Abstract
24-norursodeoxycholic acid (norUDCA), a side chain–modified ursodeoxycholic acid derivative, has dramatic therapeutic effects in experimental cholestasis and may be a promising agent for the treatment of cholestatic liver diseases. We aimed to better understand the physiologic and therapeutic properties of norUDCA and to test if they are related to its side chain length and/or relative resistance to amidation. For this purpose, Mdr2−/− mice, a model for sclerosing cholangitis, received either a standard diet or a norUDCA-, tauronorursodeoxycholic acid (tauronorUDCA)-, or dinorursodeoxycholic acid (dinorUDCA)-enriched diet. Bile composition, serum biochemistry, liver histology, fibrosis, and expression of key detoxification and transport systems were investigated. Direct choleretic effects were addressed in isolated bile duct units. The role of Cftr for norUDCA-induced choleresis was explored in Cftr−/− mice. norUDCA had pharmacologic features that were not shared by its derivatives, including the increase in hepatic and serum bile acid levels and a strong stimulation of biliary HCO3−-output. norUDCA directly stimulated fluid secretion in isolated bile duct units in a HCO3 −-dependent fashion to a higher extent than the other bile acids. Notably, the norUDCA significantly stimulated HCO3 −-output also in Cftr−/− mice. In Mdr2−/− mice, cholangitis and fibrosis strongly improved with norUDCA, remained unchanged with tauro-norUDCA, and worsened with dinorUDCA. Expression of Mrp4, Cyp2b10, and Sult2a1 was increased by norUDCA and dinorUDCA, but was unaffected by tauro-norUDCA. Conclusion: The relative resistance of norUDCA to amidation may explain its unique physiologic and pharmacologic properties. These include the ability to undergo cholehepatic shunting and to directly stimulate cholangiocyte secretion, both resulting in a HCO3−-rich hypercholeresis that protects the liver from cholestatic injury.
Due to the limited efficacy of current medical treatment of chronic diseases of the biliary tree, such as sclerosing cholangitis,1 there is an urgent need to develop novel treatment strategies and to test them in animal models for human cholangiopathies.2 Mice lacking the canalicular phosphatidylcholine floppase Mdr2 (Abcb4) (Mdr2−/− mice) develop chronic cholestatic liver disease including sclerosing cholangitis and biliary fibrosis.3,4 These unique characteristics of Mdr2−/− mice are of particular interest because increasing evidence suggests a key role of MDR3 (ABCB4, the human orthologue of rodent Mdr2) defects in human cholestatic liver diseases ranging from neonatal cholestasis to biliary cirrhosis in adults.5 More recently, MDR3 variants have been observed in patients with primary sclerosing cholangitis6 and idiopathic biliary fibrosis,7 highlighting the importance of MDR3 as a modifier gene in disease course and severity of cholangiopathies.
We have recently demonstrated that cholangitis and biliary fibrosis in Mdr2−/− mice are reversed by 24-norursodeoxycholic acid (norUDCA), a C23 homologue of ursodeoxycholic acid (UDCA) with one less methyl group in its side chain,8 suggesting that side chain–modified bile acids may represent a novel and promising treatment strategy for cholestatic liver diseases. The potential therapeutic mechanisms, hypothesized so far to explain the pharmacodynamic effects of norUDCA in Mdr2−/− mice, include increased hydrophilicity (and decreased cytotoxicity) of the circulating bile acid pool, induction of detoxification pathways and elimination routes for bile acids, as well as potential direct anti-inflammatory and anti-fibrotic effects.8 The therapeutic effects of norUDCA may be related to its side chain structure that strongly influences norUDCA metabolism.8,9 The metabolic fate of norUDCA differs substantially from that of other natural bile acids, including UDCA, because of its minimalN-acyl amidation (conjugation) with taurine or glycine8,9 together with its considerable hydroxylation and glucuronidation. In contrast to conjugated bile acids, unconjugated norUDCA is a weak organic acid with only two hydroxyl groups that can be reabsorbed from bile by cholangiocytes and subsequently be resecreted by hepatocytes thereby undergoing a cholehepatic shunt, a process that is associated with a HCO3−-rich hypercholeresis.10 Flushing of bile ducts by HCO3 −-rich bile may dilute and inactivate toxic biliary contents, thereby protecting bile ducts from further injury in Mdr2−/− mice.8 HCO3 −-rich hypercholeresis has also been described for UDCA (the parent compound of norUDCA), although this process only occurs in vitro once taurine stores have become depleted and the concentration of unconjugated UDCA prevails over that of its taurine conjugate. 11 Recent studies have shown that UDCA is also able to directly stimulate fluid secretion by cholangiocytes through Cftr-mediated ATP secretion and apical purinergic signaling,12 but the potential effects of norUDCA remain to be elucidated.
To test whether the physiologic and therapeutic properties of norUDCA are related to its relative resistance to N-acyl amidation or its side chain length, we compared norUDCA with tauro-norUDCA (a synthetic taurine conjugate of norUDCA) and dinorUDCA (a C22 homologue of UDCA and norUDCA with only three carbon atoms in its side chain) in the Mdr2−/− mouse model of sclerosing cholangitis and biliary fibrosis. Moreover, this study was designed to test the hypothesis that the pharmacologic effects of norUDCA depend on its ability to recirculate between bile ducts and hepatocytes as a consequence of cholehepatic shunting and/or its direct stimulatory effects on cholangiocyte secretion. This information should be critical for the design of novel drugs for the treatment of cholestatic liver diseases.
Materials and Methods
Animal Experiments
Mdr2−/− mice (FVB/N background) were obtained from Jackson Laboratory (Bar Harbor, ME). Eight-week-old male Mdr2−/− mice received either standard chow (Ssniff, Soest, Germany) or a diet containing norUDCA, tauro-norUDCA, or dinorUDCA (0.5% wt/wt, the dosage that corresponds approximately to 15 mg/mouse/day) over 4 weeks. Congenic B6.129P2-Cftrtm1Unc mice, which possess the S489X mutation that blocks transcription of Cftr,13 were fed with Peptamen (Nestle Clinical Nutrition, Deerfield, IL) or with Peptamen containing norUDCA (in a dosage equivalent to that used in Mdr2−/− mice) for 1 week. All animals were housed under a 12:12-hour light/dark cycle and permitted ad libitum consumption of water and diet. The experimental protocols were approved by the local Animal Care and Use Committees according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the U.S. National Academy of Sciences (National Institutes of Health publication 86-23, revised 1985).
