Abstract
EO9 (Apaziquone) is a bioreductive drug that has a chequered history. It underwent clinical trial but failed to show activity in phase II clinical trials when administered i.v. Poor drug delivery to tumours caused by a combination of rapid pharmacokinetic elimination and poor penetration through avascular tissue were the major factors responsible for EO9's poor efficacy. Based upon an understanding of why EO9 failed, a further clinical trial against patients with superficial transitional cell carcinoma of the bladder was conducted. The rationale for this was that intravesical administration directly into the bladder would circumvent the drug delivery problem, and any drug reaching the blood supply would be rapidly cleared thereby reducing the risk of systemic exposure. EO9 was well tolerated, and clinical activity against marker lesions was recorded in both phase I and II clinical trials. This article charts the pharmacological history of EO9 and discusses the potential implications that ‘the EO9 story’ has for the development of other loco-regional therapies.
Keywords: EO9, Apaziquone, Eoquin, bladder cancer, bioreductive prodrugs, NQO1, hypoxia
Introduction
EO9 (3-hydroxy-5-aziridinyl-1-methyl-2 (1H-indole-4,7-dione)prop-β-en-α-ol) is a prodrug that belongs to a class of anti-cancer agents known as bioreductive drugs. Various chemical classes of bioreductive drugs have been developed (Denny, 2004; Hay et al., 2007a; 2008; 2007b; Milbank et al., 2009; Tercel et al., 2009), and all require enzymatic reduction by various oxidoreductases in order to generate cytotoxic metabolites. This activation process is reversed in the presence of oxygen, and these agents have preferential activity against hypoxic tumour cells (Stratford and Workman, 1998; McKeown et al., 2007). Selectivity is determined by the presence of elevated levels of reductases in tumours and the absence of oxygen (Figure 1). At the time EO9 was developed, these compounds represented a shift away from the classical way anticancer drugs were developed (identifying active compounds first and then identifying mechanisms of action) towards targeted therapeutic agents that selectively exploited aspects of tumour biochemistry and physiology.
Figure 1.
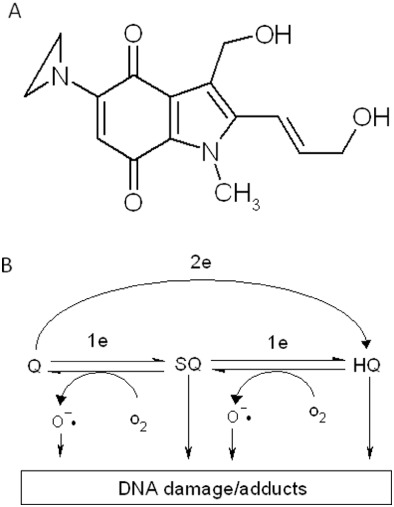
Chemical structure of EO9 (A) and scheme for possible activation of EO9 leading to DNA damage (B). 2e and 1e in panel B represent two-electron reduction (via enzymes such as NQO1) and one-electron reduction (via enzymes such as cytochrome P450 reductase) respectively. Q, SQ and HQ denote quinone (parent compound), semi-quinone (one-electron reduction product) and hydroquinone (two-electron reduction product) respectively.
EO9 was originally developed at the University of Amsterdam in the mid-1980s (Oostveen and Speckamp, 1987). The project was initially sponsored by the Dutch Cancer Society, and a series of 90 indolequinone (given the name EO) derivatives of mitomycin C (MMC) were developed, the ninth of which was EO9 (Figure 1). Preclinical and clinical evaluation was co-ordinated by the New Drug Development Office of the European Organisation for the Research and Treatment of Cancer (EORTC), and a number of laboratories belonging to the Screening and Pharmacology (SPG) and Pharmacology and Molecular Mechanism (PAMM) groups across Europe played key roles in the pharmacological evaluation of these compounds. Clinical evaluation of EO9 was halted by lack of efficacy in phase II trials (Dirix et al., 1996; Pavlidis et al., 1996). Based upon an understanding of why EO9 failed, a further phase I/II clinical trial against superficial bladder cancer using intravesical administration was commissioned in 2001 and sponsored by Spectrum Pharmaceuticals (Irvine, California). Significant anti-tumour activity was reported in the phase I/II study (Puri et al., 2006), and this was subsequently confirmed in phase II studies (van der Heijden et al., 2006). Phase III trials are currently under way, and the results are expected in the first quarter of 2012. The purpose of this article is to review the pharmacology of EO9, its preclinical and clinical history, and to discuss the potential implications that this story has for the development of other loco-regional therapies.
Pharmacology of EO9
EO9 is activated by several enzymes, the most widely studied of these is NAD(P)H:quinone oxidoreductase 1 (NQO1 or DT-diaphorase). NQO1 is a cytosolic flavoprotein that catalyses the two electron reduction of a wide range of substrates (Ernster, 1987), and its physiological function is detoxification of quinones (Cadenas, 1995). The chemistry of the side chains attached to the quinone nucleus dictates the reactivity of the reduced form (Cadenas, 1995), and in the case of EO9, it is reduced to a DNA-damaging species. In cell-free systems, reduction of EO9 by NQO1 results in the generation of DNA damage in the form of single-strand breaks (Walton et al., 1991). Catalase inhibits this process (Phillips et al., 1999), suggesting that hydrogen peroxide is formed during the redox cycling of the EO9 hydroquinone in oxygen (Butler et al., 1996; Bailey et al., 1998). Alkylation of DNA is possible via the release of hydroxyl groups at C2 and C3 as well as protonation and opening of the aziridine ring (Hargreaves et al., 2000). DNA interstrand cross-links (ICL) following the reduction of EO9 by purified rat NQO1 under hypoxic conditions have been reported (Maliepaard et al., 1995), although other groups have not observed ICLs following reduction by purified human NQO1 under aerobic conditions (Phillips, 1996). Limited information exists about the formation of mono-adducts. Other purified enzymes have been shown to reduce EO9 and induce either single-strand breaks or DNA cross-links, including xanthine oxidase (Maliepaard et al., 1995) and NADPH cytochrome P450 reductase (Bailey et al., 2001).
