Abstract
Background
Alcohol consumption has multiple biochemical consequences. Only a few of these are useful as diagnostic markers but many reflect potentially harmful or beneficial effects of alcohol. Average consumption of two to four drinks per day is associated with lower overall or cardiovascular mortality risk than either lower or higher intake. We have analysed the dose-response relationships between reported alcohol consumption and 17 biomarkers, with emphasis on intake of up to three drinks per day.
Methods
Biochemical tests were performed on serum from 8396 study participants (3750 men and 4646 women, aged 51 ± 13 years, range 18–93) who had provided information on alcohol consumption in the week preceding blood collection.
Results
GGT, ALT, AST, CDT, urate, ferritin and bilirubin showed little or no change with alcohol consumption below two to three drinks per day, but increased with higher intake. HDL-C and albumin showed increasing results, and insulin showed decreasing results, across the entire range of alcohol use. Biphasic responses, where subjects reporting one to two drinks per day had lower results than those reporting either more or less alcohol use, occurred for triglycerides, glucose, C-reactive protein, alkaline phosphatase and butyrylcholinesterase. Increasing alcohol use was associated with decreasing LDL-C in younger women, but higher LDL-C in older men.
Conclusions
Some markers show threshold relationships with alcohol, others show continuous ones, and a third group show biphasic or U-shaped relationships. Overall the biochemical sequelae of low-to-moderate alcohol use are consistent with the epidemiological evidence on morbidity and mortality.
Keywords: Alcohol, Biomarkers, Dose-response curve, Population study
Many studies have explored the relationships between alcohol intake and biochemical characteristics, either to evaluate the biological effects of alcohol use or to develop biomarkers of hazardous consumption or of relapse in alcoholics. The strongest associations have been found for gamma glutamyl transferase (GGT) and carbohydrate-deficient transferrin (CDT) (Alte et al., 2003; Conigrave et al., 2003; Scouller et al., 2000), but many other commonly measured constituents of serum (e.g. alanine aminotransferase, ALT; aspartate aminotransferase, AST; ferritin; high-density lipoprotein cholesterol, HDL-C; and urate) show highly significant associations with alcohol intake (Alatalo et al., 2009; Nagaya et al., 1999; Whitehead et al., 1996b; Whitfield et al., 2001).
Prospective epidemiological studies have examined the connection between alcohol intake and cardiovascular disease (see Ronksley et al., 2011), diabetes (Conigrave et al., 2001; Koppes et al., 2005), metabolic syndrome (Alkerwi et al., 2009) or overall mortality (Di Castelnuovo et al., 2006; Doll et al., 1994; Inoue et al., 2012; Thun et al., 1997). These have usually shown biphasic (‘U-shaped’) results, in which morbidity or mortality is lower in people who consume some alcohol than in abstainers but increases once an inflexion point at about four drinks per day for men and two for women is passed. This inflexion point is often construed as a ‘safe limit’ of alcohol intake. Conclusions about possible health benefits from alcohol have been controversial (Fillmore et al., 2006; Roerecke and Rehm, 2011, but see Fuller, 2011; Ronksley et al., 2011), and criticisms have included potential inclusion of past-drinkers who now abstain because of health problems; reliance on self-report of alcohol use; and the emphasis in most studies on middle-aged subjects at risk of cardiovascular disease when the relationship between alcohol use and mortality is likely to differ in younger people. Nevertheless, alcohol has multiple biological effects and there is no a priori reason why some of them may not be beneficial.
Any favourable effects of alcohol on cardiovascular mortality might be due to effects on known risk factors, such as HDL-C, insulin sensitivity, or coagulation and fibrinolysis. Unfavourable ones could be mediated through increased blood pressure or by changes in the number, size or atherogenicity of lipoprotein particles. The consensus from meta-analysis of experimental studies, in which volunteers take controlled amounts of alcohol, is that daily alcohol administration for a few weeks increases HDL-C and apolipoprotein A1, has no effect on LDL-C or triglycerides, decreases fibrinogen and increases adiponectin (Brien et al., 2011).
In view of the evidence that alcohol has cardioprotective effects, it is paradoxical that some markers of alcohol intake, particularly the liver enzymes gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT) and aspartate aminotransferase (AST), predict risk of cardiovascular disease, diabetes and overall mortality (Breitling et al., 2011; Fraser et al., 2007; 2009). Higher enzyme results are associated with increased risk, although the three-way relationships between alcohol, enzyme results, and risk have not been fully explained. In the population-epidemiology context, these enzymes probably measure mild hepatic steatosis and reflect the liver changes associated with metabolic syndrome. However, the risk of metabolic syndrome as defined by the combination of hypertension, dyslipidaemia, impaired glucose tolerance and obesity (Alberti et al., 2006) is decreased by alcohol; meta-analysis showed a reduction in incidence associated with up to 40 grams of alcohol per day in men and 20 grams in women (Alkerwi et al., 2009).
Many papers have assessed associations between alcohol intake and markers of systemic inflammation, most commonly by measuring serum C-reactive protein (CRP) in cross-sectional studies. At least 11 such studies have been reported since 2001; most report U-shaped relationships with alcohol, sometimes dependent on sex, BMI or APOE genotype. The alcohol intake at which CRP concentration was lowest was usually one to two drinks per day. In one experimental study (Sierksma et al., 2002) 30–40 grams of alcohol per day for three weeks resulted in a 35% decrease in CRP.
Comparison of the response of multiple biochemical markers or cardiovascular risk factors to alcohol intake, particularly to moderate alcohol intake, may allow cross-validation of the independent variable (self-reported alcohol intake) and comparison of the responses of the different dependent variables. This is best achieved by a study in which many biochemical characteristics have been measured, and compared against reliable estimates of alcohol use, rather than through comparisons across studies. We aimed to identify biomarkers or to infer changes in metabolic processes which are either presumptively favourable across the spectrum of alcohol intake or else favourable at lower levels and unfavourable at higher ones, and to define the average responses to alcohol intake as a preliminary stage before examining genetic variation in such responses.
SUBJECTS AND METHODS
Study participants
This analysis is based on a group of studies on the genetics of alcohol use and dependence, and of smoking or nicotine dependence (Heath et al., 2011; Saccone et al., 2007). These studies were approved by the Queensland Institute of Medical Research and Washington University Ethics Review Committees and participants gave informed consent. Recruitment was from the general population and was based on twins who had participated in our earlier studies, their first-degree relatives (siblings, parents or adult offspring) and their spouses or partners. Interview data were available for 16,918 people from 11,700 families; 8603 people attended for collection of blood and biochemical measurements were obtained from serum for 8396 of them. Blood was collected into plain tubes to obtain serum samples and into fluoride-oxalate tubes for measurement of plasma glucose. Samples were stored at −80°C until used. At the time of blood collection, participants filled in a retrospective alcohol diary recording the number of standard drinks (containing 10 grams of ethanol) in four categories (beer, wine, spirits and other; no distinction was made between red and white wine) consumed on each day in the past week, and a similar retrospective diary for number of cigarettes. Number of drinks was summed across days and types to give the number of drinks per week. Participants who reported any cigarette use during the week were categorised as current smokers, and number of cigarettes was used as a quantitative measure of smoking. Body mass index was calculated from self-reported weight and height.
