Abstract
Our previous studies demonstrated that 17β-estradiol (E2) rapidly induces the interaction of estrogen receptor α (ERα) with the adapter protein Shc, the translocation of ERα to the cell membrane, and the formation of dynamic membrane structures in MCF-7 breast cancer cells. The present study examined how E2 causes ERα to translocate to the region of the plasma membrane and focused on mechanisms whereby Shc and the insulin-like growth factor-1 receptor (IGF-1R) mediate this process. Shc physically interacts with IGF-1R in the plasma membrane, and E2 activates IGF-1R. We reasoned that ERα, when bound to Shc, would be directed to the region of the plasma membrane by the same processes, causing membrane translocation of Shc. We confirmed that E2 rapidly induced IGF-1R phosphorylation and demonstrated that E2 induced formation of a ternary protein complex among Shc, ERα, and IGF-1R. Knock down of Shc with a specific small inhibitory RNA decreased the association of ERα with IGF-1R by 87%, suggesting that Shc is a crucial molecule in the formation of this ternary complex. Confocal microscopy studies provided further confirmation of the functional roles of Shc and the IGF-1R in the translocation of ERα to the region of the membrane. Down-regulation of Shc, ERα, or IGF-1R with specific small inhibitory RNAs all blocked E2-induced mitogen-activated protein kinase phosphorylation. Together, our results demonstrate that Shc and IGF-1R serve as key elements in the translocation of ERα to the cell membrane and in the facilitation of ERα-mediated rapid E2 action.
Estradiol-17β (E2) plays a prominent role in mediating the maturation, proliferation, and differentiation of the mammary gland and influences the growth and development of breast cancer (1). The biological effects of E2 are mediated through the estrogen receptor (ER) acting via classical genomic events in the nucleus and by rapid nongenomic actions at the plasma membrane. Mounting evidence suggests that the nongenomic actions of E2 involve classical receptors acting at the level of the plasma membrane (2, 3). Although debated previously, current evidence favors the concept that the plasma membrane ER is the same as that locating in the nucleus, but that the membrane pool is present in much lower concentrations (4-6). Using antibodies directed against different epitopes of nuclear ERα, many laboratories, including ours, have demonstrated that the classical ERα can locate in the region of the cell membrane (7-9). However, unlike many growth factor receptors, ERα has no intrinsic transmembrane domain, raising the question how it can translocate to the plasma membrane. As a potential mechanism, we postulated that ERα might bind to Shc, which itself is translocated to the plasma membrane.
Shc binds to docking sites on many growth factor receptors in the plasma membrane, including epidermal growth factor (10), nerve growth factor (11), platelet-derived growth factor (10), and insulin-like growth factor (IGF) receptors (12). The recruitment of Shc is linked to the intracellular domains of these various activated membrane receptors. When these docking sites are activated, they recruit Shc and in turn Grb-2, SOS, RAS, RAF, and mitogen-activated protein kinase (MAPK), leading to MAPK pathway activation. We postulated that the IGF-1 receptor (IGF-1R) might be a major site for the docking of a Shc/ERα complex based on the following evidence: (i) E2 stimulates the rapid activation of the IGF-1R through its phosphorylation; (ii) ERα and IGF-1R are coexpressed, and the levels of the receptors are positively correlated in many breast cancer cells (13); (iii) crosstalk between the two receptor-mediated signaling pathways occurs and is strongly linked to the synergistic induction of cell proliferation and antiapoptosis of breast cancer cells (14); and (iv) E2 induces binding of ERα to Shc (9). Taking these data into consideration, we investigated whether Shc acts as a translocator carrying ERα to the region of the plasma membrane and binding to the transmembrane receptor IGF-1R.
