Preface
Neisseria gonorrhoeae and Neisseria meningitidis are Gram-negative bacterial pathogens that are exquisitely adapted for growth at human mucosal surfaces and for efficient transmission between hosts. One factor that is essential to neisserial pathogenesis is the interaction between the bacteria and neutrophils, which are recruited in high numbers during infection. Although this vigorous host response could simply reflect effective immune recognition of the bacteria, there is mounting evidence that in fact these obligate human pathogens manipulate the innate immune response to promote infectious processes. This Review summarizes the mechanisms used by pathogenic neisseriae to resist and modulate the antimicrobial activities of neutrophils. It also details some of the major outstanding questions about the Neisseria–neutrophil relationship and proposes potential benefits of this relationship for the pathogen.
Introduction
The genus Neisseria encompasses commensal and pathogenic species that colonize human mucosal epithelia. Commensal species, including Neisseria lactamica and Neisseria mucosa, live on the mucosal surface of the nasopharynx and rarely cause disease in healthy individuals. By contrast, the pathogenic species Neisseria gonorrhoeae and Neisseria meningitidis, both of which are closely related to commensal Neisseria spp., can induce inflammation and breach mucosal barriers (BOX 1). The pathogenic neisseriae are closely related and are therefore genetically and physiologically highly similar, but they differ in disease presentation: N. gonorrhoeae causes the prevalent sexually transmitted infection gonorrhoea, whereas N. meningitidis causes highly infectious meningococcal meningitis. However, they share the potential for long-term colonization of a single host in the absence of antibiotic therapy. They also both have the genetic flexibility to respond to changes within or between hosts, have similar high transmissibility between hosts and can increase the possibility of transmission by causing common asymptomatic infections that escape detection and treatment.
Box 1. Neisserial Disease.
Neisseria gonorrhoeae and Neisseria meningitidis cause an estimated 88 million cases of gonorrhoea and 500,000 cases of meningococcal meningitis, respectively, worldwide per year 4, 150. As obligate human bacterial pathogens, they are highly evolved for colonization of human mucosal epithelial surfaces 3, 4. Gonococci engage the epithelial surfaces encountered during sexual transmission, such as the male urethra, female cervix, the rectum and the pharynx. Whereas most urethral infections produce symptoms including dysuria and purulent discharge, infections of the cervix, rectum and pharynx are frequently asymptomatic, which complicates the diagnosis and treatment of gonorrhoea and contributes to its persistence 7. Vertical transmission during childbirth can result in neonatal conjunctivitis.
N. meningitidis colonizes the nasopharynx of ~10% of the human population, although this can be much higher during seasonal epidemics in the African ‘meningitis belt’ and during other outbreaks. Only a small percentage of individuals colonized with N. meningitidis present with the invasive diseases of meningitis and meningococcaemia 4.
The clinical hallmark of infection by the pathogenic neisseriae is a host innate immune-driven inflammatory response, characterized by a potent neutrophil influx. The subsequent tissue damage enables bacterial access to secondary anatomical sites, which promotes much of the morbidity and mortality associated with neisserial infections. For N. meningitidis, these sites include the bloodstream, the skin and the meninges, and for N. gonorrhoeae they include the fallopian tubes, heart, skin and joints. At these sites, infection by pathogenic neisseriae has adverse outcomes, including disseminated intravascular coagulation and septic shock for N. meningitidis, and pelvic inflammatory disease, dermatitis, endocarditis and arthritis for N. gonorrhoeae.
The pathogenic neisseriae express a set of common virulence determinants that allow efficient colonization, immune evasion and transmission. Reflecting their high degree of adaptation to humans, rather than produce cytotoxins or other secreted toxic products, these bacteria have evolved specialized mechanisms to promote growth and persistence in the host (TABLE 1). N. gonorrhoeae and N. meningitidis also express species- specific factors that affect their different sites of infection, transmission and disease course 1, although many of these so-called virulence determinants are also expressed by commensal organisms 2. Because it is difficult to separate the properties that facilitate colonization by the pathogenic neisseriae — properties which may be shared among all Neisseria spp. — from those that are crucial for eliciting disease, the features that define the pathogenic species remain obscure.
Table 1.
Virulence factors of the pathogenic Neisseria.
| Virulence factor | Presence in Neisseria | Function | References |
|---|---|---|---|
| Capsule | N. meningitidis | Protects against phagocytosis, antimicrobial peptides, killing by complement. Different strains produce different capsules. | 40–42, 47, 59 |
| KatA | Pathogenic and commensal Neisseria | Detoxifies H2O2. Protects against ROS. Aids infection of the mouse genital tract. | 109, 114, 125, 128 |
| FarAB | Pathogenic and commensal Neisseria | Efflux pump that protects bacteria from fatty acids. | 133 |
| FHBP | N. meningitidis and some commensals | Binds complement factor H to prevent complement-mediated killing. | 54 |
| HpuA, HpuB and HmbR | Pathogenic and commensal Neisseria | Receptors for haptoglobin-hemoglobin; iron acquisition. | 142 |
| IgA protease | Pathogenic Neisseria (and one N. lactamica isolate) | Cleaves secretory IgA. Cleaves lysosomal LAMP1 in epithelial cells. | 154–157 |
| LOS | Pathogenic and commensal Neisseria | Endotoxin. Highly stimulatory to the human immune system. Protects against antimicrobial peptides. Adheres to the asialoglycoprotein receptor on urethral cells. Important for phagocytosis by neutrophils. LOS sialylation (by the enzyme Lst) prevents complement deposition and phagocytosis by neutrophils. LOS modification by phosphoethanolamine (by the enzyme LptA) provides resistance to antimicrobial peptides and complement. Strains of the same species produce different LOS glycoforms. | 15–16, 20, 27, 47–51, 53, 59, 61, 91, 136–138 |
| LbpAB | Pathogenic and commensal Neisseria | Receptor for human lactoferrin; iron acquisition. | 142 |
| MntABC | Pathogenic Neisseria, some commensals | Quenches ROS. | 111, 123, 125 |
| MtrCDE | Pathogenic and commensal Neisseria | Efflux pump that protects bacteria from antibiotics and antimicrobial peptides; aids infection of the mouse genital tract. | 132, 134 |
| MsrAB | Pathogenic and commensal Neisseria | Repairs oxidized proteins. | 112 |
| NadA | N. meningitidis | Adhesin and invasin. | 19 |
| Ngo1686 | Pathogenic Neisseria; some commensals | Metalloproteinase that protects bacteria from ROS and non-oxidative killing by neutrophils. | 38, 39 |
| NMB0741, NMB1828 | Pathogenic and commensal Neisseria | Hypothetical proteins that protect bacteria from antimicrobial peptides. | 139 |
| NMB1436–1438 | N. meningitidis | Protects against killing by neutrophils. | 119 |
| NspA | N. meningitidis | Outer membrane protein that binds complement factor H. | 55 |
| Opa proteins | Pathogenic Neisseria and some commensals (limited repertoire) | Promote attachment and invasion of human cells, including neutrophils; bacterial aggregation; intrastrain changes in Opa expression occur as a result of gene phase variation. | 9, 11–13, 20, 36, 44–45, 59, 62–90, 124 |
| Opc | N. meningitidis | Structurally related to Opa proteins. Binds vitronectin. Promotes invasion of human cells via integrins. | 18 |
| PacA | Pathogenic Neisseria; some commensals | Acetylates peptidoglycan to protect from degradation by lysozyme. | 140 |
| Type IV pili | Pathogenic Neisseria and some commensals | Mediate attachment to various cells/tissues; microcolony formation; twitching motility; natural competence. Extensive intrastrain phase and antigenic variation occurs. | 9–10, 17, 35, 43–45, 59, 152–153 |
| Porins | Pathogenic and commensal Neisseria | Nutrient acquisition. Recognized by TLR2. Bindscomplement factors C3b, C4b and H, and complement factor 4b-binding protein. Cooperate with pili for CR3- mediated internalization. Can translocate into host cells and modulate ROS production and apoptosis. Strains of the same species can express different porins. | 17, 25, 52, 56–57, 61, 100–102, 104, 122 |
| RecN | Pathogenic and commensal Neisseria | Recombinational repair protein that protects against ROS and non-oxidative killing by neutrophils. | 38–39 |
| SOD proteins | Pathogenic and commensal Neisseria | Detoxify ROS. | 110, 113, 123 |
| TbpAB (also known as Tbp1–Tbp2) | Pathogenic and commensal Neisseria | Receptor for human transferrin; iron acquisition. | 142 |
CR3, complement receptor 3; FarAB, fatty acid resistance system; Fhbp, factor H-binding protein; HmbR, haemoglobin receptor; Hpu, haemoglobin–heptaglobin utilization; IgA, immunoglobulin A; IgAP, IgA1 protease; KatA, catalase; LAMP1, lysosome-associated membrane glycoprotein 1; LbpAB, lactoferrin-binding protein complex; LOS, lipo-oligosaccharide; MntABC, Mn(II) transport system; MsrAB, methionine sulphoxide reductase; MtrCDE, multiple transferable resistance system; NadA, neisserial adhesin A; NspA, neisserial surface protein A; Opa, opacity-associated; Opc, outer-membrane protein C; PacA, peptidoglycan O-acyltransferase; ROS, reactive oxygen species; Sod, superoxide dismutase; TbpAB, transferrin-binding protein complex; TLR2, Toll-like receptor 2.
