Abstract
The survival of cells and organisms requires proper responses to environmental signals. These responses are governed by cellular networks, which serve to process diverse environmental cues. Biological networks often contain recurring network topologies called ‘motifs’. It has been recognized that the study of such motifs allows one to predict the response of a biological network, and thus cellular behavior. However, studying a single motif in complete isolation of all other network motifs in a natural setting is difficult. Synthetic biology has emerged as a powerful approach to understanding the dynamic properties of network motifs. In addition to testing existing theoretical predictions, construction and analysis of synthetic gene circuits has led to the discovery of novel motif dynamics such as how the combination of simple motifs can lead to autonomous dynamics or how noise in transcription and translation can affect the dynamics of a motif. Here, we review developments in synthetic biology as they pertain to increasing our understanding of cellular information processing. We highlight several types of dynamic behaviors that diverse motifs can generate, including the control of input/output responses, the generation of autonomous spatial and temporal dynamics, as well as the influence of noise in motif dynamics and cellular behavior.
Keywords: systems biology, synthetic biology, gene circuit, autonomous regulation, noise
INTRODUCTION
A major goal of biology is to understand how complex interactions in biological networks give rise to specific dynamics and cellular behaviors. Biological networks are composed of recurring network topologies, or ‘motifs’, that can be combined to carry out diverse cellular functions (1, 2).
There have been two common approaches to analyzing the dynamic properties of a network motif (Figure 1a). Mathematical models with well-defined parameter sets are often used. The biological significance of theoretical predictions made by such models, however, is limited if they cannot be validated experimentally, as the parameter space chosen for simulations may not always be biologically feasible. To test modeling predictions about the dynamic properties of a network motif in its natural context, one may perturb the inputs or the molecular components that comprise the motif and examine the consequences of such perturbations. While our ability to perturb a single network motif is increasing, it is often difficult to assess the true contribution of a single network motif or its individual components to the observed dynamics and cellular behavior. When in its natural context, the vast array of networks that operate in concert with the network motif being studied may mask the true contribution of the motif to the observed dynamics.
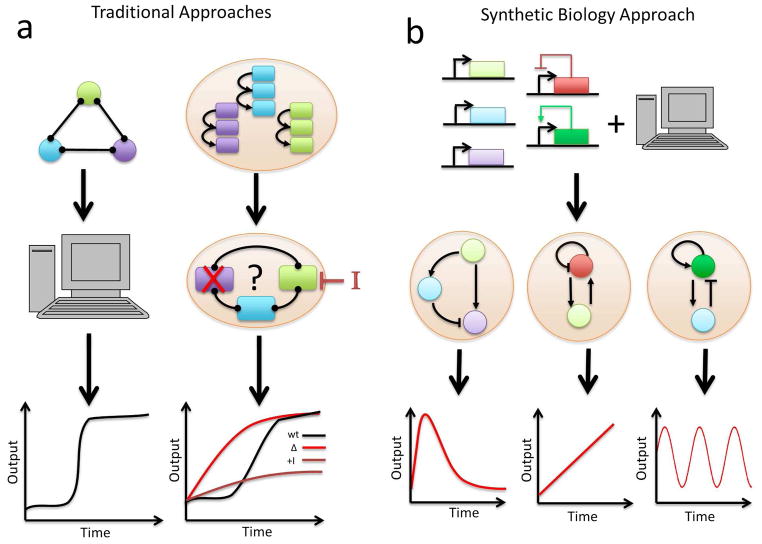
Figure 1.
Computational and experimental approaches to studying biological network motifs. a) Network dynamics can be modeled computationally (left) or in their natural context, the cell (right). Computationally, a model encompassing the key components of the motif is constructed and perturbed using well-defined parameter sets to generate variations in output. Experimentally, network components are perturbed and the output is monitored in order to elucidate the dynamic properties of each component. b) In the synthetic biology approach, components of a genetic network are combined and introduced into the cell de novo, with the design of the network motif and prediction of the behavior often guided by modeling. One can study the dynamics of a motif in relative isolation of other background processes, such as interacting networks that exist in natural systems.
Synthetic biology offers an alternative approach that can overcome these limitations. By creating, perturbing, and quantifying the dynamics of simple gene circuits that encompass a known network motif, one can gain insight into the dynamics generated by the motif (Figure 1b). This approach allows one to examine the dynamics of a motif in relative isolation from other cellular processes, providing an additional level of control. As such, one can draw more definitive conclusions regarding the dynamics of a motif by reducing interference of confounding factors, such as complex network hierarchies and unknown regulatory elements. Furthermore, in contrast to pure modeling analysis, a synthetic system operates within a biologically feasible parameter space, and thus offers additional evidence that the properties observed are biologically relevant.
In this review, we discuss recent efforts in using synthetic gene circuits to study the relationship between motif structure and function. We focus on network motifs that can generate common dynamics, including monotonic and biphasic responses, bistability, adaptation, oscillations, and pattern formation. Furthermore, we review advances in understanding how noise influences the dynamics of a network motif.
Motif control of input/output dynamics
The complexity of network motifs varies from simple one-step modulation to complex autoregulatory pathways involving multiple steps. The degrees to which variations in input can affect the output are motif-dependent. In the simplest case, a network motif will generate a monotonic response (Figure 2a), where the output increases or decreases monotonically with the input. A motif without cooperativity can convert increases in input into a gradual increase in output, leading to a graded response. In contrast, the presence of cooperativity between input functions can cause the dose-response curve to become sigmoidal in shape (3). In some cases, small changes in the input can result in sharp changes in the output, resulting in an ‘ultrasensitive’ response. The range of input values that result in a change in output is often smaller than those of a motif that does not have cooperativity.
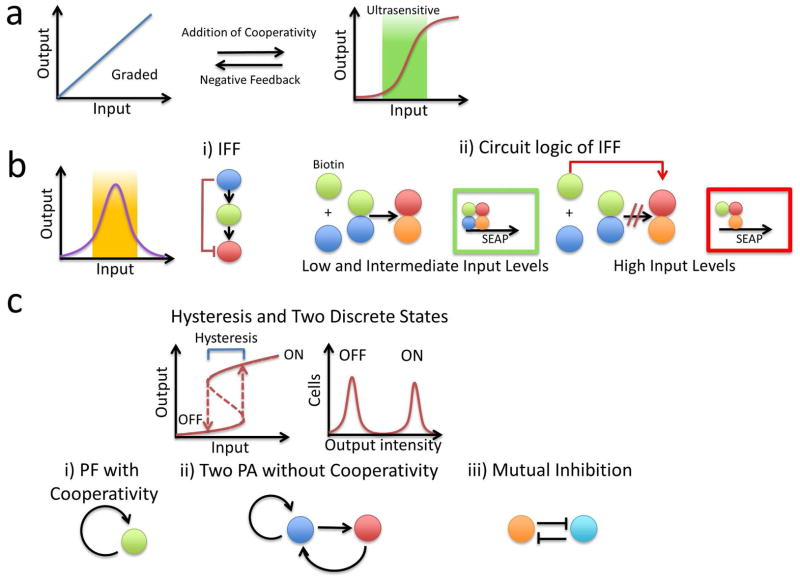
Figure 2.
Network motifs for modulating an input into different output forms. a) A monotonic response can be graded (left) or ultrasensitive (right). A graded response can be converted into an ultrasensitive response (region in green) with the incorporation of cooperativity into the circuit. An ultrasensitive response can be converted into a graded response with the incorporation of NF into the circuit. b) A bisphasic response occurs when an output first increases and then decreases with an increasing input (left panel). i) One motif that generates a biphasic response is an incoherent feedforward (IFF) loop. Here, we show an example of one type of IFF where an input activates a cascade, but also activates a negative regulator of a downstream node in the cascade (red line). ii) Weber et al. constructed an IFF loop, where increases in the input (biotin) lead to increases in the output (SEAP, left panel). However, at a high input level, the circuit becomes inhibited, thus decreasing the output (right panel). In this specific case, the input molecule (biotin) titrates transcriptionally competent complexes preventing expression of the target gene (SEAP). c) Bistability results in two discrete states (e.g., OFF and ON) and hysteresis. A bistable response requires PF with sufficient non-linearity, which can be realized by several motifs. i) A PF loop with cooperativity. ii) Combination of multiple PF loops, each being non-cooperative. iii) Mutual inhibition, which also constitutes a PF, in which two genes inhibit expression of each other.
Cooperativity can arise when the binding of multiple ‘input’ factors, such as proteins, is required to cause an output. The binding of multiple proteins to generate cooperativity is widespread in biological networks and includes the canonical example of the tetracycline inducible promoter (i.e., tet promoter) (4, 5). The tet promoter contains two operator sites that bind the tetracycline repressor (TetR) protein complex (two TetR monomers). Transcription of downstream elements under the regulation of a tet promoter is repressed only when both TetR protein complexes are bound to both sites on the promoter. This repression is removed with an appropriate chemical inducer (e.g., tetracycline or anhydrotetracycline (aTc) (6)), which binds to TetR, allowing downstream gene expression to occur. In this system, the cooperativity arises due to the dimeric nature of the TetR complex and because two of these TetR complexes are required to inhibit transcription. We note that this system has been frequently used to construct synthetic gene circuits, including several examples to be discussed.