In cell-based assays, EO9 does not behave as a classical hypoxia-targeted bioreductive drug as it also has activity against aerobic cells (Roed et al., 1989; Phillips et al., 1992; Hendriks et al., 1993; Smitskamp-Wilms et al., 1994; Plumb et al., 1994a; Collard et al., 1995). Activation of EO9 still conforms to the concept of ‘enzyme directed bioreductive therapy’ (Workman and Walton, 1990) as therapy could still be targeted at tumours that expressed high levels of NQO1. The role of NQO1 in activating EO9 to DNA-damaging species in cell-free assays is clear, but its role in determining cellular response is more complex. Under aerobic conditions, good correlations between NQO1 activity and chemosensitivity in vitro have been reported (Robertson et al., 1992; 1994; Plumb et al., 1994b; Plumb and Workman, 1994; Smitskamp-Wilms et al., 1994; Collard et al., 1995; Fitzsimmons et al., 1996). In hypoxia, however, significant potentiation of EO9's activity was only seen in cell lines that lack NQO1 activity (Plumb et al., 1994b; Plumb and Workman, 1994; Robertson et al., 1994). In cell lines where NQO1 was high, EO9 was as effective against aerobic and hypoxic cells. Mechanistically, it is likely that one electron reductases play a prominent role in the hypoxia selectivity, whereas reduction of EO9 by NQO1 is an oxygen-independent process (Workman, 1994). EO9 can therefore be used to target the hypoxic regions of NQO1-deficient tumours, whereas in NQO1-rich tumours, EO9 will target both the aerobic and hypoxic fraction. This feature of EO9's pharmacology was seen as a unique and attractive feature as it suggested that EO9 had the capacity to exhibit single-agent activity against solid tumours (Hendriks et al., 1993). These preclinical studies also suggested that EO9 might find its optimal use in combination with radiation or other drugs.
In animal tumour models, EO9 was inactive against the P388 murine leukaemia but exhibited anti-tumour activity against human tumour xenografts and the generally chemo-resistant murine adenocarcinomas of the colon (MAC) tumours (Roed et al., 1989; Hendriks et al., 1993; Collard et al., 1995). Initial evidence that in vivo response correlated with NQO1 activity (Walton et al., 1992) was not substantiated in subsequent studies where poor relationships between NQO1 activity and in vivo activity were reported (Collard et al., 1995; Cummings et al., 1998). EO9 was selected for clinical evaluation based upon its novel mechanism of action (which was distinct from MMC), its preferential activity against cells derived from solid tumours in vitro and in vivo, its ability to target both aerobic and hypoxic cells and the lack of myelosuppression in mice and rats (Hendriks et al., 1993).
Clinical evaluation
Two phase I trials started in 1992 under the auspices of the EORTC. In the first study, the maximum tolerated dose following a 3 week schedule (q3wk) of 5 min i.v. infusion was 27 mg·m−2 (Schellens et al., 1994). Bone marrow suppression was not observed, and the dose limiting toxicity was reversible proteinuria. In a second phase I trial using a weekly bolus i.v. schedule (q1wk), a maximum tolerated dose of 14 mg·m−2 was reported, and the dose limiting toxicity was again reversible proteinuria (McLeod et al., 1996; Aamdal et al., 2000). Damage to glomeruli was observed in the clinical trial, and this correlated with high levels of NQO1 in the kidney (Segura-Aguilar et al., 1994; Zappa et al., 2003). A total of three partial responses were recorded in the phase I studies: two in patients with adenocarcinoma of unknown primary site and one in a patient with bile duct cancer. Phase II clinical trials commenced in the summer of 1994, and two studies were conducted. In the first, a total of 92 patients with advanced breast, gastric, pancreatic and colorectal cancer were treated with a 5 min i.v. infusion of EO9 at a weekly dose of 12 mg·m−2 (Dirix et al., 1996). No anti-tumour activity was seen. A second study involved the treatment of 38 chemotherapy naïve patients with advanced non–small cell lung cancer (NSCLC). Two treatment schedules were evaluated; a single bolus i.v. injection at 12 mg·m−2 administered weekly and i.v. bolus injection at 22 mg·m−2 administered every 3 weeks. Dose-limiting toxicity was reversible proteinuria; and whilst stable disease was reported in thirteen patients, these studies concluded that EO9 at these doses, and schedules had no clinical activity against NSCLC (Dirix et al., 1996; Pavlidis et al., 1996).
Reasons for EO9's failure
In a critique of the design of the clinical studies conducted, Connors (1996) highlighted certain key deficiencies in clinical trial design. These included the fact that NQO1and/or hypoxia were not measured in patient tumour samples, a fact that can be partially explained by an incomplete understanding of EO9s mechanism of action in the 1990s when the trials were designed. Both these parameters are key determinants of EO9's activity, and it is conceivable that tumours lacked the appropriate biochemistry required for drug activation. This is however unlikely as high NQO1 expression and hypoxia is typically found in many solid tumours (Siegel et al., 1998; Siegel and Ross, 2000; Vaupel et al., 2001; Vaupel and Mayer, 2007). On the other hand, EO9 should have been evaluated in hypoxic tumours that lack NQO1 in combination with other modalities (such as radiotherapy) that target the aerobic fraction. Post-irradiation treatment of tumours in vivo with EO9 indicated that radiosensitization was obtained in various preclinical tumour models (Adams et al., 1992; Burd et al., 2005), but no clinical trials of this nature were conducted. In any future trials of apaziquone or other bioreductive drugs, it is therefore advised that levels of hypoxia and enzymology (particularly NQO1 in the case of apaziquone) should be measured so that they can be used as potential biomarkers to further stratify patient outcomes.
