Abstract
Acute liver failure has a high mortality unless patients receive a liver transplant; however, there are insufficient donor organs to meet the clinical need. The liver may rapidly recover from acute injury by hepatic cell regeneration given time. A bioartificial liver machine can provide temporary liver support to enable such regeneration to occur. We developed a bioartificial liver machine using human-derived liver cells encapsulated in alginate, cultured in a fluidized bed bioreactor to a level of function suitable for clinical use (performance competence). HepG2 cells were encapsulated in alginate using a JetCutter to produce ∼500 μm spherical beads containing cells at ∼1.75 million cells/mL beads. Within the beads, encapsulated cells proliferated to form compact cell spheroids (AELS) with good cell-to-cell contact and cell function, that were analyzed functionally and by gene expression at mRNA and protein levels. We established a methodology to enable a ∼34-fold increase in cell density within the AELS over 11–13 days, maintaining cell viability. Optimized nutrient and oxygen provision were numerically modeled and tested experimentally, achieving a cell density at harvest of >45 million cells/mL beads; >5×1010 cells were produced in 1100 mL of beads. This process is scalable to human size ([0.7–1]×1011). A short-term storage protocol at ambient temperature was established, enabling transport from laboratory to bedside over 48 h, appropriate for clinical translation of a manufactured bioartificial liver machine.
Key words: alginate encapsulation, fluidized bed bioreactor, HepG2 cells
Introduction
Acute liver failure can lead to death via a syndrome including hepatic encephalopathy and cerebral edema, multiorgan failure, and sepsis.1 Rapid and unpredictable progression inhibits timely identification of donor organs for transplantation. We describe a bioartificial liver (BAL) to provide support after liver injury, buying time either to allow organ procurement for transplantation or for natural regeneration to occur.
Liver-assist devices comprise artificial and bioartificial types.2 Artificial devices use physical/chemical gradients and adsorption to detoxify patients' blood. BAL devices use living cells to replace the complex repertoire of metabolic, synthetic, and detoxifying liver function. Current designs include hepatocytes in flat plate monolayers, within hollow fiber cartridges, in or on other inorganic matrices and encapsulated cells in perfused beds or suspended in scaffolds.3–15 BAL design criteria include optimized environments for hepatocyte viability and function, bidirectional mass transfer between the blood/plasma and cells, minimum dead volume, and adequate cell numbers. Since normal adult human liver contains (1–2)×1011 hepatocytes, we estimated that (0.7–1)×1011 cells constitute an appropriate biomass (30%–50% of normal), based on minimum liver mass compatible with life (10%–15% after liver resection)16; however, biomass has to perform in the hostile environment of liver failure plasma.
Many BALs (e.g., hollow-fiber cartridge-based systems) insert molecular barriers between cells and patient circulation, reducing mass transfer. Cell encapsulation in alginate, a biocompatible semipermeable hydrogel that is directly exposed to patients' plasma, provides an alternative technology, protecting cells from shear-stress during perfusion, promoting 3-dimensional culture conditions, and maximizing cell performance.17,18 We combined this with fluidized bed perfusion technology to maximize mass transfer. The cyclic microgravity environment established between vertical media-flow and the gravity of the alginate-encapsulated liver spheroids (AELSs) maximizes diffusion of metabolites between cells and perfusate. This allows rapid cell growth and spheroid formation, and, when applied clinically, maximizes exchange between biomass and patient plasma.
Alginate is a linear copolymer polysaccharide of covalently (1-4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues extracted from brown algae. The M and G residue sequence (e.g., homopolymeric M or G blocks or alternating M and G monomers) dictates viscosity and gelling properties of different alginate solutions. A medium-viscosity hydrogel provides a platform within which three-dimensional (3D) spheroidal biomass structures can grow and approximate physiological tissue architecture. AELSs exhibit better growth and up-regulated liver-specific functions compared to monolayer culture, with typical cell architecture demonstrating desmosomes, tight junctions and microvilli, high representation of endoplasmic reticulum and mitochondria, and generation of extracellular matrix reminiscent of that in normal liver.18
Potential cell sources for BALs include primary hepatocytes, established cell-lines, and stem cells. Although primary human hepatocytes initially possess the full complement of liver-specific functions, in culture they do not proliferate and rapidly lose differentiated function; they are difficult to obtain due to donor organ scarcity. Porcine hepatocytes have been used clinically in BALs, with the only controlled trial showing no benefit19 and potential biohazards existing.20–22 Cells cultured from stem cells offer exciting future prospects, but due to complexity and costs remain impractical at this time. We report here on the use of a well-differentiated human liver–derived cell line (HepG2) isolated from a hepatoblastoma that exhibits many liver-specific functions in monolayer culture,23 which can be further enhanced by specific culture techniques, such as 3D culture.
Key to clinical feasibility of any BAL is timely delivery to the patient. As BALs will be applied in the unpredicted emergency of acute liver failure, a means of storing performance-competent beads for use as needed is required. We previously demonstrated that encapsulated cells in beads can be cryopreserved;24 functional recovery of thawed beads on a large scale is as yet insufficient for BAL application. An alternative is short-term storage at ambient temperature, and in this study we developed an oxygen carrier–based method for short term (up to 48 h) AELS preservation, a time-frame suitable for delivery from a central production facility and appropriate clinical use.
We optimized conditions for growing AELSs to performance-competent cell spheroids at a scale suitable for a large animal trial, and their short-term preservation, using combined mathematical modeling and iterative experimentation. While our approach was in order to further the production of a BAL, the system is adaptable to optimize production of other cell lines for therapeutic or manufacturing purposes.
Materials and Methods
Monolayer cell culture to provide seed cells for alginate encapsulation
HepG2 cells (ECACC Wiltshire) were grown using 500-cm2 tissue culture flasks17 and modified MEM-alpha media (Gibco) supplemented with fetal calf serum (10%, PAA), insulin (0.27 IU/mL, Novo Nordisk), penicillin/streptomycin (50 μg/mL, PAA), and fungizone (1 mg/mL, PAA), to provide (5–6)×109 cells for encapsulation.
Alginate encapsulation
HepG2 cells in culture media were mixed 1:1 with Alginate (2% w/v alginate, medium viscosity [Sigma Aldrich], in 0.015 M HEPES-buffered physiological saline, pH 7.4) yielding (1.5–1.75)×106 cells/mL of solution. As a density modifier, 0.0175 g/mL 10- to 50-μm glass beads (Kisker) were added, establishing the appropriate microgravity environment when fluidizing.25,26
Beads were made using a JetCutter (GeniaLab). A stainless-steel vessel containing the stirred cell suspension was pressurized to achieve jet flow through a 350-μm nozzle at 20±1 mL/min, cut into segments mechanically by a wire cutting-disc rotating at 3300 rpm. These segments formed droplets on propulsion into polymerization solution (0.204 M CaCl2 in 0.15 M NaCl); collections did not exceed 1 h. The polymerization bath was supplemented with 70% w/v concentrated CaCl2 to replace Ca2+ ions sequestered during the gelation process. Subsequently, excess calcium was removed by three washes with Dulbecco's modified Eagle's medium (DMEM; PAA) containing 10% fetal bovine serum, antibiotics, and fungizone. Beads were collected on a 200-μm mesh, and placed in a fluidization chamber for microgravity biomass culture.
Culture of encapsulated cells
The fluidized bed bioreactor (FBB) hosting cell proliferation during the growth phase of the biomass, comprised a 15-L Fermentor (Fermac360, Electrolab), a 30-L reservoir, and a fluidization chamber.27,28 The chamber and fermentor were connected with 4.8-mm bore silicone tubing (Altec). Media perfusion was achieved (flow rate 180–350 mL/min) with a peristaltic pump (Watson-Marlow). The cell-containing alginate beads were fluidized to a bed-height of 1.56±0.1 fold expansion (mean±SD, n=9). Beads to culture-media volume ratio was kept at 1:58, with 25%, 50%, 70%, and 80% media replaced on days 4, 6, 8, and 10. Temperature (37°C) and pH (7.4) were maintained by the fermentor during the growth phase; CO2 and O2 were supplied through proportional–integral–derivative dynamic control, with design parameters to ensure oxygen levels did not drop below critical levels. Therefore, oxygen saturation in the culture medium was maintained between 15%–35%, avoiding adverse effects of hypoxia or hyperoxia on cell proliferation. During the latter part of the growth phase, additional oxygen was supplied directly inside the chamber, to ensure oxygen saturation was maintained throughout the bed under the high metabolic demand of the proliferating cells. Alpha MEM was supplemented with glucose to ∼24 mM, and 10% human plasma (National Blood Service) was added in place of fetal bovine serum during fermentation. Five amino acids (phenylalanine, cysteine, leucine, isoleucine, methionine) were supplemented to maintain their original media concentration, replenishing that consumed by the biomass.
