Abstract
Syndecan-1 is a cell surface heparan sulfate proteoglycan that binds to many mediators of disease pathogenesis. Through these molecular interactions, syndecan-1 can modulate leukocyte recruitment, cancer cell proliferation and invasion, angiogenesis, microbial attachment and entry, host defense mechanisms, and matrix remodeling. The significance of syndecan-1 interactions in disease is underscored by the striking pathological phenotypes seen in the syndecan-1 null mice when they are challenged with disease-instigating agents or conditions. This review discusses the key molecular functions of syndecan-1 in modulating the onset, progression, and resolution of inflammatory diseases, cancer, and infection.
Keywords: Syndecan, Proteoglycan, Heparan sulfate, Inflammation, Infection, Cancer
1. Introduction
Syndecans comprise a major family of cell surface heparan sulfate proteoglycans (HSPGs) (Bernfield et al., 1999; Park et al., 2000b; Couchman, 2010). Syndecans are expressed on the surface of all adherent cells and on many non-adherent cells. The mammalian syndecan family consists of 4 members, each encoded by distinct genes. Although cloning of the first syndecan, syndecan-1, was reported in 1989 (Saunders et al., 1989), its existence was known prior to this report as the Bernfield (Rapraeger and Bernfield, 1983) and Hook (Kjellen et al., 1981) groups have identified an HSPG intercalated in the plasma membrane of mouse mammary gland epithelial cells and rat hepatocytes, respectively. This HSPG was found to be expressed predominantly on the basolateral surface of epithelial cells (Rapraeger et al., 1986; Hayashi et al., 1987), bind to extracellular matrix (ECM) components, such as collagens I, III and V (Koda et al.,1985), fibronectin (Saunders and Bernfield, 1988) and thrombospondin (Sun et al., 1989), and associate with the actin cytoskeleton (Rapraeger et al., 1986). Because these properties indicated that this HSPG is well positioned to function as an anchor that stabilizes the morphology of epithelial sheets by connecting the ECM to the intracellular cytoskele-ton, the name ‘syndecan’ (from the Greek syndein, which means to bind together) was given to this HSPG. Subsequently, syndecan-2 (fibroglycan) (Marynen et al., 1989; Pierce et al., 1992), syndecan-3 (N-syndecan) (Carey et al., 1992; Gould et al., 1992), and syndecan-4 (amphiglycan/ryudocan) (David et al., 1992; Kojima et al., 1992) were identified.
Syndecans are type I transmembrane proteins, consisting of an extracellular domain where heparan sulfate (HS) chains attach distally to the plasma membrane, followed by the signature transmembrane and cytoplasmic domains which are highly homologous among the different syndecans and across species. The transmembrane domain contains a GxxxG dimerization motif and mediates both homotypic and heterotypic dimerization of syndecans (Dews and Mackenzie, 2007). The syndecan cytoplasmic domain contains several conserved signaling and scaffolding motifs, including one invariant Ser, three invariant Tyr, and a Glu-Phe-Tyr-Ala PDZ binding domain at the C-terminus (Bernfield et al., 1999; Lambaerts et al., 2009; Couchman, 2010). Syndecans are expressed on different cells and locations at different times and levels (Bernfield et al., 1992). In adult tissues, syndecan-1 is predominantly expressed by both simple and stratified epithelial cells and plasma cells, although it is expressed at a lower level in several other cell types and its expression can be induced in these cells. Syndecan-2 is typically expressed by endothelial cells and mesenchymal cells, whereas syndecan-3 expression is mostly restricted to neural crest-derived cells. Syndecan-4 is expressed ubiquitously, but it is expressed at lower levels than other co-expressed syndecans on any given cell type.
Syndecans bind to and regulate many HS- and heparin-binding molecules (Bernfield et al., 1999; Fears and Woods, 2006). However, much debate surrounds the biological significance and specificity of syndecan interactions due to the large number of potential ligands. Available data indicate that syndecans can regulate the biological activity of ligands by affecting their stability, conformation, oligomerization, or compartmentalization (Park et al., 2000b). Further, although all syndecans contain the primary ligand-binding HS chains, they show distinct temporal and spatial expression patterns (Hayashi et al., 1987; Kim et al., 1994) and, thus, are likely to function specifically in vivo. In addition, syndecans can bind to ligands through their core protein (McFall and Rapraeger, 1998; Chen et al., 2004; Hayashida et al., 2006; Dews and Mackenzie, 2007; Beauvais et al., 2009; Couchman, 2010; Purushothaman et al., 2010), suggesting that extracellular, intramembrane, and intracellular interactions mediated by the core protein are also important in the biological functions of syndecans.
On the cell surface, syndecans function primarily as coreceptors that catalyze the encounter between ligands and their respective signaling receptors (Bernfield et al., 1999; Park et al., 2000b). Syndecans can also function as soluble HSPGs as their extracellular domain, replete with all their HSchains, can be proteolytically released from the cell surface by a process known as ectodomain shedding (Jalkanen et al., 1987; Bernfield et al., 1999; Hayashida et al., 2010). Syndecan shedding is thought to be an important post-translational mechanism that regulates syndecan functions as it both rapidly reduces the amount of cell surface HS and generates soluble syndecan ectodomains that can function as autocrine or paracrine effectors. Syndecan shedding is induced in vitro by several inflammatory factors (Fitzgerald et al., 2000; Park et al., 2004; Charnaux et al., 2005; Brule et al., 2006; Chung et al., 2006; Mahtouk et al., 2007; Yang et al., 2007) and in vivo under certain pathological conditions (Kainulainen et al., 1998; Kato et al., 1998; Park et al., 2001; Yang et al., 2002; Haynes et al., 2005; Xu et al., 2005; Hayashida et al., 2008a, 2009a, 2009b; Kliment et al., 2009; Hayashida et al., 2011; Haywood-Watson et al., 2011). The majority of syndecan shedding agonists induce shedding by activating outside-in signaling pathways (Fitzgerald et al., 2000; Park et al., 2000a, 2004; Hayashida et al., 2008b), and several metalloproteinases can shed syndecan ectodomains (Li et al., 2002; Endo et al., 2003; Ding et al., 2005; Brule et al., 2006; Fears et al., 2006; Purushothaman et al., 2008; Pruessmeyer et al., 2010). These data suggest that the timing, location, and extent of syndecan shedding are regulated by a hierarchical mechanism involving shedding agonists, intracellular signaling pathways, and metalloproteinases.
Surprisingly, mice lacking syndecan-1 (Alexander et al., 2000; Park et al., 2001; Stepp et al., 2002) or syndecan-4 (Echtermeyer et al., 2001; Ishiguro et al., 2001) are healthy and fertile, and do not show major pathologies. However, dramatic pathological phenotypes emerge when both syndecan-1 null (Sdc1−/−) (Table 1) and Sdc4−/− (Echtermeyer et al., 2001; Ishiguro et al., 2001; Kon et al., 2008; Echtermeyer et al., 2009; Jiang et al., 2010; Ikesue et al., 2011) mice are challenged with disease-causing agents or conditions. These observations suggest that other syndecans or HSPGs can functionally compensate for the loss of syndecan-1 or -4 during development, but not in certain post-developmental processes, such as the pathogenesis of diseases. This review seeks to provide an overview of the key molecular functions of syndecan-1 in inflammatory diseases, cancer, and infection.
Table 1.
Partial list of syndecan-1 functions in animal and cellular models of diseases.
| Disease | Sdc1−/− mouse and Sdc1 KD cell phenotype | Target molecule | Suggested function | References |
|---|---|---|---|---|
| Inflammation | ||||
| Acute lung injury | Decreased CXCL1 in alveolar space, increased PMNs in perivascular space | CXCL1 | Generates CXCL1 chemokine gradient for transepithelial PMN migration into the alveolar space | (Li et al., 2002) |
| Allergic lung inflammation | Exaggerated Th2 response, AHR, eosinophilia, glycoprotein hypersecretion | CCL7, CCL11, CCL17 | Inhibits CC chemokine-mediated Th2 cell homing to the lung | (Xu et al., 2005) |
| Endotoxic shock | Sustained high levels of CXCL1 and CXCL2 in tissues, increased PMN accumulation, multi-organ injury, lethality | CXCL1, CXCL2 | Facilitates removal of sequestered CXCL1 and CXCL2, resolution of PMN inflammation | (Hayashida et al., 2009b) |
| Gram-positive toxic shock | Increased serum TNFα and IL-6, T cell infiltration, multi-organ injury, lethality | IFNγ, MIG | Inhibits amplification of cytokine storm by IFNγ, MIG- mediated T cell recruitment | (Hayashida et al., 2008a) |
| Protein-losing enteropathy | Increased intestinal protein leakage | IFNγ, TNFα | Inhibits IFNγ and/or TNFα | (Bode et al., 2008) |
| Colitis | Impaired mucosal repair, increased expression of inflammatory factors, leukocyte recruitment, lethality | FGF-2, ICAM-1 | Promotes intestinal epithelial wound repair, inhibits leukocyte adhesion onto endothelial cells | (Day and Forbes, 1999; Floer et al., 2010) |
| Vascular injury | Increased neointimal injury, vascular SMC proliferation | PDGF-B, PDGFRβ | Inhibits SMC PDGF-B expression and PDGFRβ activation | (Fukai et al., 2009) |
| Skin injury | Delayed wound repair, reduced keratinocyte proliferation | α9 integrins | Regulates expression and localization of α9 integrins | (Stepp et al., 2002) |
| Anti-GBM nephritis | Increased T cell influx, albuminuria, serum creatinine | Th2 cytokines and chemokines | Regulates Th1/Th2 balance | (Rops et al., 2007) |
| Allergic contact dermatitis | Increased expression of pro-inflammatory factors, leukocyte recruitment, edema formation | β2 integrins, ICAM-1 | Inhibits β2 integrin-ICAM-1 interaction | (Kharabi Masouleh et al., 2009) |
| Myocardial infarction | Increased leukocyte influx, MMP activity, cardiac dysfunction, collagen fragmentation | MMPs, adhesion molecules | Inhibits leukocyte recruitment, collagen degradation | (Vanhoutte et al., 2007) |
| Cardiac fibrosis | Reduced CTGF, collagens, cardiac dysfunction | Angiotensin II, TGFβ | Amplifies angiotensin II and TGFβ signaling | (Schellings et al., 2010) |
| Idiopathic pulmonary fibrosis | Impaired repair in epithelial cells treated with Sdc1 siRNA, reduced migration, increased proliferation in cells treated with Sdc1 ectodomain | EC-SOD, TGFβ | Promotes epithelial repair (cell surface Sdc1), inhibits epithelial repair, promotes PMN migration, TGFβ release, fibroblast proliferation (shed Sdc1) | (Kliment et al., 2009) |
| Lipoprotein metabolism | Increased TRLs in plasma | Plasma lipoproteins | Mediates uptake of hepatic and intestinal TRLs | (Stanford et al., 2009) |
| Cancer | ||||
| Tumorigenesis | Reduced Wnt-induced mammary gland hyperplasia | Wnt | Promotes Wnt signaling | (Alexander et al., 2000) |
| Cell growth and survival | Altered HGF-Met signaling when HS or Sdc1 expression is manipulated in cancer cells | HGF | Enhances HGF-Met signaling | (Derksen et al., 2002; Ramani et al., 2011) |
| Apoptosis | Increased apoptosis in cancer cells treated with Sdc1 shRNA or Sdc1 ectodomains | Growth factors | Sequesters growth factors, inhibits signaling | (Sun et al., 2008; Khotskaya et al., 2009) |
| Angiogenesis | Diminished heparanase-induced endothelial invasion when Sdc1 immunodepleted | VEGF | Activates integrins and VEGF receptors on adjacent endothelial cells | (Purushothaman et al., 2008) |
| Invasion and metastasis | Increased motility and invasion, decreased adhesion to collagen when cancer cells treated with Sdc1 siRNA | α2β1, αvβ3, αvβ5 integrins | Potentiates integrin activities | (Beauvais et al., 2009; Ishikawa and Kramer, 2010) |
| Infection (pathogen) | ||||
| HEV | Reduced viral attachment and entry in cells with reduced Sdc1 | pORF2 | Promotes viral attachment | (Kalia et al., 2009) |
| HSV | Reduced viral attachment and entry in cells with reduced Sdc1 | gB, gC, and gD | Promotes viral attachment and entry | (Bacsa et al., 2011) |
| Neisseria gonorrhoeae | Decreased invasion in cells expressing Sdc1 lacking the cytoplasmic domain | OpaHSPG | Promotes signaling required for bacterial invasion | (Freissler et al., 2000) |
| Pseudomonas aeruginosa | Reduced bacterial burden and lethality in lung and skin infection | Cationic antimicrobial factors | Inhibits antimicrobial factors | (Park et al., 2001; Haynes et al., 2005) |
| Staphylococcus aureus | Reduced corneal bacterial burden | PMNs | Inhibits extracellular killing mechanisms of PMNs | (Hayashida et al., 2011) |
| Trichinella spiralis | Similar extent of infection between Wt and Sdc1−/− mice, reduced Th2 responses | Th2 cytokines | Moderates Th2 responses | (Beiting et al., 2006) |
| Plasmodium spp. | Similar extent of infection between Wt and Sdc1−/− mice in P. yoelii infection | PfEMP1 | Promotes parasitic attachment and dissemination | (Bhanot and Nussenzweig, 2002) |
2. Inflammatory diseases
Inflammation is a fundamental host response to endogenous or exogenous agents that have the potential to cause tissue injury. The inflammatory response removes or sequesters harmful agents, and facilitates the restoration of the normal structure and function of damaged tissues. Multiple regulatory mechanisms have evolved to actively contain and resolve the inflammatory response in a timely manner to prevent excessive inflammatory tissue injury. However, when one or several of these mechanisms fail, the inflammatory response can be exaggerated or sustained, and lead to several acute and chronic diseases, such as acute lung injury, sepsis, colitis, arthritis, asthma, and fibrosis of the lung, heart, skin, kidney, and liver. Syndecan-1 binds to many factors that mediate and regulate the inflammatory response (Bartlett et al., 2007). The significance of syndecan-1 interactions in inflammatory diseases is highlighted in several studies examining the response of animals or cells whose expression or function of syndecan-1 has been experimentally altered.
