Abstract
The diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae is the prototypic member of a superfamily of transition metal ion-activated transcriptional regulators that have been isolated from Gram-positive prokaryotes. Upon binding divalent transition metal ions, the N-terminal domain of DtxR undergoes a dynamic structural organization leading to homodimerization and target DNA binding. We have used site-directed mutagenesis and NMR analysis to probe the mechanism by which apo-DtxR transits from an inactive to a fully active repressor upon metal ion binding. We demonstrate that the ancillary metal-binding site mutant DtxR(H79A) requires higher concentrations of metal ions for activation both in vivo and in vitro, providing a functional correlation to the proposed cooperativity between ancillary and primary binding sites. We also demonstrate that the C-terminal src homology 3 (SH3)-like domain of DtxR functions to modulate repressor activity by (i) binding to the polyprolyl tether region between the N- and C-terminal domains, and (ii) destabilizing the ancillary binding site, leading to full inactivation of the repressor. Finally, we show by NMR analysis that the hyperactive phenotype of DtxR(E175K) results from the stabilization of a structural intermediate in the activation process. Taken together, the data presented support a multistep model for the activation of apo-DtxR by transition metal ions.
In Corynebacterium diphtheriae, the diphtheria toxin repressor (DtxR) is an iron-sensitive transcriptional regulator that controls the expression of a number of genes, including the diphtheria toxin structural gene (tox), a number of operons involved in iron uptake and homeostasis, and outer surface lipoproteins (1–4). DtxR is the prototypic member of a large family of transition metal ion-activated repressors in Gram-positive prokaryotes (5). In the absence of divalent transition metal ions, apo-DtxR exists as an inactive monomer that is in weak equilibrium with a dimeric form (6). Whereas Fe2+ is the physiological activator of DtxR in vivo, Fe2+, Ni2+, Co2+, Cd2+, and Mn2+ have been shown to activate the aporepressor in vitro (7). Once activated, DtxR forms stable dimers, and two pairs of dimers have been shown to bind to almost opposite faces of the tox operator (toxO) sequence (6, 8).
DtxR is a 226-aa 26-kDa protein composed of two major structural domains linked by a 23-aa flexible tether that contains a proline-rich region (1, 9, 10). The N-terminal domain (residues 1–123) contains the ancillary and primary metal ion-binding sites, a canonical helix–turn–helix DNA-recognition motif, and an extensive hydrophobic surface necessary for the formation of stable dimers (9–11). We have previously shown that alanine substitution of any of the primary site residues (Met-10, Cys-102, Glu-105, and His-106) results in complete inactivation of repressor activity, whereas alanine substitution of any of the ancillary site residues (His-79, Glu-83, His-98, Glu-170, and Gln-173) results in a partial loss of activity (11, 12). Because the helical structure ends with Val-123, we consider that residue to be the distal limit of the N-terminal domain. Whereas the proline-rich region adopts a β-sheet structure in x-ray crystallographic studies (13), in solution its structure is disordered (14), supporting its inclusion with the tether. The N-terminal domain contributes all residues that constitute the primary metal ionbinding site (site 2), as well as three of five residues that constitute the ancillary metal ion-binding site (site 1) (11, 12). In contrast to x-ray crystallographic results, recent NMR solution studies demonstrate that in the absence of bound transition metal ions the N-terminal domain of apo-DtxR exists as a molten globule (15). Upon binding of transition metal ions the aporepressor undergoes a dynamic change resulting in the ordering of N-terminal domain into a stable structure, which allows for dimerization and subsequent recognition and binding to target DNA sequences (14).
The C-terminal domain of DtxR (residues 148–226) has been shown to adopt a tertiary structure similar to that of eukaryotic src homology 3 (SH3) domains (13, 14). In vitro this domain has been shown to bind a peptide carrying the amino acid sequence of the proline-rich region (residues 125–139) of the flexible tether linking the N- and C-terminal domains (14) by forming an intramolecular complex (G. P. Wylie, R. Vijayaraghavan, E. A. Bienkiewicz, J.F.L., J.R.M., and T.M.L., unpublished data). In addition, the C-terminal SH3-like domain contributes two ligands, Glu-170 and Gln-173, to the ancillary metal ion-binding site (12).
