Abstract
Can CD4+ and CD8+ “memory” T cells that are generated and maintained in the context of low-level virus persistence protect, in the absence of antibody, against a repeat challenge with the same pathogen? Although immune T cells exert effective, long-term control of a persistent γ-herpesvirus (γHV68) in Ig–/– μMT mice, subsequent exposure to a high dose of the same virus leads to further low-level replication in the lung. This lytic phase in the respiratory tract is dealt with effectively by the recall of memory T cells induced by a γHV68 recombinant (M3LacZ) that does not express the viral M3 chemokine binding protein. At least for the CD8+ response, greater numbers of memory T cells confer enhanced protection in the M3LacZ-immune mice. However, neither WT γHV68 nor the minimally persistent M3LacZ primes the T cell response to the extent that a WT γHV68 challenge fails to establish latency in the μMT mice. Memory CD4+ and CD8+ T cells thus act together to limit γHV68 infection but are unable to provide absolute protection against a high-dose, homologous challenge.
Along-term debate in the immunology community concerns the importance of antigen persistence for maintaining T cell memory (1–3). The discussion is often confused by different interpretations of the term “memory” (4). If we look at memory simply as the capacity to maintain antigen-specific, “resting” T cell numbers indefinitely, then it seems that the continued presence of the particular MHC class I or class II glycoprotein plus peptide (epitope) is certainly not required (2, 3, 5, 6). However, if memory is used in the sense of the protective immunity that might be the focus of a candidate T cell-based vaccine, then the case that continued (or sporadic) reexposure to the inducing antigen is advantageous could well have merit (7).
Acutely activated T cells deal very effectively with a homologous virus challenge (8). On the other hand, the time taken to recall “resting” memory allows an invading organism to become established (9), although the pathogen may either be cleared more rapidly or (for an agent that persists) be held to a lower “set point” (10). Although cytotoxic T cells can be induced very rapidly in vivo (11, 12), their localization to (for example) the respiratory mucosa may be delayed by the need for further activation and proliferation in the lymphoid tissue (13). Could protection be made more immediate by achieving a continuing state of enhanced lymphocyte turnover (14) and activation?
The herpesviruses (HVs) provide a natural system for analyzing immunity in the context of controlled virus persistence (15, 16). Vaccination strategies with the γHVs, like Kaposi's sarcoma virus (HHV8) and Epstein–Barr virus (EBV), can be investigated (17–22) with the murine γ-herpesvirus 68 (γHV68), a virus that has high level sequence homology with HHV8 and a pathogenesis similar to that of EBV (17–19). Respiratory exposure of C57BL/6J (B6) mice to γHV68 induces transient, lytic infection of the respiratory tract and latency in B lymphocytes and macrophages (20, 21). Virus is not normally detected by plaque assay of lung homogenates for >10–12 days after the initial challenge. Genetically disrupted μMT mice that lack both B cells and antibody (Ig–/–) also clear γHV68 from the lung and show little evidence of latency by infectious center assay (22). However, a more sensitive limiting dilution analysis (LDA) showed that γHV68 persists in macrophages and, perhaps, in other cells from Ig–/– mice after both i.p. and intranasal (i.n.) challenge (21, 23).
The present experiments ask whether the combination of CD4+ and CD8+ T cell memory (24) in mice infected once with γHV68 (25, 26) protects against superinfection with the same virus. Antibody is, of course, likely to neutralize the majority of input virus in this circumstance (27–29). Therefore, the experiments were done with Ig–/– μMT mice (30) that had been infected with either WT γHV68 or with a mutant virus (M3LacZ) that causes a normal, lytic infection in the lung, but a much lower level of latency in the lymphoid tissue of Ig+/+ controls (22). It is also the case that the protective capacity of immune CD4+ and CD8+ T cells that are maintained where there is the possibility of continued, low level antigen challenge has not (to our knowledge) been analyzed previously for any virus system.
Materials and Methods
Recombinant and WT Viruses. The WT γHV68 used was either the Cambridge University strain (γHVCam), the origin of the M3LacZ recombinant, or the Washington University isolate (γHVW) that was plaque purified from γHVCam (17, 18). The latter is the standard WT γHV68 used in our laboratory. The capacity of WT γHV68 to make the broad spectrum M3 chemokine binding protein (31, 32) was disabled by inserting the LacZ gene (M3LacZ) under a cytomegalovirus promoter (22). A recombinant (RγHV68A98.01) expressing GFP was generated (33, 34) by cloning the γHV68 genome as an infectious bacterial artificial chromosome (BAC). Virus stocks were grown in BHK-21 and 3T12 cells (American Type Culture Collection) and maintained in DMEM (GIBCO/BRL) supplemented with 10% FCS, glutamine, and gentamicin.
Mice and Infection. The Ig–/– μMT mice (30) were bred at St. Jude Children's Research Hospital, then at Charles River Breeding Laboratories. Male or female (12–16 weeks old) μMT mice were primed i.p. with 2–5 × 104 plaque-forming units (PFU) of M3LacZ or γHVCAM. After 2–5 months, both groups were anesthetized with Avertin (2,2,2-tribromoethanol) and challenged i.n. with 2 × 106 PFU of a mixture of γHVW and BAC–GFP. All mice were otherwise maintained under specific pathogen-free conditions in BL3-level containment.