2.1. Syndecan-1 in leukocyte recruitment
The acute inflammatory response, which occurs in minutes to hours, involves the coordinated delivery of plasma proteins and recruitment of leukocytes to the site of infection or injury. Although exaggerated or sustained leukocyte responses lead to unwanted tissue injury, an adequate and timely recruitment of leukocytes is essential for repair as wounds tend to heal poorly and may even lead to lethal outcomes in individuals with insufficient leukocytes (Lekstrom-Himes and Gallin, 2000). Available data indicate that syndecan-1 regulates several steps of leukocyte recruitment in non-infectious inflammatory diseases (Fig. 1).
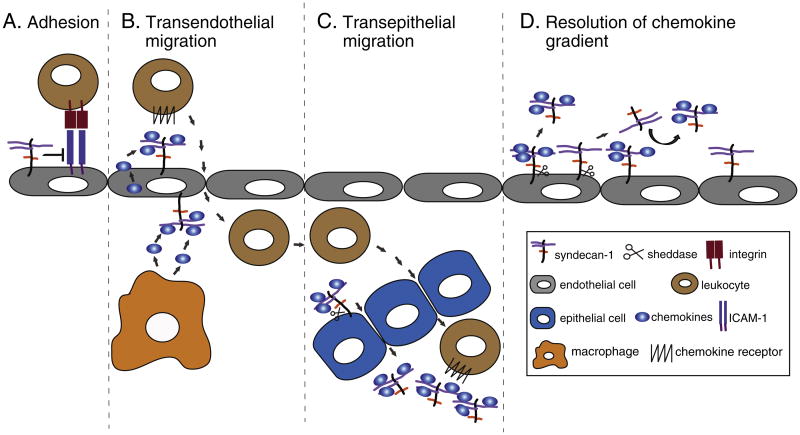
Fig. 1. Mechanisms of syndecan-1 in inflammatory diseases.
A) Syndecan-1 inhibits leukocyte adhesion to activated endothelial cells by inhibiting the β2 integrin-ICAM-1 interaction. B) Endothelial syndecan-1 binds to chemokines and generates a chemokine gradient that facilitates the transendothelial migration of leukocytes. Cell surface syndecan-1 is shown in both the basal and apical surfaces as the normal polarized expression of syndecan-1 on the basolateral surface is disrupted by injury. C) MMP-7-mediated syndecan-1 shedding in alveolar epithelial cells generates a chemokine gradient for the transepithelial migration of neutrophils. D) Syndecan-1 shedding resolves chemokine gradients by removing chemokines tethered to cell surface syndecan-1 or by displacing chemokines tethered to other HSPGs through the release of unbound syndecan-1 ectodomains.
In oxazolone-induced delayed-type hypersensitivity (DTH), a widely used model of allergic contact dermatitis, Sdc1−/− mice showed increased leukocyte recruitment and prolonged edema formation (Kharabi Masouleh et al., 2009). The expression of pro-inflammatory cytokines (TNFα, IL-6), chemokines (CCL5, CCL3), and the adhesion molecule ICAM-1 was significantly increased in Sdc1−/− mice compared to wild type (Wt) mice. Syndecan-1 expression was reduced in Wt mice during the DTH response. Further, blocking antibodies against CD18 inhibited the increased adhesion of Sdc1−/− leukocytes to ICAM-1 (Kharabi Masouleh et al., 2009). Similarly, in dextran sodium sulfate-induced colitis, an exaggerated and prolonged recruitment of leukocytes was observed in Sdc1−/− mice relative to Wt mice (Floer et al., 2010). This was accompanied by increased expression of TNFα, CCL3 and VCAM-1, impaired mucosal repair, and higher mortality, consistent with the reduced expression of syndecan-1 and impaired gut mucosal repair seen in patients with inflammatory bowel disease (Day and Forbes, 1999). Enhanced leukocyte adhesion and transendothelial migration, and increased expression of pro-inflammatory factors (e.g., MCP-1) were also observed in Sdc1−/− mice subjected to myocardial infarction by permanent ligation of the left coronary artery (Vanhoutte et al., 2007).
These data suggest that one of the primary functions of syndecan-1 in inflammation is to negatively regulate leukocyte adhesion and migration, possibly by inhibiting the interactions between leukocyte integrins and endothelial ICAM-1 and VCAM-1. Interestingly, Sdc1−/− neutrophils showed increased adhesion to endothelial cells and ICAM-1 in vitro, which were reduced to Wt levels by the addition of low molecular weight heparin (Kharabi Masouleh et al., 2009), suggesting that syndecan-1 on neutrophils negatively regulate neutrophil adhesion to endothelial cells in an HS-dependent manner. However, as evident from the usage of syndecan-1 (CD138) as a marker for plasma cells and tumors of the B cell lineage (Sanderson and Borset, 2002), leukocytes other than plasma cells do not normally express syndecan-1. Induction of syndecan-1 in activated macrophages has been reported (Yeaman and Rapraeger, 1993), but this has not been observed in other leukocytes. Thus, it is likely that syndecan-1 on endothelial cells or syndecan-1 derived from epithelial cells functions as inhibitors of leukocyte adhesion (Fig. 1A). Alternatively, the loss of syndecan-1 may have led to the generation of functionally abnormal leukocytes, but the effects of syndecan-1 on leukocyte maturation and differentiation have not been explored.
Syndecan-1 also regulates the generation and activity of chemokine gradients in inflammatory diseases (Fig. 1B). Most chemokines bind to HSPGs through discrete domains containing a stretch of positively charged amino acids (Lortat-Jacob et al., 2002; Handel et al., 2005). It is generally thought that this interaction is important in tethering the chemokines to endothelial cell surfaces at the site of infection or injury, activating the weakly bound leukocytes and inducing their firm adhesion onto the endothelium, and generating a chemokine gradient that guides the directional migration of leukocytes (Handel et al., 2005; Bao et al., 2010). However, HSPGs can also directly potentiate (Webb et al., 1993; Middleton et al., 1997; Dias-Baruffi et al., 1998) or inhibit chemokine activities (Kuschert et al., 1999), illustrating the complexity of how HSPGs regulate chemokines.
In a mouse model of bleomycin-induced acute lung injury, syndecan-1 shedding was found to facilitate the generation of a CXC chemokine gradient that directs the transepithelial migration of neutrophils (Fig. 1C) (Li et al., 2002). Here, CXCL1 binds to cell surface syndecan-1 and the complex is shed by MMP-7 into the alveolar space, generating a chemotactic gradient across the alveolar epithelium in the basal to apical direction. Hence, in both Sdc1−/− and Mmp7−/− mice, there was a significant reduction in CXCL1 levels and neutrophils in bronchoalveolar lavage fluids (BAL), whereas CXCL1 was found complexed to syndecan-1 ectodomains in Wt BAL (Li et al., 2002). This mechanism is thought to confine neutrophilic inflammation to specific sites of tissue injury where it is needed for normal repair, and to limit excessive inflammatory tissue damage.
In a mouse model of allergic lung disease, syndecan-1 ectodomains shed by intranasal allergen challenge bound to CC chemokines (CCL7, CCL11, CCL17) and inhibited the recruitment of Th2 cells to the lung, dampening the Th2 inflammatory response (Xu et al., 2005). The syndecan-1 HS chains mediate this antiinflammatory activity, as HS attenuated parameters of allergic lung disease, but syndecan-1 core proteins lacking HS did not. Consistent with this mechanism, allergen-instilled Sdc1−/− mice showed overwhelming lung inflammation compared to Wt mice (Xu et al., 2005).
Increased T cell influx was also observed in Sdc1−/− mice subjected to animal models of Gram-positive toxic shock (Hayashida et al., 2008a) and anti-glomerular basement membrane (GBM) nephritis (Rops et al., 2007). Sdc1−/− mice injected intraperitoneally with staphylococcal superantigens showed increased serum levels of TNF and IL-6, tissue levels of CXCL9, multi-organ injury, and lethality as compared to Wt mice (Hayashida et al., 2008a). T cell depletion rescued Sdc1−/− mice from Gram-positive toxic shock, indicating that increased T cell infiltration, likely mediated by CXCL9, is the cellular cause of enhanced susceptibility. Intraperitoneal injection of neutralizing anti-IFNγ antibodies partially, yet significantly reduced disease parameters in Sdc1−/− mice. Because HS binds to and inhibits IFN (Fritchley et al., 2000; Sarrazin et al., 2005), these data suggest that syndecan-1 suppresses Gram-positive toxic shock by inhibiting the capacity of IFN to induce the expression of pro-inflammatory factors (e.g., TNFα, CXCL9) and subsequent T cell infiltration. The molecular cause of increased T cell influx in the autologous phase of anti-GBM nephritis is not known, but there was increased expression of Th2 cytokines and chemokines in Sdc1−/− kidneys (Rops et al., 2007), suggesting that syndecan-1 regulates the Th1/Th2 balance in this inflammatory disease.
2.2. Syndecan-1 in the resolution of inflammation
Nonresolving inflammation is a major cause of many diseases (Serhan, 2007; Nathan and Ding, 2010). The acute inflammatory response occurs at the expense of the normal functioning of tissues as leukocytes help to heal tissues as well as to destroy them. Thus, it is imperative to rapidly halt the inflammatory response once the harmful agents are removed. In a mouse model of endotoxic shock, syndecan-1 shedding was found to facilitate the resolution of neutrophil inflammation by removing sequestered CXC chemokines in an HS-dependent manner (Hayashida et al., 2009b). Endotoxemic Sdc1−/− mice showed significantly increased multi-organ injury and dysfunction and lethality compared to endotoxemic Wt mice. Interestingly, the increased morbidity and mortality of endotoxemic Sdc1−/− mice were due to anexuberant local inflammatory response and not because of an enhanced systemic inflammatory response. High levels of CXCL1 and CXCL2 were sustained in multiple Sdc1−/− organs at later times after LPS, and this was associated with prolonged neutrophil inflammation and enhanced organ damage. Inhibition of syndecan-1 shedding in endotoxemic Wt mice inhibited the removal of tissue-associated CXCL1 and CXCL2 and impeded the resolution of neutrophilic inflammation in multiple organs, leading to more severe disease. In contrast, administration of HS facilitated the removal of tissue-associated CXCL1 and CXCL2, halted neutrophil accumulation, and attenuated multi-organ injury and lethality in endotoxemic Sdc1−/− mice.
