Abstract
Hashimoto's thyroiditis is now considered the most prevalent autoimmune disease, as well as the most common endocrine disorder. It was initially described in 1912, but only rarely reported until the early 1950s. To celebrate this centennial, we reviewed the surgical pathology archives of the Johns Hopkins hospital for cases of Hashimoto's thyroiditis, spanning the period from May 1889 to October 2012. Approximately 15,000 thyroidectomies were performed at this hospital over 124 years. The first surgical case was reported in 1942, 30 years after the original description. Then, 867 cases of Hashimoto's thyroiditis were seen from 1942 to 2012, representing 6% of all thyroidectomies. Hashimoto's thyroiditis was the sole pathological finding in 462 cases; it accompanied other thyroid pathologies in the remaining 405 cases. The most commonly associated pathology was papillary thyroid cancer, an association that increased significantly during the last two decades. The most common indication for thyroidectomy was a thyroid nodule that was cytologically suspicious for malignancy. Hashimoto's thyroiditis remains a widespread, intriguing, and multifaceted disease of unknown etiology one century after its description. Advances in the understanding of its pathogenesis and preoperative diagnosis will improve recognition and treatment of this disorder, and may one day lead to its prevention.
Introduction
Hashimoto's thyroiditis is an autoimmune disease of the thyroid gland that has a characteristic pathological appearance. The main feature is infiltration with hematopoietic cells, mainly lymphocytes, organized in lymphoid follicles that often show prominent germinal centers. Other features include transformation of normal thyrocytes into Hürthle cells in some areas, destruction and atrophy of thyrocytes in other areas, and interstitial fibrosis. These histologic findings vary significantly among patients so that a clinicopathologic spectrum of conditions, rather than a single disease, falls under the term Hashimoto's thyroiditis. This spectrum now includes the classical, fibrosing, juvenile, painless, and Hashitoxicosis variants [reviewed in (1)], as well as postpartum thyroiditis (2) and the more recently reported IgG4-related variant (3).
The classical variant was initially reported by Dr. Hakaru Hashimoto in 1912 (4). He examined the surgical specimens of four middle-aged women who had undergone thyroidectomy because of compressive symptoms, and summarized the pathological findings in an article written in German containing two Latin words in the title (struma lymphomatosa) and five microphotographs. The article went unrecognized for about two decades: only a few cases of struma lymphomatosa were reported in the literature, often eliciting considerable controversy as to whether they really represented a distinct disease or rather an early phase of Riedel's thyroiditis (5). In 1931, Graham and McCullagh used the term “Hashimoto” for the first time in the title of an article, strongly arguing that struma lymphomatosa was indeed distinct from Riedel's thyroiditis (6). In 1939, the prominent British thyroid surgeon Cecil Joll coined the term “Hashimoto disease” and used it in the title of a review he wrote about this condition (7). Since then, Hashimoto's thyroiditis has gone from being a rarity to one of the most common autoimmune diseases, as well as the most common endocrine disease. Its incidence is about 1 case per 1000 persons per year (8). The prevalence is 8 cases per 1000 when estimated from a review of published articles (9), and 46 cases per 1000 when estimated from the biochemical evidence of hypothyroidism and thyroid autoantibodies in subjects participating to the Third National Health and Nutrition Examination Survey (10).
The pathogenesis of Hashimoto's thyroiditis has elicited interest since it was first reported. Dr. Hashimoto himself speculated on possible explanations of what he saw under the microscope, eventually concluding “at present we cannot say anything definite about the cause” (4). Initial theories postulated this disease was due to infection, understandably, since infections were quite common and a large focus of clinical investigation, but no clear link with microorganisms was ever found. Other theories considered the Hashimoto goiter a premalignant condition (11). Some scholars believed the thyroid itself possessed a lymphogenic secretory capability that became hyperactive in these patients (12). Others viewed the goiter as secondary to constant anxiety and emotional unrest (13). In 1951, Hellwig proposed the colloidophagy theory, based on rodent studies performed in the late 1920s (14) and his own observations in humans (15) that macrophages exist in the thyroid gland and are capable of ingesting colloid. He postulated that thyroid macrophages that have engulfed colloid degenerate and release colloid, which then attracts lymphocytes into the thyroid (15). Finally, in the early 1950s, the field of autoimmunity began to take shape; animal models were being developed in which injection of a tissue extract was capable of reproducing a lymphocytic infiltration of that particular organ (16). This experimental approach was applied to the thyroid when, in 1956, lymphocytic infiltration of the rabbit thyroid was induced by injection of rabbit thyroid extracts (17). The horror autotoxicus dogma was dismantled and autoimmunity became recognized as an important mechanism of disease.
In the ensuing five decades, numerous studies have greatly expanded our understanding of the pathogenesis of Hashimoto's thyroiditis and helped translating research findings into clinical practice. We have known since the mid-1980s that thyroperoxidase is a dominant protein antigen targeted by the patient's immune system in Hashimoto's thyroiditis (18), and, as a result, antibodies to thyroperoxidase are now considered the most sensitive and specific biomarkers to establish this diagnosis. They also have a predictive value since their presence precedes a clinical diagnosis of Hashimoto's thyroiditis by at least 7 years (19). We have also known since 1971 (20) that Hashimoto's thyroiditis, like other autoimmune diseases, has a genetic basis. Substantial efforts have been devoted to identify the genes that predispose to Hashimoto's thyroiditis, but results have been less fruitful than expected (21). Genome-wide association studies and candidate gene approaches have identified a handful of confirmed susceptibility genes (MHC class II region, CTLA-4, PTPN22, and ARID5B) (22), each making, however, only a small contribution to the disease phenotype and through mechanisms that remain to be discovered.
To celebrate the centennial of the original description of Hashimoto's thyroiditis, we reviewed the complete surgical pathology archives of the Johns Hopkins Hospital (May 1889–October 2012) for cases of Hashimoto's thyroiditis.
Materials and Methods
Data sources
We compiled a database of all thyroidectomies performed at the Johns Hopkins Hospital (no consult slides included) using both paper and electronic records.
The paper surgical pathology records encompassed a period of 95 years, from the time the Johns Hopkins Hospital opened its doors (May 1889) to the time the recording system became electronic (March 1984). The paper archives were contained in 265 log books and comprised a total of 561,762 specimen records, of which only 10,456 (1.8%) were missing or lost. Each line in the log books reported one specimen defined by a unique identification number, date of surgery, medical record number, patient's name, age, sex, hospital location, surgeon's name, type of surgery, and gross appearance of the specimen, information that was written by hand as the specimen arrived from surgery. The last section of each line reported the final pathological diagnosis, which was added a few days later, once the pathologist had completed the microscopic analysis of the specimen. We reviewed all log books page by page to identify cases of thyroidectomy, which were then entered into a FileMaker database (FileMaker, Inc., Santa Clara, CA). We also assigned a diagnostic code to each case to classify it into one of seven disease categories: developmental lesions, inflammatory lesions, nonautoimmune goiter, autoimmune thyroid diseases (Hashimoto's thyroiditis, Riedel's thyroiditis, and Graves' disease), hyperplasia, benign tumors, and malignant tumors (Table 1). Most specimens processed during this paper period were described, in addition to the log book record indicated above, in a detailed surgical pathology report. Here the pathologist summarized the clinical history of the patient, expanded upon the gross appearance of the specimen, included the microscopic findings, and provided the final pathological diagnosis (which was then added to the specimen entry in the log book). These detailed surgical pathology reports were type-written and later scanned to microfilm. Reports of the initial years (1889–1923) have been lost, but microfilms for the 1924–1984 period were available and were consulted when the final pathological diagnosis reported in the log books was unclear or missing.