Many studies have examined individual aspects of DtxR function; however, an integrated model for its transition from an inactive aporepressor to the metal ion-bound active form of the repressor has remained incomplete. We have introduced site-directed mutations into the tether region and the SH3-like domain of both the wild-type dtxR and dtxR(E175K) (16) alleles to probe the mechanism of transition metal ion activation of the repressor. We demonstrate that the ancillary metal ion-binding site facilitates the activation of DtxR by lowering the concentration of metal ions necessary for repressor function. We show that the C-terminal SH3-like domain modulates the activity of the repressor, and we demonstrate by NMR analysis that the N-terminal domain of the hyperactive mutant DtxR(E175K) maintains a more ordered conformation than the wild-type aporepressor. Results presented here are consistent with a multistage model of repressor activation that is composed of at least three steps that mediate the conversion of inactive apo-DtxR to the fully active metal ion-bound form of the repressor.
Experimental Procedures
Construction of Mutant dtxR Genes. The QuikChange mutagenesis protocol (Stratagene) was used to mutate the dtxR gene in pROM-dtxR, pROM-dtxR(E175K), or pET-dtxR as previously described (17). In a 50-μl reaction mixture, 2.5 units of Pfu Turbo DNA polymerase and 125 ng of each mutagenic primer were added to ≈50 ng of double-stranded template DNA. Reactions were 40 μM in each dNTP and 1× in reaction buffer. Reaction products were digested with 5 units of DpnI for 1–2 h at 37°C before being used to transform competent Escherichia coli TOP10 cells. DNA preparations were made by using Wizard SV Miniprep kits (Promega), and all genes were fully sequenced by the Genetics Core Facility at Boston Medical Center. All oligonucleotides used in these studies were synthesized by GIBCO/BRL, and the sequences can be found in Table 5, which is published as supporting information on the PNAS web site.
β-Galactosidase Assays. β-Galactosidase activity was measured according to Miller (18). Cultures of E. coli DH5α-λRS45-tacPtoxO-lacZ were grown overnight in 8 ml of LB supplemented with ampicillin (Amp; 100 μg/ml) and kanamycin (Kan; 50 μg/ml) (LB/Amp/Kan) with or without 2,2′-dipyridyl (DP) at 37°C to OD600 ≈ 1. Growth was stopped by a 10-min incubation on ice. To 500 μl of culture was added 15 μl of lysis mix (200 μl of 10% SDS mixed with 400 μl of chloroform), and the mixture was vortexed for 10 sec. Twenty-five microliters of culture lysate was added to 975 μl of Z buffer (18), followed by the addition of 200 μl of o-nitrophenyl β-d-galactopyranoside (ONPG, 4 mg/ml). Reactions were incubated at 25°C for up to 60 min. The OD600 of the overnight cultures was measured, and the A550 and A420 of the reaction solution were measured. Data are shown as percentage β-galactosidase activity of the control culture that does not express DtxR and is presented as the mean ± standard deviation for four independent cultures.
Purification of DtxR(H79A). E. coli HMS174(DE3) (Novagen) cells were transformed with pET-dtxR(H79A). Cultures were grown in LB/Amp in a 15-liter New Brunswick fermenter at 37°C with aeration (10 liters of air per min) and stirring (500 rpm) until the OD600 reached ≈0.7. Isopropyl β-d-thiogalactopyranoside (IPTG) was then added to a final concentration of 1.0 mM. The culture was incubated for 3 h and the bacteria were harvested by centrifugation.
DtxR(H79A) was purified as described previously (12). Briefly, cells were lysed by sonication, and the lysates were cleared by centrifugation. After DEAE anion-exchange, Niaffinity, and Mono Q anion-exchange high-pressure liquid chromatography, wild-type and mutant repressors were ≈90% pure. EDTA was added to 10 mM and the purified repressors were then dialyzed against 20 mM Tris·HCl, pH 7.5/5 mM DTT. Aliquots of the purified proteins were stored at -80°C.
Coupled in Vitro Transcription/Translation Assays. Template DNA pJL1 for coupled in vitro transcription/translation reactions was isolated with Wizard MaxiPreps (Promega) and treated for 1–2 h with RNaseONE (Promega) at 37°C. DNA was extracted with phenol and Sevag (a 24:1 mixture of chloroform/isoamyl alcohol) and precipitated with sodium acetate and ethanol. Precipitated DNA was resuspended in water, and DNA concentrations were determined by absorbance (A260).
