Abstract
Alternative splicing of pre-mRNAs is a major contributor to proteomic diversity and to the control of gene expression in higher eukaryotic cells. For this reasons, alternative splicing is tightly regulated in different tissues and developmental stages and its disruption can lead to a wide range of human disorders. The aim of this review is to focus on the relevance of alternative splicing for muscle function and muscle disease. We begin by giving a brief overview of alternative splicing, muscle-specific gene expression and muscular dystrophy. Next, to illustrate these concepts we focus on two muscular dystrophy, myotonic muscular dystrophy and facioscapulohumeral muscular dystrophy, both associated to disruption of splicing regulation in muscle.
Keywords: repetitive DNA, epigenetics, chromatin, muscular dystrophy, alternative splicing, myotonic muscular dystrophy, facioscapulohumeral muscular dystrophy
Alternative Pre-mRNA Splicing: A Brief Overview
In eukaryotes, most protein-coding genes are split in multiple exons interrupted by introns. A typical human protein-coding gene contains 9 exons with an average size of 145 nt while the average intron size is 3,365 nt. Considering also the 5′ and 3′ untranslated regions (UTRs), the typical human gene spans close to 27 kbp. During the splicing reaction, intron sequences are removed from the primary RNA (pre-mRNA) transcript and the exons are fused together resulting in the formation of the mature messenger RNA (mRNA). Hence, more than 90% of the original pre-mRNA sequence is removed as introns. The joined exons, with some further modifications (5′ capping and 3′ polyadenylation), are then transported into the cytoplasm to be translated into proteins.
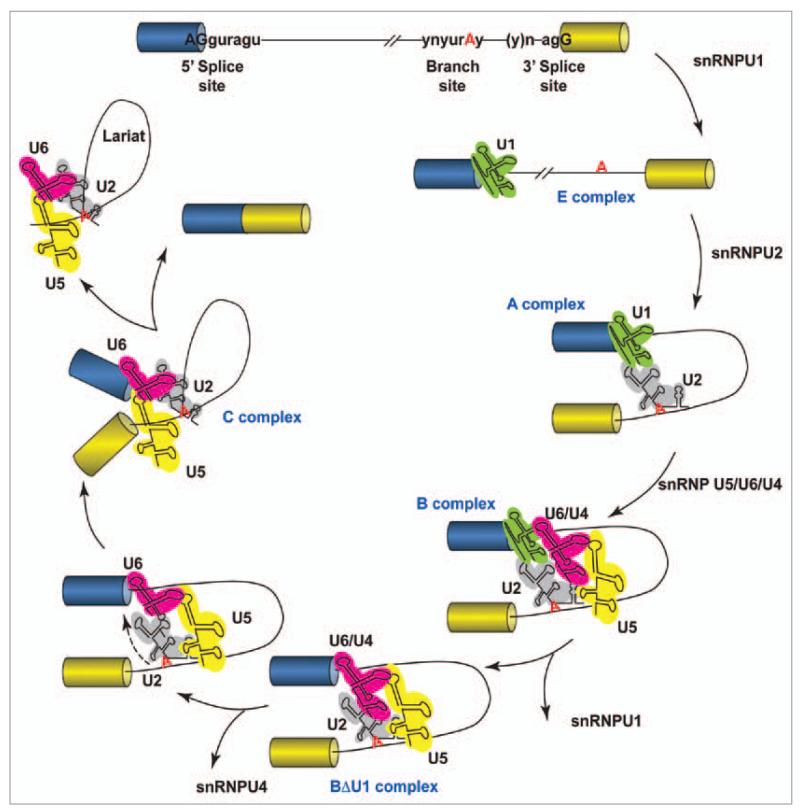
The splicing reaction is mediated by the spliceosome, a large molecular machine composed of five small nuclear RNAs (snRNPs U1, U2, U4, U5 and U6) and more than 200 polypeptides and RNA-binding proteins (Fig. 1).1
Figure 1.
Schematic representation of the splicing reaction. Two exons (boxes) are separated by an intron (line). The consensus sequences in metazoans at the 5′ splice site, branch point and 3′ splice site are as indicated, where n is any nucleotide, r is a purine, and y is a pyrimidine. The polypyrimidine tract is a pyrimidine-rich stretch located between the branch site and the 3′ splice site. The cross-intron assembly and disassembly cycle of the major spliceosome is showed. The stepwise interaction of the spliceosomal snRNPs (colored structured RNAs) in the removal of the intron from the pre-mRNA is depicted.
To meet the physiological requirements of cells and tissues, splicing must be both rapid and precise, a very demanding task considering that introns are typically much larger than exons and are strewn with translation termination codons, which would result in truncated proteins if not accurately removed. To achieve this accuracy, the splicing machinery must efficiently recognize the intron-exon boundaries in the pre-mRNA since just a single nucleotide addition or deletion at the site of exon joining will shift the reading frame with serious consequences for the protein-coding potential of the mRNA. Splicing fidelity is achieved through several intercommunicating features of pre-mRNAs cis-acting elements that distinguish exons from introns, direct the spliceosome to the correct nucleotides for the successive exon-exon joining, and serve as binding sites for the factors that regulate splicing.1 All together, these elements constitute what is known to be the ‘splicing code’, which appears to be present within and around exons2,3 and, essentially, helps to distinguish an exon from a pseudo-exon.4,5
In addition to the basal information of the ‘splicing code’, splicing regulatory proteins (hnRNPs, SR-proteins and other splicing factors) bind to pre-mRNAs cis-regulatory elements referred to as enhancers and silencers of splicing. These short elements, conventionally classified on the basis of their position as intronic (ISE, intronic splicing enhancer and ISS, intronic splicing silencer) or exonic (ESE, exonic splicing enhancer and ESS, exonic splicing silencer), can activate or suppress splice site recognition or the assembly of the spliceosome by several mechanisms.6-8
Even by this short introduction, it should be clear that the metabolic energy cost of splicing and of the surveillance necessary to eliminate imprecisely spliced mRNAs is very high. Hence, it was not evident initially why splicing arose and has been conserved with the evolution of complex organisms. It is now clear that the real payoff of splicing comes from the potential for alternative splicing.8 Alternative splicing is a mechanism by which more than one mRNA is generated from the same pre-mRNA as a consequence of differential inclusion or exclusion of exons or introns. Alternative splicing can give rise to functionally different proteins or, through alternative splicing of UTRs, can also generate mRNAs with different localization, stability, as well as efficiency of translation (Fig. 2).
Figure 2.
Major forms of alternative splicing. Exons (boxes), introns (lines) alternative and splicing process (dotted lines). (A) Constitutive splicing. (B) Alternative use of an internal cassette exon. (C) Mutually exclusive exons. (D) Intron retention. (E) Alternative 5′ splice sites. (F) Alternative 3′ splice sites. (G) Alternative polyadenylation sites. (H) Alternative promoters. (I) Alternative last exons in which one exon contains the natural stop codon while the other exon contains a premature stop codon (PTC). In many cases, these common forms can be combined to generate more complicated alternative splicing events.
Deep-sequencing surveys of gene expression in different tissues and cell lines have revealed alternative splicing as a major contributor to the generation of the increasingly complex cellular, physiological and neurological systems of higher eukaryotes.8-11 Indeed, it appears that more than 90% of human genes generate more than one transcriptional product by alternative splicing of their pre-mRNA,10,11 and a significant number of genes present a staggering variety of splice variants and alternative transcription start and termination sites.9 On the contrary, alternative splicing is far less frequent in lower eukaryotes. For example, the current estimate of alternative splicing is of about 13% in C. elegans12 and 40% in D. melanogaster.13 Collectively, these observations suggest that alternative splicing is one of the main sources of proteomic diversity in multicellular eukaryotes.
Alternative Splicing and Skeletal Muscle
Alternative splicing is often modulated in a tissue- and developmental stage-specific manner and the regulation of this process is essential for diverse cellular functions in both physiological and pathological situations.