Table 1.
Pathological Classification of 14,867 Thyroidectomies Performed at the Johns Hopkins Hospital Between May 1889 and October 2012
| Diagnosis | n | % |
|---|---|---|
| Developmental lesions (n=627) | ||
| Thyroglossal duct cyst | 598 | 4.0 |
| Aberrant thyroid | 16 | 0.1 |
| Congenital goiter | 13 | 0.1 |
| Inflammatory lesions (n=312) | ||
| Acute thyroiditis | 4 | 0.03 |
| Subacute thyroiditis | 18 | 0.1 |
| Granulomatous thyroiditis | 13 | 0.1 |
| Chronic infectious thyroiditis | 8 | 0.1 |
| Atrophy, scarring, or fibrosis | 59 | 0.4 |
| Amyloidosis of the thyroid | 3 | 0.02 |
| Chronic nonspecific lymphocytic thyroiditis | 207 | 1.4 |
| Nonautoimmune goiter (n=4998) | ||
| Simple colloid goiter (diffuse nontoxic goiter) | 1027 | 6.9 |
| Adenomatoid hyperplasia (multinodular goiter) | 3216 | 21.6 |
| oncocytic adenomatoid hyperplasia | 64 | 0.4 |
| with lymphocytic infiltration | 343 | 2.3 |
| with Hashimoto's thyroiditis | 94 | 0.6 |
| Thyroid cyst | 254 | 1.7 |
| Autoimmune thyroid diseases (n=1511) | ||
| Graves' disease | 1024 | 6.9 |
| Hashimoto's thyroiditis (isolated) | 462 | 3.1 |
| Riedel's thyroiditis | 25 | 0.2 |
| Hyperplasia (n=472) | ||
| Follicular hyperplasia | 409 | 2.8 |
| with lymphocytic infiltration | 38 | 0.3 |
| C-cell hyperplasia | 25 | 0.2 |
| Benign tumors (n=2927) | ||
| Adenoma, not otherwise categorized | 640 | 4.3 |
| Colloid adenoma | 929 | 6.3 |
| Follicular adenoma | 519 | 3.5 |
| with lymphocytic infiltration | 82 | 0.6 |
| with Hashimoto's thyroiditis | 27 | 0.2 |
| Hürthle cell adenoma | 340 | 2.3 |
| with lymphocytic infiltration | 15 | 0.1 |
| with Hashimoto's thyroiditis | 19 | 0.1 |
| Cystic adenoma | 57 | 0.4 |
| Fetal adenoma | 71 | 0.5 |
| Mixed adenoma | 162 | 1.1 |
| Papillary adenoma | 52 | 0.3 |
| Parathyroid adenoma | ||
| with Hashimoto's thyroiditis | 14 | 0.1 |
| Malignant tumors (n=3591) | ||
| Carcinoma, not otherwise categorized | 72 | 0.5 |
| Papillary carcinoma (n=2972) | 2233 | 15 |
| with lymphocytic infiltration | 508 | 3.4 |
| with Hashimoto's thyroiditis | 231 | 1.6 |
| Follicular carcinoma (n=162) | 145 | 1.0 |
| with lymphocytic infiltration | 12 | 0.1 |
| with Hashimoto's thyroiditis | 5 | 0.03 |
| Hürthle cell carcinoma (n=98) | 84 | 0.6 |
| with lymphocytic infiltration | 8 | 0.1 |
| with Hashimoto's thyroiditis | 6 | 0.04 |
| Medullary carcinoma (no.=160) | 138 | 0.9 |
| with lymphocytic infiltration | 13 | 0.1 |
| with Hashimoto's thyroiditis | 9 | 0.06 |
| Anaplastic carcinoma (n=59) | 55 | 0.4 |
| with lymphocytic infiltration | 4 | 0.03 |
| Lymphoma of the thyroid | 28 | 0.2 |
| Sarcoma of the thyroid | 4 | 0.03 |
| Metastasis to the thyroid | 28 | 0.2 |
| Paraganglioma of the thyroid | 2 | 0.01 |
| Teratoma of the thyroid | 2 | 0.01 |
| Langerhans cell histiocytosis | 1 | 0.01 |
| Solitary fibrous tumor (spindle cell tumor) | 1 | 0.01 |
| Castle diseasea | 1 | 0.01 |
| Insular carcinoma | 1 | 0.01 |
| Unknown pathological diagnosis | 188 | 1.3 |
| Normal thyroid tissue | 241 | 1.6 |
| Total | 14,867 | 100 |
Castle disease is a neoplasm that arises from ectopic intrathyroidal thymic tissue, or from intrathyroidal branchial pouch remnants.
The electronic surgical pathology records encompassed the ensuing period of 29 years, from March 1984 to the present. They comprised, as of October 30, 2012, a total of 1,422,424 specimens. Electronic records contained the full pathology report, with detailed gross appearance, microscopic findings, and final pathological diagnosis. These records were queried using the keywords “thyroid,” “Hashimoto,” or “Graves.” Cases containing these keywords were then reviewed one by one for accuracy, classified based on reanalysis of the pathology report, and combined into the same FileMaker database mentioned above.
We considered as “Hashimoto's thyroiditis” those cases that contained the word “Hashimoto” in the report and pathological findings consistent with this diagnosis. We also considered as Hashimoto's thyroiditis those reports that, despite lacking the term “Hashimoto,” featured a description containing lymphocytic infiltration of the thyroid gland with germinal center formation and Hürthle cell metaplasia. However, the presence of a simple lymphocytic infiltration was not considered indicative of Hashimoto's thyroiditis. This less-marked type of infiltration, often referred to by pathologists as “chronic nonspecific thyroiditis,” differs from that seen in Hashimoto's thyroiditis because of its focal nature, absence of germinal centers, and lack of Hürthle cells (1,23,24). For specimens collected during the electronic period, we classified this milder lymphocytic infiltration as chronic nonspecific thyroiditis when it was the only pathological feature present in the report, or added the note “with lymphocytic infiltration” when it accompanied other thyroid pathologies. For specimens collected during the paper period, we reviewed the glass slides of all records containing the terms “thyroiditis,” “lymphoid (lymphocytic) infiltration,” or “chronic inflammation” in the final diagnosis.
The study was approved by the Johns Hopkins Institutional Review Board. Statistical analyses were performed using Stata statistical software, release 12 (Stata Corp., College Station, TX).
