Abstract
HbzF from Pseudomonas alcaligenes NCIMB 9867 was purified to homogeneity as a His-tagged protein and likely a dimer by SDS-PAGE and gel filtration. This protein was demonstrated to be a novel maleylpyruvate hydrolase, catalyzing direct hydrolysis of maleylpyruvate to maleate and pyruvate, and belongs to the fumarylacetoacetate hydrolase superfamily. This study reveals the genetic determinate for the direct maleylpyruvate hydrolysis in the gentisate pathway, complementary to the well-studied maleylpyruvate isomerization route.
TEXT
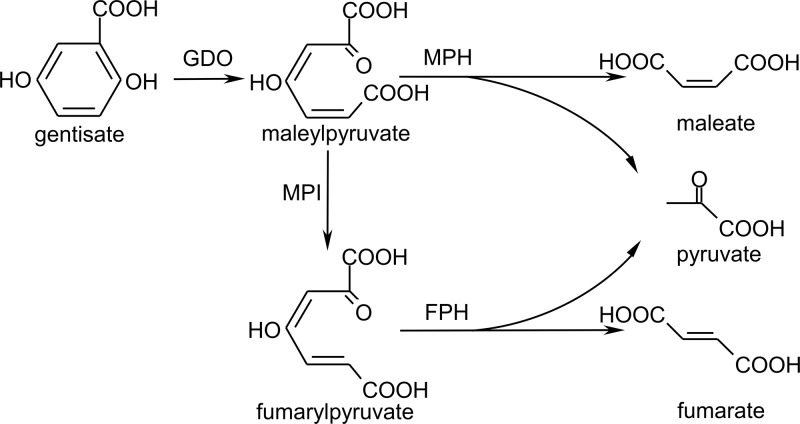
Gentisate (2,5-dihydroxybenzoate) is an important ring-cleavage intermediate present in the bacterial catabolic pathway of many aromatic compounds. In this pathway, gentisate 1,2-dioxygenase (GDO) catalyzes the ring-cleavage oxidation of gentisate to yield maleylpyruvate, which is further degraded by either isomerization to fumarylpyruvate and subsequent hydrolysis to fumarate and pyruvate (1) or direct hydrolysis to maleate and pyruvate (Fig. 1) (2). In the isomerization route, glutathione-, mycothiol-, and l-cysteine-dependent maleylpyruvate isomerases have been biochemically and genetically characterized in Gram-negative (3, 4), high-G+C Gram-positive (5, 6), and low-G+C Gram-positive (7) strains, respectively. Maleylpyruvate hydrolase, which catalyzes the direct hydrolysis of maleylpyruvate, was previously purified from Pseudomonas alcaligenes NCIMB 9867, exhibiting two polypeptides with molecular masses of 33 and 50 kDa on SDS-PAGE (8). However, neither its encoding gene nor its amino acid sequence has been reported.
Fig 1.
Two alternative routes for maleylpyruvate catabolism in the gentisate pathway. GDO, gentisate 1,2-dioxygenase; MPH, maleylpyruvate hydrolase (HbzF in this study); MPI, maleylpyruvate isomerase; and FPH, fumarylpyruvate hydrolase.
HbzF catalyzes the hydrolysis of maleylpyruvate.
An hbz gene cluster (GenBank accession number DQ394580) was recently reported to be involved in the gentisate pathway in strain NCIMB 9867, and the hbzE gene was identified to encode a strictly inducible GDO (9). Among the remaining open reading frames, hbzF was annotated to encode a maleylpyruvate isomerase (9), implying that this cluster encodes a classic isomerization route for the gentisate pathway. However, our bioinformatics analysis shows that HbzF exhibits no more than 14% identity with all functionally identified maleylpyruvate isomerases, and it belongs to the fumarylacetoacetate hydrolase superfamily (FAA hydrolase superfamily; conserved domain database accession number cl11421), which includes fumarylpyruvate hydrolase. In addition, HbzF also exhibits low identities (18 to 28%) to functionally identified fumarylpyruvate hydrolases. This stimulated our interest in investigating its biochemical function.