Materials and Methods
Cell Culture, Small Inhibitory RNA (siRNA) Transfection, and Immunoblotting. Detailed description of the reagents, tissue culture methods, and immunoblotting techniques has been described in detail (9) and is provided in detail in Supporting Text, which is published as supporting information on the PNAS web site. For the siRNA studies, a smart pool of double-stranded siRNA against Shc (P-002031-01-20), ERα (ESR1-NM-00-05), or IGF-1R (IGF1R-NM-000875) as well as nonspecific siRNA (D-001206-01-05) were obtained from Dharmacon Tech (Lafayette, CO) and used according to the manufacturer's instructions (see Supporting Materials and Methods). For the study of protein-protein interactions, the reagents and methods to detect Shc and ERα were identical to those previously described (9). IGF-1R phosphorylation and detection were carried out by immunoprecipitation of IGF-1R by using anti-IGF-1R monoclonal antibody (3B7, Santa Cruz Biotechnology) and detection of its phosphorylation status and protein expression by using antiphosphotyrosine antibody (4G10, Upstate Biotechnology, Lake Placid, NY) and anti-IGF-1R β domain antibody (C-20, Santa Cruz Biotechnology), respectively.
Confocal Analysis of Colocalization of ERα, Shc, and IGF-1R on Cell Membrane. Detailed methods regarding confocal microscopic studies were previously described in detail and are provided in Supporting Materials and Methods in Supporting Text (15). Primary antibodies used were: rabbit anti-ERα (H184, Santa Cruz Biotechnology sc-7207 diluted 1:100), mouse anti-Shc (PG-797, Santa Cruz Biotechnology sc-967 diluted 1:200), mouse anti-IGF-1R (3B7, Santa Cruz Biotechnology sc-967 diluted 1:200), and mouse antivinculin (hVIN-1, Sigma V-9131 diluted 1:200). Anti-mouse and anti-rabbit second antibodies conjugated to the fluorescent dyes Alexa 488 (green) or Alexa 633 (far red, colored blue) and phalloidin conjugated to Alexa 546 (red) were purchased from Molecular Probes. Colocalization of ERα, Shc, and IGF-1R on the cell membrane was demonstrated by the development of white color due to the overlapping of green, blue, and red pixels.
Statistical Analysis. All reported values are the means ± SEM. Statistical comparisons were determined with two-tailed Student's t tests. Results were considered statistically significant if the P value was <0.05.
Results
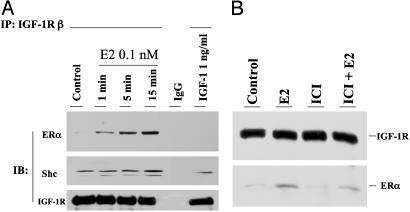
E2 Stimulates Formation of a Ternary Complex Involving Shc, ERα, and IGF-1R. We previously demonstrated that E2 induces the physical association of Shc with ERα in MCF-7 cells (9). Shc, in turn, binds to the phosphorylated tyrosin residues of IGF-1R (16). From these observations, we postulated that E2-induced ERα membrane translocation might be due to an increased Shc association with membrane receptor IGF-1R. We sought to identify this ternary protein complex by immunoprecipitation of IGF-1R and detection of coimmunoprecipitated Shc and ERα in MCF-7 cells treated with or without E2. Under basal conditions, Shc, but not ERα, associated with IGF-1R (Fig. 1A). E2 increased both ERα and Shc association with IGF-1R in a time-dependent fashion at the physiologic concentration of 0.1 nM. In contrast, IGF-1 at the dose used (1 ng/ml) had little effect on the association of ERα with IGF-1R. We further confirmed the formation of the ternary protein complex by using a reciprocal order of immunoprecipitation of Shc and then detection of both IGF-1R and ERα on Western blot (Fig. 6, which is published as supporting information on the PNAS web site). This ERα/Shc/IGF-1R complex is sensitive to disruption by the antiestrogen, ICI 182,780 (ICI), providing additional evidence that activated ERα plays an initiating role in the complex formation (Fig. 1B).
Fig. 1.
(A) E2-induced protein complex formation among ERα, Shc, and IGF-1R. MCF-7 cells were treated with vehicle, 1 ng/ml IGF-1, or E2 at 0.1 nM for the times indicated. Lysates were immunoprecipitated with IGF-1R antibody. The nonspecific monoclonal antibody (IgG) served as a negative control. (B) The effect of ICI on E2-induced protein-protein interaction was assayed by immunoprecipitation of IGF-1R and detection of ERα on Western blot.
