Abstract
Summary
People with both HIV and hepatitis C are more likely than those with HIV alone to have wrist, hip, and spine fractures. We compared hip strength between HIV/HCV-co-infected men and healthy men and found that HIV/HCV-co-infected men had decreased hip strength due to lower lean body mass.
Introduction
Hepatitis C co-infection is a risk factor for fragility fracture among HIV-infected populations. Whether bone strength is compromised in HIV/HCV-co-infected patients is unknown.
Methods
We compared dual-energy x-ray absorptiometry (DXA)-derived hip geometry, a measure of bone strength, in 88 HIV/HCV-co-infected men from the Johns Hopkins HIV Clinic to 289 men of similar age and race and without HIV or HCV from the Boston Area Community Health Survey/Bone Survey. Hip geometry was assessed at the narrow neck, intertrochanter, and shaft using hip structural analysis. Lean body mass (LBM), total fat mass (FM), and fat mass ratio (FMR) were measured by whole-body DXA. Linear regression was used to identify body composition parameters that accounted for differences in bone strength between cohorts.
Results
HIV/HCV-co-infected men had lower BMI, LBM, and FM and higher FMR compared to controls (all p<0.05). At the narrow neck, significant differences were observed between HIV/HCV-co-infected men and controls in bone mineral density, cross-sectional area, section modulus, buckling ratio, and centroid position. After adjustment for race, age, smoking status, height, and weight, only buckling ratio and centroid position remained significantly different between cohorts (all p<0.05). Substituting LBM, FM, and FMR for weight in the multivariate model revealed that differences in LBM, but not FM or FMR, accounted for differences in all narrow neck parameters between cohorts, except buckling ratio and centroid position.
Conclusion
HIV/HCV-co-infected men have compromised hip strength at the narrow neck compared to uninfected controls, which is attributable in large part to lower lean body mass.
Keywords: Body composition, Bone strength, Hepatitis C, Hip structural analysis, HIV
Introduction
With the advent of highly active antiretroviral therapy (HAART), HIV has been transformed into a chronic disease for many patients. As a result, the number of older HIV-infected persons is growing rapidly, and the importance of aging-related co-morbidities, such as osteoporosis and fracture, has increased. A meta-analysis of cross-sectional studies determined that the prevalence of osteoporosis among HIV-infected patients is 15%, which is three times greater than that of the HIV-uninfected controls [1].
The etiology of osteoporosis in HIV-infected patients is likely multifactorial. Traditional risk factors such as low body weight, hypogonadism, and smoking contribute to increased risk as do the direct effects of antiretroviral therapy and immune activation by chronic HIV infection [2–4]. In the Veterans Aging Cohort Study, HIV infection was associated with a 32% increased risk of fragility fracture (spine and hip) (HR=1.32; 95% CI 1.20–1.47) [5]. Importantly, hepatitis C co-infection has been identified as an independent risk factor for fracture in several cohorts of HIV-infected persons [6–9]. Prior studies of HIV/HCV-co-infected patients have focused on dual-energy x-ray absorptiometry (DXA) to assess bone mineral density (BMD) and thereby quantify the severity of osteoporosis and risk of fracture [1]. However, DXA-measured BMD cannot quantify bone quality, the other essential feature of osteoporosis, and therefore accounts for only about 50% of fracture risk [10]. Newer imaging techniques that measure bone strength, an important aspect of bone quality, are gaining importance in research studies in the general population. Whether bone strength is also compromised in HIV/HCV-co-infected patients is, however, unknown.
In a previous report, we found an unexpectedly high prevalence of osteoporosis in a group of HIV/HCV-co-infected persons with a median age of 50.3 years, with 26.7% having osteoporosis at the spine and 4.4% having osteoporosis at the total hip [11]. The goal of the current study was to determine whether bone strength, as measured by DXA-derived hip structural analysis, is decreased in HIV/HCV-co-infected men as compared to age- and race-matched controls. For this analysis, we focused on men since over two thirds of HIV-infected persons over the age of 50 are men, and HIV-infected men have a higher prevalence of low BMD compared to HIV-infected women of similar age [12, 13]. In addition, osteoporosis in men is underrecognized and undertreated, despite the fact that one third of hip fractures occur in men [13, 14]. A secondary objective of the analysis was to determine the relationship between bone strength and body composition. Previous studies have shown that body weight is a strong predictor of BMD [15–18]. However, it is unclear which component of body weight, either fat mass or lean mass, is critical to bone strength.
Methods
Study participants
A prospective cohort of HIV/HCV-co-infected patients was recruited from the Johns Hopkins Viral Hepatitis clinical practice and the Johns Hopkins University HIV Clinic. The primary aim of the study was to characterize liver disease progression in HIV/HCV co-infection. Between January 2007 and February 2009, 116 men underwent DXA scans in order to assess hip and spine bone mineral density and body composition. Hip geometry was also measured in this cohort by applying the hip structural analysis (HSA) program to DXA measurements. Of the 116 HIV/HCV-co-infected men, 88 had archived hip DXA data from which HSA could be performed.
For subjects within the HIV/HCV cohort, information on prescribed medications and laboratory parameters was obtained from laboratory and clinical databases. Trained personnel abstracted data on patient demographics, social practices, and clinical and laboratory parameters and prescribed antiretroviral and other medications from charts; the data were transferred electronically from the laboratory database at enrollment and subsequent 6–12-month intervals, as described previously [19]. HAART was defined as the use of a NNRTI, PI, fusion inhibitor, or integrase inhibitor. The institutional review board of each site approved the study protocol and forms, and each participant provided written informed consent.