Interestingly, splicing-sensitive microarrays and deep-sequencing analysis of alternative splicing in different human tissues reported that skeletal muscle is one of the tissues with the highest number of differentially expressed alternative exons,10,11,14 revealing a previously unappreciated degree of alternative splicing complexity in muscle-specific transcripts.
Many proteins essential for striated muscle development exist in multiple isoforms generated by alternative splicing. These include myogenic transcription factors, metabolic enzymes, and components of the myofibril.15 For example, it is known that alternatively spliced isoforms of myofibrillar protein are differentially expressed in various muscles and muscle fiber types. In addition to this complexity, it is also known that their pattern of expression varies during muscle development and is affected by neural and hormonal influences. The variable expression of myofibrillar protein isoforms is a major determinant of the differential contractile properties of individual skeletal muscle fibers.16 For example, the Cardiac Troponin T (cTNT) gene undergoes a developmentally regulated alternative splicing: cTNT exon 5 is included in the mature mRNAs in embryonic striated muscle, whereas it is skipped in the adult.17 Interestingly, the two alternative cTNT splicing isoforms confer different levels of calcium sensitivity to the myofilament, thereby affecting the contractile properties of maturing muscles.18,19
Different families of splicing factors have been discovered to regulate alternative splicing in skeletal muscle: the CELF (CUG-BP and ETR-3-like factors) family,20 the muscleblind-like (MBNL) family,21 the Fox family22 and Polypyrimidinetract-binding protein (PTB).23 In addition, different cis-acting elements that were found to be both necessary and sufficient to promote exon inclusion specifically in skeletal muscle have been also identified.24-27 Among these sequences, the muscle-specific splicing enhancer 2 (MSE2), is conserved in sequence and position in chicken and human cTNT26 and it is necessary and sufficient to promote muscle-specific cTNT exon 5 inclusion in embryonic skeletal muscle cultures when present in multiple copies.25 In several genes, a conserved CUG motif within MSE2 is located near alternative exons that undergo alternative splicing in striated muscle and is required for enhancer activity, revealing its role of muscle-specific splicing regulator.25,26 These CUG motifs are bound, in a sequence-specific manner, by CELF family members.20 Another cis-element involved in muscle-specific alternative splicing is the hexanucleotide UGCAUG.14,27 This element is specifically bounds by members of the Fox family of splicing factors: Fox-1/A2BP1, Fox-2/RBM9 and Fox-3.22 A third element enriched in exons selectively upregulated in muscle is UCUCU, which corresponds to the binding site of PTB.14,27,28 Interestingly, while CELF and Fox proteins act as positive regulators, PTB acts as a suppressor of muscle-enriched exons splicing.
Splicing as a Cause of Disease
As alternative splicing affects the majority of human genes, it is not surprising that its deregulation is often causally associated with human diseases.2,29,30
“Splicing diseases” can be broadly classified in two groups based on where the mutations are located (Fig. 3).
Figure 3.
Examples of mutations causing splicing diseases and their possible consequences. Exons (boxes), introns (lines) and mutations (stars). (A–E) Cis-acting mutations on a specific pre-mRNA could lead to complete loss of a protein, the production of a mutant protein lacking a domain or to an unbalance in the abundance of the protein isoforms encoded by the pre-mRNA. (A) Mutations disrupting either 5′ or 3′ splice sites. (B) Mutations creating cryptic 5′ or 3′ splice sites. (C) Mutations disrupting splicing regulatory sequences such as intronic (ISS) or exonic (ESS) splicing silencers or intronic (ISE) or exonic (ESE) splicing enhancers. (D) Mutations creating new ISS, ESS, ISE or ESE. (E) Mutations affecting the secondary structure of the pre-mRNA. (F) Trans-acting mutations of a particular splicing regulator could lead to aberrant splicing of several different pre-mRNAs.
The first group, representing as many as 50% of human genetic diseases caused by point mutations, contains disorders in which mutations are located within splicing regulatory sequences.31 Neurofibromatosis type 1, Fraiser’s syndrome and atypical cystic fibrosis are examples of splicing diseases caused by intronic mutations.2,30,32,33 A number of other diseases display mutations in exons. The consequence of these mutations is generally assumed to result from their effect on the protein reading frame. However, for many exon mutations it is increasingly evident that the pathogenic effect is at the level of the splicing reaction.34 For example, in the case of CFTR, the gene mutated in cystic fibrosis, one-quarter of even synonymous substitutions result in altered splicing.35 Interestingly, secondary pre-mRNA structure contributes to exon recognition and thus mutations affecting the secondary structure of the pre-mRNA can also cause aberrant splicing and disease (Fig. 3).36 In every case, the mechanisms causing altered splicing involve disruption of cis-acting elements within the affected gene. This type of mutations can be classified either as gain-of- or loss-of-splicing mutations. Gain-of-splicing mutations occur when a splicing regulatory element is enhanced or created, while loss-of-splicing mutations weaken or destroy splicing regulatory elements. As a result, these mutations alter the pre-existing ‘exon definition’ complex leading to the inappropriate skipping or inclusion of an exon, activation of cryptic splice site or intron retention (Fig. 3).37
The second group of splicing diseases is due to mutations in genes encoding for splicing regulators, either the constitutive component of the spliceosome (snRNPs and proteins) or factors that regulate alternative splicing decisions as well as associated proteins such as mRNA-export factors and transcription factors (Fig. 3).2,32,37 Importantly, these trans-acting splicing mutations can affect the expression of multiple genes at the same time (Fig. 3). Interestingly, neurodegenerative disorders are the principal clinical manifestation of RNA-binding proteins mutations. This is somewhat surprising since the mutated RNA-binding proteins are ubiquitously expressed and it is usually explained with the high prevalence of alternative splicing in the brain. Intriguingly, recent studies have shown that trans-regulatory mechanisms contribute also to several complex diseases, including cancer.38,39
In this review we will focus on two peculiar forms of muscle disease associated to aberrant alternative splicing: myotonic muscular dystrophy and facioscapulohumeral muscular dystrophy.
Muscular Dystrophy
Muscular dystrophy covers a group of genetically determined disorders causing progressive weakness and wasting of skeletal muscles.40 Although the main affected tissue in muscular dystrophies is the skeletal muscle, abnormalities can also be detected in other tissues such as cardiac muscle, brain and smooth muscle. A number of criteria are used to classify muscular dystrophies, including: age of onset, rate of disease progression, mode of inheritance, distribution of muscle weakness and genetic causes. The principal types of muscular dystrophies are: congenital, Duchenne and Becker, Emery-Dreifuss, distal, facioscapulohumeral, limb-girdle, oculopharyngeal and myotonic.
If we consider the possible molecular mechanisms, muscular dystrophies can be divided in two broad categories.
The first group, represented by congenital, Duchenne and Becker, distal and limb-girdle muscular dystrophies, contains diseases caused by mutations in genes encoding for protein components of the dystrophin-glycoprotein complex (DGC), its extracellular matrix (ECM) ligands, or enzymes involved in its post-translational modifications. The DGC is a multisubunit complex present at the sarcolemma, the muscle cell membrane, which connects the extracellular matrix to the intracellular actin cytoskeleton. A mutation in one protein component of the DGC disrupts the stability of the whole complex, which in turn makes the muscle cell membrane susceptible to contraction-induced injuries causing muscle degeneration.41,42
The second group, represented by Emery-Dreifuss, oculopharyngeal, myotonic and facioscapulohumeral muscular dystrophies, contains diseases caused by mutations affecting the activity or the expression of nuclear proteins. The molecular mechanism(s) underlying this class of muscular dystrophies are less clear. Emery-Dreifuss muscular dystrophy exists in two forms X-linked recessive (X-EMD) and autosomal dominant (AD-EMD). X-EMD is caused by mutations in the gene encoding for emerin, a protein localized to the inner nuclear membrane, whereas AD-EMD is caused by mutations in the lamin A/C gene that undergo alternative splicing to generate lamins A and C isoforms. It has been suggested that an emerin nuclear protein complex (composed of emerin, lamin A and B, and nuclear actin) exists at the nuclear envelope and that it has a dual role as (1) stabilizer of the nuclear membrane against the mechanical stresses that are generated in muscle cells during contraction and (2) in regulation of gene expression.41,43 Oculopharyngeal muscular dystrophy (OPMD) is an autosomal dominant form of slowly progressive myopathy caused by an expansion of alanine tracts in the nuclear poly(A)-binding protein N1 (PABPN1), which, together with poly(A) polymerase, regulates the elongation of mRNA poly(A) tail length.44 Expanded PABPN1 aggregates and forms intranuclear inclusions, which contain also mRNAs and other proteins. The mechanism through which expansions of PABPN1 polyalanine domain causes muscle demise is still unknown.