Data were also available from telephone interviews, conducted before the blood collection, on usual quantity and frequency of alcohol use over the past 12 months and on symptoms of alcohol dependence. Quantity and frequency data were used to estimate usual number of standard drinks per week (to be distinguished from the reported number in the past week), and symptom data were used to derive a lifetime diagnosis of DSM-IV alcohol dependence. 5716 people (out of the 8396) had quantity-frequency alcohol estimates, and 6440 had symptom data which allowed classification as positive (N = 1477) or negative (N = 4963) for lifetime alcohol dependence.
Biochemical measurements and methods
All biochemical measurements except CDT and insulin were made using Roche reagents and methods on 917 or Modular P analysers (Roche Diagnostics, Castle Hill, NSW Australia). CDT was measured by the N Latex method (Siemens Healthcare Diagnostics) on a Dade BN-II nephelometric analyzer and expressed as a percentage of total transferrin (CDT%). Insulin was measured using Abbott reagents on an Architect analyser. Results were obtained for most of the measurements for 8390–8396 of the subjects, with lower numbers for C-reactive protein (8308), glucose (6766), insulin (2147) and CDT (2008). LDL-C results were calculated using the Friedewald formula, except for 282 people who had triglyceride concentrations above 4.52 mmol/l. Because it was impractical to obtain fasting blood samples from all subjects, results for glucose and insulin were adjusted for reported time between the last meal and blood collection.
Characteristics of study participants
Means for alcohol intake and for the biomarker results are shown in Table 1. Alcohol intake in the week before blood collection was used to categorise participants into seven groups. The groups, with numbers of subjects in each, mean ages, proportions meeting lifetime alcohol dependence criteria and of current smokers, mean past-week and quantity x frequency alcohol estimates, and mean BMIs, are shown in Table 2. Cumulative frequency distributions for alcohol intake are shown in Supplementary Figure 1. Proportions of lifetime alcohol dependent subjects and current smokers were greater in the higher alcohol intake groups. BMI was not associated with alcohol intake group in men (F 6,3743 = 0.72, p = 0.633) but there was a highly significant (F 6,4639 = 27.14, p = 5.54 × 10−32) U-shaped relationship in women (Table 2, Supplementary Figure 2).
Table 1.
Characteristics of the study participants. All statistics are mean ± SD unless otherwise noted.
| Men (N = 3750) | Women (N = 4646) | |
|---|---|---|
| Age | 51.4 ± 13.4 | 50.8 ± 13.5 |
| Alcohol intake, reported number drinks in past week (median and 90th centile) | 10.0, 40.9 | 3.0, 16.0 |
| Alcohol intake, estimated usual number of drinks per week (median and 90th centile) | 8.5, 19.4 | 1.5, 10.5 |
| Lifetime DSM-IV alcohol dependence, percent | 31.5 | 15.9 |
| Current smokers, percent | 20.6 | 18.4 |
| BMI, kg/m2 | 27.1 ± 4.4 | 26.6 ± 5.6 |
| GGT, u/l | 39.2 ± 44.4 | 24.1 ± 27.2 |
| ALT, u/l | 30.8 ± 19.7 | 20.6 ± 12.9 |
| AST, u/l | 27.4 ± 11.4 | 22.4 ± 8.5 |
| CDT, percent | 1.96 ± 0.84 | 1.60 ± 0.46 |
| Urate, mmol/l | 0.346 ± 0.075 | 0.263 ± 0.071 |
| Ferritin, μg/l | 261 ± 196 | 111 ± 113 |
| HDL cholesterol, mmol/l | 1.35 ± 0.37 | 1.67 ± 0.42 |
| LDL cholesterol, mmol/l | 3.36 ± 0.91 | 3.22 ± 0.92 |
| Triglycerides, mmol/l | 2.26 ± 1.39 | 1.69 ± 0.96 |
| Glucose, mmol/l | 5.34 ± 1.84 | 4.91 ± 1.42 |
| Insulin, pmol/l | 110 ± 134 | 93 ± 136 |
| C-reactive protein, mg/l | 3.31 ± 5.91 | 4.15 ± 7.04 |
| Alkaline phosphatase, u/l | 77.6 ± 21.2 | 75.3 ± 25.0 |
| Butyrylcholinesterase, u/l | 9760 ± 1918 | 8810 ± 1924 |
| Albumin, g/l | 48.8 ± 2.9 | 47.5 ± 2.8 |
| Bilirubin, μmol/l | 9.9 ± 5.5 | 8.0 ± 4.3 |
| Urea, mmol/l | 6.76 ± 1.91 | 6.03 ± 1.74 |
Table 2.
Grouping of alcohol intake, and associations with lifetime alcohol dependence, current smoking status and BMI, for the 8396 study participants with biomarker data.
| Past-week intake group (Number of reported drinks) | N | Mean age | Percent DSM-IV AD positive | Percent current smokers | Mean past-week number of drinks | Mean Q x F number of drinks | Mean BMI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| None | 733 | 1558 | 54.8 | 54.0 | 25.3 | 11.3 | 16.2 | 15.3 | 0 | 0 | 1.64 | 1.04 | 27.34 | 27.87 |
| One or two | 235 | 684 | 51.8 | 50.4 | 17.9 | 7.9 | 16.2 | 13.7 | 1.64 | 1.54 | 1.90 | 1.56 | 27.00 | 26.62 |
| Three to five | 382 | 726 | 50.8 | 47.7 | 16.7 | 11.9 | 13.8 | 16.0 | 4.00 | 3.91 | 3.55 | 2.78 | 26.87 | 26.28 |
| Six to ten | 544 | 753 | 51.2 | 49.7 | 21.6 | 15.4 | 13.4 | 15.9 | 7.83 | 7.67 | 5.72 | 5.27 | 27.08 | 25.28 |
| Eleven to twenty | 799 | 645 | 51.6 | 49.3 | 29.0 | 23.3 | 15.9 | 25.7 | 15.09 | 14.42 | 8.70 | 8.35 | 27.20 | 25.43 |
| Twenty-one to forty | 682 | 240 | 49.4 | 48.2 | 43.8 | 45.3 | 26.8 | 38.8 | 28.66 | 26.80 | 14.35 | 13.46 | 27.01 | 25.86 |
| Over forty | 375 | 40 | 48.4 | 46.1 | 60.8 | 64.7 | 47.5 | 65.0 | 59.45 | 57.15 | 27.82 | 26.97 | 27.21 | 27.98 |
M = male, F = Female, AD = alcohol dependence (lifetime), Q x F = quantity-frequency estimate of usual weekly alcohol intake.
Data analysis
In preliminary analysis, biomarker results were subjected to multiple regression to identify effects of sex, age, BMI, alcohol intake and smoking. Biomarkers results showing skewed distributions were log-transformed. The measure used for smoking was log(N of cigarettes + 1) to allow inclusion of current non-smokers. Residuals, adjusted for all these effects except alcohol intake, were saved for further analysis.