While binding of multiple input proteins has long been recognized as a mechanism to generate ultrasensitivity, recent studies using synthetic circuits has extended our understanding of how additional motifs can generate ultrasensitivity. Molecular titration of a molecular species required for activation of transcription has been theoretically postulated to generate ultrasensitivity (7). Consider a motif consisting of an ‘active’ protein, ‘A’, that can be sequestered by a titrating molecule, ‘B’, to form an AB complex. When B is in excess of A, B acts as a sink of A, thus preventing A from driving expression of a gene. However, when the amount of A is equal to that of B, ultrasensitivity can occur, where small increases in the concentration of A lead to large increases in expression of the output gene regulated by A.
Buchler and Cross developed a synthetic circuit that validated these predictions (8). The authors created a circuit consisting of a pair of competing zinc fingers, a heterologous mammalian bZIP (CEBPα) and a dominant negative inhibitor (3HF). Binding between 3HF and CEBPα results in an inactive complex such that 3HF serves to titrate CEBPα. In contrast, binding between two CEBPα proteins results in an active complex, which activates transcription of a yellow fluorescent protein (YFP) reporter. By using promoters of various strengths or by varying the copy number of either gene, the authors could control the amount of CEBPα and 3HF. The authors observed that when 3HF was in excess of CEBPα, the YFP signal was nearly undetectable. However, when CEBPα and 3HF were in nearly equal concentrations, slight increases in CEBPα resulted in large increases in YFP expression, indicating ultrasensitivity. Increasing the level of 3HF expression resulted in an increased concentration of CEBPα required to generate the YFP output. This, in turn, increased the ultrasensitivity of the gene circuit drastically such that Hill coefficients of nearly 12 were observed. Given the ubiquity of protein sequestration in diverse cellular networks, such as those involved in generating oscillations (9) and bistability (10), molecular titration may provide a robust and efficient mechanism to generate tunable ultrasensitivity without the need for higher order protein complexes (7).
Regulatory cascades are ubiquitous in diverse organisms and are implicated in controlling numerous processes. While the ultrasensitivity and noise components of regulatory cascades have been explored theoretically (11), Hooshangi et al. used synthetic circuits to demonstrate that ultrasensitivity can arise with multi-stage transcriptional cascades. The authors created synthetic circuits consisting of transcriptional cascades with one, two, or three stages of repression (12). They observed that as the number of stages increased, the sigmoidal dose-response curves became sharper, indicating increased ultrasensitivity. Temporal analysis of their circuits revealed that increasing the number of stages in the cascade increased the response time of the network. Such dynamics have implications in how noise can affect or be affected by a transcriptional cascade, which will be discussed later. This study offers an additional example of how ultrasensitivity can be achieved without the use of multi-protein complexes and suggests a unique role for transcriptional cascades observed in natural networks, such as those involved in flagellar development in Escherichia coli (13) and sporulation in yeast (14).
Varying the concentrations of the molecular species in a transcriptional cascade can modulate the ultrasensitivity of the input/output function. Mitogen-activated protein kinase (MAPK) signaling pathways are ubiquitous in eukaryotic cells (15). Activation of a MAPK pathway can result in diverse network dynamics even within the same cell type (16). As such, understanding the dynamics of such pathways is of particular interest. One dynamic property that a MAPK signaling pathway can possess is ultrasensitivity (17). To determine the mechanisms by which ultrasensitivity can arise in such pathways, O’Shaughnessy et al. constructed a synthetic MAPK network in yeast consisting of an estradiol-inducible Raf1 protein that activates Mek1, which in turn activates Erk2 (18). The authors observed that, by varying the relative concentrations of Mek1, Erk2, and Raf1, the degree of ultrasensitivity of the cascade could be altered. Specifically, by increasing the concentration of each protein at each step (such that there was a 10-fold difference across the cascade), the degree of ultrasensitivity was nearly doubled. Decreasing the concentration of Mek1, while keeping the concentrations of both Raf1 and Erk2 constant, reduced the observed ultrasensitivity. Using a mathematical model, the authors postulated that the overall ultrasensitivity in the cascade was greater than the multiplicative accumulation of those from individual stages. That is, there is synergy between stages that enhances the overall ultrasensitivity. Furthermore, modeling suggested that varying the amount of each protein in the cascade altered the distribution of intermediate molecular species (e.g., different phosphorylation and complex states), which in turn altered the overall ultrasensitivity. Interestingly, the protein concentrations observed in a natural and highly ultrasensitive MAPK cascade found in Xenopus (19) generated high ultrasensitivity in the authors’ model (18). In contrast, protein concentrations observed in a natural MAPK cascade found in yeast (20), which exhibits low ultrasensitivity, generated low ultrasensitivity in the authors’ model (18). This study suggests that modulation of protein concentrations in a regulatory cascade can be an effective mechanism to tune its response characteristics.
Interestingly, an ultrasensitive dose-response can be converted into a graded response by using negative feedback (NF, Figure 2a). NF occurs when production of a molecular species results in the attenuation of further production of that same species. In 2009, Nevozhay et al. constructed synthetic gene circuits in Saccharomyces cerevisiae to examine the impact of NF on the input/output function of transcription induction (21). In a circuit lacking NF, green fluorescent protein (GFP) is driven by a tet promoter while TetR is constitutively expressed. Induction of this circuit results in an ultrasensitive dose-response curve. In a second circuit containing an NF loop, wherein TetR represses its own transcription and the expression of GFP, a nearly linear dose-response curve is observed. Such dynamics are also observed in natural systems. For example, when Batchelor et al. removed NF from the natural tetracycline resistant determinant from Tn10, the dose-response curve of the network was converted from near-linear to ultrasensitive (22). More recently, NF was shown to linearize an ultrasensitive response in the arabinose metabolism pathway of E. coli (23).
An incoherent feed forward loop can generate a biphasic response
In many biological systems (24), a network motif may respond to an increasing input in a more complex manner. A common property in natural systems is a biphasic response, where the output first increases and then decreases with an increasing input (Figure 2b). This property provides a mechanism for cells to respond maximally to an intermediate range of inputs. A common motif underlying a biphasic response is the incoherent feed-forward (IFF, Figure 2b(i)) loop. An IFF motif has three interactions between nodes where the signs of the indirect and direct pathways are opposite (25).
Weber et al. constructed a gene circuit in mammalian cells that consists of human placental secreted alkaline phosphatase (SEAP) gene under the control of a promoter driven by TetR and VP16 (26). In this circuit, biotin forms a complex with a VP16 transactivator domain peptide, and this complex binds to a TetR protein covalently bound to streptavidin. This complex then activates SEAP production. The authors observed a biphasic output in SEAP activity with increasing biotin concentration: the SEAP activity first increased with increasing biotin concentration until it plateaued, while a further increase in biotin concentration caused a reduction in SEAP activity. The authors proposed that, at sufficiently high biotin concentrations, the TetR-streptavidin proteins became saturated with free biotin, resulting in fewer ‘transcriptionally competent’ biotin-VP16-streptavidin-TetR complexes (Figure 2b(ii)). These results suggest that an inducer (i.e., biotin) can also act as a repressor in a concentration dependent manner – forming the basic logic of a dose-dependent IFF loop. We note that the IFF motif is also implemented in many pattern formation circuits as will be discussed below.
Bistability arises via positive feedback
The network motifs discussed in the previous sections have a common property: the output is monostable. That is, there is only one steady state for a given input level. However, some biological systems may exhibit bistability, such that two distinct steady states can be reached for a system under the same environmental condition (27). The history of a bistable system determines which steady state will be reached for a given input. This property is referred to as ‘cellular memory’ or hysteresis. Consider a bistable system with an output that can be either activated (ON state) or suppressed (OFF state). Switching from the ON state to the OFF state requires a reduction in the input signal beyond the level that is required to cause the OFF to ON transition (27). Bistable properties have been observed in network motifs governing cell cycle regulation (28–31).
A fundamental requirement for generating bistability is a positive feedback (PF) loop with sufficient nonlinearity. PF occurs when a molecular species positively regulates its own production (3). Nonlinearity in PF loops can be generated using cooperativity, which can be realized by multiple motifs (Figure 2c). Synthetic circuits yielding bistability through cooperative PF (Figure 2c(i)) have been constructed in yeast (32) and in mammalian cells (33). In the latter study, a PF circuit was created by co-cistronically expressing SEAP and a fusion protein consisting of the tetracycline-dependent activator (tTA) and a VP16 transactivator domain (33). This circuit is regulated by a hybrid tTA inducible/KRAB repressible promoter. KRAB repression is relieved by the addition of erythromycin (EM), which binds to and removes KRAB from the hybrid promoter. In the presence of EM, the tTA-VP16 fusion protein drives SEAP expression, as well as the expression of additional tTA-VP16, thus creating a PF loop. When cells containing the circuit were grown for three days in the absence of EM (the circuit started in the OFF state), 1000ng/μL of EM was required to turn on SEAP expression. In contrast, when cells containing the circuit were grown for three days in the presence of EM (the circuit started in the ON state), SEAP expression was switched OFF only when the concentration of EM was reduced below 500ng/μL. This synthetic circuit thus demonstrates the hysteretic characteristic of a bistable switch. Interestingly, the authors observed that, to generate hysteresis, the amount of tTA must be tightly controlled. A sufficiently high expression of tTA was observed to abolish hysteresis and resulted in a graded dose-response regardless of history of the cells. The authors suggested that this occurred because the hybrid promoter became biased towards binding only tTA, whereas hysteresis was generated when both tTA and KRAB could effectively compete for the same hybrid promoter. Similar effects have been observed in natural systems. For example, in the E. coli lactose catabolism network, hysteresis is abolished by the presence of mock lactose operators that titrate the lactose repressor away from the natural lactose promoter (34).