Myotonic muscular dystrophy (DM) and facioscapulohumeral muscular dystrophy (FSHD) are the two most common types of muscular dystrophy in adults. They are both characterized by progressive muscle weakness and wasting, cardiac conduction defects and, in the most severe cases, mental retardation.40,45,46 Furthermore, cataract development has been described in DM and retinal vascular disease in FSHD.
Both diseases are inherited as dominant characters and are caused by gain-of-function mutations caused by alteration of non-coding repetitive sequences. In both cases, this is causing an epigenetic alteration of neighboring genes and aberrant alternative splicing. Finally, for both diseases the number of repeats influences the severity and the age of the onset.
In DM, a triplet expansion within non-coding region of RNAs alters the activity/expression of the splicing regulators MBNL1 and CUGBP1.47 In FSHD, contraction of a DNA repeat alters the expression of the splicing regulator FRG1.48
Myotonic Dystrophy
Clinical features
Myotonic dystrophy (DM) has a worldwide incidence of 1 in 8000.32,46 Myotonic dystrophy type 1 (DM1) (OMIM 160900) result from a CTG repeat expansion in the 3′UTR of the DM protein kinase (DMPK) gene on chromosome 19q3.3.49 It is a multisystemic disorder characterized by myotonia, progressive weakness, muscle wasting, insulin resistance, cardiac conduction defects, neuropsychiatric symptoms, gonadal atrophy, and cataracts. Beyond the adult form, which is the classic form of DM1, congenital and early-onset DM1 forms also exist.
A second form of myotonic dystrophy, DM type 2 (DM2) (OMIM 602668), results from expansion of a CCTG repeat in intron 1 of the zinc finger protein 9 (ZNF9) gene on chromosome 3q21.50 DM2 shares with DM1 the autosomal dominant inheritance and the multisystem involvement. In contrast to DM1, however, proximal leg muscles are affected at onset. Moreover, while in DM1 muscle atrophy is present in type1 fibers, in DM2 type 2 fibers are affected.51
The rate of disease progression is slow. While longevity is not affected in many patients, overall life expectancy is reduced due to secondary respiratory disease, cardiovascular disease, and neoplasms. Unfortunately, no treatments are available to cure myotonic dystrophy: the existing therapy is limited to alleviate the symptoms but does nothing to stop or decrease disease progression.
Genetic mechanism
The pathophysiological mechanism for multisystem degeneration in DM is not fully understood. Several hypotheses have been proposed and the most accredited is derived from the evidence that CTG expansion in the 3′UTR of DMPK disrupts cellular processes at the chromatin, protein and RNA levels (Fig. 4).
Figure 4.
Model for the molecular consequences of triplet expansion in DM1. In healthy subjects, less than 40 CTG repeats are present in the 3′UTR of the DMPK gene and the DMPK and SIX5 genes are correctly expressed. In DM1 patients, CTG repeats are expanded to 80–1,000 repeats. This has a number of consequences. (A) Expanded repeats lead to epigenetic silencing of DMPK and SIX5 genes. (B) Long CUG repeats in the 3′UTR of the DMPK pre-mRNA titrate the RNA-binding protein MBNL1 leading to equivalent effects to MBNL1 loss-of-function. (C) Expanded CUG repeats cause phosphorylation and stabilization of the RNA-binding protein CUGBP1 leading to equivalent effects to CUGBP1 gain-of-function. (D) Altered activity/expression of MBNL1 and CUGBP1 is responsible for the alternative splicing changes observed in DM1. cTNT, IR and CLCN1 altered splicing is shown as example.
The initial theory was that the repeats expansion reduces the level of DMPK mRNA or protein resulting in DMPK haploinsufficiency in DM1 patients. Indeed, decreased expression of DMPK mRNA and protein in DM1 muscle was reported.52 To verify this hypothesis, Dmpk-knockout mice were generated but, surprisingly, they displayed only mild myopathy53 and cardiac conduction defects.54 Notably, the mice did not exhibit myotonia, which is one of the characteristic symptoms of DM1, suggesting that DMPK haploinsufficiency is not sufficient to cause DM1.
Next, it was hypothesized that the CTG repeats might influence expression of adjacent genes. The homoeodomain encoding gene SIX5 is located in this region and its mRNA level was found decreased in DM1 patients.55 Interestingly, it has been shown that repeats expansion induces the formation of a condensed chromatin structure affecting SIX5 expression.56 Nevertheless, it has to be noted that Six5-knockout mice develop only cataracts but no muscle pathology.57 Therefore decreased SIX5 expression is not the primary cause of DM1.
Altogether, these evidences clearly indicate that the multisystemic features of DM1 cannot be explained solely by DMPK or SIX5 haploinsufficiency leading to the hypothesis that DM1 is mainly an RNA-mediated disease. In fact, a number of observations suggest that an RNA gain-of-function could be the underlying molecular mechanism of DM1. First, the RNA produced from the expanded CUG repeats (CUGexp) is retained in the nuclei of affected muscle tissues forming ribonuclear inclusions precluding its export to the cytoplasm for translation into DMPK protein.58,59 Second, expression of just the DMPK 3′-UTR with expanded CTG repeats is sufficient to inhibit myogenesis in tissue culture.60 Third, transgenic mice selectively expressing 250 CTG repeats in the 3′-UTR of the human skeletal actin (HSALR) develop typical features of DM1, such as myotonia and histological abnormalities,61 demonstrating that the expression of CUG repeats independently from the DMPK locus is sufficient to exhibit major features of DM. Forth, DM2 patients, resulting from an expanded CCTG repeats in the unrelated gene ZNF9, present symptoms similar to DM1. This strongly suggests that a common RNA gain-of-function is the pathogenic mechanism at the basis of both DM1 and DM2. In summary, the mutant RNA is the predominant cause of DM1 pathogenesis, although loss of DMPK and SIX5 may also play a minor role (Fig. 4).
Molecular mechanism
An important molecular feature of DM1 is the misregulation of alternative splicing. More than twenty splicing events are mis-regulated in DM1,47 and some correlate directly with two common symptoms of the disease. For example, myotonia is due to the aberrant splicing of the skeletal muscle-specific Chloride Channel 1 (CLCN1). CLCN1 aberrant splicing produces an isoform containing premature stop codons (PTC) resulting in downregulation of CLCN1 mRNA and protein levels,62,63 which is sufficient to cause myotonia (Fig. 4). Similarly, mis-regulated splicing of Insulin Receptor 1 (IR1) in skeletal muscle results in insulin resistance and diabetes (Fig. 4).64 Interestingly, these pre-mRNAs are developmentally regulated and in DM1 the predominant alternative splicing isoform switches from the adult to the embryonic splicing pattern. For a review see ref. Lee, JE 2009.65
Current evidence indicates that mutant DMPK and ZNF9 alleles alter the activities of at least two alternative splicing factors: MBNL1 and CUGBP1.29,66
MBNL1
The expanded CUG turns into a non-correct hairpin structure67 and this different fold resembles the binding site for different splicing factors. MBNL1 was identified in a screen for proteins that specifically bound to CUGexp repeats.68 Interestingly, MBNL1 co-localizes to CUG-containing nuclear foci in DM1 cells and is depleted from the nucleoplasm raising the possibility that a MBNL1 loss-of-function could be at the basis of DM (Fig. 4).69,70 Accordingly, Mbnl1 knockout mice develop myotonia, cataracts and alternative splicing defects characteristic of DM1.71 More importantly, Mbnl1 overexpression in skeletal muscle of HSALR mice is sufficient to rescue Clcn1 splicing and myotonia.72
On the other end, Mbnl1 knockout mice do not display muscle wasting.71 Accordingly, CUGexp has MBNL1-independent effects, particularly on mRNAs encoding for extracellular matrix proteins known to have roles in other forms of muscular dystrophy.73 Moreover, while MBNL1 can be sequestered to both CUG- and CAG-containing foci with similar affinity, only CUG repeats induce abnormal splicing.74 Thus, MBNL1 loss-of-function cannot fully explain DM symptoms.