These adjusted and standardised residuals were tested for associations with alcohol in a two-step regression procedure, firstly for the linear effect of log(N of drinks +1) and then for the quadratic relationship with log(N of drinks +1) and [log(N of drinks +1)]2. The improvement in proportion of variance explained (R2) from inclusion of the quadratic term was a measure of deviation from linearity in the log(alcohol)/marker relationship. Because subjects were related and therefore not fully independent for variables affected by genetic variation, we used a Huber-White robust variance estimator (Williams, 2000) implemented in STATA, to adjust estimated standard errors and p-values for the non-independence of observations on family members.
To illustrate the shape of the alcohol/marker dose-response curves, reported alcohol intake in the week before blood collection was categorised into seven groups and the means and standard errors for the adjusted residuals were calculated for each group and plotted. Similar plots of adjusted residuals against alcohol intake grouping were made for the quantity-frequency measure, for comparison and validation purposes; and plots based on the past-week alcohol data were constructed after division of the subjects by sex, lifetime alcohol dependence history, and current smoking status.
RESULTS
Multiple regression
Results of initial analysis to define significant covariates are shown in Table 3. Most of the variables showed significant linear associations with reported alcohol intake. There were positive associations for GGT, ALT, AST, albumin, bilirubin, urea, urate, CDT, ferritin and HDL-C, and an inverse one for insulin. The strongest associations were found for CDT, GGT, HDL-C and insulin. All the biomarker results were affected by sex, and all except bilirubin, CDT and insulin by age. Smoking had independent effects on all results except butyrylcholinesterase and insulin. All results were affected by BMI, although the effect on LDL-C in men was marginal.
Table 3.
P-values from multiple regression of marker results.
| Sex | Age | BMI | Total Drinks | Smoking | ||
|---|---|---|---|---|---|---|
| GGT (log) | Beta | 0.241 | 0.123 | 0.234 | 0.241 | 0.068 |
| p | 9.31 × 10−120 | 8.50 × 10−36 | 9.04 × 10−127 | 2.62 × 10−115 | 9.33 × 10−12 | |
| ALT (log) | Beta | 0.342 | −0.074 | 0.242 | 0.105 | −0.038 |
| p | 1.24 × 10−227 | 1.34 × 10−13 | 9.83 × 10−133 | 2.74 × 10−23 | 2.00 × 10−4 | |
| AST (log) | Beta | 0.235 | 0.040 | 0.065 | 0.190 | −0.083 |
| p | 2.15 × 10−101 | 1.28 × 10−4 | 2.06 × 10−10 | 1.15 × 10−64 | 7.38 × 10−15 | |
| CDT (%) | Beta | 0.138 | 0.005 | −0.122 | 0.283 | 0.117 |
| p | 2.30 × 10−9 | NS | 3.71 × 10−9 | 2.23 × 10−31 | 8.28 × 10−8 | |
| Urate | Beta | 0.441 | 0.163 | 0.240 | 0.113 | −0.052 |
| p | <1.0 × 10−200 | 1.47 × 10−70 | 2.45 × 10−154 | 9.56 × 10−32 | 1.58 × 10−8 | |
| Ferritin (log) | Beta | 0.430 | 0.170 | 0.104 | 0.124 | 0.035 |
| p | <1.0 × 10−200 | 2.03 × 10−69 | 1.37 × 10−28 | 2.70 × 10−34 | 2.92 × 10−4 | |
| LDL-C Female | Beta | - | 0.108 | 0.133 | −0.071 | 0.070 |
| p | - | 6.50 × 10−13 | 1.47 × 10−19 | 1.60 × 10−6 | 3.41 × 10−6 | |
| LDL-C Male | Beta | - | −0.104 | 0.035 | 0.018 | 0.024 |
| p | - | 1.56 × 10−9 | 0.034 | NS | NS | |
| HDL-C | Beta | −0.448 | 0.036 | −0.278 | 0.299 | −0.110 |
| p | <1.0 × 10−200 | 1.62 × 10−4 | 6.23 × 10−189 | 2.32 × 10−185 | 6.49 × 10−30 | |
| Triglycerides (log) | Beta | 0.236 | 0.144 | 0.286 | −0.002 | 0.089 |
| p | 7.25 × 10−107 | 2.69 × 10−44 | 1.05 × 10−171 | NS | 1.24 × 10−17 | |
| Glucose | Beta | 0.112 | 0.185 | 0.141 | 0.004 | 0.055 |
| p | 3.81 × 10−19 | 4.06 × 10−53 | 7.32 × 10−33 | NS | 6.73 × 10−6 | |
| Insulin (log) | Beta | 0.135 | 0.031 | 0.301 | −0.221 | 0.012 |
| p | 4.38 × 10−9 | NS | 4.16 × 10−47 | 5.47 × 10−20 | NS | |
| Albumin | Beta | 0.195 | −0.257 | −0.140 | 0.107 | −0.028 |
| p | 2.45 × 10−72 | 2.95 × 10−130 | 6.65 × 10−43 | 2.73 × 10−22 | 0.0077 | |
| CRP (log) | Beta | −0.109 | 0.133 | 0.375 | 0.002 | 0.072 |
| p | 2.87 × 10−24 | 9.09 × 10−38 | 6.93 × 10−282 | NS | 9.54 × 10−12 | |
| Alkaline phosphatase | Beta | 0.038 | 0.232 | 0.200 | −0.024 | 0.090 |
| p | 0.00061 | 1.40 × 10−101 | 8.33 × 10−81 | 0.034 | 1.08 × 10−16 | |
| Butyrylcholinesterase | Beta | 0.229 | 0.060 | 0.267 | −0.018 | −0.019 |
| p | 2.54 × 10−97 | 8.13 × 10−9 | 4.17 × 10−145 | NS | NS | |
| Bilirubin (log) | Beta | 0.206 | −0.012 | −0.131 | 0.046 | −0.182 |
| p | 2.10 × 10−75 | NS | 9.51 × 10−36 | 5.25 × 10−5 | 1.87 × 10−61 | |
| Urea | Beta | 0.204 | 0.342 | 0.047 | −0.056 | −0.043 |
| p | 1.45 × 10−81 | 1.11 × 10−230 | 2.45 × 10−6 | 2.45 × 10−7 | 3.66 × 10−5 |
p-values > 0.05 are shown as NS, and p-values less than the Bonferroni-corrected critical value of 5.9 × 10−4 are shown in scientific notation. Sex is coded as female = 0, male = 1; Total Drinks is the number of drinks reported in the week before blood collection (not log-transformed); and the Smoking variable is log(N of cigarettes + 1).
Effects of low to moderate alcohol intake
The results from the initial multiple regression represent the linear effects across the observed range of alcohol intake. The two-stage regression analysis on the standardised residuals of marker results after adjustment for sex, age, BMI and log(N of cigarettes + 1) showed highly significant quadratic associations with alcohol intake (deviations from linearity) for all markers except insulin and albumin (Table 4). More detailed examination of alcohol-biomarker relationships within the low- to moderate-intake range was done using plots of the means and standard errors for the marker results for the seven groups defined by reported alcohol intake in the week before blood collection. Results are shown in Figures 1, 2, 3, 4, 5 and 6. There was good correspondence between the expected marker values, calculated from the regression coefficients, and the observed means (Supplementary Figure 3).