The nonlinearity required for generating bistability can also be achieved by coupling multiple non-cooperative PF loops (Figure 2c(ii)). In 2011, Palani et al. constructed a synthetic ligand/receptor complex with two coupled PF loops in yeast, each being non-cooperative (35). The first PF loop consists of the plant cytokinin isopentenyl (IP) adenine responsive receptor, AtCRE, driven by a hybrid promoter that is activated by the yeast transcription factor, SKN7. Binding of IP to the AtCRE receptor results in phosphorylation and activation of SKN7 such that it drives additional expression of the AtCRE receptor. The second PF loop consists of an SKN7-inducible promoter that, upon binding to phosphorylated SKN7, drives expression of additional SKN7. GFP is placed under the regulation of the SKN7 inducible promoter to serve as a reporter. The authors confirmed that both PF loops were non-cooperative when activated separately. When coupled, however, the two PF loops generated bistability such that the dose-response curve displayed a high degree of ultrasensitivity (Hill coefficient of ~20) and hysteresis. When the authors removed the AtCRE PF loop by placing AtCRE under a constitutive promoter, ultrasensitivity, hysteresis, the amount of fluorescence at steady state, and the region of bistability were significantly reduced. Removal of both PF loops resulted in a reduction in ultrasensitivity and the amount of fluorescence at steady state as well as the abolishment of bistability. The authors proposed that coupled PF may serve as a unique platform in which to engineer and modulate synthetic circuits. It is also interesting to speculate that cells may utilize coupled non-cooperative PF to tune their response to external stimuli.
Tan et al. also observed that coupled non-cooperative PF loops can generate bistability (36). In their study, the first PF loop consists of a mutant T7 RNA polymerase that activates its own expression. Activation by the mutant T7 RNAP was shown to be non-cooperative (36), similar to the wild-type T7 RNAP (37, 38). The second PF loop is generated when the metabolic burden associated with circuit activation results in growth retardation, which facilitate accumulation of intracellular molecules (growth serves to dilute these molecules). The coupling of both PF loops was sufficient to generate hysteresis for appropriate system parameters. While this study also serves as additional evidence that coupled non-cooperative PF loops can generate bistability, it also demonstrates that motifs can interact with the host physiology, thus creating unique dynamics.
Bistability can also arise due to mutual inhibition (which in itself constitutes a PF loop) (27), when, for example, two genes (A and B) suppress expression of each other. With appropriate parameters, such a system can generate two stable steady states: one where A is expressed while B is repressed and a second where B is expressed while A is repressed (Figure 2c(iii)). A pioneering synthetic bistable switch constructed in E. coli, termed the toggle switch (39), builds around such a motif. Topologically similar motifs have also been engineered in mammalian cells (40). Such motifs have been identified in many biological processes, including a cell-fate decision network involved in Caenorhabditis elegans development (41).
Increasing complexity in motif design can lead to autonomous dynamics
Integrating different network motifs allows for construction of networks that generate more complex dynamics. To this end, construction of synthetic circuits that yield autonomous dynamics has provided insight into how such composite motifs govern diverse dynamics observed in natural systems such as adaptation, oscillations, and pattern formation.
Cellular adaptation occurs via negative feedback and incoherent feed-forward loops
Adaptation, in the context of network dynamics, refers to a transient response to a sustained input. An increase in the input results in initial excitation of the output followed by its return to the pre-stimulated level, even as the input persists. This results in pulse-like dynamics, in which there is a change in the output followed by a gradual return to the pre-stimulated level. Adaptation is observed in a number of natural biological phenomena such as during bacterial chemotaxis (42) and during the maintenance cellular homeostasis (43).
Barkai and Leibler (44) proposed that NF can robustly generate adaptation. In their theoretical study, the authors suggested that an activator molecule drives expression of an output as well as a second compound that catalyzes the reversible modification of the activator to an inactive state, thus serving as an inhibitor. Maithreye et al. used a synthetic circuit to examine the effect of NF on adaptive dynamics (45). The authors constructed a NF circuit where TetR represses its own transcription via a tet promoter, while GFP acts as the circuit readout. Activation of this circuit resulted in an initial increase in GFP fluorescence (i.e., overshoot), which gradually decayed towards the pre-stimulated state. Interestingly, by introducing a time delay into the NF (the authors increased the distance between TetR and the tet promoter via a DNA spacer), the circuit produced a significantly larger transient overshoot of GFP fluorescence as compared to the circuit with TetR placed next to the promoter. As such, this study indicates that a critical requirement to generate a strong adaptive response by NF is a sufficient time delay (Figure 3a(i)). Interestingly, experimental rewiring of an endogenous adaptive network in Bacillus subtilis has confirmed the role that NF plays in adaptive responses (46). When the NF loop of the B. subtilis competency network was bypassed, adaptive dynamics were compromised.
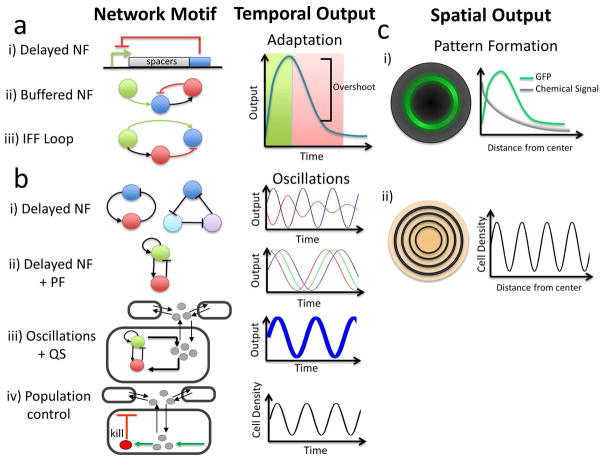
Figure 3.
Network motifs that generate complex autonomously regulated dynamics. a) Adaptation in cellular networks can be generated using two different motifs. i) Increasing the time delay in NF results in a greater overshoot. ii) NF with a buffer node where the delay is created by the output serving as a “buffer” between the input activator and the inhibitor. iii) An IFF loop where fast activation is coupled with delayed downstream inhibition. b) Several motifs can result in oscillatory dynamics. i) Delayed NF results in less robust oscillations. ii) Interlinking PF with delayed NF results in more robust and tunable, yet unsynchronized, oscillations. iii) Coupling cell-to-cell communication with an intracellular oscillator results in synchronized oscillations. iv) Coupling intracellular gene expression with population growth and survival results in a different type of population oscillations, where cell density oscillates with intracellular gene expression. c) Motifs capable of generating spatial pattern formation. i) An IFF loop generates a biphasic response to a chemical signal gradient, which results in a pulse of gene expression (e.g., GFP) in the spatial domain. ii) Oscillations in high and low cell density result in spatially defined waves.
Recently, Ma et al. explored all possible combinations of three-node motifs and determined that one of two minimal motifs are necessary for an adaptive response (47). One motif consists of a NF loop with a “buffer” node. In this motif, the input activates expression of an output, which in turn activates the expression of its inhibitor (thus serving as a “buffer” between the input and inhibitor, Figure 3a(ii)). The second motif consists of an IFF loop, where an input activates expression of a compound as well as an inhibitor of the same compound, resulting in downstream inhibition (Figure 3a(iii)).
Indeed, both of these motifs have been shown to produce adaptation in synthetic circuits. Bashor et al. created a synthetic circuit that consisted of a NF loop with a buffer node (48). Their synthetic circuit consists of a MAPK pathway that artificially recruits proteins to the scaffold protein, Ste5. When the input is sensed (i.e., the alpha mating pheromone), it triggers the sequential phosphorylation of three proteins (Ste11, Ste7 and Fus3) localized on the Ste5 scaffold. Sequential phosphorylation drives the expression of two proteins: GFP, which serves as an output, and Msg5, which serves as an inhibitor of phosphorylated Fus3 and thus creates a NF loop. Indeed, this synthetic circuit generated adaptation in GFP expression. By tuning the degree of NF, the authors observed that weakening the NF resulted in a larger overshoot of GFP, but that the rate at which GFP expression was reduced from the overshoot was significantly slower. As such, this study demonstrates that a NF loop with a buffer node can produce adaptation and that the overshoot and decay of the signal are dependent upon the degree of NF.
IFF loops have also been implemented in synthetic gene circuits to generate adaptation. An early study examining this property was that by Basu et al. who engineered a pulse-generation circuit (49). Here, the circuit input is a quorum-sensing (QS) signal, acyl-homoserine lactone (AHL). AHL complexes with LuxR, an AHL receptor protein, to activate the expression of the GFP output (under control of a LuxPRCI promoter) and a repressor compound, CI (under the control of the LuxPR promoter). CI binds to the LuxPRCI promoter to inhibit expression of GFP. This leads to fast activation of GFP by AHL (when complexed to LuxR) and delayed inhibition of GFP by CI, resulting in pulse-like dynamics in the GFP output. By varying the amount of AHL or the rate of increase of AHL, the overshoot and duration of GFP expression could be modulated. In the latter property, increasing the rate of input led to a higher overshoot of GFP expression with a shorter delay. Using a mathematical model, the authors suggested that when the AHL increase rate was high, GFP and CI were initially expressed at high levels, leading to a large overshoot and a short delay. However, since CI was in abundance, GFP expression was shut down quickly. This study highlights the importance of the input dynamics in adaptive dynamics while also setting the foundation for a later study in pattern formation.
Network motifs underlying sustained, tunable, and synchronized oscillations
Oscillatory dynamics are found in a number of biological networks, including the circadian rhythm of cyanobacteria (50), the Xenopus cell cycle (51), and the NF-κB immune response (52). The earliest synthetic circuit capable of oscillations was made from three transcriptional repressors that formed a closed loop of inhibition, termed the repressilator (53). While the repressilator generated sinusoidal oscillations and represented a tremendous leap forward in the controllability of cellular behavior, the oscillations were noisy and lacked tunability.