Sampling. Mice were anesthetized, the axillary artery was sectioned, and the blood was collected into heparin. Inflammatory cells were recovered from the infected respiratory tract by bronchoalveolar lavage (BAL) and the lungs were snap-frozen in liquid nitrogen and stored at –70°C for plaque assay. Single-cell preparations were made from the mediastinal lymph nodes (MLN) and spleen, whereas the BAL cells were adhered to plastic for 1 h at 37°C to remove macrophages and monocytes.
Flow Cytometry. Lymphocytes were suspended in ice-cold PBS–BSA (0.1%)/azide (0.01%), and stained on ice (30 min) with conjugated mAbs supplied by Pharmingen. Spleen and MLN populations were enriched for the CD8+ set by in vitro depletion with mAbs to I-Ab (M5/114.15.2) and CD4 (GK1.5), followed by sheep-anti-rat Ig and sheep-anti-mouse Ig-coated magnetic beads (Dynal, Oslo). Virus-specific CD8+ T cells were stained with phycoerythrin (PE)-labeled tetrameric complexes (25) of H-2Db+p56 (Dbp56) or H-2Kb+79 (Kbp79) at room temperature followed by anti-CD8+-Tricolor (Caltag, South San Francisco, CA). Unenriched cells were stained with anti-CD8-FITC and anti-CD4-PE to determine the T cell phenotype. The cells were analyzed on a FACScan by using cellquest software (Becton Dickinson). Numbers were calculated from the total cell count, the percentage CD8+ and the percentage CD8+ tetramer+.
Virus Titration. Lytic infection in the lung was measured by 6d plaque assay of clarified lung homogenates on 3T12 cells (20). Plaques from mice that had been challenged with BAC–GFP were first counted in a fluorescence microscope before staining with Giemsa, or processing for the detection of M3LacZ. The LDA for latent virus (21, 23) used single cell suspensions plated as 24 replicates per serial 3× dilution on primary murine embryonic fibroblasts in 96-well, flat-bottomed plates. These were incubated for 14 days, then 100 μl of the supernatant from each well was plated on 3T12 cells, incubated for 5 days, and analyzed for cytopathic effect. Frequencies were determined by applying the Poisson formula to the number of negative cultures per dilution, then expressed as reciprocal log10 values and analyzed by Student's t test.
The 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) assay was used to check for reactivated virus in plaques from M3LacZ primed μMT mice that had been depleted of both CD4+ and CD8+ T cells (see below) before or after challenge with γHVW+BAC–GFP. Briefly, the plaques were fixed with 2% formaldehyde for 5 min, the fixative was removed, the cells were washed once and stained with PBS containing 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, and 1 mg/ml of the chromogenic substrate X-Gal (Roche Diagnostics). The plates were incubated for 12 h at 37°C before counting the blue plaques resulting from the X-Gal staining of the β-galactosidase reaction product.
Depletion of CD4+ and CD8+ T Cells. The Ig–/– μMT mice were injected i.p. every second day for at least 6 days with 0.5-ml aliquots of ascitic fluid containing the GK1.5 mAb to CD4 and/or the 2.43.1 mAb to CD8α (23, 35). After sampling, various lymphocyte populations were stained with non-cross-reactive mAbs (Pharmingen) to CD4 (RM4–4) and CD8β (53–5.8) to check the efficiency of depletion by flow cytometry (23, 35).
Results
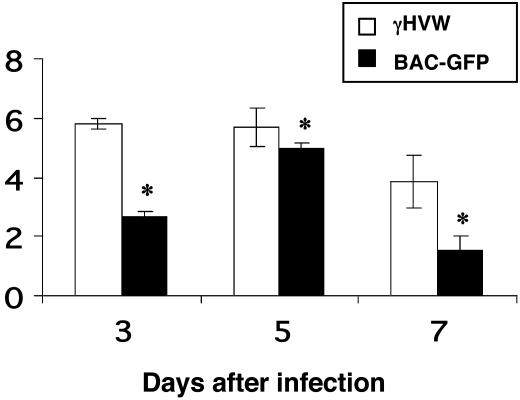
The Challenge System. The intent was to show whether primed CD4+ and CD8+ T cells in mice persistently infected with a γHV can prevent superinfection. The aim had been to use the green fluorescence marker of the BAC–GFP to determine whether any recovered virus originated from the primary or secondary inoculum. However, preliminary challenge experiments proved disappointing, as the growth profile for BAC–GFP in vivo was much less consistent than that for the WT γHVW (Fig. 1 and data not shown). We thus decided on a strategy of challenging with equal parts (total 2 × 106 PFU) of BAC–GFP and γHVW. Detection of at least a few “green plaques” establishes that the input virus has indeed become established. This is not essential for the mice given M3LacZ, which gives “blue plaques” by X-Gal staining.
Fig. 1.
Replication of the γHVW and BAC–GFP viruses in the lungs of female Ig–/– μMT mice. The 10- to 12-week-old mice were infected i.n. with 106 PFU of γHVW or BAC–GFP, then lungs were collected from three individuals (per group) on d3, d5, and d7, homogenized, and assayed for plaque formation on 3T12 cells. The plaques were fixed and stained with Giemsa (γHVW) or counted under a fluorescence microscope (BAC–GFP). Virus titers are expressed as log10 PFU/ml. The asterisk designates values that are significantly different (P ≤ 0.05).
