 10.1128/IAI.01066-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapaque N, Walzer T, Méresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. J Immunol. 2009;182(7):4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirby AC, Yrlid U, Wick MJ. The innate immune response differs in primary and secondary Salmonella infection. J Immunol. 2002;169(8):4450–4459. doi: 10.4049/jimmunol.169.8.4450. [DOI] [PubMed] [Google Scholar]
- 23.Mittrücker H-W, Raupach B, Köhler A, Kaufmann SHE. Cutting edge: Role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164(4):1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 24.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(-/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68(1):46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Ohdan H, Manilay JO, Sykes M. NK cell tolerance in mixed allogeneic chimeras. J Immunol. 2003;170(11):5398–5405. doi: 10.4049/jimmunol.170.11.5398. [DOI] [PubMed] [Google Scholar]
- 27.Gillard GO, et al. Thy1+ NK [corrected] cells from vaccinia virus-primed mice confer protection against vaccinia virus challenge in the absence of adaptive lymphocytes. PLoS Pathog. 2011;7(8):e1002141. doi: 10.1371/journal.ppat.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosmaraki EE, et al. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol. 2001;31(6):1900–1909. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176(3):1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 30.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214(1):47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 31.Carotta S, Pang SHM, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117(20):5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 32.Andrews DM, Smyth MJ. A potential role for RAG-1 in NK cell development revealed by analysis of NK cells during ontogeny. Immunol Cell Biol. 2010;88(2):107–116. doi: 10.1038/icb.2009.94. [DOI] [PubMed] [Google Scholar]
- 33.Kupz A, et al. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8⁺ T cells. Nat Immunol. 2012;13(2):162–169. doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- 34.Griggs ND, Smith RA. Adoptive transfer of natural killer cell activity in B6D2F1 mice challenged with Salmonella typhimurium. Cell Immunol. 1991;135(1):88–94. doi: 10.1016/0008-8749(91)90256-b. [DOI] [PubMed] [Google Scholar]
- 35.Pegram HJ, Jackson JT, Smyth MJ, Kershaw MH, Darcy PK. Adoptive transfer of gene-modified primary NK cells can specifically inhibit tumor progression in vivo. J Immunol. 2008;181(5):3449–3455. doi: 10.4049/jimmunol.181.5.3449. [DOI] [PubMed] [Google Scholar]
- 36.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro- Salmonella vaccines. Microb Pathog. 1992;13(6):477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 37.Menager N, et al. Fcgamma receptors are crucial for the expression of acquired resistance to virulent Salmonella enterica serovar Typhimurium in vivo but are not required for the induction of humoral or T-cell-mediated immunity. Immunology. 2007;120(3):424–432. doi: 10.1111/j.1365-2567.2006.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.al-Ramadi BK, Fernandez-Cabezudo MJ, Ullah A, El-Hasasna H, Flavell RA. CD154 is essential for protective immunity in experimental salmonella infection: Evidence for a dual role in innate and adaptive immune responses. J Immunol. 2006;176(1):496–506. doi: 10.4049/jimmunol.176.1.496. [DOI] [PubMed] [Google Scholar]
- 39.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61(9):3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paterson GK, Cone DB, Peters SE, Maskell DJ. Redundancy in the requirement for the glycolytic enzymes phosphofructokinase (Pfk) 1 and 2 in the in vivo fitness of Salmonella enterica serovar Typhimurium. Microb Pathog. 2009;46(5):261–265. doi: 10.1016/j.micpath.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Hoves S, Trapani JA, Voskoboinik I. The battlefield of perforin/granzyme cell death pathways. J Leukoc Biol. 2010;87(2):237–243. doi: 10.1189/jlb.0909608. [DOI] [PubMed] [Google Scholar]
- 42.Cowley SC, et al. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med. 2005;202(2):309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn PL, North RJ. Resolution of primary murine listeriosis and acquired resistance to lethal secondary infection can be mediated predominantly by Thy-1+ CD4- CD8- cells. J Infect Dis. 1991;164(5):869–877. doi: 10.1093/infdis/164.5.869. [DOI] [PubMed] [Google Scholar]
- 44.Uzonna JE, Kaushik RS, Zhang Y, Gordon JR, Tabel H. Experimental murine Trypanosoma congolense infections. II. Role of splenic adherent CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4+8- and CD3+Thy1.2+ TCR-alpha beta- gamma delta- CD4-8- cells in the production of IL-4, IL-10, and IFN-gamma and in trypanosome-elicited immunosuppression. J Immunol. 1998;161(11):6189–6197. [PubMed] [Google Scholar]
- 45.Johnson LL, VanderVegt FP, Havell EA. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect Immun. 1993;61(12):5174–5180. doi: 10.1128/iai.61.12.5174-5180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bregenholt S, Berche P, Brombacher F, Di Santo JP. Conventional alpha beta T cells are sufficient for innate and adaptive immunity against enteric Listeria monocytogenes. J Immunol. 2001;166(3):1871–1876. doi: 10.4049/jimmunol.166.3.1871. [DOI] [PubMed] [Google Scholar]
- 47.Andersson A, Dai WJ, Di Santo JP, Brombacher F. Early IFN-gamma production and innate immunity during Listeria monocytogenes infection in the absence of NK cells. J Immunol. 1998;161(10):5600–5606. [PubMed] [Google Scholar]
- 48.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457(7229):557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7(5):507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 50.Lee S-J, et al. Temporal expression of bacterial proteins instructs host CD4 T cell expansion and Th17 development. PLoS Pathog. 2012;8(1):e1002499. doi: 10.1371/journal.ppat.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martín-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5(12):1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 52.Gordon MA, et al. Invasive non-typhoid salmonellae establish systemic intracellular infection in HIV-infected adults: An emerging disease pathogenesis. Clin Infect Dis. 2010;50(7):953–962. doi: 10.1086/651080. [DOI] [PubMed] [Google Scholar]
- 53.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herring CD, Glasner JD, Blattner FR. Gene replacement without selection: regulated suppression of amber mutations in Escherichia coli. Gene. 2003;311:153–163. doi: 10.1016/s0378-1119(03)00585-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.