Although the precise manner in which syndecan-1 shedding facilitates the removal of tissue-associated CXCL1 and CXCL2 is unclear, the newly synthesized CXC chemokines may be tethered to syndecan-1 on the endothelial cell surface, and syndecan-1 shedding may release the sequestered chemokines, resulting in dispersion of the CXC chemokine gradient for neutrophil infiltration (Fig. 1D). Also, because syndecan-1 is expressed in excess of CXCL1 and CXCL2, syndecan-1 shedding may release large amounts of unbound syndecan-1ectodomains that can displace CXC chemokines tethered to endothelial syndecan-1 and other HSPGs (Fig. 1D). Altogether, these findings suggest that syndecan-1 shedding is an important post-translational mechanism that confines, attenuates, or resolves inflammation by modulating HS-binding pro-inflammatory factors.
2.3. Syndecan-1 in matrix remodeling
Remodeling of the ECM is critical in the restoration of the normal structure and function of injured tissues. Fibrogenic factors induce the synthesis of matrix components, and epithelial, mesenchymal, and inflammatory cells assemble the developing ECM using cross-linking enzymes, matrix receptors, and matrix-degrading enzymes. Syndecan-1 has been shown to modulate matrix assembly in several models of inflammatory diseases. Sdc1−/− mice subjected to myocardial infarction showed abnormal infarct healing, associated with the assembly of a disorganized matrix with a predominance of smaller and fragmented collagen fibers (Vanhoutte et al., 2007). Increased inflammatory cells in Sdc1−/− mice contributed to increased MMP-2 and MMP-9 activity and degradation of tissue transglutaminase by MMP-2 (Vanhoutte et al., 2007). Consistent with the role of tissue transglutaminase in collagen fiber assembly (Lorand and Graham, 2003), these deficiencies led to impaired collagen cross-linking and increased cardiac dilatation and failure in Sdc1−/− mice. Further, overexpression of syndecan-1 reduced inflammation and protected against cardiac dilatation and failure in Sdc1−/− mice (Vanhoutte et al., 2007), suggesting that syndecan-1 plays a key role in the normal remodeling of injured cardiac tissues.
However, syndecan-1 can also exacerbate cardiac fibrosis, as seen in a mouse model of angiotensin II-induced cardiac fibrosis (Schellings et al., 2010). Syndecan-1 expression was increased predominantly in the fibrotic regions of the left ventricle after angiotensin II treatment. The loss of syndecan-1 protected mice against angiotensin II-induced cardiac dysfunction and fibrosis by blunting the effects of angiotensin II and TGFβ1 on cardiac expression of connective tissue growth factor (CTGF) and collagens I and III. HS chains of syndecan-1 are important as protamine decreased CTGF and collagen I levels, while recombinant syndecan-1 without HS did not affect CTGF levels (Schellings et al., 2010). Further, HS binds to angiotensin II (Koppel et al., 2003) and the loss of syndecan-1 mitigated cardiac dysfunction and fibrosis in a Smad2-dependent manner (Schellings et al., 2010). These findings suggest that syndecan-1 may promote cardiac fibrosis by amplifying angiotensin II and TGFβ1 signaling in an HS-dependent manner.
Once solubilized, syndecan-1 ectodomains can exhibit functions similar to or different from cell surface syndecan-1. In idiopathic pulmonary fibrosis (IPF), syndecan-1 expression is increased in fibrotic lungs and syndecan-1 ectodomains are increased in BAL and lung homogenates from IPF patients (Kliment et al., 2009). Syndecan-1 ectodomains are also increased in BAL and lung homogenates in extracellular superoxide dismutase (EC-SOD) null mice subjected to asbestos- or bleomycin-induced lung fibrosis (Kliment et al., 2009), consistent with the protective role of EC-SOD in oxidative stress-induced syndecan-1 shedding (Kliment and Oury, 2011). Interestingly, cell surface syndecan-1 was found to promote alveolar re-epithelialization, whereas syndecan-1 ectodomains shed by oxidative stress increased neutrophil chemotaxis and inhibited re-epithelialization (Kliment etal., 2009). Syndecan-1 ectodomains also increased fibroblast proliferation and the release of TGFβ1 (Kliment et al., 2009). Thus, cell surface syndecan-1 apparently attenuates fibrosis by promoting repair, whereas syndecan-1 ectodomain promotes fibrosis by inhibiting epithelial repair and up-regulating fibrotic factors and processes. The underlying mechanism of how this is accomplished is not known. Because reactive oxygen species (ROS) can bind to and modify HS (Rees et al., 2005), syndecan-1 ectodomains shed by oxidative stress may exhibit different HS structures and function from those of membrane-bound syndecan-1. Further, because EC-SOD directly binds to HS and heparin (Sandstrom et al., 1992; Kliment et al., 2008), this anti-oxidant may not only protect syndecan-1 from shedding but also from modification by ROS. Future studies directed at defining the structure: function relationship of oxidized syndecan-1 HS should address these potentially important mechanisms in fibrotic diseases.
2.4. Other functions of syndecan-1 in inflammatory diseases
Syndecan-1 has also been shown to regulate protein leakage in the intestinal epithelium (Bode et al., 2008), smooth muscle cell (SMC) proliferation in vascular injury (Fukai et al., 2009), keratinocyte proliferation in dermal injury (Stepp et al., 2002), and lipoprotein metabolism (Stanford et al., 2009). Protein-losing enteropathy (PLE) is characterized by excessive efflux of plasma proteins into the intestinal lumen, and is often associated with viral infections, increased IFNγ and TNFα levels, and a pro-inflammatory state (Lenz et al., 2003). Loss of syndecan-1 or HS from the basolateral surface of intestinal cells was found to be closely associated with PLE episodes, whereas both were induced when the disease resolved (Bode et al., 2008). More importantly, Sdc1−/− and Sdc1−/− intestinal epithelium showed increased basal protein leakage and were susceptible to protein loss upon IFNγ and TNFα challenge and increased venous pressure (Bode et al., 2008). Because HS/heparin can bind to and inhibit IFNγ (Fritchley et al., 2000; Sarrazin et al., 2005) and TNFα (Lantz et al., 1991), syndecan-1 may potentially attenuate protein leakage by blocking the activities of these pro-inflammatory cytokines in an HS-dependent manner. Addition of 2-O- and 3-O-desulfated heparin rescued the pathological phenotypes (Bode et al., 2008), suggesting that these sulfate modifications in syndecan-1 HS are not required. However, precisely how loss of syndecan-1 or HS leads to increased intestinal epithelial leakage is unclear as epithelial tight junctions were determined to be intact at the EM level (Bode et al., 2008). Perhaps syndecan-1 is required for the maintenance of normal epithelial morphology and of other epithelial junctions, such as adherens junctions, as syndecan-1 expression has been linked to E-cadherin expression and loss of syndecan-1 induces an epithelial to mesenchymal transition (EMT)-like process in mammary gland epithelial cells (Kato et al., 1995). Alternatively, because IFNγ and TNFα synergize to induce syndecan-1 shedding (Henry-Stanley et al., 2006) and because HS has been proposed as an impermeable molecule that seals epithelial gaps (Watson et al., 2005), syndecan-1 ectodomains shed by the pro-inflammatory cytokines may serve this function in preventing PLE.
Arterial injury induces SMC proliferation and migration, and intimal accumulation of cells and ECM. Syndecan-1 was found to regulate these processes in a mouse model of ligation-induced carotid artery injury (Fukai et al., 2009). Sdc1−/− mice developed a larger neointimal lesion after injury compared to Wt mice that developed little to no lesion. Injured Sdc1−/− arteries also showed a significant increase in SMCs in both the media and intima, suggesting that the primary function of syndecan-1 in arterial injury is to inhibit abnormal SMC proliferation. Interestingly, cultured Sdc1−/− SMCs showed increased proliferation in response to PDGF-BB, thrombin, FGF-2, EGF and serum, and this was accompanied by increased mRNA levels of PDGF-B chain. DNA synthesis in Sdc1−/− SMCs treated with thrombin, PDGF-BB, or serum was inhibited by the down-regulation of PDGF-BB or PDGFRβ. Because HS can inhibit arterial SMC proliferation and migration (Bingley et al., 1998), these findings suggest that HS chains of syndecan-1 regulate SMC proliferation in arterial injury by inhibiting PDGF signaling and possibly signaling by other HS-binding growth factors, such as FGF-2 and EGF. In contrast, keratinocyte proliferation was reduced in Sdc1−/− mice in a model of dermabrasion (Stepp et al., 2002). This was accompanied by reduced localization of a9 integrin in the injured Sdc1−/− epidermis. A subsequent study showed that TGFβ1 increases the cell surface expression of α2β1, αvβ6, αvβ8 and α6β4 integrins in cultured Wt keratinocytes, but not in Sdc1−/− keratinocytes (Stepp et al., 2007). These data suggest that syndecan-1 modulates keratinocyte proliferation by regulating integrin expression and the cross-talk between integrins and growth factor signaling.
Elevated plasma triglyceride is a key risk factor for atherosclerosis. Syndecan-1 was identified as the primary HSPG receptor that mediates hepatic clearance of triglyceride-rich lipoproteins (TRLs) in mice (Stanford et al., 2009). Sdc1−/− mice showed increased plasma levels of TRLs after fasting and delayed clearance of TRLs during consumption of normal dietary fat. This was specific to the loss of syndecan-1, as Sdc3−/− and Sdc4−/− mice did not show these phenotypes and adenovirus-mediated syndecan-1 overexpression rescued Sdc1−/− mice from the pathological phenotypes (Stanford et al., 2009). The abundant expression of syndecan-1 on the microvilli of hepatocyte basal membranes suggests that syndecan-1 is well positioned to capture circulating TRLs. Binding of TRLs to the HS chains of syndecan-1 could occur through electrostatic interactions between negatively charged sulfate and carboxyl groups with positively charged groups in apolipoproteins or lipases (Stanford et al., 2009), and TRLs may be internalized when syndecan-1 undergoes endocytosis (Fuki et al., 1997).
3. Cancer
Syndecan-1 expression is dysregulated in many cancers, including carcinomas of the prostate (Kiviniemi et al., 2004), breast (Lendorf et al., 2011), pancreas (Juuti et al., 2005), ovary (Kusumoto et al., 2010), and colon (Hashimoto et al., 2008). Low syndecan-1 expression in tissue is associated with a worse prognosis in head and neck (Anttonen et al., 1999), lung (Anttonen et al., 2001) and colorectal (Lundin et al., 2005) cancers, whereas high levels of shed syndecan-1 in serum correlate with a poor prognosis in lung cancer (Joensuu et al., 2002) and myeloma (Seidel et al., 2000b). These findings suggest that syndecan-1, like many other proteoglycans, has important functions in tumor biology (Iozzo and Sanderson, 2011). Indeed, accumulating evidence suggests that syndecan-1 modulates several key processes of tumorigenesis, such as cancer cell proliferation and apoptosis, angiogenesis, and metastasis (Fig. 2).
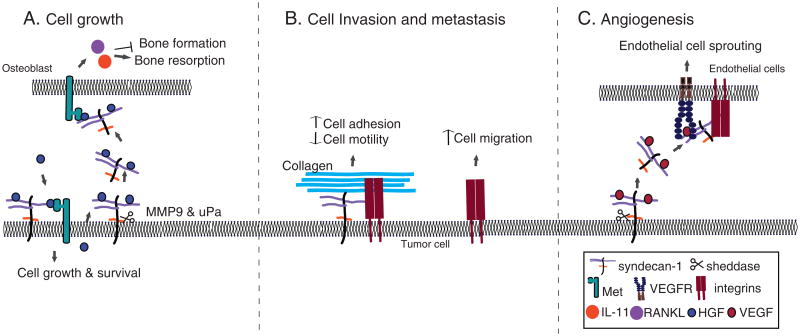
Fig. 2. Mechanisms of syndecan-1 in cancer.
A) In myeloma, cell surface syndecan-1 binds to HGF via HS chains and promotes Met activation, leading to myeloma cell growth and survival. Shed syndecan-1 also binds to and presents HGF to osteoblasts. This results in the activation of IL-11 and upregulation of RANKL, leading to inhibition of bone formation and promotion of bone resorption. B) Cell surface syndecan-1 mediates cancer cell adhesion to the ECM and retards cancer cell migration. C) Syndecan-1 shedding releases the VEGF-syndecan-1 ectodomain complex, which binds to and activates VEGFRs on endothelial cells. Syndecan-1 can also activate αvβ3 integrin, which mediates the activation of VEGF signaling. This transactivation mechanism stimulates endothelial cell sprouting and subsequent angiogenesis.