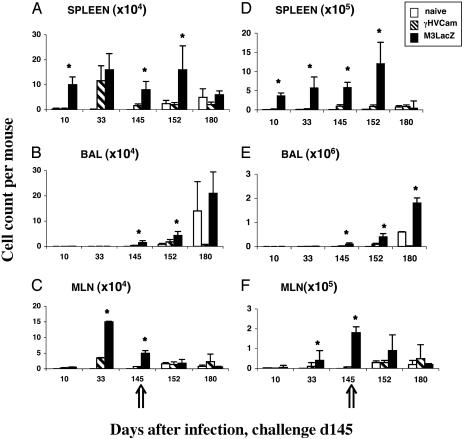
The CD8+ T Cell Response in Ig–/– Mice. Groups of Ig–/– μMT mice were injected i.p. with 104 PFU of either γHVCam or M3LacZ. The well established i.p. route (17) was used to minimize the establishment of γHV68 latency or macrophage activation in the lung, as evidenced by the lack of virus-specific CD8+ T cell localization to that site by day 10 (d10) or d33 after infection (BAL results, Fig. 2 B and E). Somewhat surprisingly, priming with M3LacZ rather than WT γHV68 led to much higher numbers of both CD8+Dbp56+ and CD8+Kbp79+ T cells in spleen and MLN populations assayed at d10, d33, and d145 after infection (Fig. 2 A, C, D, and F). Furthermore, the CD8+Kbp79+ set tended to be at higher prevalence than the CD8+Dbp56+ population. Although γHV68 latency is poorly understood, indirect evidence from conventional Ig+/+ mice suggests that the Kbp79 epitope is expressed for much longer than Dbp56, which tends to be prominent early.
Fig. 2.
The virus-specific CD8+ T cell response in μMT mice that were previously uninfected (naïve) or primed i.p. (2 × 104 PFU) with γHVCam or M3LacZ before i.n. challenge on d145 with a 1:1 mixture of γHVW and BAC–GFP (2 × 106 PFU). (A–C) The number of CD8+Dbp56+ T cell calculated from the percentage of cells staining and the total cell counts (data not shown). (D–F) The values for the CD8+Kbp79+ set. The results are expressed as mean ± SD. The asterisk designates differences that were significant at P ≤ 0.05.
A few CD8+Dbp56+ and CD8+ Kbp79+ T cells were detected in the BAL on d145 after the initial priming with virus (Fig. 2 B and E), but the T cell numbers in the lymphoid tissue were high (Fig. 2 A, C, D, and F) and activated CD8+ T cells tend to localize to the lung lumen sampled by BAL in the absence of any respiratory disease (36). The i.n. BAC–GFP/γHVW challenge on d145 (Fig. 2) established in the respiratory tract of the naïve mice (Table 1), and low levels of virus were also recovered 5 days later from four of five mice that had been given γHVCam. However, no viral replication was detected in the lungs of those primed with M3LacZ (Table 1).
Table 1. Virus in lung after i.n. challenge of persistently infected and naïve Ig-/- mice.
| Virus titer (log10 PFU/ml)
|
|||
|---|---|---|---|
| Day | Naïve | γHVCam | M3LacZ |
| 5 | 5.3 ± 0.7 (5/5) | 2.1 ± 1.0 (4/5) | NoCPE (0/5) |
| 6 | 4.7 ± 4.1 (4/4) | 1.3 ± 0.8 (2/4) | NoCPE (0/4) |
| 7 | 3.9 ± 3.5 (3/5) | No CPE (0/3) | NoCPE (0/3) |
The Ig-/- μMT mice were given 2 × 104 PFU of the γHVCam or M3LacZ viruses 2-3 months previously, then challenged i.n. with 2 × 106 PFU of a 1:1 mixture of the γHVW+BAC-GFP viruses. The naïve controls were age-matched, previously uninfected mice. Lung homogenates were assayed on 3T12 cells, and virus titers were expressed as mean ± SD log10/ml. The x/y values in parentheses give the numbers of mice with detectable virus in lung. The GFP+ plaques were counted under a fluorescence microscope before staining with Giemsa. Approximately 1.4% (3.7 ± 0.5 log10 PFU/ml) of the plaques detected on d5 in the naïve mice were GFP+, with the comparable values in the γHVCam-primed mice ranging from 5% to 12% (0.7 ± 0.9 log10 PFU/ml). No GFP+ virus was detected after d5. CPE, cytopathic effect.
The naïve controls developed typical (25), primar y CD8+Dbp56+ and CD8+Kbp79+ responses, although virus-specific CD8+ T cells numbers were not greatly boosted in the MLN and spleen of the previously exposed mice (Fig. 2 A, C, D, and F) and the cell counts in (particularly) the spleen of the M3LacZ-primed group seemed to fall by d15 after challenge (d180, Fig. 2 A and D), whereas the opposite trend was seen for the BAL (d180, Fig. 2 B and E). Overall, the higher levels of CD8+ T cell memory (Fig. 2) in the M3LacZ-immune group correlated with greater resistance (Table 1), although it also seemed that the challenge either diminished the magnitude of the memory CD8+ T cell populations in the lymphoid tissue of the M3LacZ-immune mice or caused them to migrate to sites like the respiratory tract (d180, Fig. 2 A and D and B and E). Why this should happen in the absence of obvious viral replication in the lung (Table 1) is not clear.