CUGBP1
Another RNA-binding protein involved in DM1 is CUGBP1.75 Unlike MBNL1, CUGBP1 binds to single-stranded CUG repeats75 and its binding is not proportional to the length of the RNA repeats. Moreover, CUGBP1 does not colocalize with CUGexp RNA in the nuclear foci.69,76,77 CUGBP1 steady-state protein levels are increased in DM1 patients26,78 and in mouse models containing expanded repeats within the DMPK 3′-UTR gene.79,80 Apparently, the mutant RNA activates the PKC signalling pathway through an unknown mechanism, that in turn induce CUGBP1 hyperphosphorylation and stabilization (Fig. 4).81 Notably, mice overexpressing CUGBP1 reproduce histopathological, functional and splicing changes observed in DM1 mouse models and DM1 patients.82
While MBNL1 nuclear levels increase during development, CUGBP1 nuclear levels decrease.70,83 Notably, a number of developmentally regulated alternative splicing events are modulated by MBNL1 and CUGBP1 in an antagonistic fashion,83 supporting the hypothesis that altered level and localization of these splicing factors are responsible for the adult to fetal alternative splicing switch observed in DM1 tissues (Fig. 4).64,84
Finally, MBNL1 and CUGBP1 are involved in a number of other regulatory pathways beside splicing, including mRNA turnover, localization and translation suggesting that triplet expanded RNAs in DM can also impact gene expression at several levels.85,86
Facioscapolohumeral Muscular Dystrophy
Clinical features
FSHD (OMIM 158900) is characterized by wasting and atrophy of a selected group of skeletal muscles. As its name suggests, weakness first arises in facial mimic and shoulder girdle muscles.87 Later, the disease may progress to abdominal muscles leading to abdominal protrusion and spine lordosis.88 In more severe cases, muscle degeneration reaches the foot dorsiflexor muscle and the pelvic girdle impairing the ability to walk. The majority of FSHD patients show the first symptoms in the second decade of life, but age of onset can vary from childhood to fifty years old.89 In general, the earlier is the age of onset the more severe is the pathology. For example, patients that display the first symptoms during childhood will probably need wheelchair in adulthood. Interestingly, several authors90,91 noted a mild gender effect. Males are generally more severely affected than women: women tend to be more often asymptomatic, have a slightly later onset, and a somewhat milder course of the disease.91 Several extra-muscular manifestations are presents in FSHD patients. Subclinical high tone hearing loss and retinal telangiectasias have been described as part of the disease, occurring in 75% and 60% of affected individuals, respectively.91-94 Mental impairment (mental retardation, autism and seizure behavior) has been described in severely affected FSHD infants.40,87 Cardiac defects and respiratory insufficiency are observed,95 together with muscle pain96 and fatigue.97
FSHD displays an extremely high degree of variability in terms of onset, progression and severity of the phenotype. Although the molecular basis of this heterogeneity is not fully understood, several evidences suggest that it derives from the interplay of complex genetic and epigenetic events.48
Currently, no therapeutic treatments to either stop progression or reverse muscle weakness and atrophy is available for FSHD.
Genetic mechanism
FSHD is an autosomal dominant disorder and it is of considerable interest for the unique nature of the molecular mutation, which is a deletion within a large, complex DNA tandem array located in 4q35.98 The locus involved in this rearrangement consists in a series of tandemly arranged 3.3 kbp repeat sequences, named D4Z4 (Fig. 5). In more than 95% of FSHD cases, the disease is described as the loss of an integral number of repeats.91 The number of D4Z4 repeats is highly polymorphic and varies between 11 and 150 in the normal population.99 Instead, FSHD patients present only 1 to 10 D4Z4 copies (Fig. 5).98,100 Interestingly, the number of D4Z4 repeats is inversely correlated to the age of onset and the severity of the disease.87
Figure 5.
Model for the alternative splicing consequences of D4Z4 repeats deletion in FSHD. In healthy subjects, more than 10 D4Z4 repeats are present at the 4q35 sub-telomere and the FRG1 gene is correctly expressed. In FSHD patients, D4Z4 repeats are deleted leaving 1-10. This leads to the epigenetic overexpression of FRG1. Increased FRG1 expression is responsible for the alternative splicing changes observed in FSHD.
D4Z4-like repeats are present on several other subtelomeric regions throughout the genome. In particular, D4Z4 repeats in chromosome 10 share 99% identity with 4q35 repeats and are equally polymorphic.101 Nevertheless, only contractions on chromosome 4 are pathogenic. Moreover, hybrid alleles (a mix of 4q35- and 10q26-type D4Z4 repeats) can be observed both in 4q35 and in 10q26.102 Importantly, while the contraction of 4q35-type repeats on chromosome 10 does not cause FSHD,102 contraction of 10q26-type repeats on chromosome 4 can lead to FSHD.91 These observations suggest that FSHD is not caused by alteration of the specific sequence composition of the D4Z4 repeats but rather by their structural organization in the 4q region and that 4q35 regions outside the D4Z4 repeats are relevant for the pathogenesis of FSHD.103
Molecular mechanism
A number of evidences strongly indicate that the contraction of D4Z4 repeats causes a drastic alteration in the chromatin organization of the 4q35 region.48 As a result, in normal individuals the FSHD locus displays a repressed chromatin conformation while in FSHD patients the region became more open and this might favor gene expression (Fig. 5). In fact, overexpression of several 4q35 genes has been reported in FSHD patients104-107 although with some controversy.108-110
For the purpose of this review, we will uniquely focus on the 4q35 gene FRG1.