Coupled in vitro transcription/translation reactions were performed as previously described (17). One microgram of pJL1 was added to 20 μl of premix and 5 μl of complete amino acid mixture. One microgram of purified DtxR (or mutant DtxR) protein, DP, and/or activating metal ion solutions were added as noted, and the reaction mixture was brought up to a final volume of 35 μl with water. Reaction mixtures were incubated at room temperature for 10–12 min to allow for DtxR activation and operator binding, and then 15 μl of S30 extracts was added. Reaction mixtures were incubated for 1 h at 37°C. To halt in vitro synthesis, reaction tubes were placed on ice for 10 min. Samples of each reaction were diluted in luciferase dilution buffer and luciferase activity was measured by using a Turner Designs 20/20 luminometer. All reactions were performed in quadruplicate.
NMR Studies. Two-dimensional 1H,15N-correlation spectra (19) were collected with sweep widths of 8,333.33 Hz (512 complex points) and 1,650 Hz (140 complex points) in the 1H and 15N dimensions, respectively, on a 500-MHz three-channel Varian Unityplus instrument equipped with waveform generators and pulsed-field gradient accessories. Samples of apo-DtxR and apo-DtxR(E175K) were prepared at 0.8 mM protein concentration in 10 mM Hepes buffer (pH 6.8)/10 mM DTT, and 0.56 mM 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) was added as a chemical shift reference.
Results
Activation of the Ancillary Site Mutant DtxR(H79A) Requires a Higher Concentration of Transition Metal Ions Than Wild-Type DtxR. A recurring question in the transition metal ion-induced activation of DtxR concerns the relationship(s) between the primary and ancillary metal ion-binding sites. It is known that an intact primary site (M10, C102, E105, H106, the backbone carbonyl of C102, and a structural water molecule) is essential for repressor activity (11). In contrast, alanine substitution for any of the ancillary site residues (H79, E83, H98, E170, and Q173) does not abolish repressor activity in vivo; however, the small phenotypic changes associated with these mutations are accompanied by an altered mobility of the 32P-labeled toxO probe by electrophoretic mobility-shift assay in vitro (11, 12).
Because the N-terminal domain of apo-DtxR has been shown to have the properties of a molten globule (15), we reasoned that transition metal ion occupation of the ancillary binding site might facilitate the structural transitions required for the formation of the primary binding site. If this were the case, one would anticipate ancillary site mutants of DtxR to require higher concentrations of transition metal ions to induce activation of repressor function. This hypothesis is based on the assumption that even as a molten globule, the N-terminal domain of the aporepressor would transiently adopt a conformation that presents an intact primary site capable of binding an activating transition metal ion. Once the primary site became occupied the structure of the N-terminal domain would then be stabilized in an activated conformation.
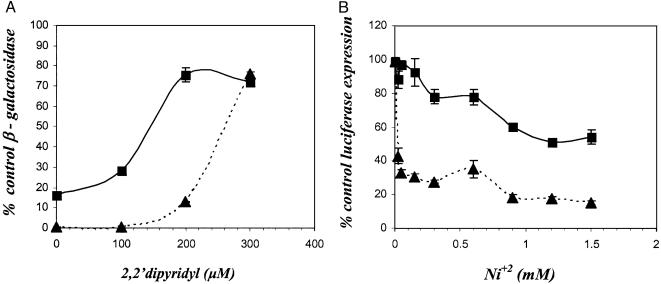
To test this hypothesis, we compared the sensitivity of DtxR and the ancillary metal ion-binding site mutant DtxR(H79) to inactivation in vivo by the chelator DP. The reporter strain E. coli DH5α-λRS45-tacPtoxO-lacZ (17) was transformed separately with the plasmids pROM, pROM-dtxR, and pROM-dtxR(H79A). Strains were grown overnight in LB/Amp/Kan medium supplemented with increasing concentrations of DP and then assayed for reporter gene expression. As shown in Fig. 1A, transformants expressing DtxR(H79A) were significantly more sensitive to DP-induced inactivation than were transformants expressing the wild-type repressor.
Fig. 1.