Activation of IGF-1R Is Involved in E2 Rapid Action in MCF-7 Cells. The time-dependent effects of E2 on IGF-1R phosphorylation were tested by using cell extracts prepared at various times after treatment. E2 stimulated IGF-1R phosphorylation as early as 3 min with the maximum effect at 15 min (Fig. 7A, which is published as supporting information on the PNAS web site). As a positive control, IGF-1 at 1 ng/ml also strongly stimulated IGF-1R phosphorylation in MCF-7 cells. To further study the effects of other signaling molecules on E2-induced IGF-1R activation, we used a series of selective inhibitors, AG1024 for IGF-1R phosphorylation (17), ICI for ERα, and PP2 for Src, respectively. As shown in Fig. 7B, which is published as supporting information on the PNAS web site, E2 greatly increased IGF-1R phosphorylation, which was blocked by all three inhibitors, indicating that ERα and c-Src are upstream components of IGF-1R activation in E2 rapid action. Taken together, our results demonstrated that IGF-1R is rapidly activated by E2 treatment in MCF-7 cells, a process regulated by ERα and c-Src.
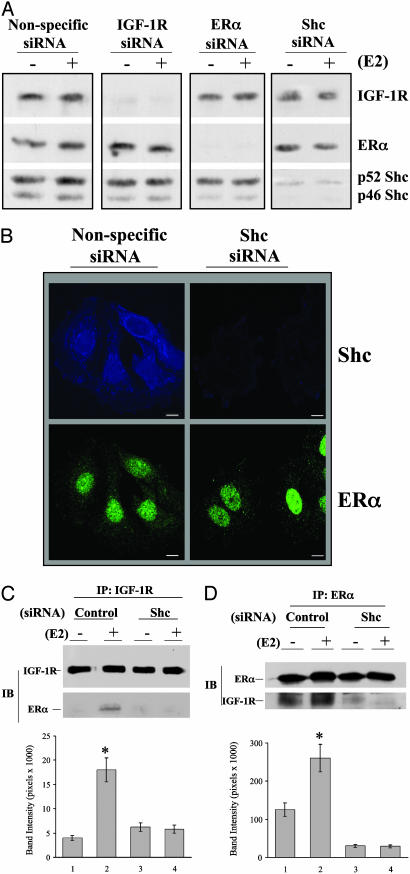
Protein Silencing and the Role of Shc in the Ternary Complex Formation. We determined whether the physical association among Shc, ERα, and IGF-1R was necessary for E2 to exert functional effects and specifically knocked down each component with siRNA technology. When cells were transfected with the siRNA directed against IGF-1R, the levels of this protein were decreased 24-48 h after transfection and reached <5-10% when compared with levels of control proteins (Fig. 2A). The siRNA against ERα exerted similar specific effects. When MCF-7 cells were transfected with the siRNA against Shc, the levels of both p52 and p46 isoforms were equally decreased, suggesting that both p52 and p46 mRNA of Shc were equally targeted by the siRNA. Decreased Shc protein expression was documented only in the cells expressing siRNA against Shc but not in cells expressing siRNA against either IGF-1R or ERα, demonstrating the specificity of the siRNA. This was also the case for siRNAs against IGF-1R or ERα. We then used confocal microscopy techniques to demonstrate specificity of knock down of Shc on ERα expression in two-color immunofluorescence staining image (Fig. 2B). The result clearly shows that siRNA against Shc only decreased Shc protein level without altering the level of ERα expression, as indicated by nuclear ERα staining. To further confirm the specificity of the siRNAs, we probed the polyvinylidene difluoride membrane with two unrelated proteins, insulin receptor substrate 1 and vitamin D receptor, and found no decrease in their levels (Fig. 8, which is published as supporting information on the PNAS web site). Taken together, these experiments demonstrated that siRNAs against IGF-1R, ERα, and Shc were specific and effectively down-regulated the selective target proteins in MCF-7 cells.
Fig. 2.