Table 4.
Regression analysis. Coefficients from linear and quadratic regression of biomarker results (adjusted for se x, age, BMI and log(N of cigarettes + 1)) on log(N of Drinks + 1).
| Linear
|
Quadratic
|
Proportion of variance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BDrinks | pDrinks | BDrinks | pDrinks | BDrinks-squared | pDrinks-squared | R2 linear | R2 quadratic | R2 change | |
| GGT (log) | 0.2814 | 1.79 × 10−37 | −0.5156 | 4.13 × 10−15 | 0.5353 | 5.91 × 10−34 | 2.5% | 4.9% | 2.4% |
| ALT (log) | 0.1142 | 8.32 × 10−8 | −0.3087 | 4.11 × 10−7 | 0.2841 | 1.02 × 10−12 | 0.4% | 1.1% | 0.7% |
| AST (log) | 0.2186 | 6.87 × 10−24 | −0.3716 | 3.27 × 10−9 | 0.3963 | 3.00 × 10−20 | 1.5% | 2.8% | 1.3% |
| CDT% | 0.3558 | 1.99 × 10−16 | −0.2375 | 0.1004 | 0.3259 | 5.0 × 10−4 | 3.7% | 4.6% | 0.9% |
| Urate | 0.1878 | 4.81 × 10−19 | −0.1376 | 0.0242 | 0.2185 | 2.45 × 10−8 | 1.1% | 1.5% | 0.4% |
| Ferritin (log) | 0.1813 | 3.65 × 10−20 | −0.2159 | 3.10 × 10−4 | 0.2668 | 4.54 × 10−13 | 1.0% | 1.6% | 0.6% |
| HDL-C | 0.5253 | 2.17 × 10−133 | 0.1122 | 0.0618 | 0.2774 | 7.85 × 10−13 | 8.7% | 9.3% | 0.6% |
| LDL-C (Male) | 0.0849 | 0.0046 | 0.1996 | 0.034 | −0.0700 | 0.205 | 0.2% | 0.3% | 0.1% |
| LDL-C (Female) | −0.1595 | 7.13 × 10−7 | 0.0398 | 0.670 | −0.1595 | 0.024 | 0.6% | 0.7% | 0.1% |
| Triglycerides (log) | −0.4556 | 0.0309 | −0.2335 | 1.3 × 10−4 | 0.1262 | 0.0019 | 0.1% | 0.2% | 0.1% |
| Glucose | −0.0455 | 0.0611 | −0.2821 | 5.3 × 10−5 | 0.1615 | 2.40 × 10−4 | 0.1% | 0.3% | 0.2% |
| Insulin (log) | −0.3333 | 1.35 × 10−17 | −0.2658 | 0.0322 | −0.0368 | 0.582 | 3.3% | 3.3% | 0 |
| Albumin | 0.2057 | 3.66 × 10−23 | 0.1135 | 0.066 | 0.062 | 0.118 | 1.3% | 1.3% | 0 |
| CRP (log) | −0.0628 | 0.0021 | −0.2949 | 9.48 × 10−7 | 0.1557 | 3.00 × 10−5 | 0.1% | 0.3% | 0.2% |
| Alkaline Phosphatase | −0.1544 | 2.26 × 10−13 | −0.4992 | 6.61 × 10−15 | 0.2315 | 3.09 × 10−9 | 0.7% | 1.2% | 0.5% |
| Butyrylcholinesterase | −0.0584 | 0.0065 | −0.2056 | 0.0018 | 0.0989 | 0.021 | 0.1% | 0.2% | 0.1% |
| Bilirubin (log) | 0.1297 | 9.98 × 10−10 | 0.3282 | 2.44 × 10−7 | −0.1333 | 0.001 | 0.5% | 0.7% | 0.2% |
| Urea | −0.0392 | 0.055 | 0.1662 | 0.009 | −0.1379 | 3.60 × 10−4 | 0.1% | 0.2% | 0.1% |
p-values are based on use of the robust estimator as described in the text.
Figure 1.
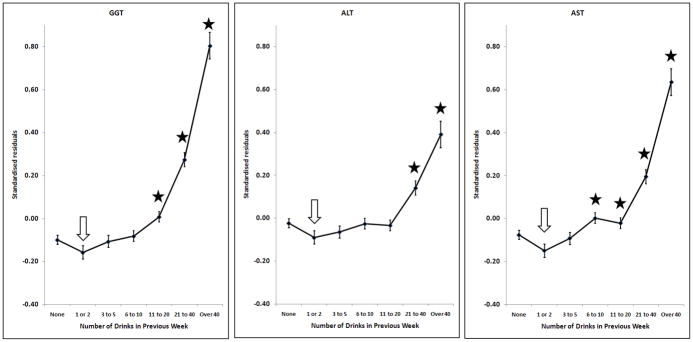
Effects of alcohol on log10GGT, log10ALT, log10AST; adjusted for sex, age, BMI and smoking. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Figure 2.
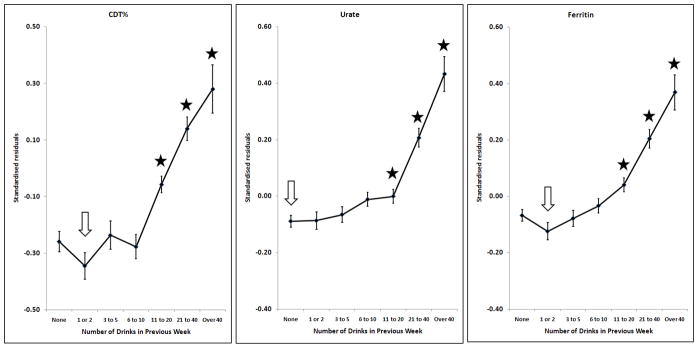
Effects of alcohol on CDT, urate, log10Ferritin; adjusted for sex, age, BMI and smoking. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Figure 3.
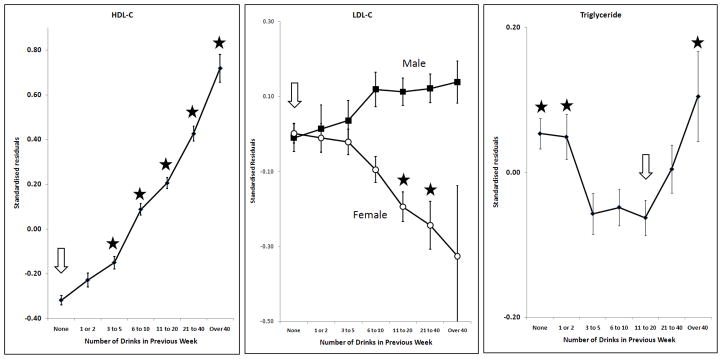
Effects of alcohol on HDL-C, LDL-C, log10Triglyceride; adjusted for sex (except for LDL-C), age, BMI and smoking. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Figure 4.