Synthesis of norUDCA (3α,7β-Dihydroxy-5β-cholan-23-oic Acid), tauro-norUDCA [3α,7β-Dihydroxy- 5β-cholan-23-oic Acid N-(2-Sulfoethyl)amide, norUDC-tau], and dinorUDCA (3α,7β-Dihydroxy-5β-cholan-22-oic Acid, bisnorUDCA)
The compounds studied (Supporting Fig. 1) were synthesized from UDCA as described.14–16 Unconjugated bile acids were pure by thin layer chromatography; tauro-norUDCA was pure by high-pressure liquid chromatography.
Analysis of Bile Flow and Composition
Bile flow and composition were determined as described.8 Before harvesting, mice were anesthetized with tribromoethanol (Avertin) 250 mg/kg body weight intraperitoneally. The common bile duct was ligated, and the gallbladder was cannulated for collection of bile. After a 10-minute equilibration period, bile was collected for 1 hour in test tubes under mineral oil for determination of HCO3−, total carbon dioxide and pH using an automatic blood gas analyzer (AVL 995 hp, AVL, Graz, Austria; COBAS Mira plus, Roche). For bile flow measurements and further bile analysis, bile was collected for the following 30 minutes in preweighed test tubes. Bile flow was determined gravimetrically and normalized to liver weight as described.17 Biliary glutathione concentration was determined after protein precipitation using 5% metaphosphoric acid (Glutathione Assay Kit; Calbiochem, La Jolla, CA) according to the manufacturer’s instructions. Biliary bile acid concentration (of 3α-hydroxy bile acids) was measured enzymatically using a colorimetric bile acid kit (Ecoline S+, Holzheim, Germany).
Intrahepatic Mouse Bile Duct Units and Assessment of Ductular Secretion via Video-Optical Planimetry
Isolated bile duct units (IBDUs)—sealed fragments of isolated bile ductules with retained polarity that can secrete into a closed lumen—were prepared, purified, and cultured as described.18–20 Secretion in IBDUs is determined by measuring expansion of their lumen over time using video-optical planimetry as an indicator of the ductular secretion rate, as described.18–20 After a 5-minute baseline period, IBDUs were exposed to bile acids (at 250 µM concentration) for up to 30 minutes, and the luminal area was measured every 5 minutes via video-optical planimetry. Serial images of the IBDUs were acquired as described.12 To determine whether secretion depends on HCO3−, IBDUs were perfused with either HCO3−-free 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) buffer or HCO3−-containing Krebs-Ringer buffer.
Bile Acid Analysis
Bile acids were extracted from liver homogenate, serum, and bile and analyzed using electrospray ionization mass spectrometry and gas chromatography mass spectrometry as described.8,21
Routine Serum Biochemistry
Alanine aminotransferase and alkaline phosphatase were analyzed with a Hitachi 917 analyzer (Boehringer Mannheim, Mannheim, Germany).
Liver Histology
For conventional light microscopy, formalin-fixed livers were embedded in paraffin, and 4-µm thick sections were stained with hematoxylin-eosin and Sirius red staining. The sections were coded and examined by a pathologist (C. L.) who was unaware of the animals’ genotype and treatment.
Immunohistochemistry for Keratin 19
As described, keratin 19 (K19) immunohistochemistry was performed on cryosections with monoclonal rat anti– Troma-III antibody (dilution 1:100, Developmental Studies Hybridoma Bank) developed under the auspices of the National Institute of Child Health and Human Development (NICHID) and maintained at the University of Iowa (Iowa City, IA).22
Western Blotting
α-Smooth muscle actin (α-SMA) and K19 protein levels were determined in liver homogenates (30 µg protein) via western blotting as described8,23 using monoclonal mouse antibody against α-SMA (dilution, 1:1,500; Sigma-Aldrich, Vienna, Austria) and monoclonal rat K19 (dilution, 1:1,500; Developmental Studies Hybridoma Bank). Blots were reprobed with an anti–β-actin antibody (dilution, 1:5,000; Sigma-Aldrich, Vienna, Austria) to confirm the specificity of the observed changes in transporter protein levels.
Determination of Hepatic Hydroxyproline Content
To quantify liver fibrosis, hepatic hydroxyproline was measured as described.24
Messenger RNA Analysis
Total hepatic RNA was isolated and reverse-transcribed into complementary DNA as described.21 In brief, total RNA was isolated using TRIzol Reagent (Invitrogen, Austria) and reverse-transcribed into complementary DNA by using the GeneAmp Gold RNA PCR Core Kit (Applied Biosystems, Vienna, Austria) according to the manufacturer’s instructions. Real-time PCR was performed on a GeneAmp 7900 Sequence Detection System with GeneAmp 7900 SDS software (Applied Biosystems, CA). Polymerase chain reaction was performed in a 20-µL reaction mixture containing TaqMan or SYBR Universal PCR Master Mix (Applied Biosystems, CA). Polymerase chain reaction products were checked via gel electrophoresis for correct size and quality. All data were normalized to 18s ribosomal RNA. The TaqMan oligonucleotides were as follows: Mrp4 forward primer 5′-TTAGATGGGCCTCTGGTTC-3′, reverse primer 5′-GCCCACAATTCCAACCTTT-3′, probe 5′-ACTGCGCTCATCAAGTCCAGGG-3′ (Gen-Bank accession number W54702); Cyp2b10 forward primer 5′-CAATGGGGAACGTTGGAAGA-3′, reverse primer 5′-TGATGCACTGGAAGAGGAAC-3′, probe 5′-TTCGTAGATTCTCTCTGGCCACCATGAGA-3′ (GenBank accession number NM_009998); Sult2a1 forward primer 5′-GGAAGGACCACGACTCATAAC-3′, reverse primer 5′-GATTCTTCACAAGGTTTGTGTTACC-3′, probe 5′-CCCATCCATCTCTTCTCCAAGTCTTTCTTCAG-3′ (GenBank accession number L02335). 18sRNA was determined using SYBR Green Mastermix and forward primer 5′-GTAACCCGTTGAACCCCATT-3′, reverse primer 5′-CCATCCAATCGGTAGTAGCG-3′ (GenBank accession number X00686).
Statistical Analysis
Data are reported as the arithmetic mean ± standard deviation of reported animals in each group. Statistical analysis was performed using SPSS software. The data were analyzed with a nonparametric Mann-Whitney U test. A P value <0.05 was considered significant.