Results
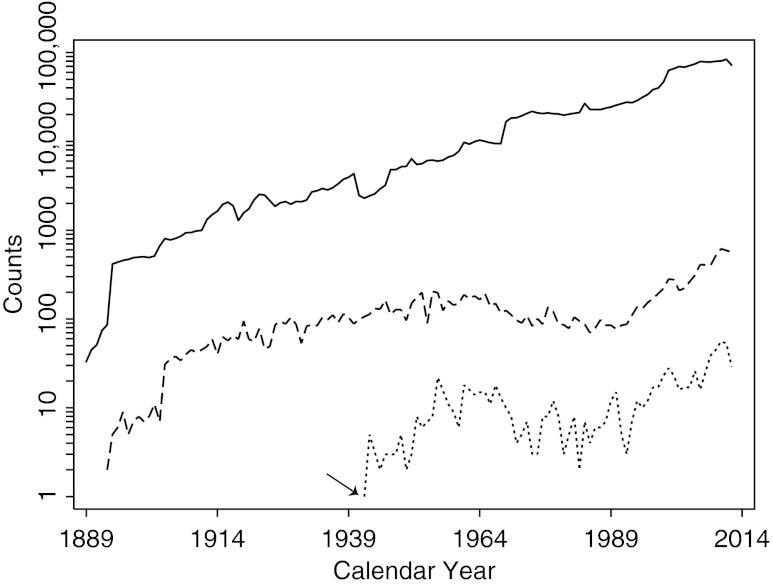
A total of 14,867 thyroidectomies were performed at the Johns Hopkins Hospital between May 1889 and October 2012 (Table 1). Following the initial years, the number of yearly thyroidectomies oscillated around 100 (108±38) for eight decades (1914–1994), and then increased 3-fold (330±157) in more recent times (1995–2012). Thyroidectomies (Fig. 1, dashed line) represented overall less than 1% of the total (1,984,186) surgical pathology specimens (Fig. 1, solid line) processed by the department of pathology.
FIG. 1.
Annual number of Hashimoto's thyroiditis cases (dotted line) among the thyroidectomies (dashed line) performed at the Johns Hopkins Hospital between May 1889 and October 2012. The numbers of all surgical specimens processed by the Department of Pathology (solid line) is also shown. The arrow indicates year 1942, the time when the term “Hashimoto thyroiditis” was first used in a pathology report at this hospital.
The term “Hashimoto” first appeared in the Johns Hopkins surgical pathology archives in 1942 (Fig. 1, arrow by the dotted line), 30 years after the original publication by Dr. Hashimoto. It was a specimen from a 37-year-old woman who underwent bilateral thyroidectomy for compressive goiter. The pathologist opened his microscopic description by writing “the appearance of this thyroid is very unusual” and concluded it with “the appearance of this gland is compatible with the entity described by Hashimoto in 1912.” Before this specimen, however, we identified three cases, one in 1928, one in 1935, and the third in 1939, featuring descriptive terms that suggested to us a diagnosis of Hashimoto's thyroiditis, such as “extraordinary lymphoid infiltration in the thyroid.” Review of the original glass slides showed they were indeed cases of full-blown Hashimoto's thyroiditis. They all showed massive lymphocytic infiltration organized into follicles with prominent germinal centers, and an almost complete transformation of the remaining thyroid follicles into Hürthle cells.
A total of 867 cases of Hashimoto's thyroiditis were identified from 1942 to October 2012, representing approximately 6% of all (14,867) thyroid surgical specimens (Tables 1 and 2), a prevalence nearly identical to the one recently reported in Krakow, Poland (452 cases of 7545 thyroidectomies) (25). Approximately half of our cases (n=462, Table 2) were isolated, meaning that the report indicated Hashimoto's thyroiditis as the sole pathological finding. Of them, we were able to derive the reason for the thyroidectomy in 167 cases operated after 1984 (Table 2). The majority (100 of 167, 60%) were patients with a nodular goiter and a cytology suspicious for malignancy. A third (52 of 167, 31%) were patients with a goiter causing symptoms from compression of airways, esophagus, or laryngeal nerve. A minority were cases operated upon patient's request (12 of 167, 7%), or because of an initial phase of hyperthyroidism clinically interpreted as Graves' disease that was refractory to medical treatment (3 of 167, 2%).
Table 2.
Classification of 867 Cases of Hashimoto's Thyroiditis Operated at the Johns Hopkins Hospital Between 1942 and October 2012
| n | % | |
|---|---|---|
| Isolated Hashimoto's thyroiditis (n=462) | ||
| Operated for nodule with suspicious cytology | 100 | 11.5 |
| Operated for compressive symptoms | 52 | 6.0 |
| Operated upon patient's request | 12 | 1.4 |
| Operated for refractory hyperthyroidism | 3 | 0.3 |
| Reason for thyroidectomy unknown | 295 | 34 |
| Hashimoto's thyroiditis found in association with (n=405) | ||
| Papillary thyroid cancer | 231 | 26.6 |
| Multinodular goiter | 94 | 10.8 |
| Follicular adenoma | 27 | 3.1 |
| Hürthle cell adenoma | 19 | 2.2 |
| Parathyroid adenoma | 14 | 1.6 |
| Medullary thyroid cancer | 9 | 1.0 |
| Hürthle cell carcinoma | 6 | 0.7 |
| Follicular thyroid cancer | 5 | 0.6 |
| Total | 867 | 100 |
Isolated (n=462) and associated cases (n=405) are classified according to the reason for thyroidectomy.
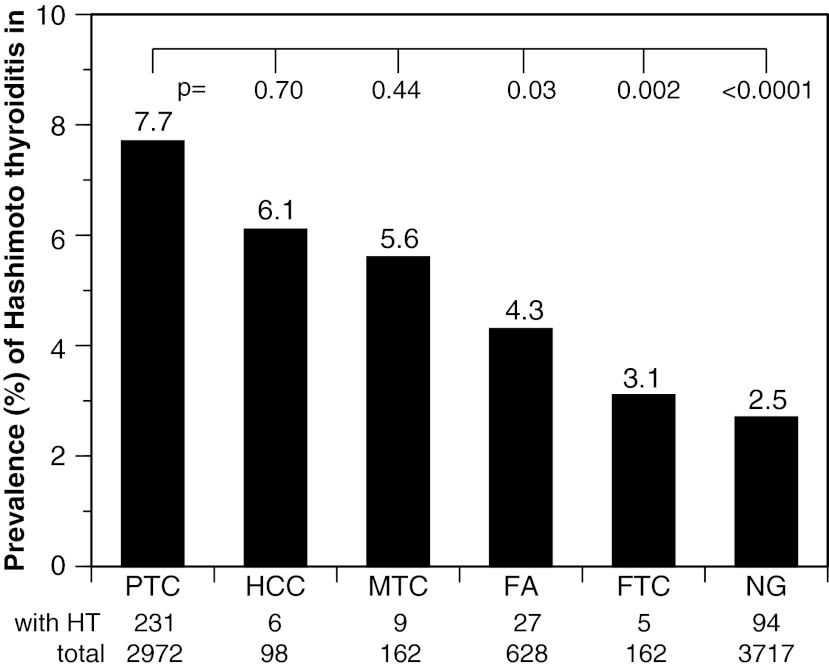
The remaining half of the Hashimoto's thyroiditis cases (n=405) were those in which Hashimoto's thyroiditis was found incidentally in association with other thyroid pathologies (Table 2). The most common associated condition was papillary thyroid cancer. This cancer was found in 231 of the total 867 Hashimoto's thyroiditis cases (26.6%, Table 2), a prevalence that is very similar to the one recently reported in Krakow (106 of 452, 24%) (25). The coexistence of Hashimoto's thyroiditis with papillary cancer (231 of 2972 total papillary cancer cases, 7.7%) was significantly greater than the coexistence with a multinodular goiter (94 of 3717, 2.5%, p<0.0001 by the chi-squared test), follicular carcinoma (5 of 162, 3.1%, p=0.03), or follicular adenoma (27 of 628, 4.3%, p=0.002; Fig. 2). However, it was not a unique feature of papillary thyroid cancer, since a similar prevalence was found for the coexistence with Hürthle cell carcinoma (6 of 98, 6.1%, p=0.70) and medullary thyroid cancer (9 of 162, 5.6%, p=0.44; Fig. 2).