Given the high degree of adaptation of the pathogenic neisseriae to humans, it is noteworthy that the bacteria avoid the host adaptive immune system (BOX 2) and trigger a potent innate immune response involving neutrophils (also known as polymorphonuclear leukocytes (PMNs)). Infection stimulates the recruitment of neutrophils to the urogenital tract and cerebrospinal fluid (CSF), sites that are normally free of these cells, and the presence of copious numbers of neutrophils at sites of infection is the primary clinical manifestation of disease 3, 4. As the first responders of the immune system to infection or injury, neutrophils possess cytoplasmic granules that contain many antimicrobial enzymes, peptides and reactive chemicals 5. These cells either take up microorganisms by phagocytosis into phagolysosomes, which then fuse with the granules to kill the microorganisms inside, or kill microorganisms extracellularly via granule exocytosis or release of DNA-rich neutrophil extracellular traps 6. The prevailing hypothesis emphasized in medical microbiology is that humans are especially adept at recognizing and responding to neisserial infections, and this vigorous innate immune response accounts for much of the host damage that accompanies gonorrhoea and meningococcal meningitis (BOX 1). But, despite this potent inflammatory response, neutrophils do not clear these infections; viable bacteria can be cultured from CSF in patients with meningococcal meningitis and from gonorrhoeal secretions, in which neutrophils are plentiful. Moreover, individuals with neutrophil immunodeficiencies are not at increased risk of neisserial infection 3, 4. These observations suggest that, instead, the recruitment of neutrophils by pathogenic neisseriae may not be especially deleterious to the bacteria and may in fact enhance the disease-causing potential of these pathogens. In this Review, we describe the interaction between the pathogenic neisseriae, N. gonorrhoeae and N. meningitidis, and neutrophils, including the recruitment of neutrophils during neisserial infections, resistance of the bacteria to the extracellular and intracellular antibacterial arsenal of neutrophils, and bacterial modulation of neutrophil phagocytosis, oxidant production and apoptosis. We propose a model whereby the interaction between pathogenic neisseriae and neutrophils promotes, rather than blocks, the infection cycle.
Box 2. Interactions of pathogenic neisseriae with the adaptive immune system.
Infection by the pathogenic species Neisseria gonorrhoeae and Neisseria meningitidis is poorly controlled by the adaptive immune system owing to high-frequency antigenic variation of three major surface antigens: lipo-oligosaccharide (LOS), opacity-associated (Opa) proteins and type IV pili 59. Variation of LOS and Opa proteins is mediated by the random switching on and off (phase variation) of accessory LOS glycosyltransferases or complete opa genes. Both switches occur via changes in nucleotide repeat numbers during DNA replication, altering the reading frame of the affected gene and subsequent protein production 65, 151. Pili vary by gene conversion between silent storage copies of variant pilin information (the multiple copies of pilS) and the single expressed pilin gene (pilE) 151; pili also phase vary through changes in nucleotide repeat number in the pilC genes (which maintain pilus expression on the bacterial surface) 153. Thus, every population of pathogenic Neisseria spp. has subpopulations of bacteria that express antigenically distinct surface structures. It is assumed that antibodies reacting with any of these three antigens could block infection, providing the selective pressure for evolution of such complex antigenic and phase variation mechanisms. However, switching of LOS, Opa protein or pilus expression states also alters bacterial interactions with host cells such as neutrophils (see main text). N. gonorrhoeae has the genetic capacity to generate much more diversity in these surface antigens than N. meningitidis, presumably reflecting the greater chance of gonococcal transmission and reinfection. Whereas certain N. meningitidis capsular polysaccharides elicit a protective antibody-mediated immune response in immunized individuals — and these polysaccharides are the basis of the current quadrivalent vaccines —the presence of a capsule composed uniquely of sialic acid on serogroup B N. meningitidis makes this serogroup poorly immunogenic and refractory to vaccine-mediated protective immunity4.
The pathogenic neisseriae also directly modulate adaptive immune functions. N. gonorrhoeae and N. meningitidis secrete an immunoglobulin A1 (IgA1) protease that can directly inactivate certain classes of secretory IgA in mucosal secretions 154. Protease expression may prevent IgA-mediated opsonization of the bacteria to block phagocytosis through IgA receptors on neutrophils and other cells, and to prevent complement-mediated lysis. IgA1 protease also cleaves the human lysosome-associated membrane glycoprotein 1 (LAMP1)155, 156, which localizes to the lysosomes of cells, including neutrophils. However, the role of IgA1 protease in infection with pathogenic neisseriae is enigmatic, as an IgA1 protease-mutant N. gonorrhoeae retains infectivity in the male urethral-challenge model 157. The pathogenic neisseriae also downregulate CD4+ T cell function 143 and kill B cells 144 in CEACAM1-dependent manners. Along with the inability of live pathogenic neisseriae to fully activate antigen-presenting cells such as dendritic cells 158, these results imply that during infections with pathogenic neisseriae there is a localized immune suppression that would contribute to long-term bacterial persistence in the host.
Initiation of infection and initial immune detection
Host cell binding
An essential first step in initiating human infection is colonization of the target mucosal epithelium (FIG. 1). The pathogenic neisseriae infect diverse mucosal surfaces, including the urethra, cervix, fallopian tubes, rectum, nasopharynx and conjunctiva 3, 4, 7. Type IV pili mediate the initial attachment to the apical surface of epithelial cells at most mucosal surfaces, after which opacity-associated proteins (Opa proteins) drive intimate adherence and internalization 8. In male volunteers inoculated with N. gonorrhoeae, only piliated bacteria efficiently colonize and produce symptomatic infection 9, 10, and the majority of the N. gonorrhoeae cells isolated from the male urethra and from the female genital tract during the proliferative stage of the menstrual cycle express Opa proteins 11–13. Additional adhesins and invasins allow the pathogenic neisseriae to infect particular epithelial cell subsets. For example, lipo-oligosaccharide (LOS) with lacto-N-neotetraose at its terminus can bind the asialoglycoprotein receptor on urethral cells; the pilus and the porins interact cooperatively with complement receptor 3 (CR3) on cervical epithelium; and ‘moonlighting’ bacterial proteins such as ribosomal protein L12 bind the lutropin receptor on endometrial cells 14–17. Adhesins specific to N. meningitidis, including outer-membrane protein C (Opc) and neisserial adhesin A (NadA), target receptors on epithelial and other cells that the bacterium encounters after penetration of the nasopharynx, including those in the bloodstream and meninges 18, 19.
Figure 1. Neutrophil recruitment to sites of infection by the pathogenic neisseriae.

Although pathogenic neisseriae colonize diverse mucosal surfaces in the human body, we have only a limited understanding of how differences in these diverse environments create challenges and opportunities for the bacteria 4, 7. Pathogenic neisseriae use type IV pili, opacity-associated (Opa) proteins and other adhesins to colonize and occasionally invade the mucosal epithelium. Bacterial products such as lipo-oligosaccharide (LOS), peptidoglycan and lipoproteins, which are released either as free compounds or in outer-membrane vesicles (small red circles), stimulate NOD-like receptor (NLR) and Toll-like receptor (TLR) family pattern recognition receptors (green) on epithelial cells and immune cells such as dendritic cells (DCs), macrophages (Mϕ) and T cells (T¢). As a result, gradients of pro-inflammatory cytokines, including interleukin-6 (IL-6), IL-8, IL-1β, interferon-γ (IFNγ) and IL-17, are established. The cytokines recruit neutrophils (yellow) and induce their migration across the epithelium, where they bind and phagocytose the bacteria.
Detection by the innate immune system
Like many other bacteria, pathogenic neisseriae are detected by mucosal epithelial cells and by the sentinel immune cells in the epithelium, which may include T helper 17 (TH17) cells, macrophages and dendritic cells 20–22. These cells can detect infection or injury via the membrane-associated Toll-like receptor (TLR) and cytoplasmic NOD-like receptor (NLR) families of pattern recognition receptors 23, 24, and they therefore participate in the recognition of pathogenic neisseriae. LOS is a potent activator of the TLR4–CD14 complex, and porins and lipoprotein H.8 engage TLR2 25–27. Furthermore, peptidoglycan fragments in outer-membrane vesicles are recognized by NLRs in the cytoplasm of epithelial cells 28. As a consequence, infection by pathogenic neisseriae promotes local release of interleukin-8 (IL-8), IL-6, tumour necrosis factor, IL-1β and other cytokines, creating a microenvironment that efficiently recruits and activates neutrophils 29–33 (Figure 1). Movement of neutrophils to the site of infection follows a well-described series of events, starting with increased adhesion to the vasculature, followed by transendothelial migration and crossing of subepithelial tissues and ending with entry into the infected epithelium 34. In male volunteers experimentally infected with N. gonorrhoeae, neutrophil recruitment to the urethra occurred, on average, 2–3 days after infection, and the appearance of neutrophils in the urine was rapidly followed by the onset of dysuria and other symptoms consistent with acute disease 13, 35. A similar timing of neutrophil recruitment has been observed in the mouse genital tract model of gonococcal infection 36. By contrast, the timing of neutrophil recruitment to the meninges in response to N. meningitidis is less well established, but the appearance of neutrophils in the CSF is used to help diagnose meningitis caused by N. meningitidis and other bacteria 4.
Survival of neisseriae after neutrophil exposure
Neutrophils have potent intracellular and extracellular antimicrobial activities. Neutrophil extracellular traps and reactive oxygen species (ROS) combat extracellular microorganisms, whereas bacteria that are taken up by neutrophils are transported into a phagosome that contains ROS, degradative enzymes and antimicrobial peptides. In vivo, neutrophils are closely associated with both pathogenic neisseriae: gonorrhoeal exudates and the CSF from patients with meningococcal meningitis reveal bacteria attached to and inside neutrophils. However, as these fluids contain viable bacteria, pathogenic neisseriae must possess mechanisms that allow them to survive both the intracellular and extracellular antimicrobial mechanisms of neutrophils. As described below, pathogenic neisseriae can modulate phagocytosis by neutrophils, but even those bacteria that are internalized can circumvent the intracellular antibacterial activities of the host.
Resistance to extracellular antimicrobial mechanisms
Neutrophils kill adherent microorganisms through localized granule exocytosis and extracellular production of ROS 37. Neutrophils also produce neutrophil extracellular traps, which capture and inactivate bacteria at a distance from the neutrophil surface 6. Despite these mechanisms, neutrophils are not especially adept at killing extracellular bacteria; when N. gonorrhoeae is incubated in the presence of neutrophils in vitro, >80% of extracellular bacteria remain viable, and neutrophils in which phagocytosis is inhibited possess a limited ability to kill N. gonorrhoeae 38. At least one N. gonorrhoeae virulence factor, the metalloproteinase NGO1686 (which is also encoded in meningococcal genomes), protects the bacterium from extracellular killing by neutrophils 38, 39. This observation supports the contention that N. gonorrhoeae, and potentially N. meningitidis, can withstand the extracellular antimicrobial activities of neutrophils when phagocytosis has not occurred.