Results
norUDCA and Its Side Chain Derivatives Have Differing Effects on Bile Flow and Composition
To test whether side chain modification of norUDCA influenced bile flow and composition in a cholestatic mouse model, we fed Mdr2−/− mice, a model for spontaneous sclerosing cholangitis and biliary fibrosis,4 with nor-bile acids. In contrast to the significant increase of bile flow and biliary output of bile acids, HCO3− and glutathione by norUDCA treatment in Mdr2−/− mice8 (Table 1), tauro-norUDCA had no effect on glutathione output, and the effects on HCO3− output were much less pronounced (Table 1). dinorUDCA altered neither bile flow nor bile acid, HCO3−, or glutathione output (Table 1). Similar to Mdr2−/− mice, norUDCA also stimulated bile flow and bicarbonate output in corresponding wild-type animals as reported.8 Of note, stimulation of biliary HCO3− output in wild-type animals was significantly higher under norUDCA (241.2 ± 45.3 nmol/g liver weight−1/minute−1) compared with tauro-norUDCA (123.6 ± 47.0 nmol/g liver weight−1/minute−1) and untreated controls (44.3 ± 6.5 nmol/g liver weight−1/min −1). These differences between norUDCA and tauronorUDCA in their ability to induce a HCO3−-rich bile flow both in normal and cholestatic conditions could be secondary to the potential of norUDCAto undergo cholehepatic shunting10 and/or stimulate HCO3−-rich choleresis at the level of the bile ducts11.
Table 1.
Bile Flow and Composition in Mdr2−/− Mice under nor-Bile Acid Treatment
| Variable | Control (n = 8) |
norUDCA (n = 8) |
T-norUDCA (n = 8) |
dinorUDCA (n = 4) |
|---|---|---|---|---|
| Bile flow (µL/gLW−1/min−1) | 2.1 ± 0.2 | 3.4 ± 0.4* | 3.1 ± 0.5* | 1.9 ± 0.2† |
| Bile acid output (nmol/gLW−1/min−1) | 10.1 ± 2.5 | 21.7 ± 7.4* | 18.5 ± 4.9* | 10.8 ± 2.0† |
| Bicarbonate output (nmol/gLW−1/min−1) | 56.4 ± 4.0 | 104.4 ± 22.3* | 76.8 ± 12.5*,† | 50.8 ± 6.7† |
| Glutathione output (nmol/gLW−1/min−1) | 3.9 ± 1.2 | 6.5 ± 1.0* | 4.1 ± 0.7*,† | 4.7 ± 1.1† |
Values are expressed as the mean ± standard deviation. Control, standard chow-fed animals; norUDCA, norUDCA–fed animals; T-norUDCA, tauro-norUDCA–fed animals; dinorUDCA, dinorUDCA–fed animals.
P < 0.05 versus control.
P < 0.05 versus norUDCA.
norUDCA Significantly Increases Fluid Secretion in IBDUs in a Partially HCO3−-Dependent Manner
To directly address the choleretic potential of side chain–shortened derivatives of UDCA at the cholangiocyte level, we perfused mouse IBDUs in vitro with norUDCA, tauro-norUDCA, or dinorUDCA and measured luminal expansion rates as a marker of cholangiocellular bile secretion.12 IBDUs perfused with UDCA were used as an internal control as reported.12 In HCO3−-containing media, norUDCA significantly increased cholangiocyte fluid secretion in IBDUs above the levels obtained by perfusion with equimolar amounts of tauro-norUDCA and UDCA, while the stimulation of the secretion by dinorUDCA was the lowest (Table 2). In HCO3−-free conditions, bile acid–induced cholangiocyte secretion was drastically reduced, but not abolished. The stimulation of cholangiocyte secretion in the absence of HCO3− was similar for both norUDCA and tauro-norUDCA in contrast to previously mentioned differences in HCO3−-containing medium (Table 2). These results strongly argue for the concept that norUDCA directly stimulates fluid and HCO3− secretion in cholangiocytes in addition to stimulating canalicular bile flow.
Table 2.
Effect of nor-Bile Acids on Fluid Secretion in IBDUs using HCO3− - Containing and HCO3−-Free Buffer
| Variable | % Increase in Luminal Area after 30 Minutes |
n | P value |
|---|---|---|---|
| HCO3 buffer | |||
| Control | 12 ± 5 | 9 | |
| UDCA 250 µM | 42 ± 13 | 9 | <0.001* |
| norUDCA 250 µM | 65 ± 21 | 33 | <0.001* |
| <0.01‡ | |||
| T-norUDCA 250 µM | 49 ± 18 | 18 | <0.001* |
| <0.05† | |||
| NS‡ | |||
| dinorUDCA 250 µM | 29 ± 12 | 18 | <0.01* |
| <0.001† | |||
| <0.05‡ | |||
| HCO3−-free HEPES buffer | |||
| Control | 15 ± 5 | 8 | |
| norUDCA 250 µM | 31 ± 11 | 10 | <0.01* |
| T-norUDCA 250 µM | 33 ± 7 | 8 | <0.01* |
| NS† | |||
| dinorUDCA 250 µM | 21 ± 12 | 8 | NS* |
| NS† |
Values are expressed as the mean ± standard deviation.
Abbreviations: HEPES, 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid; NS, not significant.
P < 0.05 versus control.
P < 0.05 versus norUDCA 250 µM.
P < 0.05 versus UDCA 250 µM.
norUDCA-Induced Stimulation of Biliary HCO3−Output In Vivo Is Partly Cftr-Independent
To further understand norUDCA’s mechanisms of action and to test whether norUDCA-induced HCO3−-rich hyper-choleresis is Cftr-dependent, we fed wild-type and Cftr knockout (Cftr−/−) mice with norUDCA for 1 week. In wild-type littermates, norUDCA significantly increased HCO3− output (5.2-fold) and bile flow (3.2-fold) (Table 3). In Cftr−/− mice norUDCA also stimulated HCO3− output, while the increase in bile flow failed to reach statistical significance (P=0.053). Of note, the degree of stimulation of HCO3− output (5.8-fold) and bile flow (3.3-fold) by norUDCA in Cftr−/− mice was similar to that achieved in wild-type mice. In line with these in vivo findings, norUDCA also stimulated fluid secretion in IBDUs from Cftr−/− mice, although to a lower extent compared with IBDUs from wild-type animals (data not shown). Collectively, these finding suggest that norUDCA is able to generate a biliary HCO3− secretion also in conditions characterized by defective Cftr. Of interest, the stimulation of glutathione output by norUDCA in wild-type animals was abrogated in Cftr−/− mice (Table 3). Furthermore, the amount of bile acid output was lower in Cftr−/− mice, a finding consistent with the production of less alkaline bile in Cftr-defective animals.