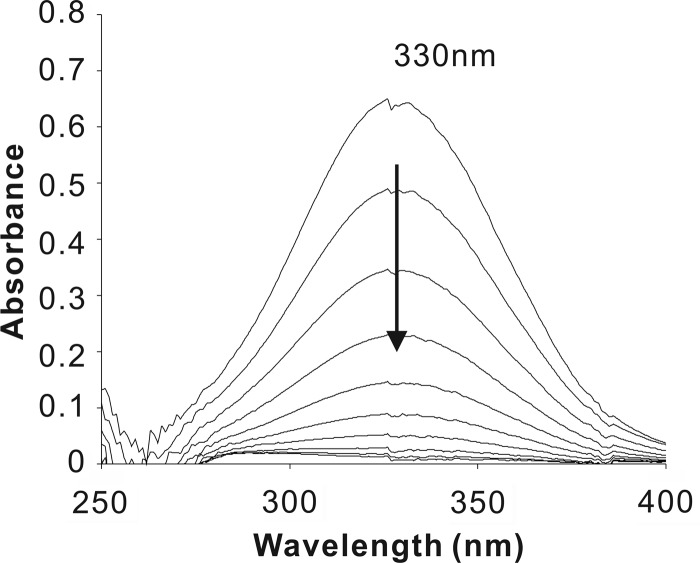
The hbzF gene was amplified from strain NCIMB 9867 genomic DNA using the primer pair F-22b-F (5′-GAC CAT ATG CAA TCG AGT CGG TTG CTC C-3′) and F-22b-R (5′-AAA CTC GAG CAC CGA GCG GAT GGC AAT-3′), with NdeI and XhoI sites incorporated into their respective 5′ ends. The amplified hbzF fragment was cloned into pET22b (Novagen, Madison, WI), in frame with the His6 coding sequence to produce pET22b-hbzF, resulting in the expression of a C-terminal His6-tagged HbzF. Escherichia coli BL21(DE3) cells carrying pET22b-hbzF were grown in lysogeny broth at 37°C to an optical density at 600 nm of 0.6 and then induced for 8 h by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside at 16°C. Cell extracts were prepared by sonication in an ice-water bath for 60 cycles of 5 s with 5-s intervals, after which cell debris was removed by centrifugation at 13,000 × g for 1 h at 4°C. Maleylpyruvate (λmax [wavelength of maximum absorption] = 330 nm) and fumarylpyruvate (λmax = 340 nm) were prepared as described previously (10). After the addition of 5 μg cell extracts to 60 μM maleylpyruvate, a rapid disappearance at 330 nm was observed on a spectrophotometer (Fig. 2), indicating the presence of maleylpyruvate hydrolase activity. The same cell extracts containing HbzF did not catalyze the hydrolysis of fumarylpyruvate, and no activity was detected during the same procedure performed with cell extracts of E. coli BL21(DE3) harboring pET22b with no insert.
Fig 2.
Spectrophotometric changes during the transformation of maleylpyruvate catalyzed by cell extracts of E. coli BL21(DE3)[pET22b-hbzF] expressing HbzF.
Purification and biochemical properties of HbzF.
HbzF-His6 was overexpressed and purified by Ni Sepharose High Performance (GE Healthcare, Uppsala, Sweden) affinity chromatography. When 55 mg of cell extract protein with a specific activity of 1.2 U/mg was applied to 4 ml of affinity chromatography beads, 2.4 mg of HbzF-His6 was purified with a specific activity of 2.0 U/mg. A single band at an apparent molecular mass of 33 kDa was detected by SDS-PAGE (Fig. 3), and its native molecular mass, as determined on gel filtration chromatography, was 77 kDa by a previously described method (11), indicating that the native HbzF was likely a homodimeric protein.