3.1. Syndecan-1 in cancer cell proliferation and apoptosis
Syndecan-1 can affect tumorigenesis by regulating mediators of tumor cell survival and proliferation (e.g., oncogenes, growth factors). For example, Sdc1−/− mice were protected against Wnt-1 induced mammary tumorigenesis (Alexander et al., 2000). Further, syndecan-1 ectodomains, but not syndecan-1 core protein devoid of HS, potentiated the activity of Wingless, a Drosophila Wnt-1 homologue, in transgenic Drosophila S2 cells expressing the Wnt receptor DFz2 in a dose-dependent manner (Alexander et al., 2000). These findings suggest that syndecan-1 functions as a coreceptor for Wnt signaling in an HS-dependent manner. A subsequent study suggested that syndecan-1 affects the capacity of Wnt to induce the accumulation of mammary progenitor cells (Liu et al., 2004), but it is not known if syndecan-1 similarly regulates Wnt signaling in other target tissues of Wnt.
Hepatocyte growth factor (HGF) binds to the HS chains of syndecan-1 on myeloma cells (Seidel et al., 2000a; Derksen et al., 2002). The signaling receptor for HGF is Met, and Met signaling has been implicated in the growth, survival, and spread of various cancers (Birchmeier et al., 2003; Christensen et al., 2005). Importantly, the interaction of HGF with syndecan-1 potentiated Met signaling, which involves the activation of PI3 kinase-protein kinase B and Ras-MAP kinase pathways (Derksen et al., 2002). Syndecan-1 shedding adds another dimension to the regulation of HGF-Met signaling in myeloma biology (Fig. 2A). Heparanase induced syndecan-1 shedding in myeloma cells through the upregulation of MMP-9 and urokinase-type plasminogen activator (uPA), both of which can act as a syndecan-1 sheddase (Purushothaman et al., 2008). Heparanase also induced HGF expression in myeloma cells, and newly synthesized HGF associated with syndecan-1 ectodomains and the HGF-syndecan-1 ectodo-main complex enhanced Met signaling in osteoblasts and stromal cells (Ramani et al., 2011). This paracrine Met signaling activated IL-11 and upregulated the receptor activator of NF-κB ligand (RANKL) in osteoblast-like Saos2 cells. IL-11 inhibits bone formation (Hughes and Howells, 1993) and supports bone resorption (Shaughnessy et al., 2002). Further, heparin enhances IL-11 signaling through activation of ERK MAP kinase (Rajgopal et al., 2006), suggesting that syndecan-1 functions in an HS-dependent manner. Together, these data suggest that syndecan-1 synergizes with heparanase to promote myeloma bone disease by activating the HGF-Met-IL-11-RANKL signaling axis.
The tumor microenvironment provides a conductive milieu for the growth and progression of tumor cells. Syndecan-1 expression is elevated in the reactive stroma of breast carcinoma tissue (Stanley et al., 1999). Invasive breast cancer cells induced the expression of syndecan-1 in mouse embryonic fibroblasts (MEFs) in vitro, but this was not observed with normal or less invasive cell lines. Moreover, syndecan-1 expressing MEFs enhanced the growth of breast cancer cell lines in co-culture and promoted breast carcinoma progression in vivo (Maeda et al., 2004), both in an HS-dependent manner. Syndecan-1 expression in reactive stromal fibroblasts could store and present heparin-binding growth factors such as FGFs, HGF, and EGFs to cancer cells. Indeed, shedding of syndecan-1 ectodomains from stromal fibroblasts stimulated tumor cell growth through the activation of FGF2 and SDF1 signaling (Su et al., 2007). A subsequent study identified membrane type 1 matrix metalloproteinase (MT1-MMP) as the stromal syndecan-1 sheddase in breast carcinoma (Su et al., 2008). These studies reveal an interesting mechanism where tumor cells utilize syndecan-1 shedding to derive growth factors from the neighboring stroma to maintain its growth conductive environment.
Syndecan-1 was also found to regulate tumor cell apoptosis. Syndecan-1 knock-down in myeloma cells induced growth arrest and apoptosis (Khotskaya et al., 2009). This could be due to diminished pro-survival signals as a result of reduced levels of cell surface HSPG coreceptors for growth factor signaling. However, several studies suggest that syndecan-1 can also directly promote tumor cell apoptosis. For example, recombinant human syndecan-1 ectodomains induced apoptosis in MCF-7 breast cancer cells (Sun et al., 2008). Syndecan-1 expression was determined to be upregulated through n−3 polyunsaturated fatty acids (n−3 PUFA)-activated peroxisome proliferator-activated receptor γ (PPARγ) signaling, which promoted apoptosis in breast cancer cells. Docosahexaenoic acid (DHA,an n−3 PUFA)-mediated upregulation of syndecan-1 expression and direct addition of syndecan-1 ectodomains also led to apoptosis of cultured human prostate cancer cells (Hu et al., 2010).
It is not clear how both cell surface and shed syndecan-1 can stimulate growth and survival in myeloma cells and yet also elicit apoptosis in other cancer cells. Perhaps this apparent paradox could be explained by tumor cell-specific requirements for growth factor signaling. In addition, the bell-shaped activity curve of HS in regulating HS-binding ligands may explain the opposing functions of syndecan-1 in tumor cell apoptosis. HS generally serves as a scaffold that brings both ligand and its binding partner to proximity. In other words, HS potentiates the interaction by increasing the local concentration of the ligand and the bell-shaped curve may be concave or convex depending on whether HS-binding is inhibitory or stimulatory for the ligand. Thus, at either ends of the optimal HS concentration, HS activity is low or absent and will not potentiate growth factor signaling. Further, the tumor microenvironment is likely unique to the distinct tumors, and HS chains of syndecan-1 may be differentially modified to either promote or attenuate tumor cell growth. Indeed, both growth promoting and growth inhibiting sequences are present in HS (Liu et al., 2002a). Whether one or several of these mechanisms control the growth-modulating activity of syndecan-1 in cancer remains to be explored.
3.2. Syndecan-1 in cancer cell invasion and metastasis
Metastasis of cancer cells is a multi-step process that involves cell adhesion and migration and ECM degradation (Hanahan and Weinberg, 2011). Cell surface syndecan-1 is thought to promote cell adhesion to the ECM and retard cancer cell migration. For example, head and neck squamous cell carcinoma (HNSCC) cells expressing high levels of syndecan-1 migrated poorly and were less invasive in collagen I matrices as compared to HNSCC cells expressing lower levels of syndecan-1 (Ishikawa and Kramer, 2010). Loss of syndecan-1 diminished cell adhesion to collagen substrates but enhanced cell motility and invasion through 3-D collagen gels. Inhibition of the α2β1 integrin by anti-α2 monoclonal antibodies blocked attachment of syndecan-1 silenced HNSCC cells to collagen I (Ishikawa and Kramer, 2010), suggesting that syndecan-1 is a coreceptor for α2β1 binding to collagen I.
Integrins are key regulators of cell adhesion, proliferation, and migration (Hynes et al., 2002; Desgrosellier and Cheresh, 2010; Rathinam and Alahari, 2010). There is ample evidence suggesting that syndecan-1 cooperates with integrins in mediating these cellular processes (Fig. 2B) (Iba et al., 2000; Morgan et al., 2007; Ogawa et al., 2007; Gama-de-Souza et al., 2008; Vuoriluoto et al., 2008; Beauvais et al., 2009; Ishikawa and Kramer, 2010). Syndecan-1 can potentiate the activation and signaling of αvβ3 and αvβ5 integrins through the engagement of αv ligands such as vitronectin (Beauvais et al., 2009). Integrins and syndecan-1 can also form a dynamic linkage between the ECM and cytoskeleton, allowing for cell migration that involves formation of cell adhesion at the leading edge and disruption of cell adhesion at the rear edge, together with active polymerization and depolymerization of the actin cytoskeleton (Ridley, 2011). Syndecan-1 gene silencing reduced α2β1 integrin-dependent activation of FAK that led to reduced HNSCC cell adhesion to collagen I (Ishikawa and Kramer, 2010). In addition, gene silencing of syndecan-1 in HNSCC cells reduced levels of active RhoA but enhanced Rac1 and triggered filopodia and lamellopodia formation at the cell's leading edge (Ishikawa and Kramer, 2010). These data suggest that integrin and syndecan-1 function cooperatively to stabilize focal adhesions and actin cytoskeleton, resulting in restricted cell locomotion. These findings also suggest that the loss of cell surface syndecan-1, seen in many carcinomas, favors acquisition of the meta-static phenotype by cancer cells.
The spatiotemporal expression pattern of syndecan-1 also allows it to differentially modulate tumor cell migration. Cell surface syndecan-1 inhibited, whereas syndecan-1 ectodomains promoted the invasion of MCF-7 breast cancer cells into Matrigel (Nikolova et al., 2009). Similarly, cell surface syndecan-1 inhibited migration of HT1080 fibrosarcoma cells on collagen while syndecan-1 ectodomains stimulated HT1080 cell migration (Endo et al., 2003). Treatment with MMP inhibitors increased syndecan-1 expression on the surface of HT1080 cells, concurrent with the formation of actin stress fibers that led to inhibition of cell migration (Endo et al., 2003). Similar to carcinoma cells, the loss of cell surface syndecan-1 likely leads to reduced cell adhesion and spreading, thereby allowing the sarcoma cells to acquire a more motile and invasive phenotype. These findings suggest that membrane-bound syndecan-1 and soluble syndecan-1 may play unique roles at different stages of cancer progression, and provide insights into targeting cell surface vs. shed syndecan-1 in cancer therapy.
Epithelial–stromal interactions are crucial in the acceleration of carcinoma growth and progression. During neoplastic transformation, stromal fibroblasts and the ECM are altered to allow a more supportive microenvironment for cancer progression. Syndecan-1 is aberrantly expressed by fibroblasts in the surrounding stroma of infiltrating breast carcinoma (Stanley et al., 1999; Maeda et al., 2004). In addition to supporting breast carcinoma growth and angiogenesis, increased stromal syndecan-1 expression altered fibronectin production and ECM organization (Yang et al., 2011). The ECM produced by syndecan-1-negative fibroblasts consisted of an intersecting meshwork, which was in stark contrast to the parallel pattern of fibronectin and collagen I fibers produced by syndecan-1-positive fibroblasts (Yang et al., 2011). Importantly, the structurally abnormal ECM produced by the syndecan-1-expressing fibroblasts promoted breast carcinoma cell motility. How stromal syndecan-1 modulates ECM assembly is not fully understood, but syndecan-1 may modulate fibronectin matrix assembly directly or together with integrins (Stepp et al., 2010), as was observed for syndecan-2 (Klass et al., 2000) and syndecan-4 (Midwood et al., 2004).
3.3. Syndecan-1 in angiogenesis
Tumor angiogenesis generates new vascular beds that provide nutrients and oxygen for the highly metabolic tumor mass. Syndecan-1 can bind to pro-angiogenic factors like FGF-2 and VEGF, and subsequently present these factors to their respective receptors on endothelial cells to initiate endothelial invasion and budding. The broader functional implications of syndecan-1 in angiogenesis may also be to allow soluble syndecan-1 ectodomains with bound pro-angiogenic factors to foster angiogenesis at premetastatic niches. For example, in myeloma, shedding of syndecan-1 ectodomains by heparanase facilitated endothelial invasion and subsequent angiogenesis (Fig. 2C) (Purushothaman et al., 2010). Heparanase also upregulated HGF and VEGF in myeloma cells, and syndecan-1 ectodomains bound to VEGF and presented VEGF to endothelial cells (Purushothaman et al., 2008, 2010). Binding of syndecan-1 ectodomains to αvβ3 and αvβ5 integrins is apparently necessary for its pro-angiogenic function, as a short inhibitory peptide that mimics the syndecan-1 ectodomain core protein (synstatin) abrogated syndecan-1 interactions with both integrins and inhibited endothelial cell invasion as well as tumor growth in vivo (Beauvais et al., 2009; Purushothaman et al., 2010).