Table 3.
Bile Flow and Composition in Wild-Type and Cftr−/− Mice with or without norUDCA Treatment
| Wild-Type |
Cftr−/− |
|||
|---|---|---|---|---|
| Variable | Control (n = 4) |
norUDCA (n = 4) |
Control (n = 3) |
norUDCA (n = 5) |
| Bile flow (µL/gLW−1/min−1) | 0.8 ± 0.2 | 2.6 ± 0.6* | 0.6 ± 0.4 | 2.0 ± 0.8 |
| Bile acid output (nmol/gLW−1/min−1) | 17.9 ± 4.7 | 34.4 ± 9.2 | 10.9 ± 7.9 | 18.9 ± 6.0† |
| Bicarbonate output (nmol/gLW−1/min−1) | 24.3 ± 6.0 | 127.2 ± 40.5* | 14.3 ± 6.5 | 83.2 ± 39.3* |
| Glutathione output (nmol/gLW−1/min−1) | 3.1 ± 0.7 | 5.3 ± 1.2* | 2.5 ± 2.1 | 2.7 ± 1.2† |
Mice were fed a control diet or a diet containing norUDCA for 1 week. Values are expressed as the mean ± standard deviation.
P < 0.05 wild-type norUDCA versus wild-type control, Cftr−/− norUDCA versus Cftr−/− control.
P < 0.05 Cftr−/− control versus wild-type control, Cftr−/− norUDCA versus wild-type norUDCA.
norUDCA and tauro-norUDCA Feeding Increases Biliary Bile Acid Hydrophilicity
To investigate whether the replacement of hydrophobic (cytotoxic) bile acids in phospholipid-depleted bile of Mdr2−/− mice by more hydrophilic ones may contribute to the therapeutic mechanisms of norUDCA, we next determined the bile acid composition in Mdr2−/− mice treated with norUDCA and its analogues (Table 4). Bile acid feeding did not increase biliary bile acid concentrations compared with controls. Bile acids were efficiently conjugated with taurine in untreated Mdr2−/− mice, as no unconjugated bile acids were present. In contrast, unconjugated bile acids were present in all three bile acid–fed groups. In mice receiving norUDCA, about the half of bile acids were present in unconjugated form. In animals receiving tauro-norUDCA, 14% were not taurine– conjugated, and in animals receiving dinorUDCA only 7% of biliary bile acids were unconjugated. The presence of taurine-conjugated bile acids in mice receiving norUDCA can be explained by the ability of the ileal transport system to absorb and thereby conserve the small fraction of norUDCA that undergoes taurine conjugation. Conversely, the presence of unconjugated bile acids in mice receiving tauro-norUDCA results from tauro-norUDCA undergoing deconjugation by intestinal bacteria with absorption of norUDCA. The failure of dinorUDCA to alter biliary bile acid composition is remarkable. It suggests that dinorUDCA is poorly absorbed by the gastrointestinal tract. The only major biotransformation of any of the bile acid was 6β-hydroxylation to form β-muricholic or nor-β-muricholic acid (nor-β-MCA). Electrospray ionization mass spectrometry could also confirm the excretion of minor amounts of nonamidated norUDCA- and nor-β-MCA-glucuronides and sulfates.
Table 4.
Bile Acid Concentration in Serum, Liver, and Bile: Biliary Bile Acid Composition In Mdr2−/− Mice under nor-Bile Acid Treatment
| Variable | Control (n = 6) |
norUDCA (n = 6) |
T-norUDCA (n = 6) |
dinorUDCA (n = 4) |
|---|---|---|---|---|
| Serum (µmol/L) | 57 ± 12 | 313 ± 80* | 95 ± 41† | 60 ± 10† |
| Liver (nmol/g) | 69 ± 27 | 307 ± 81* | 106 ± 40† | 109 ± 45† |
| Bile (mmol/L) | 4.3 ± 1.4 | 4.2 ± 2.2 | 3.8 ± 2.3 | 2.9 ± 0.5 |
| Biliary bile acids (rel. %) | ||||
| Taurine-amidated | ||||
| Cholic | 36.9 ± 0.6 | 8.1 ± 0.9 | 12.1 ± 2.1 | 35.4 ± 0.9 |
| Allocholic | <0.1 | <0.1 | <0.1 | 2.3 ± 0.9 |
| Chenodeoxycholic | 3.9 ± 0.6 | <0.1 | <0.1 | 3.9 ± 0.3 |
| Ursodeoxycholic | 0.6 ± 0.3 | 0.8 ± 0.4 | 1.1 ± 0.6 | 0.6 ± 0.3 |
| β -Muricholic | 40.2 ± 1.7 | 4.4 ± 0.7 | 3.8 ± 0.6 | 34.0 ± 0.9 |
| α -Muricholic | 3.4 ± 0.6 | 1.3 ± 1.1 | 0.3 ± 0.2 | 8.4 ± 0.9 |
| ω -Muricholic | 6.2 ± 1.6 | 0.6 ± 0.4 | 0.6 ± 0.4 | 5.7 ± 0.3 |
| norUDCA | <0.1 | 23.1 ± 1.2 | 56.2 ± 5.5 | <0.1 |
| norβ-Muricholic | <0.1 | 11.0 ± 1.1 | 11.7 ± 1.9 | <0.1 |
| Glucuronidated, non-amidated | ||||
| nordiol | <0.1 | 5.6 ± 2.3 | 2.4 ± 2.1 | <0.1 |
| nortriol | <0.1 | 3.1 ± 1.5 | 0.8 ± 0.7 | <0.1 |
| Non-amidated, non-glucuronidated | ||||
| norUDCA | <0.1 | 13.2 ± 0.9 | 0.2 ± 1.4 | <0.1 |
| norβ -Muricholic | <0.1 | 17.1 ± 2.4 | 0.4 ± 0.4 | <0.1 |
| norol-one | <0.1 | 9.0 ± 2.3 | 8.4 ± 2.7 | <0.1 |
| nordiol-sulfate | <0.1 | 1.5 ± 1.1 | 0.4 ± 0.4 | <0.1 |
| dinorUDCA | <0.1 | <0.1 | <0.1 | 6.4 ± 0.5 |
| dinorol-one | <0.1 | <0.1 | <0.1 | 0.4 ± 0.3 |
Control, standard chow-fed animals. T-norUDCA, T-norUDCA-fed animals. Values are expressed as the mean ± standard deviation. Values do not add up to 100% because trace bile acids (1%) are not shown.