Prevention of phagocytosis
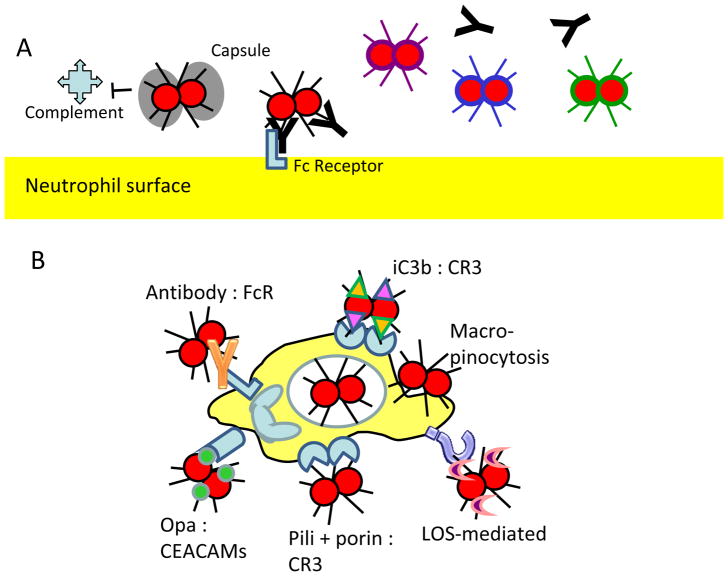
The pathogenic neisseriae avoid phagocytosis by neutrophils in three ways: by preventing their binding to the neutrophil surface, by limiting the deposition of opsonic antibody or complement on the bacterial surface and by varying antigenic surface structures to evade humoral immunity (Fig. 2a).
Figure 2. Interactions of pathogenic neisseriae with neutrophils.
A. Avoidance of phagocytosis. Capsular polysaccharides (bacterium with black hatched or grey surface) prevent deposition of complement on Neisseria meningitidis and subsequent phagocytosis by neutrophils. Continual changes in surface antigens (bacteria with different color surfaces) allow neisseriae to avoid triggering the generation of effective opsonic antibodies that would facilitate phagocytosis.
B. Induction of phagocytosis. Neisseriae can be phagocytosed by host cells using six different routes, which can be receptor dependent or receptor independent: immunoglobulin G (IgG)-opsonized bacteria bind the Fcγ receptor (FcR); proteolytically inactive C3b (iC3b)-opsonized bacteria bind complement receptor 3 (CR3); pili and porin proteins cooperatively bind CR3; neisserial opacity-associated (Opa) proteins bind CEACAMs; lipo-oligosaccharide (LOS) binds unknown receptors on neutrophils; and receptor-independent macropinocytosis can also occur.
C. Protection against neutrophil-mediated antibacterial activities. Neutrophils produce reactive oxygen species (ROS) through the phagocyte NADPH oxidase and myeloperoxidase (MPO). Neisserial proteins such as catalase (KatA), superoxide dismutase (Sod) proteins, the Mnii transport system (MntABC) and l-glutamate transporter (GltT) detoxify or quench ROS. The bacteria also repair proteins damaged by ROS through methionine sulphoxide reductase (MsrAB), and repair DNA damaged by ROS through recombinational (RecA), nucleotide excision (the Uvr proteins) and base excision (MutY) repair of DNA. The pathogenic neisseriae also suppress neutrophil-mediated production of ROS through porins and other mechanisms that are dependent on live bacteria.
D. Bacterial defences against non-oxidative factors from neutrophils. Antimicrobial factors that are independent of ROS include antimicrobial peptides (AMPs), proteases, lysozyme and acid. The multiple transferable resistance system (MtrCDE) and fatty acid resistance system (FarAB) in pathogenic neisseriae remove some of these products from the bacterial cytosol. Furthermore, bacterial lipopolysaccharide transporter periplasmic protein A (LptA) and peptidoglycan O-acyltransferase (PacA) modify LOS and peptidoglycan (PG), respectively, to protect the bacteria from these factors. The bacterial proteins NGO1686, RecN, NMB0741 and NMB1828 also protect the bacteria against non-oxidative factors, but their protective mechanisms are unknown. Specific locations of molecules associated with the bacterial envelope (PG, LptA, LOS, NGO1686, MtrCDE, FarAB and MisS) are not shown. HOCl, hypochlorous acid.
As phagocytosis requires close apposition between the neutrophil and bacterial membranes, one effective strategy to avoid phagocytosis is to physically block this interaction. N. meningitidis produces a polysaccharide capsule that prevents phagocytosis by increasing the negative charge of the bacterial surface 40. Many different capsular serotypes have been described for N. meningitidis, six of which are associated with epidemic disease (A, B, C, W-135, X and Y) 41. N. gonorrhoeae does not produce a capsule. Interestingly, capsule expression in N. meningitidis can be turned on and off during the bacterial life cycle, suggesting that there are points in the life cycle at which capsule production is disadvantageous, such as when inside a host cell 41, 42. It was originally thought that the type IV pili of pathogenic neisseriae, which extend several micrometres from the bacterial outer membrane, were antiphagocytic 43, but most research has refuted this idea 44, 45.
Efficient phagocytosis of bacteria is driven by opsonization with antibody and/or complement. Therefore, bacteria can avoid phagocytosis by preventing antibody or complement deposition. The meningococcal capsule masks bacterial surface antigens that can be recognized by antibodies, complement or lectin-type phagocytic receptors. This masking not only blocks opsonophagocytosis but also confers resistance to complement-mediated killing 46, 47. The pathogenic neisseriae also prevent antibody deposition by incorporating sialic acid (N-acetylneuraminic acid) into their surface structures. As sialic acid is also found on the surface of mammalian cells, it is not antigenic to the immune system. Because antibodies are not raised against sialylated moieties on bacterial surface structures, sialylation inhibits opsonophagocytosis of the bacteria. Sialic acid is the major component of the serogroup B N. meningitidis capsule and is also part of the capsules of serogroup C, W-135 and Y N. meningitidis 41. Furthermore, the terminal galactose residue of LOS from pathogenic neisseriae is sialylated by the α-2,3-sialyltransferase, encoded by lst 48. Reflecting the high degree of adaptation of the pathogenic neisseriae to humans, the activated sialic acid substrate for the sialyltransferase, cytidine monophospho-N-acetylmuramic acid, is produced by human cells, not the bacteria 49. Bacteria with sialylated LOS have enhanced survival in the presence of human and mouse neutrophils 50 and are also more resistant to complement-mediated killing 51.
The pathogenic neisseriae also limit complement-mediated phagocytosis by binding the complement cascade inhibitors complement factor H and complement factor 4b-binding protein (C4BP). Complement factor H directly interacts with the N. gonorrhoeae porin PorB1A (encoded by porB) and sialylated LOS, and N. meningitidis also produces complement factor H-binding lipoprotein and neisserial surface protein A (NspA) 52–55. C4BP binds to the PorA porin of N. meningitidis (encoded by porA) and to both PorB1A and PorB1B (also encoded by porB) of N. gonorrhoeae, contributing to bacterial serum resistance 56, 57. The association between variants of the human complement factor H gene and serious meningococcal disease 58, and the observation that individuals lacking terminal complement components (such as C6) are more susceptible to meningococcal infection 4, underscores the importance of evading complement deposition in neisserial pathogenesis.
The pathogenic neisseriae avoid antibody-mediated opsonization by varying the antigenic surface structures. N. gonorrhoeae and N. meningitidis undergo high-frequency variation in the expression and composition of LOS, Opa proteins and type IV pili (Box 2) 59. By continually varying the antigens that are presented to the host immune system, the bacteria stay one step ahead of the humoral immune response, thereby preventing antibody binding to the bacterial surface and subsequent phagocytosis through immunoglobulin receptors.
Phagocytosis of neisseriae
Although the pathogenic neisseriae can avoid phagocytosis in various ways, numerous bacteria are detected inside neutrophils from patients with gonorrhoea or meningococcal meningitis (in fact, the original name for N. meningitidis was Diplococcus intracellularis 4). Experimental evidence suggests that both opsonized and non-opsonized bacteria can be phagocytosed by neutrophils (FIG. 2b). Generally, when a particle engages immunoglobulin or complement receptors on neutrophils, it is internalized into a phagosome that fuses with cytoplasmic granules. This produces an acidified phagolysosome containing ROS, degradative enzymes and antimicrobial peptides, a toxic combination 60. Although the pathogenic neisseriae have evolved extensive mechanisms for evasion of complement and antibodies, as described above, convalescent serum from patients with meningococcal meningitis contains Neisseria spp.-reactive antibodies, and complement factors C3b and C4b can be fixed on the bacterial surface 4, 61. These findings suggest that a fraction of the pathogenic neisseriae is subjected to opsonophagocytosis during infection.
In the absence of opsonization, the outer-membrane Opa proteins mediate internalization of the pathogenic neisseriae by neutrophils. N. meningitidis and N. gonorrhoeae encode up to 11 and five different Opa proteins in their genomes, respectively 62–64; most research has focused on the gonococcal Opa proteins. Each Opa-encoding gene independently undergoes phase variation through a pentameric repeat in the DNA sequence encoding the signal peptide, such that there are both Opa+ and Opa− bacteria in the population, and some Opa+ bacteria express multiple Opa proteins 65. The majority of Opa proteins are recognized by one or more of the human CEACAM receptors (these Opa proteins are referred to here as OpaCEA proteins), a more restricted subset binds heparan sulphate proteoglycans (referred to here as OpaHS proteins) and some Opa proteins engage both types of protein 66. N. gonorrhoeae expressing defined OpaCEA proteins undergoes enhanced phagocytosis and killing 45, 67–70. Moreover, heterologous expression of OpaCEA proteins in E. coli is sufficient to promote phagocytosis by neutrophils 71, and N. gonorrhoeae outer membrane vesicles containing Opa proteins, or purified Opa proteins reconstituted in liposomes or detergent micelles, inhibit the interaction of homologous Opa+ bacteria with neutrophils 72. Thus OpaCEA proteins are an important mediator of neutrophil-mediated phagocytosis of pathogenic neisseriae.