Fig 3.
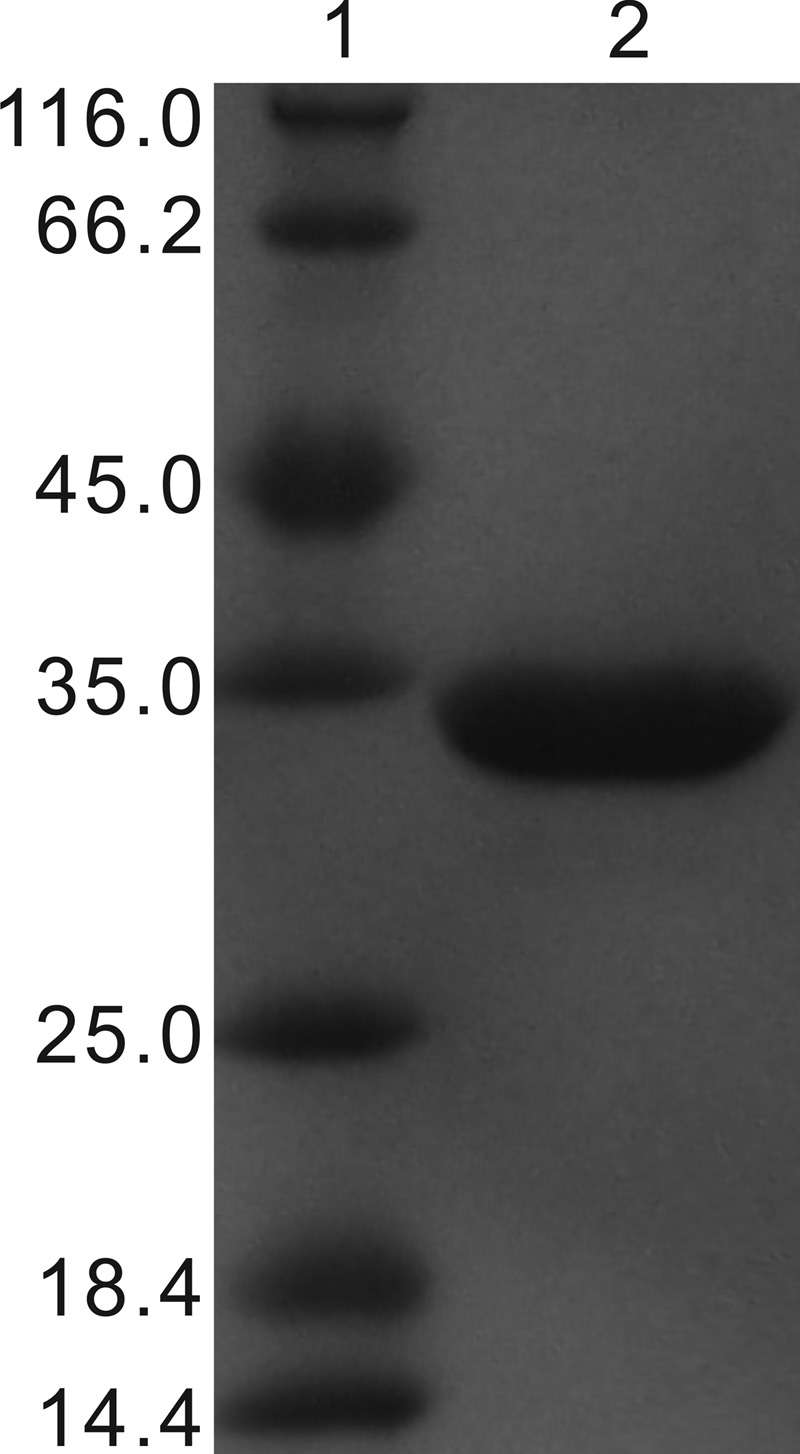
SDS-PAGE of purified HbzF-His6. Lane 1, molecular mass standards (molecular masses are indicated on the left in kDa); lane 2, 5 μg purified HbzF-His6.