FIG. 2.
Prevalence of the coexistence of Hashimoto's thyroiditis (HT) with papillary thyroid cancer (PTC), Hürthle cell cancer (HCC), medullary thyroid cancer (MTC), follicular adenoma (FA), follicular thyroid cancer (FTC), and nodular goiter (NG). p-Values compare by chi-squared the coexistence of HT and PTC to the coexistence with other thyroid pathologies.
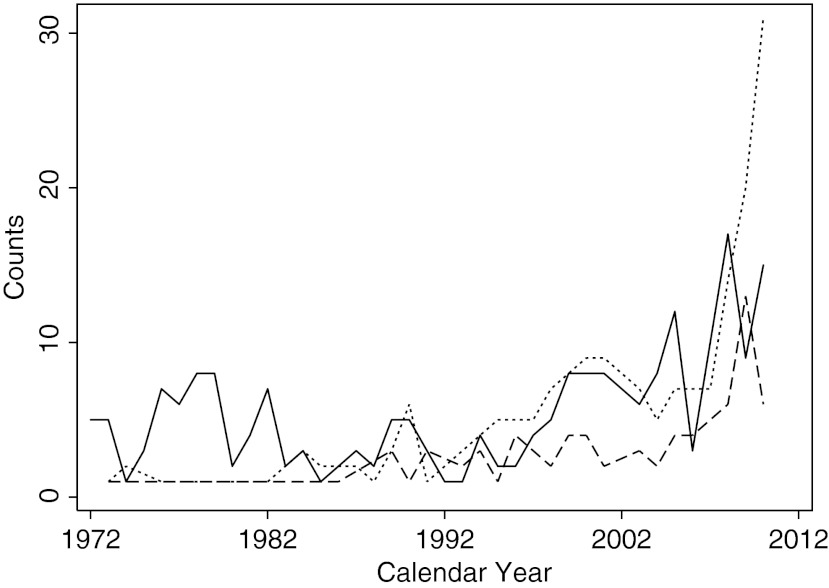
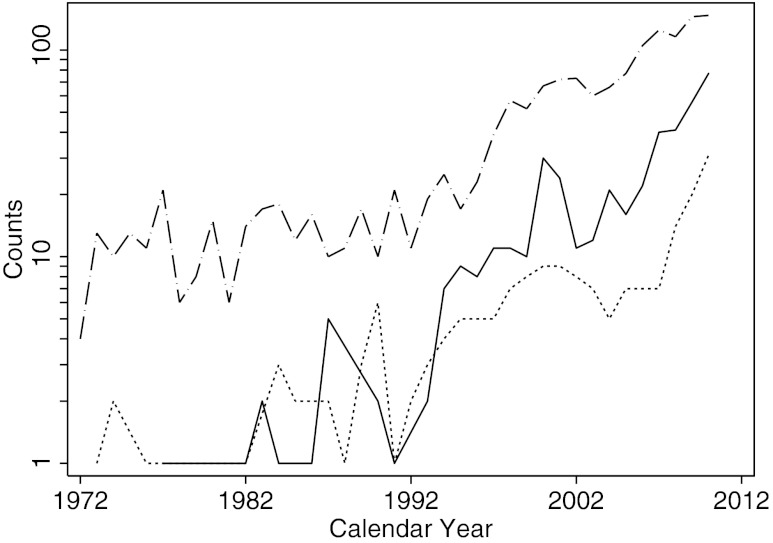
The number of yearly Hashimoto's cases (Fig. 1, dotted line) has changed considerably over time. Following the initial 53 years of surgeries (1889–1942) in which basically no case was reported, the incidence increased significantly over the ensuing 25 years (1943–1967, beta coefficient for trend [β]=0.63, p<0.0001). It then remained fairly constant for the next 25 years (1968–1992, β=−0.02, p=0.791), before increasing again over the past two decades (1993–2012, β=1.76, p<0.0001). There were, in fact, 207 cases reported during the first period (for an average of 8 cases per year), 172 cases in the second period (average of 7 yearly cases), and 488 in the last two decades (24 cases per year). The main contributor to the recent increase in incidence was Hashimoto's thyroiditis associated with papillary thyroid cancer (Figs. 3 and 4, dotted line). Interestingly, the increased yearly counts of isolated papillary thyroid cancer (Fig. 4, dashed line) were accompanied not only by increases in cases associated with Hashimoto's thyroiditis (Figs. 3 and 4, dotted line), but also by increases in cases of papillary thyroid cancer associated with the less prominent lymphocytic infiltration sometimes referred to as chronic nonspecific thyroiditis (Fig. 4, solid line).
FIG. 3.
Annual numbers of HT cases associated with PTC (dotted line), HT cases associated with multinodular goiter (dashed line), and isolated cases of HT (solid line).
FIG. 4.
Annual numbers of isolated PTC (dashed line), PTC associated with HT (dotted line), and PTC associated with chronic nonspecific lymphocytic thyroiditis (solid line).