The CEACAMs are members of the immunoglobulin superfamily of cell adhesion receptors and mediate intercellular associations that are important for tissue structure, neovascularization and insulin responsiveness; importantly, CEACAMs are used as receptors by non-neisserial human-specific bacterial pathogens that live at mucosal surfaces, including Moraxella catarrhalis and Haemophilus influenzae 73. It has been proposed that CEACAM3, which is expressed only by neutrophils and other granulocytes, evolved to assist neutrophils in clearing these bacteria 74–76. CEACAM1 and CEACAM6 are also expressed by neutrophils 77–81, and human CEACAM1, CEACAM3, CEACAM5 and CEACAM6 all bind neisserial OpaCEA proteins through an interaction of their amino-terminal immunoglobulin variable-like domain with the hypervariable loops of OpaCEA 82, 83. CEACAM1, CEACAM3 and CEACAM6 possess multiple extracellular immunoglobulin-like domains, and each protein transduces different intracellular signals. Membrane-spanning isoforms of CEACAM1 have two cytoplasmic immunotyrosine inhibitory motifs (ITIMs) that block cytokine production and proliferation in T cells 84. By contrast, transmembrane isoforms of CEACAM3 have a cytoplasmic immunotyrosine activation motif (ITAM) that stimulates kinase-dependent signalling events in neutrophils, culminating in phagocytosis, ROS production and killing of OpaCEA+ N. gonorrhoeae 87–89. The glycophosphatidylinositol (GPI)-anchored CEACAM6 and ‘short’ isoforms of CEACAM1 and CEACAM3 require transmembrane CEACAMs or other receptors to transduce signals 73, 84. Intriguingly, CEACAM1 and CEACAM6 potentiate, rather than impede, CEACAM3 signalling in neutrophils infected with high numbers of N. gonorrhoeae 88. This observation helps explain why N. gonorrhoeae that binds only CEACAM1 promotes ROS production 89 and bacterial killing 90 by neutrophils.
OpaCEA+ N. gonorrhoeae survives less well after exposure to neutrophils in vitro than its Opa− counterpart 68, 69. However, Opa+ N. gonorrhoeae is commonly recovered from the urethra of men with uncomplicated gonorrhoea and from the cervix of women in the proliferative phase of the menstrual cycle, two sites where neutrophils are also commonly found during neisserial infection, suggesting that at least some Opa+ bacteria survive neutrophil surveillance despite recognition by CEACAM3 11–13. At least two possibilities could explain the in vivo selection for Opa+ neisseriae despite their increased sensitivity to killing by neutrophils. The first possibility is that the selective pressure to maintain Opa expression for bacterial binding and uptake by epithelial cells outweighs the detrimental effect of Opa expression on survival after exposure to neutrophils. With this in mind, it is intriguing that a subset of neisserial OpaCEA proteins are recognized by other human CEACAMs but not by CEACAM3 79, 81. This suggests that the pathogenic neisseriae have evolved these specific Opa proteins to maintain the capacity for mediating epithelial colonization while avoiding clearance by neutrophils through CEACAM3 76. As Opa expression is phase variable, a proportion of the bacterial population can avoid CEACAM3-dependent phagocytosis and killing by neutrophils, ensuring persistence of some bacteria in the human host. The second possibility, as discussed below and suggested by in vitro evidence, is that not all internalized Opa+ neisseriae are killed by neutrophils. The pathogenic neisseriae may in fact exploit OpaCEA protein expression to drive their own uptake into neutrophils, which may provide the bacteria with advantages inside the host.
As well as the Opa proteins facilitating phagocytosis of non-opsonized neisseriae, adherent and chemokine-primed primary human neutrophils have been shown to internalize non-opsonized Opa− N. gonorrhoeae 38. Opsonin-independent, Opa-independent phagocytosis of the pathogenic neisseriae may occur in three ways. First, neutrophils may use an unknown receptor to bind to N. meningitidis producing lacto-N-neotetraose-containing LOS 91. Second, pathogenic neisseriae may engage CR3 through a cooperative interaction between type IV pili and the porin PorB1B, as described for the N. gonorrhoeae interaction with cervical epithelial cells 17. Third, the pathogenic neisseriae may be small enough (less than 1 μm in diameter) to be internalized by receptor-independent macropinocytosis.
It is clear that the surface variability of the pathogenic neisseriae contributes to complex interactions with neutrophils that can either activate or inhibit phagocytosis and antibacterial activities. It will require systematic investigation with defined variants and mutants to determine how the expression of different bacterial surface ligands alters phagocytosis and whether these interactions dictate different fates for the bacteria within neutrophils (see below).
Neisserial survival inside neutrophils
Phagocytosis by neutrophils is generally a dead end for bacteria. However, a fraction of the pathogenic neisseriae seems to survive and replicate inside neutrophils. Electron microscopy studies revealed the presence of electron-dense, intact bacteria inside neutrophils from inflammatory exudate44, 92–94, and through the use of membrane-impermeant antibiotics it was shown that the number of intracellular N. gonor-rhoeae cells and the number of bacteria per intracellular phagosome inside neutrophils increase over time 95–97. This occurs for both serum-opsonized, Opa+ and non-opsonized, Opa− N. gonorrhoeae, suggesting that the route of entry does not affect the eventual replication of the bacteria inside cells 38, 98. Although it is formally possible that the increase in bacterial numbers inside neutrophils is due to continual phagocytosis of live extracellular bacteria, and the increase in numbers of bacteria per phagosome results from fusion between phagosomes, these findings strongly suggest that N. gonorrhoeae, and potentially also N. meningitidis, survives and replicates inside neutrophils. Pathogenic neisseriae may persist inside neutrophils as a result of their intrinsic resistance to neutrophil antimicrobial factors and by interfering with the release of these factors.
Preventing PMN apoptosis
As neutrophils are terminally differentiated cells with a half-life of several hours, internalization by these cells could be considered a dead end for Neisseria spp., unless the lifespan of these immune cells could be increased. To this end, the pathogenic neisseriae modulate the apoptosis programmes of neutrophils and of other human cells. Experiments primarily using cultured immortalized epithelial cells have revealed both pro-and anti-apoptotic effects of pathogenic neisseriae 99–102. Modulation of apoptosis has been linked to translocation of neisserial porin to the mitochondrion, a process which may require other factors, such as Omp85 103. However, the mechanism by which the translocated porin modulates apoptosis remains unknown 102, 104. Modulation of apoptosis by a porin may also occur in neutrophils, as infection by N. gonorrhoeae extends the lifetime of some primary human neutrophils by delaying spontaneous apoptosis 33, 105, in contrast to phagocytosis of most bacteria, which triggers apoptosis 106. This lengthening of the neutrophil lifespan by pathogenic neisseriae supports the idea that these bacteria can modulate neutrophils to gain an advantage during infection of the human host.
Disarming neutrophils
Whether bacteria are inside or outside neutrophils, there are two basic mechanisms that can be invoked to explain survival of the pathogenic neisseriae among neutrophils. First, the bacteria could possess inherent resistance to neutrophil antimicrobial factors. There is strong genetic and biochemical evidence that specific gene products can resist neutrophil factors, but in many cases these protective gene products have not been directly evaluated in the context of intact neutrophils. Second, the bacteria could respond to and downregulate the antimicrobial activities of neutrophils — for example, by delaying neutrophil apoptosis and modulating release of neutrophil antimicrobial products. Given the high degree of host adaptation by the pathogenic neisseriae, it is not surprising that there is evidence that both mechanisms can act to promote bacterial survival after exposure to neutrophils.
Neisserial resistance to neutrophil-mediated oxidative damage
One of the best described antimicrobial properties of neutrophils is the oxidative burst. Activated neutrophils assemble the multisubunit NADPH oxidase enzyme on the phagosomal or plasma membrane 107 (Fig. 2c). NADPH oxidase generates superoxide, which spontaneously or catalytically dismutates to hydrogen peroxide; in turn, hydrogen peroxide is used by the neutrophil granule enzyme myeloperoxidase to produce hypochlorous acid. These ROS are microbicidal owing to their ability to damage lipids, proteins and nucleic acids 108.
To defend against ROS, the pathogenic neisseriae encode several proteins that directly detoxify these compounds (including catalase, cytochrome c peroxidase and superoxide dismutase) or quench them (such as the MnII transport system (MntABC)), or that repair oxidatively damaged DNA and proteins (such as MutY, RecA, the Uvr system and MsrAB) 109–117 (Fig. 2C). In addition, N. meningitidis counters ROS by using L-glutamate transporter (GltT) to acquire L-glutamate, which is converted to glutathione and helps maintain cytoplasmic redox potential during ROS exposure 118. Additional proteins that protect against ROS include the gonococcal metalloproteinase NGO1686 and the proteins encoded in the NMB1436–1438 operon in N. meningitidis 39, 119. In N. gonorrhoeae str. FA1090, transcription of many of these genes is increased following exposure to sublethal concentrations of oxidants, suggesting the presence of transcription-regulatory circuits that protect the bacteria from oxidative stress 39. The pathogenic neisseriae also possess at least three means of inhibiting the production of ROS by neutrophils. Exposure to lactate, a by-product of neutrophil glycolysis, enhances bacterial consumption of molecular oxygen, which depletes the substrate for NADPH oxidase and thus blunts the oxidative burst 120. Viable Opa− N. gonorrhoeae can also suppress ROS production through a process that requires metabolically active bacteria and direct contact with neutrophils 121. Finally, purified porin can translocate into cells and, subsequently, into the mitochondria by a poorly understood mechanism to block ROS production 122.
Despite the presence of many bacterial antioxidant defences, there is mounting evidence that ROS have a minimal role in neutrophil killing of Neisseria spp. during infection. Mutants lacking antioxidant gene products, singly or in combination, survive neutrophil exposure similarly to wild-type bacteria 38, 123. Moreover, chemical or genetic inhibition of neutrophil NADPH oxidase 38, 68, 124, 125, or incubation with neutrophils in anoxic conditions 126, does not affect bacterial survival after exposure to human or mouse neutrophils. Although some Opa+ bacteria induce ROS production by neutrophils, NADPH oxidase activity does not affect bacterial survival in neutrophils 38, 124. Even the survival of gonococcal NGO1686 and recN mutants, which are significantly more sensitive to ROS and to the antibacterial activities of neutrophils than their wild-type counterparts, is unaffected by NADPH oxidase inhibition 38. Together, these observations show that neutrophil-mediated activity against pathogenic neisseriae and the production of ROS are separable phenotypes. Notably, there are other host sources of ROS besides neutrophils. Several N. gonorrhoeae strains with mutations in genes encoding antioxidant proteins have survival defects in primary human cervical epithelial cells and in the mouse genital tract, implying that these cells and tissues produce ROS 126, 127. Commensal lactobacilli that are present in the female genital tract can also generate hydrogen peroxide, although evidence from mouse models, as well as studies using human secretions, suggests that this source of ROS does not significantly affect gonococcal survival 128, 129.