P < 0.05 versus control.
P < 0.05 versus norUDCA.
norUDCA Is Highly Enriched in Liver and Serum of Mdr2−/− Mice
As shown in Table 4, concentrations of bile acids in serum and liver were 4 to 5 times higher in the norUDCA group compared with controls, whereas bile acid levels did not change after tauro-norUDCA and dinorUDCA feeding (Table 4). Similar to bile, 43% to 50% of liver and serum norUDCA and its metabolites were nonamidated. The composition of serum and liver bile acids in the other groups did not differ significantly from that found in the bile (data not shown).
norUDCA Is Superior to tauro-norUDCA and dinorUDCA in Improving Cholestatic Liver Injury in Mdr2−/− Mice
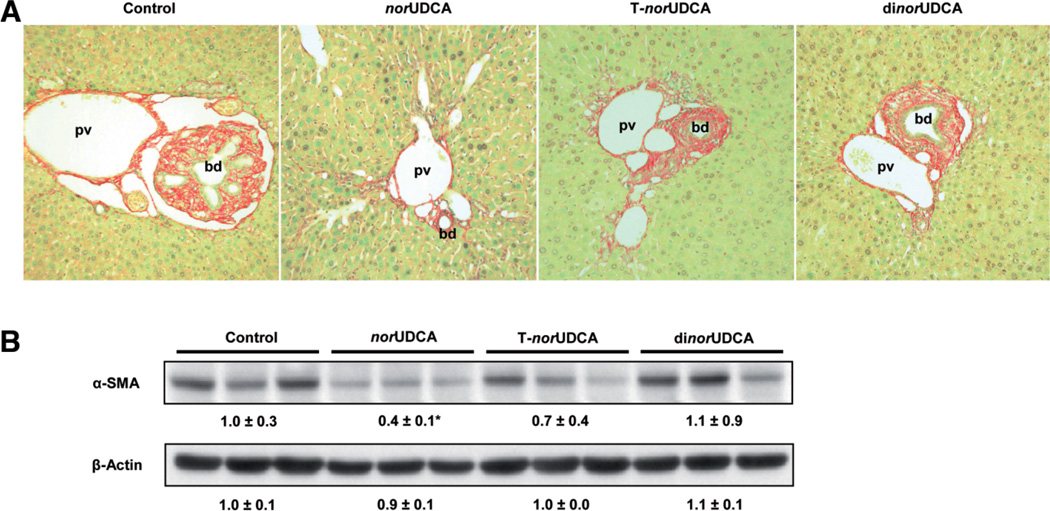
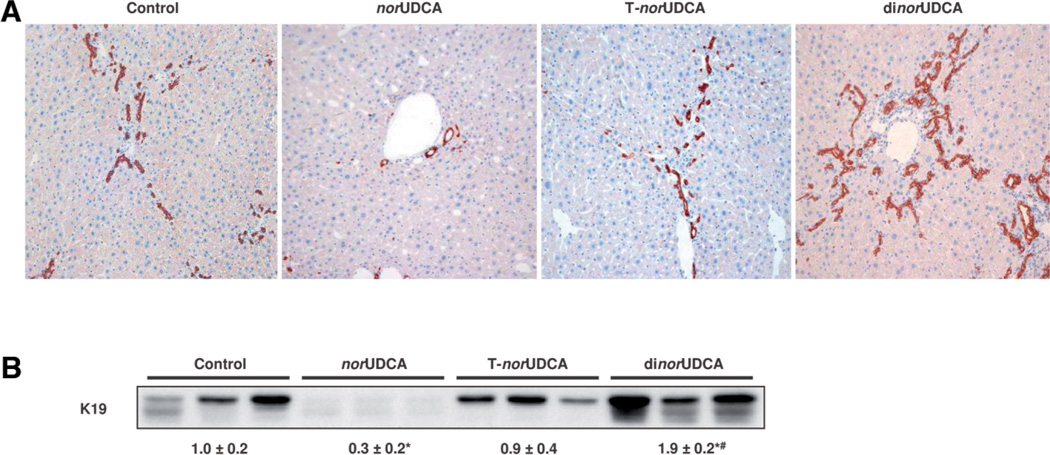
We next determined the impact of these differential pharmacologic mechanisms on the therapeutic efficacy of the nor-bile acids on cholestatic liver injury in Mdr2−/− mice. norUDCA feeding significantly improved serum alanine aminotransferase and alkaline phosphatase levels (Table 5) and liver histology (Fig. 1 and Supporting Fig. 2) as described.8 In contrast to norUDCA, the reduction of alanine aminotransferase and alkaline phosphatase levels in response to tauronorUDCA feeding was less pronounced and dinorUDCA treatment worsened liver biochemistry (Table 5). Periductal fibrosis on hematoxlin-eosin and Sirius red staining was markedly reduced by norUDCA treatment (Fig. 1A and Supporting Fig. 2); in agreement, α-SMA protein levels were reduced as well (Fig. 1B). In contrast, tauronorUDCA and dinorUDCA had only negligible effects on α-SMA expression levels (Fig. 1B). Increased hepatic hydroxyproline content (205 ± 61 µg/g liver) in Mdr2−/− mice, reflecting liver fibrosis, was significantly reduced by norUDCA treatment (129 ± 25 µg/g liver), whereas tauro-norUDCA and dinorUDCA did not have a significant effect on liver hydroxyproline levels (172 ± 47 and 187 ± 96 µg/g liver, respectively). norUDCA feeding reversed the ductular reaction, as evidenced by significantly lower hepatic K19 protein levels, serving as a specific cholangiocyte marker (Fig. 2A). On the other hand, tauro-norUDCA had no effect on the ductular reaction (Fig. 2A), and dinorUDCA increased ductular mass, most likely as a result of aggravation of liver injury. Western blotting for K19 (Fig. 2B) demonstrated a significantly reduced expression by norUDCA, no effects by tauronorUDCA, and an increase in K19 protein mass by dinorUDCA (Fig. 2B).
Table 5.
Effects of nor-Bile Acids on Liver Enzymes in Mdr2−/− Mice
| Variable | Control (n = 10) |
norUDCA (n = 8) |
T-norUDCA (n = 9) |
dinorUDCA (n = 7) |
|---|---|---|---|---|
| ALT (U/L) | 395 ± 101 | 272 ± 35* | 331 ± 100 | 903 ± 177*,† |
| AP (U/L) | 212 ± 33 | 170 ± 22* | 188 ± 34 | 940 ± 175*,† |
Control, standard chow-fed animals; norUDCA, norUDCA–fed animals; T-norUDCA, tauro-norUDCA–fed animals; dinorUDCA, dinorUDCA–fed animals. Values are expressed as the mean ± standard deviation.