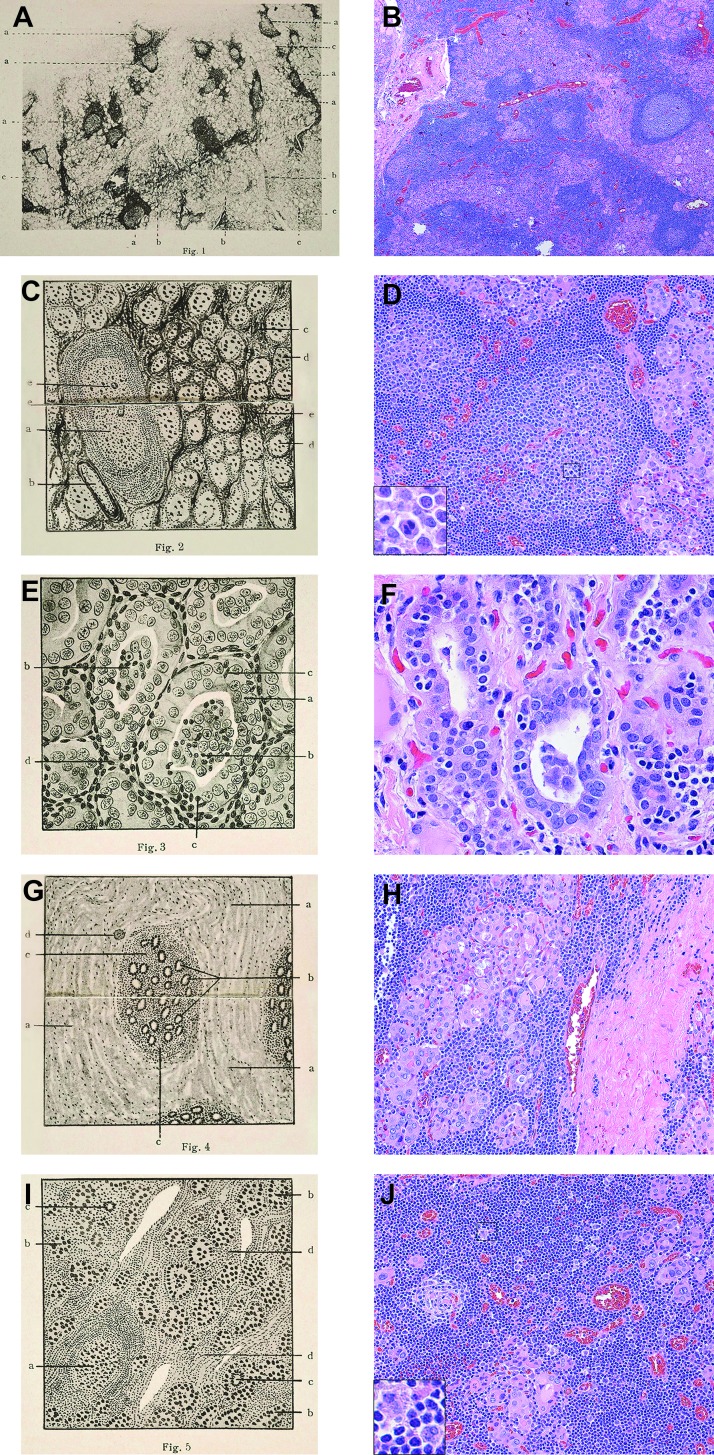
The modern-day pathological features of Hashimoto's thyroiditis (Fig. 5B, D, F, H, J) are nearly identical to the ones originally reported by Dr. Hashimoto in 1912 (Fig. 5A, C, E, G, I). At low power (Fig. 5A, B), the most striking feature is the infiltration of the thyroid gland with lymphocytes. They often organize into bona fide lymphoid follicles with germinal centers, assuming the compartmentalization typically observed in lymph nodes and spleen. At high power, it is easy to identify lesions of the interstitium and lesions of the thyroid cells. The key interstitial lesion is the infiltration with hematopoietic cells, mainly represented by lymphocytes, but also plasma cells and some macrophages (Fig. 5C, D), overall conferring a pale and gray-tan color to the cut surface. Lymphocytes are seen to come in contact with thyrocytes, and in some instances even penetrate into the cytoplasm of the thyrocyte, a phenomenon known as emperipolesis (Fig. 5E, F). The other interstitial feature is the increase in connective tissue fibers (Fig. 5G, H), which impart a firm consistency to the gland. In the fibrosing variant of Hashimoto's thyroiditis, this interstitial fibrosis is extensive, still remaining contained within the thyroid capsule (in contrast to what is seen in Riedel's thyroiditis). Lesions of the thyrocytes vary in composition and intensity from one section of the thyroid to another. In some areas, thyroid cells are atrophic and encircle small follicles with scanty colloid. In other areas (Fig. 5E, F), thyroid cells appear enlarged, with a granular and deeply eosinophilic cytoplasm and a hyperchromatic nucleus, and are commonly called Hürthle cells.
FIG. 5.
Histopathological features of Hashimoto's thyroiditis. Left panels: Microphotographs originally presented by Dr. Hashimoto in 1912. Right panels: Corresponding hematoxylin and eosin (H&E) histopathological features. (Legends for the left panels are our translation of the original legends.) (A) Low-power view showing pronounced lymphoid follicles within the thyroid parenchyma. a, lymphoid follicles with germinal center; b, diffusely hypertrophic interstitium; c, atrophic thyroid follicles containing scarce colloid. (B) A 20× magnification showing similar features as those described in (A). (C) Interstitial changes. a, lymphoid follicle; b, blood vessel; c, nest of lymphocytes; d, abnormal thyroid follicles showing atrophy, ill-defined borders, and scarce colloid; e, nuclear division. (D) A 64× magnification illustrating features similar to (C). The inset (252×) depicts a cell undergoing nuclear division. (E) Thyroid follicle changes. a, thyrocytes are bold, stratified in some regions, and with undefined boundaries; b, the colloidal space contains infiltrating leukocytes; c, leukocytes migrating through the follicular wall; d, mononuclear cells in the interstitium. (F) A 160× magnification showing the metaplastic transformation of the thyroid epithelium we now refer to as Hürthle cells, as well as the penetration of lymphocytes into the thyroid cell, named emperipolesis. (G) Marked proliferation of the interstitial connective tissue. a, hypertrophic connective tissue; b, small atrophic thyroid follicles with near-absent colloid; c, pronounced mononuclear (lymphocytic) infiltration; d, blood vessel. (H) A 64× magnification showing abundant fibrosis, small remaining thyroid follicles surrounded by lymphocytes. (I) Notable changes. a, lymphoid follicle with germinal center; b, thyroid follicles with scanty colloid and dense appearance; c, multinucleated giant cell; d, interstitial proliferation with mononuclear infiltration. (J) A 64× magnification summarizing features illustrated in previous panels, as well as a rare multinucleated giant cell (inset).
Discussion
Hashimoto's thyroiditis is now the most common autoimmune disease in humans (9,26), and the most frequent cause of hypothyroidism (27,28). Its diagnosis is typically established on clinical grounds from typical clinical features, reduced echogenicity of the thyroid parenchyma on thyroid ultrasound, and the presence of serum autoantibodies against key thyroid antigens (mainly thyroperoxidase). Its treatment is symptomatic and based on the administration of synthetic thyroxine to correct the hypothyroidism when present and/or to decrease thyroid volume by lowering serum thyrotropin concentrations. In contemporary practice, pathology is rarely needed to establish a diagnosis of Hashimoto's thyroiditis, and surgery is seldom used as a form of treatment (29–31). Yet, analysis of surgical pathology records over a prolonged period of time provides insights about the natural history of the disease, as well as the practice of medicine.
The first interesting finding of our study is the changing trend in the incidence of surgical Hashimoto's thyroiditis. The disease was not described during the first 53 years of the hospital's operations (1889–1942), even though approximately 2500 thyroidectomies were performed. This absence could be, in part, explained by an information bias, since the disease was unknown to the medical community for a great part of this initial period. However, even after reviewing the glass slides from all cases in which thyroid lymphocytes were mentioned in the surgical pathology report, we identified only 3 cases of Hashimoto's thyroiditis. This low prevalence period (3 of 2500, 0.12%, in 50 years) may suggest the introduction of an environmental change that caused the appearance of, or increased incidence of, Hashimoto's thyroiditis.