One reason that the oxidative burst may be dispensable for the antibacterial activities of neutrophils against Neisseria spp. is that the bacteria use multiple mechanisms to defend themselves against ROS. We speculate that in early stages of infection by the pathogenic neisseriae, when few bacteria are present and most are viable, host ROS production is minimal and antioxidant proteins protect the bacteria sufficiently. However, more ROS are produced when the number of Opa+ bacteria increases and a sizable population of non-growing bacteria forms, leading to increases in the levels of inflammatory products such as LOS. We hypothesize that the ROS produced by neutrophils and other host cells acts as a signal to the bacteria that inflammation has commenced and, in response, the bacteria initiate a transcriptional programme that is protective against oxidative and non-oxidative host damage. As support for this hypothesis, pretreatment of N. gonorrhoeae with hydrogen peroxide enhances bacterial survival in primary human neutrophils independently of the oxidative burst 38. Thus, the pathogenic neisseriae exploit the oxidative arsenal of the host by using it to induce the production of additional defences against neutrophils.
Neisserial resistance to neutrophil-mediated non-oxidative damage
As oxidative products are dispensable for neutrophil-mediated antimicrobial activities against pathogenic neisseriae, other, non-oxidative antibacterial factors of neutrophils must act on the bacteria, although the presence of viable neisseriae in the presence of neutrophils implies that these factors are not sufficient to clear infection. The non-oxidative antibacterial factors in neutrophils include degradative enzymes (such as lysozyme, elastase and cathepsin G), cationic peptides (such as defensins and the cathelicidin LL-37) and the vesicular proton-ATPase, which lowers the pH of the phagosome 130, 131. Many of these factors are also present at mucosal surfaces and have important functions in protecting the host. For example, LL-37 is present in mucosal secretions, and the female genital tract has a low pH.
Many proteins of pathogenic neisseriae protect the bacteria after exposure to non-oxidative antibacterial factors in vitro. The multiple transferable resistance system (MtrCDE) and fatty acid resistance system (FarAB) efflux pumps play a prominent part in conferring resistance to antibiotics, free fatty acids, detergents and antimicrobial peptides 132, 133. Gonococcal mutants lacking the MtrCDE efflux pump do not colonize the mouse genital tract as well as wild-type bacteria 134, but it is not known whether this is due to non-oxidative functions of genital neutrophils, increased sensitivity of the mutants to the antimicrobial peptides in mucosal secretions or both. The MisRS (also known as PhoPQ) two-component regulatory system, which promotes resistance to antimicrobial peptides in Neisseria spp. and other pathogens 135, directly and indirectly regulates various genes in Neisseria spp. One of the gene products in the MisRS regulon, lipopolysaccharide transporter periplasmic protein (LptA), adds a phosphoethanolamine moiety to LOS 136, 137. Meningococcal LptA confers resistance to antimicrobial peptides as well as complement 138. Other gene products that defend pathogenic neisseriae against non-oxidative factors include the meningococcal proteins NMB0741 and NMB1828, which provide protection against cathelicidin 139, and peptidoglycan O-acyltransferase (PacA), which O-acetylates peptidoglycan to render it resistant to lysozyme 140. In addition, the N. meningitidis capsule confers resistance to antimicrobial peptides 42.
One of the major gaps in our understanding of the interactions between neutrophils and pathogenic neisseriae is how the virulence properties that defend the bacteria against non-oxidative factors in vitro affect bacterial survival after neutrophil challenge. To date, only the gonococcal NGO1686 and RecN proteins have been shown to promote bacterial survival after neutrophil exposure independently of NADPH oxidase activity 38, and how these proteins mediate their effect is not currently understood. Many other proteins confer resistance to non-oxidative antimicrobial factors derived from neutrophils, but their role in protecting the bacteria from clearance by neutrophils remains unexplored.
Neutrophils may promote neisserial pathogenesis
Successful pathogens create a niche in their hosts for nutrient acquisition and a means of effective transmission to new individuals, all the while limiting deleterious effects on the health of the host. As bacteria that are well adapted to their host, the pathogenic neisseriae possess unique mechanisms to evade and subvert host immune responses. On the basis of this adaptation, we posit that these bacteria actively recruit neutrophils for intra-and interhost dissemination, in a mechanism similar to that proposed for Staphylococcus aureus 141. As described above, the pathogenic neisseriae meet several criteria that would support this contention: neutrophils are recruited in unusually high numbers at sites of infection, the bacteria survive and replicate in and around neutrophils, and the bacteria use dedicated proteins to defend against killing mechanisms in neutrophils and modulate neutrophil activities. These pathogenic mechanisms have not necessarily evolved to maximize the survival of any individual bacterial cell, but probably function on a population-wide scale, enabling a subset of bacteria to undergo replication, dissemination and transmission (FIG. 3). As elaborated on below, we speculate that these pathogens actively associate with neutrophils to help themselves acquire nutrients, to create a protective niche from the immune system and to allow transmission to tissues deeper in the body and even to other hosts.
Figure 3. Model for the role of neutrophils in the dissemination and spread of pathogenic neisseriae.
Perturbations in the epithelium during neutrophil infiltration enhance the influx of serum and associated nutrients for extracellular neisseriae. Neisseriae that have been phagocytosed by neutrophils may be able to access nutrients from inside the phagosome. Intracellular neisseriae avoid surveillance by humoral immunity. Attachment or phagocytosis by motile neutrophils promotes bacterial movement to deeper or ascendant tissues and transmission to new hosts.
Nutrient acquisition
The pathogenic neisseriae reside primarily on the surface of epithelial cells, where the limited nutrient flow in mucosal secretions restricts bacterial replication. As part of the inflammatory response to infection by pathogenic Neisseria spp., neutrophil influx causes leakage of serum components and damage to surrounding tissues, both of which would liberate nutrients for extracellular bacteria. Phagocytosis of the bacteria by neutrophils may allow pathogenic neisseriae access to intracellular nutrient pools, including concentrated sources of iron, such as holotransferrin and lactoferrin, for which these bacteria have receptors142.
Creating a protective niche
When inside neutrophils, the bacteria reside within phagosomes, which protect the pathogens from humoral immune surveillance. Neutrophils are not thought to function as antigen-presenting cells, so persistence inside neutrophils may also protect the bacteria from potentially toxic sources of cell-mediated immunity, such as cytotoxic T lymphocytes. Of course, phagocytosis of a dead, infected neutrophil by a macrophage would represent a dead end for the bacteria inside and would amplify the humoral immune response. However, infection by pathogenic neisseriae can prolong the neutrophil lifespan, and these bacteria provide a general immune suppression to dampen adaptive immune responses 143, 144. These two features would contribute to the persistence of the pathogens when in association with neutrophils.
Access to deeper and ascendant tissues
A small fraction of the pathogenic neisseriae transit across epithelial and endothelial monolayers, but in general these bacteria are not particularly motile; the one organelle associated with motility, the type IV pilus, in fact counters dissemination by driving the formation of bacterial microcolonies that enhance attachment to host tissues 145. The presence of neutrophils would facilitate bacterial access to secondary sites of infection in two ways. First, the influx of neutrophils and the associated inflammatory milieu produces local tissue damage, creating breaches in epithelial integrity through which the bacteria could pass 146. Second, as recently suggested, neutrophils themselves may carry viable bacteria to new sites 147. Activated neutrophils could carry N. meningitidis throughout the nasopharyngeal epithelium or aid N. gonorrhoeae ascension to the upper reproductive tract. Retrograde (tissue-to-bloodstream) movement of neutrophils in sterile injury has been reported 148; if this were to occur during infection with a pathogenic neisserial species, neutrophils could also transport the bacteria into the bloodstream to aid dissemination. Neutrophil-driven movement of neisseriae has not yet been visualized, but it would promote the main outcomes of N. meningitidis infections — meningococcaemia and meningitis — and would also lead to disseminated gonococcal infection and associated arthritis, endocarditis and dermatitis.
Transmission to new hosts
N. gonorrhoeae survives poorly, if at all, outside the human body, whereas capsulated N. meningitidis can survive for a limited duration on fomites and solid surfaces 3, 4. It is tempting to speculate that neutrophils serve as a conduit for transfer of meningococci and gonoccoci to receptive hosts. Although there is no direct evidence for transfer of neutrophils between individuals, there is a possibility that mechanisms in addition to the transfer of free bacteria are important for the high frequencies of transmission between hosts — for instance, in the transfer of N. gonorrhoeae from women to men. Taking this into account, the acute inflammation associated with gonorrhoea could be a response to foreign neutrophils as well as to the bacteria3.
Conclusions
Many crucial questions about the interactions between pathogenic neisseriae and neutrophils remain to be addressed. For instance, how do the bacteria survive and replicate in a cell type that is optimized for antibacterial function? How does the plethora of proteins that protects the bacteria from purified host-derived components in vitro contribute to persistence during infection? What are the mechanisms by which pathogenic neisseriae modulate neutrophil functions (ROS production, release of antimicrobial components and apoptosis)? This last question is especially intriguing considering that virulence-associated secretion systems and production of exotoxins are highly reduced or absent in these bacteria 149. Defining the ultimate fate of the bacteria during neutrophil infection will require a system for following the entire infection process, from initial exposure to establishment of chronic infection. As ethical considerations preclude the parallel examination of Neisseria spp. within neutrophils in the human host, animal models, including transgenic mouse models, will probably provide the appropriate system 36. Given the recent exciting developments in the study of the interactions between pathogenic Neisseria spp. and neutrophils, it is likely that advances in the genetics and genomics of Neisseria spp., neutrophil cell biology and in vivo systems for investigating innate immune function will contribute to refining and addressing these questions in the near future.