P < 0.05 versus control.
P < 0.05 versus norUDCA.
Fig. 1.
norUDCA is more effective than tauro-norUDCA and dinorUDCA in reducing biliary fibrosis in Mdr2−/− mice. (A) Sirius red staining of liver in standard chow-fed (control), norUDCA-fed, tauro-norUDCA-fed (T-norUDCA), and dinorUDCA-fed Mdr2−/− mice. Compared with striking reduction of fibrosis with periductal collagen fibers (red) in norUDCA-treated Mdr2−/− mice, the effects of tauro-norUDCA and dinorUDCA were much weaker (magnification ×20). bd, bile duct; pv, portal vein. (B) Western blot analysis of hepatic α-SMA protein levels (marker for activated myofibroblasts) in liver homogenates of standard chow-fed (control), norUDCA-fed, tauro-norUDCA-fed (T-norUDCA), and dinorUDCA-fed Mdr2−/− mice. Densitometry data are expressed as n-fold change relative to standard chow-fed Mdr2−/− mice. Values are expressed as the mean ± standard deviation. Note a significant decrease in α-SMA protein levels by norUDCA treatment. *P < 0.05 versus control.
Fig. 2.
norUDCA, but not tauro-norUDCA and dinorUDCA, reduce ductular proliferation in Mdr2−/− mice. (A) Immunohistochemistry of cholangiocytes using an anti-K19 antibody in standard chow-fed (control), norUDCA-fed, tauro-norUDCA-fed (T-norUDCA), and dinorUDCA-fed Mdr2−/− mice (magnification ×20). Ductular proliferation in standard chow-fed Mdr2−/− mice was reduced by norUDCA and remained unchanged after tauro-norUDCA. dinorUDCA even increased ductular proliferation in Mdr2−/− mice. (B) Western blot analysis of hepatic K19 protein levels in liver homogenates of standard chow-fed (control), norUDCA-fed, tauro-norUDCA-fed (T-norUDCA), and dinorUDCA-fed Mdr2−/− mice. Densitometry data are expressed as n-fold change relative to standard chow-fed Mdr2−/− mice. Values are expressed as the mean ± standard deviation. Note the significant decrease in K19 protein levels by norUDCA and significant increase by dinorUDCA. *P < 0.05 versus control. #P < 0.05 versus norUDCA.
Effects of norUDCA and Its Side Chain Derivatives on Bile Acid Detoxification and Alternative Export
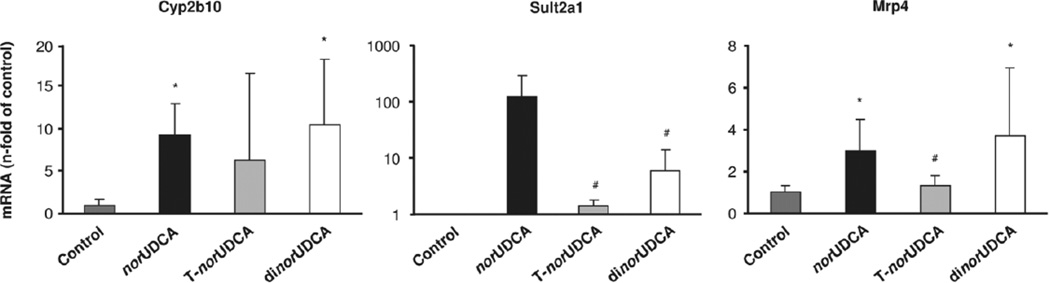
To study the possible role of induction of bile acid detoxifying enzymes and adaptive transporter expression, we determined messenger RNA expression levels of key phase I (Cyp2b10) and phase II (Sult2a1) detoxifying enzymes as well as phase III export system Mrp4, because these gene products have been identified as key targets of norUDCA.8 Cyp2b10, Sult2a1, and Mrp4 were highly up-regulated in response to norUDCA treatment (Fig. 3), whereas induction by tauro-norUDCA was less pronounced. In contrast to its toxic effects on cholestatic liver injury, dinorUDCA feeding resulted in a robust increase of Cyp2b10, Sult2a1, and Mrp4 (Fig. 3).
Fig. 3.
Effects of nor-bile acids on hepatic messenger RNA expression of phase I and II detoxification enzymes and alternative bile acid transporter. Relative messenger RNA expression of Cyp2b10, Sult2a1, and Mrp4 of standard chow-fed (control), norUDCA-fed, tauro-norUDCA-fed (T-norUDCA), and dinorUDCA-fed Mdr2−/− mice. Values are expressed as n-fold change compared with the control group (mean ± standard deviation of five animals). norUDCA and dinorUDCA induced both detoxification enzymes Cyp2b10 and Sult2a1 as well as alternative export pump Mrp4. The effects of T-norUDCA were less pronounced. *P < 0.05 versus control. #P < 0.05 versus norUDCA.
Discussion
norUDCA has been shown to cure liver injury in Mdr2−/− mice.8 Comparing different norUDCA analogues, we now show that the therapeutic properties of norUDCA in the Mdr2−/− mouse model for sclerosing cholangitis and portal fibrosis are unique and not shared by its analogues tauro-norUDCA and dinorUDCA. Most importantly, we demonstrate that the unique pharmacodynamic effects of norUDCA depend on its relative resistance to taurine conjugation, its ability to undergo cholehepatic shunting with stimulation of canalicular bile flow, and its direct stimulation of cholangiocyte secretion.