In addition to concerns about the design of the clinical trials, attention also focused on whether or not drug delivery to tumours was impaired. The factors that determine how much drug is delivered to tumours can be broadly grouped into supply (extent of tumour vasculature and pharmacokinetics), flux (the drug's ability to penetrate through multiple layers of cells) and metabolism/sequestration of the drug within cells or the extracellular matrix (Minchinton and Tannock, 2006). In mice, the half-life was 1.9 ± 0.1 min, and the AUC was 4.8 µg min·mL−1 following i.v. administration of EO9 at 12 mg·kg−1; and in male Sprague–Dawley rats, the half-life was 3.0 ± 0.2 min with an AUC of 6.2 µg min·mL−1 following an i.v. dose of 3 mg·kg−1 (Workman et al., 1992). The rapid clearance and extremely short half-life of EO9 in rodents was replicated in man, with half-lives ranging from 0.8 to 19 min at the maximum tolerated dose of 27 mg·m−2 administered i.v. (Schellens et al., 1994). Similar preclinical and clinical data were reported by other groups (Bibby et al., 1993b; McLeod et al., 1996).
Given these data, it was clear that the supply of EO9 to tumours is likely to be impaired by its poor pharmacokinetics. To some extent, poor pharmacokinetics can be offset if the ‘flux’ of drugs through avascular tissue is good, but early studies using three-dimensional multilayered post-confluent cultures and multi-cell spheroids suggested that drug penetration barriers may exist (Bibby et al., 1993a; Pizao et al., 1993). Resistance in these models could be due to a multitude of reasons, including low cell proliferation rates, reduced extracellular pH, reduced nutrient status, etc. EO9 is however preferentially active against cells in acidic extracellular pH (Phillips et al., 1992), is able to kill confluent monolayer cultures (Phillips and Clayton, 1997) and is active against hypoxic cells. In 1996, Cowan et al. (1996) described an assay that could quantify the rate at which drugs crossed multi-cell layers in vitro. EO9 is able to cross DLD-1 human colorectal cancer multi-cell layers, but in comparison with tirapazamine (a nitroimidazole based bioreductive drug), its penetration rate is slow (Phillips et al., 1998). This study concluded that when EO9's rapid pharmacokinetic elimination is taken into consideration, EO9 would only penetrate a few microns from a blood vessel within its pharmacokinetic life span, and this is the probable reason for its failure to demonstrate efficacy in the clinic (Phillips et al., 1998).
Whilst inadequate drug delivery to tumours appears to be a plausible explanation, the question remains as to why EO9 is active against preclinical tumour models (Hendriks et al., 1993) but inactive in clinical trials, although its pharmacokinetics are similar in rodents and humans. A critical review of the preclinical studies however reveals that the magnitude of anti-tumour response observed was low with specific growth delays of only a few days reported. This level of activity would be acceptable if EO9 functioned purely as a hypoxia-targeted agent as cytotoxic effects against hypoxic cells would be masked by the continued growth of the aerobic fraction of cells. In NQO1-rich tumours, however, EO9 would target the aerobic fraction of cells as well, and in this case, a much greater level of activity would be expected. EO9 does induce some responses in preclinical tumours, and partial responses and stable disease were seen in phase I and II studies (Schellens et al., 1994; Pavlidis et al., 1996), so some EO9 was reaching the tumour. Direct intra-tumoural injection of EO9 resulted in improved anti-tumour activity (Loadman et al., 2002), supporting the fact that sub-optimal concentrations of EO9 were reaching the tumours following systemic administration.
Clinical evaluation of EO9 against superficial bladder cancer
Based upon this understanding of why EO9 failed, investigators were presented with two options: to develop analogues of EO9 that retained its good pharmacodynamics but had improved pharmacokinetics (Phillips, 1996; Phillips et al., 1999; 2004; Loadman et al., 2002) or utilize EO9's bad pharmacokinetics to gain therapeutic advantage. In this latter case, loco-regional administration of drug would circumvent the problems of drug delivery, and if the drug could be retained at this site for long periods, improved penetration into the tumour would occur. Furthermore, any drug that reached the systemic circulation would be rapidly cleared, thereby reducing the risk of systemic toxicity. Superficial transitional cell carcinoma (TCC) of the bladder provided a suitable clinical model to test this hypothesis as intravesical administration of chemotherapy or immunotherapy following transurethral resection (TUR) is an established mode of treatment (Hall et al., 2007). Following studies that demonstrated that TCC of the bladder expressed the key biochemical machinery required to activate EO9 (Figure 2) (Choudry et al., 2001; Basu et al., 2004), a phase I/II pilot study commenced in 2004.
Figure 2.