FRG1 (FSHD Region Gene 1)
FRG1 was the first candidate gene for FSHD identified in the region nearby the D4Z4 repeats.111 FRG1 is an interesting candidate for the pathogenesis of FSHD. FRG1 was found overexpressed in FSHD patients.104 Intriguingly, D4Z4 deletion at 4q35 is associated with alteration in chromatin loops involving the FRG1 promoter.112,113 As a result, an enhancer located in the 4q subtelomer strongly interacts with the FRG1 promoter specifically in FSHD113 possibly explaining the reported overexpression of FRG1 in FSHD.113,114 Significantly, individuals carrying deletions of D4Z4 and FRG1 are normal, suggesting that FRG1 is required for FSHD.115 Accordingly, FRG1 overexpression in transgenic mice causes the development of an FSHD-phenotype.116 Notably, recent Xenopus results supported an FRG1 role in muscular and extra-muscular manifestations of FSHD.117,118
While FRG1 is highly conserved from non-vertebrate to vertebrate,119 its aminoacid sequence does not display homology and similarities with known proteins.111
FRG1 is a nuclear protein localized in the nucleolus, Cajal bodies and in nuclear speckles.119,120 Interestingly, FRG1 has been repeatedly co-purified with the C-complex of the human spliceosome.121-123 Moreover, FRG1 was identified in C. elegans and Zebrafish clusters of coregulated genes involved in ribosomal and mRNA biogenesis.124,125 Finally, SMN and PABPN1, two proteins involved in RNA metabolism, were identified as binding partners of FRG1.120 Intriguingly, SMN and PABPN1 are themselves involved in neuromuscular disorders.126 Indeed, OPMD is caused by alanine expansions in the PABPN1 protein leading it to form intranuclear inclusions,127,128 whereas Spinal Muscular Atrophy (SMA) is caused by mutations in SMN, which is involved in spliceosome biogenesis.129
Collectively, these evidences are highly suggestive for an active role of FRG1 in alternative splicing regulation. Accordingly, aberrant alternative splicing of several genes has been described in mice and cells overexpressing FRG1, and in FSHD patients (Fig. 5).116,130
Transgenic mice selectively overexpressing FRG1 in skeletal muscle develop a pathology with physiological, histological and molecular features analogous to FSHD patients.116 While all the different transgenic lines analyzed displayed myopathic changes, the severity of the muscular dystrophy was proportional to the FRG1 expression level.116 Aberrant alternative splicing patterns of Myotubularin Related protein 1 (MTMR1) and Troponin T type 3 (TNNT3) pre-mRNAs was present in muscle of FRG1 mice and in FSHD muscle cells and these alterations precede the onset of the muscular disease.116 Moreover, the extent of aberrant alternative splicing correlated with the level of FRG1 overexpression.116 Significantly, aberrant alternative splicing of the same genes was not detected in mice overexpressing other two 4q35 genes, such as FRG2 and ANT1.116 Interestingly, the alternative splicing observed in FRG1 mice is not a secondary effect of the dystrophic process, as the splicing alterations were present in 4-week old FRG1 mice (an age at which they do not display any histological and ultrastructural sign of dystrophic alterations) and more importantly they were absent in mdx mice (the mouse model for Duchenne muscular dystrophy).116 Moreover, the alteration of TNNT3 and MTMR1 alternative splicing was a direct and specific effect of FRG1 overexpression since it was detected in C2C12 muscle cells overexpressing FRG1.116 Interestingly, TNNT and MTMR1 aberrant splicing has been also described in myotonic muscular dystrophy.63,71
Recently, Davidovic and its colleagues reported altered expression of alternative splicing isoforms derived from Fragile X Related Protein 1 (FXR1) gene in FSHD patients.130 Moreover, reduced stability of FXR1 mRNA transcripts, leading to a reduced expression of muscle specific FXR1 isoforms, was also described. Based on this, the authors suggested that FRG1 could regulate different levels of mRNA metabolism, including splicing and stability of RNAs.130
FXR1 belongs to the fragile X related (FXR) family of RNA-binding proteins and Fxr1 null mice display a disruption of the skeletal muscle cellular architecture.131 Moreover, complete or partial inactivation of fxr1 in Xenopus perturbs normal myogenesis.132 Finally, fxr1 reduction in Zebrafish causes muscular dystrophy.133 Interestingly, altered FXR1 splicing was reported also in DM.80 Hence, FXR1 splicing deregulation might be relevant to both DM and FSHD pathogenesis and could contribute to the molecular mechanisms causing these related muscular dystrophies.
Conclusion
Myotonic muscular dystrophy and facioscapulohumeral muscular dystrophy are the two most common types of muscular dystrophy in adults. They have a number of similarities. They are autosomal dominant disorders that, unlike the majority of genetic diseases, are not caused by mutations in protein coding genes but are caused by gain-of-function mutations caused by alterations of non-coding repetitive sequences. These alterations are associated to both an epigenetic alteration in the expression of the genes mapping in the disease locus and to aberrant alternative splicing of several pre-mRNAs.
While the molecular mechanism responsible for aberrant splicing in myotonic muscular dystrophy is relatively clear, the precise mechanism responsible for splicing alterations in FSHD is unknown. The current data suggest that altered splicing in FSHD is due to altered expression of FRG1 but the mechanism of action of FRG1 is largely unknown. Further work is required to investigate if FRG1 binds RNA and change directly splicing dynamics or if it regulates the activity or the expression of splicing factors.
Acknowledgements
Due to space limitations, we apologize with our colleagues for the vastly incomplete documentation of their important contribution to the research described in this review. We thank Dr. Emanuele Buratti and Dr. Matteo Ruggiu for critical reading of the manuscript. Research in Dr. Gabellini laboratory is made possible by support provided by the European Research Council (ERC), the Muscular Dystrophy Association of USA (MDA), the European Alternative Splicing Network of Excellence (EURASNET), a Jaya Motta private donation and the FSH Society, Inc. Davide Gabellini is a Dulbecco Telethon Institute Assistant Scientist.
Abbreviations
- ANT1
adenine nucleotide translocator 1
- A2BP1
ataxin 2-binding protein 1
- CFTR
cystic fibrosis transmembrane conductance regulator
- Clcn1
chloride channel 1
- CUGBP1
CUG-binding protein 1
- cTNT
cardiac troponin T
- DGC
dystrophin-glycoprotein complex
- DM
myotonic dystrophy
- DMPK
DM protein kinase
- ECM
extracellular matrix
- EMD
emery-dreifuss muscular dystrophy
- ESE
exonic splicing enhancer
- ESS
exonic splicing silencer
- FRG1
FSHD region gene 1
- FRG2
FSHD region gene 2
- FSHD
facioscapulohumeral muscular dystrophy
- FXR1
fragile x related protein 1
- HSA
human skeletal actin
- IR1
insulin receptor 1
- ISE
intronic splicing enhancer
- ISS
intronic splicing silencer
- MBNL1
muscleblind-like 1
- MTMR1
myotubularin related protein 1
- OPMD
oculopharyngeal muscular dystrophy
- PABPN1
poly(A)-binding protein N1
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
- TNNT3
troponin T type 3
References
- 1.Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–18. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–61. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Burge CB. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA. 2008;14:802–13. doi: 10.1261/rna.876308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu XD. Towards a splicing code. Cell. 2004;119:736–8. doi: 10.1016/j.cell.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XH, Chasin LA. Computational definition of sequence motifs governing constitutive exon splicing. Genes Dev. 2004;18:1241–50. doi: 10.1101/gad.1195304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–54. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licatalosi DD, Darnell RB. RNA processing and its regulation: global insights into biological networks. Nat Rev Genet. 2010;11:75–87. doi: 10.1038/nrg2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsen TW, Graveley BR. Expansion of the eukaryotic proteome by alternative splicing. Nature. 2010;463:457–63. doi: 10.1038/nature08909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–63. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 10.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–5. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 11.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–6. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillier LW, Reinke V, Green P, Hirst M, Marra MA, Waterston RH. Massively parallel sequencing of the polyadenylated transcriptome of C. elegans. Genome Res. 2009;19:657–66. doi: 10.1101/gr.088112.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolc V, Gauhar Z, Mason C, Halasz G, van Batenburg MF, Rifkin SA, et al. A gene expression map for the euchromatic genome of Drosophila melanogaster. Science. 2004;306:655–60. doi: 10.1126/science.1101312. [DOI] [PubMed] [Google Scholar]