The therapeutic effects of norUDCA are highly specific. Conjugation with taurine (resulting in tauronorUDCA), as well as further side chain shortening (i.e., dinorUDCA) resulted in a marked reduction or even complete abrogation of its therapeutic properties, respectively. Our data indicate that the effectiveness of norUDCA in comparison with the other UDCA derivatives correlates with its ability to induce a hydrophilic, HCO3−-rich choleresis of both canalicular and ductal origin that may flush the injured bile ducts.8 Previous findings by Yoon et. al. on the choleretic properties of norUDCA in three rodent species10 indicate that norUDCA may undergo cholehepatic shunting. This multistep process includes reabsorption of the unconjugated protonated, lipophilic bile acid by cholangiocytes, and transport into the periductal capillary plexus, which drains into sinusoids leading to hepatocellular reuptake and canalicular re-excretion into bile. A key physicochemical property of bile acids that undergo cholehepatic shunting is that they are weak organic acids that are easily protonated and are able to cross cell membranes rapidly, presumably by a passive mechanism. Taurine conjugation results in a bile acid being present as a charged anion that can only undergo cholehepatic shunting via apical sodium- dependent bile acid transporter Asbt,25 as taurine conjugation renders bile acids membrane-impermeable, thus limiting the HCO3− generation in bile. Cholehepatic shunting of bile acids may therefore ensue when taurine stores are depleted or conjugation-resistant bile acids are administered.10 In our experimental conditions, the concept of cholehepatic shunting is further supported by the abundance of unconjugated norUDCA in the liver. These data therefore underline the concept that the therapeutic superiority of norUDCA may indeed be related to its relative resistance to conjugation, as well as its capacity to undergo cholehepatic shunting and to stimulate canalicular bile flow and HCO3−-rich hypercholeresis.
As shown previously, UDCA, the parent compound of norUDCA, directly stimulates fluid secretion in isolated cholangiocytes through a Cftr-dependent secretion of ATP that, in turn, interacts with apical purinergic receptors, which then stimulate Ca2+-dependent Cl− channels to secrete Cl−. The secreted Cl− is subsequently exchanged with HCO3− by the apical anion exchanger AE2.12 To study whether norUDCA has direct choleretic effects on cholangiocytes, we measured the secretory effects of the three bile acids by quantifying luminal expansion in IBDUs. In line with the choleretic effect observed in vivo, norUDCA had the strongest effect on ductular secretion in IBDUs. Secretory effects of nor-bile acids were for the most part HCO3−-dependent. Incubation of IBDUs with a HCO3 −-free buffer reduced norUDCA-stimulated fluid secretion by more than half, by one third for tauro-norUDCA and minimally for dinorUDCA. Investigating the mechanisms of these additional HCO3−-independent mechanisms was, however, beyond the scope of the present study.
Further experiments with norUDCA in Cftr−/− mice now indicate that norUDCA-induced HCO3− secretion is partly Cftr-independent. This stimulation of biliary HCO3− excretion by norUDCA via an at least partly Cftr-independent mechanism should encourage the use of norUDCA as a potential therapeutic strategy in cystic fibrosis-associated hepatobiliary disease. Reduced bile acid output in norUDCA-treated Cftr−/− mice is likely due to the lower biliary pH that promotes protonation and reabsorption of norUDCA. This observation also provides an explanation as to why hypercholeretic bile acids directly stimulate HCO3− secretion in cholangiocytes. It is attractive to speculate that the mechanisms could have evolved to improve the biliary disposal of potentially toxic weak organic acid compounds that otherwise would continue to recirculate in the liver. In fact, HCO3− secretion by bile ducts would be predicted to reduce their protonation and increase the biliary disposal of cholephiles.
The unique combination of cholehepatic shunting (which induces canalicular bile flow) and direct induction of a HCO3 −-dependent secretion from cholangiocytes8 may exert a flushing effect on the injured biliary tree, likely removing and inactivating toxic compounds. In contrast to the beneficial choleretic effects of norUDCA, UDCA-induced choleresis has been shown to result in disruption of cholangioles and aggravation of liver injury in the Mdr2−/− model for sclerosing cholangitis.4 The underlying mechanism for these puzzling differences remains to be resolved, but could include differences in the capability of directly stimulating ductular secretion. Nevertheless, future clinical trials should take into account potential detrimental norUDCA effects in the context of frank biliary obstruction.
The effects of different nor-bile acids on liver fibrosis were closely linked to their effects on ductular reaction, which has been postulated to trigger biliary fibrosis in mice and humans.22,26,27 Also in our studies the amount of liver fibrosis in treated animals correlated with the amount of ductular reaction. norUDCA reduced both ductular proliferation and biliary fibrosis. Although norUDCA reversed only the pathologically enhanced ductular hyperproliferation in Mdr2−/− mice and never induced ductopenia in wild-type animals, we cannot definitely rule out the theoretical risk for development of ductopenia in humans with vanishing bile duct syndromes. Future clinical pilot trials should therefore consider only patients without advanced ductopenia. In comparison to norUDCA, dinorUDCA-treated Mdr2−/− mice showed a brisk induction of the ductular reaction that likely reflects a regenerative response to increased liver injury, as suggested by significantly increased serum aminotransferases. In contrast to the findings in Mdr2−/− mice, wild-type mice fed with the same dosage of dinorUDCA with similar hepatic enrichment had induced bile flow, but did not show any alterations in liver enzymes or liver histology excluding a general toxic effect (data not shown).
Bile acids are able to directly stimulate their metabolism and export by the induction of hepatic phase I and phase II detoxification systems as well as by induction of basolateral and apical bile acid transporters.28 These mechanisms, promoting detoxification of bile acids to more water-soluble derivatives and facilitating their excretion via the kidney, could counteract the effects of cholestasis and liver injury.28 In line with a previous study,8 norUDCA feeding resulted in a robust induction of phase I and phase II detoxification enzymes, explaining the increased appearance of polyhydroxylated, sulfated, and glucuronidated norUDCA metabolites in bile, serum, and urine in mice and in humans.8,9 Similar biotransformation patterns seem to be common to other nor-bile acids as shown previously for norchenodeoxycholic and dinorchenodeoxycholic acid, both being amidation-resistant and glucuronidated in the hamster and the rat.29 Taurine conjugation abrogated the effects of norUDCA on induction of Cyp2b10, Sult2a1, and the basolateral export pump Mrp4. Astonishingly, dinorUDCA effects on expression of detoxifying and export systems were comparable with those of norUDCA, despite its toxic effect in Mdr2−/− mice. Therefore, the observed induction of detoxifying and alternative export systems is not likely to represent a central therapeutic mechanism, and could rather support a secondary response to injury or to the accumulation of nor-bile acids.
In conclusion, this comprehensive analysis of the differential physiologic and therapeutic mechanisms of norUDCA and its derivatives tauro-norUDCA and dinorUDCA clearly demonstrates the therapeutic superiority of norUDCA in an animal model for sclerosing cholangitis and biliary fibrosis (Supporting Table 1). The differential effects of the various nor-bile acids depend on specific properties that affect their ability to undergo cholehepatic shunting, thereby stimulating canalicular bile flow as well as by directly stimulating fluid secretion by cholangiocytes. These data may have high relevance for the design of novel pharmacologic treatments for cholangiopathies and liver diseases in general.