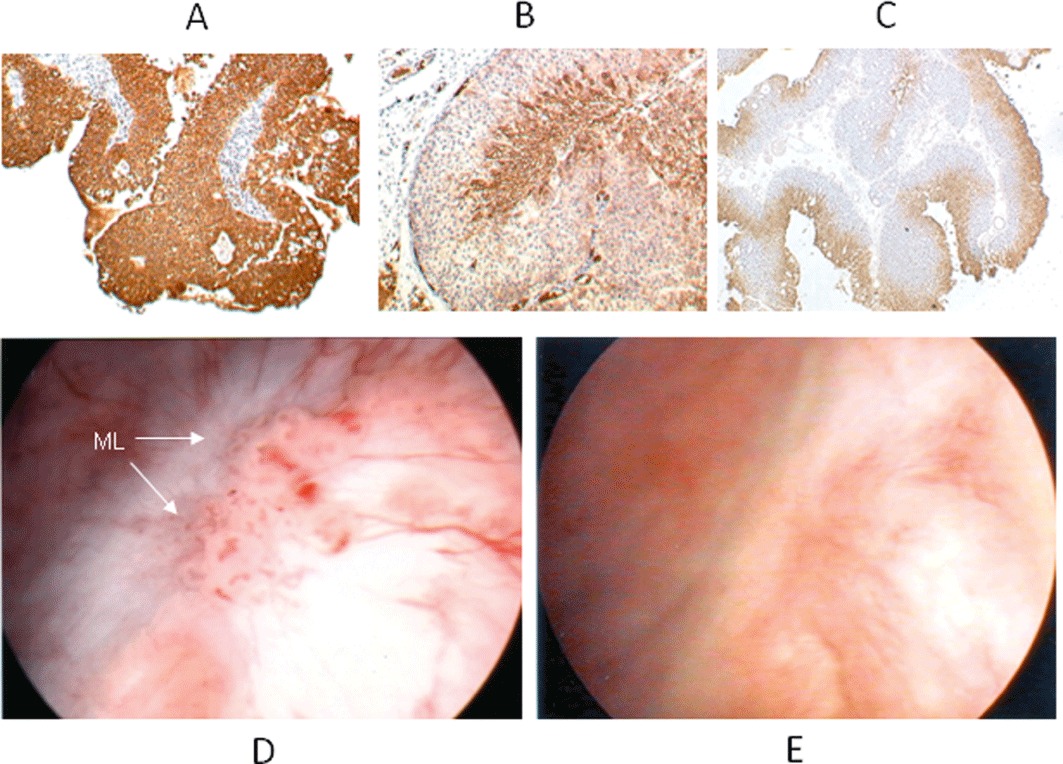
Representative immunohistochemical analysis of superficial human TCC of bladder expressing high levels of NQO1 (A), the glucose transporter GLUT1 (B) and pimonidazole-treated tumour (C). GLUT1 and pimonidazole have been used as endogenous and exogenous markers of hypoxia respectively (Rademakers et al., 2011). The brown staining in panels A to C represents areas of positive staining. Appearance of marker lesion (ML) in situ before (D) and after (E) a 6 week course of EO9 administered intravesically once a week at a dose of 4 mg/40 mL.
The purpose of this trial was to establish the dose of EO9 (now renamed EOquin by the sponsor Spectrum Pharmaceuticals, which was later changed to apaziquone) that could be safely administered intravesically and obtain evidence of efficacy against a marker lesion left in situ at TUR (Puri et al., 2006; Gofrit et al., 2010). Six patients with multi-focal superficial TCC of the bladder received increasing doses of EO9 (0.5–16 mg/40 mL) administered intravesically, which was retained within the bladder for 1 h. EO9 was well tolerated at doses up to 4 mg/40 mL, with grade 2 and 3 dysuria and haematuria being observed at doses at or above 8 mg/40 mL. No EO9 could be detected in plasma (Puri et al., 2006). A further six patients received EO9 at 4 mg/40 mL once a week for 6 weeks, which was well tolerated in all cases. Analysis of EO9 in the urine at the end of the instillation demonstrated that the concentration increased linearly with dose and that therapeutically effective concentrations were being achieved (Puri et al., 2006). At 4 mg/40 mL (100 µg·mL−1), the concentration of EO9 in the urine at the end of the1 h instillation was 72. 2 ± 11.8 µM (20.79 µg·mL−1), which represents a significant increase in drug exposure parameters compared to those reported following i.v. administration (Table 1). A total of 8 out of 12 patients had complete remission as defined by complete loss of the marker lesion, negative cytology and histology at the site of the lesion (Puri et al., 2006). Representative images of the marker lesion in situ before and after EO9 are presented in Figure 2.
Table 1.
Summary of pharmacokinetic parameters following the i.v. administration of EO9 (5 min infusion) and intravesical administration (1 h instillation) at maximum tolerated doses
| Route of administration | Dose | Cmax (µg·mL−1) | Cmax (µM) | AUC (µM.h) | T½ (min) |
|---|---|---|---|---|---|
| I.v.* | 27 mg·m−2 | 1.418 ± 0.911 | 4.92 ± 3.16 | 0.468 | 7.8 ± 5.6 |
| Intravesical# | 4 mg/40 mL | 100.00 | 347.2 | 204.8 | 36.6 |
The values shown following i.v. administration refer to plasma levels, whereas following intravesical administration, values quoted are in the levels in urine
Data obtained from Schellens et al. (1994).
Data obtained from Puri et al. (2006).
Phase II studies were conducted using an identical study design to the phase I/II pilot study, and each patient received six weekly intravesical instillations of EO9 at 4 mg/40 mL, with the first instillation starting 2 weeks after TUR (van der Heijden et al., 2006). EO9 was well tolerated, with no systemic side effects and grades 1 to 3 dysuria and haematuria being the most common local side effects. Of a total of 45 patients with superficial TCC of the bladder, 30 (67%) patients had complete response as defined by complete macro- and microscopic elimination of a marker lesion (van der Heijden et al., 2006). Recurrence-free rates were good in comparison with the results of other ablative studies (Hendricksen et al., 2009; Jain et al., 2009). EO9 was reformulated in 2007 (van der Schoot et al., 2007a; 2008; van der Schoot et al., 2007b), and it is currently undergoing phase III clinical evaluation in several centres across North America and Europe. Additionally, studies where EO9 was administered within 24 h of TUR, which is the standard recommended treatment for superficial TCC of the bladder (Sylvester et al., 2004), demonstrated that EO9 was well tolerated and has a good safety profile (Hendricksen et al., 2008).
Conclusions and future prospects
EO9 has had a chequered history, but by understanding the reasons why it failed, EO9 has been transformed from a clinically inactive drug to one that has efficacy against superficial bladder cancer. The outcome of the phase III clinical trials is eagerly anticipated, but even so, the ‘EO9 story’ demonstrates that compounds with poor systemic pharmacokinetics can be effectively used in a loco-regional setting. In the case of EO9, direct intravesical administration circumvented the drug delivery problems encountered following i.v. administration and resulted in high concentrations of drug that are confined within the bladder. In this setting, EO9's poor systemic pharmacokinetics were advantageous as any drug that reached the bloodstream was rapidly cleared. Whilst this review has focused specifically on EO9, our experience with EO9 has potentially significant implications for the development of loco-regional therapies in general. We suggest that compounds with good pharmacodynamics, but poor systemic pharmacokinetics could be valuable therapeutic agents for treating cancers in a loco-regional setting.