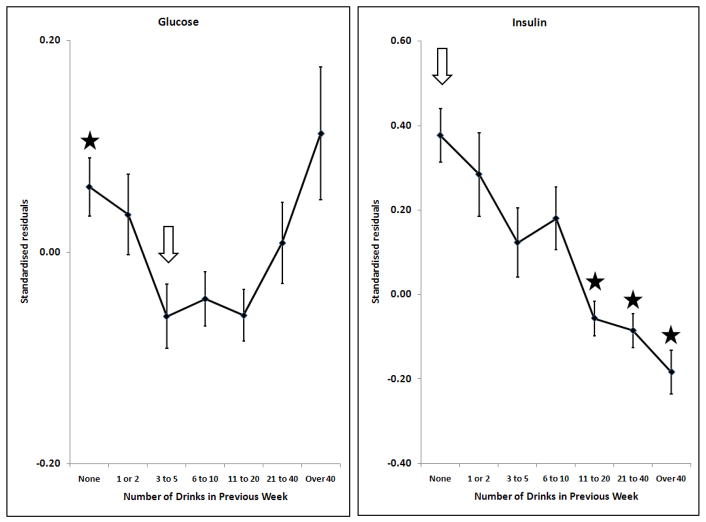
Effects of alcohol on glucose, log10Insulin; adjusted for sex, age, BMI, smoking and time since last meal. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Figure 5.
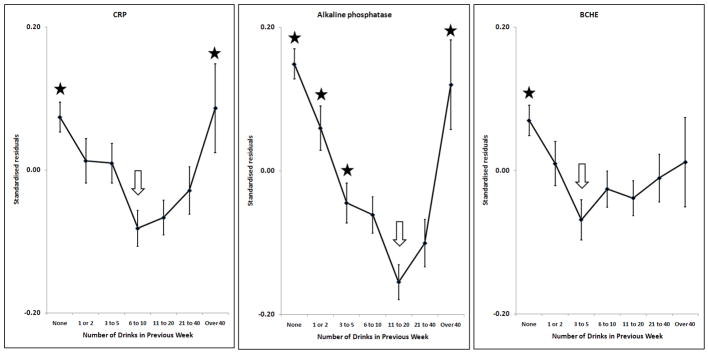
Effects of alcohol on log10CRP, alkaline phosphatase, butyrylcholinesterase; adjusted for sex, age, BMI and smoking. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Figure 6.
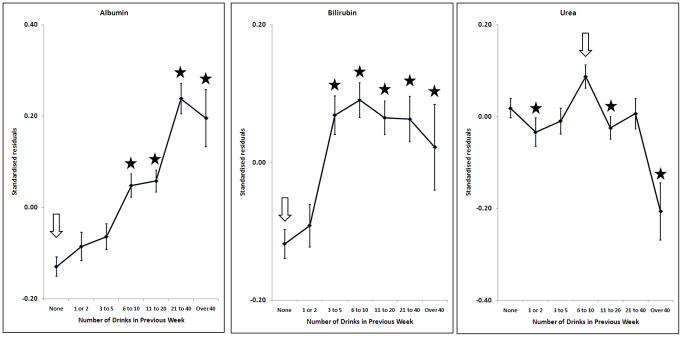
Effects of alcohol on albumin, log10Bilirubin, urea; adjusted for sex, age, BMI and smoking. Points and error bars show mean ± SEM. The arrow shows the reference group and stars indicate the groups which are significantly different from it after allowing for multiple comparisons (p < (0.05/6) = 0.0083).
Many markers showed little or no change with increasing alcohol intake until a threshold level was exceeded. This was found for GGT, ALT, AST, CDT, urate and ferritin (Figures 1 and 2). In general, the threshold was at around two drinks per day. However, some markers were affected by any reported alcohol consumption above zero; this occurred for HDL-C (Figure 3) and insulin (Figure 4), with the former increasing and the latter decreasing across the entire range of alcohol intake. Albumin also increased across all alcohol intake groups (Figure 6). Other variables showed decreases across the lowest intake groups but increased with larger amounts of alcohol. This ‘U-shaped’ pattern of alcohol effects was seen for triglycerides (Figure 3), glucose (Figure 4), alkaline phosphatase, butyrylcholinesterase and CRP (Figure 5).
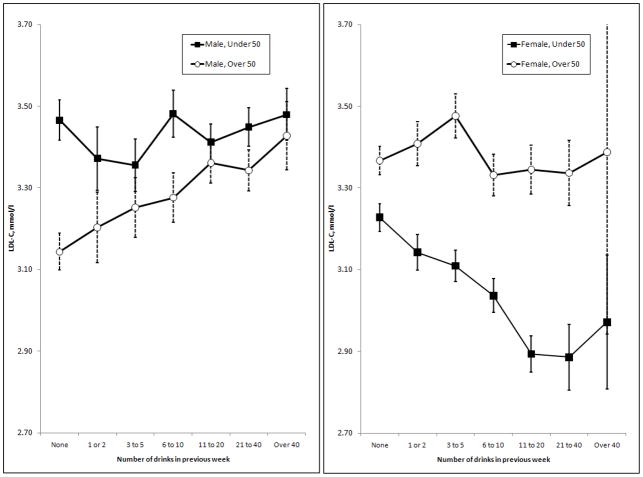
Other effects were more complex; the response of CDT to alcohol varied with body mass index, as reported previously (Whitfield et al., 2008). The relationship between alcohol and LDL-C differed by sex (Figure 3). Initial results showed significantly significant inverse relationship between alcohol and LDL-C in women but there was little effect in men. Because effects might differ between pre- and post-menopausal women, we repeated the analysis after dividing subjects by age (below or above 50) as well as sex. In men, alcohol had little effect on LDL-C in the younger group but increased it in the older group; in women, alcohol decreased LDL-C in the younger group but had little effect in the older group (Figure 7).
Figure 7.
Associations between alcohol intake and calculated LDL-C in men and women. In each panel, the results are divided by age of the subjects, into those aged less than 50 and those aged 50 or more. Error bars show standard errors. Linear regression showed significant effects of alcohol only in the older men (p = 0.0045) and younger women (p = 7.86 × 10−9).
The alcohol-biomarker relationships when the quantity-frequency measure of alcohol intake was used instead of past-week intake are shown in Supplementary Figure 4. Comparisons of the effects of alcohol on the biomarker means by sex, lifetime alcohol dependence, and smoking status gave consistent results across categories, shown in Supplementary Figures 5 to 7.
Comparison of two measures of alcohol intake
Most study participants had provided information on their usual frequency and quantity of alcohol use. This was used to provide a validation of the past-week intake data; the sex-adjusted correlation between these two measures, using the log(N of drinks + 1) transformation, was 0.76. However the number of drinks per week estimated from the usual quantity and frequency responses was lower than that reported for the week before blood collection. This is illustrated in Supplementary Figure 1; the cumulative frequency distributions level out at lower values for the quantity-frequency measure and at higher alcohol intake the past-week number of drinks was approximately double the quantity-frequency estimate. However, the shape of the relationships between the biomarker results and the quantity-frequency estimate of alcohol use were essentially the same as for the past-week estimate (Supplementary Figure 4). In particular, glucose, triglycerides, CRP and alkaline phosphatase still showed U-shaped relationships with alcohol when this measure was used.