Samples of media and beads throughout the experiment determined cell function, viability, cell proliferation, glucose consumption, and lactate synthesis. After 11 days of culture AELSs were harvested and prepared for an animal preclinical trial (publication submitted).
Cell viability
One milliliter of beads (n=5) containing cells were washed twice in phosphate-buffered saline (PBS; Lonza) and stained using 0.0128 mg/mL fluorescein diacetate (FDA) and 0.0256 mg/mL propidium iodide (PI) vital dyes, and viability was quantified under fluorescence microscopy using Lucia Imaging software, as described.24
Cell number
Beads were washed twice in Hank's balanced salt solution. Cell spheroids were liberated from alginate with 16 mM EDTA, pH 7.4, pelleted, resuspended in PBS and disaggregated using a 23-G needle followed by automated cell nuclei quantitation (Nucleocounter, Sartorius-Stedim).
Lactate production and glucose consumption
Lactate production and glucose consumption were quantified by enzyme-linked oxygen-rate measurement using a GM-7 Micro-Stat analyzer (Analox) with a Clark-type amperometric oxygen electrode.
Synthetic function
Protein synthesis was monitored by measurement of alpha-fetoprotein (AFP) release as this specific protein of HepG2 cells is not present in the fresh frozen plasma (FFP) that comprises 10% of the culture media. AFP was measured by sandwich enzyme-linked immunosorbent assay (standards from Applichem), with primary and secondary AFP antibodies (Abcam ab10071+ab10072).
Gene expression and protein arrays
RNA was prepared from AELSs at the time of optimal cell function and compared with RNA prepared from the same number of monolayer cells. Microarray analysis was performed after first and second strand cDNA synthesis, biotin labeling of mRNA targets with in vitro transcription, and cRNA fragmentation to 35–200 base targets, using an Affymetrix U95Av2 human genechip. Relevant genes that showed significant changes were further interrogated by Western blotting and, where possible, functional analysis. Protein lysates (8 mg) were also prepared from these two cell conditions (encapsulated vs. monolayer) and subjected to immunoanalysis using the Powerblot antibody-array (Becton Dickinson), and investigation of protein oxidation using the OxyBlot™ kit (Chemicon International) detecting carbonyl groups (aldehydes and ketones) on proteins that occur at and modify the side chains of lysine, arginine, proline, or threonine residues and form cysteine disulfide bonds as a result of several types of oxidative damage. The carbonyl groups in the protein side chain were derivatized to 2,4-dinitrophenylhydrazone (DNP-hydrazone) by reaction with 2,4-dinitrophenylhydrazine (DNPH) and separated by polyacrylamide gel electrophoresis, followed by Western blotting. Oxidized proteins were revealed by an anti-DNP antibody and quantified on a scanning densitometer.
Bead dimensions
AELSs (250 μL) were washed twice in PBS and loaded onto 2-mm-deep slides. Phase contrast images (Lucia Image Software at 4× magnification) captured ∼80–100 beads. Total alginate bead volume was calculated from measured average bead-diameter, and cell-density data. The fraction of beads occupying a determined space (solid-phase porosity: ɛS) compared to media fraction (liquid-phase porosity: ɛL) was empirically estimated by establishing volume of liquid phase (Vliquid), total volume including the beads (Vtotal), using the relationships, ɛL=Vliquid/Vtotal and ɛS=1 – ɛL.
Biomass preservation
After the proliferation phase, AELSs were stored at ambient temperature for 48 h in sealed T175 culture flasks with perfluorodecalin (PFC; F2 Chemicals) and culture medium. PFC was autoclaved and oxygenated prior to use by bubbling 100% oxygen for 10 min; media contained 25 mM HEPES, pH 7.4 (Invitrogen, 15630). To develop optimal conditions for ambient storage, AELSs were stored at different ratios of PFC/culture media with different headspace volumes. An antioxidant mixture comprising 0.85 mM Trolox (Sigma, 238813), 500 IU/mL Catalase (Sigma, c9322) and 3 mM N-acetyl cysteine (Sigma, a8199) was trialed for efficacy.
Amino acid concentration
Concentrations of essential amino acids in the medium were measured serially and sampled during the fermentation phase and at the end of the PFC storage to explore depletion.
Media samples were protein depleted and homogenized; 5 nmol norleucine (internal standard) and loading buffer (60 μL) were added to 10 μL of sample, which was then injected onto an amino acid analyzer (Biochrom 30). Ion-exchange chromatography (sodium system) eluted amino acids with a series of buffers over pH 3.2–6.45 range. Peak detection was achieved by mixing the eluate with ninhydrin at 135°C and measuring absorbance at 570 and 440 nm. Quantitation used Chromeleon software and calibration curves for each amino acid.29
Statistics
Analysis of variance and Student's t-test assessed significance of data (Excel, GraphPad Prism), and mean±SEM or SD is reported.
Results
Cell growth and viability in 3D environment
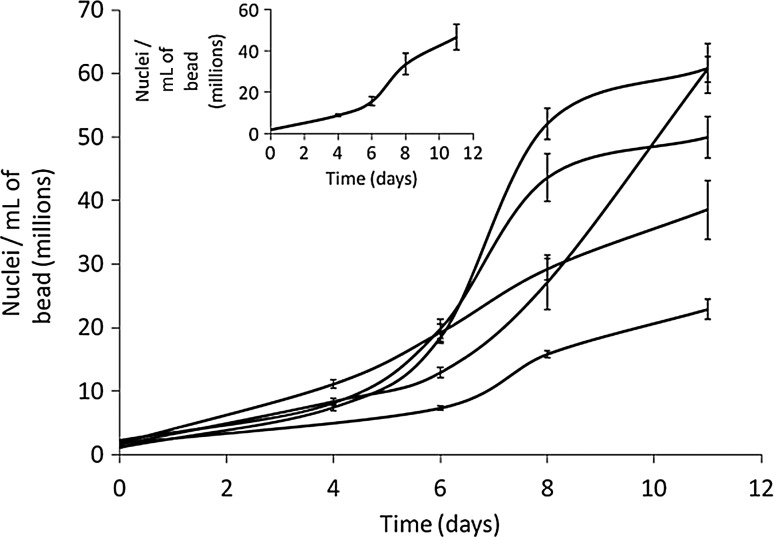
HepG2 cells were cultured in the alginate matrix under a fluidized regime. A cell density of (46.57±6.3)×106 cells/mL of alginate bead at harvest (mean±SEM, n=5) was achieved over the growth phase (Fig. 1), a total biomass of (5.43±0.7)×1010 cells (SEM, n=5). Media supplementation with human FFP promoted high cell proliferation, and later, differentiated liver function during 11 days' cultivation. A transient decrease in viability assessed by propidium iodide was recorded immediately after encapsulation (viabilities at 83.44±6.2%). By day 1 (less than 24 h after encapsulation) viability increased to >93% (93.85%±2.3%), a small but significant difference (p<0.05). This is likely to reflect a transient alteration in membrane integrity since in contrast in 24 h the total number of cells visualized had not changed; day 0: 2.44±0.4 and day 1: (1.94±0.1)×106 nuclei/mL, p=0.08, nor had the viable cell number, p>0.3. Figure 2 reflects the viability after encapsulation and at harvest on day 11.
FIG. 1.
Cell density in beads during proliferation phase of the biomass (n=5, mean±SEM). Inset: Average proliferation, (46.57±6.3)×106 cells/mL at harvest (SEM).