Loco-regional chemotherapy is emerging as an important adjunct to surgery and systemic chemotherapy in selected patients with certain types of cancer (Ceelen and Flessner, 2010; Lu et al., 2010). In addition, as early detection strategies for cancer become more effective, there is a case for using loco-regional chemotherapy as an adjunct to surgical excision. Therapeutic agents used for loco-regional therapies are typically conventional chemotherapeutic drugs that are widely used to treat systemic disease. These can be effective but the success of this approach has been restricted by local toxicity, inadequate drug penetration and systemic toxicity caused by drugs leaking out from the site of administration into the systemic circulation (Masters et al., 1990; 1996; Lu et al., 2010). In terms of selecting drugs for use in a loco-regional setting, experience with EO9 has shown that compounds with poor systemic pharmacokinetics can be locally efficacious without the systemic toxic side effects observed when administered i.v. In the context of the majority of drug discovery programs, compounds with poor systemic pharmacokinetics are typically rejected during the process of lead compound optimisation and currently reside at the back of the shelves of medicinal chemists. Based upon our experience with EO9, we suggest that many of these compounds should be revisited and re-evaluated as potential loco-regional therapies.
Acknowledgments
The authors would like to thank Mr Rajiv Puri, consultant urologist at Bradford Royal Infirmary, for provision of the images of marker lesions before and after treatment with EO9 (Figure 2).
Glossary
- AUC
area under the curve
- EORTC
European Organisation for the Research and Treatment of Cancer
- ICL
interstrand cross-link
- NQO1
NAD(P)H:quinone oxidoreductase-1
- NSCLC
non–small cell lung cancer; TCC, transitional cell carcinoma of the bladder
- TUR
trans-urethral resection
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Aamdal S, Lund B, Koier I, Houten M, Wanders J, Verweij J. Phase I trial with weekly EO9, a novel bioreductive alkylating indoloquinone, by the EORTC Early Clinical Study Group (ECSG) Cancer Chemother Pharmacol. 2000;45:85–88. doi: 10.1007/PL00006748. [DOI] [PubMed] [Google Scholar]
- Adams GE, Stratford IJ, Edwards HS, Bremner JC, Cole S. Bioreductive drugs as post-irradiation sensitizers: comparison of dual function agents with SR 4233 and the mitomycin C analogue EO9. Int J Radiat Oncol Biol Phys. 1992;22:717–720. doi: 10.1016/0360-3016(92)90510-o. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Lewis AD, Knox RJ, Patterson LH, Fisher GR, Workman P. Reduction of the indoloquinone anticancer drug EO9 by purified DT-diaphorase: a detailed kinetic study and analysis of metabolites. Biochem Pharmacol. 1998;56:613–621. doi: 10.1016/s0006-2952(97)00661-8. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Lewis AD, Patterson LH, Fisher GR, Knox RJ, Workman P. Involvement of NADPH: cytochrome P450 reductase in the activation of indoloquinone EO9 to free radical and DNA damaging species. Biochem Pharmacol. 2001;62:461–468. doi: 10.1016/s0006-2952(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Basu S, Brown JE, Flannigan GM, Gill JH, Loadman PM, Martin SW, et al. Immunohistochemical analysis of NAD(P)H:quinone oxidoreductase and NADPH cytochrome P450 reductase in human superficial bladder tumours: relationship between tumour enzymology and clinical outcome following intravesical mitomycin C therapy. Int J Cancer. 2004;109:703–709. doi: 10.1002/ijc.20005. [DOI] [PubMed] [Google Scholar]
- Bibby MC, Cronin BP, Phillips RM. Evaluation of the cytotoxicity of the indolequinone EO9 in a human colon adenocarcinoma model. Int J Oncol. 1993a;3:661–666. doi: 10.3892/ijo.3.4.661. [DOI] [PubMed] [Google Scholar]
- Bibby MC, Sleigh NR, Loadman PM, Double JA. Potentiation of EO9 anti-tumour activity by hydralazine. Eur J Cancer. 1993b;29A:1033–1035. doi: 10.1016/s0959-8049(05)80218-7. [DOI] [PubMed] [Google Scholar]
- Burd R, Lavorgna SN, Lenaz L, Chawla S, Reddy G, Wachsberger R, et al. Tumour radiosensitization by apaziquone (EO9, EOquin) Proc Am Assoc Cancer Res. 2005;46 abstract number 1454. [Google Scholar]
- Butler J, Spanswick VJ, Cummings J. The autoxidation of the reduced forms of EO9. Free Radic Res. 1996;25:141–148. doi: 10.3109/10715769609149919. [DOI] [PubMed] [Google Scholar]
- Cadenas E. Antioxidant and prooxidant functions of DT-diaphorase in quinone metabolism. Biochem Pharmacol. 1995;49:127–140. doi: 10.1016/s0006-2952(94)00333-5. [DOI] [PubMed] [Google Scholar]
- Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7:108–115. doi: 10.1038/nrclinonc.2009.217. [DOI] [PubMed] [Google Scholar]
- Choudry GA, Hamilton Stewart PA, Double JA, Krul MRL, Naylor B, Flannigan GM, et al. A novel styrategy for NQO1 (NAD(P)H:quinone oxidoreductase, EC1.6.99.2) mediated therapy of bladder cancer based on the pharmacologocal properties of EO9. Br J Cancer. 2001;85:1137–1146. doi: 10.1054/bjoc.2001.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard J, Matthew AM, Double JA, Bibby MC. EO9: relationship between DT-diaphorase levels and response in vitro and in vivo. Br J Cancer. 1995;71:1199–1203. doi: 10.1038/bjc.1995.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors TA. Bioreductive agents, hypoxic cells and therapy. Eur J Cancer. 1996;32A:1833–1834. doi: 10.1016/0959-8049(96)00305-x. [DOI] [PubMed] [Google Scholar]