Maleylpyruvate hydrolase activity was measured by the decrease of absorption at 330 nm. The molar extinction coefficient of maleylpyruvate was taken as 13,000 M−1 cm−1 (3). One unit of maleylpyruvate hydrolase activity was defined as the amount required for the disappearance of 1 μmol of maleylpyruvate per min at 23°C. Specific activities are expressed as units per milligram of protein, and the values are expressed as means ± standard deviations calculated from triplicate assays. Protein concentration was determined according to the Bradford method, with bovine serum albumin as the standard. For kinetic analysis, three independent sets of experiments were performed with at least six substrate concentrations ranging from 10 to 150 μM. The purified HbzF-His6 displayed typical Michaelis-Menten kinetics, and its Km value was 19.9 ± 0.8 μM for maleylpyruvate, calculated by nonlinear regression analysis (Origin 8.0). This is similar to the Km value (14 μM) of maleylpyruvate hydrolase purified from strain NCIMB 9867 (8). Maximum activity of HbzF-His6 was observed at pH 7.6 when tested in 50 mM Tris-HCl buffer and its optimal activity exhibited at 30°C. Partial inhibition of HbzF-His6 was observed when the concentration of Ni2+, Cd2+, Co2+, or Cu2+ was 50 or 100 μM, and complete inhibition was evident when 1 mM these ions were present. Addition of Mn2+ at a concentration of 50 or 100 μM enhanced its activity by 30%. These biochemical properties are generally consistent with those of the purified maleylpyruvate hydrolase described previously (8). After 20 days storage at −80°C with 50% glycerol, the enzyme retained 62% of its original activity, and 27% of the activity remained after 50 days. When stored at 4°C with 5% glycerol, 50% and 21% activities remained after 10 and 40 days, respectively.
Identification of products formed from HbzF-catalyzed maleylpyruvate hydrolysis.
Both maleate and pyruvate were identified as products of maleylpyruvate hydrolysis in the system containing purified HbzF-His6 by comparison with standards on a high-performance liquid chromatography (HPLC) system as described previously (3), with 5 mM H2SO4 as the eluant at a flow rate of 0.6 ml/min. Their retention times were 8.3 and 9.2 min, respectively, which could be easily distinguished from that of fumarate (retention time, 15.6 min). In a quantitative-analysis system containing 200 μM maleylpyruvate and 10 μg purified HbzF-His6, almost equimolar amounts of maleate (178 μM) and pyruvate (189 μM) were produced following the completion of maleylpyruvate hydrolysis determined by spectrophotometer, after incubation at 30°C for 30 min. This indicated the stoichiometric production of maleate and pyruvate from maleylpyruvate. During the analysis by liquid chromatography-mass spectrometry (LC-MS), performed with an UltiMate 3000 RSLC series system (Dionex, Sunnyvale, CA) coupled with a Bruker micrOTOF-Q II mass spectrometer (Bruker Daltonics, Bremen, Germany), two compounds appeared at retention times of 1.4 and 2.3 min, giving an [M-H]− ion at m/z 87.0086 and an [M-H]− ion at 115.0040, corresponding to pyruvate and maleate standards, at m/z 87.0096 and 115.0038, respectively.
The hbzF gene is cotranscribed with hbzE, and 3-hydroxybenzoate enhanced its transcription.