Consequences of Depleting CD4+ and CD8+ T Cells from Immune Mice. It thus seems that prior infection via the i.p. route substantially (γHVCam) or, perhaps, completely (M3LacZ) protects Ig–/– μMT mice against respiratory challenge with a high dose mixture of γHVW and BAC–GFP. The next step was to show that this was indeed mediated by immune CD4+ and/or CD8+ T cells, and was not caused by some form of viral interference. The latter seemed unlikely, because the lack of an early inflammatory response (d10 and d33, Fig. 2) in the BAL after i.p. exposure to either γHVCam or M3LacZ would suggest that there was no significant virus replication in the respiratory tract. Even so, the possibility that protection is mediated by some factor other than CD4+ and CD8+ T cells merited investigation.
The Ig–/– mice were infected i.p. with γHVCam or M3LacZ, left for 2 months, treated with monoclonal antibodies (mAbs) to CD4 and/or CD8 (23) for 4 days before i.n. challenge with the mixture of γHVW and BAC–GFP, then assayed after a further 5 days (Table 2). Flow cytometric analysis (data not shown) of MLN, spleen, BAL, and blood confirmed that one or both T cell subsets had been completely eliminated by the time of sampling.
Table 2. Consequences of T cell depletion for the establishment of challenge virus in naïve and primed Ig-/- mice.
| mAb depletion
|
Virus recovery from naïve and primed mice
|
Virus titers (log10 PFU/ml) and priming
|
||||||
|---|---|---|---|---|---|---|---|---|
| CD4 | CD8 | Virus | Naïve | γHVCam | M3LacZ | Naïve | γHVCam | M3LacZ |
| - | - | Total | 6/6 | 6/6 | 0/6 | 4.9 ± 0.2 | 2.3 ± 0.5 | 0 |
| GFP+ | 6/6 | 3/6 | 0/6 | 2.4 ± 0.2 | 1.12 ± 0.3 | 0 | ||
| + | - | Total | 6/6 | 5/6 | 5/6 | 3.9 ± 0.3 | 2.5 ± 0.5 | 1.6 ± 0.5 |
| GFP+ | 6/6 | 1/6 | 0/6 | 2.3 ± 0.3 | 1.1 ± 0.3 | 0 | ||
| - | + | Total | 6/6 | 6/6 | 3/6 | 4.6 ± 0.3 | 3.1 ± 0.2 | 1.1 ± 0.6 |
| GFP+ | 6/6 | 0/6 | 0/6 | 2.7 ± 0.4 | 0 | 0 | ||
| + | + | Total | 6/6 | 6/6 | 6/6 | 5.7 ± 0.2 | 5.1 ± 0.2 | 3.8 ± 0.1 |
| GFP+ | 6/6 | 6/6 | 6/6 | 3.5 ± 0.1 | 3.4 ± 0.3 | 1.8 ± 0.1 | ||
The μMT mice were primed i.p. with 1 × 104 PFU of γHVCam or M3LacZ for 2 months. They were then dosed i.p. with the GK1.5 mAb to CD4 and/or the 2.43.1 mAb to CD8 three times before and every second day after i.n. challenge with 5 × 106 PFU of 1:1 γHVW+BAC-GFP. Age-matched mice were used as naïve controls. Lungs were harvested on d5, homogenized, and assayed for PFU. The GFP+ plaques were counted by immunofluorescence before fixing and staining with Giemsa. The results are expressed as log10 mean ± SE from six mice.
Analysis of un-depleted, control (CD4+8+) mice at the d5 time point confirmed our earlier finding (Table 1) that the extent of protection by M3LacZ was greater than that conferred by persistent infection with γHVCam (Table 2). By this time (10 days after beginning the mAb treatment) all of the CD4+ and CD8+ T cells had been eliminated from the spleen, MLN, BAL, and blood (data not shown). Although depleting either CD4+ or CD8+ T cells led to diminished protection in the mice persistently infected with M3LacZ, removing both T cells subsets had an additive effect (Table 2). Even so, the titers in the CD4–8– groups were still significantly lower in those that had been given M3LacZ, perhaps reflecting that the larger numbers of virus-specific CD8+ T cells in these mice (Fig. 2) had not been completely removed from all tissue sites by the time of challenge, which was 5 days before sampling and fluorescence-activated cell sorter analysis (data not shown). An alternative is that some other effector, possibly the γδ T cell (37), is preferentially engaged in the mice infected with M3LacZ.
Both “green” (BAC–GFP) and normal virus were recovered from the respiratory tract of each member of the naïve group (Table 2). This was also true for every γHVCam- and M3LacZ-primed mouse that had been treated with mAbs to both CD4 and CD8. Even so, although the outgrowth of “green” plaques from the lungs of all CD4–8– mice (Table 2) showed that the challenge inoculum indeed became established, it is also possible that some of the “clear” plaquing virus recovered from these mice was γHVCam or M3LacZ that had reactivated from latency. Low titers (generally <102) of γHVCam were found previously in the lungs of persistently infected μMT mice after a prolonged course (14 days) of such double depletion. All of the lung homogenates from the CD4–8–M3LacZ group were thus replated and checked (after X-Gal staining) for the presence of “blue” M3LacZ virus. This was detected in three of six mice at plaque counts of 60–600 per ml. The majority of the virus isolated from the CD4–8– mice (Table 2) must, therefore, have been derived from the challenge inoculum. Furthermore, the fact that M3LacZ could be induced to reemerge in some mice subsequent to T cell depletion, although it could not be found in lungs from the CD4+8+ controls, confirms that M3LacZ establishes latency in these B cell-deficient Ig–/– mice.