(A) Expression of β-galactosidase in recombinant E. coli strains that carry lacZ under the control of the DtxR-sensitive hybrid promoter/operator tacPtoxO in the absence and presence of increasing concentrations of DP. E. coli DH5α-λRS45-tacPtoxO-lacZ/pROM served as the control. ▴, E. coli DH5α-λRS45-tacPtoxO-lacZ/pPOM-DtxR, which carries wild-type DtxR. ▪, E. coli DH5α-λRS45-tacPtoxO-lacZ/pROM-DtxR(H79A), which carries the ancillary metal ion-binding site mutant DtxR(H79A). (B) S30 extracts of E. coli were programmed with 1 μg of pJL-1 DNA for the coupled transcription/translation of luciferase expression from a tacPtoxO/luc transcriptional fusion. All in vitro reaction mixtures contained 55 μM DP and 1 μg of either DtxR (▴) or DtxR(H79A) (▪). Increasing concentrations of NiCl2 were added to activate repressor function. After incubation, luciferase activity expressed in vitro for each Ni2+ concentration was measured. In both A and B reactions for each point were performed in quadruplicate. The data are presented as the mean ± standard deviation.
To further test this hypothesis, both DtxR and DtxR(H79A) were purified to >90% homogeneity (data not shown). By electrophoretic mobility-shift analysis, both repressors were active in binding a 32P-labeled toxO probe as previously demonstrated (data not shown). We then compared the transition metal ion-induced activation of purified DtxR and DtxR(H79A) by using a coupled in vitro transcription/translation assay. In this assay, E. coli S30 extracts are programmed with pJL1 DNA, which carries the reporter gene luciferase under the control of a tacPtoxO hybrid promoter operator (17). Assays were performed in the presence of 55 μM DP, a chelator concentration that completely inactivates both DtxR and DtxR(H79A), and increasing concentrations of Ni2+. As shown in Fig. 1B, the ancillary site mutant DtxR(H79A) requires significantly higher concentrations of added Ni2+ than that of the wild-type repressor to become even partially activated. These results support the contention that the two metal ion-binding sites of wild-type DtxR function in concert (20). This observation is also supported by earlier x-ray crystallographic analyses of DtxR that have shown an extensive network of salt bridges and hydrogen bonds between the primary and ancillary metal ion-binding sites (11).
The data presented above suggest that metal ion binding in the ancillary site facilitates the activation of DtxR by inducing conformational change(s) in the N-terminal domain which, in turn, result in the organization of the primary metal ion-binding site. Recent observations have shown that the SH3-like domain residues E170 and Q173 serve as functional ancillary site ligands (12). In the case of DtxR(E175K), the single SH3-like domain mutation clearly plays an essential role in establishing the hyperactive phenotype.
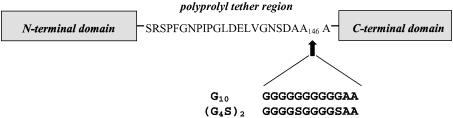
In-Frame Extension of the Tether Between the N- and C-Terminal Domains of DtxR Affects Metal Ion Activation of the Repressor. Because several observations suggest that the C-terminal SH3-like domain is able to modulate the activation of DtxR, we reasoned that in-frame insertions into the 23-aa flexible tether might decrease repressor activity. To test this hypothesis, we introduced a unique NotI restriction endonuclease site at the C terminus of the tether region in the wild-type dtxR allele. The NotI site was then used to independently insert oligonucleotides encoding the in-frame insertion of an additional 12-aa sequence (G10 or G4SG4S, each followed by two A residues) after A146 in DtxR and DtxR(E175K)(Fig. 2).
Fig. 2.
Schematic diagram of the in-frame insertions made within the tether region of DtxR. The insertion of a 12-aa polypeptide is predicted to increase the length of the tether region between the N- and C-terminal domains by ≈50%.
When grown under iron-replete conditions, individual transformants that carried in-frame insertions in the tether region of DtxR displayed little phenotypic differences from the wild-type repressor (Table 1). In contrast, under iron-limiting conditions, all of the insertion sequences allowed for the complete derepression of β-galactosidase to levels similar to the level observed in the absence of DtxR. These results suggest that the tether region between the N- and C-terminal domains functions, at least in part, to modulate repressor activity under iron-limiting conditions.