Acknowledgments
Research in A.K.C.’s laboratory is supported by the US National Institutes of Health (NIH) grant R00 TW008042 and by the Thomas F. and Kate Miller Jeffress Memorial Trust. Work in H.S.S.’s laboratory is supported by NIH grants R37 AI033493 and R01 AI044239.
Glossary
- Adhesins
Molecules that are expressed on a cell to mediate adherence to another surface
- Apoptosis
Programmed cell death of host cells. The rate and extent of apoptosis in neutrophils and other cells can be influenced by bacterial infection
- Capsule
The polysaccharide coating found on N. meningitidis that alters the bacterial cell surface and its interactions with other cells and molecules
- CEACAM (Carcinoembryonic antigen-related cell adhesion molecule) receptors
A family of host proteins that are expressed on a variety of cell types to mediate intercell communication. They serve as receptors for opacity-associated (Opa) proteins
- Commensal
Describing the relationship between two organisms in which one organism benefits while the other is unaffected
- Complement
A system of >25 proteins that recognize foreign objects and target them for destruction or phagocytosis. The complement proteins undergo sequential proteolytic events, resulting in activation of the membrane attack complex, a pore that forms on the surface of the foreign object to rupture it. Complement proteins such as C3b and C4b are recognized by complement receptors, and this triggers phagocytosis of the complement-opsonized particle
- Cytokines
Secreted proteins that are produced by cells of the immune system and by non-immune cells that can recognize infection, injury or inflammation. Chemokines are a class of chemotactic cytokines which recruit immune cells to specific locations in the body
- Dysuria
Pain while urinating. Common in men with acute gonorrhoea
- Granulocytes
Innate immune cells with a cytoplasm that is morphologically distinguished by the presence of granules. Neutrophils, eosinophils and basophils are all granulocytes
- Lipo-oligosaccharide (LOS)
The main component of the outer leaflet of the outer membrane of Gram-negative bacteria. LOS is similar to lipopolysaccharide (LPS) but lacks the long sugar chain (the O antigen). Pathogenic neisseriae produce different LOS isotypes owing to phase variation of glucosyltransferases for LOS
- Neutrophil extracellular traps (NETs)
Chromatin-based structures that are coated with granule proteins and are released from neutrophils to entrap microorganisms. There is currently some debate about whether neutrophil extracellular traps are released from dying neutrophils only, or whether live neutrophils can also produce them
- Opacity-associated proteins
A family of proteins expressed on the surface of pathogenic neisseriae. The assortment of Opa proteins expressed by the bacteria constantly changes owing to phase variation of the opa genes. Opa proteins mediate bacterium–bacterium and bacterium–host cell interactions, and some alter the characteristics of neisserial colonies grown on solid media
- Opsonic
Coating a particle to facilitate its uptake by phagocytes. Common opsonins are immunoglobulins (antibodies) and complement
- Oxidative burst
The production of reactive oxygen species (ROS) through the action of the NADPH oxidase enzyme
- Pattern recognition receptors
A family of proteins that recognize families of foreign objects, including evolutionarily conserved products of microorganisms, such as lipopolysaccharide and peptidoglycan. Common pattern recognition receptors include proteins of the Toll-like receptor family and the NOD-like receptor family
- Phagocytosis
A crucial process by which the immune system clears foreign objects, invading microorganisms and dead or dying cells. Phagocytic cells of the immune system include neutrophils and macrophages; dendritic cells can also undergo phagocytosis
- Phagolysosomes
Intracellular compartments that are capable of degrading material ingested by phagocytic cells. In neutrophils, the phagolysosome is formed when the early phagosome fuses with granules that carry antimicrobial products
- Pilus
A fibre that extends from the bacterial cell surface to mediate adherence to a host cell
- Porin
Bacterial outer-membrane, channel-forming proteins. Neisserial porins have been reported to translocate into human cells and affect mitochondrial membrane potential, cell lifespan and the neutrophil-mediated oxidative burst
- Reactive oxygen species (ROS)
A family of chemicals that are oxidized versions of molecular oxygen, including hydrogen peroxide, superoxide and hydroxyl radicals. ROS are produced by neutrophils via the action of NADPH oxidase and exert antimicrobial activities by damaging lipids, carbohydrates, proteins and nucleic acids
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Contributor Information
Alison K. Criss, Email: akc2r@virginia.edu.
H. Steven Seifert, Email: h-seifert@northwestern.edu.
References
- 1.Schielke S, Frosch M, Kurzai O. Virulence determinants involved in differential host niche adaptation of Neisseria meningitidis and Neisseria gonorrhoeae. Med Microbiol Immunol. 2010;199:185–96. doi: 10.1007/s00430-010-0150-5. [DOI] [PubMed] [Google Scholar]
- 2.Marri PR, et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One. 2010;5:e11835. doi: 10.1371/journal.pone.0011835. In this investigation, the authors sequence the genomes of multiple commensal and pathogenic neisseriae to show that supposed ‘virulence’ genes are present in many commensal species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiesner PJ, Thompson SE., 3rd Gonococcal diseases. Dis Mon. 1980;26:1–44. doi: 10.1016/s0011-5029(80)80002-2. [DOI] [PubMed] [Google Scholar]
- 4.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27 (Suppl 2):B71–7. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 6.Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687–96. doi: 10.1111/j.1462-5822.2006.00792.x. An excellent overview of the methods used by neutrophils to kill intracellular and extracellular microorganisms. [DOI] [PubMed] [Google Scholar]
- 7.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–81. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merz AJ, So M. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–57. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 9.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. J Bacteriol. 1963;85:1274–9. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson J, et al. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–57. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–40. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988;168:2121–9. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerse AE, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–20. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spence JM, Clark VL. Role of ribosomal protein L12 in gonococcal invasion of Hec1B cells. Infect Immun. 2000;68:5002–10. doi: 10.1128/iai.68.9.5002-5010.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol. 2001;42:659–72. doi: 10.1046/j.1365-2958.2001.02666.x. [DOI] [PubMed] [Google Scholar]
- 16.Edwards JL, Apicella MA. The role of lipooligosaccharide in Neisseria gonorrhoeae pathogenesis of cervical epithelia: lipid A serves as a C3 acceptor molecule. Cell Microbiol. 2002;4:585–98. doi: 10.1046/j.1462-5822.2002.00212.x. [DOI] [PubMed] [Google Scholar]
- 17.Edwards JL, et al. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 2002;4:571–84. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 18.Virji M, et al. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–95. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 19.Capecchi B, et al. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol Microbiol. 2005;55:687–98. doi: 10.1111/j.1365-2958.2004.04423.x. [DOI] [PubMed] [Google Scholar]
- 20.Makepeace BL, Watt PJ, Heckels JE, Christodoulides M. Interactions of Neisseria gonorrhoeae with mature human macrophage opacity proteins influence production of proinflammatory cytokines. Infect Immun. 2001;69:1909–13. doi: 10.1128/IAI.69.3.1909-1913.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzai O, et al. Carbohydrate composition of meningococcal lipopolysaccharide modulates the interaction of Neisseria meningitidis with human dendritic cells. Cell Microbiol. 2005;7:1319–34. doi: 10.1111/j.1462-5822.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 22.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol. 2010;3:312–21. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geddes K, Magalhaes JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8:465–79. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 24.Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388:621–5. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 25.Massari P, et al. Cutting edge: Immune stimulation by Neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–7. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 26.Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kappaB activation in epithelial cells in a Toll-like receptor 2-dependent manner. J Biol Chem. 2003;278:46252–60. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- 27.Zughaier SM, et al. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect Immun. 2004;72:371–80. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaparakis M, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–85. doi: 10.1111/j.1462-5822.2009.01404.x. References 25–28 identify the products of pathogenic neisseriae that activate human TLR and NLR family receptors to modulate immune activation. [DOI] [PubMed] [Google Scholar]
- 29.Waage A, et al. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–67. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsey KH, et al. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–91. doi: 10.1093/infdis/172.1.186. References 29 and 30 identify the cytokines released during human infection with pathogenic neisseriae, including cytokines that coordinate neutrophil influx. [DOI] [PubMed] [Google Scholar]
- 31.Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–8. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christodoulides M, et al. Interaction of Neisseria meningitidis with human meningeal cells induces the secretion of a distinct group of chemotactic, proinflammatory, and growth-factor cytokines. Infect Immun. 2002;70:4035–44. doi: 10.1128/IAI.70.8.4035-4044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen A, Seifert HS. Neisseria gonorrhoeae-mediated inhibition of apoptotic signalling in polymorphonuclear leukocytes. Infect Immun. 2011 doi: 10.1128/IAI.01267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–43. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 35.Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J Clin Invest. 1994;93:2744–9. doi: 10.1172/JCI117290. This article and reference 13 report the timing and numbers of neutrophils recruited to the male urethra in response to experimental N. gonorrhoeae infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–708. doi: 10.1128/iai.67.11.5699-5708.1999. This groundbreaking study describes the development of a genetically tractable model system for examining infection by the pathogenic Neisseria spp.: genital infection of female mice by N. gonorrhoeae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacy P, Eitzen G. Control of granule exocytosis in neutrophils. Front Biosci. 2008;13:5559–70. doi: 10.2741/3099. [DOI] [PubMed] [Google Scholar]
- 38.Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cell Microbiol. 2009;11:1074–87. doi: 10.1111/j.1462-5822.2009.01308.x. This report shows that ROS do not participate in the antibacterial activity of human neutrophils against N. gonorrhoeae, and that up to 50% of phagocytosed bacteria seem to be viable inside neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stohl EA, Criss AK, Seifert HS. The transcriptome response of Neisseria gonorrhoeae to hydrogen peroxide reveals genes with previously uncharacterized roles in oxidative damage protection. Mol Microbiol. 2005;58:520–32. doi: 10.1111/j.1365-2958.2005.04839.x. This article identified the first N. gonorrhoeae gene products that protect the bacteria from killing by human neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarvis GA, Vedros NA. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–80. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frosch M, Vogel U. Structure and genetics of the meningococcal capsule. In: Frosch M, Maiden MCJ, editors. Handbook of Meningococcal Disease: Infection Biology, Vaccination, Clinical Management. Wiley-VCH Verlag GmbH & Co.KGaA; Weinheim, Germany: 2006. [Google Scholar]