Induction of Latency After Superinfection of M3LacZ-Primed μMT Mice. The preceding experiments (Fig. 2 and Tables 1 and 2) showed that persistent infection of Ig–/– mice with M3LacZ maintains a state of continuing CD4+ and CD8+ T cell priming that is sufficient to minimize any lytic infection in the lung after a second, high-dose respiratory challenge with WT γHV68. The next question was whether these M3LacZ-immune mice were also protected against the development of further latent infection. This was determined by LDA: the data are expressed as reciprocal log10 frequencies, so the lower the number (spleen latency titer, Table 3) the greater the prevalence of latently infected cells.
Table 3. Further establishment of latency after secondary challenge of M3LacZ-primed Ig-/- mice.
| M3LacZ priming*
|
γHV68 challenge
|
T cell depletion
|
Lung titer, PFU†
|
Spleen latency
|
||
|---|---|---|---|---|---|---|
| Line | Reciprocal LDA titer‡ | Percent X-Gal+§¶ | ||||
| 1 | - | + | - | 0 | 3.9 ± 0.1 | 0 |
| 2 | - | + | + | 3.1 ± 0.5 | 3.2 ± 0.8 | 0 |
| 3 | + | - | - | 0 | 4.7 ± 0.6¶ | 100 |
| 4 | + | + | - | 0 | 3.7 ± 0.6 | 23 ± 23 |
| 5 | + | + | + | 3.6 ± 0.6 | 2.8 ± 0.3 | 29 ± 15 |
The mice were infected i.p. with 1 × 104 PFU of M3LacZ, then challenged i.n. 2 months later with 5 × 106 PFU of 1:1 γHVW+BAC-GFP, together with naïve (-) controls. After another month, groups of naive and primed challenged mice were depleted for 10 days by the i.p. inoculation of mAbs to CD4 and CD8.
Mean ± SD log10 per ml for groups of five mice. No “green” BAC-GFP or “blue” M3LacZ was detected.
Log10 reciprocal frequency of infected spleen cells detected by LDA. The BAC-GFP “green” virus was found in only three of five naïve, virus-challenged T cell-depleted mice.
Percentage of positive cells containing M3LacZ-derived “blue” plaques.
Virus was detected in four of five mice.
The Ig–/– mice were thus infected i.p. with M3LacZ and rested for 2 months, when the majority (together with naïve controls) were challenged i.n. with the mixture of γHVW+BAC–GFP. After a further month, they were treated for 10 days with mAbs to CD4 and CD8 and assayed for the presence of virus. Virus titers in the lung are expressed as PFU (Table 3), whereas the reciprocal LDA frequencies define viral persistence in the spleen (Table 3). Control spleens from i.n. challenged mice that were not immunized with M3LacZ reactivated latent virus in LDA cultures, but lytic virus was only detected in the lungs of those that were also T cell depleted (Table 3, lines 1 and 2). Similarly, the reactivation of “green” BAC–GFP virus in spleen LDA cultures from three of five mice in the unprimed, CD4–8– challenge group (Table 3, line 2) established that the BAC–GFP can establish latency, although with low efficiency when compared to WT γHVW.
The mice given M3LacZ i.p. that were neither T cell-depleted nor challenged reactivated “blue” (X-Gal staining) virus from the spleen, but not the respiratory tract (Table 3, line 3). Similarly, although no virus was found in the lungs of the challenged, un-depleted M3LacZ-immune group, the titers in spleen were increased 10x and 80% of the recovered virus showed the “clear plaque” characteristic of the challenge inoculum (Table 3, line 4). The level of latent infection was 8 times higher again (70% “clear”) when these mice were T cell depleted (Table 3, line 5). Furthermore, “clear” virus was now detected in the lung (Table 3, line 5) at a titer comparable to that found in comparably treated mice that were not primed with M3LacZ (Table 3, line 2). It was also apparent that the persistent M3LacZ is being controlled by an ongoing T cell response. The numbers of spleen cells giving “blue plaques” in the secondarily challenged group increased 10 times in the CD4–8– group (Table 3, compare lines 4 and 5).
The present analysis thus tells us that M3LacZ establishes controlled latency in the lymphoid tissue of these B cell-deficient, Ig–/–, μMT mice (L3–5, Table 3). This had been shown previously for WT γHVW, but not for M3LacZ which, by the infectious center assay that seems mainly to measure infected B cells (38), is only found at a very low level in Ig+/+ mice (ref. 22 and data not shown). The LDA is known to detect persistent γHVW macrophages (27), a likely site of M3LacZ maintenance in the Ig–/– group. The findings for the spleen also show that the M3LacZ-immune mice can be superinfected with WT γHVW (Table 3, lines 4 and 5). In addition, the fact that the lytic virus can be isolated from the lungs of these recovered (then T cell depleted) mice, makes it likely that the secondary γHVW challenge may have led to virus persistence for at least a month in (perhaps) lung macrophages (Table 3, line 5).