- Cowan DS, Hicks KO, Wilson WR. Multicellular membranes as an in vitro model for extravascular diffusion in tumours. Br J Cancer Suppl. 1996;27:S28–S31. [PMC free article] [PubMed] [Google Scholar]
- Cummings J, Spanswick VJ, Gardiner J, Ritchie A, Smyth JF. Pharmacological and biochemical determinants of the antitumour activity of the indoloquinone EO9. Biochem Pharmacol. 1998;55:253–260. doi: 10.1016/s0006-2952(97)00265-7. [DOI] [PubMed] [Google Scholar]
- Denny WA. Prospects for hypoxia-activated anticancer drugs. Curr Med Chem Anticancer Agents. 2004;4:395–399. doi: 10.2174/1568011043352812. [DOI] [PubMed] [Google Scholar]
- Dirix LY, Tonnesen F, Cassidy J, Epelbaum R, ten Bokkel Huinink WW, Pavlidis N, et al. EO9 phase II study in advanced breast, gastric, pancreatic and colorectal carcinoma by the EORTC Early Clinical Studies Group. Eur J Cancer. 1996;32A:2019–22. doi: 10.1016/0959-8049(96)00226-2. [DOI] [PubMed] [Google Scholar]
- Ernster L. DT Diaphorase: a historical review. Chem Scr. 1987;27A:1–13. [Google Scholar]
- Fitzsimmons SA, Workman P, Grever M, Paull K, Camalier R, Lewis AD. Reductase enzyme expression across the National Cancer Institute Tumor cell line panel: correlation with sensitivity to mitomycin C and EO9. J Natl Cancer Inst. 1996;88:259–269. doi: 10.1093/jnci/88.5.259. [DOI] [PubMed] [Google Scholar]
- Gofrit ON, Zorn KC, Shikanov S, Steinberg GD. Marker lesion experiments in bladder cancer–what have we learned? J Urol. 2010;183:1678–1684. doi: 10.1016/j.juro.2009.12.104. [DOI] [PubMed] [Google Scholar]
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Hargreaves RH, Hartley JA, Butler J. Mechanisms of action of quinone-containing alkylating agents: DNA alkylation by aziridinylquinones. Front Biosci. 2000;5:E172–E180. doi: 10.2741/hargreav. [DOI] [PubMed] [Google Scholar]
- Hay MP, Hicks KO, Pruijn FB, Pchalek K, Siim BG, Wilson WR, et al. Pharmacokinetic/pharmacodynamic model-guided identification of hypoxia-selective 1,2,4-benzotriazine 1,4-dioxides with antitumor activity: the role of extravascular transport. J Med Chem. 2007a;50:6392–6404. doi: 10.1021/jm070670g. [DOI] [PubMed] [Google Scholar]
- Hay MP, Pchalek K, Pruijn FB, Hicks KO, Siim BG, Anderson RF, et al. Hypoxia-selective 3-alkyl 1,2,4-benzotriazine 1,4-dioxides: the influence of hydrogen bond donors on extravascular transport and antitumor activity. J Med Chem. 2007b;50:6654–6664. doi: 10.1021/jm701037w. [DOI] [PubMed] [Google Scholar]
- Hay MP, Hicks KO, Pchalek K, Lee HH, Blaser A, Pruijn FB, et al. Tricyclic [1,2,4]triazine 1,4-dioxides as hypoxia selective cytotoxins. J Med Chem. 2008;51:6853–6865. doi: 10.1021/jm800967h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden AG, Moonen PM, Cornel EB, Vergunst H, de Reijke TM, van Boven E, et al. Phase II marker lesion study with intravesical instillation of apaziquone for superficial bladder cancer: toxicity and marker response. J Urol. 2006;176:1349–1353. doi: 10.1016/j.juro.2006.06.007. discussion 1353. [DOI] [PubMed] [Google Scholar]
- Hendricksen K, Gleason D, Young JM, Saltzstein D, Gershman A, Lerner S, et al. Safety and side effects of immediate instillation of apaziquone following transurethral resection in patients with nonmuscle invasive bladder cancer. J Urol. 2008;180:116–120. doi: 10.1016/j.juro.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Hendricksen K, van der Heijden AG, Cornel EB, Vergunst H, de Reijke TM, van Boven E, et al. Two-year follow-up of the phase II marker lesion study of intravesical apaziquone for patients with non-muscle invasive bladder cancer. World J Urol. 2009;27:337–342. doi: 10.1007/s00345-009-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks HR, Pizao PE, Berger DP, Kooistra KL, Bibby MC, Boven E, et al. EO9: a novel bioreductive alkylating indoloquinone with preferential solid tumour activity and lack of bone marrow toxicity in preclinical models. Eur J Cancer. 1993;29A:897–906. doi: 10.1016/s0959-8049(05)80434-4. [DOI] [PubMed] [Google Scholar]
- Jain A, Phillips RM, Scally AJ, Lenaz G, Beer M, Puri R. Response of multiple recurrent TaT1 bladder cancer to intravesical apaziquone (EO9): comparative analysis of tumor recurrence rates. Urology. 2009;73:1083–1086. doi: 10.1016/j.urology.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Loadman PM, Bibby MC, Phillips RM. Pharmacological approach towards the development of indolequinone bioreductive drugs based on the clinically inactive agent EO9. Br J Pharmacol. 2002;137:701–709. doi: 10.1038/sj.bjp.0704916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Wang J, Wientjes MG, Au JL. Intraperitoneal therapy for peritoneal cancer. Future Oncol. 2010;6:1625–1641. doi: 10.2217/fon.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown SR, Cowen RL, Williams KJ. Bioreductive drugs: from concept to clinic. Clin Oncol (R Coll Radiol) 2007;19:427–442. doi: 10.1016/j.clon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- McLeod HL, Graham MA, Aamdal S, Setanoians A, Groot Y, Lund B. Phase I pharmacokinetics and limited sampling strategies for the bioreductive alkylating drug EO9. EORTC Early Clinical Trials Group. Eur J Cancer. 