Protein silencing and the role of Shc in protein complex formation in MCF-7 cells. (A) Cells were transfected with nonspecific or specific siRNA for 2 days and then treated with vehicle or 0.1 nM E2 for 15 min for additional MAPK phosphorylation assay. Polyvinylidene difluoride membranes were probed with the specific antibodies to detect the protein expression of IGF-1R, ERα, and Shc. (B) Confocal microscopy study of siRNA expression in MCF-7 cells. Cells, transfected with or without siRNA against Shc, were grown on cover slips and then subjected to immunofluorescence staining for Shc (blue) and ERα (green), as described in Materials and Methods. (C and D) The role of Shc in mediating the interaction of ERα and IGF-1R. Cells transfected with siRNA as indicated were treated with vehicle or E2 at 0.1 nM. The IGF-1R and ERα interaction was assayed by immunoprecipitation of IGF-1R and detection of ERα on Western blot (C) or by immunoprecipitation of ERα and detection of IGF-1R on Western blot (D). The data are from three experiments combined, and only one experiment is shown. *, P < 0.05 comparison of E2-treated cells with vehicle control.
We next tested whether Shc is required for the physical interaction between IGF-1R and ERα. MCF-7 cells were transfected with or without specific siRNAs against Shc for 2 days, followed by treatment with vehicle or E2. The interaction of ERα with IGF-1R was first assayed by immunoprecipitation of IGF-1R and detection of ERα on Western blot (Fig. 2C). Expression of the nonspecific siRNA did not block E2-induced enhancement of ERα/IGF-1R association. In marked contrast, expression of the siRNA against Shc blocked this association by 87%. To confirm this observation, a reciprocal order of antibodies was used. Fig. 2D shows that IGF-1R is coimmunoprecipitated with ERα, which was increased by E2 treatment in control siRNA transfected cells. Down-regulation of Shc blocked both basal level as well as E2-induced ERα/IGF-1R interaction. Together, our data demonstrated that Shc plays an important role in MCF-7 breast cancer cells by linking ERα to the membrane receptor IGF-1R.
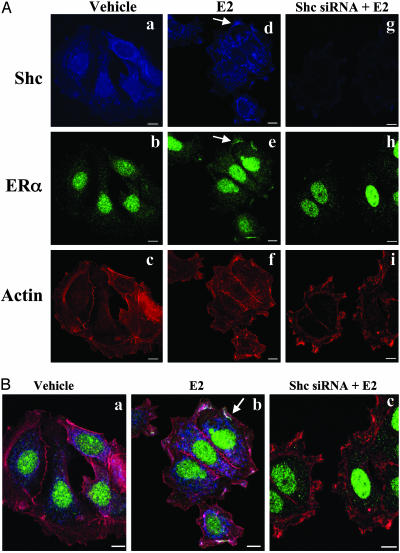
Functional Role of Shc in Mediating the Membrane Effects of ERα We focused on the colocalization of Shc and ERα in response to E2 in the cells with or without Shc siRNA transfection. Fig. 3A represents nonmerged individual stained images of Shc, ERα, and actin. Fig. 3 Left, Center, and Right are images of vehicle-treated cells, E2-treated cells, and cells treated by E2 after Shc down-regulation by siRNA, respectively. Under basal conditions, Shc is mainly expressed in the cytoplasm (Fig. 3Aa) and ERα mainly in the nucleus with minimal scattered staining in the cytoplasm (Fig. 3Ab). In marked contrast, E2 rapidly translocates Shc to the region of the plasma membrane as the arrow pointed (Fig. 3Ad). E2 appeared also to translocate ERα into the region of the plasma membrane (Fig. 3Ae). As further evidence of the efficacy of the siRNA against Shc to knock down this adaptor protein, the blue staining representing Shc decreased by 90% (Fig. 3Ag). Shc knock down strikingly abrogated the ability of E2 to translocate ERα to the region of the plasma membrane. This did not result from down-regulation of ERα itself, as evidenced by the persistence of nuclear ERα staining (Fig. 3Ah).
Fig. 3.