- 42.Spinosa MR, et al. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect Immun. 2007;75:3594–603. doi: 10.1128/IAI.01945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thongthai C, Sawyer WD. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973;7:373–9. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King G, James JF, Swanson J. Studies on gonococcus infection. XI. Comparison of in vivo and vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis. 1978;137:38–43. doi: 10.1093/infdis/137.1.38. This historic work compares neutrophils in urethral gonorrhoeal secretions with peripheral neutrophils from the same individuals to show that both cell populations ingest N. gonorrhoeae. The work also shows that both piliated and non-piliated bacteria are phagocytosed by neutrophils. [DOI] [PubMed] [Google Scholar]
- 45.Virji M, Heckels JE. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986;132:503–12. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- 46.Jack DL, et al. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J Immunol. 1998;160:1346–53. [PubMed] [Google Scholar]
- 47.Kahler CM, et al. The (alpha2-->8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–47. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert M, et al. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–6. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- 49.Nairn CA, et al. Cytidine 5′-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988;134:3295–306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Jerse AE. Alpha-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect Immun. 2006;74:4094–103. doi: 10.1128/IAI.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith H, Parsons NJ, Cole JA. Sialylation of Neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog. 1995;19:365–77. doi: 10.1006/mpat.1995.0071. [DOI] [PubMed] [Google Scholar]
- 52.Ram S, et al. Binding of complement factor H to loop 5 of porin protein 1A: a molecular mechanism of serum resistance of nonsialylated Neisseria gonorrhoeae. J Exp Med. 1998;188:671–80. doi: 10.1084/jem.188.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ram S, et al. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–52. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madico G, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–10. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis LA, et al. The meningococcal vaccine candidate Neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ram S, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–95. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jarva H, Ram S, Vogel U, Blom AM, Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174:6299–307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 58.Davila S, et al. Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet. 2010;42:772–6. doi: 10.1038/ng.640. [DOI] [PubMed] [Google Scholar]
- 59.Virji M. Pathogenic Neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7:274–86. doi: 10.1038/nrmicro2097. An excellent review on the virulence-associated, antigenically variable surface structures of the pathogenic Neisseria. [DOI] [PubMed] [Google Scholar]
- 60.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes Infect. 2003;5:1299–306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Lewis LA, et al. Defining targets for complement components C4b and C3b on the pathogenic Neisseriae. Infect Immun. 2008;76:339–50. doi: 10.1128/IAI.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dempsey JA, Litaker W, Madhure A, Snodgrass TL, Cannon JG. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J Bacteriol. 1991;173:5476–86. doi: 10.1128/jb.173.17.5476-5486.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aho EL, Dempsey JA, Hobbs MM, Klapper DG, Cannon JG. Characterization of the opa (class 5) gene family of Neisseria meningitidis. Mol Microbiol. 1991;5:1429–37. doi: 10.1111/j.1365-2958.1991.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 64.Bhat KS, et al. The opacity proteins of Neisseria gonorrhoeae strain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 65.Murphy GL, Connell TD, Barritt DS, Koomey M, Cannon JG. Phase variation of gonococcal protein II: regulation of gene expression by slipped-strand mispairing of a repetitive DNA sequence. Cell. 1989;56:539–47. doi: 10.1016/0092-8674(89)90577-1. [DOI] [PubMed] [Google Scholar]
- 66.Sadarangani M, Pollard AJ, Gray-Owen SD. Opa proteins & CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol Rev. 2011 doi: 10.1111/j.1574-6976.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 67.King GJ, Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978;21:575–84. doi: 10.1128/iai.21.2.575-584.1978. This study is the first to identify outer-membrane proteins — later found to be Opa proteins — that mediate interaction of N. gonorrhoeae with neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rest RF, Fischer SH, Ingham ZZ, Jones JF. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982;36:737–44. doi: 10.1128/iai.36.2.737-744.1982. The authors of this report use human neutrophils infected with N. gonorrhoeae expressing defined Opa proteins to correlate the Opa–neutrophil interaction with reduced bacterial survival and enhanced ROS production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fischer SH, Rest RF. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988;56:1574–9. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 1993;12:641–50. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Belland RJ, Chen T, Swanson J, Fischer SH. Human neutrophil response to recombinant Neisserial Opa proteins. Mol Microbiol. 1992;6:1729–37. doi: 10.1111/j.1365-2958.1992.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 72.Naids FL, Belisle B, Lee N, Rest RF. Interactions of Neisseria gonorrhoeae with human neutrophils: studies with purified PII (Opa) outer membrane proteins and synthetic. Opa peptides Infect Immun. 1991;59:4628–35. doi: 10.1128/iai.59.12.4628-4635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–71. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billker O, et al. Distinct mechanisms of internalization of Neisseria gonorrhoeae by members of the CEACAM receptor family involving Rac1- and Cdc42-dependent and -independent pathways. EMBO J. 2002;21:560–71. doi: 10.1093/emboj/21.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmitter T, Agerer F, Peterson L, Munzner P, Hauck CR. Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J Exp Med. 2004;199:35–46. doi: 10.1084/jem.20030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pils S, Gerrard DT, Meyer A, Hauck CR. CEACAM3: an innate immune receptor directed against human-restricted bacterial pathogens. Int J Med Microbiol. 2008;298:553–60. doi: 10.1016/j.ijmm.2008.04.005. References 74–76 describe and support the concept that CEACAM3 evolved as a granulocyte receptor for engulfment and killing of human-specific bacteria such as the pathogenic neisseriae. [DOI] [PubMed] [Google Scholar]
- 77.Chen T, Gotschlich EC. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci U S A. 1996;93:14851–6. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med. 1997;185:1557–64. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–45. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virji M, Makepeace K, Ferguson DJ, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic Neisseriae. Mol Microbiol. 1996;22:941–50. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 81.Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun. 1997;65:2353–61. doi: 10.1128/iai.65.6.2353-2361.1997. References 77–81 identify members of the CEACAM family of receptors as the receptors (on neutrophils and other cells) that act as binding partners for a subset of neisserial Opa proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bos MP, Kuroki M, Krop-Watorek A, Hogan D, Belland RJ. CD66 receptor specificity exhibited by Neisserial Opa variants is controlled by protein determinants in CD66 N-domains. Proc Natl Acad Sci U S A. 1998;95:9584–9. doi: 10.1073/pnas.95.16.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Virji M, Watt SM, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–39. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 84.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–46. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 85.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–80. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 86.Booth JW, et al. Phosphatidylinositol 3-kinases in carcinoembryonic antigen-related cellular adhesion molecule-mediated internalization of Neisseria gonorrhoeae. J Biol Chem. 2003;278:14037–14045. doi: 10.1074/jbc.M211879200. [DOI] [PubMed] [Google Scholar]
- 87.Sarantis H, Gray-Owen SD. The specific innate immune receptor CEACAM3 triggers neutrophil bactericidal activities via a Syk kinase-dependent pathway. Cell Microbiol. 2007;9:2167–2180. doi: 10.1111/j.1462-5822.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 88.Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cell adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun. 2012;80:345–358. doi: 10.1128/IAI.05702-11. The authors of this work express selected CEACAMs, alone or in combination, in mouse promyelocytic cells to show that each receptor initiates different signalling events in response to N. gonorrhoeae. CEACAM1 and CEACAM6 are found to enhance CEACAM3 signals in neutrophils, an unexpected finding given the inhibitory role of CEACAM1 in other cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- 90.McCaw SE, Liao EH, Gray-Owen SD. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect Immun. 2004;72:2742–2752. doi: 10.1128/IAI.72.5.2742-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Estabrook MM, Zhou D, Apicella MA. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect Immun. 1998;66:1028–36. doi: 10.1128/iai.66.3.1028-1036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ovcinnikov NM, Delektorskij VV. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Br J Vener Dis. 1971;47:419–39. doi: 10.1136/sti.47.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farzadegan H, Roth IL. Scanning electron microscopy and freeze-etching of gonorrhoeal urethral exudate. Br J Vener Dis. 1975;51:83–91. doi: 10.1136/sti.51.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Apicella MA, et al. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis. 1996;173:636–46. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 95.Casey SG, Veale DR, Smith H. Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J Gen Microbiol. 1979;113:395–8. doi: 10.1099/00221287-113-2-395. [DOI] [PubMed] [Google Scholar]
- 96.Casey SG, Veale DR, Smith H. Intracellular survival of Neisseria gonorrhoeae in human urethral exudate. FEMS Microbiol Lett. 1980;8:97–100. [Google Scholar]