Effects of past alcohol dependence
Potential effects of lifetime alcohol dependence status on the biomarkers, alone or in combination with current alcohol intake, were examined. This is particularly relevant to the group reporting no alcoholic drinks in the seven days before blood collection, and it can be seen from Table 2 that the lifetime prevalence of AD was higher in the zero-drinks category than in the ‘1 or 2 drinks’ category. This difference was significant (stratified by sex, G2 = 9.68, 2 df, two-tailed p = 0.0079). Arising from this, we considered whether there was a significant difference between biomarker means for alcohol-dependence-positive and alcohol-dependence-negative people in the zero-drinks category, but none of the differences reached significance at p < 0.05 (Supplementary Figure 6).
Effects of current smoking
Although current smoking status had significant effects on many of the markers (Table 3), the alcohol/marker relationships were essentially parallel in the smokers and non-smokers (Supplementary Figure 7).
Comparison of beer and wine
We compared GGT and HDL-C results from participants who reported consumption of only wine, or only beer, in the seven days before blood collection in order to test whether the effects of alcohol depend on beverage. There was some confounding of preferred beverage with sex, because the beer-only drinkers were mostly (83%) men while the wine-only drinkers were mostly (78%) women. Once this was taken into account, the effects of alcohol from wine or from beer on GGT or on HDL-C were essentially the same (data not shown).
DISCUSSION
We concentrate on results which involve small amounts of alcohol, show significantly biphasic (U- or J-shaped) relationships, or have a bearing on the long-term health effects of alcohol.
Effects of low to moderate alcohol consumption
Many of the variables tested show a pattern of little change with increasing alcohol intake at lower levels, below about two drinks (20 grams of alcohol) per day, and an increase in average values above that. This applies to the liver function tests GGT, ALT and AST, and also to CDT, urate and ferritin. This suggests that alcohol has little effect below a threshold, but the threshold is marker-specific and there is no evidence of a threshold for HDL-C or insulin.
For the lipids related to cardiovascular risk, the situation is complex. HDL-C increases with increasing alcohol intake even across the low-intake categories, from those who report no alcohol in the previous week to an average of one drink (10 grams of alcohol) per day. This trend continues across the entire spectrum of alcohol intake. For LDL-C, the associations with alcohol intake differ between men and women, and by age. The apparent effects of alcohol in younger women and older men may not be causal, and we cannot exclude effects from lifestyle or other confounders. For triglycerides, there was a decrease in the mean with increasing alcohol intake up to one to two drinks a day (10–20 grams per day) but an increase for the categories equivalent to four or more drinks per day.
There is a substantial literature about associations between alcohol intake and plasma lipids in cross-sectional population studies, largely focused on implications for cardiovascular risk. The response of HDL-C is the most consistent feature, and there is some evidence that the higher HDL-C associated with greater alcohol use is accompanied by an increase in reverse cholesterol transport (Kralova Lesna et al., 2010). Overall, high HDL-C is associated with lower cardiovascular risk but doubts have been raised about the role of HDL-C in mediating alcohol’s effects on cardiovascular disease. Adjusting for HDL-C results in a recent prospective study on fatal coronary heart disease made little difference to the Hazard Ratios associated with alcohol use (Magnus et al., 2011). Against this, results from other studies have shown that adjustment for HDL-C does attenuate alcohol’s relationship with cardiovascular mortality (Langer et al., 1992; Mukamal et al., 2005), which suggests that the reduction in HDL-C is important.
Recent meta-analysis of experimental studies in which volunteers took known amounts of alcohol (Brien et al., 2011) showed significant and dose-dependent increases in HDL-C, but no significant effects overall for LDL-C or triglycerides. In our data, triglycerides were lowest in people reporting between three and twenty drinks per week, or 4 to 30 grams of alcohol per day. There have been previous reports of U-shaped alcohol-trigycerides associations in cross-sectional population studies, (Kato et al., 2003; Tolstrup et al., 2009; Whitehead et al., 1996a) with lowest mean triglyceride values at about 20 grams of alcohol per day.
Turning to glucose homeostasis, insulin concentration decreased with increasing alcohol consumption across the entire range and the linear association between alcohol intake and insulin concentration (β = −0.33, Table 4) was one of the strongest we found. Small amounts of alcohol were associated with lower glucose concentration, but reported consumption of over 20 drinks per week was associated with a reversal of this trend. This suggests that low alcohol intake (compared to no alcohol) increases insulin sensitivity and circulating insulin concentration decreases in response, but at higher levels of alcohol use insulin secretion is insufficient and glucose concentrations increase. There may also be differences in diet or other unmeasured lifestyle factors in the high-alcohol-intake subjects which could increase glucose concentrations. Our results are consistent with most studies which have assessed serum insulin or insulin resistance in humans. Observational studies have found that higher alcohol consumption is associated with greater insulin sensitivity or decreased serum insulin (Fueki et al., 2007; Kawamoto et al., 2009; Sung et al., 2007). Experimental studies with humans taking 25 or 30 grams of alcohol per day for 6 or 8 weeks gave consistent but not conclusive results, with one showing significant reductions in fasting insulin and insulin resistance (Joosten et al., 2008) while the other showed only a trend towards improved insulin sensitivity (Kim et al., 2009). Animal studies support the hypothesis that alcohol improves insulin sensitivity (Hong et al., 2009), and suggest that this is mediated by increased expression of anti-inflammatory factors in adipose tissue (Paulson et al., 2010).
The only marker of inflammation which we measured was C-reactive protein. Concentrations were lowest in people reporting around 10 grams of alcohol (one drink) per day, with higher results associated with both lower and higher alcohol use. Although this association between alcohol and CRP was U-shaped and significant, it was not particularly strong, accounting for only 0.3% of the variance in log-transformed CRP concentration. Most cross-sectional studies have shown lower mean CRP concentrations in drinkers taking up to 20 grams of alcohol per day compared to abstainers; several have also found higher CRP in excessive drinkers (Raum et al., 2007; Volpato et al., 2004; Wang et al., 2008) and those which d id not (Albert et al., 2003; Levitan et al., 2005) were limited by the small number of excessive drinkers and a rather low top category of alcohol intake. Some experimental studies using 20–40 grams of alcohol daily for 3–4 weeks (Sacanella et al., 2007; Sierksma et al., 2002) showed a decrease in CRP, while others using similar amounts of alcohol (Rajdl et al., 2007; Retterstol et al., 2005) have found small but non-significant increases.
Alkaline phosphatase also showed a highly significant U-shaped relationship with alcohol use. Few previous studies of alcohol and alkaline phosphatase could be found, although one (Whitfield et al., 1978) found an increase in the prevalence of abnormally high alkaline phosphatase as quantity of alcohol per drinking day increased. The possibility of multiple effects of alcohol with differing thresholds is particularly relevant for alkaline phosphatase, because its activity in serum results from bone, liver and intestinal isoenzymes. For example, high alcohol intake might increase liver alkaline phosphatase and account for the upward part of the U-shaped relationship, while smaller amounts of alcohol could be associated with decreases in bone or intestinal sources. This question cannot be resolved with our data. For butyrylcholinesterase, there was a significant biphasic component as shown in the quadratic regression analysis (p = 0.009). This marker was included because of its strong association with obesity and other components of metabolic syndrome. Albumin increased with increasing alcohol intake; there was no indication that excessive intake reduced its hepatic synthesis in this population-based study. Alcohol had no effect on urea except in the extreme group of people taking over 40 drinks per week, where there was a decrease. This may be due to low protein intake, but there is no direct evidence on this point.