Shc involvement in E2-induced ERα membrane association in MCF-7 cells. Cells were transfected with or without siRNA directed against Shc. Two days later, cells were treated with vehicle or E2 (0.1 nM) for 15 min and then subjected to immunofluorescence staining with anti-Shc anti-ERα antibodies. Actin was stained with phalloidin, indicating filamentous cell membrane. (A) The nonmerged images show Shc in blue, ERα in green, and cell membrane in red. (Left) The vehicle-treated cells stained with Shc, ERα, and actin. (Center and Right) Estrogen-treated cells with or without siRNA of Shc expression. (B) Colocalization of ERα, Shc, and actin on cell membrane is shown in three color-merged images. To highlight the specific changes, the arrows illustrate one example of the effects in single cells. Close inspection reveals multiple changes not marked by arrows.
To provide further evidence of protein colocalization and cell morphologic changes in response to E2, we used a three-color merged image technique (Fig. 3B). Under basal conditions (Fig. 3Ba), cells show fewer ruffles on the membrane. Much of the Shc staining was actually cytoplasmic rather than membranous, as indicated by the blue color. ERα was seen primarily in the nucleus but also expressed in cytoplasm. E2 treatment stimulated Shc and ERα membrane region association where they colocalized along the cell membrane, as indicated by the white color development because of the overlapping of blue/green/red colors (Fig. 3Bb). In many instances, ERα seemed to be collected specifically on the upper surface of extending filopodia rather than on the more basal extending edge. Expression of siRNA directed against Shc greatly reduced Shc protein expression (Fig. 3Bc), which also abolished ERα membrane association induced by E2. A striking finding was rapid induction of E2 induced cell morphologic changes, such as ruffles and filopodia as shown in Fig. 3Bb. Blockade of ERα membrane association by down-regulation of Shc did not abrogate the E2-stimulated cell morphologic changes (Fig. 3Bc), indicating that the morphologic changes are not regulated by the ERα/Shc membrane association. Collectively, these results demonstrated that Shc as a molecule mediates ERα rapid membrane association, and this ERα/Shc membrane association is not involved in E2-induced specific changes on cell morphology.
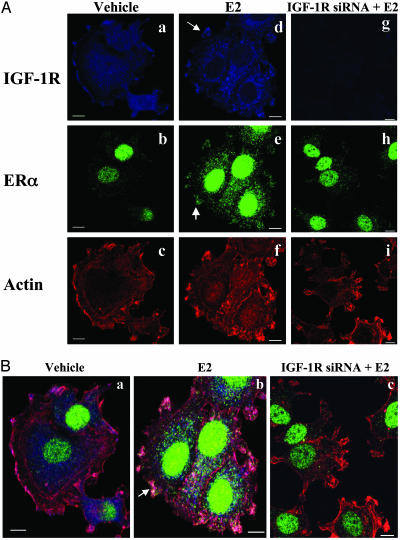
IGF-1R Is a Membrane Protein for Shc/ERα Binding. To test whether IGF-1R is a docking protein on the cell membrane for ERα/Shc association, a confocal microscopy experiment was performed by immunostaining of IGF-1R (blue), ERα (green), and phalloidin (blue). IGF-1R is expressed focally along the cell membrane (Fig. 4A) as well as in the cytoplasm (Fig. 4Aa). E2 treatment had little effect on IGF-1R distribution (Fig. 4Ad) but induced the ERα membrane translocation (arrow in Fig. 4Ae). Expression of IGF-1R siRNA greatly down-regulated IGF-1R protein expression in the cells (Fig. 4Ag), as indicated by decreased blue color. Down-regulation of IGF-1R clearly blocked ERα membrane translocation in the cells (Fig. 4 Ah and Bc), confirming the role of IGF-1R on ERα membrane association. E2 rapidly induced cell motile structure formation (Fig. 4Ab), compared with vehicle treatment (Fig. 4Ba). At the same time, down-regulation of IGF-1R had little effect on the E2-induced cell morphologic changes (Fig. 4Bc). Together, our data demonstrated that both Shc and IGF-1R are involved in ERα membrane association in MCF-7 cells but not in E2 action on cell motile structure development.
Fig. 4.
IGF-1R involvement in E2-induced ERα membrane association in MCF-7 cells. Cells cultured on coverslips were transfected with or without siRNA against IGF-1R for 2 days and then treated with vehicle or E2 at 0.1 nM for 15 min. After fixation, cells were immunofluorescently stained with anti-IGF-1R (blue) anti-ERα (green) antibodies. Actin was stained with phalloidin (red), indicating filamentous cell membrane. Both nonmerged (A) and merged (B) images are shown.