Modeling has been used to explore the design principles behind robust and sustained oscillations. It has been predicted that oscillations can arise due to NF with a sufficient time-delay (Figure 3b(i)) (53–56) and that the inclusion of PF can lead to additional robustness and tunability (57, 58). Specifically, Tsai et al. showed that relaxation-type oscillators, those containing NF coupled with PF on the element that activates its inhibitor (Figure 3b(ii)), are able to generate oscillations across a wide range of parameters than those without PF (59).
Stricker et al. engineered a synthetic relaxation-type oscillator that could generate robust and tunable oscillations in E. coli (60). Their circuit consists of an activator (AraC), a repressor (LacI) and a circuit output (GFP) all under the regulation of three separate Plac/ara-1 promoters. Given that the Plac/ara-1 promoter is activated by AraC and repressed by LacI, the circuit consists of a PF loop (AraC activating its own expression) and a NF loop (LacI inhibiting expression of araC). The circuit generated fast, robust, and tunable (by temperature, media composition, or inducer levels) oscillations in up to 99% of cells. When the PF loop was removed, the resulting NF-only circuit continued to generate oscillations, but these oscillations were less robust and significantly less tunable. While this study confirms that a minimal requirement for oscillations is NF, it also demonstrates that the addition of PF adds tunability, regularity, and robustness to the oscillations. We note that a synthetic circuit, implemented in mammalian cells, composed of an interlinked PF loop and a time-delayed NF loop, was also able to generate robust and tunable oscillations (61). Indeed, interlinked PF and NF loops have been shown to underlie oscillatory dynamics in natural networks including in the Xenopus cell cycle (62).
In the aforementioned synthetic oscillators, the oscillations were not synchronized across cells. Several theoretical studies have focused on design strategies to synchronize cellular oscillations. McMillen et al. proposed that coupling a relaxation-type oscillator with intercellular signaling (via a QS module) could generate cell synchronization (Figure 3b(iii)) (63). Furthermore, Garcia-Ojalvo et al. proposed an alternative design where the repressilator was coupled with a QS module to achieve synchronized oscillations (64). A synthetic circuit able to generate synchronized oscillations via a QS module was recently constructed (65). In this circuit, three separate AHL responsive promoters drive the expression of three genes: luxI (an enzyme that synthesizes AHL), aiiA (catalyzes the degradation of AHL), and GFP. The circuit logic is as follows: LuxI synthesizes AHL, which serves to activate the production of additional LuxI, thus forming a PF loop. AHL also drives the expression of aiiA, which serves to degrade AHL and thus acts as a NF loop. By using freely diffusible AHL to regulate the circuit dynamics, AHL globally and simultaneously regulates the circuit dynamics in each cell. As such, synchronized oscillations were observed. Interestingly, by modulating the rate at which AHL was removed from the system (i.e., increasing the flow rate in their microfluidic system) both the period and amplitude of the oscillations could be changed. The achievement of synchronized oscillations opened the door to the recent design of a macroscopic bacterial clock, whose synchronized oscillations across a large population of bacterial cells could be used to sense arsenic via an oscillatory readout (66).
A second, but similar, method to generate synchronized oscillations is through molecular entrainment, where oscillations, produced from a self-sustaining oscillatory network, are modulated by an external signal (67). By entraining the aforementioned synthetic relaxation-type oscillator (60), Mondragon-Palomino et al. were able generate more synchronized oscillations (68). The authors modulated the expression of the synthetic circuit by periodically changing the concentration of the circuit inducer (i.e., arabinose) thus serving as a mechanism of entrainment. Using a mathematical model, the authors predicted regions of entrainment where the oscillations became frequency locked. Interestingly, the authors demonstrated that the range of entrainment, where the ratio between the period of the inducer and the period of the natural oscillatory signal is close to 1, increased with the strength of the PF loop. As entrainment devices are observed in natural systems (67), this study demonstrates that the inclusion of PF in naturally entrained networks may serve to allow single cells to adapt synchronously to complex environments (69).
While density sensing (by QS or other means) has been used to realize cell synchronization, it has also been used to generate population oscillations in a completely different manner, where population growth and survival is directly coupled with intracellular dynamics (Figure 3b(iv)) (70, 71). In particular, using a previously engineered population control circuit (72), Balagadde et al. demonstrated long-term, robust population oscillations that lasted for several hundred hours (73), by using a novel microfluidic device (microchemostat). In this case, by coupling gene expression with cell growth and death, cell density becomes part of the programmed dynamics. As such, this eliminates the need to separately control cell growth, as has to be done in circuits that rely on synchronization of oscillators in individual cells. An important distinction in these aforementioned population oscillators is that cell-to-cell variability is a critical component for the generation of oscillations (e.g., cells should not all die at the same time). In synchronized oscillators, however, cell-to-cell communication primarily acts to reduce cell-to-cell variability.
We note that the progression of oscillators over the past 12 years has aided in illuminating principles underlying natural oscillating motifs and could potentially be of use in better understanding disease states associated with malfunctioning oscillatory systems (74, 75), including the basis of neurological disorders and disease (76).
Synthetic circuits for studying cellular pattern formation
By adapting the circuitry underlying temporal fluctuations to the spatial domain, synthetic circuits that generate patterns can be constructed. Cellular pattern formation occurs via the accumulation of positional information that instructs cells to respond in different ways within a tissue or other spatially defined habitat (77). Pattern formation is observed in numerous biological contexts, including during the development of the Drosophila embryo (78), during formation of feather patterns in birds (79), and in the spatial pattern of somite boundaries in vertebrates (80).
The mechanisms of natural pattern formation can be divided into two classes. In the first class, a chemical signal produced from a defined source forms a concentration gradient that acts on a homogeneous distribution of cells in a concentration-dependent manner (81). Here, the chemical signal acts to specify gene expression changes and cell fate selection (82, 83). In the second class, the system begins with homogenous initial conditions that self-organize upon a symmetry breaking stimulus, achieved by short-range activation coupled with long-range inhibition (84, 85). Indeed, this mechanism of pattern formation is observed in many natural systems (86–89). While questions as to how a continuous gradient of chemical signals is transformed into changes in gene expression remain unanswered (90), a number of synthetic circuits have shed light on such processes.
A synthetic circuit that produces a biphasic response (see above) can create patterns when adapted to the spatial domain (Figure 3c(i)). In 2005, Basu et al. employed this concept to engineering a multicellular system capable of spatial pattern formation in E. coli (91). In this system, ‘sender’ cells produce and release AHL, which serves as the input to an IFF circuit carried by ‘receiver cells’. The circuit output (GFP) was nearly undetectable at low and high AHL concentrations due to two separate repressors that inhibit GFP expression, which act at low and high AHL concentrations, respectively. However, at intermediate AHL concentrations, GFP was strongly expressed. When the receiver cells were plated as a lawn on an agar plate, and a population of sender cells was placed at a single point in the receiver cell lawn, GFP expression from the receiver population produced a ring pattern. Here, AHL produced from the sender population created a gradient in the agar plate, thus leading to differential expression of GFP. The authors showed that by changing the degradation rate of a repressor in the IFF circuit (i.e., LacI), the position and time of emergence of the GFP ring could be modulated. Furthermore, by varying the positioning of the sender cell population(s), the authors created different patterns of GFP expression. As such, this study highlights the importance of dynamics of the individual motif components, as well as spatial positioning of the source providing the chemical signal, in controlling pattern formation. A similar pattern formation network employing an IFF motif to generate a band-pass response in the spatial domain was also constructed in mammalian cells (92). IFFs have been found to be a core motif in pattern formation, including the gap gene network, which is responsible for pattern formation in the Drosophila embryo (93).
Recently, Sohka et al. (94) engineered a synthetic circuit containing an IFF motif capable of pattern formation in response to three chemical signals, ampicillin (Amp), isopropyl-β-D-thio-galactoside (IPTG), and tetracycline. Their circuit consists of an IPTG-inducible β-lactamase (BLA) gene that catalyzes the hydrolysis of Amp. Both the tetracycline resistance gene (tetC) and GFP are controlled by an ampC promoter, which is induced by the cell wall intermediate aM-pentapeptide (aM-PP). Treatment with sub-lethal concentrations of Amp leads to partial cell wall breakdown and accumulation of aM-PP, which can be reduced with the expression of BLA. For a given concentration of Amp and tetracycline, the circuit logic is as follows: at low levels of IPTG, insufficient BLA is produced and thus the cells are killed by Amp. However, at a sufficiently high level of IPTG, insufficient aM-PP accumulates in the cell (i.e., as Amp is hydrolyzed by BLA). As such, the ampC promoter is not triggered, expression of tetC is not activated, and the cells are killed by tetracycline. Only at intermediate concentrations of IPTG, and thus intermediate expression levels of BLA, do the cells grow and express GFP. When grown on an agar plate, cells containing this circuit produced a ring of GFP expression, the position and size of which was modulated by varying the concentrations of IPTG, Amp, and tetracycline in the plates. The authors predict that such a mechanism, where two overlapping gradients form a pattern, may be used in natural systems to form linear patterns.