The environmental factor most commonly discussed in the thyroid community is iodine. Iodine is ingested from a variety of sources and its intake is currently assessed by urinary concentration. Urinary iodine values of 100–199 μg/L (150–249 in pregnancy) are considered indicative of adequate iodine intake (32). There are no U.S. population data on urinary iodine before 1970, but landmark studies by David Marine during the World War I period showed a high prevalence of goiter in the Great Lakes area and the serious consequences of iodine deficiency (33,34), marking de facto the beginning of iodine supplementation in this country. Urinary iodine was first assessed on a population basis during the first NHANES survey (1971–1974), and found to be elevated with a median concentration of 320 μg/L (35). This concentration then decreased to the normal range in the third NHANES survey (1988–1994, 145 μg/L) (35), and remained normal in NHANESs 2001–2002 (168 μg/dL), 2003–2004 (160 μg/dL), 2005–2006 (164 μg/dL), and 2007–2008 (164 μg/dL) [from Table 1 in (36)]. More recent epidemiological studies support a connection between dietary iodine and Hashimoto's thyroiditis. For example, Teng and colleagues analyzed cross-sectionally three regions in China with low (84 μg/dL), more than adequate (243 μg/dL), and excessive (651 μg/dL) iodine intake, reporting a cumulative incidence of autoimmune thyroiditis of 0.2%, 1%, and 1.3%, respectively (37,38). Similar results were obtained by Bjergved et al., who analyzed longitudinally thyroid function and antibodies in Denmark before (1997–98) and after (2008–10) the introduction of a mandatory program for iodization of salt. The authors found a rising incidence of hypothyroidism (elevated serum thyrotropin), which was accompanied by the appearance/increase in thyroperoxidase antibodies (39). Nevertheless, despite epidemiological studies and animal experiments clearly indicating that more dietary iodine increases the incidence of autoimmune thyroiditis (40,41), the mechanism for the proimmunogenic effect of iodine remains to be explained (42).
The association between Hashimoto's thyroiditis and papillary thyroid cancer was responsible for the significant increase in the number of surgical Hashimoto's thyroiditis cases observed during the last two decades (1993–2012) in our study. This association, documented since 1955 (43), remains mysterious. Scholars have debated as to whether it represents the mere co-occurrence of two relatively common conditions, which are ascertained now more frequently due to the increased usage of thyroid ultrasound, or if instead it indicates a true cause–effect relationship. In the case of the latter scenario, it is unclear whether cancer or autoimmunity comes first. The clinical consequences of this association have also been debated, although most recent studies suggest that papillary thyroid cancer follows a more benign course when accompanied by Hashimoto's thyroiditis (44,45). Our analysis provides some insights into this association. It shows that the increased incidence of papillary thyroid cancer observed in recent years (46–49), and reflected in our data, was paralleled by increases in both the form of papillary thyroid cancer associated with Hashimoto's thyroiditis and in the one associated with the milder form of lymphocytic infiltration referred to as chronic nonspecific thyroiditis. These findings suggest that papillary thyroid cancer is the initial lesion, which then induces a lymphocytic infiltration that in some patients progresses to assume the features of full-blown Hashimoto's thyroiditis, whereas in others remains at the stage of chronic nonspecific thyroiditis. Rather than being part of the same causal pathway, the lymphocytic infiltration of the thyroid thus seems to be the consequence of the neoplastic transformation of the thyroid cell. This view is also supported by our observation that the coexistence of Hashimoto's thyroiditis was not unique to papillary thyroid cancer, but also found at similar frequencies for Hürthle cell carcinoma and medullary thyroid cancer.
It is interesting to reflect on the reasons for which patients with Hashimoto's thyroiditis today undergo thyroidectomy, since the disease is largely a medical disease. The most common reason in our series was a thyroid nodule with a cytology suspicious for malignancy, in keeping with previous reports (29). These patients had a long-standing history of Hashimoto's thyroiditis, were usually on thyroxine replacement, and developed thyroid nodules with time. As shown in the results section, interstitial fibrosis is one of the key pathological features, and thus, nodularity is to be expected in Hashimoto glands. Fine needle aspiration of these nodules shows a polymorphic population of lymphoid cells (small mature lymphocytes, larger activated lymphocytes, and occasional plasma cells) typically accompanied by Hürthle cells, which may show anisonucleosis and nuclear atypia. Lymphocytes are often seen to make intimate contact with groups of thyroid cells, a feature that has recently been considered useful to distinguish Hashimoto's thyroiditis from thyroid neoplasms (50). Some aspirates, however, lack lymphoid cells and consist almost exclusively of Hürthle cells. In these instances, the cytopathologist cannot determine with certainty whether the Hürthle cells are those found in Hashimoto's thyroiditis or instead are those found in other oncocytic lesions of the thyroid, such as the oncocytic adenomatoid nodule, Hürthle cell adenoma, or Hürthle cell carcinoma (51). The cytopathologist uses the term “atypia of undetermined significance” (52) to indicate cytological features that are neither definitely benign nor definitely malignant. Although the recommendation of the Bethesda reporting system for thyroid cytopathology for atypia of undetermined significance is conservative management (53), many patients are referred to a surgeon, and then undergo thyroidectomy. Thus, even one century after the original description, there are still patients who might be spared from a thyroidectomy if a more accurate preoperative diagnosis of Hashimoto's thyroiditis is established. This is especially relevant if we consider that thyroidectomy in patients with Hashimoto's thyroiditis causes more surgical complications, both transient and permanent, than thyroidectomy performed for other pathologies (54).
Finally, it is interesting to contemplate the ontogeny and significance of the Hürthle cells, by some authors considered to be the wild card (55) in the pathogenesis of Hashimoto's thyroiditis, traditionally focused on the infiltrating lymphocytes rather than on the thyroid cells themselves. Hürthle cells represent a form of metaplasia where thyrocytes become enlarged and display a pink, granular cytoplasm and a hyperchromatic nucleus with a prominent nucleolus (56). The cytoplasmic granularity is caused by the increased number of mitochondria that appear abnormal in size, shape, and content on analysis by electron microscopy (57). We reported that mice genetically engineered to produce interferon gamma in the thyroid develop Hürthle cells by inducing the expression of an immunoproteasome component called LMP2, and that pharmacologic blockade of LMP2 ameliorated the Hürthle phenotype (58), overall highlighting the immunoproteasome as a novel therapeutic target for Hürthle cell lesions. The role of the immunoproteasome has been recently confirmed in a mouse model of thyroiditis induced by iodine where selective blockade of LMP7 decreased thyroiditis severity (59). We more recently reported in interferon gamma transgenic mice of different genetic backgrounds that the presence of Hürthle cells has functional implications, since they are the strongest predictor of hypothyroidism (60).
In conclusion, Hashimoto's thyroiditis remains an intriguing and multifaceted disease one century after its original description. Improvements in the preoperative diagnosis, advances in the understanding of disease pathogenesis, and development of novel therapies based on mechanisms rather than symptoms will improve the quality of life for this patient population.