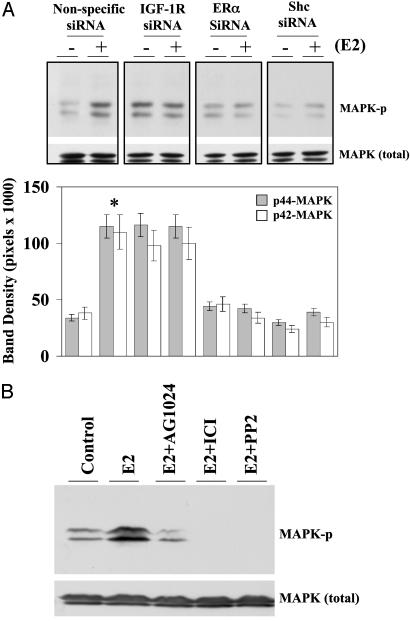
ERα Membrane Association Is Directly Correlated to E2-Induced MAPK Phosphorylation. We examined the effect of E2 on MAPK phosphorylation in cells in which Shc, ERα, or IGF-1R was selectively knocked down. The rapid induction of MAPK activation served as the biologic endpoint reflecting functionality. As shown in Fig. 5A, treatment with physiologic concentrations of E2 at 0.1 nM resulted in a 3.4-fold increase of MAPK phosphorylation in control siRNA transfected cells. The siRNAs against IGF-1R, ERα, or Shc specifically blocked E2-induced MAPK phosphorylation, indicating the involvement of IGF-1R, Shc, and ERα in E2 action on MAPK. Elevated basal MAPK phosphorylation was seen in the cells expressing siRNA against IGF-1R. To further confirm the involvement of IGF-1R on E2-induced MAPK activation, a specific IGF-1R tyrosine kinase inhibitor AG1024 was tested along with other inhibitors. Fig. 5B shows that E2 greatly increased MAPK phosphorylation, which was blocked by AG1024, ICI, and PP2, indicating that IGF-1R, ERα, and c-Src are all upstream components regulating MAPK in E2 rapid action. AG 1024 also blocked cell proliferation as biologic evidence for the role of IGF-1R in E2-induced cell proliferation (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 5.
Evidence of the involvement of ERα, Shc, and IGF-1R in E2-induced MAPK phosphorylation. (A) Down-regulation of ERα, Shc, and IGF-1R blocked E2-induced MAPK phosphorylation. Cells were transfected with specific and nonspecific siRNAs. At day 2, cells were treated with vehicle or E2 at 0.1 nM for 15 min, and then whole-cell extracts were prepared and analyzed by Western blotting as described in Materials and Methods. Polyvinylidene difluoride membranes were probed with antidual phosphotyrosine MAPK antibody. *, P < 0.05 comparison of E2-treated cells with the vehicle-treated control in each siRNA transfected group. (B) Selective pathway inhibitors on E2-induced MAPK phosphorylation. Cells were challenged with vehicle or 0.1 nM E2 for 15 min in the presence of the inhibitors, as indicated.
Discussion
ERα mediates the effects of E2 at the level of the nucleus through classic genomic actions on transcription and additionally initiates rapid nongenomic events via membrane-associated interactions. The present study examined the mechanism by which the ERα can translocate to the region on or near the plasma membrane. We postulated that the adaptor protein Shc serves as the translocator by binding to ERα and then carrying this receptor to Shc-binding sites of IGF-1R located on the cell membrane. Thus ERα translocates to the plasma membrane region via the same mechanisms that allow Shc to locate there. Our previous studies demonstrated that E2 induced direct interaction of ERα with Shc and caused translocation of ERα to the region of the plasma membrane (9). Our current findings provide several lines of evidence to support our hypothesis regarding ERα/Shc/IGF-1R interactions leading to ERα membrane association. We demonstrated that Shc and IGF-1R are strictly required for translocation of ERα to the membrane region and for E2 induction of MAPK activation. Immunoprecipitation and confocal localization studies demonstrated that E2 causes the formation of ternary protein complexes involving Shc, ERα, and the IGF-1R. More importantly, selective knock down of Shc or IGF-1R with siRNA technology or use of the antiestrogen, ICI, disrupts the ternary complexes. Knock down of Shc abrogates the ability of E2 to translocate ERα to the membrane region and to activate MAPK. As evidence of biologic function, an inhibitor of IGF-1R phosphorylation blocks E2-induced cell proliferation (Fig. 9). Taken together, our findings demonstrate a previously undescribed function of Shc that links ERα to the region of the cell membrane by binding to IFG-1R.