HS fragments generated by heparanase can stimulate melanoma angiogenesis in vivo (Roy and Marchetti, 2009). However, there is a clear need for an intact syndecan-1 ectodomain in heparanase-induced angiogenesis in myelomas. Rat aortas were grown in Matrigel in the presence of medium conditioned either by heparanase-low or heparanase-high myeloma cells. Addition of syndecan-1 ectodomains and VEGF to medium conditioned by low heparanase-expressing myeloma cells (low syndecan-1 ectodomain levels) enhanced endothelial sprouting. Conversely, immunodepletion of syndecan-1 from the conditioned medium of high heparanase-expressing myeloma cells (high syndecan-1 ectodomain) reduced endothelial sprouting (Purushothaman et al., 2010), indicating that an intact syndecan-1 ectodomain promotes angiogenesis.
Increased syndecan-1 expression in stromal fibroblasts is observed in several carcinomas, such as those of the breast (Stanley et al., 1999; Maeda et al., 2004), stomach (Wiksten et al., 2001), and thyroid (Barbareschi et al., 2003). In a xenograft model of human breast carcinoma cells and syndecan-1-transfected fibroblasts implantation into mice, stromal syndecan-1 expression was associated with significantly elevated microvessel density and larger vessel area (Maeda et al., 2006). Expression of syndecan-1 in stromal fibroblasts of human breast carcinomas also correlated significantly with high microvessel density and larger vessel area (Maeda et al., 2006). These findings raise the possibility that syndecan-1 in the reactive stroma may sequester pro-angiogenic factors and increase the local concentration of these factors to promote angiogenesis.
4. Infectious diseases
Syndecan-1, as the major cell surface HSPG of epithelial cells, is thought to be targeted by microbial pathogens especially during the early phase of infection. Several studies suggest that syndecan-1 is subverted in several steps of infection, including the initial attachment and subsequent entry of pathogens into host cells, and inhibition of host defense mechanisms (Fig. 3). Evidence that these syndecan-1 functions are indeed important in the pathogenesis of infectious diseases is provided by studies using animal and cell culture-based models of infection, where it was observed that loss or reduction of syndecan-1 enables mice or cells to significantly resist infection by several viral and bacterial pathogens (Table 1). The underlying molecular mechanisms have yet to be precisely defined, but several studies have revealed interesting details of how both cell surface and shed syndecan-1 can promote pathogenesis through distinct molecular mechanisms.
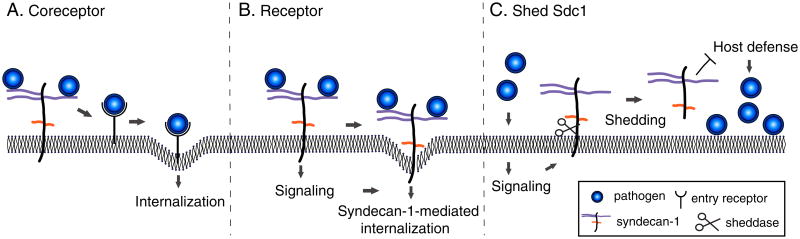
Fig. 3. Mechanisms of syndecan-1 in infectious diseases.
A) Syndecan-1 is subverted by many microbial pathogens as a coreceptor that localizes pathogens to the host cell surface and promotes their interactions with specific entry receptors. B) Select pathogens, such as N. gonorrhoeae, can use cell surface syndecan-1 as a direct entry receptor by stimulating intracellular signaling mediated by the syndecan-1 cytoplasmic domain. C) Several bacterial pathogens induce syndecan-1 shedding and exploit the capacity of syndecan-1 ectodomains to inhibit the antibacterial mechanisms of neutrophils and antimicrobial peptides.
4.1. Cell surface syndecan-1 in pathogen attachment and entry
A wide variety of pathogens, including viruses, bacteria and parasites, use cell surface HSPGs as attachment receptors (Rostand and Esko, 1997; Spillmann, 2001; Rusnati and Urbinati, 2009; Bartlett and Park, 2010). For example, hepatitis E virus (HEV) appears to target syndecan-1 for its attachment to host cells (Kalia et al., 2009). HEV is a positive-sense, non-enveloped single-stranded RNA virus that causes hepatitis E, a recently described inflammatory liver disease of humans. HEV uses its major capsid protein pORF2 to bind to hepatoma cells, and this is significantly inhibited by heparinase III treatment and syndecan-1 knock-down (Kalia et al., 2009). Syndecan-4 knockdown or treatment with phosphatidylinositol-specific phospholipase C (PI-PLC) that releases GPI-anchored glypicans from the cell surface did not inhibit pORF2 binding to hepatoma cells (Kalia et al., 2009). These results suggest that HEV binds specifically to the HS chains of syndecan-1 for its attachment to host cells.
Further evidence supporting the role of syndecan-1 as a viral attachment receptor comes from a study examining its role in a cell culture-based model of human papillomavirus (HPV) infection. HPV is a non-enveloped, double-stranded DNA virus that infects skin and mucosal epithelial cells, and hijacks the host cell cycle to cause both benign and malignant epithelial tumors. Induced expression of syndecan-1 in erythroleukemia cells significantly increased HPV attachment and internalization compared to the induced expression of syndecan-4 or glypican-1. (Shafti-Keramat et al., 2003). Further, HPV attachment and internalization were inhibited by heparinase III treatment of host cells and addition of antibodies against the HPV capsid protein L1 (Shafti-Keramat et al., 2003). L1 binding to cell surface HSPGs induces a conformational change in the minor capsid protein L2, exposing residues in L2 that bind to a secondary entry receptor, which mediates HPV internalization (Richards et al., 2006; Day et al., 2008). These observations suggest a mechanism where HPV attaches to host cells via the L1-syndecan-1 interaction, which leads to the activation of L2 and L2-mediated HPV entry.
Other viral pathogens that target epithelial cells have also been shown to bind to cell surface HSPGs for their initial attachment to host cells (Spillmann, 2001; Bartlett and Park, 2010), suggesting that syndecan-1 functions prominently in many viral infections. The molecular details of syndecan-1-virus interactions have yet to be clearly defined, but because viruses bind to the HS moiety of HSPGs, structural features of syndecan-1 HS may be important. For example, initial studies revealed that the alphaherpesviruses Herpes simplex virus (HSV)-1 and HSV-2 bind to HSPGs using glycoproteins gB and gC and that these interactions mediate viral attachment but not entry (WuDunn and Spear, 1989; Laquerre et al., 1998). Gene silencing studies showed that both syndecan-1 and -2 are important in HSV-1 attachment (Bacsa et al., 2011). However, a rare form of HS containing 3-O-sulfated glucosamine residues was found to induce gD-mediated HSV-1 internalization (Shukla et al., 1999; Liu et al., 2002b). Soluble 3-O-sulfated HS was also found to trigger HSV-1 entry into CHO cells (Tiwari et al., 2007), suggesting that syndecan-1 may directly mediate HSV internalization if its HS chains are modified to contain sufficient 3-O-sulfated HS domains to potentiate gD signaling.
Human immunodeficiency virus (HIV), the etiologic agent of AIDS, binds to HSPGs on macrophages, dendritic cells, spermatozoa, endothelial cells, and epithelial cells primarily through gp120 (Saphire et al., 2001; Argyris et al., 2003; Bobardt et al., 2003; de Witte et al., 2007; Ceballos et al., 2009; Smith et al., 2010). HIV binds to K562 human leukemic cells transfected with syndecan-1, but not to non-transfected K562 cells, which are deficient in syndecans (Saphire et al., 2001), indicating that syndecan-1 can mediate the attachment of HIV to host cells. Further, syndecan-1 expression was increased in the gut of HIV-1-infected individuals and this was associated with increased viral translocation across the gut epithelium (Smith et al., 2010). However, several syndecans can apparently mediate HIV attachment to host cells. HIV binding to Burkitt lymphoma-derived Namalwa B cells was significantly increased when these cells were transfected with syndecan-1, -2, -3, or -4 (Bobardt et al., 2003), and syndecan-3 was shown to be the major HIV-1 attachment receptor on dendritic cells (de Witte et al., 2007). These findings suggest that syndecans expressed on target host cells of HIV may contain unique HS structures that HIV preferentially binds to or that syndecans are expressed in cellular compartments where HIV preferentially infects. Alternatively, syndecans may be physically and/or functionally linked (e.g., via syndecan cytoplasmic domain signaling) to HIV entry receptors, such as CD4, DC-SIGN, and mannose receptors. These mechanisms have yet to be explored.
The role of the syndecan cytoplasmic domain in microbial pathogenesis was directly examined in a cell culture model of Neisseria gonorrhoeae infection (Freissler et al., 2000). Along with Chlamydia trachomatis, N. gonorrhoeae is one of the most common causative agents of bacterial sexually transmitted diseases in the US. N. gonorrhoeae binds to syndecan-1 and -4 through its outer membrane protein OpaHSPG as overexpression of syndecan-1 or -4 in HeLa cells increased N. gonorrhoeae infection (Freissler et al., 2000). Interestingly, N. gonorrhoeae attached but did not invade HeLa cells expressing syndecan-1 or -4 lacking the cytoplasmic domain. Furthermore, a syndecan-4 mutant lacking the syndecan-4-specific dimerization motif in the cytoplasmic domain that binds to PKC and phosphatidylinositol 4,5-bisphosphate, and a syndecan-4 mutant lacking the C-terminal Glu-Phe-Tyr-Ala PDZ binding domain did not support Neisseria invasion (Freissler et al., 2000). Mutagenesis studies with the syndecan-1 cytoplasmic domain was not performed, but syndecan-1 also contains the Glu-Phe-Tyr-Ala PDZ binding motif, suggesting that PDZ proteins that bind to the cytoplasmic domain of syndecans, such as syntenin (Grootjans et al., 1997), may be important in facilitating gonococcal invasion. In addition, Neisseria binding to cell surface HSPGs induces a signaling pathway that involves phosphatidylcholine-specific phospholipase C, diacylglycerol, acidic sphingomyelinase, and ceramide (Grassmé et al., 1997). Whether the cytoplasmic domain of syndecans also interacts with these signaling molecules remains to be determined. Regardless, these observations indicate that syndecan-1 can serve as an entry receptor for N. gonorrhoeae invasion (Fig. 3B).
4.2. Syndecan-1 shedding in microbial pathogenesis and host defense
Pseudomonas aeruginosa (Park et al., 2000a), Staphylococcus aureus (Park et al., 2004), Streptococcus pneumoniae (Chen et al., 2007), and Bacillus anthracis (Popova et al., 2006) have been shown to secrete factors that induce syndecan-1 shedding. Pathogen-induced ectodomain shedding is not restricted to syndecan-1, as several pathogens induce the ectodomain shedding of a variety of inflammatory mediators from the host cell surface (Vollmer et al., 1996; Walev et al., 1996, 2000; Schmidtchen et al., 2001; Lemjabbar and Basbaum, 2002), presumably to modulate the host environment to favor pathogenesis. P. aeruginosa is a Gram-negative bacterium associated with infections of the skin, urinary tract, and lung. P. aeruginosa induces syndecan-1 shedding through LasA (Park et al., 2000a), which is a known virulence factor of P. aeruginosa in animal models of lung infection (Woods et al., 1982; Blackwood et al., 1983). LasA activates a PTK-regulated, metalloproteinase-mediated shedding mechanism of host cells to enhance syndecan-1 shedding (Park et al., 2000a). P. aeruginosa-induced syndecan-1 shedding is a virulence mechanism as newborn Sdc1−/− mice significantly resisted intranasal lung infection with P. aeruginosa compared to newborn Wt mice (Park et al., 2001). Intranasal administration of syndecan-1 ectodomain or HS enhanced bacterial virulence in Sdc1−/− mice, whereas inhibition of syndecan-1 shedding attenuated virulence in Wt mice. Further, P. aeruginosa did not bind to syndecan-1 and excess heparin had no effect on the adhesion of P. aeruginosa to lung epithelial cells in vitro. Syndecan-1 ectodomains were also detected in BAL after intranasal infection with P. aeruginosa or instillation of LasA, and syndecan-1 ectodomains inhibited the antibacterial activity of several cationic antimicrobial peptides in vitro (Park et al., 2001). Adult Sdc1−/− ice also showed significantly attenuated P. aeruginosa sepsis following thermal injury (Haynes et al., 2005). Together, these findings suggest that the subversion of syndecan-1 shedding to inhibit host defense is an important virulence mechanism of P. aeruginosa.