In an in vitro model of pattern formation, Isalan et al. (95) engineered a synthetic transcriptional network that mimicked Bicoid-mediated activation of gap genes Hunchback, Giant, and Krüppel, which are involved in Drosophila embryonic pattern formation (93). Products from these genes interact to modulate each other’s expression, mostly through repression. The authors created plastic chambers that were filled with a transcription/translation mixture to allow gene expression. In their circuit, each gene in the network is covalently bound to magnetic beads, the positions of which can be modulated within the chamber. The synthetic circuit consists of three transcriptional repressors derived from artificial zinc finger DNA-binding domains, termed A, B, and C (analogous to Hunchback, Giant, and Krüppel, respectively) all of which are activated via a polymerase (either T7 or SP6), which is analogous to Bicoid. In this network, as in the natural network, A represses B, and both A and B repress C, thus creating an IFF loop. To mimic the distribution of transcriptional activity in the Drosophila embryo, both T7 polymerase and beads containing gene A were placed at the poles of the chamber, while the SP6 polymerase and beads containing genes B and C were placed throughout the chamber. This network arrangement generated a pattern in which A was highly expressed at the poles of the chamber, C was highly expressed in the middle, and B was highly expressed between the areas of high A and C expression. By comparing the expression pattern of this network to a network without repression and a network engineered to have mutual repression of all genes, the authors determined that more repression resulted in sharper patterning. This study further demonstrates that an IFF motif could be an underlying network for pattern formation, and that the additional connectivity in the motif may generate more well-defined patterns.
The previously described synthetic pattern formation networks fall into the first class of pattern formation networks. Liu et al. designed a pattern formation network belonging to the second class of pattern formation mechanisms (96). In their circuit, a motility control module is coupled to a QS module. At high cell densities (and thus high AHL concentrations), a complex between LuxR and AHL drives the expression of the CI repressor protein. CI inhibits the expression of the motility control gene, cheZ, resulting in a non-motile phenotype. In contrast, at low cell density, CI is not activated, allowing expression of cheZ and thus a motile phenotype. When cells containing this circuit were inoculated into the center of a semi-solid agar plate, this network resulted in a radial pattern of alternating white (high cell density, non-motile phenotype) and dark (low cell density, motile phenotype) stripes, that appeared as two traveling-waves that moved outward from the initial inoculum (Figure 3c(ii)). Interestingly, by decreasing the maximum cellular motility, via the addition of an aTc inducible copy of the CI repressor, the authors varied the number of stripes observed. While the previous networks adapt an IFF motif to the spatial domain to generate spatial pulses, this network adapts oscillations (between motile and non-motile phenotypes) to the spatial domain to generate spatial waves. Adding to the synthetic pattern formation networks that have been successfully constructed, a recent theoretical study has uncovered a number of other motifs that generate pattern formation, some of which have been confirmed to exist in natural systems (97).
In addition to using diffusible molecules to generate patterns, circuits can be engineered to respond to light, adding another layer of control in pattern formation. In particular, by coupling light-responsive regulatory elements with chemical sensing, Tabor and colleagues have created a synthetic edge detector using E. coli cells (98). The light-responsive component of their circuit consists of a protein modified to contain a photoreceptor domain from blue-green algae, which, in the presence of light, does not induce expression of the ompC promoter. Conversely, in the absence of light, the photoreceptor binds to the ompC promoter and induces expression of luxI and the CI repressor. As an output, lacZ, whose product cleaves a substrate in the medium to form a black pigment, is placed under a promoter that is repressed by CI but activated by AHL (when AHL is present at a sufficiently high local density). As such, bacteria in the dark produce AHL (but cannot respond to it due to inhibition of lacZ via CI), which diffuses across the light/dark interface. Cells in the light, which do not produce AHL or CI, form the pigment (via LacZ) but only when a sufficiently high amount of AHL is present. As such, black pigment is only produced at the dark/light interface, thus creating an edge detector. In contrast to previous pattern forming networks, each cell within the isogenic population responds appropriately to local signals without the need for positional information and global coordination. The authors note that such a system could be used as a model for studying design principles that govern natural edge detection processes, such as image formation by the retina (99).
The role of noise in generating unique network dynamics
Stochastic fluctuations in different cellular components or variations in environmental signals can contribute to cell-to-cell variability in gene expression (or ‘noise’) (100). Even a genetically identical population of cells can exhibit substantial variation in cellular behavior. Such variability has been observed frequently in synthetic circuits, initially leading to questions regarding the controllability and predictability of such circuits. Recent studies have adopted synthetic circuits to further examine the role of noise on network motif dynamics (101–103).
NF, without a significant time delay, has been suggested to reduce variability in gene expression and enhance the ability by a system to resist perturbations (104) (Figure 4a(i)). This notion has been validated using a simple circuit consisting of TetR fused to a GFP (circuit readout), placed under the regulation of a tet promoter (105). As such, the TetR-GFP fusion protein serves to repress its own expression, constituting NF. The authors observed that this circuit reduced variability in gene expression in comparison to a circuit where the NF was disrupted (by mutations to TetR). Interestingly, when the degree of repression was reduced through the addition of aTc (which inhibits the ability of TetR to bind to the tet promoter), the average amount of noise increased with an increasing reduction in NF strength. We note that NF may be involved in reducing noise in QS regulated gene expression in Vibrio harveyi (103).
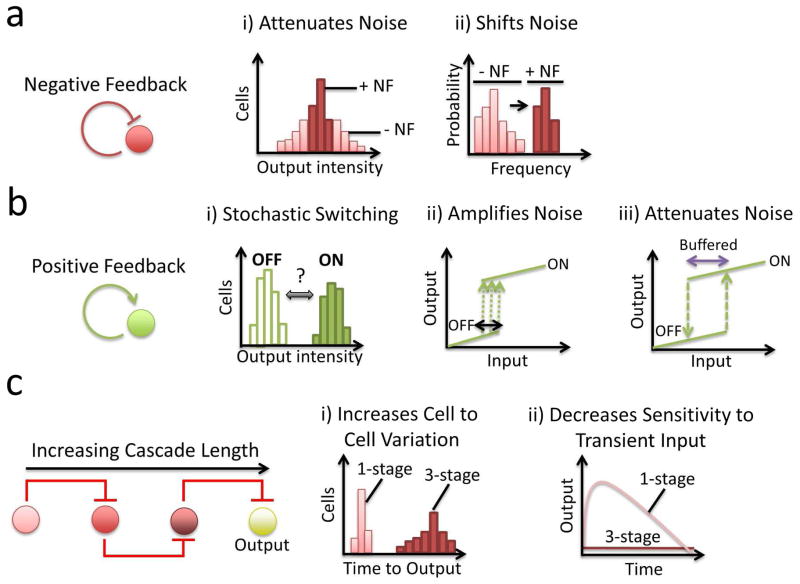
Figure 4.
Motif dynamics can modulate or can be modulated by noise. a) NF can attenuate noise. i) Noise will introduce a large distribution of cells that vary in the degree of output for a given input. In the presence of NF, noise is significantly reduced, thus reducing the size of the distribution. ii) NF accomplishes this by shifting the noise frequency range into higher frequencies, which may be more effectively filtered out by regulatory cascades. b) PF can either attenuate or enhance noise. i) PF can lead to stochastic switching between two distinct outputs. ii) PF can enhance the effect of noise. Small fluctuations (green arrows) near an area of ultrasensitivity (black line) can result in a sharp transition from the OFF to the ON state. iii) PF can attenuate noise if the system contains hysteresis. Here, noise-mediated decreases in the input level do not cause a transition from the ON to the OFF state. As such, the system is buffered against noise (purple arrow). c) Increasing the length of a transcriptional cascade has a dual effect on noise (left panel). i) By increasing the length of the cascade, the cell-to-cell variability in the time to observe an output increases. ii) However, longer cascades can filter out transient increases in input. When the cascade is long (i.e., 3-stage), a transient burst of input is insufficient to activate the output. In contrast, when the cascade is short (i.e., 1-stage), a transient burst of input will result in an output.
In contrast to Becskei and Serrano (105), Dublanche et al. found that noise is best suppressed at intermediate amounts of repression via NF (106). The authors constructed three gene circuits: the first consists of a TetR-GFP fusion protein repressing itself (i.e., regulated by a tet promoter, TG-nf)); the second consists of a tet promoter-regulated TetR that also represses GFP via a second tet promoter (T+G-nf); the third has no NF, where GFP is repressed by a consititutively expressed TetR (T+G). The authors observed that when the circuits contained NF (i.e., TG-nf and T+G-nf), the total noise of the system at all repression levels (i.e., concentrations of aTc) was reduced as compared to when NF was not present (i.e., T+G), thus confirming previous findings (105). Without NF (i.e., T+G), unimodal population distributions were observed at high or low concentrations of aTc (indicating less noise in GFP expression), while a bimodal distribution of GFP was observed at intermediate concentrations of aTc (indicating large variability in GFP expression). Using a mathematical model, the authors hypothesized that cell-to-cell variation in plasmid copy number could explain this bimodal trend observed at intermediate induction levels.
Interestingly, the TG-nf circuit produced the least amount of noise at intermediate aTc concentrations (and therefore intermediate degrees of repression). Again, based on modeling, the authors proposed that, when repression was very tight, slight increases in plasmid copy number allowed transient leaky expression of GFP, as insufficient TetR was produced in the short term to allow effective repression of the newly aquired plasmid copies. In contrast, with a low amount of repression, the NF loop was disrupted due to an insufficient amount of TetR protein that could participate in NF. This resulted in an increased amount of noise in GFP fluorescence. As such, this study reveals a critical quantitative property of NF, the strength of repression required for noise reduction. Since fluctuations in the plasmid copy number are an extrinsic source of noise in the NF circuit, the study serves to validated a previous theoretical prediction (107) that NF can reduce noise caused by extrinsic sources.