We compared the cohort of HIV/HCV-co-infected men to a subset of male subjects enrolled in the Boston Area Community Health Survey/Bone Survey (BACH/Bone), a cohort with similar demographic parameters as the HIV/HCV cohort, but without HIV or chronic hepatitis. The BACH/Bone Survey is a cross-sectional observational study of musculoskeletal health in 1,219 (of 1,877 eligible, 65% response rate) randomly selected black, Hispanic, and white male Boston, MA, residents aged 30 to 79 years [20, 21]. Data were collected from Sept. 2002 to June 2005. For the purposes of this analysis, of the 1,219 men enrolled in the BACH/Bone survey, 438 were excluded because of Hispanic ancestry (a demographic group not represented in the HIV/HCV cohort), history of HIV or hepatitis B or C medication use, and unusable HSA data. Of the 781 remaining eligible subjects, 246 were excluded because their ages were outside of the range of ages found among the HIV/HCV-co-infected men. Of the 535 remaining eligible subjects, 239 Caucasian men were excluded after selecting a random sample of Caucasian participants to reflect the proportion of Caucasians in the HIV/HCV cohort. Of the 296 remaining eligible subjects, 7 were excluded because their BMIs were outside of the range of BMIs found in the HIV/HCV-co-infected men. The remaining 289 eligible men were included in the analysis.
Laboratory evaluations
Patients in the HIV/HCV cohort had standard laboratory assessments performed by licensed clinical laboratories, including CD4 cell count and plasma HIV RNA level (reverse-transcriptase polymerase chain reaction) within 6 months of DXA (CD4 median, 13 days; IQR, −22, 41; RNA median, 13 days; IQR, −31, 44).
Body composition and hip structural analysis
Body mass index was defined as weight (kilograms) divided by height (meters) squared. In both of the cohorts, height was measured using a wall-mounted stadiometer. Weight was assessed while the participant was wearing minimal clothing. Site-specific DXA at the hip was performed to assess bone mineral content (BMC) and BMD. Whole-body DXA was performed to measure total body and regional fat mass and nonfat mass. Fat mass ratio, a measure of fat distribution, was calculated by dividing trunk fat mass by lower extremity fat mass [22]. Lean body mass was calculated by subtracting BMC from fat-free mass [23]. Participants in the HIV/HCV cohort were evaluated using a Hologic 4500A machine with QDA software version 9.03 (Hologic Inc., Waltham, MA). Men in the BACH/Bone cohort were assessed with a Hologic QDR 4500W (Hologic Inc., Waltham, MA). To ensure comparability of hip geometry parameters, a calibration phantom was scanned at both scanner sites, and the resulting calibrations were incorporated into the HSA analysis.
Hip geometry parameters for both groups were derived using the HSA program, which has been extensively described elsewhere [24, 25]. HSA processing for both cohorts was completed by trained staff at the Johns Hopkins University School of Medicine under the supervision of one of the authors (TJB). HSA parameters in BACH/Bone have been described previously [26]. In brief, the HSA software uses the DXA scan image to generate profiles of pixel values traversing the proximal femur at three locations where fracture is likely: the narrow neck (NN), intertrochanter (IT), and shaft [26, 27]. The NN is the narrowest width of the femoral neck, and it typically corresponds to the conventional DXA femoral neck region, an area at high risk for osteoporotic fracture. The IT lies along the angle bisector defined by the neck and shaft axes. The shaft is located at a point 1.5 cm distal to the midpoint of the lesser trochanter [26, 27]. The HSA program analyzes cross-sectional portions of bone at each of these three locations, using individual lines of pixels traversing the bone axis. For each location, the HSA program computes the following parameters: (1) areal BMD (grams per square centimeter); (2) bone cross-sectional area (square centimeters), an expression of cortical tissue surface area of bone in the cross section and an index of axial strength; (3) centroid position, a measure of the center of mass of bone across the profile and expressed as the ratio of the distance from the medial bone margin to the center of mass divided by the total subperiosteal width; (4) cross-sectional modulus (Z, cubic centimeters), a measure of bending strength calculated by dividing the cross-sectional moment of inertia (CSMI) by dmax, which is the maximum distance from the centroid to the medial or lateral cortical margin; (5) estimated average cortical thickness, centimeters; and (6) buckling ratio (BR), derived from dmax divided by the estimated mean cortical thickness and an indicator of cortical stability under compressive loads [21, 27].
The primary outcomes were HSA parameters, specifically BMD, cross-sectional area, section modulus, cortical thickness, centroid position, and buckling ratio, at the narrow neck and intertrochanteric regions, areas of the hip that are commonly fractured. The secondary outcomes were the aforementioned HSA parameters at the shaft region, an uncommon site of fragility fractures.
Statistical analysis
Demographic data and DXA-derived body composition parameters were compared between the HIV/HCV and BACH/Bone cohort using chi-squared tests and t tests as appropriate. Mean site-specific HSA parameters at the intertrochanter, narrow neck, and shaft regions were compared between cohorts using t tests. Adjusted means, accounting for age, smoking status, race, weight, and height, were estimated using generalized linear models. A series of multivariable linear regression models were generated to estimate differences in HSA parameters between the two cohorts at the three regions. All models included age, smoking status, race, and height given their hypothesized associations with the outcomes of interest and differences across the two study sites. The baseline model also included a term for weight. In a stepwise fashion, components of body composition were substituted for weight in order to determine which specific aspects of body composition accounted for differences in HSA parameters between the cohorts. First, lean mass was substituted for weight in the multivariable model. Next, all three components of weight, total fat, fat distribution (as fat mass ratio), and lean mass, were included in the multivariable model. Two-sided p values <0.05 were considered statistically significant. All analyses were performed using SAS, version 9.1 (Cary, NC, USA).