Potential confounders
Biphasic or U-shaped relationships with alcohol intake were found for triglycerides, glucose, CRP, alkaline phosphatase and butyrylcholinesterase. As with the mortality and morbidity data, these could potentially be due to inclusion of past-drinkers in the zero-alcohol group. We consider this unlikely, because there are no significant biomarker differences between lifetime-AD-positive and lifetime-AD-negative people in the zero-alcohol group. Confining the regression analysis performed on all subjects (Table 3) to people who had never met the DSM-IV criteria for alcohol dependence still gave significant quadratic terms for triglycerides, CRP, alkaline phosphatase and butyrylcholinesterase (p = 0.0014, 0.0011, 0.0018 and 0.021, respectively) but not for glucose (p = 0.473). Similarly, the U-shaped effects do not depend on the association between higher alcohol intake and smoking. The possible effects of inaccurate reporting of alcohol intake are mitigated by the availability of two measures of alcohol intake, the number of drinks in the week before blood collection and the usual quantity and frequency measure. These were highly correlated, as shown in previous studies (Whitfield et al., 2004). At high alcohol intake, it seems that people underestimated their usual frequency or quantity of alcohol use and the past-week estimate may be more accurate, but both measures of alcohol intake showed similar relationships with the biomarkers. In addition to these considerations, any errors in alcohol intake assessment will apply to all the alcohol-marker relationships and could not account for the differences where some are essentially linear, some show thresholds and others show U-shaped curves.
Limitations
Our results are subject to some limitations. We have insufficient information on morbidity and mortality to be able to connect alcohol, the biomarkers, and clinical outcomes into an integrated account so this paper is restricted to the observed alcohol-biomarker relationships. It was not feasible to obtain all blood samples in the fasting state, so additional variation is introduced for at least insulin and glucose and to a lesser extent for triglycerides and LDL-C. However this is unlikely to generate false associations with alcohol use, nor to distort the shape of the alcohol-marker dose-response curves.
Conclusions
It is clear that variation in drinking behaviour is associated with variation in many biochemical markers and metabolic processes. There may be common pathways from alcohol to several of the observed changes, but the differences in the alcohol-marker dose-response curves indicate that there is not a single pathway for all of them. There are changes which are probably harmful, and others which are probably beneficial. The pattern of potentially beneficial effects of alcohol on HDL-C and insulin increasing progressively as consumption increases, while the negative effects show thresholds, is consistent with net U- or J-shaped effects of alcohol on health. Questions for future research include whether genetic variation contributes to differences in responses to alcohol use, and how far the biomarker responses to alcohol predict clinical outcomes such as liver damage or cardioprotection.
Supplementary Material
Acknowledgments
We are grateful to the participants in this study for their time and trouble. We also wish to acknowledge the contributions of project staff at Queensland Institute of Medical Research, under the leadership of Anjali Henders and Leanne Wallace, to sample processing and biobanking, and of Jole Bojovic and Veronica Dy at Royal Prince Alfred Hospital, Sydney, to biomarker measurements.
Support: Subject recruitment and interviews, and blood collection and processing, were supported by grants AA013321, AA013326, DA012854 and AA013320 from the US National Institutes of Health to ACH, NGM, PAFM, and the late Richard Todd, MD PhD. Biomarker measurement was supported by AA014041 to JBW. GWM is supported by the National Health and Medical Research Council of Australia Fellowship Scheme. MLP received support from the US National Institutes of Health grant DA019951.
References
- Alatalo P, Koivisto H, Puukka K, Hietala J, Anttila P, Bloigu R, Niemela O. Biomarkers of liver status in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2009;44:199–203. doi: 10.1093/alcalc/agn099. [DOI] [PubMed] [Google Scholar]
- Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Alkerwi A, Boutsen M, Vaillant M, Barre J, Lair ML, Albert A, Guillaume M, Dramaix M. Alcohol consumption and the prevalence of metabolic syndrome: a meta-analysis of observational studies. Atherosclerosis. 2009;204:624–635. doi: 10.1016/j.atherosclerosis.2008.10.036. [DOI] [PubMed] [Google Scholar]
- Alte D, Ludemann J, Piek M, Adam C, Rose HJ, John U. Distribution and dose response of laboratory markers to alcohol consumption in a general population: results of the study of health in Pomerania (SHIP) J Stud Alcohol. 2003;64:75–82. doi: 10.15288/jsa.2003.64.75. [DOI] [PubMed] [Google Scholar]
- Breitling LP, Claessen H, Drath C, Arndt V, Brenner H. Gamma-glutamyltransferase, general and cause-specific mortality in 19,000 construction workers followed over 20 years. J Hepatol. 2011;55:594–601. doi: 10.1016/j.jhep.2010.12.029. [DOI] [PubMed] [Google Scholar]
- Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98(Suppl 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Hu BF, Camargo CA, Jr, Stampfer MJ, Willett WC, Rimm EB. A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes. 2001;50:2390–2395. doi: 10.2337/diabetes.50.10.2390. [DOI] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Hall E, Wheatley K, Gray R. Mortality in relation to consumption of alcohol: 13 years’ observations on male British doctors. BMJ. 1994;309:911–918. doi: 10.1136/bmj.309.6959.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore KM, Kerr WC, Stockwell TR, Chikritzhs TN, Bostrom A. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies. Addict Res Theory. 2006;14:101–132. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–2735. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- Fueki Y, Miida T, Wardaningsih E, Ito M, Nakamura A, Takahashi A, Hanyu O, Tsuda A, Saito H, Hama H, Okada M. Regular alcohol consumption improves insulin resistance in healthy Japanese men independent of obesity. Clin Chim Acta. 2007;382:71–76. doi: 10.1016/j.cca.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Fuller TD. Moderate Alcohol Consumption and the Risk of Mortality. Demography. 2011;48:1105–1125. doi: 10.1007/s13524-011-0035-2. [DOI] [PubMed] [Google Scholar]
- Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou YL, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PA, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiatry. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Smith RR, Harvey AE, Nunez NP. Alcohol consumption promotes insulin sensitivity without affecting body fat levels. Int J Obes (Lond) 2009;33:197–203. doi: 10.1038/ijo.2008.266. [DOI] [PubMed] [Google Scholar]
- Inoue M, Nagata C, Tsuji I, Sugawara Y, Wakai K, Tamakoshi A, Matsuo K, Mizoue T, Tanaka K, Sasazuki S, Tsugane S Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan. Impact of alcohol intake on total mortality and mortality from major causes in Japan: a pooled analysis of six large-scale cohort studies. J Epidemiol Community Health. 2012;66:448–456. doi: 10.1136/jech.2010.121830. [DOI] [PubMed] [Google Scholar]