Acknowledgments
We dedicate this work to the memory of Professor Aldo Pinchera, a truly larger-than-life figure who dedicated his life to promoting the study and awareness of thyroid diseases. We thank our colleagues at the Johns Hopkins University School of Medicine: Drs. Paul Ladenson and David Cooper for critically reading the manuscript, and Drs. William Westra and Justin Bishop for improving the classification presented in Table 1. The study was supported by NIH grant DK55670 to P.C.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.LiVolsi VA. The pathology of autoimmune thyroid disease: a review. Thyroid. 1994;4:333–339. doi: 10.1089/thy.1994.4.333. [DOI] [PubMed] [Google Scholar]
- 2.Jansson R. Totterman TH. Sallstrom J. Dahlberg PA. Intrathyroidal and circulating lymphocyte subsets in different stages of autoimmune postpartum thyroiditis. J Clin Endocrinol Metab. 1984;58:942–946. doi: 10.1210/jcem-58-5-942. [DOI] [PubMed] [Google Scholar]
- 3.Kakudo K. Li Y. Taniguchi E. Mori I. Ozaki T. Nishihara E. Matsuzuka F. Miyauchi A. IgG4-related disease of the thyroid gland. Endocr J. 2011;59:273–281. doi: 10.1507/endocrj.ej11-0309. [DOI] [PubMed] [Google Scholar]
- 4.Hahsimoto H. Zur Kenntniss der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa) Arch Klin Chir. 1912;97:219–248. [Google Scholar]
- 5.Sawin CT. Hakaru Hashimoto (1881–1934) and his disease. Endocrinologist. 2001;11:73–76. [Google Scholar]
- 6.Graham A. McCullagh EP. Atrophy and fibrosis associated with lymphoid tissue in the thyroid. Struma lymphomatosa (Hashimoto) Arch Surg. 1931;22:548–567. [Google Scholar]
- 7.Joll CA. The pathology, diagnosis, and treatment of Hashimoto's disease (struma lymphomatosa) Brit J Surg. 1939;27:351–389. [Google Scholar]
- 8.Vanderpump MP. Tunbridge WM. French JM. Appleton D. Bates D. Clark F. Grimley Evans J. Hasan DM. Rodgers H. Tunbridge F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson DL. Gange SJ. Rose NR. Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 10.Hollowell JG. Staehling NW. Flanders WD. Hannon WH. Gunter EW. Spencer CA. Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 11.Patterson H. Starkey G. The clinical aspects of chronic thyroiditis. Ann Surg. 1948;128:756–759. [PubMed] [Google Scholar]
- 12.Williamson GS. Pearse IH. Lymphadenoid goitre and its clinical significance. Brit Med J. 1929;1:4–5. doi: 10.1136/bmj.1.3548.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davison TC. Letton AH. Hashimoto's disease. J Clin Endocrinol. 1949;9:980–986. doi: 10.1210/jcem-9-10-980. [DOI] [PubMed] [Google Scholar]
- 14.Loeb L. Gray SH. The effect of the oral administration of potassium iodide and thyroid substance on the mitotic proliferation and structure of acini in the thyroid gland in guinea pigs. Am J Pathol. 1928;4:257–270. [PMC free article] [PubMed] [Google Scholar]
- 15.Hellwig A. Colloidophagy in the human thyroid gland. Science. 1951;113:725–726. doi: 10.1126/science.113.2947.725. [DOI] [PubMed] [Google Scholar]
- 16.Voisin G. Delaunay A. Barber M. Testicular lesions induced in the guinea pig by iso- and auto-sensitization. Ann Inst Pasteur (Paris) 1951;81:48–63. [PubMed] [Google Scholar]
- 17.Rose NR. Witebsky E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956;76:417–427. [PubMed] [Google Scholar]
- 18.McLachlan SM. Rapoport B. Thyroid peroxidase as an autoantigen. Thyroid. 2007;17:939–948. doi: 10.1089/thy.2007.0169. [DOI] [PubMed] [Google Scholar]
- 19.Hutfless S. Matos P. Talor MV. Caturegli P. Rose NR. Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease. J Clin Endocrinol Metab. 2011;96:E1466–E1471. doi: 10.1210/jc.2011-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vladutiu AO. Rose NR. Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science. 1971;174:1137–1139. doi: 10.1126/science.174.4014.1137. [DOI] [PubMed] [Google Scholar]
- 21.Simmonds MJ. Gough SC. The search for the genetic contribution to autoimmune thyroid disease: the never ending story? Brief Funct Genomics. 2011;10:77–90. doi: 10.1093/bfgp/elq036. [DOI] [PubMed] [Google Scholar]
- 22.Tomer Y. Hasham A. Davies TF. Stefan M. Concepcion E. Keddache M. Greenberg DA. Fine mapping of loci linked to autoimmune thyroid disease identifies novel susceptibility genes. J Clin Endocrinol Metab. 2013;98:E144–E152. doi: 10.1210/jc.2012-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetsch E. Chronic non-specific thyroiditis. Am J Surg. 1951;82:71–75. doi: 10.1016/0002-9610(51)90298-x. [DOI] [PubMed] [Google Scholar]
- 24.Kurashima C. Hirokawa K. Focal lymphocytic infiltration in thyroids of elderly people. Histopathological and immunohistochemical studies. Surv Synth Pathol Res. 1985;4:457–466. doi: 10.1159/000156996. [DOI] [PubMed] [Google Scholar]
- 25.Konturek A. Barczynski M. Wierzchowski W. Stopa M. Nowak W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2012 2012 Oct 26; doi: 10.1007/s00423-012-1021-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper GS. Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 27.Delemer B. Aubert JP. Nys P. Landron F. Bouee S. An observational study of the initial management of hypothyroidism in France: the ORCHIDEE study. European J Endocrinol. 2012;167:817–823. doi: 10.1530/EJE-11-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51. doi: 10.1093/bmb/ldr030. [DOI] [PubMed] [Google Scholar]
- 29.Gyory F. Lukacs G. Juhasz F. Mezosi E. Szakall S. Vegh T. Math J. Balazs G. Surgically treated Hashimoto's thyroiditis. Acta Chir Hung. 1999;38:243–247. [PubMed] [Google Scholar]
- 30.Shih ML. Lee JA. Hsieh CB. Yu JC. Liu HD. Kebebew E. Clark OH. Duh QY. Thyroidectomy for Hashimoto's thyroiditis: complications and associated cancers. Thyroid. 2008;18:729–734. doi: 10.1089/thy.2007.0384. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CG., Jr. Rutledge RG. Surgical intervention in chronic (Hashimoto's) thyroiditis. Ann Surg. 1981;193:769–776. doi: 10.1097/00000658-198106000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson CA. Zimmermann MB. Skeaff S. Pearce EN. Dwyer JT. Trumbo PR. Zehaluk C. Andrews KW. Carriquiry A. Caldwell KL. Egan SK. Long SE. Bailey RL. Sullivan KM. Holden JM. Betz JM. Phinney KW. Brooks SP. Johnson CL. Haggans CJ. Summary of an NIH workshop to identify research needs to improve the monitoring of iodine status in the United States and to inform the DRI. J Nutr. 2012;142:1175S–1185S. doi: 10.3945/jn.111.156448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimball OP. Marine D. The prevention of simple goiter in man. Second paper. 1918. Nutrition. 1992;8:200–204. discussion 205–206. [PubMed] [Google Scholar]