In terms of the specific mechanism by which NF reduces noise, theoretical analysis has suggested that NF serves to shift the noise frequency into a higher range, which may be more effectively filtered out by gene networks (108). Using a circuit similar to that by Becskei and Serrano (i.e., TetR represses expression of itself and GFP), Austin et al. demonstrated that NF indeed causes such a shift (Figure 4a(ii)) (109). When the authors compared the noise frequency spectrum of the circuit with NF to a similar circuit not carrying NF, they demonstrated that NF served to shift the low-frequency noise to higher frequencies. Interestingly, the magnitude of frequency shift depended upon the strength of the NF, which could be controlled by varying the growth rate of the cells. At a high growth rate, TetR was diluted through cell division at a high rate. As such, for a given concentration of aTc, most of the TetR in each cell was bound to aTc, thus weakening the NF. At a low growth rate, abundant TetR was present in each cell, but the repression curve became saturated, lowering the overall strength of the NF. An intermediate growth rate led to an intermediate strength of NF, which resulted in the highest degree of noise frequency shift. Here, sufficient TetR dimer existed to allow NF, but the abundance of TetR was insufficient to saturate the repression curve. As such, in addition to confirming that NF shifts the noise frequency, the study also demonstrates how a non-intuitive factor (i.e., cell division) can influence the ability of NF to shift the noise frequency.
PF can either amplify or attenuate the effects of noise, depending on the input parameters (Figure 4b). Theoretical analysis (110) has suggested that the transition between bistable states, mediated through PF, could be induced by noise. Small fluctuations in transcription could be amplified through PF, thus triggering the switching between two states. In 2001, Becskei et al. constructed a simple PF circuit in yeast using the doxycycline-inducible rtTA transcription factor fused to a GFP (32). Expression of this construct is controlled via an rtTA-inducible promoter, thus allowing rtTA to activate its own expression and form a PF loop. At an intermediate level of induction via doxycycline, the authors observed that the majority of yeast colonies initiated from a single OFF cell grew as a mixture of OFF (i.e., no GFP fluorescence) and ON (i.e., GFP fluorescence) cells. Using a mathematical model, the authors suggested that the transition from the OFF to the ON state could be stochastic due to noise in transcription. Interestingly, the authors did not observe the transition from ON to OFF. This could suggest that the circuit might have a hysteretic component, which would serve to reduce the amount of noise in GFP expression. In this scenario, once the cells are in the ON state, the transition to the OFF state is more difficult. Noise-induced transitions through a PF motif have been observed in natural systems, including competency in B. subtillis (111–113) and lactose metabolism in E. coli (114).
Recent work has suggested that noise can induce bimodality via a PF motif that does not contain cooperativity. To and Maheshri (115) constructed two non-cooperative circuits in S. cerevisiae consisting of tTA that binds to and activate its own expression via a tet promoter. This circuit is regulated by two variants of the tet promoter: one consists of a single tTA-binding site; the other consists of seven tangent tTA-binding sites. YFP under the regulation of a separate tet promoter serves as the circuit readout. When tested experimentally, the circuit containing one tTA-binding site produced a unimodal distribution of YFP, the intensity of which increased with increasing PF strength. In contrast, the circuit with seven tTA binding sites produced a bimodal distribution of YFP. The authors demonstrated that the bimodality of the circuit with seven tTA binding sites resulted from stochastic expression of tTA that occurs at a low burst frequency (i.e., the number of transcriptional events during the lifetime of an mRNA or a protein (116)) and a large burst size (i.e., the quantity of mRNA or protein produced per transcriptional event). When this occurred, a sufficiently high burst size was able to drive the PF motif into the high expression state. However because the half-life of tTA was short, tTA degraded before the next burst, and thus allowed the cells to enter the low expression state. These observations thus verified previous theoretical predictions (117, 118) that appropriate levels of burst frequency and size can lead to bimodality in a non-cooperative PF motif. We note that a non-cooperative PF involved in HIV escape from latency has been observed to produce a transient bimodal distribution (119).
While transcriptional cascades can increase the ultrasensitivity of a network (see ‘Input/output dynamics’), they can also affect the amount of noise in a network (Figure 4c) (12). Hooshangi et al. demonstrated that as the number of stages of a transcriptional cascade increased, the variability in the output at intermediate inputs increased (12). Furthermore, and as mentioned previously, an increase in the number of stages in the cascade resulted in an increase in the amount of time required for the cascade to become activated. This unique feature also had a direct effect on noise; the increased time delay of the cascades with more stages also caused a transient increase in the cell-to-cell variability in the time that was required for the circuit to be become activated (Figure 4c(i)). By modeling, the authors suggested that noise from each repression step in the cascade was amplified as the number of stages in the cascade increased, leading to increased cell-to-cell variability in longer cascades.
From another perspective, theoretical analysis has suggested that longer cascades could also effectively filter out noise in the input (2). Transient fluctuations in components that activate a pathway would be more easily filtered out in a long cascade, as they would fail to completely activate each step in the cascade. In essence, a long cascade can act as a low-pass filter. Hooshangi et al. observed that when circuits consisting of one or two transcriptional stages were induced with a short pulse (i.e., 5 minutes) of aTc, both circuits were activated (Figure 4c(ii)) (12). In contrast, when the three-stage circuit was pulsed for the same amount of time, it was not activated. A longer pulse (i.e., 45 minutes) was sufficient to activate the three-stage circuit, but the time required to observe circuit activation was longer, and the circuit output was less intense, as compared to activation of the one- or two-stage circuits. As such, while longer cascades amplify output noise at intermediate levels of network induction, they also attenuate input noise by filtering out short-lived input signals.
CONCLUDING REMARKS
By constructing and perturbing synthetic circuits, synthetic biologists have gained insight into the dynamic properties of diverse network motifs that underlie cellular functions. These engineered motifs have evolved from relatively simple topologies consisting of one-step autoregulation to interlinked topologies consisting of multiple motifs to form more complex, autonomous dynamics. The next level of complexity is to integrate populations of different cellular networks into multicellular systems (120). Indeed, a number of synthetic multicellular systems have been constructed (e.g., (73), and design of such systems is ongoing. Along the same lines of using synthetic gene circuits to address questions in systems biology, integrated multicellular networks could potentially be used as models to address questions in ecology and evolution (121).
KEY CONCEPTS.
Motif
A recurring network topology that is often combined with additional motifs to form a network. In using a synthetic biology approach to studying cellular information processing, one will often focus on the study of a single motif, using a synthetic gene circuit, and how it contributes to the behavior of an entire network.
Negative feedback
When a molecular species negatively regulates its own expression, either by acting as a repressor of its own gene expression or by activating expression of a downstream compound that represses its expression. That is, production of a molecular species results in the attenuation of further production of that same species.
Positive feedback
When a molecular species positively regulates its own production. That is, the production of a molecular species will lead to additional production of the same species.
Ultrasensitivity
In contrast to a graded response, which can often be described using a Michaelis-Menten type equation, an ultrasensitive response occurs when small changes in the input can lead to large changes in the output. Often, a Hill equation is used to describe an ultrasensitive response.
Noise
Cell-to-cell variability in gene expression due to stochastic fluctuations in cellular components (such as polymerases, ribosomes, nucleotides, etc.) or variations in environmental signals.
Incoherent feed-forward loop
Where a compound serves as both an activator and a repressor of a downstream target. Often, activation and repression events occur on different time scale, and outputs may be time- or dose-dependent.
Acknowledgments
FUNDING SOURCES
We are supported by the National Institutes of Health (1P50GM081883, 1R01-CA118486, and 1R01-GM098642), a DuPont Young Professorship, a National Science Foundation CAREER award, and a David and Lucile Packard Fellowship.