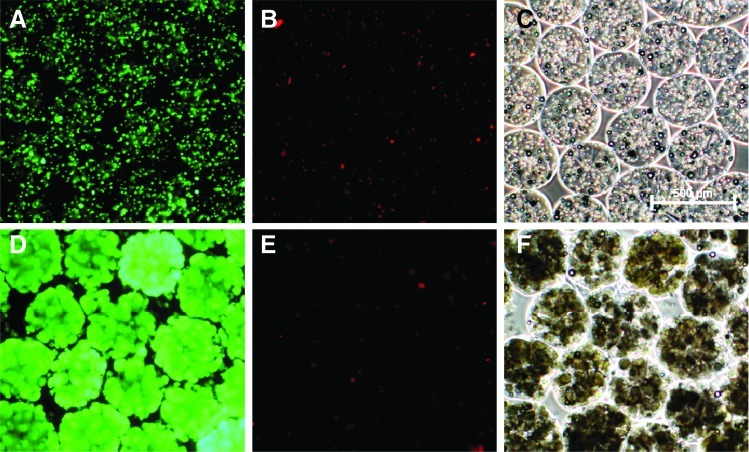
FIG. 2.
Viability and phase contrast microscopy of HepG2s encapsulated in alginate beads. (A, D) Fluorescein diacetate (FDA)-stained viable cells. (B, E) Propidium iodide (PI)-stained dead cells. (C, F) Phase contrast images. (A–C) Day of encapsulation, (D–F) after 10 days of proliferation in fluidized bed bioreactor.
Biomass performance
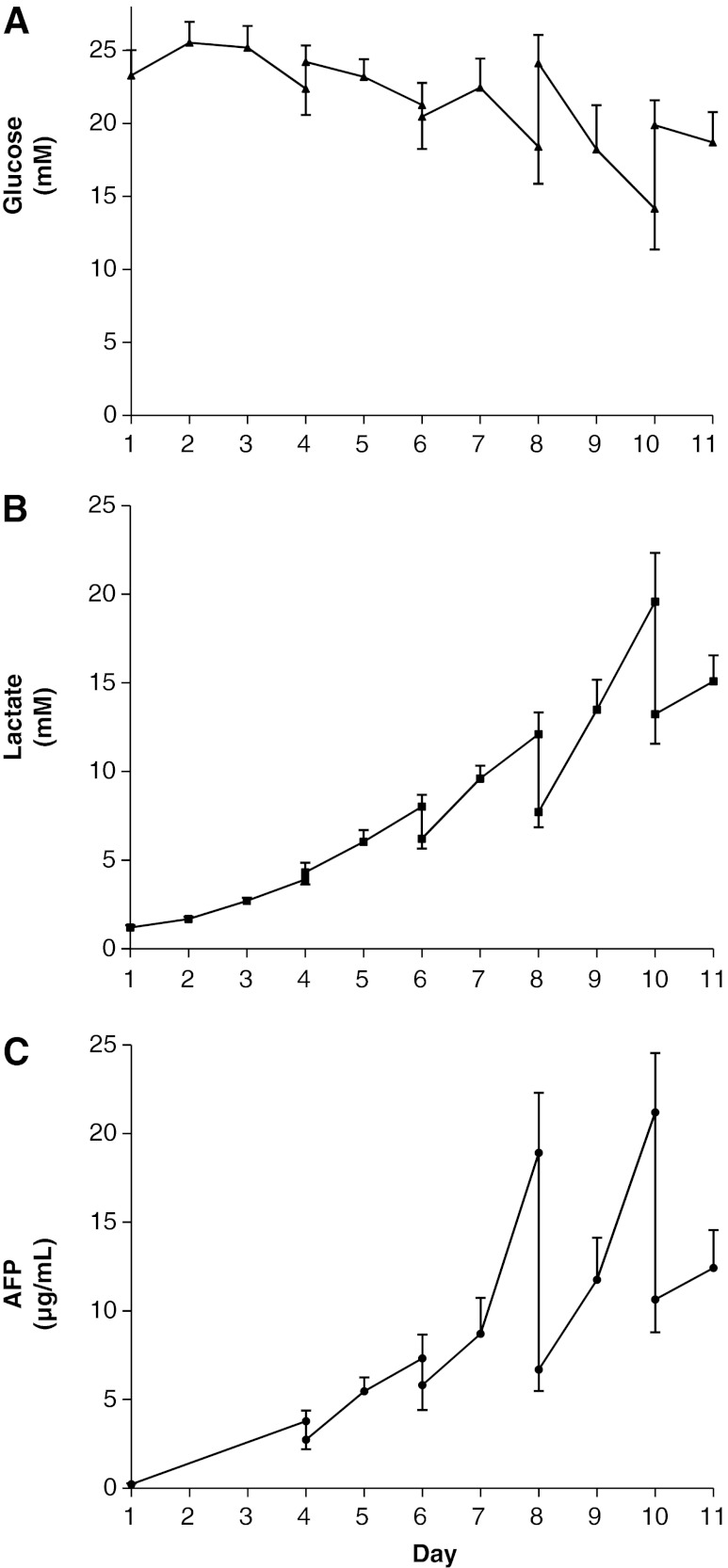
Glucose concentration decreased over time, despite interventional glucose supplementation, but was maintained>15 mM over the exponential growth period (Fig. 3A). Glucose consumption rate, expressed as micromoles per million cells per day, decreased over the proliferation period (14.3±7.96 on day 4; 6.43±2.09 on day 6; 9.01±2.76 on day 8; mean±SEM, n=5). By harvest, consumption dropped to 2.87±0.54 (mean±SEM, n=5), concomitant with the decrease in proliferation. Lactate accumulated in the culture medium, consistent with the metabolic profile of this cell line (Fig. 3B).30 Lactate build-up was minimized by media replacement on days 4, 6, 8, and 10. Sustained AFP production was observed over the proliferation period (Fig. 3C).
FIG. 3.
Metabolite levels during culture of proliferating biomass. (A) Glucose concentration during proliferation phase (n=5, mean±SEM), peaks match media changes on days 4, 6, 8, and 10). (B) Lactate profile: Lactate concentration during proliferation (n=5, mean±SEM). Falls in lactate correspond to media changes (days 4, 6, 8, and 10). (C) Alpha-fetoprotein (AFP) concentration; vertical lines represent samples before and after media change.
Gene and protein expression
RNA microarrays and protein powerblot arrays indicated alginate-encapsulated cells expressed cytoprotective pathways to a greater extent than monolayer-cultured cells. Thus, protein oxidation measured by Western blotting of protein carbonyl groups (OxyBlot™), indicated protection by alginate-encapsulation and culture (n=2): (monolayer 26.1 and 35.5 vs. AELS 9.27 and 7.86 densitometric units). When stressed by hypoxia and reperfusion, this protection persisted (monolayer 43.47 and 46.14 vs. AELS 24.16 and 30.24). Heat-shock protein 70 (HSP70), catalase, and MHC class I were increased in AELS cf. monolayers by 3.46-, 2.44-, and 3.2-fold, respectively, at mRNA levels, while HSP47 and RNA polymerase II were decreased by 3.15- and 2.38-fold, respectively. By Western blotting MAPKp49 (8.36 vs. 9.75) and ST1/Hop-p60 (7.70 vs. 13.99) were decreased in AELSs while p38delta was unchanged cf. monolayer; these data were confirmed by Powerblot array. Hemoxygenase 1 (HO-1) was dramatically increased in AELSs (13.37 vs. 5.50) while HO-2 was unchanged (2.89 vs. 2.98). The Powerblot array was further interrogated for adaptive- and cellular-stress proteins (Table 1) demonstrating increases in eight and decreases in two proteins associated with adaptive stress, and an increase in cathepsin D29, a protein involved in tissue remodeling. CDC42, a Rho protein that activates the protein kinase MEKK1 was decreased as was HSF4-38, a heat-shock factor that mediates the transcription of heat-shock proteins when HSP70 or 90 are decreased. This provides more confidence in the microarray data, which showed an increase in HSP70. Catalase specific activity was increased: AELS 18±1 mU/min per mg of protein vs. monolayer 8±1 mU/min per mg of protein.
Table 1.