Supplementary Material
Acknowledgment
The authors thank Dr. W. Erwa (Graz, Austria) and colleagues for performing liver function tests. The authors also thank Dr. T. Claudel (Graz, Austria) for stimulating discussions and helpful suggestions and P. De and P. Komala (Bangalore, India) for synthesizing the UDCA derivatives. The rat anti-Troma-III antibody developed by Dr. R. Kemler, Max-Planck Institute, Freiburg, Germany, was obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHID, and maintained by the University of Iowa (Iowa City, IA).
Supported by grant P19118-B05 from the Austrian Science Foundation and a GEN-AU project grant (GZ 200.139/1-VI/1/2006) from the Austrian Ministry for Science (both to M. T.) and by the Swedish Research Council and The Swedish Medical Association (to H. U. M). E. H. was also supported by the Ph.D. program of the Medical University of Graz. M. S., C. S., and R. F. were supported by Fondazione S. Martino, Bergamo; National Institutes of Health Grant DK079005; and Yale University Liver Center (National Institutes of Health Grant DK34989). R. F. was also supported by Cystic Fibrosis Foundation (CFF-FIOROT0810). C. S. is a recipient of an ALF/AASLD Liver Scholar Award. The synthetic work was supported by a grant from JNCASR, Bangalore (to U. M.). A. F. H. was supported by National Institutes of Health Grant DK64891.
The Medical University of Graz has filed the patent for the use of norUDCA in the treatment of liver diseases, and Michael Trauner, Alan Hofmann, and Peter Fickert are listed as inventors (publication number WO2006119803).
Abbreviations
- dinorUDCA
dinorursodeoxycholic acid
- IBDU
isolated bile duct unit
- K19
keratin 19
- norUDCA
24-norursodeoxycholic acid
- α-SMA
α-smooth muscle actin
- tauro-norUDCA
tauronorursodeoxycholic acid
- UDCA
ursodeoxycholic acid
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ishibashi H, Komori A, Shimoda S, Gershwin ME. Guidelines for therapy of autoimmune liver disease. Semin Liver Dis. 2007;27:214–226. doi: 10.1055/s-2007-979472. [DOI] [PubMed] [Google Scholar]
- 2.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, et al. Ursodeoxycholic acid aggravates bile infarcts in bile ductligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 4.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 6.Melum E, Boberg KM, Franke A, Bergquist A, Hampe J, Schreiber S, et al. Variation in the MDR3 gene influences disease progression in PSC patients and disease susceptibility in epistatic interaction with polymorphism in OST-alpha gene. HEPATOLOGY. 2007;46:265A. [Google Scholar]
- 7.Ziol M, Barbu V, Rosmorduc O, Frassati-Biaggi A, Barget N, Hermelin B, et al. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology. 2008;135:131–141. doi: 10.1053/j.gastro.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann AF, Zakko SF, Lira M, Clerici C, Hagey LR, Lambert KK, et al. Novel biotransformation and physiological properties of norursodeoxycholic acid in humans. HEPATOLOGY. 2005;42:1391–1398. doi: 10.1002/hep.20943. [DOI] [PubMed] [Google Scholar]
- 10.Yoon YB, Hagey LR, Hofmann AF, Gurantz D, Michelotti EL, Steinbach JH. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 11.Strazzabosco M, Sakisaka S, Hayakawa T, Boyer JL. Effect of UDCA on intracellular and biliary pH in isolated rat hepatocyte couplets and perfused livers. Am J Physiol. 1991;260:G58–G69. doi: 10.1152/ajpgi.1991.260.1.G58. [DOI] [PubMed] [Google Scholar]
- 12.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 13.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 14.Schteingart CD, Hofmann AF. Synthesis of 24-nor-5 beta-cholan-23-oic acid derivatives: a convenient and efficient one-carbon degradation of the side chain of natural bile acids. J Lipid Res. 1988;29:1387–1395. [PubMed] [Google Scholar]
- 15.Tserng KY, Hachey DL, Klein PD. An improved procedure for the synthesis of glycine and taurine conjugates of bile acids. J Lipid Res. 1977;18:404–407. [PubMed] [Google Scholar]
- 16.Huffman JW, Sobti RR. Synthetic approaches to steroidal alkaloids II (1). Preparation and reactions of 12-oxo-dinorcholanic acids. Steroids. 1970;16:755–770. doi: 10.1016/s0039-128x(70)80153-2. [DOI] [PubMed] [Google Scholar]
- 17.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Pojer C, Zenz R, et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology. 2001;121:170–183. doi: 10.1053/gast.2001.25542. [DOI] [PubMed] [Google Scholar]
- 18.Spirli C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 19.Spirli C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology. 2003;124:737–753. doi: 10.1053/gast.2003.50100. [DOI] [PubMed] [Google Scholar]
- 20.Mennone A, Alvaro D, Cho W, Boyer JL. Isolation of small polarized bile duct units. Proc Natl Acad Sci U S A. 1995;92:6527–6531. doi: 10.1073/pnas.92.14.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner M, Fickert P, Zollner G, Fuchsbichler A, Silbert D, Tsybrovskyy O, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 22.Fickert P, Stoger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trauner M, Arrese M, Soroka CJ, Ananthanarayanan M, Koeppel TA, Schlosser SF, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 24.Jamall IS, Finelli VN, Que Hee SS. A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Anal Biochem. 1981;112:70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 25.Alpini G, Glaser S, Baiocchi L, Francis H, Xia X, Lesage G. Secretin activation of the apical Na_-dependent bile acid transporter is associated with cholehepatic shunting in rats. HEPATOLOGY. 2005;41:1037–1045. doi: 10.1002/hep.20653. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Munoz D, Castellano-Megias VM, Romero-Gomez M. Expression of bcl-2 in ductular proliferation is related to periportal hepatic stellate cell activation and fibrosis progression in patients with autoimmune cholestasis. Dig Liver Dis. 2007;39:262–266. doi: 10.1016/j.dld.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Clouston AD, Powell EE, Walsh MJ, Richardson MM, Demetris AJ, Jonsson JR. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. HEPATOLOGY. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 28.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 29.Yeh HZ, Schteingart CD, Hagey LR, Ton-Nu HT, Bolder U, Gavrilkina MA, et al. Effect of side chain length on biotransformation, hepatic transport, and choleretic properties of chenodeoxycholyl homologues in the rodent: studies with dinorchenodeoxycholic acid, norchenodeoxycholic acid, and chenodeoxycholic acid. HEPATOLOGY. 1997;26:374–385. doi: 10.1002/hep.510260218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.