1996;32A:1518–1522. doi: 10.1016/0959-8049(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Wolfs A, Groot SE, de Mol NJ, Janssen LH. Indoloquinone EO9: DNA interstrand cross-linking upon reduction by DT-diaphorase or xanthine oxidase. Br J Cancer. 1995;71:836–839. doi: 10.1038/bjc.1995.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters JR, McDermott BJ, Jenkins WE, Fenwick E, Shah PJ, Mundy AR, et al. ThioTEPA pharmacokinetics during intravesical chemotherapy and the influence of Tween 80. Cancer Chemother Pharmacol. 1990;25:267–273. doi: 10.1007/BF00684884. [DOI] [PubMed] [Google Scholar]
- Masters JR, McDermott BJ, Harland S, Bibby MC, Loadman PM. ThioTEPA pharmacokinetics during intravesical chemotherapy: the influence of dose and volume of instillate on systemic uptake and dose rate to the tumour. Cancer Chemother Pharmacol. 1996;38:59–64. doi: 10.1007/s002800050448. [DOI] [PubMed] [Google Scholar]
- Milbank JB, Stevenson RJ, Ware DC, Chang JY, Tercel M, Ahn GO, et al. Synthesis and evaluation of stable bidentate transition metal complexes of 1-(chloromethyl)-5-hydroxy-3-(5,6,7-trimethoxyindol-2-ylcarbonyl)-2,3-dihy dro-1H-pyrrolo[3,2-f]quinoline (seco-6-azaCBI-TMI) as hypoxia selective cytotoxins. J Med Chem. 2009;52:6822–6834. doi: 10.1021/jm9008746. [DOI] [PubMed] [Google Scholar]
- Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- Oostveen EA, Speckamp WN. Mitomycin analogues 1. Indolequinones as potent bisalkylating agents. Tetrahedron. 1987;43:255–262. [Google Scholar]
- Pavlidis N, Hanauske AR, Gamucci T, Smyth J, Lehnert M, te Velde A, et al. A randomized phase II study with two schedules of the novel indoloquinone EO9 in non-small-cell lung cancer: a study of the EORTC Early Clinical Studies Group (ECSG) Ann Oncol. 1996;7:529–531. doi: 10.1093/oxfordjournals.annonc.a010645. [DOI] [PubMed] [Google Scholar]
- Phillips RM. Bioreductive activation of a series of analogues of 5-aziridinyl-3-hydroxymethyl-1-methyl-2-[1H-indole-4, 7-dione] prop-beta-en-alpha-ol (EO9) by human DT-diaphorase. Biochem Pharmacol. 1996;52:1711–1718. doi: 10.1016/s0006-2952(96)00521-7. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Clayton MR. Plateau-phase cultures: an experimental model for identifying drugs which are bioactivated within the microenvironment of solid tumours. Br J Cancer. 1997;75:196–201. doi: 10.1038/bjc.1997.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RM, Hulbert PB, Bibby MC, Sleigh NR, Double JA. In vitro activity of the novel indoloquinone EO-9 and the influence of pH on cytotoxicity. Br J Cancer. 1992;65:359–364. doi: 10.1038/bjc.1992.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RM, Loadman PM, Cronin BP. Evaluation of a novel in vitro assay for assessing drug penetration into avascular regions of tumours. Br J Cancer. 1998;77:2112–2119. doi: 10.1038/bjc.1998.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RM, Naylor MA, Jaffar M, Doughty SW, Everett SA, Breen AG, et al. Bioreductive activation of a series of indolequinones by human DT-diaphorase: structur-activity relationships. J Med Chem. 1999;42:4071–4080. doi: 10.1021/jm991063z. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Jaffar M, Maitland DJ, Loadman PM, Shnyder SD, Steans G, et al. Pharmacological and biological evaluation of a series of substituted 1,4-naphthoquinone bioreductive drugs. Biochem Pharmacol. 2004;68:2107–2116. doi: 10.1016/j.bcp.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Pizao PE, Peters GJ, Van Ark-Otte J, Smets LA, Smitskamp-Wilms E, Winograd B, et al. Cytotoxic effects of anticancer agents on subconfluent and multilayered postconfluent cultures. Eur J Cancer. 1993;29A:1566–1573. doi: 10.1016/0959-8049(93)90296-r. [DOI] [PubMed] [Google Scholar]
- Plumb JA, Workman P. Unusually marked hypoxic sensitization to indoloquinone EO9 and mitomycin C in a human colon-tumour cell line that lacks DT-diaphorase activity. Int J Cancer. 1994;56:134–139. doi: 10.1002/ijc.2910560124. [DOI] [PubMed] [Google Scholar]
- Plumb JA, Gerritsen M, Milroy R, Thomson P, Workman P. Relative importance of DT-diaphorase and hypoxia in the bioactivation of EO9 by human lung tumor cell lines. Int J Radiat Oncol Biol Phys. 1994a;29:295–299. doi: 10.1016/0360-3016(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Plumb JA, Gerritsen M, Workman P. DT-diaphorase protects cells from the hypoxic cytotoxicity of indoloquinone EO9. Br J Cancer. 1994b;70:1136–1143. doi: 10.1038/bjc.1994.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri R, Palit V, Loadman PM, Flannigan M, Shah T, Choudry GA, et al. Phase I/II Pilot Study of Intravesical Apaziquone (EO9) for Superficial Bladder Cancer. J Urol. 2006;176:1344–1348. doi: 10.1016/j.juro.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Rademakers SE, Lok J, van der Kogel AJ, Bussink J, Kaanders JH. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1alpha, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 2011;11:167–177. doi: 10.1186/1471-2407-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson N, Stratford IJ, Houlbrook S, Carmichael J, Adams GE. The sensitivity of human tumour cells to quinone bioreductive drugs: what role for DT-diaphorase? Biochem Pharmacol. 1992;44:409–412. doi: 10.1016/0006-2952(92)90429-m. [DOI] [PubMed] [Google Scholar]