S. aureus is an important Gram-positive bacterial pathogen of humans that causes a wide variety of diseases including keratitis, impetigo, pneumonia, osteomyelitis, endocarditis, toxic shock syndrome, and sepsis. S. aureus induces syndecan-1 shedding through its cytotoxic virulence factors α-toxin and β-toxin (Park et al., 2004). Similar to P. aeruginosa LasA, both α-toxin and β-toxin induce syndecan-1 shedding by activating the PTK-dependent, metalloproteinase-mediated shedding mechanism of host cells (Park et al., 2004). The physiological significance of S.aureus-induced syndecan-1 shedding was examined in a mouse model of scarified corneal infection (Hayashida et al., 2011). Scarified Sdc1−/− corneas significantly resisted S. aureus infection compared to Wt corneas that express abundant syndecan-1 in their epithelium. S. aureus did not bind to syndecan-1 and did not require syndecan-1 to colonize cultured corneal epithelial cells, but it induced syndecan-1 shedding in corneal epithelial cells both in vitro and in vivo. Topical administration of purified syndecan-1 ectodomains or HS significantly increased, whereas inhibition of syndecan-1 shedding significantly decreased the bacterial burden in corneal tissues. Further, depletion of neutrophils in the resistant Sdc1−/− mice increased the corneal bacterial burden to that of the susceptible Wt mice, suggesting that syndecan-1 moderates neutrophils to promote infection. Interestingly, neutrophils infiltrated similarly into infected Wt and Sdc1−/− corneas. However, syndecan-1 ectodomains and HS significantly inhibited the killing of S. aureus by isolated neutrophils. These observations suggest that S. aureus exploits the capacity of syndecan-1 ectodomains to inhibit neutrophil-mediated bacterial killing mechanisms in an HS-dependent manner topromote its pathogenesis in the cornea. Whether α-toxin and/or β-toxin induce syndecan-1 shedding during S. aureus corneal infection remains to be determined, but both toxins are established virulence factors in animal models of S. aureus keratitis (O'Callaghan et al., 1997; Girgis et al., 2005), suggesting that syndecan-1 shedding may mediate the capacity of these exotoxins to enhance S. aureus virulence in the cornea.
However, because many pathogens bind to cell surface HSPGs for their attachment and entry, it is not clear why syndecan-1 shedding is not a host defense mechanism that rapidly down-regulates microbial attachment sites. In fact, P. aeruginosa binds to HSPGs on the basolateral surface of polarized epithelial cells (Bucior et al., 2010), which is where syndecan-1 is expressed, and S. aureus shows increased adherence to ARH-77 cells overexpressing syndecan-1 (Henry-Stanley et al., 2005). Perhaps only highly effective pathogens that possess redundant mechanisms for attachment, such as S. aureus, can use syndecan-1 shedding to promote their pathogenesis. Alternatively, some pathogens may exploit both cell surface and shed syndecan-1 to attach to host cells and to inhibit host defense mechanisms. Whether other bacterial pathogens that possess the capacity to induce syndecan-1 shedding, such as S. pneumoniae and B. anthracis, subvert this host mechanism to promote their pathogenesis remains to be determined.
5. Concluding remarks
Studies using animal models of diseases have provided clear indications that syndecan-1 plays an important role in the development of inflammatory diseases, cancer, and infection. Syndecan-1 is a critical cofactor in the pathogenesis of these diseases as Sdc1−/− mice only show dramatic pathological phenotypes when challenged with disease-causing agents or conditions, and do not show spontaneous pathologies. In general, syndecan-1 attenuates non-infectious inflammatory diseases by inhibiting leukocyte adhesion onto the activated endothelium, reducing the expression and inhibiting the activity of pro-inflammatory factors, confining leukocyte infiltration to specific sites of tissue injury, or by removing sequestered chemokines and facilitating the resolution of inflammation. In contrast, in cancer and infectious diseases, syndecan-1 functions are mostly pro-pathogenic. Syndecan-1 enhances oncogene and growth factor signaling, inhibits cancer cell apoptosis, and promotes angiogenesis. However, syndecan-1 can also attenuate these processes depending on the type of tumor, site of action, or the mechanisms that it regulates. In infectious diseases, syndecan-1 clearly promotes pathogenesis as it mediates the attachment and entry of pathogens into host cells and inhibits host defense mechanisms. Moreover, loss of syndecan-1 is a gain of function mutation that increases the resistance of mice to several bacterial infections. These data suggest that one of the crucial functions of mammalian syndecan-1 in vivo is to assure the adequate and correct functioning of inflammation. However, this beneficial function of syndecan-1 comes at a price as certain cancer cells and microbial pathogens have either adapted or evolved to take advantage of syndecan-1 for their pathogenesis. The underlying mechanisms that govern the physiological vs. pathological functions of syndecan-1 are not known, but the unique spatiotemporal expression pattern of syndecan-1 likely mediates its prominent functions in many diseases. Future studies aimed at defining the molecular details of how syndecan-1 expression and shedding are turned on or off, and how syndecan-1 is targeted to specific cellular and tissue compartments during pathogenesis are anticipated to reveal new therapeutic targets for inflammatory diseases, cancer, and infection.
Acknowledgments
We thank our colleagues in the Park laboratory for helpful discussions. We apologize for not citing studies that may have been relevant but was omitted due to space limitations. Our research described in this review is supported by NIH Grants HL094613 and HL107472.
References
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- Anttonen A, Kajanti M, Heikkila P, Jalkanen M, Joensuu H. Syndecan-1 expression has prognostic significance in head and neck carcinoma. Br J Cancer. 1999;79:558–564. doi: 10.1038/sj.bjc.6690088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttonen A, Heikkila P, Kajanti M, Jalkanen M, Joensuu H. High syndecan-1 expression is associated with favourable outcome in squamous cell lung carcinoma treated with radical surgery. Lung Cancer. 2001;32:297–305. doi: 10.1016/s0169-5002(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Argyris EG, Acheampong E, Nunnari G, Mukhtar M, Williams KJ, Pomerantz RJ. Human immunodeficiency virus type 1 enters primary human brain micro-vascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J Virol. 2003;77:12140–12151. doi: 10.1128/JVI.77.22.12140-12151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacsa S, Karasneh G, Dosa S, Liu J, Valyi-Nagy T, Shukla D. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J Gen Virol. 2011;92:733–743. doi: 10.1099/vir.0.027052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Moseman EA, Saito H, Petryniak B, Thiriot A, Hatakeyama S, Ito Y, Kawashima H, Yamaguchi Y, Lowe JB, von Andrian UH, Fukuda M. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, Veronese S, Caffo O, Lucenti A, Palma PD, Galligioni E, Doglioni C. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 2003;98:474–483. doi: 10.1002/cncr.11515. [DOI] [PubMed] [Google Scholar]
- Bartlett AH, Park PW. Proteoglycans in host–pathogen interactions: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2010;12:e5. doi: 10.1017/S1462399409001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett AH, Hayashida K, Park PW. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol Cells. 2007;24:153–166. [PubMed] [Google Scholar]
- Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 2009;206:691–705. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiting DP, Park PW, Appleton JA. Synthesis of syndecan-1 by muscle cells is an early response to infection with Trichinella spiralis but is not essential for larval development or immune modulation. Infect Immun. 2006;74:1941–1943. doi: 10.1128/IAI.74.3.1941-1943.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Nussenzweig V. Plasmodium yoelii sporozoites infect syndecan-1 deficient mice. Mol Biochem Parasitol. 2002;123:143–144. doi: 10.1016/s0166-6851(02)00132-9. [DOI] [PubMed] [Google Scholar]
- Bingley JA, Hayward IP, Campbell JH, Campbell GR. Arterial heparan sulfate proteoglycans inhibit vascular smooth muscle cell proliferation and phenotype change in vitro and neointimal formation in vivo. J Vasc Surg. 1998;28:308–318. doi: 10.1016/s0741-5214(98)70167-3. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Blackwood LL, Stone RM, Iglewski BH, Pennington JE. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect Immun. 1983;39:198–201. doi: 10.1128/iai.39.1.198-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity. 2003;18:27–39. doi: 10.1016/s1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- Bode L, Salvestrini C, Park PW, Li J, Esko JD, Yamaguchi Y, Murch S, Freeze HH. Heparan sulfate and syndecan-1 are essential in maintaining murine and human intestinal barrier function. J Clin Invest. 2008;118:229–238. doi: 10.1172/JCI32335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, Gattegno L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect Immun. 2010;78:939–953. doi: 10.1128/IAI.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DJ, Evans DM, Stahl RC, Asundi VK, Conner KJ, Garbes P, Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992;117:191–201. doi: 10.1083/jcb.117.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos A, Remes Lenicov F, Sabatte J, Rodriguez Rodrigues C, Cabrini M, Jancic C, Raiden S, Donaldson M, Agustin Pasqualini R, Jr, Marin-Briggiler C, Vazquez-Levin M, Capani F, Amigorena S, Geffner J. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J Exp Med. 2009;206:2717–2733. doi: 10.1084/jem.20091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnaux N, Brule S, Chaigneau T, Saffar L, Sutton A, Hamon M, Prost C, Lievre N, Vita C, Gattegno L. RANTES (CCL5) induces a CCR5-dependent accelerated shedding of syndecan-1 (CD138) and syndecan-4 from HeLa cells and forms complexes with the shed ectodomains of these proteoglycans as well as with those of CD44. Glycobiology. 2005;15:119–130. doi: 10.1093/glycob/cwh148. [DOI] [PubMed] [Google Scholar]
- Chen L, Klass C, Woods A. Syndecan-2 regulates transforming growth factor-beta signaling. J Biol Chem. 2004;279:15715–15718. doi: 10.1074/jbc.C300430200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hayashida A, Bennett AE, Hollingshead SK, Park PW. Streptococcus pneumoniae sheds syndecan-1 ectodomains through ZmpC, a metalloproteinase virulence factor. J Biol Chem. 2007;282:159–167. doi: 10.1074/jbc.M608542200. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005;225:1–26. doi: 10.1016/j.canlet.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Chung MC, Popova TG, Millis BA, Mukherjee DV, Zhou W, Liotta LA, Petricoin EF, Chandhoke V, Bailey C, Popov SG. Secreted neutral metalloproteases of Bacillus anthracis as candidate pathogenic factors. J Biol Chem. 2006;281:31408–31418. doi: 10.1074/jbc.M605526200. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- David G, van der Schueren B, Marynen P, Cassiman JJ, van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol. 1992;118:961–969. doi: 10.1083/jcb.118.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R, Forbes A. Heparin, cell adhesion, and pathogenesis of inflammatory bowel disease. Lancet. 1999;354:62–65. doi: 10.1016/S0140-6736(98)09267-8. [DOI] [PubMed] [Google Scholar]
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J Virol. 2008;82:4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci U S A. 2007;104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PW, Keehnen RM, Evers LM, van Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood. 2002;99:1405–1410. doi: 10.1182/blood.v99.4.1405. [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews IC, Mackenzie KR. Transmembrane domains of the syndecan family of growth factor coreceptors display a hierarchy of homotypic and heterotypic interactions. Proc Natl Acad Sci U S A. 2007;104:20782–20787. doi: 10.1073/pnas.0708909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Baruffi M, Pereira-da-Silva G, Jamur MC, Roque-Barreira MC. Heparin potentiates in vivo neutrophil migration induced by IL-8. Glycoconj J. 1998;15:523–526. doi: 10.1023/a:1006995222189. [DOI] [PubMed] [Google Scholar]