Results
Description of study population
The demographic and clinical characteristics of the two study populations are presented in Table 1. By design, the cohorts were similar in their age and race distributions (HIV/HCV cohort: 51.6 years vs BACH/Bone survey 50.7 years; 86% African–American). The proportion of participants with a past or current history of smoking was also similar between the two cohorts.
Table 1.
Characteristics of participants in the HIV/HCV cohort and BACH/Bone cohort at the time of DXA
| HIV/HCV cohort (n=88) |
BACH/Bone cohort (n=289) |
p value | |
|---|---|---|---|
| Mean age in years (SD) | 51.6 (6.3) | 50.7 (8.6) | 0.31 |
| Race, black (%) | 76 (86.4) | 250 (86.5) | 0.97 |
| Height, cm (SD) | 176.1 (6.5) | 175.2 (6.6) | 0.25 |
| Mean BMI, g/cm2 (SD) | 25.4 (4.8) | 28.2 (4.9) | <0.0001 |
| Mean lean body mass, kg (SD) | 56.2 (8.5) | 59.2 (8.7) | 0.005 |
| Mean fat mass, kg (SD) | 16.56 (8.6) | 21.59 (9.07) | <0.0001 |
| Mean fat ratio (SD) | 1.87 (0.82) | 1.67 (0.48) | 0.03 |
| Smoking status at DXA (%) | 0.16 | ||
| Current | 45 (52.3) | 132 (45.7) | |
| Former | 11 (12.8) | 64 (22.2) | |
| Never | 30 (34.9) | 93 (32.2) | |
| HIV RNA <400 at DXA (%) | 64 (80.0) | ||
| CD4 >200 at DXA (%) | 69 (87.3) | ||
| History of HAART (%) | 73 (84.9) | ||
| Current HAART (%) | 53 (61.6) | ||
| Mean cumulative years on HAART (SD) | 6.08 (3.98) | ||
| Significant liver fibrosis (Metavir ≥2) (%) | 32 (39.0) |
BMI body mass index, DXA dual-energy x-ray absorptiometry, HIV human immunodeficiency virus, RNA ribonucleic acid, HAART highly active antiretroviral therapy
The mean BMI was lower in the HIV/HCV cohort than the BACH/Bone survey (25.4 vs 28.2 kg/m2, p<0.0001). In addition, lean body mass and fat mass were significantly lower in the HIV/HCV cohort compared to the BACH/Bone survey. In contrast, fat mass ratio, a measure of relative adiposity of the trunk, was greater in the HIV/HCV cohort than the BACH/Bone survey. Within the HIV/HCV cohort, 87.3% had a CD4 count >200 cells/mm3, 80% had an HIV RNA level <400 copies/mL, 61.6% were receiving HAART at the time of DXA, and 84.9% had ever received HAART. Cumulative exposure to HAART within the HIV/HCV cohort was 6.08 years (standard deviation, 3.98 years).
Body composition and hip structural analysis
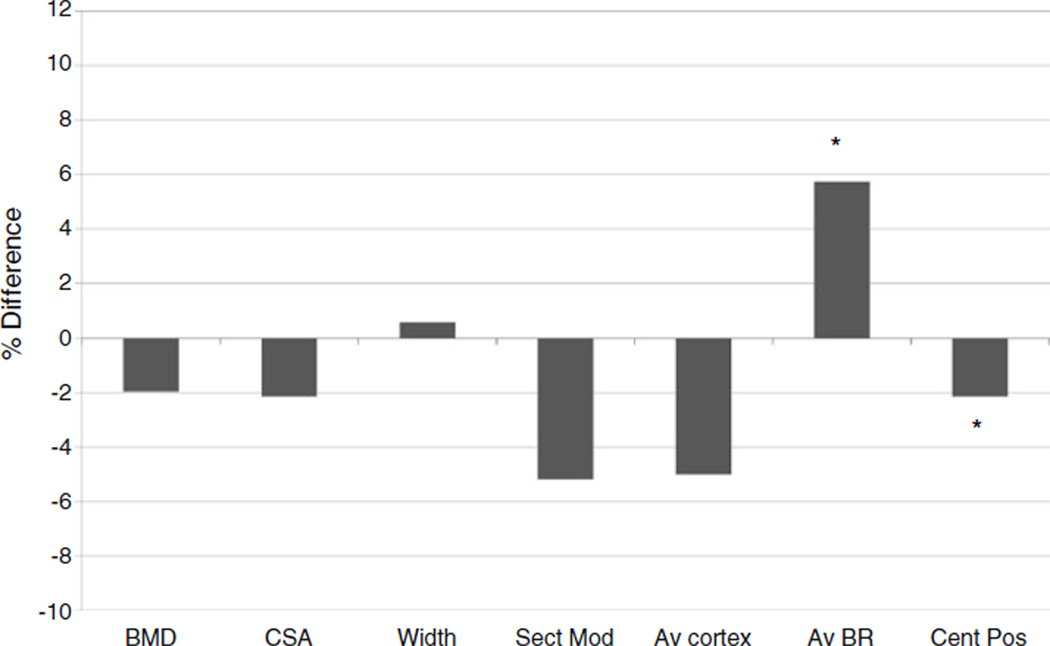
Hip structural analysis parameters at the intertrochanter, narrow neck, and shaft, stratified by cohort, are presented in Table 2. At the intertrochanter, there were no differences in HSA parameters between cohorts in the unadjusted model or after adjustment for race, age, smoking status, height, and weight. At the narrow neck, mean BMD, cross-sectional area (CSA), section modulus, AV cortex, and centroid position were significantly lower in HIV/HCV-co-infected men in the model, whereas AV buckling ratio was significantly higher in the HIV/HCV cohort. After adjustment for race, age, smoking status, height, and weight, only AV buckling ratio and centroid position remained significantly different between the two cohorts (Fig. 1). At the shaft, the HIV/HCV cohort had significantly lower BMD, CSA, and section modulus. After adjustment for race, age, smoking status, height, and weight, these differences were no longer statistically significant. After adjusting for race, age, smoking status, height, and weight, significant differences between cohorts were observed only at the narrow neck; therefore, the remainder of the analyses focused on HSA parameters at this location.