Powerblot Analysis
| Catalog or product no. | Confidence level | Fold change | Function | |
|---|---|---|---|---|
| Adaptive stress proteins | ||||
| p115 | P67420 | 5 | +2.09 | Vesicular transport from the ER to Golgi |
| Plectin −144 | P92020 | 5 | +3.04 | Filament binding protein-crosslinking in cytoskeleton |
| E-cadherin | C377020 | 5 | +1.98 | Calcium-dependent adhesion molecule; increase reduces invasive carcinoma, important for epithelial junction formation |
| Fibronectin | F14420 | 5 | +4.03 | Extracellular matrix protein via integrin binds to collage and attachment to cell, cellular signaling |
| HSP60 | H99020 | 4 | +2.01 | Heat shock protein (HSP), constitutively expressed in normal and apoptotic cells |
| Annexin IV | A29920 | 4 | +2.44 | Family of calcium and phospholipid binding proteins |
| Adaptin alpha | A43920 | 4 | +2.21 | Recruits membrane proteins, vesicular transport |
| REF-1 | R64820 | 4 | +2.51 | Redox factor. Increase by AP-1 DNA binding, DNA repair |
| HRF | H42020 | 5 | −6.37 | Histamine releasing factor |
| Nip-1 | N79420 | 4 | −1.95 | Pro-apoptotic proteins, target proteins to mitochondria |
| Cellular stress response protein | ||||
| Cathepsin D-29 | C47620 | 5 | +9.2 | Tissue remodeling in response to estrogen |
| CDC42 | C70820 | 5 | −4.3 | Rho protein, activates MEKK1 |
| HSF4 –38 | H65520 | 5 | −4.25 | Heat shock factor, mediates transcription of HSPs, increases when there is a decrease of HSP70, 90 |
| Cell cycle proteins | ||||
| KAP-27 | K32120 | 4 | +3.55 | Cdk-associated phosphatase, cell cycle regulation |
| NBS1 | N10720 | 4 | +2.67 | Nijmejin breakage syndrome-complex with Rad 50 and MRE11, important for both DNA damage and repair, and telomere length maintenance |
| Bub3 cl.31 | B11520 | 5 | −3.46 | Sensing kinetochore attachment to microtubules during prometaphase to metaphase transition |
| GCAP-1 | G54220 | 4 | −6.74 | Guanylate cyclase binding protein, cell cycle progression |
| NEK2-46 | N52120 | 4 | −2.71 | Nima related kinase, conditions, controls entry of cells into S phase and mitosis |
| IAK1-46 | I71320 | 4 | −2.68 | Mammalian chromosome segregation |
The most significant results are shown, grouped into biological functions. Confidence levels 5 and 4 are shown as most significant, as a result of reproducibility of results and fold increases. Stress proteins increased in three-dimensional (3D) cultures cf. monolayer culture suggesting an adaptive response to enhanced performance. Comparison of cell-cycle proteins in AELS vs. monolayer, showing that the majority of cell-cycle proteins decreased, since doubling time of monolayer HepG2 cells increased from ∼24 h to 2–3 days when proliferating in 3D culture.
There were increases in two cell cycle–associated proteins; KAP-27, a cdk-associated phosphatase cell cycle regulator by 3.55-fold, and NBS1, a part of the DNA repair/telomere length maintenance mechanism by 2.67-fold. Five proteins were decreased: Bub3 cl.31, 3.46-fold; GCAP-1, 6.74-fold; NEK2-46, 2.71-fold; IAK1-46, 2.68-fold; and cyclin-B, 2.26-fold. Proliferation was assessed immunocytochemically using antibodies to endogenous Ki67 and to exogenously added BRDU. Staining was evident throughout the liver cell spheroids, in ∼50% of the cells, irrespective of their position within the spheroid; moreover, there were no necrotic centers, as assessed by hematoxylin and eosin (HE) staining (data not shown).
Nutrient provision
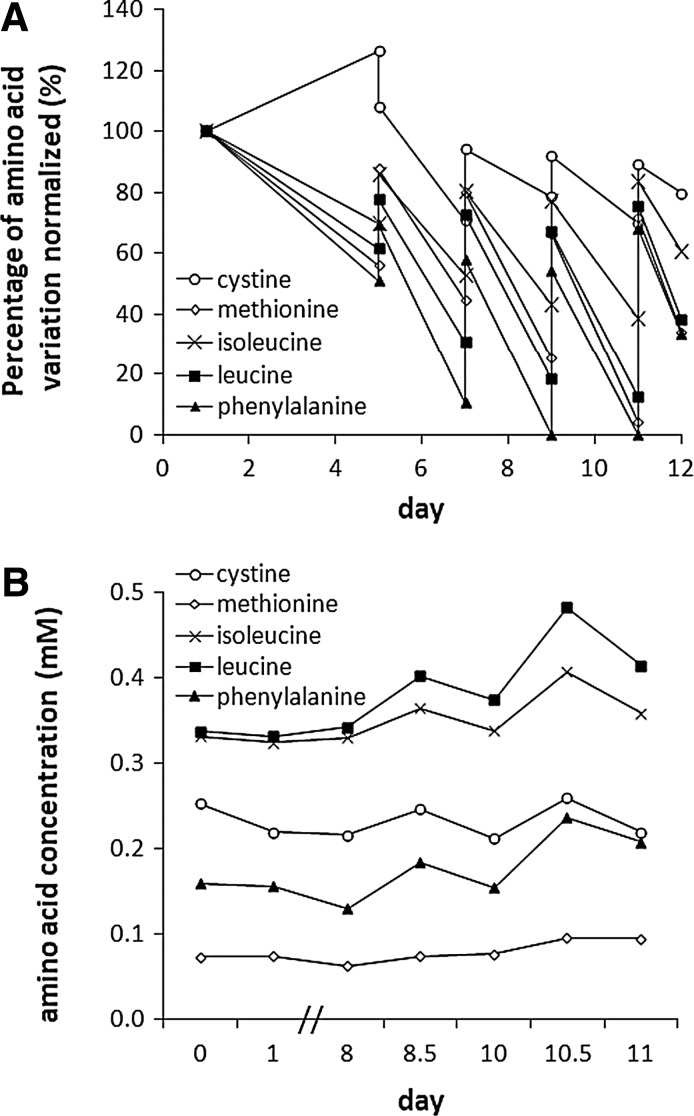
Amino acid levels over 12 days in the bioreactor media initially showed a marked decline in five essential amino acids: leucine, isoleucine, cystine, methionine, and phenylalanine, despite regular medium changes. All but cystine had dropped below 60% of the starting concentration by day 6 and continued to decline, with phenylalanine being totally consumed on days 9 and 11. These data informed a mathematical model, predicting amino acid consumption of AELSs during fermentation; this was fine-tuned via iterative empirical measurements. Figure 4A shows data for all amino acids whose concentration fell during the proliferation stage of biomass culture. After establishing the maximum concentration that could be used without toxicity to the cells for each (Supplementary Fig. S1), leucine, isoleucine, cysteine, methionine, and phenylalanine were added daily from a stock concentration of 100 mM, thus maintaining these key depleted amino acids (Fig. 4B) at ≥60% of initial levels.
FIG. 4.
Amino acid levels during biomass production. (A) Amino acid levels during 12 days of biomass cultivation. Graph shows consumption of five key amino acids being only partially replenished by media changes on days 5, 7, 9, and 11. (B) Successful maintenance of depleted amino acids during fermentation. Control was by use of a model relating cell proliferation and amino acid consumption to predict media supplementation; in all cases the minimum amino acid concentrations remained above 60% of initial value.
Alginate bead physical parameters.
Average bead size after encapsulation was 528±69.6 μm (mean±SD, n=558) and after harvest 566±74 μm (mean±SD, n=473; no significant difference). The average number of cells per bead immediately after encapsulation was 320±30.4 (mean±SEM, n=5) and 10,868±1971 (men±SEM, n=5) at harvest—an approximately 34-fold increase in cells/bead. There were ∼4400 beads per milliliter of biomass. Beads occupied 42% of the total volume (ɛS) when settled.