Discussion
The failure to induce T cell responses that protect completely against the establishment of latency after superinfection of these M3LacZ-primed Ig–/– mice with a high dose of WT γHV68 is in accord with the results of previous experiments. The massive numbers of CD8+Dbp56+ T cells generated in Ig+/+ mice immunized by a prime/boost regime with recombinant influenza and vaccinia viruses blocked much of the lytic phase in the lung after i.n. challenge with WT γHV68, but the extent of persistence in the spleen was (within 3 weeks) no different from that in unprimed controls (39). Similar findings were recorded for Ig+/+ mice immunized with γHV68 DNA or peptides, including a CD4+ T cell target (40) and an epitope associated with latency (41). In addition, Ig+/+ mice primed with a vaccinia recombinant expressing a γHV68 surface glycoprotein, which would be expected to generate virus-specific Ig and, perhaps, immune CD4+ T cells, also showed evidence of viral persistence after challenge (27). A recent study (29) using Ig+/+ mice infected with a mutant γHV68 that does not express a v-cyclin gene required for virus reactivation supported the idea of Ig-mediated protection against further challenge, although, in the presence of antibody, it was not possible to show a requirement for immune CD8+ T cells and no attempt was made to address the possible role of the CD4+ subset. Only a small amount of virus may need to “sneak through,” as the extent of latency in the long-term seems to be minimally dependent on the inducing dose (42).
The present findings establish that persistent infection with the M3LacZ mutant primes CD4+ and CD8+ T cell memory to the extent that Ig–/– μMT mice are substantially protected against superinfection of the respiratory epithelium with WT γHVW. The degree of immune control is greater than that found in mice that were first infected with WT virus, although it is apparent that the γHVW challenge still becomes established in the lymphoid tissue of the M3LacZ-primed mice. The M3LacZ has been modified by the insertion of LacZ so that it no longer makes the γHV68 M3 chemokine-binding protein (22, 31, 32, 43). The absence of M3 may increase the magnitude of the inflammatory response after γHV68 challenge, but the effect is not large (32). The more complete protection afforded by M3LacZ correlated with the presence of larger numbers of virus-specific CD8+ memory T cells, particularly the CD8+ Kbp79+ population. That result was surprising, as M3LacZ persists at much lower levels than γHVW in conventional Ig+/+ mice and, unlike the WT virus, cannot be detected by infectious center assay (22). The correlation between protection and memory CD8+ T cell numbers does, however, seem to be true also for other viruses (9, 39, 44).
A formal possibility for the difference in the CD8+Dbp56+ and CD8+Kbp79+ lymphocyte counts for the mice primed with WT γHV68 and M3LacZ is that the presence of more latent virus in the γHVCam-infected group is associated with a measure of virus-specific CD8+ T cell depletion (45, 46). However, everything that we know about the interaction between γHV68 persistence and reactivation on the one hand, and CD8+ T cell numbers on the other, would suggest the contrary. Previous studies comparing long-term CD8+ T cell responses for MHC class II+/+ and II–/– mice infected with WT γHV68 indicate that both the CD8+Dbp56+ and CD8+Kbp79+ sets are maintained at greater frequency (and turn over at a higher rate) until the terminal stages of the persistent, lytic infectious process characteristic of the CD4+ T cell-deficient MHC class II–/– animals (14, 47). Postexposure boosting of the MHC class II–/– mice with the p56-vaccinia recombinant massively increased virus-specific CD8+ T cells numbers, but only slightly delayed the onset of lethal disease (48).
Both CD4+ and CD8+ T cell memory contributed to the protective effect in the M3LacZ-primed Ig–/– mice. Other experiments have shown that the recalled, γHV68-specific CD8+ T cells probably operate by cytotoxicity (49). Although it is possible that CD40-dependent (50) CD4+ T help promotes CD8+ CTL function via the intermediate (51, 52) of the activated antigen-presenting cell, it is also clear that CD4+ effectors limit the γHV68 infectious process directly by an IFN γ-dependent mechanism (23). Mice that lack optimally functioning CD4+ T cells as a consequence of disruption of either the CD40 ligand or the MHC class II (I-Ab–/–) glycoprotein show persistent (although reduced) production of lytic virus in the respiratory tract, and may die (I-Ab–/–) after 100 days or so from an acute onset wasting syndrome (20, 53).
The most intriguing aspect of these experiments is thus that a mutant virus (M3LacZ) that lacks a broad-spectrum chemokine binding protein was surprisingly effective at inducing long-term protection and memory. The M3LacZ virus is clearly under continuing T cell-mediated control in these Ig–/– mice. Other experiments with CD28–/– mice indicate that passive antibody transfer limits the reactivation of latent WT γHV68 after such T cell depletion (28). In the Ig–/– mice, the combined effect of the absence of both neutralizing Ig and any inhibitory constraints imposed by the M3 chemokine binding protein (32, 54, 55) on T cell recruitment and localization to the secondary lymphoid tissue could act to enhance a continuing process of low-level stimulation. Whether antibody acts to inhibit the immunepotentiating effect of low-level virus persistence has yet to be analyzed for the γHV68 model.
A further possibility is that the greater response to M3LacZ in the μMT mice could to some extent reflect the presentation of LacZ peptides in antigen-presenting cells that are also expressing γHV epitopes. It could be argued that concurrent LacZ-specific CD4+ T help promotes the γHV68-specific CD8+ T cell response, although it is not obvious why this would be advantageous as CD4+ T cell-mediated immunity to γHV68 is normally substantial (23, 26). Also, neither the size nor the long-term quality of the virus-specific CD8+ T cell populations in CD4 T cell-deficient MHC class II–/– mice are obviously compromised when compared to those in the MHC class II+/+ controls (48, 56, 57).