Footnotes
CONFLICT OF INTEREST
The authors wish to declare that they do not have any conflicts of interest
References
- 1.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 2.Shen-Orr SS, Milo R, Mangan S, Alon U. Network motifs in the transcriptional regulation network of Escherichia coli. Nature genetics. 2002;31:64–68. doi: 10.1038/ng881. [DOI] [PubMed] [Google Scholar]
- 3.Alon U. An introduction to systems biology: Design principles of biological circuits. CRC Press, Taylor and Fracis Group; London, UK: 2007. [Google Scholar]
- 4.Meier I, Wray L, Hillen W. Differential regulation of the Tn10-encoded tetracycline resistance genes tetA and tetR by the tandem tet operators O1 and O2. EMBO J. 1988;7:567–572. doi: 10.1002/j.1460-2075.1988.tb02846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillen W, Schollmeier K, Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. Journal of Molecular Biology. 1984;172:185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- 6.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the tetR/O and araC/I1-I2 regulatory elements. Nucleic Acids Research. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchler NE, Louis M. Molecular Titration and Ultrasensitivity in Regulatory Networks. Journal of Molecular Biology. 2008;384:1106–1119. doi: 10.1016/j.jmb.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 8.Buchler NE, Cross FR. Protein sequestration generates a flexible ultrasensitive response in a genetic network. Molecular Systems Biology. 2009;5 doi: 10.1038/msb.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nature Review Genetics. 2001;2:702–715. doi: 10.1038/35088576. [DOI] [PubMed] [Google Scholar]
- 10.Dubnau D, Losick R. Bistability in bacteria. Molecular Microbiology. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- 11.Thattai M, van Oudenaarden A. Attenuation of noise in ultrasensitive signaling cascades. Biophysical journal. 2002;82:2943–2950. doi: 10.1016/S0006-3495(02)75635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooshangi S, Thiberge S, Weiss R. Ultrasensitivity and noise propagation in a synthetic transcriptional cascade. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3581–3586. doi: 10.1073/pnas.0408507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalir S, McClure J, Pabbaraju K, Southward C, Ronen M, Leibler S, Surette MG, Alon U. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science. 2001;292:2080–2083. doi: 10.1126/science.1058758. [DOI] [PubMed] [Google Scholar]
- 14.Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. The transcriptional program of sporulation in budding yeast. Science. 1998;282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 15.Avruch J. MAP kinase pathways: The first twenty years. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:1150–1160. doi: 10.1016/j.bbamcr.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 17.Huang CY, Ferrell JE. Ultrasensitivity in the mitogen-activated protein kinase cascade. Proceedings of the National Academy of Sciences. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Shaughnessy EC, Palani S, Collins JJ, Sarkar CA. Tunable signal processing in synthetic MAP kinase cascades. Cell. 2011;144:119–131. doi: 10.1016/j.cell.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrell JE., Jr Tripping the switch fantastic: how a protein kinase cascade can convert graded inputs into switch-like outputs. Trends in Biochemical Sciences. 1996;21:460–466. doi: 10.1016/s0968-0004(96)20026-x. [DOI] [PubMed] [Google Scholar]
- 20.Ghaemmaghami S, Huh W-K, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 21.Nevozhay D, Adams RM, Murphy KF, Josic K, Balazsi G. Negative autoregulation linearizes the dose-response and suppresses the heterogeneity of gene expression. Proceedings of the National Academy of Sciences. 2009;106:5123–5128. doi: 10.1073/pnas.0809901106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batchelor E, Silhavy TJ, Goulian M. Continuous control in bacterial regulatory circuits. Journal of Bacteriology. 2004;186:7618–7625. doi: 10.1128/JB.186.22.7618-7625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madar D, Dekel E, Bren A, Alon U. Negative auto-regulation increases the input dynamic-range of the arabinose system of Escherichia coli. BMC Systems Biology. 2011;5:111. doi: 10.1186/1752-0509-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong JV, Yao G, Nevins JR, You L. Viral-mediated noisy gene expression reveals biphasic E2f1 response to MYC. Molecular cell. 2011;41:275–285. doi: 10.1016/j.molcel.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proceedings of the National Academy of Sciences. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber W, Stelling, Rimann M, Keller B, Daoud-El Baba M, Weber CC, Aubel D, Fussenegger M. A synthetic time-delay circuit in mammalian cells and mice. Proceedings of the National Academy of Sciences. 2007;104:2643–2648. doi: 10.1073/pnas.0606398104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrell JE. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Current opinion in cell biology. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 28.Xiong W, Ferrell JE. A positive-feedback-based bistable ‘memory module’ that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 29.Sha W, Moore J, Chen K, Lassaletta AD, Yi C-S, Tyson JJ, Sible JC. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proceedings of the National Academy of Sciences. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nature Cell Biology. 2008;10:476–482. doi: 10.1038/ncb1711. [DOI] [PubMed] [Google Scholar]
- 31.Pomerening JR, Sontag ED, Ferrell JE. Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nature Cell Biology. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 32.Becskei A, Seraphin B, Serrano L. Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. EMBO J. 2001;20:2528–2535. doi: 10.1093/emboj/20.10.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer BP, Fussenegger M. Hysteresis in a synthetic mammalian gene network. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9517–9522. doi: 10.1073/pnas.0500345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozbudak EM, Thattai M, Lim HN, Shraiman BI, van Oudenaarden A. Multistability in the lactose utilization network of Escherichia coli. Nature. 2004;427:737–740. doi: 10.1038/nature02298. [DOI] [PubMed] [Google Scholar]
- 35.Palani S, Sarkar CA. Synthetic conversion of a graded receptor signal into a tunable, reversible switch. Molecular Systems Biology. 2011;7 doi: 10.1038/msb.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan C, Marguet P, You L. Emergent bistability by a growth-modulating positive feedback circuit. Nature Chemical Biology. 2009;5:842–848. doi: 10.1038/nchembio.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin CT, Coleman JE. Kinetic analysis of T7 RNA polymerase-promoter interactions with small synthetic promoters. Biochemistry. 1987;26:2690–2696. doi: 10.1021/bi00384a006. [DOI] [PubMed] [Google Scholar]
- 38.Jia Y, Kumar A, Patel SS. Equilibrium and stopped-flow kinetic studies of interaction between T7 RNA polymerase and its promoters measured by protein and 2-aminopurine fluorescence changes. Journal of Biological Chemistry. 1996;271:30451–30458. doi: 10.1074/jbc.271.48.30451. [DOI] [PubMed] [Google Scholar]
- 39.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–342. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 40.Kramer BP, Viretta AU, Baba MD-E, Aubel D, Weber W, Fussenegger M. An engineered epigenetic transgene switch in mammalian cells. Nature Biotechnology. 2004;22:867–870. doi: 10.1038/nbt980. [DOI] [PubMed] [Google Scholar]
- 41.Johnston RJ, Chang S, Etchberger JF, Ortiz CO, Hobert O. MicroRNAs acting in a double-negative feedback loop to control a neuronal cell fate decision. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12449–12454. doi: 10.1073/pnas.0505530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg HC, Tedesco PM. Transient response to chemotactic stimuli in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Samad H, Goff JP, Khammash M. Calcium homeostasis and parturient hypocalcemia: an integral feedback perspective. Journal of Theoretical Biology. 2002;214:17–29. doi: 10.1006/jtbi.2001.2422. [DOI] [PubMed] [Google Scholar]
- 44.Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–917. doi: 10.1038/43199. [DOI] [PubMed] [Google Scholar]
- 45.Maithreye R, Sarkar RR, Parnaik VK, Sinha S. Delay-induced transient increase and heterogeneity in gene expression in negatively auto-regulated gene circuits. PLoS One. 2008;3:e2972. doi: 10.1371/journal.pone.0002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suel GM, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 47.Ma WZ, Trusina A, El-Samad H, Lim WA, Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009;138:760–773. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bashor CJ, Helman NC, Yan S, Lim WA. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science. 2008;319:1539–1542. doi: 10.1126/science.1151153. [DOI] [PubMed] [Google Scholar]
- 49.Basu S, Mehreja R, Thiberge S, Chen MT, Weiss R. Spatiotemporal control of gene expression with pulse-generating networks. Proceedings of the National Academy of Sciences. 2004;101:6355–6360. doi: 10.1073/pnas.0307571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakahira Y, Katayama M, Miyashita H, Kutsuna S, Iwasaki H, Oyama T, Kondo T. Global gene repression by KaiC as a master process of prokaryotic circadian system. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:881–885. doi: 10.1073/pnas.0307411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, Edwards SW, McDowell HP, Unitt JF, Sullivan E, Grimley R, Benson N, Broomhead D, Kell DB, White MR. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 53.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000;403:335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 54.Bratsun D, Volfson D, Tsimring LS, Hasty J. Delay-induced stochastic oscillations in gene regulation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14593–14598. doi: 10.1073/pnas.0503858102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishna S, Jensen MH, Sneppen K. Minimal model of spiky oscillations in NF-kappaB signaling. Proceedings of the National Academy of Sciences. 2006;103:10840–10845. doi: 10.1073/pnas.0604085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rateitschak K, Wolkenhauer O. Intracellular delay limits cyclic changes in gene expression. Mathematical Biosciencesi. 2007;205:163–179. doi: 10.1016/j.mbs.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Hasty J, Dolnik M, Rottschafer V, Collins JJ. Synthetic gene network for entraining and amplifying cellular oscillations. Physical Review Letters. 2002;88:148101. doi: 10.1103/PhysRevLett.88.148101. [DOI] [PubMed] [Google Scholar]
- 58.Barkai N, Leibler S. Biological rhythms - Circadian clocks limited by noise. Nature. 2000;403:267–268. doi: 10.1038/35002258. [DOI] [PubMed] [Google Scholar]