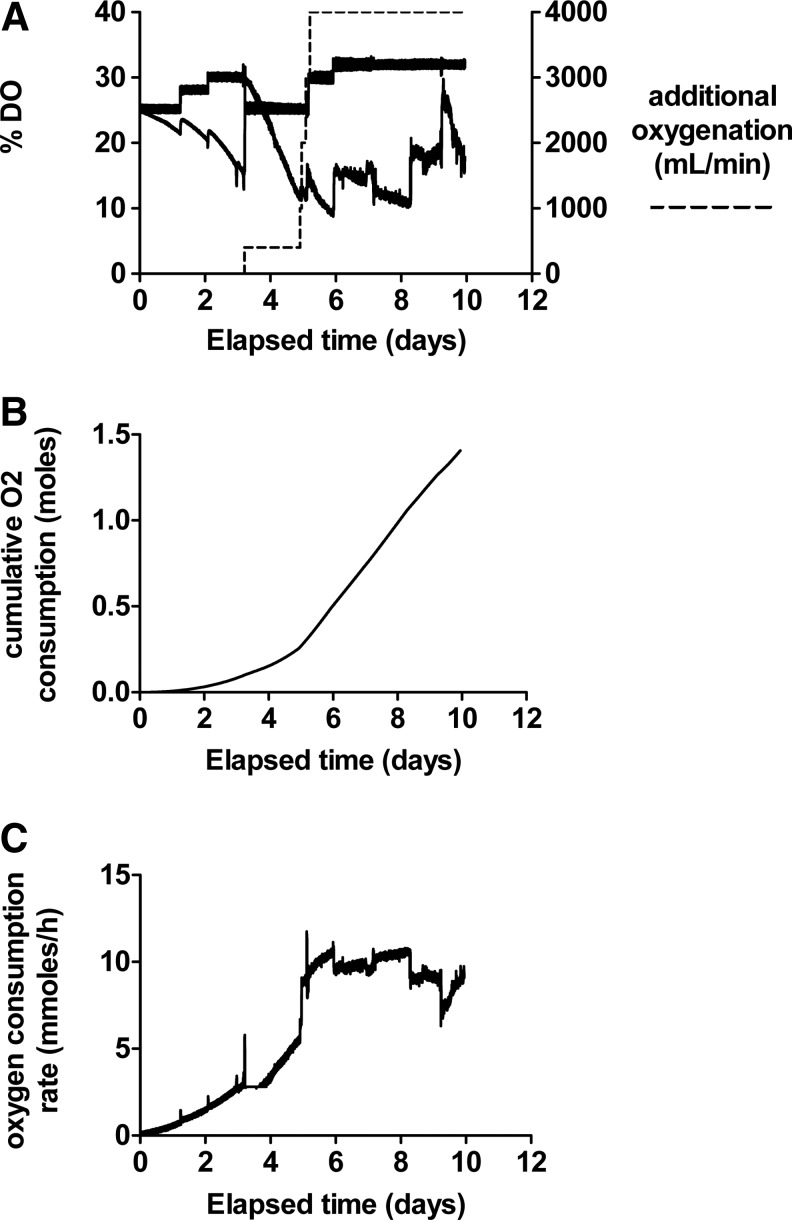
Oxygen consumption
Dissolved oxygen (DO) levels during cell proliferation were measured in the reservoir (probe: DO1) and also downstream of the biomass (probe: DO2), measuring oxygen depletion by proliferating cells and controlling oxygenation by increasing DO setpoint. To prevent hyperoxic toxicity to biomass, oxygen delivery at the inlet to the growth chamber was ≤35%. Additional oxygen was supplied directly within the growth chamber by a thin membrane oxygenator. Figure 5 illustrates the oxygenation parameters and consumption of a representative experiment.
FIG. 5.
Oxygenation of a typical fluidized bed bioreactor experiment over 11 days. (A) Dissolved oxygen (DO)1 (dO2 in fermentor), DO2 (dO2 immediately after the biomass); additional oxygenation (dashed line) supplied directly to the biomass chamber. (B) Cumulative oxygen consumption of biomass. (C) Oxygen consumption in millimoles per hour.
Biomass preservation at room temperature
Preliminary data showed a lack of headspace (air) above the AELS suspension in cell flasks resulted in poor AELS viability even after PFC supplementation. Increasing headspace to 50% improved viability to >80%. When AELS to medium ratio was 1:1, viability was 80%, improving to 95% when the volume of medium was increased to 10 times that of beads with an antioxidant cocktail. Viable cell numbers improved by approximately 23% over 48 h compared with control. Essential amino acid concentrations at the end of storage were all sufficiently high, except for valine. The chosen optimized protocol for bead to media to PFC ratio was 1:10:1, headspace was 50%, antioxidants and valine (1.965 mM) added. Cell viability was maintained (85.4%±12.3%; mean±SD, n=9 after 48 h; compared to a starting value of 93%±8.1%; mean±SD, n=9; no significant difference, p>0.05), and proliferation continued during storage such that viable cell numbers were not decreased.
Discussion
The clinical imperative
To date only organ transplantation successfully replaces the failed human liver. Controlled clinical trials of bio-artificial liver and artificial liver devices have not shown improved survival. A review from Carpentier et al.31 summarized the bioartificial device data, while two artificial device trials based on albumin dialysis have been more recently reported.32,33 The justification for providing temporary liver support derives both from current surgical practice and extensive animal work, which have repeatedly demonstrated that there is a critical mass of liver that is required for survival. Currently this is exemplified by “small for size” liver scenarios that affect both resectional surgery for tumors and living-donor liver transplantation; in these cases insufficient liver mass may be present postsurgery for adequate liver function and greater confirmed functional biomass would clearly be of considerable value.
Our hypothesis is that HepG2 cells cultured in an optimized form can replace deficient liver function adequately to allow adequate time for liver repair to take place or to buy time for an organ donor to be identified.
Fulfilling the functional requirements
In vitro data indicate that these cells do express a panel of Phase I and Phase 2 detoxifying enzymes when cultured in 3D format in our system (AELSs), although at a lower level than primary cells. Additionally, they metabolize ammonia, and they produce new transport proteins, notably albumin. Clearly each individual metabolic detoxifying pathway above is likely to be supplemented proportionately to the number of cells over which a patient's plasma is perfused.
The provision of freshly synthesized albumin also merits note. In particular, many nonbiological liver machine studies are based on a form of albumin dialysis. While none so far has improved survival, there is symptomatic relief in some patients, which is associated with the removal of certain toxins by the albumin. However, an issue that has clearly emerged is the source and thus biological efficacy of the albumin in such systems, and its fitness-for-purpose as a carrier protein to aid detoxification. Commercially isolated or prepared albumin has an altered affinity for toxins that makes it considerably less effective than native albumin; the loss of function being attributed in part to the preservatives (e.g., cacodylate) used in their preparation. An advantage of our system is that the HepG2 cells are continuously making endogenous nascent albumin and indeed, we have demonstrated that in our system the per cell production is equivalent to that calculated from human in vivo data. This nascent albumin has obviously never been exposed to preservatives or other manufacturing processes that adversely affect the toxin-binding characteristics of commercial albumin.
Providing adequate functioning biomass requires innovation in cell culture conditions and scale, bioreactor technology, and preservation and transport parameters, and we have addressed all of these.