- 14.Castle JC, Zhang C, Shah JK, Kulkarni AV, Kalsotra A, Cooper TA, et al. Expression of 24,426 human alternative splicing events and predicted cis regulation in 48 tissues and cell lines. Nat Genet. 2008;40:1416–25. doi: 10.1038/ng.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladd AN, Charlet N, Cooper TA. The CELF family of RNA binding proteins is implicated in cell-specific and developmentally regulated alternative splicing. Mol Cell Biol. 2001;21:1285–96. doi: 10.1128/MCB.21.4.1285-1296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 17.Cooper TA, Ordahl CP. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985;260:11140–8. [PubMed] [Google Scholar]
- 18.McAuliffe JJ, Gao LZ, Solaro RJ. Changes in myofibrillar activation and troponin C Ca2+ binding associated with troponin T isoform switching in developing rabbit heart. Circ Res. 1990;66:1204–16. doi: 10.1161/01.res.66.5.1204. [DOI] [PubMed] [Google Scholar]
- 19.Godt RE, Fogaca RT, Silva IK, Nosek TM. Contraction of developing avian heart muscle. Comp Biochem Physiol Comp Physiol. 1993;105:213–8. doi: 10.1016/0300-9629(93)90197-c. [DOI] [PubMed] [Google Scholar]
- 20.Barreau C, Paillard L, Méreau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie. 2006;88:515–25. doi: 10.1016/j.biochi.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Pascual M, Vicente M, Monferrer L, Artero R. The Muscleblind family of proteins: an emerging class of regulators of developmentally programmed alternative splicing. Differentiation. 2006;74:65–80. doi: 10.1111/j.1432-0436.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 22.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawicka K, Bushell M, Spriggs KA, Willis AE. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochem Soc Trans. 2008;36:641–7. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 24.Ryan KJ, Cooper TA. Muscle-specific splicing enhancers regulate inclusion of the cardiac troponin T alternative exon in embryonic skeletal muscle. Mol Cell Biol. 1996;16:4014–23. doi: 10.1128/mcb.16.8.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper TA. Muscle-specific splicing of a heterologous exon mediated by a single muscle-specific splicing enhancer from the cardiac troponin T gene. Mol Cell Biol. 1998;18:4519–25. doi: 10.1128/mcb.18.8.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science. 1998;280:737–41. doi: 10.1126/science.280.5364.737. [DOI] [PubMed] [Google Scholar]
- 27.Sugnet CW, Srinivasan K, Clark TA, O’Brien G, Cline MS, Wang H, et al. Unusual intron conservation near tissue-regulated exons found by splicing microarrays. PLoS Comput Biol. 2006;2:4. doi: 10.1371/journal.pcbi.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh R, Valcarcel J, Green MR. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–6. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- 29.Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–93. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tazi J, Bakkour N, Stamm S. Alternative splicing and disease. Biochim Biophys Acta. 2009;1792:14–26. doi: 10.1016/j.bbadis.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Bigas N, Audit B, Ouzounis C, Parra G, Guigo R. Are splicing mutations the most frequent cause of hereditary disease? FEBS Lett. 2005;579:1900–3. doi: 10.1016/j.febslet.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 32.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–37. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 33.Baralle D, Baralle M. Splicing in action: assessing disease causing sequence changes. J Med Genet. 2005;42:737–48. doi: 10.1136/jmg.2004.029538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagani F, Baralle FE. Genomic variants in exons and introns: identifying the splicing spoilers. Nat Rev Genet. 2004;5:389–96. doi: 10.1038/nrg1327. [DOI] [PubMed] [Google Scholar]
- 35.Pagani F, Raponi M, Baralle FE. Synonymous mutations in CFTR exon 12 affect splicing and are not neutral in evolution. Proc Natl Acad Sci USA. 2005;102:6368–72. doi: 10.1073/pnas.0502288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buratti E, Baralle FE. Influence of RNA secondary structure on the pre-mRNA splicing process. Mol Cell Biol. 2004;24:10505–14. doi: 10.1128/MCB.24.24.10505-10514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Blanco MA, Baraniak AP, Lasda EL. Alternative splicing in disease and therapy. Nat Biotechnol. 2004;22:535–46. doi: 10.1038/nbt964. [DOI] [PubMed] [Google Scholar]
- 38.Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M. Mapping complex disease traits with global gene expression. Nat Rev Genet. 2009;10:184–94. doi: 10.1038/nrg2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghigna C, Valacca C, Biamonti G. Alternative splicing and tumor progression. Curr Genomics. 2008;9:556–70. doi: 10.2174/138920208786847971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emery AE. The muscular dystrophies. Lancet. 2002;359:687–95. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 41.Cohn RD, Campbell KP. Molecular basis of muscular dystrophies. Muscle Nerve. 2000;23:1456–71. doi: 10.1002/1097-4598(200010)23:10<1456::aid-mus2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 43.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics and mechanotransduction. Circ Res. 2008;102:1307–18. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abu-Baker A, Rouleau GA. Oculopharyngeal muscular dystrophy: recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies. Biochim Biophys Acta. 2007;1772:173–85. doi: 10.1016/j.bbadis.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Tawil R. Facioscapulohumeral muscular dystrophy. Neurotherapeutics. 2008;5:601–6. doi: 10.1016/j.nurt.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheeler TM. Myotonic dystrophy: therapeutic strategies for the future. Neurotherapeutics. 2008;5:592–600. doi: 10.1016/j.nurt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ranum LP, Cooper TA. RNA-Mediated Neuromuscular Disorders. Annu Rev Neurosci. 2006;29:259–77. doi: 10.1146/annurev.neuro.29.051605.113014. [DOI] [PubMed] [Google Scholar]
- 48.Neguembor M, Gabellini D. In Junk We Trust: Repetitive DNA, Epigenetics and Facioscapulohumeral Muscular Dystrophy. Epigenomics. 2010;2:271–87. doi: 10.2217/epi.10.8. [DOI] [PubMed] [Google Scholar]
- 49.Cho D, Tapscott S. Myotonic dystrophy: Emerging mechanisms for DM1 and DM2. Biochimica et Biophysica Acta (BBA) 2006;17772:195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–7. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 51.Vihola A, Bassez G, Meola G, Zhang S, Haapasalo H, Paetau A, et al. Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology. 2003;60:1854–7. doi: 10.1212/01.wnl.0000065898.61358.09. [DOI] [PubMed] [Google Scholar]
- 52.Fu YH, Friedman DL, Richards S, Pearlman JA, Gibbs RA, Pizzuti A, et al. Decreased expression of myotoninprotein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993;260:235–8. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- 53.Jansen G, Groenen PJ, Bachner D, Jap PH, Coerwinkel M, Oerlemans F, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13:316–24. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- 54.Berul CI, Maguire CT, Aronovitz MJ, Greenwood J, Miller C, Gehrmann J, et al. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J Clin Invest. 1999;103:1–7. doi: 10.1172/JCI5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton CA, Wymer JP, Simmons Z, McClain C, Moxley RT., 3rd Expansion of the myotonic dystrophy CTG repeat reduces expression of the flanking DMAHP gene. Nat Genet. 1997;16:407–9. doi: 10.1038/ng0897-407. [DOI] [PubMed] [Google Scholar]