- Ding K, Lopez-Burks M, Sanchez-Duran JA, Korc M, Lander AD. Growth factor-induced shedding of syndecan-1 confers glypican-1 dependence on mitogenic responses of cancer cells. J Cell Biol. 2005;171:729–738. doi: 10.1083/jcb.200508010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtermeyer F, Bertrand J, Dreier R, Meinecke I, Neugebauer K, Fuerst M, Lee YJ, Song YW, Herzog C, Theilmeier G, Pap T. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Fears CY, Gladson CL, Woods A. Syndecan-2 is expressed in the microvasculature of gliomas and regulates angiogenic processes in microvascular endothelial cells. J Biol Chem. 2006;281:14533–14536. doi: 10.1074/jbc.C600075200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3 sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floer M, Gotte M, Wild MK, Heidemann J, Gassar ES, Domschke W, Kiesel L, Luegering A, Kucharzik T. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am J Pathol. 2010;176:146–157. doi: 10.2353/ajpath.2010.080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissler E, Meyer auf der Heyde A, David G, Meyer TF, Dehio C. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol. 2000;2:69–82. doi: 10.1046/j.1462-5822.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- Fritchley SJ, Kirby JA, Ali S. The antagonism of interferon-gamma (IFN-gamma) by heparin: examination of the blockade of class II MHC antigen and heat shock protein-70 expression. Clin Exp Immunol. 2000;120:247–252. doi: 10.1046/j.1365-2249.2000.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai N, Kenagy RD, Chen L, Gao L, Daum G, Clowes AW. Syndecan-1: an inhibitor of arterial smooth muscle cell growth and intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2009;29:1356–1362. doi: 10.1161/ATVBAHA.109.190132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki IV, Kuhn KM, Lomazov IR, Rothman VL, Tuszunski GP, Iozzo RV, Swenson TL, Fisher EA, Williams KJ. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J Clin Invest. 1997;100:1611–1622. doi: 10.1172/JCI119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama-de-Souza LN, Cyreno-Oliveira E, Freitas VM, Melo ES, Vilas-Boas VF, Moriscot AS, Jaeger RG. Adhesion and protease activity in cell lines from human salivary gland tumors are regulated by the laminin-derived peptide AG73, syndecan-1 and beta1 integrin. Matrix Biol. 2008;27:402–419. doi: 10.1016/j.matbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Girgis DO, Sloop GD, Reed JM, O'Callaghan RJ. Effects of toxin production in a murine model of Staphylococcus aureus keratitis. Invest Ophthalmol Vis Sci. 2005;46:2064–2070. doi: 10.1167/iovs.04-0897. [DOI] [PubMed] [Google Scholar]
- Gould SE, Upholt WB, Kosher RA. Syndecan 3: a member of the syndecan family of membrane-intercalated proteoglycans that is expressed in high amounts at the onset of chicken limb cartilage differentiation. Proc Natl Acad Sci U S A. 1992;89:3271–3275. doi: 10.1073/pnas.89.8.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmé H, Gulbins E, Brenner B, Ferlinz K, Sandhoff K, Harzer K, Lang F, Meyer TF. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/s0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci U S A. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans — as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Skacel M, Adams JC. Association of loss of epithelial syndecan-1 with stage and local metastasis of colorectal adenocarcinomas: an immunohistochemical study of clinically annotated tumors. BMC Cancer. 2008;8:185. doi: 10.1186/1471-2407-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hayashi M, Jalkanen M, Firestone JH, Trelstad RL, Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987;35:1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Johnston DR, Goldberger O, Park PW. Syndecan-1 expression in epithelial cells is induced by TGF-beta through a PKA-dependent pathway. J Biol Chem. 2006;281:24365–24374. doi: 10.1074/jbc.M509320200. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Chen Y, Bartlett AH, Park PW. Syndecan-1 is an in vivo suppressor of Gram-positive toxic shock. J Biol Chem. 2008a;283:19895–19903. doi: 10.1074/jbc.M801614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Stahl PD, Park PW. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J Biol Chem. 2008b;283:35435–35444. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida A, Bartlett AH, Foster TJ, Park PW. Staphylococcus aureus beta-toxin induces acute lung injury through syndecan-1. Am J Pathol. 2009a;174:509–518. doi: 10.2353/ajpath.2009.080394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009b;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Bartlett AH, Chen Y, Park PW. Molecular and cellular mechanisms of ectodomain shedding. Anat Rec. 2010;293:925–937. doi: 10.1002/ar.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida A, Amano S, Park PW. Syndecan-1 promotes Staphylococcus aureus corneal infection by counteracting neutrophil-mediated host defense. J Biol Chem. 2011;285:3288–3297. doi: 10.1074/jbc.M110.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes A, III, Ruda F, Oliver J, Hamood AN, Griswold JA, Park PW, Rumbaugh KP. Syndecan-1 shedding contributes to Pseudomonas aeruginosa sepsis. Infect Immun. 2005;73:7914–7921. doi: 10.1128/IAI.73.12.7914-7921.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang W, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6:e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Stanley MJ, Hess DJ, Erlandsen SL, Wells CL. Ability of the heparan sulfate proteoglycan syndecan-1 to participate in bacterial translocation across the intestinal epithelial barrier. Shock. 2005;24:571–576. doi: 10.1097/01.shk.0000184286.95493.78. [DOI] [PubMed] [Google Scholar]
- Henry-Stanley MJ, Zhang B, Erlandsen SL, Wells CL. Synergistic effect of tumor necrosis factor-alpha and interferon-gamma on enterocyte shedding of syndecan-1 and associated decreases in internalization of Listeria monocytogenes and Staphylococcus aureus. Cytokine. 2006;34:252–259. doi: 10.1016/j.cyto.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Hu Y, Sun H, Owens RT, Gu Z, Wu J, Chen YQ, O'Flaherty JT, Edwards IJ. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia. 2010;12:826–836. doi: 10.1593/neo.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes FJ, Howells GL. Interleukin-11 inhibits bone formation in vitro. Calcif Tissue Int. 1993;53:362–364. doi: 10.1007/BF01351844. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K, Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harb Symp Quant Biol. 2002;67:143–153. doi: 10.1101/sqb.2002.67.143. [DOI] [PubMed] [Google Scholar]
- Iba K, Albrechtsen R, Gilpin B, Frohlich C, Loechel F, Zolkiewska A, Ishiguro K, Kojima T, Liu W, Langford JK, Sanderson RD, Brakebusch C, Fassler R, Wewer UM. The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to beta1 integrin-dependent cell spreading. J Cell Biol. 2000;149:1143–1156. doi: 10.1083/jcb.149.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikesue M, Matsui Y, Ohta D, Danzaki K, Ito K, Kanayama M, Kurotaki D, Morimoto J, Kojima T, Tsutsui H, Uede T. Syndecan-4 deficiency limits neointimal formation after vascular injury by regulating vascular smooth muscle cell proliferation and vascular progenitor cell mobilization. Arterioscler Thromb Vasc Biol. 2011;31:1066–1074. doi: 10.1161/ATVBAHA.110.217703. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, Kusugami K, Saito H, Muramatsu T. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem. 2001;276:47483–47488. doi: 10.1074/jbc.M106268200. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kramer RH. Sdc1 negatively modulates carcinoma cell motility and invasion. Exp Cell Res. 2010;316:951–965. doi: 10.1016/j.yexcr.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen M, Rapraeger A, Saunders S, Bernfield M. Cell surface proteoglycan of mouse mammary epithelial cells is shed by cleavage of its matrix-binding ectodomain from its membrane-associated domain. J Cell Biol. 1987;105:3087–3096. doi: 10.1083/jcb.105.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, Li Y, Tager AM, Goetinck PF, Luster AD, Noble PW. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest. 2010;120:2049–2057. doi: 10.1172/JCI38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, Leppa S. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210–5217. [PubMed] [Google Scholar]
- Juuti A, Nordling S, Lundin J, Louhimo J, Haglund C. Syndecan-1 expression — a novel prognostic marker in pancreatic cancer. Oncology. 2005;68:97–106. doi: 10.1159/000085702. [DOI] [PubMed] [Google Scholar]
- Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- Kalia M, Chandra V, Rahman SA, Sehgal D, Jameel S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J Virol. 2009;83:12714–12724. doi: 10.1128/JVI.00717-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Wang H, Kainulainen V, Fitzgerald ML, Ledbetter S, Ornitz DM, Bernfield M. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4:691–697. doi: 10.1038/nm0698-691. [DOI] [PubMed] [Google Scholar]
- Kharabi Masouleh B, Ten Dam GB, Wild MK, Seelige R, van der Vlag J, Rops AL, Echtermeyer FG, Vestweber D, van Kuppevelt TH, Kiesel L, Gotte M. Role of the heparan sulfate proteoglycan syndecan-1 (CD138) in delayed-type hypersensitivity. J Immunol. 2009;182:4985–4993. doi: 10.4049/jimmunol.0800574. [DOI] [PubMed] [Google Scholar]
- Khotskaya YB, Dai Y, Ritchie JP, MacLeod V, Yang Y, Zinn K, Sanderson RD. Syndecan-1 is required for robust growth, vascularization, and metastasis of myeloma tumors in vivo. J Biol Chem. 2009;284:26085–26095. doi: 10.1074/jbc.M109.018473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi J, Kallajoki M, Kujala I, Matikainen MT, Alanen K, Jalkanen M, Salmivirta M. Altered expression of syndecan-1 in prostate cancer. APMIS. 2004;112:89–97. doi: 10.1111/j.1600-0463.2004.apm1120202.x. [DOI] [PubMed] [Google Scholar]
- Kjellen L, Pettersson I, Hook M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981;78:5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass CM, Couchman JR, Woods A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J Cell Sci. 2000;113:493–506. doi: 10.1242/jcs.113.3.493. [DOI] [PubMed] [Google Scholar]
- Kliment CR, Oury TD. Extracellular superoxide dismutase protects cardiovascular syndecan-1 from oxidative shedding. Free Radic Biol Med. 2011;50:1075–1080. doi: 10.1016/j.freeradbiomed.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliment CR, Tobolewski JM, Manni ML, Tan RJ, Enghild J, Oury TD. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid Redox Signal. 2008;10:261–268. doi: 10.1089/ars.2007.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda JE, Rapraeger A, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem. 1985;260:8157–8162. [PubMed] [Google Scholar]
- Kojima T, Shworak NW, Rosenberg RD. Molecular cloning and expression of two distinct cDNA-encoding heparan sulfate proteoglycan core proteins from a rat endothelial cell line. J Biol Chem. 1992;267:4870–4877. [PubMed] [Google Scholar]
- Kon S, Ikesue M, Kimura C, Aoki M, Nakayama Y, Saito Y, Kurotaki D, Diao H, Matsui Y, Segawa T, Maeda M, Kojima T, Uede T. Syndecan-4 protects against osteopontin-mediated acute hepatic injury by masking functional domains of osteopontin. J Exp Med. 2008;205:25–33. doi: 10.1084/jem.20071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel H, Yard BA, Christ M, Wehling M, van der Woude FJ. Modulation of angiotensin II-mediated signalling by heparan sulphate glycosaminoglycans. Nephrol Dial Transplant. 2003;18:2240–2247. doi: 10.1093/ndt/gfg376. [DOI] [PubMed] [Google Scholar]
- Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- Kusumoto T, Kodama J, Seki N, Nakamura K, Hongo A, Hiramatsu Y. Clinical significance of syndecan-1 and versican expression in human epithelial ovarian cancer. Oncol Rep. 2010;23:917–925. doi: 10.3892/or_00000715. [DOI] [PubMed] [Google Scholar]
- Lambaerts K, Wilcox-Adelman SA, Zimmermann P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opin Cell Biol. 2009;21:662–669. doi: 10.1016/j.ceb.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M, Thysell H, Nilsson E, Olsson I. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest. 1991;88:2026–2031. doi: 10.1172/JCI115530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–1714. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- Lemjabbar H, Basbaum C. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat Med. 2002;8:41–46. doi: 10.1038/nm0102-41. [DOI] [PubMed] [Google Scholar]
- Lendorf ME, Manon-Jensen T, Kronqvist P, Multhaupt HA, Couchman JR. Syndecan-1 and syndecan-4 are independent indicators in breast carcinoma. J Histochem Cytochem. 2011;59:615–629. doi: 10.1369/0022155411405057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz D, Hambsch J, Schneider P, Hausler HJ, Sauer U, Hess J, Tarnok A. Protein-losing enteropathy in patients with Fontan circulation: is it triggered by infection? Crit Care. 2003;7:185–190. doi: 10.1186/cc2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Liu D, Shriver Z, Venkataraman G, El Shabrawi Y, Sasisekharan R. Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. Proc Natl Acad Sci USA. 2002a;99:568–573. doi: 10.1073/pnas.012578299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shriver Z, Pope RM, Thorp SC, Duncan MB, Copeland RJ, Raska CS, Yoshida K, Eisenberg RJ, Cohen G, Linhardt RJ, Sasisekharan R. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein D. J Biol Chem. 2002b;277:33456–33467. doi: 10.1074/jbc.M202034200. [DOI] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci USA. 2002;99:1229–1234. doi: 10.1073/pnas.032497699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin M, Nordling S, Lundin J, Isola J, Wiksten JP, Haglund C. Epithelial syndecan-1 expression is associated with stage and grade in colorectal cancer. Oncology. 2005;68:306–313. doi: 10.1159/000086969. [DOI] [PubMed] [Google Scholar]