Physical advantages of the system
Hepatocyte function is affected by cell shape and by the presence of extracellular matrix. Alginate has many advantages as an exogenous matrix, being a natural product harvested globally to commercial scale and being relatively bio-inert, with purified preparations nonantigenic in the patient.34,35 Its semipermeable nature in the polymerized form provides a protective environment and a structure for cell growth and adherence suitable for epithelial cells. As a 1% hydrogel (i.e., 99% fluid),26 it allows ready release of cell-synthesized substances into the surrounding milieu, and free passage of small molecules through the gel to the cells. We described elsewhere the favorable effects when cells are encapsulated in alginate, generating 3D colonies of polarized cells with excellent morphology, locally generated extracellular matrix proteins, and up-regulated functionality.17,36–39 Encapsulation, using the JetCutter technology we describe, allows efficient production of a cell mass adequate to charge a bioreactor for human use in <3 h, convenient and feasible for industrial production. Calcium ion concentration is critical during encapsulation and culture. This affects polymerization via covalently bonding adjacent alginate chains, modulating permeability, size, shape, and rigidity of the beads. Calcium is also hygroscopic and can cause bead swelling, but our technique of stabilizing calcium concentrations resulted in stable bead size, indicating bead integrity throughout encapsulation and culture.40
Alginate encapsulation minimizes shear stress to cells,41 advantageous in the context of a fluidized bed perfusion system. The fluidizing motion does not affect mechanical strength of alginate beads.25 Feasibility of an FBB based on alginate beads was proven by mathematical modeling and empirical testing27 using encapsulated cells immediately after encapsulation without prior culture. Here we extended that approach to include prolonged culture allowing cell proliferation, development of cell-to-cell contacts, and the critical benefits of 3D culture increasing per cell performance over time. The microgravity, rather than static regime also up-regulates per cell functionality.36
The choice of biomass
Using HepG2 cells in BALs has caused controversy. They are a tumor cell line, albeit derived from a well-differentiated hepatoblastoma. Notably one clone derived from the original cell line has been used in an FDA-approved clinical trial in man.15 We previously referred to the known deficiencies in some hepatic functions (e.g., absent functional ornithine transcarbamylase in the urea cycle),42 although other pathways of ammonia assimilation are present. HepG2 cells have two modes of amino acid transport, the mode used by primary hepatocytes (System N) and that used by tumor cells (System ASC, sodium-dependent amino acid transporter) resulting in rapid glutamine uptake several fold faster than primary hepatocytes alone—offering an explanation of how ammonia, incorporated into glutamine, may be lowered by this system.43,44
Further, other detoxifying functions, such as those associated with cytochrome P450 enzymes, when up-regulated by the 3D culture compared with monolayer culture, rise to levels within the lower quartile of cultured primary human hepatocytes.45 We and others have identified many genes that are up-regulated in 3D culture compared with monolayer.46–48 Some metabolic pathways appear even more favorable than primary hepatocytes for use in a BAL; for example, ATP generation from aerobic glycolysis in the presence of adequate oxygen and normal mitochondria49 may confer an advantage in liver failure plasma since such metabolically switched cells are resistant to xenobiotics and to compounds causing mitochondrial uncoupling and dysfunction. Moreover, synthetic, detoxifying functions and viability were maintained after perfusion for 6–8 h in liver failure plasma in previous work.37
The microarray data presented here indicate that HepG2 cells cultured in alginate beads demonstrate adaptive and potentially beneficial stress responses.50 Catalase and glutathione-S-transferase, both involved in antioxidant defense51,52 were increased, and stress-inducible phosphoprotein ST1/Hopp60 decreased. Expression of the molecular chaperone HSP70 increased, potentially preventing denaturation of existing cellular proteins and promoting the initial folding of nascent proteins.53,54 Another cytoprotective pathway, HO-1,55 was increased in AELSs. Moreover, a decrease in lipid peroxidation and protein oxidation in encapsulated cells suggests the milieu within the alginate polymer reduced cellular stress associated with increased function.
It has been estimated that between 10% and 30% of the liver cell mass is sufficient for adequate replacement of liver activity.56 Previous BALs may have failed partly due to an underestimation of the required cell numbers for liver support.16 Here we demonstrated the expansion of HepG2 numbers to a level suitable for use in an animal trial and demonstrate that the processes described regularly generates 40–50 billion cells 25%–50% of that in normal adult liver,57 in an alginate scaffold occupying less than 1700 cm3, with high cell viabilities.
Achieving these cell numbers required a protocol to address nutrient deficiency, toxic chemical build-up, and metabolite diffusion in the bioreactors. Mass transfer models were coupled with Monod kinetics to describe oxygen diffusion and cell proliferation. Oxygen diffusivity values are derived from Gerontas et al.58; oxygen uptake rates are derived from fluidized bed experimental data. Regions of high oxygen level (DOT=35%) seen at the base of the vessel drop to ∼8% DOT in region of biomass, increasing again in upper cell-free zone, thus at all times exceeding the levels seen in areas of venous draining (lowest oxygen) in the liver. Our oxygen consumption on a per cell basis compares favorably at 0.038 fmol/sec per cell with those data derived from the AMC bioreactor (0.0166 fmol/sec per cell) 59 and even more so from the hollow fiber cartridge charged with primary human hepatocytes from Mueller et al.60 at 0.0028 fmol/sec per cell. Depletion of the five most required amino acids was adequately prevented by intermittent supplementation regimes based on modeling or iterative experimental confirmation; this approach is simpler, reduces cost, and is more readily applicable in bioreactor technology than would be large-scale complete media changes.
Biochemical studies monitoring metabolism suggested sequential change during progression of the AELSs cultured over time. The rise in glucose concentration during days 1–2 is consistent with gluconeogenesis by hepatic cells, before proliferation enters the log-phase and glucose consumption increases; a later fall in consumption towards the end of culture suggests a change from a proliferative toward a more differentiated phenotype. AFP production similarly changed over time; production of this protein is characteristic of less-differentiated hepatocytes, cf. normal mature adult hepatocytes, which preferentially produce albumin. AFP progressively accumulated up to 8 days and then decreased compatible with greater differentiation of the cells. Others have also shown a decrease in AFP production accompanied by albumin secretion on 3D culture of liver cells suggesting a switch from proliferation to differentiation.61
The cells are performing aerobic glycolysis in which situation lactate accumulation does not reflect hypoxia. There is no reason to expect preferential and thus potentially toxic lactate accumulation in the center of the beads (as might be the case in a solid tissue mass) for several reasons. Each bead is only ∼500 μm in diameter, with only a 250-μm pathway from surface to center, and is a 1% hydrogel without any outside membrane, such that oxygen will be adequately transferred to the center. Further, HE sections of beads did not show any central necrosis, and proliferation of cells was not diminished in the central area.
Comparison with hollow fiber–based systems
The system described here differs in several respects from the ELAD device, which also used a human liver-derived cell line, C3A, a subclone of HepG2 cells. The major differences between the ELAD device and our system reside in (1) directly facilitated mass transfer between the cells and the patient's plasma, (2) the ability to accurately quantify cell number, viability, and function, both synthetic and metabolic, in the biomass to provide validation for the bioartificial liver, and (3) the improved per cell performance the alginate-encapsulated liver cells demonstrate. ELAD uses a hollow fiber cartridge charged with C3A cells inoculated on one side of a hollow fiber membrane, with a 100-kDa plasma filtrate passing through the fibers. Even with a plasma filtrate, the high protein concentration of plasma is likely to cause a degree of membrane fouling and pore blockage. This is in direct contrast to our system using cells encapsulated in a 1% porous hydrogel without a diffusion limiting membrane, perfused by whole plasma, that allows even large molecules such as fibrinogen (MW 340 kDA) to pass freely. Moreover, since each functional unit is a ∼0.5-mm diameter bead and each exists in its own milieu of plasma, the degree of mass transfer as a whole is extremely high.26 We have previously published functional data on AELSs in a BAL format (e.g., bilirubin conjugation, protein synthetic function, glucose consumption)37 and most recently the preclinical results in a porcine model of ischemic acute liver failure.62
Preservation at ambient temperature
Preservation of cells in AELSs during storage and transport to the recipient is a considerable logistical challenge for future clinical application. While successful cryopreservation of performance competent encapsulated liver cells would provide an indefinite shelf life, functional recovery of cryopreserved cells at the clinical scale is not yet sufficient. We therefore developed a protocol for storage of beads at ambient temperature over 48 h as a time useful for transport of the biomass to the bedside. Most recently we successfully used the system, prior to testing in an animal model, to allow transport to a site distant to AELS production.62 Although metabolism is reduced at mild hypothermia (ambient temperature) compared with 37°C, provision of oxygen is necessary and was achieved by supplementing the AELS suspension with oxygen-saturated PFC, such as is used for oxygen delivery in organ preservation.63–65 Preservation of function during PFC-supported transport required optimization of headspace to medium ratio, allowing for adequate gaseous exchange, cell-bead to medium ratios, and essential amino acid concentrations. Finally, as hypothermia can result in oxidative stress, known to be damaging to encapsulated liver cells,66–68 viable cells numbers were improved using antioxidants with this technique.
Viability was successfully maintained for AELSs over 48 h at ambient temperature, and as far as we are aware, it is the first time this approach has been utilized for storage of any encapsulated cells, providing transport of competent beads from GMP production sites to end-user clinics. It may also be applicable for other regenerative medicine applications.