- 34.Marine D. Kimball OP. The prevention of simple goiter in man. A survey of the incidence and types of thyroid enlargements in the schoolgirls of Akron (Ohio), from the 5th to the 12th grades, inclusive—the plan of prevention proposed. 1917. J Lab Clin Med. 1990;115:128–136. [PubMed] [Google Scholar]
- 35.Hollowell JG. Staehling NW. Hannon WH. Flanders DW. Gunter EW. Maberly GF. Braverman LE. Pino S. Miller DT. Garbe PL. DeLozier DM. Jackson RJ. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994) J Clin Endocrinol Metab. 1998;83:3401–3408. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- 36.Caldwell KL. Makhmudov A. Ely E. Jones RL. Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid. 2011;21:419–427. doi: 10.1089/thy.2010.0077. [DOI] [PubMed] [Google Scholar]
- 37.Teng W. Shan Z. Teng X. Guan H. Li Y. Teng D. Jin Y. Yu X. Fan C. Chong W. Yang F. Dai H. Yu Y. Li J. Chen Y. Zhao D. Shi X. Hu F. Mao J. Gu X. Yang R. Tong Y. Wang W. Gao T. Li C. Effect of iodine intake on thyroid diseases in China. New Engl J Med. 2006;354:2783–2793. doi: 10.1056/NEJMoa054022. [DOI] [PubMed] [Google Scholar]
- 38.Teng X. Shan Z. Chen Y. Lai Y. Yu J. Shan L. Bai X. Li Y. Li N. Li Z. Wang S. Xing Q. Xue H. Zhu L. Hou X. Fan C. Teng W. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–950. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 39.Bjergved L. Jorgensen T. Perrild H. Carle A. Cerqueira C. Krejbjerg A. Laurberg P. Ovesen L. Bulow Pedersen I. Rasmussen LB. Knudsen N. Predictors of change in serum TSH after iodine fortification: an 11-year follow-up to the DanThyr study. J Clin Endocrinol Metab. 2012;97:4022–4029. doi: 10.1210/jc.2012-2508. [DOI] [PubMed] [Google Scholar]
- 40.Allen EM. Appel MC. Braverman LE. The effect of iodide ingestion on the development of spontaneous lymphocytic thyroiditis in the diabetes-prone BB/W rat. Endocrinology. 1986;118:1977–1981. doi: 10.1210/endo-118-5-1977. [DOI] [PubMed] [Google Scholar]
- 41.Rasooly L. Rose NR. Saboori AM. Ladenson PW. Burek CL. Iodine is essential for human T cell recognition of human thyroglobulin. Autoimmunity. 1998;27:213–219. doi: 10.3109/08916939808993833. [DOI] [PubMed] [Google Scholar]
- 42.Carayanniotis G. Molecular parameters linking thyroglobulin iodination with autoimmune thyroiditis. Hormones (Athens) 2011;10:27–35. doi: 10.14310/horm.2002.1290. [DOI] [PubMed] [Google Scholar]
- 43.Dailey ME. Lindsay S. Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 44.Cunha LL. Ward LS. Concurrent lymphocytic thyroiditis is associated to less aggressive papillary thyroid carcinomas. Eur Arch Otorhinolaryngol. 2012;269:699–700. doi: 10.1007/s00405-011-1764-y. [DOI] [PubMed] [Google Scholar]
- 45.Huang BY. Hseuh C. Chao TC. Lin KJ. Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto's thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011;22:144–149. doi: 10.1007/s12022-011-9164-9. [DOI] [PubMed] [Google Scholar]
- 46.Blomberg M. Feldt-Rasmussen U. Andersen KK. Kjaer SK. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer. 2012;131:2360–2366. doi: 10.1002/ijc.27497. [DOI] [PubMed] [Google Scholar]
- 47.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enewold LR. Zhou J. Devesa SS. Berrington de Gonzalez A. Anderson WF. Zahm SH. Stojadinovic A. Peoples GE. Marrogi AJ. Potter JF. McGlynn KA. Zhu K. Thyroid cancer incidence among active duty U.S. military personnel, 1990–2004. Cancer Epidemiol Biomarkers Prev. 2011;20:2369–2376. doi: 10.1158/1055-9965.EPI-11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNally RJ. Blakey K. James PW. Gomez Pozo B. Basta NO. Hale J. Increasing incidence of thyroid cancer in Great Britain, 1976–2005: age-period-cohort analysis. Eur J Epidemiol. 2012;27:615–622. doi: 10.1007/s10654-012-9710-x. [DOI] [PubMed] [Google Scholar]
- 50.Harvey AM. Truong LD. Mody DR. Diagnostic pitfalls of Hashimoto's/lymphocytic thyroiditis on fine-needle aspirations and strategies to avoid overdiagnosis. Acta Cytol. 2012;56:352–360. doi: 10.1159/000338738. [DOI] [PubMed] [Google Scholar]
- 51.Yang GC. Schreiner AM. Sun W. Can abundant colloid exclude oncocytic (Hürthle cell) carcinoma in thyroid fine needle aspiration? Cytohistological correlation of 127 oncocytic (Hürthle cell) lesions. Cytopathology. 2012 2012 Jun 6; doi: 10.1111/j.1365-2303.2012.00988.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Cibas ES. Ali SZ. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 53.Baloch ZW. LiVolsi VA. Asa SL. Rosai J. Merino MJ. Randolph G. Vielh P. DeMay RM. Sidawy MK. Frable WJ. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–437. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 54.McManus C. Luo J. Sippel R. Chen H. Is thyroidectomy in patients with Hashimoto thyroiditis more risky? J Surg Res. 2012;178:529–532. doi: 10.1016/j.jss.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuart A. The changing scene in Hashimoto's disease: a review. Med Hypotheses. 2011;77:424–426. doi: 10.1016/j.mehy.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Mete O. Asa SL. Oncocytes, oxyphils, Hürthle, and Askanazy cells: morphological and molecular features of oncocytic thyroid nodules. Endocr Pathol. 2010;21:16–24. doi: 10.1007/s12022-009-9102-2. [DOI] [PubMed] [Google Scholar]
- 57.Nesland JM. Sobrinho-Simoes MA. Holm R. Sambade MC. Johannessen JV. Hürthle-cell lesions of the thyroid: a combined study using transmission electron microscopy, scanning electron microscopy, and immunocytochemistry. Ultrastruct Pathol. 1985;8:269–290. doi: 10.3109/01913128509141518. [DOI] [PubMed] [Google Scholar]
- 58.Kimura HJ. Chen CY. Tzou SC. Rocchi R. Landek-Salgado MA. Suzuki K. Kimura M. Rose NR. Caturegli P. Immunoproteasome overexpression underlies the pathogenesis of thyroid oncocytes and primary hypothyroidism: studies in humans and mice. PloS One. 2009;4:e7857. doi: 10.1371/journal.pone.0007857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagayama Y. Nakahara M. Shimamura M. Horie I. Arima K. Abiru N. Prophylactic and therapeutic efficacies of a selective inhibitor of the immunoproteasome for Hashimoto's thyroiditis, but not for Graves' hyperthyroidism, in mice. Clin Exp Immunol. 2012;168:268–273. doi: 10.1111/j.1365-2249.2012.04578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iwama S. De Remigis A. Bishop JA. Kimura HJ. Caturegli P. Hurthle cells predict hypothyroidism in interferon-gamma transgenic mice of different genetic backgrounds. Endocrinology. 2012;153:4059–4066. doi: 10.1210/en.2012-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]