Table 1. β-Galactosidase expression by E. coli strains carrying wild-type and mutant forms of DtxR grown under iron-replete and iron-limiting conditions.
| β-Galactosidase, % of control
|
||
|---|---|---|
| Repressor | LB/Amp/Kan | LB/Amp/Kan + 300 μM DP |
| DtxR | 0.32 ± 0.04 | 81.0 ± 2.25 |
| DtxR(E175K) | 0.25 ± 0.04 | 30.7 ± 1.61 |
| DtxR(G10) | 0.76 ± 0.07 | 95.9 ± 3.41 |
| DtxR(G4S)2 | 0.43 ± 0.12 | 97.5 ± 5.62 |
| DtxR(E175K,G10) | 0.65 ± 0.73 | 22.3 ± 0.42 |
Alanine Substitution Mutations Within the Polyprolyl Region of the Tether Are Suppressed in the dtxR(E175K) Background. We then turned to a site-directed mutational analysis of the polyprolyl region of the tether in the hyperactive mutant repressor DtxR(E175K). Given the position of E175 relative to the two SH3-like domain ancillary site ligands E170 and Q173, we envisioned the E175K substitution to function proximal to, but not within, this binding site. Furthermore, we reasoned that the E175K substitution might stabilize a partially active intermediate of DtxR in its transit from the apo- to the fully active metal ion-bound form. Were this the case, one would anticipate that effects associated with mutations within the polyprolyl region would be suppressed in the DtxR(E175K) background.
As shown in Table 2, the independent introductions of S126A, P127A, P131A, and P133A mutations into the polyprolyl region of the tether of DtxR(E175K) have little measurable effect on repressor activity when cultures are grown in iron-rich medium. However, introduction of these mutations in the wild-type DtxR background results in partial inactivation of repressor activity. Thus, under iron-replete conditions, the E175K substitution is able to suppress the effects of these mutations seen in the wild-type repressor. Under iron-limiting conditions, the S126A, P127A, and P133A mutations in the DtxR(E175K) background were indistinguishable from those made in wild-type DtxR. Only DtxR(P131A) and DtxR(P131A,E175K) were found to be statistically different (P < 0.01, by paired t test).
Table 2. In vivo activity of repressor with alanine substitutions within the polyprolyl tether region of wild-type DtxR and DtxR(E175K).
| β-Galactosidase, % of control
|
||
|---|---|---|
| Mutation | LB/Amp/Kan | LB/Amp/Kan + 300 μM DP |
| DtxR | 0.13 ± 0.02 | 69.0 ± 2.0 |
| DtxR(E175K) | 0.14 ± 0.04 | 13.4 ± 1.8 |
| DtxR(S126A) | 65.3 ± 1.0 | 105 ± 10 |
| DtxR(E175K,S126A) | 9.1 ± 0.6 | 94.8 ± 2.1 |
| DtxR(P127A) | 34.0 ± 2.4 | 96.0 ± 11.0 |
| DtxR(E175K,P127A) | 3.9 ± 0.2 | 92.1 ± 3.6 |
| DtxR(P131A) | 1.8 ± 0.1 | 86.0 ± 5.0 |
| DtxR(E175K,P131A) | 0.20 ± 0.1 | 51.0 ± 3.4 |
| DtxR(P133A) | 35.6 ± 5.2 | 101 ± 8.0 |
| DtxR(E175K,P133A) | 3.8 ± 0.3 | 95.5 ± 9.9 |
These observations suggest that the polyprolyl region in the tether between the N- and C-terminal domains of DtxR serves a downstream function in addition to being the target site of SH3-like domain binding. Because it has been shown that the tether region forms many hydrogen bonds with α-helices that constitute the dimerization domain of DtxR (14, 21), these interactions are likely to stabilize active dimers of DtxR.
The Proline-Rich Region of the Tether as Trigger for Repressor Activation. To isolate the SH3-like domain effects, a stable premature polypeptide chain termination mutant of DtxR, DtxRΔSH3, has been isolated and characterized. As shown in Table 3, DtxRΔSH3 functions as a metal ion-dependent repressor, albeit less active than the wild-type repressor in vivo. Because DtxRΔSH3 retains P133 in its tether region, we examined the role of this proline in overall repressor activity. Based on the hypothesis that the polyprolyl region of the tether serves only as a binding site for the SH3-like domain, the introduction of P133A mutation in DtxRΔSH3 should have no additional effect. To our surprise, the introduction of the P133A mutation in DtxRΔSH3 results in an almost complete loss of residual repressor activity (Table 3). These results strongly suggest that the polyprolyl region of DtxR plays a greater role in repressor activation and activity.