- 56.Otten AD, Tapscott SJ. Triplet repeat expansion in myotonic dystrophy alters the adjacent chromatin structure. Proc Natl Acad Sci USA. 1995;92:5465–9. doi: 10.1073/pnas.92.12.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klesert TR, Cho DH, Clark JI, Maylie J, Adelman J, Snider L, et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25:105–9. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- 58.Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128:995–1002. doi: 10.1083/jcb.128.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis BM, McCurrach ME, Taneja KL, Singer RH, Housman DE. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci USA. 1997;94:7388–93. doi: 10.1073/pnas.94.14.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amack JD, Paguio AP, Mahadevan MS. Cis and trans effects of the myotonic dystrophy (DM) mutation in a cell culture model. Hum Mol Genet. 1999;8:1975–84. doi: 10.1093/hmg/8.11.1975. [DOI] [PubMed] [Google Scholar]
- 61.Mankodi A, Logigian E, Callahan L, McClain C, White R, Henderson D, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289:1769–73. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 62.Charlet BN, Savkur RS, Singh G, Philips AV, Grice EA, Cooper TA. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell. 2002;10:45–53. doi: 10.1016/s1097-2765(02)00572-5. [DOI] [PubMed] [Google Scholar]
- 63.Mankodi A, Takahashi MP, Jiang H, Beck CL, Bowers WJ, Moxley RT, et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell. 2002;10:35–44. doi: 10.1016/s1097-2765(02)00563-4. [DOI] [PubMed] [Google Scholar]
- 64.Savkur RS, Philips AV, Cooper TA. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet. 2001;29:40–7. doi: 10.1038/ng704. [DOI] [PubMed] [Google Scholar]
- 65.Lee JE, Cooper TA. Pathogenic mechanisms of myotonic dystrophy. Biochem Soc Trans. 2009;37:1281–6. doi: 10.1042/BST0371281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Rourke JR, Swanson MS. Mechanisms of RNA-mediated disease. J Biol Chem. 2009;284:7419–23. doi: 10.1074/jbc.R800025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Napierala M, Krzyzosiak WJ. CUG repeats present in myotonin kinase RNA form metastable “slippery” hairpins. J Biol Chem. 1997;272:31079–85. doi: 10.1074/jbc.272.49.31079. [DOI] [PubMed] [Google Scholar]
- 68.Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–48. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang H, Mankodi A, Swanson MS, Moxley RT, Thornton CA. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet. 2004;13:3079–88. doi: 10.1093/hmg/ddh327. [DOI] [PubMed] [Google Scholar]
- 70.Lin X, Miller JW, Mankodi A, Kanadia RN, Yuan Y, Moxley RT, et al. Failure of MBNL1-dependent postnatal splicing transitions in myotonic dystrophy. Hum Mol Genet. 2006;15:2087–97. doi: 10.1093/hmg/ddl132. [DOI] [PubMed] [Google Scholar]
- 71.Kanadia RN, Johnstone KA, Mankodi A, Lungu C, Thornton CA, Esson D, et al. A muscleblind knockout model for myotonic dystrophy. Science. 2003;302:1978–80. doi: 10.1126/science.1088583. [DOI] [PubMed] [Google Scholar]
- 72.Kanadia RN, Shin J, Yuan Y, Beattie SG, Wheeler TM, Thornton CA, et al. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci USA. 2006;103:11748–53. doi: 10.1073/pnas.0604970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Du H, Cline MS, Osborne RJ, Tuttle DL, Clark TA, Donohue JP, et al. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nat Struct Mol Biol. 2010;17:187–93. doi: 10.1038/nsmb.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho TH, Savkur RS, Poulos MG, Mancini MA, Swanson MS, Cooper TA. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J Cell Sci. 2005;118:2923–33. doi: 10.1242/jcs.02404. [DOI] [PubMed] [Google Scholar]
- 75.Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, et al. Identification of a (CUG) n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–14. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fardaei M, Larkin K, Brook JD, Hamshere MG. In vivo co-localisation of MBNL protein with DMPK expanded-repeat transcripts. Nucleic Acids Res. 2001;29:2766–71. doi: 10.1093/nar/29.13.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mankodi A, Teng-Umnuay P, Krym M, Henderson D, Swanson M, Thornton CA. Ribonuclear inclusions in skeletal muscle in myotonic dystrophy types 1 and 2. Ann Neurol. 2003;54:760–8. doi: 10.1002/ana.10763. [DOI] [PubMed] [Google Scholar]
- 78.Timchenko NA, Cai ZJ, Welm AL, Reddy S, Ashizawa T, Timchenko LT. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J Biol Chem. 2001;276:7820–6. doi: 10.1074/jbc.M005960200. [DOI] [PubMed] [Google Scholar]
- 79.Mahadevan MS, Yadava RS, Yu Q, Balijepalli S, Frenzel-McCardell CD, Bourne TD, et al. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat Genet. 2006;38:1066–70. doi: 10.1038/ng1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Orengo JP, Chambon P, Metzger D, Mosier DR, Snipes GJ, Cooper TA. Expanded CTG repeats within the DMPK 3′ UTR causes severe skeletal muscle wasting in an inducible mouse model for myotonic dystrophy. Proc Natl Acad Sci USA. 2008;105:2646–51. doi: 10.1073/pnas.0708519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koshelev M, Sarma S, Price RE, Wehrens XH, Cooper TA. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum Mol Genet. 2010;19:1066–75. doi: 10.1093/hmg/ddp570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB, et al. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci USA. 2008;105:20333–8. doi: 10.1073/pnas.0809045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho TH, Charlet BN, Poulos MG, Singh G, Swanson MS, Cooper TA. Muscleblind proteins regulate alternative splicing. EMBO J. 2004;23:3103–12. doi: 10.1038/sj.emboj.7600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Lee JE, Wilusz J, Wilusz CJ. The RNA-binding protein CUGBP1 regulates stability of tumor necrosis factor mRNA in muscle cells: implications for myotonic dystrophy. J Biol Chem. 2008;283:22457–63. doi: 10.1074/jbc.M802803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osborne RJ, Lin X, Welle S, Sobczak K, O’Rourke JR, Swanson MS, et al. Transcriptional and post-transcriptional impact of toxic RNA in myotonic dystrophy. Hum Mol Genet. 2009;18:1471–81. doi: 10.1093/hmg/ddp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pandya S, King WM, Tawil R. Facioscapulohumeral dystrophy. Phys Ther. 2008;88:105–13. doi: 10.2522/ptj.20070104. [DOI] [PubMed] [Google Scholar]
- 88.Shahrizaila N, Wills AJ. Significance of Beevor’s sign in facioscapulohumeral dystrophy and other neuromuscular diseases. J Neurol Neurosurg Psychiatry. 2005;76:869–70. doi: 10.1136/jnnp.2004.052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Maarel SM, Frants RR, Padberg GW. Facioscapulohumeral muscular dystrophy. Biochim Biophys Acta. 2007;1772:186–94. doi: 10.1016/j.bbadis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Tonini MM, Passos-Bueno MR, Cerqueira A, Matioli SR, Pavanello R, Zatz M. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD) Neuromuscul Disord. 2004;14:33–8. doi: 10.1016/j.nmd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Tawil R, Van Der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34:1–15. doi: 10.1002/mus.20522. [DOI] [PubMed] [Google Scholar]
- 92.Fitzsimons RB, Gurwin EB, Bird AC. Retinal vascular abnormalities in facioscapulohumeral muscular dystrophy. A general association with genetic and therapeutic implications. Brain. 1987;110:631–48. doi: 10.1093/brain/110.3.631. [DOI] [PubMed] [Google Scholar]
- 93.Padberg GW, Brouwer OF, de Keizer RJ, Dijkman G, Wijmenga C, Grote JJ, et al. On the significance of retinal vascular disease and hearing loss in facioscapulohumeral muscular dystrophy. Muscle Nerve. 1995;2:73–80. [PubMed] [Google Scholar]
- 94.Trevisan CP, Pastorello E, Tomelleri G, Vercelli L, Bruno C, Scapolan S, et al. Facioscapulohumeral muscular dystrophy: hearing loss and other atypical features of patients with large 4q35 deletions. Eur J Neurol. 2008;15:1353–8. doi: 10.1111/j.1468-1331.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- 95.Wohlgemuth M, van der Kooi EL, van Kesteren RG, van der Maarel SM, Padberg GW. Ventilatory support in facioscapulohumeral muscular dystrophy. Neurology. 2004;63:176–8. doi: 10.1212/01.wnl.0000133126.86377.e8. [DOI] [PubMed] [Google Scholar]
- 96.Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2008;89:320–8. doi: 10.1016/j.apmr.2007.08.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kalkman JS, Schillings ML, van der Werf SP, Padberg GW, Zwarts MJ, van Engelen BG, et al. Experienced fatigue in facioscapulohumeral dystrophy, myotonic dystrophy and HMSN-I. J Neurol Neurosurg Psychiatry. 2005;76:1406–9. doi: 10.1136/jnnp.2004.050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, Dauwerse HG, et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet. 1992;2:26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]