Thus, we developed a scalable system based on a simple, ∼0.5-mm-diameter cell-containing bead as the unit of function. The use of a cell-line confers reliability in proliferation; the application of a fluidized bed perfusion enabled good mass transfer, and the system is a straightforward concept with potential for highly complex applications. We have already used this system successfully in a large animal model of acute liver failure in pigs (Supplementary Fig. S2), demonstrating improvement in a number of clinically important parameters of liver function.62
Supplementary Material
Acknowledgments
We thank NIHR, Liver Group Charity, Weston Foundation, Eranda Foundation, Peter Stebbings Memorial Charity, and Steele Charitable Trust.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ostapowicz G. Fontana RJ. Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier B. Gautier A. Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690–1702. doi: 10.1136/gut.2008.175380. [DOI] [PubMed] [Google Scholar]
- 3.Gerlach J. Kloppel K. Schauwecker HH, et al. Use of hepatocytes in adhesion and suspension cultures for liver support bioreactors. Int J Artif Organs. 1989;12:788–792. [PubMed] [Google Scholar]
- 4.Liu JJ. Chen BS. Tsai TF, et al. Long term and large-scale cultivation of human hepatoma Hep G2 cells in hollow fiber bioreactor. Cultivation of human hepatoma Hep G2 in hollow fiber bioreactor. Cytotechnology. 1991;5:129–139. doi: 10.1007/BF00365429. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita T. Ijima H. Koide N, et al. High albumin production by multicellular spheroids of adult rat hepatocytes formed in the pores of polyurethane foam. Appl Microbiol Biotechnol. 1991;36:324–326. doi: 10.1007/BF00208150. [DOI] [PubMed] [Google Scholar]
- 6.Shatford RA. Nyberg SL. Meier SJ, et al. Hepatocyte function in a hollow fiber bioreactor: a potential bioartificial liver. J Surg Res. 1992;53:549–557. doi: 10.1016/0022-4804(92)90253-v. [DOI] [PubMed] [Google Scholar]
- 7.Rozga J. Williams F. Ro MS, et al. Development of a bioartificial liver: properties and function of a hollow-fiber module inoculated with liver cells. Hepatology. 1993;17:258–265. [PubMed] [Google Scholar]
- 8.Bader A. Knop E. Frühauf N, et al. Reconstruction of liver tissue in vitro: geometry of characteristic flat bed, hollow fiber, and spouted bed bioreactors with reference to the in vivo liver. Artif Organs. 1995;19:941–950. doi: 10.1111/j.1525-1594.1995.tb02456.x. [DOI] [PubMed] [Google Scholar]
- 9.Naruse K. Sakai Y. Nagashima I, et al. Comparisons of porcine hepatocyte spheroids and single hepatocytes in the non-woven fabric bioartificial liver module. Int J Artif Organs. 1996;19:605–609. [PubMed] [Google Scholar]
- 10.Flendrig LM. la Soe JW. Jörning GG, et al. In vitro evaluation of a novel bioreactor based on an integral oxygenator and a spirally wound nonwoven polyester matrix for hepatocyte culture as small aggregates. J Hepatol. 1997;26:1379–1392. doi: 10.1016/s0168-8278(97)80475-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohshima N. Yanagi K. Miyoshi H. Packed-bed type reactor to attain high density culture of hepatocytes for use as a bioartificial liver. Artif Organs. 1997;21:1169–1176. doi: 10.1111/j.1525-1594.1997.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsuura T. Kawada M. Hasumura S, et al. High density culture of immortalized liver endothelial cells in the radial-flow bioreactor in the development of an artificial liver. Int J Artif Organs. 1998;21:229–234. [PubMed] [Google Scholar]
- 13.Busse B. Gerlach JC. Bioreactors for hybrid liver support: historical aspects and novel designs. Ann NY Acad Sci. 1999;875:326–339. doi: 10.1111/j.1749-6632.1999.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 14.Shito M. Kim NH. Baskaran H, et al. In vitro and in vivo evaluation of albumin synthesis rate of porcine hepatocytes in a flat-plate bioreactor. Artif Organs. 2001;25:571–578. doi: 10.1046/j.1525-1594.2001.025007571.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellis AJ. Hughes RD. Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE. Putnam CW. Groth CG, et al. Alopecia, ascites, and incomplete regeneration after 85 to 90 per cent liver resection. Am J Surg. 1975;129:587–590. doi: 10.1016/0002-9610(75)90323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil M. Shariat-Panahi A. Tootle R, et al. Human hepatocyte cell lines proliferating as cohesive spheroid colonies in alginate markedly upregulate both synthetic and detoxificatory liver function. J Hepatol. 2001;34:68–77. doi: 10.1016/s0168-8278(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 18.Selden C. Khalil M. Hodgson H. Three dimensional culture upregulates extracellular matrix protein expression in human liver cell lines—a step towards mimicking the liver in vivo? Int J Artif Organs. 2000;23:774–781. [PubMed] [Google Scholar]
- 19.Demetriou AA. Brown RS Jr. Busuttil RW, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–667. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruhauf JH. Mertsching H. Giri S, et al. Porcine endogenous retrovirus released by a bioartificial liver infects primary human cells. Liver Int. 2009;29:1553–1561. doi: 10.1111/j.1478-3231.2009.02087.x. [DOI] [PubMed] [Google Scholar]
- 21.Baquerizo A. Mhoyan A. Kearns-Jonker M, et al. Characterization of human xenoreactive antibodies in liver failure patients exposed to pig hepatocytes after bioartificial liver treatment: an ex vivo model of pig to human xenotransplantation. Transplantation. 1999;67:5–18. doi: 10.1097/00007890-199901150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Baquerizo A. Mhoyan A. Shirwan H, et al. Xenoantibody response of patients with severe acute liver failure exposed to porcine antigens following treatment with a bioartificial liver. Transplant Proc. 1997;29:964–965. doi: 10.1016/s0041-1345(96)00330-2. [DOI] [PubMed] [Google Scholar]
- 23.Knowles BB. Howe CC. Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 24.Massie I. Selden C. Hodgson H, et al. Cryopreservation of encapsulated liver spheroids for a bioartificial liver: reducing latent cryoinjury using an ice nucleating agent. Tissue Eng Part C. 2011;17:765–774. doi: 10.1089/ten.TEC.2010.0394. [DOI] [PubMed] [Google Scholar]
- 25.David B. Barbe L. Barthes-Biesel D, et al. Mechanical properties of alginate beads hosting hepatocytes in a fluidized bed bioreactor. Int J Artif Organs. 2006;29:756–763. doi: 10.1177/039139880602900805. [DOI] [PubMed] [Google Scholar]
- 26.David B. Dore E. Jaffrin MY, et al. Mass transfers in a fluidized bed bioreactor using alginate beads for a future bioartificial liver. Int J Artif Organs. 2004;27:284–293. doi: 10.1177/039139880402700404. [DOI] [PubMed] [Google Scholar]
- 27.Legallais C. Dore E. Paullier P. Design of a fluidized bed bioartificial liver. Artif Organs. 2000;24:519–525. doi: 10.1046/j.1525-1594.2000.06510.x. [DOI] [PubMed] [Google Scholar]
- 28.Legallais C. Jaffrin MY. A feasibility study of a filtration type autotransfusion device. J Biomed Eng. 1993;15:143–147. doi: 10.1016/0141-5425(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 29.Spackman DH. Stein WH. Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem. 1958;30:1190–1206. [PubMed] [Google Scholar]
- 30.Blackard WG. Clore JN. Powers LP. A stimulatory effect of FFA on glycolysis unmasked in cells with impaired oxidative capacity. Am J Physiol. 1990;259:E451–E456. doi: 10.1152/ajpendo.1990.259.3.E451. [DOI] [PubMed] [Google Scholar]
- 31.Carpentier B. Gautier A. Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690–1702. doi: 10.1136/gut.2008.175380. [DOI] [PubMed] [Google Scholar]
- 32.Rifai K. Kribben A. Gerken G, et al. Extracorporeal liver support by fractionated plasma separation and adsorption (Prometheus®) in patients with acute-on-chronic liver failure (Helios Study): a prospective randomized controlled multicenter study. J Hepatol. 2010;52:S3. [Google Scholar]