Treating persistently infected Ig+/+ mice with mAbs to CD4 and CD8 failed to cause detectable viral reactivation (35), whereas the same experiment with these Ig–/– animals greatly increased the numbers of infected cells in the spleen. It seems that virus-specific antibody and CD4+ and CD8+ T cells all play a part in the control of γHV68 infection, although it may be the case that neither cellular nor humoral immunity can prevent superinfection after an aggressive challenge with WT virus (28, 29, 39). Even so, these experiments point to the extreme difficulty of achieving sterilizing, T cell-mediated immunity that confers absolute protection against further virus challenge.
Acknowledgments
We thank Vicki Henderson for help with the manuscript and Gabriela Diaz with technical assistance. These experiments were supported by National Institutes of Health Grant CA21765, Wellcome Trust Grant 059601 (to S.E.), Deutsche Forschungsgemeinschaft Grants Ad 121/2-1 (to H.A.) and SFB 455 (to U.H.K.), and ALSAC. S.A. was supported by American Cancer Society Grant PF-98-162-02-MBC, and P.C.D. is supported by a Burnet Fellowship of the National Health and Medical Research Council (Australia).
Abbreviations: dn, day n; HV, herpesvirus; LDA, limiting diluting analysis; i.n., intranasal(ly); BAC, bacterial artificial chromosome; PFU, plaque-forming unit; BAL, bronchoalveolar lavage; MLN, mediastinal lymph node; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside.
References
- 1.Oehen, S., Waldner, H., Kundig, T. M., Hengartner, H. & Zinkernagel, R. M. (1992) J. Exp. Med. 176, 1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou, S., Hyland, L., Ryan, K. W., Portner, A. & Doherty, P. C. (1994) Nature 369, 652–654. [DOI] [PubMed] [Google Scholar]
- 3.Lau, L. L., Jamieson, B. D., Somasundaram, T. & Ahmed, R. (1994) Nature 369, 648–652.7516038 [Google Scholar]
- 4.Doherty, P. C. (2000) Philos. Trans. R. Soc. London B 355, 361–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murali-Krishna, K., Lau, L. L., Sambhara, S., Lemonnier, F., Altman, J. & Ahmed, R. (1999) Science 286, 1377–1381. [DOI] [PubMed] [Google Scholar]
- 6.Swain, S. L. (2000) Philos. Trans. R. Soc. London B 355, 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinkernagel, R. M. (2002) Curr. Opin. Immunol. 14, 523–536. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, P. C., Allan, W., Boyle, D. B., Coupar, B. E. & Andrew, M. E. (1989) J. Infect. Dis. 159, 1119–1122. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, J. P., Doherty, P. C., Branum, K. C. & Riberdy, J. M. (2000) J. Virol. 74, 11690–11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letvin, N. L., Barouch, D. H. & Montefiori, D. C. (2002) Annu. Rev. Immunol. 20, 73–99. [DOI] [PubMed] [Google Scholar]
- 11.Mueller, S. N., Jones, C. M., Smith, C. M., Heath, W. R. & Carbone, F. R. (2002) J. Exp. Med. 195, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber, D. L., Wherry, E. J. & Ahmed, R. (2003) J. Immunol. 171, 27–31. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, K. J., Riberdy, J. M., Christensen, J. P., Altman, J. D. & Doherty, P. C. (1999) Proc. Natl. Acad. Sci. USA 96, 8597–8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belz, G. T. & Doherty, P. C. (2001) J. Virol. 75, 4435–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein, G. (1994) Cell 77, 791–793. [DOI] [PubMed] [Google Scholar]
- 16.Rickinson, A. B. & Moss, D. J. (1997) Annu. Rev. Immunol. 15, 405–431. [DOI] [PubMed] [Google Scholar]
- 17.Virgin, H. W. & Speck, S. H. (1999) Curr. Opin. Immunol. 11, 371–379. [DOI] [PubMed] [Google Scholar]
- 18.Nash, A. A., Dutia, B. M., Stewart, J. P. & Davison, A. J. (2001) Philos. Trans. R. Soc. London B 356, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doherty, P. C., Christensen, J. P., Belz, G. T., Stevenson, P. G. & Sangster, M. Y. (2001) Philos. Trans. R. Soc. London B 356, 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardin, R. D., Brooks, J. W., Sarawar, S. R. & Doherty, P. C. (1996) J. Exp. Med. 184, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weck, K. E., Kim, S. S., Virgin, H. I. & Speck, S. H. (1999) J. Virol. 73, 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridgeman, A., Stevenson, P. G., Simas, J. P. & Efstathiou, S. (2001) J. Exp. Med. 194, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen, J. P., Cardin, R. D., Branum, K. C. & Doherty, P. C. (1999) Proc. Natl. Acad. Sci. USA 96, 5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seder, R. A. & Ahmed, R. (2003) Nat. Immunol. 4, 835–842. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson, P. G., Belz, G. T., Altman, J. D. & Doherty, P. C. (1999) Eur. J. Immunol. 29, 1059–1067. [DOI] [PubMed] [Google Scholar]