- Robertson N, Haigh A, Adams GE, Stratford IJ. Factors affecting sensitivity to EO9 in rodent and human tumour cells in vitro: DT-diaphorase activity and hypoxia. Eur J Cancer. 1994;30A:1013–1019. doi: 10.1016/0959-8049(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Roed H, Aabo K, Vindelov L, Spang-Thomsen M, Christensen IB, Hansen HH. In vitro and in vivo evaluation of the indoloquinone EO-9 (NSC 382 459) against human small cell carcinoma of the lung. Eur J Cancer Clin Oncol. 1989;25:1197–1201. doi: 10.1016/0277-5379(89)90415-x. [DOI] [PubMed] [Google Scholar]
- Schellens JH, Planting AS, van Acker BA, Loos WJ, de Boer-Dennert M, van der Burg ME, et al. Phase I and pharmacologic study of the novel indoloquinone bioreductive alkylating cytotoxic drug E09. J Natl Cancer Inst. 1994;86:906–912. doi: 10.1093/jnci/86.12.906. [DOI] [PubMed] [Google Scholar]
- van der Schoot SC, Nuijen B, Flesch FM, Gore A, Mirejovsky D, Lenaz L, et al. Development of a bladder instillation of the indoloquinone anticancer agent EO-9 using tert-butyl alcohol as lyophilization vehicle. AAPS PharmSciTech. 2007a;8:E61. doi: 10.1208/pt0803059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schoot SC, Vainchtein LD, Beijnen JH, Gore A, Mirejovsky D, Lenaz L, et al. EO-9 bladder instillations: formulation selection based on stability characteristics and in vitro simulation studies. Int J Pharm. 2007b;329:135–141. doi: 10.1016/j.ijpharm.2006.08.031. [DOI] [PubMed] [Google Scholar]
- van der Schoot SC, Vainchtein LD, Nuijen B, Gore A, Mirejovsky D, Lenaz L, et al. Purity profile of the indoloquinone anticancer agent EO-9 and chemical stability of EO-9 freeze dried with 2-hydroxypropyl-beta-cyclodextrin. Pharmazie. 2008;63:796–805. [PubMed] [Google Scholar]
- Segura-Aguilar J, Cremades A, Llombart-Bosch A, Monsalve E, Ernster L, Romero FJ. Activity and immunohistochemistry of DT-diaphorase in hamster and human kidney tumours. Carcinogenesis. 1994;15:1631–1636. doi: 10.1093/carcin/15.8.1631. [DOI] [PubMed] [Google Scholar]
- Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000;29:246–253. doi: 10.1016/s0891-5849(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Siegel D, Franklin WA, Ross D. Immunohistochemical detection of NAD(P)H:quinone oxidoreductase in human lung and lung tumors. Clin Cancer Res. 1998;4:2065–2070. [PubMed] [Google Scholar]
- Smitskamp-Wilms E, Peters GJ, Pinedo HM, van Ark-Otte J, Giaccone G. Chemosensitivity to the indoloquinone EO9 is correlated with DT-diaphorase activity and its gene expression. Biochem Pharmacol. 1994;47:1325–1332. doi: 10.1016/0006-2952(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Stratford IJ, Workman P. Bioreductive drugs into the next millennium. Anticancer Drug Des. 1998;13:519–528. [PubMed] [Google Scholar]
- Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. quiz 2435. [DOI] [PubMed] [Google Scholar]
- Tercel M, Atwell GJ, Yang S, Stevenson RJ, Botting KJ, Boyd M, et al. Hypoxia-activated prodrugs: substituent effects on the properties of nitro seco-1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one (nitroCBI) prodrugs of DNA minor groove alkylating agents. J Med Chem. 2009;52:7258–7272. doi: 10.1021/jm901202b. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001;28:29–35. doi: 10.1016/s0093-7754(01)90210-6. [DOI] [PubMed] [Google Scholar]
- Walton MI, Smith PJ, Workman P. The role of NAD(P)H: quinone reductase (EC 1.6.99.2, DT-diaphorase) in the reductive bioactivation of the novel indoloquinone antitumor agent EO9. Cancer Commun. 1991;3:199–206. doi: 10.3727/095535491820873164. [DOI] [PubMed] [Google Scholar]
- Walton MI, Bibby MC, Double JA, Plumb JA, Workman P. DT-diaphorase activity correlates with sensitivity to the indoloquinone EO9 in mouse and human colon carcinomas. Eur J Cancer. 1992;28A:1597–1600. doi: 10.1016/0959-8049(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Workman P. Enzyme-directed bioreductive drug development revisited: a commentary on recent progress and future prospects with emphasis on quinone anticancer agents and quinone metabolizing enzymes, particularly DT-diaphorase. Oncol Res. 1994;6:461–475. [PubMed] [Google Scholar]
- Workman P, Walton MI. Enzyme directed bioreductive drug development. In: Adams GE, Breccia A, Fielden EM, Wardman P, editors. Selective Activation of Drugs by Redox Processes. New York: Plenum Press; 1990. pp. 173–191. [Google Scholar]
- Workman P, Binger M, Kooistra KL. Pharmacokinetics, distribution, and metabolism of the novel bioreductive alkylating indoloquinone EO9 in rodents. Int J Radiat Oncol Biol Phys. 1992;22:713–716. doi: 10.1016/0360-3016(92)90509-g. [DOI] [PubMed] [Google Scholar]
- Zappa F, Ward T, Pedrinis E, Butler J, McGown A. NAD(P)H: quinone oxidoreductase 1 expression in kidney podocytes. J Histochem Cytochem. 2003;51:297–302. doi: 10.1177/002215540305100304. [DOI] [PubMed] [Google Scholar]