- Maeda T, Alexander CM, Friedl A. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 2004;64:612–621. doi: 10.1158/0008-5472.can-03-2439. [DOI] [PubMed] [Google Scholar]
- Maeda T, Desouky J, Friedl A. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 2006;25:1408–1412. doi: 10.1038/sj.onc.1209168. [DOI] [PubMed] [Google Scholar]
- Mahtouk K, Hose D, Raynaud P, Hundemer M, Jourdan M, Jourdan E, Pantesco V, Baudard M, De Vos J, Larroque M, Moehler T, Rossi JF, Reme T, Goldschmidt H, Klein B. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood. 2007;109:4914–4923. doi: 10.1182/blood-2006-08-043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marynen P, Zhang J, Cassiman JJ, Van den Berghe H, David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989;264:7017–7024. [PubMed] [Google Scholar]
- McFall AJ, Rapraeger AC. Characterization of the hight affinity cell-binding domain in the cell surface proteoglycan syndecan-4. J Biol Chem. 1998;273:28270–28276. doi: 10.1074/jbc.273.43.28270. [DOI] [PubMed] [Google Scholar]
- Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell. 2004;15:5670–5677. doi: 10.1091/mbc.E04-08-0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Nikolova V, Koo CY, Ibrahim SA, Wang Z, Spillmann D, Dreier R, Kelsch R, Fischgrabe J, Smollich M, Rossi LH, Sibrowski W, Wulfing P, Kiesel L, Yip GW, Gotte M. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis. 2009;30:397–407. doi: 10.1093/carcin/bgp001. [DOI] [PubMed] [Google Scholar]
- O'Callaghan RJ, Callegan MC, Moreau JM, Green LC, Foster TJ, Hartford OM, Engel LS, Hill JM. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. The short arm of laminin gamma2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin beta4 chain. Mol Biol Cell. 2007;18:1621–1633. doi: 10.1091/mbc.E06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PW, Pier GB, Preston MJ, Goldberger O, Fitzgerald ML, Bernfield M. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J Biol Chem. 2000a;275:3057–3064. doi: 10.1074/jbc.275.5.3057. [DOI] [PubMed] [Google Scholar]
- Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem. 2000b;275:29923–29926. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M, Chen Y. Activation of syndecan-1 ectodomain shedding by Staphylococcus aureus alpha-toxin and beta-toxin. J Biol Chem. 2004;279:251–258. doi: 10.1074/jbc.M308537200. [DOI] [PubMed] [Google Scholar]
- Pierce A, Lyon M, Hampson IN, Cowling GJ, Gallagher JT. Molecular cloning of the major cell surface heparan sulfate proteoglycan from rat liver. J Biol Chem. 1992;267:3894–3900. [PubMed] [Google Scholar]
- Popova TG, Millis B, Bradburne C, Nazarenko S, Bailey C, Chandhoke V, Popov SG. Acceleration of epithelial cell syndecan-1 shedding by anthrax hemolytic virulence factors. BMC Microbiol. 2006;6:8–24. doi: 10.1186/1471-2180-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, Hoettecke N, Schmidt B, Sechi A, Uhlig S, Ludwig A. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem. 2010;285:555–564. doi: 10.1074/jbc.M109.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Chen L, Yang Y, Sanderson RD. Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J Biol Chem. 2008;283:32628–32636. doi: 10.1074/jbc.M806266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, Sanderson RD. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood. 2010;115:2449–2457. doi: 10.1182/blood-2009-07-234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgopal R, Butcher M, Weitz JI, Shaughnessy SG. Heparin synergistically enhances interleukin-11 signaling through up-regulation of the MAPK pathway. J Biol Chem. 2006;281:20780–20787. doi: 10.1074/jbc.M600169200. [DOI] [PubMed] [Google Scholar]
- Ramani VC, Yang Y, Ren Y, Nan L, Sanderson RD. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J Biol Chem. 2011;286:6490–6499. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger AC, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983;258:3632–3636. [PubMed] [Google Scholar]
- Rapraeger A, Jalkanen M, Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986;103:2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- Rees MD, Pattison DI, Davies MJ. Oxidation of heparan sulphate by hypochlorite: role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem J. 2005;391:125–134. doi: 10.1042/BJ20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006;103:1522–1527. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Rops AL, Gotte M, Baselmans MH, van den Hoven MJ, Steenbergen EJ, Lensen JF, Wijnhoven TJ, Cevikbas F, van den Heuvel LP, van Kuppevelt TH, Berden JH, van der Vlag J. Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int. 2007;72:1204–1215. doi: 10.1038/sj.ki.5002514. [DOI] [PubMed] [Google Scholar]
- Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Marchetti D. Cell surface heparan sulfate released by heparanase promotes melanoma cell migration and angiogenesis. J Cell Biochem. 2009;106:200–209. doi: 10.1002/jcb.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnati M, Urbinati C. Polysulfated/sulfonated compounds for the development of drugs at the crossroad of viral infection and oncogenesis. Curr Pharm Des. 2009;15:2946–2957. doi: 10.2174/138161209789058156. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Borset M. Syndecan-1 in B lymphoid malignancies. Ann Hematol. 2002;81:125–135. doi: 10.1007/s00277-002-0437-8. [DOI] [PubMed] [Google Scholar]
- Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75:9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Bonnaffe D, Lubineau A, Lortat-Jacob H. Heparan sulfate mimicry: a synthetic glycoconjugate that recognizes the heparin binding domain of interferon-gamma inhibits the cytokine activity. J Biol Chem. 2005;280:37558–37564. doi: 10.1074/jbc.M507729200. [DOI] [PubMed] [Google Scholar]
- Saunders S, Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol. 1988;106:423–430. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders S, Jalkanen M, O'Farrell S, Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellings MW, Vanhoutte D, van Almen GC, Swinnen M, Leenders JJ, Kubben N, van Leeuwen RE, Hofstra L, Heymans S, Pinto YM. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension. 2010;55:249–256. doi: 10.1161/HYPERTENSIONAHA.109.137885. [DOI] [PubMed] [Google Scholar]
- Schmidtchen A, Frick I, Björck L. Dermatan sulfate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- Seidel C, Borset M, Hjertner O, Cao D, Abildgaard N, Hjorth-Hansen H, Sanderson RD, Waage A, Sundan A. High levels of soluble syndecan-1 in myeloma-derived bone marrow: modulation of hepatocyte growth factor activity. Blood. 2000a;96:3139–3146. [PubMed] [Google Scholar]
- Seidel C, Sundan A, Hjorth M, Turesson I, Dahl IM, Abildgaard N, Waage A, Borset M. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000b;95:388–392. [PubMed] [Google Scholar]
- Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy SG, Walton KJ, Deschamps P, Butcher M, Beaudin SM. Neutralization of interleukin-11 activity decreases osteoclast formation and increases cancellous bone volume in ovariectomized mice. Cytokine. 2002;20:78–85. doi: 10.1006/cyto.2002.1981. [DOI] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Schacker TW, Reilly CS, Haase AT. A role for syndecan-1 and claudin-2 in microbial translocation during HIV-1 infection. J Acquir Immune Defic Syndr. 2010;55:306–315. doi: 10.1097/QAI.0b013e3181ecfeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83:811–817. doi: 10.1016/s0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MJ, Stanley MW, Sanderson RD, Zera R. Syndecan-1 expression is induced in the stroma of infiltrating breast carcinoma. Am J Clin Pathol. 1999;112:377–383. doi: 10.1093/ajcp/112.3.377. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DD, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, Yuspa SH. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–2863. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Daley WP, Bernstein AM, Pal-Ghosh S, Tadvalkar G, Shashurin A, Palsen S, Jurjus RA, Larsen M. Syndecan-1 regulates cell migration and fibronectin fibril assembly. Exp Cell Res. 2010;316:2322–2339. doi: 10.1016/j.yexcr.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G, Blaine SA, Qiao D, Friedl A. Shedding of syndecan-1 by stromal fibroblasts stimulates human breast cancer cell proliferation via FGF2 activation. J Biol Chem. 2007;282:14906–14915. doi: 10.1074/jbc.M611739200. [DOI] [PubMed] [Google Scholar]
- Su G, Blaine SA, Qiao D, Friedl A. Membrane type 1 matrix metalloproteinase-mediated stromal syndecan-1 shedding stimulates breast carcinoma cell proliferation. Cancer Res. 2008;68:9558–9565. doi: 10.1158/0008-5472.CAN-08-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem. 1989;264:2885–2889. [PubMed] [Google Scholar]
- Sun H, Berquin IM, Owens RT, O'Flaherty JT, Edwards IJ. Peroxisome proliferator-activated receptor gamma-mediated up-regulation of syndecan-1 by n−3 fatty acids promotes apoptosis of human breast cancer cells. Cancer Res. 2008;68:2912–2919. doi: 10.1158/0008-5472.CAN-07-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V, O'Donnell C, Copeland RJ, Scarlett T, Liu J, Shukla D. Soluble 3-O-sulfated heparan sulfate can trigger herpes simplex virus type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells. J Gen Virol. 2007;88:1075–1079. doi: 10.1099/vir.0.82476-0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortes V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- Vollmer P, Walev I, Rose-John S, Bhakdi S. Novel pathogenic mechanism of microbial metalloproteinases: liberation of membrane-anchored molecules in biologically active form exemplified by studies with the human interleukin-6 receptor. Infect Immun. 1996;64:3646–3651. doi: 10.1128/iai.64.9.3646-3651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuoriluoto K, Jokinen J, Kallio K, Salmivirta M, Heino J, Ivaska J. Syndecan-1 supports integrin alpha2beta1-mediated adhesion to collagen. Exp Cell Res. 2008;314:3369–3381. doi: 10.1016/j.yexcr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Walev I, Vollmer P, Palmer M, Bhakdi S, Rose-John S. Pore-forming toxins trigger shedding of receptors for interleukin 6 and lipopolysaccharide. Proc Natl Acad Sci USA. 1996;93:7882–7887. doi: 10.1073/pnas.93.15.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Tappe D, Glubins E, Bhakdi S. Streptolysin O-permeabilized granulocytes shed L-selectin concomitantly with ceramide generation via neutral sphingomyelinase. J Leukoc Biol. 2000;68:865–872. [PubMed] [Google Scholar]
- Watson AJ, Chu S, Sieck L, Gerasimenko O, Bullen T, Campbell F, McKenna M, Rose T, Montrose MH. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912. doi: 10.1053/j.gastro.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Webb LMC, Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A. Binding of heparan sulfate or heparin enhances neutrophil responses to IL-8. Proc Natl Acad Sci USA. 1993;90:7158–7162. doi: 10.1073/pnas.90.15.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiksten JP, Lundin J, Nordling S, Lundin M, Kokkola A, von Boguslawski K, Haglund C. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int J Cancer. 2001;95:1–6. doi: 10.1002/1097-0215(20010120)95:1<1::aid-ijc1000>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Woods DE, Cryz SJ, Friedman RL, Iglewski BH. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982;36:1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WuDunn D, Spear PG. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Viro. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Park PW, Kheradmand F, Corry DB. Endogenous attenuation of allergic lung inflammation by syndecan-1. J Immunol. 2005;174:5758–5765. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yaccoby S, Liu W, Langford JK, Pumphrey CY, Theus A, Epstein J, Sanderson RD. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002;100:610–617. doi: 10.1182/blood.v100.2.610. [DOI] [PubMed] [Google Scholar]
- Yang Y, Macleod V, Miao HQ, Theus A, Zhan F, Shaughnessy JD, Jr, Sawyer J, Li JP, Zcharia E, Vlodavsky I, Sanderson RD. Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Biol Chem. 2007;282:13326–13333. doi: 10.1074/jbc.M611259200. [DOI] [PubMed] [Google Scholar]
- Yang N, Mosher R, Seo S, Beebe D, Friedl A. Syndecan-1 in breast cancer stroma fibroblasts regulates extracellular matrix fiber organization and carcinoma cell motility. Am J Pathol. 2011;178:325–335. doi: 10.1016/j.ajpath.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Rapraeger AC. Post-transcriptional regulation of syndecan-1 expression by cAMP in peritoneal macrophages. J Cell Biol. 1993;122:941–950. doi: 10.1083/jcb.122.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]