Table 2.
Mean (standard error) HSA outcome measures by cohort
| Crude mean (STE) | Adjusted mean (STE)a | |||
|---|---|---|---|---|
| HIV/HCV | BACH/Bone | HIV/HCV | BACH/Bone | |
| Intertrochanter | ||||
| BMD, g/cm2 | 1.00 (0.02) | 1.02 (0.01) | 1.02 (0.02) | 1.02 (0.01) |
| CSA, cm2 | 5.59 (0.12) | 5.77 (0.06) | 5.70 (0.10) | 5.74 (0.05) |
| Width, cm | 5.89 (0.04) | 5.94 (0.03) | 5.87 (0.04) | 5.94 (0.02) |
| Sect mod, cm3 | 5.31 (0.13) | 5.37 (0.06) | 5.36 (0.11) | 5.36 (0.06) |
| Av cortex, cm | 0.42 (0.01) | 0.43 (0.005) | 0.43 (0.01) | 0.43 (0.005) |
| Av BR | 8.22 (0.26) | 7.98 (0.10) | 7.99 (0.20) | 8.04 (0.10) |
| Cent pos | 0.44 (0.002) | 0.44 (0.001) | 0.44 (0.002) | 0.44 (0.001) |
| Narrow neck | ||||
| BMD, g/cm2 | 0.97 (0.02)* | 1.03 (0.01)* | 1.00 (0.02) | 1.02 (0.01) |
| CSA, cm2 | 3.14 (0.07)* | 3.31 (0.04)* | 3.22 (0.06) | 3.29 (0.03) |
| Width, cm | 3.41 (0.02) | 3.38 (0.02) | 3.40 (0.03) | 3.38 (0.02) |
| Sect mod, cm3 | 1.62 (0.04)* | 1.75 (0.03)* | 1.65 (0.05) | 1.74 (0.03) |
| Av cortex, cm | 0.19 (0.004)* | 0.20 (0.002)* | 0.19 (0.004) | 0.20 (0.002) |
| Av BR | 10.30 (0.28)* | 9.34 (0.14)* | 9.97 (0.24)* | 9.43 (0.13)* |
| Cent pos | 0.46 (0.002)* | 0.47 (0.001)* | 0.46 (0.002)* | 0.47 (0.001)* |
| Shaft | ||||
| BMD, g/cm2 | 1.60 (0.03)* | 1.67 (0.01)* | 1.65 (0.03) | 1.66 (0.01) |
| CSA, cm2 | 4.82 (0.11)* | 5.11 (0.04)* | 4.98 (0.08) | 5.07 (0.04) |
| Width, cm | 3.17 (0.02) | 3.22 (0.01) | 3.18 (0.02) | 3.22 (0.01) |
| Sect mod, cm3 | 2.76 (0.07)* | 2.92 (0.03)* | 2.84 (0.05) | 2.90 (0.02) |
| Av cortex, cm | 0.61 (0.02) | 0.64 (0.007) | 0.63 (0.01) | 0.63 (0.006) |
| Av BR | 2.85 (0.11) | 2.67 (0.04) | 2.72 (0.08) | 2.70 (0.04) |
| Cent pos | 0.49 (0.001) | 0.49 (0.0006) | 0.50 (0.001) | 0.49 (0.0006) |
HSA hip structural analysis, STE standard error, BMD bone mineral density, CSA cross-sectional area, Sect mod sectional modulus, Av BR average buckling ratio, Cent pos centroid position
p<0.05
Adjusted for race, age, smoking status, height, and weight
Fig. 1.
Percent difference in narrow neck hip structural analysis parameters, HIV/HCV cohort vs BACH/Bone cohort, after adjustment for race, age, smoking status, height, and weight. *p<0.05, BMD bone mineral density, CSA cross-sectional area, Sect Mod section modulus, Av cortex average cortical thickness, Av BR average buckling ratio, Cent Pos centroid position
Table 3 shows the relationship between narrow neck HSA parameters and components of body composition. After adjustment for lean mass (model 1), only average buckling ratio and centroid position remained significantly different between the cohorts, indicating that differences in BMD, CSA, section modulus, and cortical thickness between cohorts were largely explained by lower lean mass among the HIV/HCV cohort. In model 2, all three components of weight, total fat mass, fat mass ratio, and lean mass, were included in the multivariable linear regression model. This analysis resulted in insignificant differences between cohorts for most HSA parameters, with the exception of AV buckling ratio and centroid position. Of the body composition parameters, only lean mass, but not fat mass or fat mass ratio, remained significantly associated with all HSA parameters.