Extraction of total RNA, reverse transcription (RT)-PCR, and real-time PCR were carried out as described previously (7). It was reported that the hbzE-encoded GDO was strictly inducible by 3-hydroxybenzoate, gentisate, or 2,5-xylenol (9, 12). Since hbzF was located upstream of hbzE, RT-PCR analysis of total RNA isolated from a 3-hydroxybenzoate-grown culture of strain NCIMB 9867 was carried out using a primer pair, FE-RT-F (5′-GGC AGA ACG CCA ATA CCA-3′) and FE-RT-R (5′-GCA ACA CCG AAC GCA ACG-3′), that spanned across the hbzF and hbzE genes. The correctly sized amplified product (Fig. 4) indicates that hbzF is cotranscribed along with hbzE, and hbzF-encoded maleylpyruvate hydrolase is also strictly inducible by 3-hydroxybenzoate. Relative quantification analysis was carried out using the 2−ΔΔCT method (13), with a 154-bp fragment of the 16S rRNA gene of strain NCIMB 9867 as an internal control. The calculated result from real-time quantitative PCR suggested that, 2 h after 3-hydroxybenzoate induction, hbzF was transcribed at a level 16-fold higher than in the noninduced cells.
Fig 4.
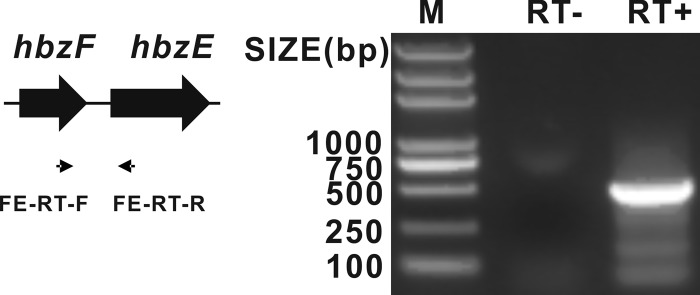
Agarose gel electrophoresis of RT-PCR analysis of total RNA obtained from 3-hydroxybenzoate-induced cells of P. alcaligenes NCIMB 9867. Primers (FE-RT-F and FE-RT-R) used in this experiment are indicated by small horizontal arrows, with a PCR product of 459 bp. Lane M, DNA marker; lane RT−, product of PCR carried out without RT; and lane RT+, product of PCR carried out with RT.
Phylogenetic analysis of HbzF.
The amino acid sequence of HbzF was aligned using CLUSTAL W (14) with other functionally identified FAA hydrolase superfamily proteins and also with maleylpyruvate isomerases. A distance neighbor-joining tree by a previously described method (15) was constructed using the MEGA5 package (16) based on the aligned sequences. As shown in Fig. 5, HbzF is located in an unrelated branch with maleylpyruvate isomerases and most closely related to the hydrolases for different substrates. However, experimental evidence showed that recombinant HbzF had no hydrolase activity against fumarylpyruvate. The native maleylpyruvate hydrolase purified from this strain also did not catalyze the hydrolysis of fumarylpyruvate (8).
Fig 5.
Phylogenetic relationship analysis of HbzF with other members of the FAA hydrolase superfamily and maleylpyruvate isomerases by generating a distance neighbor-joining tree. Accession numbers of the proteins are listed on the right. The bootstrap confidence limits (expressed as percentages) based on 10,000 resampled data sets are indicated at the nodes. HbzF is indicated by a star.
In conclusion, we have demonstrated that the hbzF gene from Pseudomonas alcaligenes NCIMB 9867 encodes a functional maleylpyruvate hydrolase. The kinetic parameter and biochemical properties are generally consistent with those of the strictly inducible maleylpyruvate hydrolase from this strain (8, 17). These results lead us to speculate that the purified maleylpyruvate hydrolase from strain NCIMB 9867 (8) was encoded by hbzF and the 50-kDa band in the previous study was a contaminating polypeptide. This study, for the first time, reveals the genetic determinate for the direct maleylpyruvate hydrolysis in the gentisate pathway, complementary to the well-studied maleylpyruvate isomerization route (3, 5, 7).
Nucleotide sequence accession number.
The 16S rRNA gene of strain NCIMB 9867 has been deposited in GenBank under the accession number JX867714.