Table 3. β-Galactosidase activity of E. coli reporter strains carrying wild-type DtxR, DtxRΔSH3, and DtxRΔSH3(P133A).
| β-Galactosidase, % of control
|
|
|---|---|
| Repressor | (LB/Amp/Kan) |
| DtxR | 0.09 ± 0.03 |
| DtxRΔSH3 | 21.5 ± 1.1 |
| DtxRΔSH3(P133A) | 95.7 ± 3.7 |
The E175K Mutation in DtxR Stabilizes a Structural Intermediate in the Activation Cascade. The mechanism by which the E175K mutation in DtxR leads to a hyperactive phenotype is not known. One explanation is that the ε-amino moiety of K175 may form a salt bridge to the N-terminal domain of the repressor in a position proximal to the ancillary metal ion-binding site (e.g., E19, E20, E21, or E35). To examine the role of electrostatic interactions in the hyperactive phenotype of DtxR(E175K), we introduced a series of amino acid substitutions at position 175 by site-directed mutagenesis. As shown in Table 4, when individual transformants are grown in iron-rich medium, the activity of all of the mutant repressors is essentially the same. However, when these transformants are grown in iron-limiting medium, the activity of each mutant repressor was found to fall into one of three classes. For example, wild-type DtxR and DtxR(E175D), which carry negatively charged residues at this position, are similarly inactivated by DP. In contrast, DtxR(E175K) and DtxR(E175R) both display a hyperactive phenotype, and DtxR(E175A) and DtxR(E175Q) display a phenotype that is intermediate between the wild-type and positively charged residues at position 175. Taken together, these observations support the hypothesis that the E175K and E175R mutations stabilize a “half-activated” conformation of the repressor.
Table 4. β-Galactosidase expression by E. coli strains carrying wild-type and mutant forms of DtxR with various amino acids at position 175 grown under iron-replete and iron-limiting conditions.
| β-Galactosidase, % of control
|
||
|---|---|---|
| Amino acid at position 175 | LB/Amp/Kan | LB/Amp/Kan + 300 μM DP |
| E* | 0.24 ± 0.12 | 48.4 ± 2.83 |
| D | 0.41 ± 0.23 | 48.7 ± 2.92 |
| K | 0.20 ± 0.10 | 8.71 ± 0.25 |
| R | 0.16 ± 0.03 | 8.93 ± 1.08 |
| Q | 0.14 ± 0.01 | 21.2 ± 0.31 |
| A | 0.12 ± 0.05 | 24.6 ± 1.61 |
Wild type
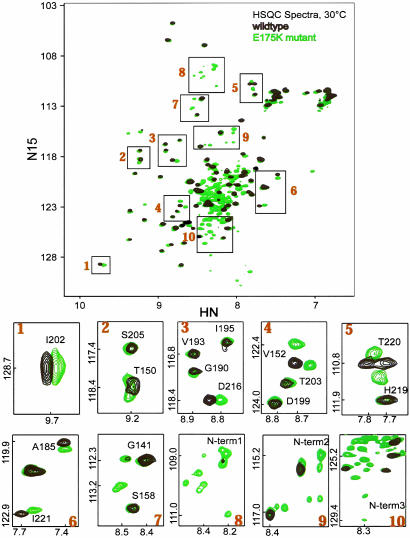
Further support for this interpretation comes from the comparison of the 1H,15N heteronuclear single quantum correlation (HSQC) NMR spectra of wild type DtxR and DtxR(E175K) (Fig. 3). As noted previously, the backbone amide resonances from the N-terminal domain of the aporepressor are broadened by conformational exchange on the intermediate time scale and are not observed (15). In contrast, the apo-DtxR(E175K) spectrum shows many resonances that must arise from the N-terminal domain. The boxes in Fig. 3 Lower (numbered 1–10) show the apo-DtxR and apo-DtxR(E175K) in higher expansion for better comparison. Compared with the wild-type repressor, DtxR(E175K) shows specific changes in the SH3-like domain resonances. For example, D216 is affected by the mutation but V193 is not. Similarly, V152 shifts in DtxR(E175K) relative to V152 in the wild type. The appearance of resonances from the N-terminal domain indicates a change in the conformational averaging such that the amide resonances are no longer broadened beyond detection. Furthermore, the chemical shifts of these resonances (boxes 8–10) indicate the formation of stabilized secondary and tertiary structure that is not present in the wild-type aporepressor. Taking these observations together, we infer that the E175K mutation alters the interactions between the N-terminal and SH3-like domains such that there are regions of the SH3-like domain that are affected by the mutation, and that the apo-N-terminal domain adopts a more ordered conformation.
Fig. 3.