Table 3.
Crude and adjusted mean differences (standard error) in hip structural analysis parameters between cohorts
| Unadjusted model | Model 1a | Model 2a | |||||
|---|---|---|---|---|---|---|---|
| Cohortb | Cohortb | Lean mass (kg) | Cohortb | Total fat (kg) | Fat ratio | Lean mass (kg) | |
| Narrow neck | |||||||
| BMD, g/cm2 | −0.063 (0.023)* | −0.032 (0.021) | 0.0087 (0.0012)* | −0.038 (0.021) | 0.00072 (0.0012) | 0.032 (0.016) | 0.0078 (0.0015)* |
| CSA, cm2 | −0.17 (0.079)* | −0.088 (0.071) | 0.033 (0.004)* | −0.10 (0.073) | 0.0024 (0.0042) | 0.087 (0.056) | 0.030 (0.0050)* |
| Width, cm | 0.029 (0.035) | 0.012 (0.35) | 0.0047 (0.002)* | 0.017 (0.036) | −0.00072 (0.0021) | −0.027 (0.027) | 0.0056 (0.0024)* |
| Sect mod, cm3 | −0.13 (0.061)* | −0.094 (0.057) | 0.020 (0.0033)* | −0.11 (0.059) | 0.00031 (0.0034) | 0.057 (0.045) | 0.019 (0.0040)* |
| Av cortex, cm | −0.013 (0.0047)* | −0.0067 (0.0044) | 0.0018 (0.0002)* | −0.0080 (0.0045) | 0.00014 (0.00026) | 0.0066 (0.0035) | 0.0016 (0.00031)* |
| Av BR | 0.96 (0.29)* | 0.56 (0.28)* | −0.085 (0.016)* | 0.64 (0.29)* | −0.0011 (0.017) | −0.42 (0.22) | −0.072 (0.020)* |
| Cent pos | −0.0096 (0.0023)* | −0.0082 (0.0024)* | 0.0004 (0.0001)* | −0.0087 (0.0024)* | −0.00011 (0.00014) | 0.00053 (0.0019) | 0.00044 (0.00017)* |
HSA hip structural analysis, STE standard error, BMD bone mineral density, CSA cross-sectional area, Sect mod sectional modulus, Av cortex average cortical thickness, Av BR average buckling ratio, Cent pos centroid position. Mean difference between HIV/HCV cohort and BACH/Bone cohort
p<0.05
Adjusted for race, age, smoking status, and height
Difference [beta (standard error)] in HSA parameters in the HIV/HCV vs BACH/Bone cohorts
We conducted supplemental analyses to investigate further the relationship between body composition and HSA parameters in each cohort. In adjusting for total fat and fat mass ratio individually and simultaneously, HSA parameters remained significantly different between the two cohorts (data not shown). We also found that the relationship between lean mass and HSA parameters was similar in magnitude between the two cohorts. Given that lower extremity lipoatrophy is associated with decreased BMD of the hip in HIV-infected men on HAART [28], we investigated the relationship between lipoatrophy and HSA parameters in our cohort of HIV/HCV-co-infected men and found that lower extremity fat was not associated with HSA parameters (data not shown). Additionally, among the HIV/HCV cohort, narrow neck HSA parameters were not associated with CD4 cell count, specific antiretroviral therapies, the degree of HIV suppression, or liver disease severity (data not shown).
Discussion
In this cross-sectional study comparing a predominantly African–American, HIV/HCV-co-infected cohort of men in Baltimore to a healthy control population in Boston of similar age and race, we found that the HIV/HCV-co-infected men have compromised bone strength (as measured by hip geometry parameters) at the shaft and narrow neck regions. Although there are considerable differences in all body composition parameters between the cohorts, our analyses reveal that lean mass exerts the predominant influence on lower hip strength among men with HIV/HCV co-infection. This is consistent with an earlier study of the BACH cohort used as a reference population in the present study [23]. However, even after adjustment for lean mass, residual differences in buckling ratio and centroid position at the narrow neck remain between the two cohorts, suggesting that other factors besides lean mass lead to compromised hip strength in this cohort.
Recent studies have shown that fracture prevalence is higher among HIV-infected men compared to non-HIV-infected men for vertebral, hip, and wrist fractures, sites typically associated with osteoporosis [29]. In addition, multiple studies have shown that hepatitis C co-infection is independently associated with an increased risk of osteoporosis and fracture [6–9, 30]. Prior work in our HIV/HCV-co-infected cohort showed an increased prevalence of osteoporosis, with 5% having osteoporosis of the total hip, 6% at the femoral neck, and 25% at the spine [31]. Additionally, a cross-sectional study of HIV-infected persons found that mean femoral neck BMD Z-scores were lower among hepatitis C-co-infected women compared to HIV-monoinfected women [32].
In order to determine whether hip strength is also compromised in HIV/HCV co-infection, we used HSA in its first application, to our knowledge, in any HIV-infected population. HSA extracts geometric strength information from DXA scans of the hip at three sites, the intertrochanter, narrow neck, and shaft. The intertrochanteric region lies across the angle bisector of the neck–shaft axes and is a common site of fracture in elderly persons [26]. The proximal shaft, an uncommon fracture site, is composed purely of cortical bone and is the site of aging-related subperiosteal expansion [25]. The narrow neck incorporates both trabecular and cortical bone and is a commonly fractured site [25].