- 59.Tsai TY, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE., Jr Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–129. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stricker J, Cookson S, Bennett MR, Mather WH, Tsimring LS, Hasty J. A fast, robust and tunable synthetic gene oscillator. Nature. 2008;456:516–519. doi: 10.1038/nature07389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M. A tunable synthetic mammalian oscillator. Nature. 2009;457:309–312. doi: 10.1038/nature07616. [DOI] [PubMed] [Google Scholar]
- 62.Pomerening JR, Kim SY, Ferrell JE., Jr Systems-level dissection of the cell-cycle oscillator: bypassing positive feedback produces damped oscillations. Cell. 2005;122:565–578. doi: 10.1016/j.cell.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 63.McMillen D, Kopell N, Hasty J, Collins JJ. Synchronizing genetic relaxation oscillators by intercell signaling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:679–684. doi: 10.1073/pnas.022642299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Ojalvo J, Elowitz MB, Strogatz SH. Modeling a synthetic multicellular clock: repressilators coupled by quorum sensing. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10955–10960. doi: 10.1073/pnas.0307095101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danino T, Mondragon-Palomino O, Tsimring L, Hasty J. A synchronized quorum of genetic clocks. Nature. 2010;463:326–330. doi: 10.1038/nature08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prindle A, Samayoa P, Razinkov I, Danino T, Tsimring LS, Hasty J. A sensing array of radically coupled genetic ‘biopixels’. Nature. 2012;481:39–44. doi: 10.1038/nature10722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature Review Genetics. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mondragon-Palomino O, Danino T, Selimkhanov J, Tsimring L, Hasty J. Entrainment of a population of synthetic genetic oscillators. Science. 2011;333:1315–1319. doi: 10.1126/science.1205369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rand DA, Shulgin BV, Salazar D, Millar AJ. Design principles underlying circadian clocks. Journal of The Royal Society Interface. 2004;1:119–130. doi: 10.1098/rsif.2004.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balagadde FK, You L, Hansen CL, Arnold FH, Quake SR. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science. 2005;309:137–140. doi: 10.1126/science.1109173. [DOI] [PubMed] [Google Scholar]
- 71.Marguet P, Tanouchi Y, Spitz E, Smith C, You L. Oscillations by minimal bacterial suicide circuits reveal hidden facets of host-circuit physiology. PloS one. 2010;5:e11909. doi: 10.1371/journal.pone.0011909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.You L, Cox RS, 3rd, Weiss R, Arnold FH. Programmed population control by cell-cell communication and regulated killing. Nature. 2004;428:868–871. doi: 10.1038/nature02491. [DOI] [PubMed] [Google Scholar]
- 73.Balagadde FK, Song H, Ozaki J, Collins CH, Barnet M, Arnold FH, Quake SR, You L. A synthetic Escherichia coli predator-prey ecosystem. Molecular Systems Biology. 2008;4 doi: 10.1038/msb.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu YH, Fischer DF, Kalsbeek A, Garidou-Boof ML, van der Vliet J, van Heijningen C, Liu RY, Zhou JN, Swaab DF. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the “master clock”. Faseb J. 2006;20:1874–1876. doi: 10.1096/fj.05-4446fje. [DOI] [PubMed] [Google Scholar]
- 75.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fussenegger M. Synthetic biology: Synchronized bacterial clocks. Nature. 2010;463:301–302. doi: 10.1038/463301a. [DOI] [PubMed] [Google Scholar]
- 77.Wolpert L. One hundred years of positional information. Trends in genetics : TIG. 1996;12:359–364. doi: 10.1016/s0168-9525(96)80019-9. [DOI] [PubMed] [Google Scholar]
- 78.Simpson-Brose M, Tresiman J, Desplan C. Synergy between the hunchback and bicoid morphogens is required for anterior patterning in Drosophila. Cell. 1994;78:855–865. doi: 10.1016/s0092-8674(94)90622-x. [DOI] [PubMed] [Google Scholar]
- 79.Noramly S, Morgan B. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 80.Pourquie O. The segmentation clock: Converting embryonic time into spatial pattern. Science. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- 81.Lewis J, Slack JM, Wolpert L. Thresholds in development. Journal of Theoretical Biology. 1977;65:579–590. doi: 10.1016/0022-5193(77)90216-8. [DOI] [PubMed] [Google Scholar]
- 82.Driever W, Nusslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988;54:83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 83.Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. doi: 10.1016/0092-8674(88)90183-3. [DOI] [PubMed] [Google Scholar]
- 84.Turing AM. The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 1952;237:37–72. doi: 10.1098/rstb.2014.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- 86.Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314:1447–1450. doi: 10.1126/science.1130088. [DOI] [PubMed] [Google Scholar]
- 87.Nakamasu A, Takahashi G, Kanbe A, Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamasu A, Takahashi G, Kanbe A, Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proceedings of the National Academy of Sciences. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133:385–394. doi: 10.1242/dev.02238. [DOI] [PubMed] [Google Scholar]
- 91.Basu S, Gerchman Y, Collins CH, Arnold FH, Weiss R. A synthetic multicellular system for programmed pattern formation. Nature. 2005;434:1130–1134. doi: 10.1038/nature03461. [DOI] [PubMed] [Google Scholar]
- 92.Greber D, Fussenegger M. An engineered mammalian band-pass network. Nucleic Acids Research. 2010;38:e174. doi: 10.1093/nar/gkq671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivera-Pomar R, Jackle H. From gradients to stripes in Drosophila embryogenesis: filling in the gaps. Trends in genetics : TIG. 1996;12:478–483. doi: 10.1016/0168-9525(96)10044-5. [DOI] [PubMed] [Google Scholar]
- 94.Sohka T, Heins R, Phelan R, Greisler J, Townsend C, Ostermeier M. An externally tunable bacterial band-pass filter. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10135–10140. doi: 10.1073/pnas.0901246106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Isalan M, Lemerle C, Serrano L. Engineering gene networks to emulate Drosophila embryonic pattern formation. PLoS Biology. 2005;3:e64. doi: 10.1371/journal.pbio.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu C, Fu X, Liu L, Ren X, Chau CK, Li S, Xiang L, Zeng H, Chen G, Tang LH, Lenz P, Cui X, Huang W, Hwa T, Huang JD. Sequential establishment of stripe patterns in an expanding cell population. Science. 2011;334:238–241. doi: 10.1126/science.1209042. [DOI] [PubMed] [Google Scholar]
- 97.Cotterell J, Sharpe J. An atlas of gene regulatory networks reveals multiple three-gene mechanisms for interpreting morphogen gradients. Molecular Systems Biology. 2010;6:425. doi: 10.1038/msb.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tabor JJ, Salis HM, Simpson ZB, Chevalier AA, Levskaya A, Marcotte EM, Voigt CA, Ellington AD. A synthetic genetic edge detection program. Cell. 2009;137:1272–1281. doi: 10.1016/j.cell.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maturana HR, Frenk S. Directional movement and horizontal edge detectors in the pigeon retina. Science. 1963;142:977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- 100.Swain PS, Elowitz MB, Siggia ED. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proceedings of the National Academy of Sciences. 2002;99:12795–12800. doi: 10.1073/pnas.162041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raj A, van Oudenaarden A. Nature, Nurture, or Chance: Stochastic Gene Expression and Its Consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balazsi Gb, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–925. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Savageau MA. Comparison of classical and autogenous systems of regulation in inducible operons. Nature. 1974;252:546–549. doi: 10.1038/252546a0. [DOI] [PubMed] [Google Scholar]
- 105.Becskei A, Serrano L. Engineering stability in gene networks by autoregulation. Nature. 2000;405:590–593. doi: 10.1038/35014651. [DOI] [PubMed] [Google Scholar]
- 106.Dublanche Y, Michalodimitrakis K, Kummerer N, Foglierini M, Serrano L. Noise in transcription negative feedback loops: simulation and experimental analysis. Molecular Systems Biology. 2006;2 doi: 10.1038/msb4100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- 108.Simpson ML, Cox CD, Sayler GS. Frequency domain analysis of noise in autoregulated gene circuits. Proceedings of the National Academy of Sciences. 2003;100:4551–4556. doi: 10.1073/pnas.0736140100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Austin DW, Allen MS, McCollum JM, Dar RD, Wilgus JR, Sayler GS, Samatova NF, Cox CD, Simpson ML. Gene network shaping of inherent noise spectra. Nature. 2006;439:608–611. doi: 10.1038/nature04194. [DOI] [PubMed] [Google Scholar]
- 110.Hasty J, Pradines J, Dolnik M, Collins JJ. Noise-based switches and amplifiers for gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2075–2080. doi: 10.1073/pnas.040411297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suel G, Garcia-Ojalvo J, Liberman LM, Elowitz MB. An excitable gene regulatory circuit induces transient cellular differentiation. Nature. 2006;440:545–550. doi: 10.1038/nature04588. [DOI] [PubMed] [Google Scholar]
- 112.Suel GM, Kulkarni RP, Dworkin J, Garcia-Ojalvo J, Elowitz MB. Tunability and Noise Dependence in Differentiation Dynamics. Science. 2007;315:1716–1719. doi: 10.1126/science.1137455. [DOI] [PubMed] [Google Scholar]
- 113.Maamar Hd, Raj A, Dubnau D. Noise in Gene Expression Determines Cell Fate in Bacillus subtilis. Science. 2007;317:526–529. doi: 10.1126/science.1140818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Choi PJ, Cai L, Frieda K, Xie XS. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science. 2008;322:442–446. doi: 10.1126/science.1161427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.To T-L, Maheshri N. Noise can induce bimodality in positive transcriptional feedback loops without bistability. Science. 2010;327:1142–1145. doi: 10.1126/science.1178962. [DOI] [PubMed] [Google Scholar]
- 116.Maheshri N, O’Shea EK. Living with noisy genes: how cells function reliably with inherent variability in gene expression. Annual Review of Biophysics and Biomolecular Structure. 2007;36:413–434. doi: 10.1146/annurev.biophys.36.040306.132705. [DOI] [PubMed] [Google Scholar]
- 117.Friedman N, Cai L, Xie XS. Linking Stochastic Dynamics to Population Distribution: An Analytical Framework of Gene Expression. Physical Review Letters. 2006;97:168302. doi: 10.1103/PhysRevLett.97.168302. [DOI] [PubMed] [Google Scholar]
- 118.Karmakar R, Bose I. Positive feedback, stochasticity and genetic competence. Physical Biology. 2007;4:29. doi: 10.1088/1478-3975/4/1/004. [DOI] [PubMed] [Google Scholar]
- 119.Weinberger LS, Shenk T. An HIV feedback resistor: auto-regulatory circuit deactivator and noise buffer. PLoS Biology. 2006;5:e9. doi: 10.1371/journal.pbio.0050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith R. Design principles and applications of engineered microbial consortia. Acta Horticulturae. 2011;905:63–69. [Google Scholar]
- 121.Tanouchi Y, Smith RP, You L. Engineering microbial systems to explore ecological and evolutionary dynamics. Current Opinion in Biotechnology. 2012;23:1–7. doi: 10.1016/j.copbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]