- 33.Banares R. Nevens F. Larsen FS, et al. Extracorporeal liver support with the molecular adsorbent recirculating system (MARS) in patients with acute-on-chronic liver failure (AOCLF). The Relief Trial. J Hepatol. 2010;52:S459–S460. [Google Scholar]
- 34.Menard M. Dusseault J. Langlois G, et al. Role of protein contaminants in the immunogenicity of alginates. J Biomed Mater Res B Appl Biomater. 2010;93:333–340. doi: 10.1002/jbm.b.31570. [DOI] [PubMed] [Google Scholar]
- 35.Klock G. Pfeffermann A. Ryser C, et al. Biocompatibility of mannuronic acid-rich alginates. Biomaterials. 1997;18:707–713. doi: 10.1016/s0142-9612(96)00204-9. [DOI] [PubMed] [Google Scholar]
- 36.Coward SM. Selden C. Mantalaris A, et al. Proliferation rates of HepG2 cells encapsulated in alginate are increased in a microgravity environment compared with static cultures. Artif Organs. 2005;29:152–158. doi: 10.1111/j.1525-1594.2005.29026.x. [DOI] [PubMed] [Google Scholar]
- 37.Coward SM. Legallais C. David B, et al. Alginate-encapsulated HepG2 cells in a fluidized bed bioreactor maintain function in human liver failure plasma. Artif Organs. 2009;33:1117–1126. doi: 10.1111/j.1525-1594.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 38.Damelin LH. Coward S. Kirwan M, et al. Fat-loaded HepG2 spheroids exhibit enhanced protection from pro-oxidant and cytokine induced damage. J Cell Biochem. 2007;101:723–734. doi: 10.1002/jcb.21229. [DOI] [PubMed] [Google Scholar]
- 39.Selden C. Shariat A. McCloskey P, et al. Three-dimensional in vitro cell culture leads to a marked upregulation of cell function in human hepatocyte cell lines—an important tool for the development of a bioartificial liver machine. Ann NY Acad Sci. 1999;875:353–363. doi: 10.1111/j.1749-6632.1999.tb08517.x. [DOI] [PubMed] [Google Scholar]
- 40.Thu B. Bruheim P. Espevik T, et al. Alginate polycation microcapsules. II. Some functional properties. Biomaterials. 1996;17:1069–1079. doi: 10.1016/0142-9612(96)85907-2. [DOI] [PubMed] [Google Scholar]
- 41.Dore E. Legallais C. A new concept of bioartificial liver based on a fluidized bed bioreactor. Ther Apher. 1999;3:264–267. doi: 10.1046/j.1526-0968.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- 42.Mavri-Damelin D. Eaton S. Damelin LH, et al. Ornithine transcarbamylase and arginase I deficiency are responsible for diminished urea cycle function in the human hepatoblastoma cell line HepG2. Int J Biochem Cell Biol. 2007;39:555–564. doi: 10.1016/j.biocel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Bode BP. Souba WW. Modulation of cellular proliferation alters glutamine transport and metabolism in human hepatoma cells. Ann Surg. 1994;220:411–422. doi: 10.1097/00000658-199410000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bode BP. Fuchs BC. Hurley BP, et al. Molecular and functional analysis of glutamine uptake in human hepatoma and liver-derived cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1062–G1073. doi: 10.1152/ajpgi.00031.2002. [DOI] [PubMed] [Google Scholar]
- 45.Wilkening S. Stahl F. Bader A. Comparison of primary human hepatocytes and hepatoma cell line HEPG2 with regard to their biotransformation properties. Drug Metab Dispos. 2003;31:1035–1042. doi: 10.1124/dmd.31.8.1035. [DOI] [PubMed] [Google Scholar]
- 46.Khaoustov VI. Risin D. Pellis NR, et al. Microarray analysis of genes differentially expressed in HEPG2 cells cultured in simulated microgravity: preliminary report. In Vitro Cell Dev Biol Anim. 2001;37:84–88. doi: 10.1290/1071-2690(2001)037<0084:MAOGDE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 47.Shimada M. Yamashita Y. Tanaka S, et al. Characteristic gene expression induced by polyurethane foam/spheroid culture of hepatoma cell line, Hep G2 as a promising cell source for bioartificial liver. Hepatogastroenterology. 2007;54:814–820. [PubMed] [Google Scholar]
- 48.Thomas A. Hodgson HJF. Selden C. Cytochrome P450 activity in HepG2 cells is significantly chemically enhanced and can be maintained in the absence of inducer. BASL. 2007:44. [Google Scholar]
- 49.Marroquin LD. Hynes J. Dykens JA, et al. Circumventing the crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- 50.Nilsson HO. Blom J. Abu Al-Soud W, et al. Effect of cold starvation, acid stress, and nutrients on metabolic activity of Helicobacter pylori. Appl Environ Microbiol. 2002;68:11–19. doi: 10.1128/AEM.68.1.11-19.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai J. Cederbaum AI. Adenovirus-mediated overexpression of catalase in the cytosolic or mitochondrial compartment protects against cytochrome P450 2E1-dependent toxicity in HepG2 cells. J Biol Chem. 2001;276:4315–4321. doi: 10.1074/jbc.M008895200. [DOI] [PubMed] [Google Scholar]
- 52.Bai JX. Rodriguez AM. Melendez JA, et al. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 53.Papp E. Nardai G. Soti C, et al. Molecular chaperones, stress proteins and redox homeostasis. Biofactors. 2003;17:249–257. doi: 10.1002/biof.5520170124. [DOI] [PubMed] [Google Scholar]
- 54.Patel B. Khaliq A. JarvisEvans J, et al. Hypoxia induces HSP 70 gene expression in human hepatoma (HEP G2) cells. Biochem Mol Biol Int. 1995;36:907–912. [PubMed] [Google Scholar]
- 55.Poss KD. Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen JW. Hassanein T. Bhatia SN. Advances in bioartificial liver devices. Hepatology. 2001;34:447–455. doi: 10.1053/jhep.2001.26753. [DOI] [PubMed] [Google Scholar]
- 57.Imamura H. Kawasaki S. Shiga J, et al. Quantitative evaluation of parenchymal liver cell volume and total hepatocyte number in cirrhotic patients. Hepatology. 1991;14:448–453. [PubMed] [Google Scholar]
- 58.Gerontas S. Farid SS. Hoare M. Windows of operation for bioreactor design for the controlled formation of tissue-engineered arteries. Biotechnol Prog. 2009;25:842–853. doi: 10.1002/btpr.140. [DOI] [PubMed] [Google Scholar]
- 59.Poyck PP. Hoekstra R. van Wijk AC, et al. Functional and morphological comparison of three primary liver cell types cultured in the AMC bioartificial liver. Liver Transpl. 2007;13:589–598. doi: 10.1002/lt.21090. [DOI] [PubMed] [Google Scholar]
- 60.Mueller D. Tascher G. Müller-Vieira U, et al. In-depth physiological characterization of primary human hepatocytes in a 3D hollow-fiber bioreactor. J Tissue Eng Regen Med. 2011;5:e207–218. doi: 10.1002/term.418. [DOI] [PubMed] [Google Scholar]
- 61.Schmelzer E. Triolo F. Turner ME, et al. Three-dimensional perfusion bioreactor culture supports differentiation of human fetal liver cells. Tissue Eng Part A. 2010;16:2007–2016. doi: 10.1089/ten.TEA.2009.0569. [DOI] [PubMed] [Google Scholar]
- 62.Selden C. Erro E. Spearman W, et al. Towards a clinical bioartificial liver machine based on human liver cells. Int J Artif Organs. 2012;35(8):555–587. [Google Scholar]
- 63.Matsumoto S. Clinical application of perfluorocarbons for organ preservation. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:75–82. doi: 10.1081/bio-200046698. [DOI] [PubMed] [Google Scholar]
- 64.Gioviale MC. Damiano G. Palumbo VD, et al. Pancreatic islets from non-heart-beating donor pig: two-layer preservation method in an in vitro porcine model. Int J Artif Organs. 2011;34:519–525. doi: 10.5301/IJAO.2011.8465. [DOI] [PubMed] [Google Scholar]
- 65.Hosgood SA. Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89:1169–1175. doi: 10.1097/TP.0b013e3181da6064. [DOI] [PubMed] [Google Scholar]
- 66.Vairetti M. Griffini P. Pietrocola G, et al. Cold-induced apoptosis in isolated rat hepatocytes: protective role of glutathione. Free Radic Biol Med. 2001;31:954–961. doi: 10.1016/s0891-5849(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 67.Huang H. Salahudeen AK. Cold induces catalytic iron release of cytochrome P-450 origin: a critical step in cold storage-induced renal injury. Am. J Transpl. 2002;2:631–639. doi: 10.1034/j.1600-6143.2002.20708.x. [DOI] [PubMed] [Google Scholar]
- 68.Karhumaki P. Tiitinen SL. Turpeinen H, et al. Inhibition of ERK 1/2 activation by phenolic antioxidants protects kidney tubular cells during cold storage. Transplantation. 2007;83:948–953. doi: 10.1097/01.tp.0000259249.24268.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.