These parameters have been associated with fracture risk in multiple cohorts of the general population. The Study of Osteoporotic Fractures (SOF) and the Women's Health Initiative (WHI), for example, have shown that HSA parameters can independently predict hip fracture [27, 33]. Specifically, average buckling ratio predicted hip fracture at the shaft and intertrochanter among WHI participants, and cortical thickness and average buckling ratio predicted narrow neck fracture among SOF subjects [27, 33]. Additionally, a decrease by 1 SD (16.6% of mean value) in CSA increased the risk of incident hip fracture by 1.80–1.93, depending on which covariates were included in the model [27, 33]. The Osteoporotic Fracture in Men Study found that low bending strength (estimated by peripheral quantitative computed tomography derived strength–strain index and CSMI) was strongly and significantly associated with fracture risk [34]. Therefore, the decreased narrow neck axial strength (CSA) and bending strength (section modulus) observed among the HIV/HCV cohort may translate into an increased risk of hip fracture with aging.
The most important factor that explained all of the differences in HSA parameters at the shaft and most of the differences at the narrow neck between the two cohorts was the lower lean mass in the HIV/HCV-co-infected men. Decreased weight and lean body mass are common among HIV-infected persons and can occur early in asymptomatic HIV infection [35]. The loss of lean body mass, which reflects muscle mass, can also be seen in chronic liver disease, including chronic viral hepatitis [36, 37], and may be due to alterations in chronic inflammation, gonadal status, and IGF-1 production.
Existing evidence suggests that lean mass and its associated mechanical load on bone confers beneficial effects on bone mineral content and bone mineral density [21, 38]. Specifically, larger lean mass transmits larger mechanical loading on the skeleton ultimately resulting in increased BMC and BMD [38]. Therefore, it follows that lower lean mass would translate into reduced BMD and hip bone strength as was our finding at the femoral shaft and narrow neck. This finding is consistent with our work in the Study of HIV, Injection Drug Use, Nutrition and Endocrinology which found that lower lean mass was associated with lower whole-body BMD among men in this underserved urban cohort [11]. Our findings likewise echo those of Rosenthall et al., who found that lean mass was a determinant of lower extremity BMC among HIV-infected males on HAART [28]. Notably, the loss of lean body mass may be partially reversible with the use of potent antiretroviral therapy; specifically, men randomized to efavirenz or zidovudine and lamivudine had significant increases in fat-free mass after 64 weeks of therapy [39]. Reductions in lean body mass associated with uncontrolled HIV disease are not fully restored with effective suppression of viral replication. The reasons for this are undefined but may be due to residual immune activation [35], which may be more pronounced in co-infected patients. Future studies should include HIV-monoinfected and hepatitis C-monoinfected comparison groups to better understand the individual contributions of HIV infection and hepatitis C infection on systemic inflammation, the effect on lean mass, and the consequential reductions in hip strength.
We also examined whether differences in HSA parameters were related to differences in fat mass or fat distribution. Existing evidence has not fully resolved the impact of fat mass on BMD, with some studies reporting an inverse relationship between fat mass and bone mass and others reporting a positive relationship [23, 28, 38, 40]. In our study, HIV/HCV men had lower fat mass, and decreased fat mass was associated with increased BMD, CSA, section modulus, average cortical thickness, and centroid position and decreased average buckling ratio at the intertrochanter, narrow neck, and shaft (data not shown). However, after accounting for lean mass, the impact of fat mass on hip geometry parameters at all three sites became insignificant. Similarly, we did not find any association between relative central fat distribution and HSA parameters at any of the three regions assessed, although the HIV/HCV men had a higher fat mass ratio. Relative central fat accumulation is common among HIV-infected persons and may be associated with lower BMD [41, 42]. DXA, however, cannot distinguish between subcutaneous and visceral fat, and further studies should use a more specific measurement of central fat accumulation.
The observed differences in narrow neck average buckling ratio and centroid position were not fully explained by differences in lean mass between the two cohorts. We did not find any association between HSA parameters and CD4 cell count, HAART exposure, degree of HIV viral load suppression, or severity of hepatitis C infection (as liver fibrosis). Additional mechanisms which should be investigated in future studies include physical activity, diet, socioeconomic status, and lower peak bone mass which were not able to be assessed in the study. Additionally, the effect of chronic immune activation, past illicit drug use, alcohol use, and hypogonadism, which may mediate the differences in buckling ratio and centroid position between the cohorts, should be a target of further investigation.
Our study has several limitations, including the absence of comparable data on behaviors and exposures in both cohorts that may impact bone health, such as physical activity, nutrition, socioeconomic status, or intravenous drug use. We also do not have HIV or hepatitis B or C antibody status or viral load to confirm the absence of infection among the BACH/Bone survey participants. The observation that geometry differences were not present at the intertrochanter is somewhat puzzling although this may be a statistical power issue since unadjusted CSA, section modulus, and buckling ratio trended in the same direction as at the narrow neck. It is possible that these differences may be more apparent in a larger sample. Additionally, the hip structural analysis program is derived from DXA, which, as a two-dimensional imaging modality, was not originally designed to measure geometry. Therefore, small changes in femur positioning during DXA can have a large effect on the dimensions from which geometry parameters are measured; these uncertainties will average out in large studies, but will likely be more meaningful in an individual patient [24]. DXA images can also be blurry, making it hard to define the edge margins, which is particularly important in assessing hip geometry parameters [24].