- Joosten MM, Beulens JW, Kersten S, Hendriks HF. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia. 2008;51:1375–1381. doi: 10.1007/s00125-008-1031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato I, Kiyohara Y, Kubo M, Tanizaki Y, Arima H, Iwamoto H, Shinohara N, Nakayama K, Fujishima M. Insulin-mediated effects of alcohol intake on serum lipid levels in a general population: the Hisayama Study. J Clin Epidemiol. 2003;56:196–204. doi: 10.1016/s0895-4356(02)00578-4. [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Kohara K, Tabara Y, Miki T, Ohtsuka N, Kusunoki T, Abe M. Alcohol consumption is associated with decreased insulin resistance independent of body mass index in Japanese community-dwelling men. Tohoku J Exp Med. 2009;218:331–337. doi: 10.1620/tjem.218.331. [DOI] [PubMed] [Google Scholar]
- Kim SH, Abbasi F, Lamendola C, Reaven GM. Effect of moderate alcoholic beverage consumption on insulin sensitivity in insulin-resistant, nondiabetic individuals. Metabolism. 2009;58:387–392. doi: 10.1016/j.metabol.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- Kralova Lesna I, Suchanek P, Stavek P, Poledne R. May alcohol-induced increase of HDL be considered as atheroprotective? Physiol Res. 2010;59:407–413. doi: 10.33549/physiolres.931769. [DOI] [PubMed] [Google Scholar]
- Langer RD, Criqui MH, Reed DM. Lipoproteins and blood pressure as biological pathways for effect of moderate alcohol consumption on coronary heart disease. Circulation. 1992;85:910–915. doi: 10.1161/01.cir.85.3.910. [DOI] [PubMed] [Google Scholar]
- Levitan EB, Ridker PM, Manson JE, Stampfer MJ, Buring JE, Cook NR, Liu S. Association between consumption of beer, wine, and liquor and plasma concentration of high-sensitivity C-reactive protein in women aged 39 to 89 years. Am J Cardiol. 2005;96:83–88. doi: 10.1016/j.amjcard.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Magnus P, Bakke E, Hoff DA, Hoiseth G, Graff-Iversen S, Knudsen GP, Myhre R, Normann PT, Naess O, Tambs K, Thelle DS, Morland J. Controlling for high-density lipoprotein cholesterol does not affect the magnitude of the relationship between alcohol and coronary heart disease. Circulation. 2011;124:2296–2302. doi: 10.1161/CIRCULATIONAHA.111.036491. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Jensen MK, Gronbaek M, Stampfer MJ, Manson JE, Pischon T, Rimm EB. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation. 2005;112:1406–1413. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Yoshida H, Takahashi H, Matsuda Y, Kawai M. Dose-response relationships between drinking and serum tests in Japanese men aged 40–59 years. Alcohol. 1999;17:133–138. doi: 10.1016/s0741-8329(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Paulson QX, Hong J, Holcomb VB, Nunez NP. Effects of body weight and alcohol consumption on insulin sensitivity. Nutr J. 2010;9:14. doi: 10.1186/1475-2891-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdl D, Racek J, Trefil L, Siala K. Effect of white wine consumption on oxidative stress markers and homocysteine levels. Physiol Res. 2007;56:203–212. doi: 10.33549/physiolres.930936. [DOI] [PubMed] [Google Scholar]
- Raum E, Gebhardt K, Buchner M, Schiltenwolf M, Brenner H. Long-term and short-term alcohol consumption and levels of C-reactive protein. Int J Cardiol. 2007;121:224–226. doi: 10.1016/j.ijcard.2006.08.104. [DOI] [PubMed] [Google Scholar]
- Retterstol L, Berge KE, Braaten O, Eikvar L, Pedersen TR, Sandvik L. A daily glass of red wine: does it affect markers of inflammation? Alcohol Alcohol. 2005;40:102–105. doi: 10.1093/alcalc/agh132. [DOI] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. Ischemic heart disease mortality and morbidity rates in former drinkers: a meta-analysis. Am J Epidemiol. 2011;173:245–258. doi: 10.1093/aje/kwq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacanella E, Vazquez-Agell M, Mena MP, Antunez E, Fernandez-Sola J, Nicolas JM, Lamuela-Raventos RM, Ros E, Estruch R. Down-regulation of adhesion molecules and other inflammatory biomarkers after moderate wine consumption in healthy women: a randomized trial. Am J Clin Nutr. 2007;86:1463–1469. doi: 10.1093/ajcn/86.5.1463. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, Agrawal A, Dick DM, Heath AC, Todorov AA, Maunu H, Heikkila K, Morley KI, Rice JP, Todd RD, Kaprio J, Peltonen L, Martin NG, Goate AM, Madden PA. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. Am J Hum Genet. 2007;80:856–866. doi: 10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scouller K, Conigrave KM, Macaskill P, Irwig L, Whitfield JB. Should we use carbohydrate-deficient transferrin instead of gamma-glutamyltransferase for detecting problem drinkers? A systematic review and metaanalysis. Clin Chem. 2000;46:1894–1902. [PubMed] [Google Scholar]
- Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56:1130–1136. doi: 10.1038/sj.ejcn.1601459. [DOI] [PubMed] [Google Scholar]
- Sung KC, Kim SH, Reaven GM. Relationship among alcohol, body weight, and cardiovascular risk factors in 27,030 Korean men. Diabetes Care. 2007;30:2690–2694. doi: 10.2337/dc07-0315. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337:1705–1714. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Tolstrup JS, Gronbaek M, Nordestgaard BG. Alcohol intake, myocardial infarction, biochemical risk factors, and alcohol dehydrogenase genotypes. Circ Cardiovasc Genet. 2009;2:507–514. doi: 10.1161/CIRCGENETICS.109.873604. [DOI] [PubMed] [Google Scholar]
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Fellin R, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–612. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Tung TH, Yin WH, Huang CM, Jen HL, Wei J, Young MS. Effects of moderate alcohol consumption on inflammatory biomarkers. Acta Cardiol. 2008;63:65–72. doi: 10.2143/AC.63.1.2025334. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Robinson D, Allaway SL. The effects of cigarette smoking and alcohol consumption on blood lipids: a dose-related study on men. Ann Clin Biochem. 1996a;33:99–106. doi: 10.1177/000456329603300201. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Robinson D, Allaway SL. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in men. Ann Clin Biochem. 1996b;33:530–535. doi: 10.1177/000456329603300607. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Dy V, Madden PA, Heath AC, Martin NG, Montgomery GW. Measuring carbohydrate-deficient transferrin by direct immunoassay: factors affecting diagnostic sensitivity for excessive alcohol intake. Clin Chem. 2008;54:1158–1165. doi: 10.1373/clinchem.2007.101733. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Hensley WJ, Bryden D, Gallagher H. Some laboratory correlates of drinking habits. Ann Clin Biochem. 1978;15:297–303. doi: 10.1177/000456327801500171. [DOI] [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Heath AC, Powell LW, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25:1037–1045. [PubMed] [Google Scholar]
- Whitfield JB, Zhu G, Madden PA, Neale MC, Heath AC, Martin NG. The genetics of alcohol intake and of alcohol dependence. Alcohol Clin Exp Res. 2004;28:1153–1160. doi: 10.1097/01.alc.0000134221.32773.69. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.