- 97.Casey SG, Shafer WM, Spitznagel JK. Neisseria gonorrhoeae survive intraleukocytic oxygen-independent antimicrobial capacities of anaerobic and aerobic granulocytes in the presence of pyocin lethal for extracellular gonococci. Infect Immun. 1986;52:384–9. doi: 10.1128/iai.52.2.384-389.1986. References 95–97 provide the first evidence for N. gonorrhoeae replication inside human neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–7. doi: 10.1128/IAI.73.4.1971-1977.2005. The authors of this article develop an in vitro infection assay with N. gonorrhoeae that yields evidence for bacterial survival and replication inside adherent primary human neutrophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Binnicker MJ, Williams RD, Apicella MA. Infection of human urethral epithelium with Neisseria gonorrhoeae elicits an upregulation of host anti-apoptotic factors and protects cells from staurosporine-induced apoptosis. Cell Microbiol. 2003;5:549–60. doi: 10.1046/j.1462-5822.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 100.Deghmane AE, et al. Differential modulation of TNF-alpha-induced apoptosis by Neisseria meningitidis. PLoS Pathog. 2009;5:e1000405. doi: 10.1371/journal.ppat.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kepp O, et al. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS Pathog. 2009;5:e1000348. doi: 10.1371/journal.ppat.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 2003;5:99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 103.Kozjak-Pavlovic V, Ott C, Gotz M, Rudel T. Neisserial omp85 protein is selectively recognized and assembled into functional complexes in the outer membrane of human mitochondria. J Biol Chem. 2011;286:27019–26. doi: 10.1074/jbc.M111.232249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muller A, et al. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 2000;19:5332–43. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simons MP, Nauseef WM, Griffith TS, Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol. 2006;8:1780–90. doi: 10.1111/j.1462-5822.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- 106.Witko-Sarsat V, Pederzoli-Ribeil M, Hirsch E, Sozzani S, Cassatella MA. Regulating neutrophil apoptosis: new players enter the game. Trends Immunol. 2011;32:117–24. doi: 10.1016/j.it.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–15. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 108.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–32. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 109.Johnson SR, Steiner BM, Cruce DD, Perkins GH, Arko RJ. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect Immun. 1993;61:1232–8. doi: 10.1128/iai.61.4.1232-1238.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilks KE, et al. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect Immun. 1998;66:213–7. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–86. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 112.Skaar EP, et al. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A. 2002;99:10108–13. doi: 10.1073/pnas.152334799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis. 2004;190:136–47. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- 114.Soler-Garcia AA, Jerse AE. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb Pathog. 2004;37:55–63. doi: 10.1016/j.micpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 115.Davidsen T, Bjoras M, Seeberg EC, Tonjum T. Antimutator role of DNA glycosylase MutY in pathogenic Neisseria species. J Bacteriol. 2005;187:2801–9. doi: 10.1128/JB.187.8.2801-2809.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stohl EA, Seifert HS. Neisseria gonorrhoeae DNA recombination and repair enzymes protect against oxidative damage caused by hydrogen peroxide. J Bacteriol. 2006;188:7645–51. doi: 10.1128/JB.00801-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.LeCuyer BE, Criss AK, Seifert HS. Genetic characterization of the nucleotide excision repair system of Neisseria gonorrhoeae. J Bacteriol. 2010;192:665–73. doi: 10.1128/JB.01018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tala A, et al. Glutamate utilization promotes meningococcal survival in vivo through avoidance of the neutrophil oxidative burst. Mol Microbiol. 2011;81:1330–42. doi: 10.1111/j.1365-2958.2011.07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grifantini R, et al. Characterization of a novel Neisseria meningitidis Fur and iron-regulated operon required for protection from oxidative stress: utility of DNA microarray in the assignment of the biological role of hypothetical genes. Mol Microbiol. 2004;54:962–79. doi: 10.1111/j.1365-2958.2004.04315.x. This study identifies the first N. meningitidis gene products found to protect the bacterium from killing by primary human neutrophils. [DOI] [PubMed] [Google Scholar]
- 120.Britigan BE, Klapper D, Svendsen T, Cohen MS. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J Clin Invest. 1988;81:318–24. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Criss AK, Seifert HS. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol. 2008;10:2257–70. doi: 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lorenzen DR, et al. Neisseria gonorrhoeae porin modifies the oxidative burst of human professional phagocytes. Infect Immun. 2000;68:6215–22. doi: 10.1128/iai.68.11.6215-6222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seib KL, et al. Investigation of oxidative stress defenses of Neisseria gonorrhoeae by using a human polymorphonuclear leukocyte survival assay. Infect Immun. 2005;73:5269–72. doi: 10.1128/IAI.73.8.5269-5272.2005. This work uses N. gonorrhoeae carrying mutations in multiple antioxidant genes to show that neutrophil-mediated killing of gonococci is independent of ROS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frangipane JV, Rest RF. Anaerobic growth of gonococci does not alter their Opa-mediated interactions with human neutrophils. Infect Immun. 1992;60:1793–9. doi: 10.1128/iai.60.5.1793-1799.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu H, Soler-Garcia AA, Jerse AE. A strain-specific catalase mutation and mutation of the metal-binding transporter gene mntC attenuate Neisseria gonorrhoeae in vivo but not by increasing susceptibility to oxidative killing by phagocytes. Infect Immun. 2009;77:1091–102. doi: 10.1128/IAI.00825-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu HJ, et al. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect Immun. 2005;73:8444–8. doi: 10.1128/IAI.73.12.8444-8448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seib KL, et al. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol. 2007;63:54–68. doi: 10.1111/j.1365-2958.2006.05478.x. [DOI] [PubMed] [Google Scholar]
- 128.Muench DF, et al. Hydrogen peroxide-producing lactobacilli inhibit gonococci in vitro but not during experimental genital tract infection. J Infect Dis. 2009;199:1369–78. doi: 10.1086/597390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.O’Hanlon DE, Lanier BR, Moench TR, Cone RA. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:120. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Levy O. Antimicrobial proteins and peptides: anti-infective molecules of mammalian leukocytes. J Leukoc Biol. 2004;76:909–25. doi: 10.1189/jlb.0604320. [DOI] [PubMed] [Google Scholar]
- 131.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9:781–95. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A. 1998;95:1829–33. doi: 10.1073/pnas.95.4.1829. This report describes the cloning and characterization of the gonococcal MtrCDE efflux pump and its crucial role in neisserial defence against cationic antimicrobial peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee EH, Shafer WM. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol Microbiol. 1999;33:839–45. doi: 10.1046/j.1365-2958.1999.01530.x. [DOI] [PubMed] [Google Scholar]
- 134.Jerse AE, et al. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003;71:5576–82. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson CR, et al. Generation and characterization of a PhoP homologue mutant of Neisseria meningitidis. Mol Microbiol. 2001;39:1345–55. doi: 10.1111/j.1365-2958.2001.02324.x. [DOI] [PubMed] [Google Scholar]
- 136.Tzeng YL, et al. The MisR/MisS two-component regulatory system influences inner core structure and immunotype of lipooligosaccharide in Neisseria meningitidis. J Biol Chem. 2004;279:35053–62. doi: 10.1074/jbc.M401433200. [DOI] [PubMed] [Google Scholar]
- 137.Newcombe J, et al. Phenotypic and transcriptional characterization of the meningococcal PhoPQ system, a magnesium-sensing two-component regulatory system that controls genes involved in remodeling the meningococcal cell surface. J Bacteriol. 2005;187:4967–75. doi: 10.1128/JB.187.14.4967-4975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lewis LA, et al. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect Immun. 2009;77:1112–20. doi: 10.1128/IAI.01280-08. In this investigation, different modifications of neisserial LOS, including the addition of phosphoethanolamine by LptA, were shown to differentially affect bacterial resistance to complement and cationic antimicrobial peptides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frigimelica E, Bartolini E, Galli G, Grandi G, Grifantini R. Identification of 2 hypothetical genes involved in Neisseria meningitidis cathelicidin resistance. J Infect Dis. 2008;197:1124–32. doi: 10.1086/533456. [DOI] [PubMed] [Google Scholar]
- 140.Dillard JP, Hackett KT. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 2005;73:5697–705. doi: 10.1128/IAI.73.9.5697-5705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9:215–22. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 142.Rohde KH, Dyer DW. Mechanisms of iron acquisition by the human pathogens Neisseria meningitidis and Neisseria gonorrhoeae. Front Biosci. 2003;8:d1186–218. doi: 10.2741/1133. [DOI] [PubMed] [Google Scholar]
- 143.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–36. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 144.Pantelic M, et al. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun. 2005;73:4171–9. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Merz AJ, Enns CA, So M. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol Microbiol. 1999;32:1316–32. doi: 10.1046/j.1365-2958.1999.01459.x. [DOI] [PubMed] [Google Scholar]
- 146.Zen K, Parkos CA. Leukocyte-epithelial interactions. Curr Opin Cell Biol. 2003;15:557–64. doi: 10.1016/s0955-0674(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 147.Soderholm N, Vielfort K, Hultenby K, Aro H. Pathogenic Neisseria Hitchhike on the Uropod of Human Neutrophils. PLoS One. 2011;6:e24353. doi: 10.1371/journal.pone.0024353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–9. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Ulsen P, Tommassen J. Protein secretion and secreted proteins in pathogenic Neisseriaceae. FEMS Microbiol Rev. 2006;30:292–319. doi: 10.1111/j.1574-6976.2006.00013.x. [DOI] [PubMed] [Google Scholar]
- 150.WHO. Fact Sheet RH. Vol. 11.14. Geneva, Switzerland: 2011. Emergence of multi-drug resistant Neisseria gonorrhoeae – Threat of global rise in untreatable sexually transmitted infections. [Google Scholar]
- 151.Jennings MP, et al. The genetic basis of the phase variation repertoire of lipopolysaccharide immunotypes in Neisseria meningitidis. Microbiology. 1999;145 (Pt 11):3013–21. doi: 10.1099/00221287-145-11-3013. [DOI] [PubMed] [Google Scholar]
- 152.Cahoon LA, Seifert HS. Focusing homologous recombination: pilin antigenic variation in the pathogenic. Neisseria Mol Microbiol. (201) doi: 10.1111/j.1365-2958.2011.07773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jonsson AB, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 1991;10:477–88. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Blake MS, Swanson J. Studies on gonococcus infection. XVI. Purification of Neisseria gonorrhoeae immunoglobulin A1 protease. Infect Immun. 1978;22:350–8. doi: 10.1128/iai.22.2.350-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hauck CR, Meyer TF. The lysosomal/phagosomal membrane protein h-lamp-1 is a target of the IgA1 protease of Neisseria gonorrhoeae. FEBS Lett. 1997;405:86–90. doi: 10.1016/s0014-5793(97)00163-4. [DOI] [PubMed] [Google Scholar]
- 156.Ayala P, Lin L, Hopper S, Fukuda M, So M. Infection of epithelial cells by pathogenic Neisseriae reduces the levels of multiple lysosomal constituents. Infect Immun. 1998;66:5001–7. doi: 10.1128/iai.66.10.5001-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johannsen DB, Johnston DM, Koymen HO, Cohen MS, Cannon JG. A Neisseria gonorrhoeae immunoglobulin A1 protease mutant is infectious in the human challenge model of urethral infection. Infect Immun. 1999;67:3009–13. doi: 10.1128/iai.67.6.3009-3013.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jones HE, Uronen-Hansson H, Callard RE, Klein N, Dixon GL. The differential response of human dendritic cells to live and killed Neisseria meningitidis. Cell Microbiol. 2007;9:2856–69. doi: 10.1111/j.1462-5822.2007.01001.x. [DOI] [PubMed] [Google Scholar]