In conclusion, we found that HIV/HCV-co-infected men, in this middle-aged population of predominantly African–American subjects, had compromised bone strength in the proximal femur, mostly attributable to lower lean mass compared to non-infected matched controls. Ideally, future studies would target therapeutic agents that could increase axial strength and bending strength and reduce buckling ratio in order to stabilize the hip. Existing evidence suggests that calcium supplementation and physical activities, such as low-impact hip flexing and activities of daily living, may achieve this aim of improved hip geometry parameters [43, 44]. Additionally, programs aimed at increasing lean mass would likely lead to increased hip bone strength and decreased fracture risk. Further investigation into the etiology and clinical consequences of the residual differences in buckling ratio and centroid position in HIV/HCV-co-infected men is warranted.
Acknowledgments
Financial support for this study came from K24DA00432, DA-11602, DA-16065, and 2R37DA013806 from the National Institute on Drug Abuse, AA016893 from the National Institute on Alcohol Abuse and Alcoholism, K23 AT002862 (TTB) from the National Center for Complementary and Alternative Medicine, grant HS 07–809 from the Agency for Healthcare Policy and Research and the Clinical Research Unit at the Johns Hopkins Medical Institutions, M01RR-02719. The project described was supported by grant number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and by award number R01AG020727 from the National Institute on Aging (NIA), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR, NIA, or NIH.
Footnotes
Conflicts of interest The Hip Structure Analysis program used in this study and developed by TJB was licensed by Johns Hopkins University to Hologic Inc. All authors have no conflict of interest.
Contributor Information
V. Walker Harris, Email: vwalker4@jhmi.edu, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
C. G. Sutcliffe, Johns Hopkins School of Public Health, Baltimore, MD, USA
A. B. Araujo, New England Research Institutes, Watertown, MA, USA
G. R. Chiu, New England Research Institutes, Watertown, MA, USA
T. G. Travison, Boston University, Boston, MA, USA
S. Mehta, Johns Hopkins School of Public Health, Baltimore, MD, USA
M. S. Sulkowski, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Y. Higgins, Johns Hopkins University School of Medicine, Baltimore, MD, USA
D. L. Thomas, Johns Hopkins University School of Medicine, Baltimore, MD, USA
A. S. Dobs, Johns Hopkins University School of Medicine, Baltimore, MD, USA
T. J. Beck, Whiting School of Engineering, Johns Hopkins University, Baltimore, MD, USA
T. T. Brown, Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 3.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tebas P, Umbleja T, Dube M. Initiation of ART is associated with bone loss independent of the specific ART regimen: results of ACTG A5005s. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles. 2007. [Google Scholar]
- 5.Womack JA, Goulet JL, Gibert C, Brandt C, Chang CC, Gulanski B, Fraenkel L, Mattocks K, Rimland D, Rodriguez-Barradas MC, Tate J, Yin MT, Justice AC. Veterans Aging Cohort Study Project Team (2011) Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0017217. e17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin F, Duval X, Le Moing V, Piroth L, Al Kaied F, Massip P, Villes V, Chene G, Raffi F. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23:1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dao CYB, Buchacz K, Baker R, Brooks J. Higher and increasing rates of fracture among HIV-infected persons in the HIV outpatient study compared to the general US population, 1994–1998. Abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 8.Volk J, Localio R, Newcomb C, Yang Y, Hennessy S, Kostman J, Tebas P, Leonard M, Lo Re V., III Risk of fractures associated with HIV/hepatitis C coinfection. Abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 9.Yin MT, Kendall MA, Wu X, Tassiopoulos K, Huang JS, Shane E, Hochberg M, McComsey GA. Incidence and determinants of fracture in HIV+ individuals on ART in the ALLRT Study. Abstracts of the 18th Conference on Retroviruses and Opportunistic Infections; Boston. 2011. [Google Scholar]
- 10.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Maouche D, Xu X, Cofrancesco J, Jr, Dobs AS, Brown TT. Prevalence of low bone mineral density in a low-income inner-city population. J Bone Miner Res. 2011;26:388–396. doi: 10.1002/jbmr.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cazanave C, Dupon M, Lavignolle-Aurillac V, Barthe N, Lawson-Ayayi S, Mehsen N, Mercie P, Morlat P, Thiebaut R, Dabis F. Reduced bone mineral density in HIV-infected patients: prevalence and associated factors. AIDS. 2008;22:395–402. doi: 10.1097/QAD.0b013e3282f423dd. [DOI] [PubMed] [Google Scholar]
- 13.Paul SM, Martin RM, Lu SE, Lin Y. Changing trends in human immunodeficiency virus and acquired immunodeficiency syndrome in the population aged 50 and older. J Am Geriatr Soc. 2007;55:1393–1397. doi: 10.1111/j.1532-5415.2007.01295.x. [DOI] [PubMed] [Google Scholar]
- 14.Olszynski WP, Shawn Davison K, Adachi JD, Brown JP, Cummings SR, Hanley DA, Harris SP, Hodsman AB, Kendler D, McClung MR, Miller PD, Yuen CK. Osteoporosis in men: epidemiology, diagnosis, prevention, and treatment. Clin Ther. 2004;26:15–28. doi: 10.1016/s0149-2918(04)90002-1. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Black D. Bone mass measurements and risk of fracture in Caucasian women: a review of findings from prospective studies. Am J Med. 1995;98:24S–28S. doi: 10.1016/s0002-9343(05)80041-5. [DOI] [PubMed] [Google Scholar]