Two-dimensional 1H,15N heteronuclear single quantum correlation (HSQC) NMR spectra of wild-type apo-DtxR (black) and apo-DtxR(E175K) (green) at 30°C collected at 500-MHz proton frequency. Areas in numbered boxes in Upper are enlarged and shown in Lower 1–10.
Discussion
We have shown that the ancillary site mutant DtxR(H79A) requires higher concentrations of metal ion for repressor activation both in vivo and in vitro. These results suggest that once a metal ion is bound in the ancillary metal ion-binding site there is a partial stabilization of N-terminal domain structure and an increase the affinity of the nascent primary site through cooperative interactions. This interpretation is consistent with previous transition metal ion-binding data, which demonstrated that the intact ancillary site of the primary site mutant DtxR(M10A) has a higher affinity for metal ion than the intact primary site of ancillary site mutant DtxR(H79A) (20). However, it should be noted that these measurements were unable to assess the affinity of the primary site for transition metal ions when the ancillary site was already occupied.
Nonetheless, occupation of the primary metal ion-binding site appears to be necessary to induce the changes in N-terminal domain structure that are required for DtxR homodimerization and DNA recognition. The apparent cooperative relationship between the two metal-binding sites suggests that occupancy of the ancillary metal ion-binding site induces some level of proximal organization of the repressor that is sufficient to increase the apparent affinity of the primary site. We also have shown by comparative 1H,15N NMR spectra of wild-type DtxR and the hyperactive DtxR(E175K) that the mutant repressor retains a more ordered conformation in the absence of activating transition metal ions. These results are consistent with DtxR(E175K) being in a partially activated conformation, even in the absence of transition metal ions.
In addition, we have probed by site-directed mutagenesis the role of the polyprolyl region of the tether that links the N- and SH3-like C-terminal domains of DtxR. This analysis has clearly suggested that the polyprolyl region has at least two functions: (i) it acts as the target site of SH3-like domain binding, and (ii) it stabilizes the dimerization domain through the formation of hydrogen bonds.
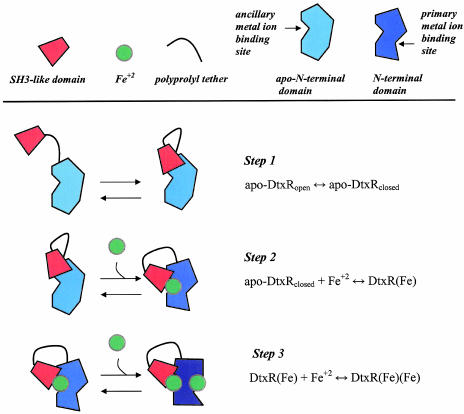
Taken together, these observations support a three-step model for the conversion of the inactive apo-form of DtxR to its fully active metal ion-bound form of the repressor (Fig. 4). Because the in-frame insertion of 12 amino acid residues into the tether region of DtxR results in complete inactivation of repressor activity only in the absence of added transition metal ions, we conclude that apo-DtxR may transit between an “open” and “closed” conformation (step 1). Once a transition metal ion is bound in the ancillary binding site of the closed form of the repressor, it becomes further stabilized through the SH3-like domain ligands E170 and Q173 (step 2). These interactions appear to induce the protein structure changes that result in the formation of the primary metal ion-binding site. In the case of the hyperactive mutants DtxR(E175K) or DtxR(E175R), even in the absence of transition metal ions, electrostatic interactions between the positively charged moieties of these residues with the N-terminal domain (e.g., E19, E20, E21, or E35) are likely to give rise to a partially ordered structure similar to that formed by the wild type repressor with an occupied ancillary metal ion-binding site. Finally, in the case of either the wild-type repressor or the hyperactive mutants, the binding of a transition metal ion to the primary binding site likely results in the formation of fully activated DtxR (step 3) capable of homodimerization and target DNA sequence recognition.
Fig. 4.
A proposed three-step model of the activation of apo-DtxR by transition metal ions. The on–off equilibrium of the SH3-like C-terminal domain (red trapezoid) with its proline-rich binding target occurs in the absence of an activating transition metal ion in step 1. The first activating transition metal ion (green circle) is coordinated at the ancillary metal ion-binding site, which is completed by the donation of two ligands (E170 and Q173) from the SH3-like C-terminal domain (step 2). This step is likely to require the release of the SH3-like binding from the polyprolyl region in the tether between the N- and C-terminal domains. Metal ion occupation of the ancillary metal ion-binding site induces a further structural organization leading to the formation of the primary metal ion-binding site. Finally, the second transition metal ion is bound in the primary metal ion-binding site (step 3), leading to the structural organization of the N-terminal domain necessary for dimerization and DNA recognition. The increase in tertiary structure in the N-terminal domain from the apo- to the fully active repressor is represented schematically by a deepening in blue shading of the N-terminal domain.