- 99.Wijmenga C, van Deutekom JC, Hewitt JE, Padberg GW, van Ommen GJ, Hofker MH, et al. Pulsed-field gel electrophoresis of the D4F104S1 locus reveals the size and the parental origin of the facioscapulohumeral muscular dystrophy (FSHD)-associated deletions. Genomics. 1994;19:21–6. doi: 10.1006/geno.1994.1006. [DOI] [PubMed] [Google Scholar]
- 100.van Deutekom JC, Wijmenga C, van Tienhoven EA, Gruter AM, Hewitt JE, Padberg GW, et al. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum Mol Genet. 1993;2:2037–42. doi: 10.1093/hmg/2.12.2037. [DOI] [PubMed] [Google Scholar]
- 101.Rossi M, Ricci E, Colantoni L, Galluzzi G, Frusciante R, Tonali PA, et al. The Facioscapulohumeral muscular dystrophy region on 4qter and the homologous locus on 10qter evolved independently under different evolutionary pressure. BMC Med Genet. 2007;8:8. doi: 10.1186/1471-2350-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Greef JC, Frants RR, van der Maarel SM. Epigenetic mechanisms of facioscapulohumeral muscular dystrophy. Mutat Res. 2008;647:94–102. doi: 10.1016/j.mrfmmm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tupler R, Gabellini D. Molecular basis of facioscapulohumeral muscular dystrophy. Cell Mol Life Sci. 2004;61:557–66. doi: 10.1007/s00018-003-3285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gabellini D, Green MR, Tupler R. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell. 2002;110:339–48. doi: 10.1016/s0092-8674(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 105.Rijkers T, Deidda G, van Koningsbruggen S, van Geel M, Lemmers RJ, van Deutekom JC, et al. FRG2, an FSHD candidate gene, is transcriptionally upregulated in differentiating primary myoblast cultures of FSHD patients. J Med Genet. 2004;41:826–36. doi: 10.1136/jmg.2004.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laoudj-Chenivesse D, Carnac G, Bisbal C, Hugon G, Bouillot S, Desnuelle C, et al. Increased levels of adenine nucleotide translocator 1 protein and response to oxidative stress are early events in facioscapulohumeral muscular dystrophy muscle. J Mol Med. 2005;83:216–24. doi: 10.1007/s00109-004-0583-7. [DOI] [PubMed] [Google Scholar]
- 107.Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, Qian H, et al. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc Natl Acad Sci USA. 2007;104:18157–62. doi: 10.1073/pnas.0708659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang G, Yang F, van Overveld PG, Vedanarayanan V, van der Maarel S, Ehrlich M. Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Hum Mol Genet. 2003;12:2909–21. doi: 10.1093/hmg/ddg323. [DOI] [PubMed] [Google Scholar]
- 109.Winokur ST, Chen YW, Masny PS, Martin JH, Ehmsen JT, Tapscott SJ, et al. Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum Mol Genet. 2003;12:2895–907. doi: 10.1093/hmg/ddg327. [DOI] [PubMed] [Google Scholar]
- 110.Klooster R, Straasheijm K, Shah B, Sowden J, Frants R, Thornton C, et al. Comprehensive expression analysis of FSHD candidate genes at the mRNA and protein level. Eur J Hum Genet. 2009;17:1615–24. doi: 10.1038/ejhg.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Deutekom JC, Lemmers RJ, Grewal PK, van Geel M, Romberg S, Dauwerse HG, et al. Identification of the first gene (FRG1) from the FSHD region on human chromosome 4q35. Hum Mol Genet. 1996;5:581–90. doi: 10.1093/hmg/5.5.581. [DOI] [PubMed] [Google Scholar]
- 112.Petrov A, Pirozhkova I, Carnac G, Laoudj D, Lipinski M, Vassetzky YS. Chromatin loop domain organization within the 4q35 locus in facioscapulohumeral dystrophy patients versus normal human myoblasts. Proc Natl Acad Sci USA. 2006;103:6982–7. doi: 10.1073/pnas.0511235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pirozhkova I, Petrov A, Dmitriev P, Laoudj D, Lipinski M, Vassetzky Y. A functional role for 4qA/B in the structural rearrangement of the 4q35 region and in the regulation of FRG1 and ANT1 in facioscapulohumeral dystrophy. PLoS One. 2008;3:3389. doi: 10.1371/journal.pone.0003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bodega B, Ramirez GD, Grasser F, Cheli S, Brunelli S, Mora M, et al. Remodeling of the chromatin structure of the facioscapulohumeral muscular dystrophy (FSHD) locus and upregulation of FSHD-related gene 1 (FRG1) expression during human myogenic differentiation. BMC Biol. 2009;7:41. doi: 10.1186/1741-7007-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tupler R, Berardinelli A, Barbierato L, Frants R, Hewitt JE, Lanzi G, et al. Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy. J Med Genet. 1996;33:366–70. doi: 10.1136/jmg.33.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gabellini D, D’Antona G, Moggio M, Prelle A, Zecca C, Adami R, et al. Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1. Nature. 2006;439:973–7. doi: 10.1038/nature04422. [DOI] [PubMed] [Google Scholar]
- 117.Hanel ML, Wuebbles RD, Jones PL. Muscular dystrophy candidate gene FRG1 is critical for muscle development. Dev Dyn. 2009;238:1502–12. doi: 10.1002/dvdy.21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wuebbles RD, Hanel ML, Jones PL. FSHD region gene 1 (FRG1) is crucial for angiogenesis linking FRG1 to facioscapulohumeral muscular dystrophy-associated vasculopathy. Dis Model Mech. 2009;2:267–74. doi: 10.1242/dmm.002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Koningsbruggen S, Dirks RW, Mommaas AM, Onderwater JJ, Deidda G, Padberg GW, et al. FRG1P is localised in the nucleolus, Cajal bodies and speckles. J Med Genet. 2004;41:46. doi: 10.1136/jmg2003.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Koningsbruggen S, Straasheijm KR, Sterrenburg E, de Graaf N, Dauwerse HG, Frants RR, et al. FRG1P-mediated aggregation of proteins involved in pre-mRNA processing. Chromosoma. 2007;116:53–64. doi: 10.1007/s00412-006-0083-3. [DOI] [PubMed] [Google Scholar]
- 121.Jurica MS, Licklider LJ, Gygi SR, Grigorieff N, Moore MJ. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. Rna. 2002;8:426–39. doi: 10.1017/s1355838202021088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Makarov EM, Makarova OV, Urlaub H, Gentzel M, Will CL, Wilm M, et al. Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science. 2002;298:2205–8. doi: 10.1126/science.1077783. [DOI] [PubMed] [Google Scholar]
- 123.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;12:1231–45. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim SK, Lund J, Kiraly M, Duke K, Jiang M, Stuart JM, et al. A gene expression map for Caenorhabditis elegans. Science. 2001;293:2087–92. doi: 10.1126/science.1061603. [DOI] [PubMed] [Google Scholar]
- 125.Trede NS, Medenbach J, Damianov A, Hung LH, Weber GJ, Paw BH, et al. Network of coregulated spliceosome components revealed by zebrafish mutant in recycling factor p110. Proc Natl Acad Sci USA. 2007;104:6608–13. doi: 10.1073/pnas.0701919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Licatalosi DD, Darnell RB. Splicing Regulation in Neurologic Disease. Neuron. 2006;52:93–101. doi: 10.1016/j.neuron.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 127.Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18:164–7. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 128.Calado A, Tome FM, Brais B, Rouleau GA, Kuhn U, Wahle E, et al. Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum Mol Genet. 2000;9:2321–8. doi: 10.1093/oxfordjournals.hmg.a018924. [DOI] [PubMed] [Google Scholar]
- 129.Briese M, Esmaeili B, Sattelle DB. Is spinal muscular atrophy the result of defects in motor neuron processes? Bioessays. 2005;27:946–57. doi: 10.1002/bies.20283. [DOI] [PubMed] [Google Scholar]
- 130.Davidovic L, Sacconi S, Bechara EG, Delplace S, Allegra M, Desnuelle C, et al. Alteration of expression of muscle specific isoforms of the fragile X related protein 1 (FXR1P) in facioscapulohumeral muscular dystrophy patients. J Med Genet. 2008;45:679–85. doi: 10.1136/jmg.2008.060541. [DOI] [PubMed] [Google Scholar]
- 131.Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, et al. Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet. 2004;13:1291–302. doi: 10.1093/hmg/ddh150. [DOI] [PubMed] [Google Scholar]
- 132.Huot ME, Bisson N, Davidovic L, Mazroui R, Labelle Y, Moss T, et al. The RNA-binding protein fragile X-related 1 regulates somite formation in Xenopus laevis. Mol Biol Cell. 2005;16:4350–61. doi: 10.1091/mbc.E05-04-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van’t Padje S, Chaudhry B, Severijnen LA, van der Linde HC, Mientjes EJ, Oostra BA, et al. Reduction in fragile X related 1 protein causes cardiomyopathy and muscular dystrophy in zebrafish. J Exp Biol. 2009;212:2564–70. doi: 10.1242/jeb.032532. [DOI] [PubMed] [Google Scholar]