ACKNOWLEDGMENTS
This study was supported by the National Key Basic Research Program of China (973 Program; grants 2012CB725202 and 2012CB721003) and by the National Natural Science Foundation of China (grant 31271333).
We thank Nan Hu at Burker Daltonics, Beijing representative office, for assistance with the LC-MS analysis.
Footnotes
Published ahead of print 30 November 2012
REFERENCES
- 1. Lack L. 1959. The enzymic oxidation of gentisic acid. Biochim. Biophys. Acta 34:117–123 [DOI] [PubMed] [Google Scholar]
- 2. Hopper DJ, Chapman PJ, Dagley S. 1968. Enzymic formation of D-malate. Biochem. J. 110:798–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou NY, Fuenmayor SL, Williams PA. 2001. nag genes of Ralstonia (formerly Pseudomonas) sp. strain U2 encoding enzymes for gentisate catabolism. J. Bacteriol. 183:700–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jeon CO, Park M, Ro HS, Park W, Madsen EL. 2006. The naphthalene catabolic (nag) genes of Polaromonas naphthalenivorans CJ2: evolutionary implications for two gene clusters and novel regulatory control. Appl. Environ. Microbiol. 72:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng J, Che YS, Milse J, Yin YJ, Liu L, Ruckert C, Shen XH, Qi SW, Kalinowski J, Liu SJ. 2006. The gene ncgl2918 encodes a novel maleylpyruvate isomerase that needs mycothiol as cofactor and links mycothiol biosynthesis and gentisate assimilation in Corynebacterium glutamicum. J. Biol. Chem. 281:10778–10785 [DOI] [PubMed] [Google Scholar]
- 6. Liu TT, Xu Y, Liu H, Luo S, Yin YJ, Liu SJ, Zhou NY. 2011. Functional characterization of a gene cluster involved in gentisate catabolism in Rhodococcus sp. strain NCIMB 12038. Appl. Microbiol. Biotechnol. 90:671–678 [DOI] [PubMed] [Google Scholar]
- 7. Liu TT, Zhou NY. 2012. Novel L-cysteine-dependent maleylpyruvate isomerase in the gentisate pathway of Paenibacillus sp. strain NyZ101. J. Bacteriol. 194:3987–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayly RC, Chapman PJ, Dagley S, Diberardino D. 1980. Purification and some properties of maleylpyruvate hydrolase and fumarylpyruvate hydrolase from Pseudomonas alcaligenes. J. Bacteriol. 143:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yeo CC, Tan CL, Gao XL, Zhao B, Poh CL. 2007. Characterization of hbzE-encoded gentisate 1,2-dioxygenase from Pseudomonas alcaligenes NCIMB 9867. Res. Microbiol. 158:608–616 [DOI] [PubMed] [Google Scholar]
- 10. Shen XH, Jiang CY, Huang Y, Liu ZP, Liu SJ. 2005. Functional identification of novel genes involved in the glutathione-independent gentisate pathway in Corynebacterium glutamicum. Appl. Environ. Microbiol. 71:3442–3452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang JJ, Liu H, Xiao Y, Zhang XE, Zhou NY. 2009. Identification and characterization of catabolic para-nitrophenol 4-monooxygenase and para-benzoquinone reductase from Pseudomonas sp. strain WBC-3. J. Bacteriol. 191:2703–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeo CC, Wong MVM, Feng YM, Song KP, Poh CL. 2003. Molecular characterization of an inducible gentisate 1,2-dioxygenase gene, xlnE, from Pseudomonas alcaligenes NCIMB 9867. Gene 312:239–248 [DOI] [PubMed] [Google Scholar]
- 13. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 14. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 16. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laa Poh C, Bayly RC. 1980. Evidence for isofunctional enzymes used in m-cresol and 2,5-xylenol degradation via the gentisate pathway in Pseudomonas alcaligenes. J. Bacteriol. 143:59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]