In the reverse direction, inactivation of DtxR is likely to proceed with metal ion disassociation from the primary binding site first. If this were the case, then interactions between residue 175 in the SH3-like domain with the N-terminal domain of the repressor would likely be extremely important in maintaining the partially ordered structure necessary for maintaining the half-activation state of the hyperactive mutant repressors. In the case of the wild-type repressor, loss of metal ion from both the primary and ancillary binding sites would be expected to allow the N-terminal domain to return to a fully inactive molten globule state.
Supplementary Material
Acknowledgments
We are grateful to the National High Magnetic Field Laboratory for partial support of this work. This work was supported by U.S. Public Health Service Grant AI-21628 from the National Institute of Allergy and Infectious Diseases.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DP, 2,2′-dipyridyl; DtxR, diphtheria toxin repressor; SH3, src homology 3; Amp, ampicillin; Kan, kanamycin.
References
- 1.Boyd, J., Oza, M. & Murphy, J. R. (1990) Proc. Natl. Acad. Sci. USA 87, 5968-59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitt, M. P. & Holmes, R. K. (1991) Infect. Immun. 59, 1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt, M. P. (1997) Infect. Immun. 65, 4634-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt, M. P., Talley, B. G. & Holmes, R. K. (1997) Infect. Immun. 65, 5364-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hantke, K. (2001) Curr. Opin. Microbiol. 4, 172-177. [DOI] [PubMed] [Google Scholar]
- 6.Tao, X., Zeng, H. & Murphy, J. R. (1995) Proc. Natl. Acad. Sci. USA 92, 6803-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao, X. & Murphy, J. R. (1992) J. Biol. Chem. 267, 21761-21764. [PubMed] [Google Scholar]
- 8.White, A, Ding, X, vanderSpek, J. C., Murphy, J. R. & Ringe, D. (1998) Nature 394, 502-506. [DOI] [PubMed] [Google Scholar]
- 9.Schiering, N., Tao, X., Zeng, H., Murphy, J. R., Petsko, G. A. & Ringe, D. (1995) Proc. Natl. Acad. Sci. USA 92, 9843-9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qui, X., Verlinde, C. L., Zhang, S., Schmitt, M. P., Holmes, R. K. & Hol, W. G. (1995) Structure 3, 87-100. [DOI] [PubMed] [Google Scholar]
- 11.Ding, X., Zeng, H., Schiering, N., Ringe, D. & Murphy, J. R. (1996) Nat. Struct. Biol. 3, 382-387. [DOI] [PubMed] [Google Scholar]
- 12.Love, J. F., vanderSpek, J. C. & Murphy, J. R. (2003) J. Bacteriol. 185, 2251-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pohl, E., Holmes, R. K. & Hol, W. G. (1999) J. Mol. Biol. 292, 653-667. [DOI] [PubMed] [Google Scholar]
- 14.Wang, G., Wylie, G. P., Twigg, P. D., Caspar, D. L., Murphy, J. R. & Logan, T. (1999) Proc. Natl. Acad. Sci. USA 96, 6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Twigg, P. D., Parthasarathy, G., Guerrero, L., Logan, T. & Caspar, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun, L., vanderSpek, J. & Murphy, J. R. (1998) Proc. Natl. Acad. Sci. USA 95, 14985-14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Love, J. F. & Murphy, J. R. (2002) J. Microbiol. Methods 51, 63-72. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. (1992) Experiments in Molecular Genetics (Cold Spring Harbor Lab. Press, Plainview, NY).
- 19.Kay, L. E., Keifer, P. & Saarinen, T. (1992) J. Am. Chem. Soc. 114, 10663-10665. [Google Scholar]
- 20.Spiering, M. M., Ringe, D., Murphy, J. R. & Marletta, M. A. (2003) Proc. Natl. Acad. Sci. USA 100, 3808-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu, X., Pohl, E., Holmes, R. K. & Hol, W. G. J. (1996) Biochemistry 35, 12292-12302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.