- 16.De Laet C, Oden A, Johansson H, Johnell O, Jonsson B, Kanis JA. The impact of the use of multiple risk indicators for fracture on case-finding strategies: a mathematical approach. Osteoporos Int. 2005;16:313–318. doi: 10.1007/s00198-004-1689-z. [DOI] [PubMed] [Google Scholar]
- 17.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–169. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 18.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res. 1992;7:547–553. doi: 10.1002/jbmr.5650070511. [DOI] [PubMed] [Google Scholar]
- 19.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–S41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 20.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 21.Travison TG, Beck TJ, Esche GR, Araujo AB, McKinlay JB. Age trends in proximal femur geometry in men: variation by race and ethnicity. Osteoporos Int. 2008;19:277–287. doi: 10.1007/s00198-007-0497-7. [DOI] [PubMed] [Google Scholar]
- 22.Bonnet E, Delpierre C, Sommet A, Marion-Latard F, Herve R, Aquilina C, Labau E, Obadia M, Marchou B, Massip P, Perret B, Bernard J. Total body composition by DXA of 241 HIV-negative men and 162 HIV-infected men: proposal of reference values for defining lipodystrophy. J Clin Densitom. 2005;8:287–292. doi: 10.1385/jcd:8:3:287. [DOI] [PubMed] [Google Scholar]
- 23.Travison TG, Araujo AB, Esche GR, Beck TJ, McKinlay JB. Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res. 2008;23:189–198. doi: 10.1359/JBMR.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck T. Measuring the structural strength of bones with dual-energy X-ray absorptiometry: principles, technical limitations, and future possibilities. Osteoporos Int. 2003;14(Suppl 5):S81–S88. doi: 10.1007/s00198-003-1478-0. [DOI] [PubMed] [Google Scholar]
- 25.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 26.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix AZ, Beck TJ, Cauley JA, Lewis CE, Bassford T, Jackson R, Wu G, Chen Z. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthall L, Falutz J. Bone mineral and soft-tissue changes in AIDS-associated lipoatrophy. J Bone Miner Metab. 2005;23:53–57. doi: 10.1007/s00774-004-0541-z. [DOI] [PubMed] [Google Scholar]
- 29.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin MT, Shi Q, Hoover DR, Anastos K, Sharma A, Young M, Levine A, Cohen MH, Shane E, Golub ET, Tien PC. Fracture incidence in HIV-infected women: results from the Women' Interagency HIV Study. Aids. 2010;24:2679–2686. doi: 10.1097/QAD.0b013e32833f6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Maouche D, Mehta Shruti, Sutcliffe C, Higgins Y, Torbenson M, Moore R, Thomas DL, Sulkowski M, Brown TT. Controlled HIV viral replication, not liver disease severity associated with low bone mineral density in HIV/HCV co-infection. J Hepatol. 2011 doi: 10.1016/j.jhep.2011.01.035. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Re V, 3rd, Guaraldi G, Leonard MB, Localio AR, Lin J, Orlando G, Zirilli L, Rochira V, Kostman JR, Tebas P. Viral hepatitis is associated with reduced bone mineral density in HIV-infected women but not men. AIDS. 2009;23:2191–2198. doi: 10.1097/QAD.0b013e32832ec258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, Cummings SR. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA. Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26:63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shikuma CM, Zackin R, Sattler F, Mildvan D, Nyangweso P, Alston B, Evans S, Mulligan K. Changes in weight and lean body mass during highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1223–1230. doi: 10.1086/424665. [DOI] [PubMed] [Google Scholar]
- 36.George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, Karvat A, Bhatia SJ, Shah S, Menon PS, Shah N. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol. 2009;15:3516–3522. doi: 10.3748/wjg.15.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neff GW, O'Brien CB, Shire NJ, DeManno A, Kahn S, Rideman E, Safdar K, Madariaga J, Rudich SR. Topical testosterone treatment for chronic allograft failure in liver transplant recipients with recurrent hepatitis C virus. Transplant Proc. 2004;36:3071–3074. doi: 10.1016/j.transproceed.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dube MP, Parker RA, Mulligan K, Tebas P, Robbins GK, Roubenoff R, Grinspoon SK. Effects of potent antiretroviral therapy on free testosterone levels and fat-free mass in men in a prospective, randomized trial: A5005s, a substudy of AIDS Clinical Trials Group Study 384. Clin Infect Dis. 2007;45:120–126. doi: 10.1086/518620. [DOI] [PubMed] [Google Scholar]
- 40.Reid IR. Fat and bone. Arch Biochem Biophys. 2010;503:20–27. doi: 10.1016/j.abb.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 41.Brown TT, Ruppe MD, Kassner R, Kumar P, Kehoe T, Dobs AS, Timpone J. Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab. 2004;89:1200–1206. doi: 10.1210/jc.2003-031506. [DOI] [PubMed] [Google Scholar]
- 42.Huang JS, Rietschel P, Hadigan CM, Rosenthal DI, Grinspoon S. Increased abdominal visceral fat is associated with reduced bone density in HIV-infected men with lipodystrophy. AIDS. 2001;15:975–982. doi: 10.1097/00002030-200105250-00005. [DOI] [PubMed] [Google Scholar]
- 43.Kaptoge S, Jakes RW, Dalzell N, Wareham N, Khaw KT, Loveridge N, Beck TJ, Reeve J. Effects of physical activity on evolution of proximal femur structure in a younger elderly population. Bone. 2007;40:506–515. doi: 10.1016/j.bone.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 44.Nurzenski MK, Briffa NK, Price RI, Khoo BC, Devine A, Beck TJ, Prince RL. Geometric indices of bone strength are associated with physical activity and dietary calcium intake in healthy older women. J Bone Miner Res. 2007;22:416–424. doi: 10.1359/jbmr.061115. [DOI] [PubMed] [Google Scholar]