- 26.Christensen, J. P. & Doherty, P. C. (1999) J. Virol. 73, 4279–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart, J. P., Micali, N., Usherwood, E. J., Bonina, L. & Nash, A. A. (1999) Vaccine 17, 152–157. [DOI] [PubMed] [Google Scholar]
- 28.Kim, I. J., Flano, E., Woodland, D. L. & Blackman, M. A. (2002) J. Immunol. 168, 3958–3964. [DOI] [PubMed] [Google Scholar]
- 29.Tibbetts, S. A., McClellan, J. S., Gangappa, S., Speck, S. H. & Virgin, H. W. T. (2003) J. Virol. 77, 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. (1991) Nature 350, 423–426. [DOI] [PubMed] [Google Scholar]
- 31.Parry, C. M., Simas, J. P., Smith, V. P., Stewart, C. A., Minson, A. C., Efstathiou, S. & Alcami, A. (2000) J. Exp. Med. 191, 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Berkel, V., Levine, B., Kapadia, S. B., Goldman, J. E., Speck, S. H. & Virgin, H. W. (2002) J. Clin. Invest. 109, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler, H., Messerle, M., Wagner, M. & Koszinowski, U. H. (2000) J. Virol. 74, 6964–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adler, H., Messerle, M. & Koszinowski, U. H. (2001) J. Virol. 75, 5692–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson, P. G., Cardin, R. D., Christensen, J. P. & Doherty, P. C. (1999) J. Gen. Virol. 80, 477–483. [DOI] [PubMed] [Google Scholar]
- 36.Sprent, J. (1973) Cell Immunol. 7, 10–39. [DOI] [PubMed] [Google Scholar]
- 37.Carding, S. R. & Egan, P. J. (2000) Immunol. Rev. 173, 98–108. [DOI] [PubMed] [Google Scholar]
- 38.Sunil-Chandra, N. P., Efstathiou, S. & Nash, A. A. (1992) J. Gen. Virol. 73, 3275–3279. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson, P. G., Belz, G. T., Castrucci, M. R., Altman, J. D. & Doherty, P. C. (1999) Proc. Natl. Acad. Sci. USA 96, 9281–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, L., Usherwood, E. J., Blackman, M. A. & Woodland, D. L. (1999) J. Virol. 73, 9849–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usherwood, E. J., Ward, K. A., Blackman, M. A., Stewart, J. P. & Woodland, D. L. (2001) J. Virol. 75, 8283–8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tibbetts, S. A., Loh, J., Van Berkel, V., McClellan, J. S., Jacoby, M. A., Kapadia, S. B., Speck, S. H. & Virgin, H. W. T. (2003) J. Virol. 77, 7696–7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Berkel, V., Barrett, J., Tiffany, H. L., Fremont, D. H., Murphy, P. M., McFadden, G., Speck, S. H. & Virgin, H. I. (2000) J. Virol. 74, 6741–6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doherty, P. C. & Christensen, J. P. (2000) Annu. Rev. Immunol. 18, 561–592. [DOI] [PubMed] [Google Scholar]
- 45.Moskophidis, D., Lechner, F., Pircher, H. & Zinkernagel, R. M. (1993) Nature 362, 758–761. [DOI] [PubMed] [Google Scholar]
- 46.Wherry, E. J., Blattman, J. N., Murali-Krishna, K., van der Most, R. & Ahmed, R. (2003) J. Virol. 77, 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson, P. G., Belz, G. T., Altman, J. D. & Doherty, P. C. (1998) Proc. Natl. Acad. Sci. USA 95, 15565–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belz, G. T., Stevenson, P. G., Castrucci, M. R., Altman, J. D. & Doherty, P. C. (2000) Proc. Natl. Acad. Sci. USA 97, 2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Topham, D. J., Cardin, R. C., Christensen, J. P., Brooks, J. W., Belz, G. T. & Doherty, P. C. (2001) J. Gen. Virol. 82, 1971–1981. [DOI] [PubMed] [Google Scholar]
- 50.Sarawar, S. R., Lee, B. J., Reiter, S. K. & Schoenberger, S. P. (2001) Proc. Natl. Acad. Sci. USA 98, 6325–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett, S. R., Carbone, F. R., Karamalis, F., Miller, J. F. & Heath, W. R. (1997) J. Exp. Med. 186, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ridge, J. P., Di Rosa, F. & Matzinger, P. (1998) Nature 393, 474–478. [DOI] [PubMed] [Google Scholar]
- 53.Sangster, M. Y., Topham, D. J., D'Costa, S., Cardin, R. D., Marion, T. N., Myers, L. K. & Doherty, P. C. (2000) J. Immunol. 164, 1820–1828. [DOI] [PubMed] [Google Scholar]
- 54.Rice, J., de Lima, B., Stevenson, F. K. & Stevenson, P. G. (2002) Eur. J. Immunol. 32, 3481–3487. [DOI] [PubMed] [Google Scholar]
- 55.Jensen, K. K., Chen, S. C., Hipkin, R. W., Wiekowski, M. T., Schwarz, M. A., Chou, C. C., Simas, J. P., Alcami, A. & Lira, S. A. (2003) J. Virol. 77, 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu, H., Andreansky, S., Diaz, G., Hogg, T. & Doherty, P. C. (2002) J. Immunol. 168, 3477–3483. [DOI] [PubMed] [Google Scholar]
- 57.Belz, G. T., Liu, H., Andreansky, S., Doherty, P. C. & Stevenson, P. G. (2003) J. Gen. Virol. 84, 337–341. [DOI] [PubMed] [Google Scholar]