Members of the steroid hormone family classically act through transcriptional regulation at the nuclear level (18). However, increasing evidence suggests that all of these hormones also exert rapid membrane-mediated effects on their target cells (2, 19-22). Controversy exists regarding the identity of the membrane receptor proteins and specifically whether they are classical receptors or other binding proteins (6, 8, 9) Down-regulation of ERα by the selective siRNA in the present study clearly demonstrates the role of ERα itself in mediating the effects of E2 on MAPK activation. Li et al. (23) have described a role for truncated forms of ERα on the membrane, as reviewed in detail.
Transmembrane receptors for growth factors, such as IGF-1 and epidermal growth factor receptor (EGFR), are believed to be integral components in the growth response to E2, but the specific steps are unclear. The tyrosine phosphorylation and activation of IGF-1R induced by E2 were reported in uterine epithelial cells and ER-transfected COS-7 cells (24-26). Previously, we demonstrated strong interaction between ERα and Shc through N-terminal ERα and PTB/SH2 domains of Shc in MCF-7 cells (9). Shc is a well-defined molecule, which mediates growth factor mitogenic actions by activation of the MAPK pathway (27). Activation of IGF-1R will recruit Shc binding on the receptors (28). Regarding IGF-1R, it is noteworthy that a large amount of evidence pointed out that Y950 of IGF-1R is a major site for Shc binding, phosphorylation, and activation in response to IGF-1 (16, 29). IGF-1R functions as a membrane-docking site that recruits the adapter protein Shc after the receptor is activated. Nevertheless, more complicated mechanisms exist for the involvement of IGF-1R, because phosphorylated IGF-1R also recruits the p85 regulatory domain of phosphatidylinositol 3-kinase binding to the receptor. This domain has been reported to physically associate with ERα in human vascular endothelial cells and MCF-7 cells (30, 31). This might explain our biochemical findings that down-regulation of Shc decreased the level of ERα associated with IGF-1R but did not completely eliminate ERα and IGF-1R interaction.
EGFR, in a fashion analogous to IGF-1R, is also a transmembrane tyrosine kinase receptor that has been engaged in different processes critical for cell proliferation, survival, and tumor invasion. E2 has been reported to activate MAPK by activation of matrix metalloproteinases-2 and -9, heparin-binding EGF release, and activation of EGFR (7, 32). The interaction between the signaling pathways of ERα and the EGFR is known to contribute to the biological effects of E2. Experiments show that EGF antibody prevents E2-induced vaginal and uterine growth (33), implying that crosstalk from ERα to the EGFR at the membrane may be physiologically important. The EGFR signaling pathway is important in the development of reproductive tissues (34, 35). The requirement for EGFR on E2-induced ERα-membrane association is currently under investigation in our laboratory.
The present study demonstrated a dramatic dissociation between the requirement of Shc for MAPK activation and for dynamic membrane changes. Specifically, knock down of Shc blocked MAPK activation by E2 but not dynamic membrane changes (Figs. 3Bc and 4Bc). Our working hypothesis to explain this dichotomy of effects is that an ERα/Shc/IGF-1R complex in the plasma membrane may be needed to activate MAPK. On the other hand, the presence of ERα in the cytoplasm contiguous with dynamic membrane changes may be sufficient to induce dynamic membrane changes. Based on our findings, we propose a model for E2-induced ERα membrane association and its function on MAPK activation (Fig. 10, which is published as supporting information on the PNAS web site). In this model, liganded ERα interacts with Shc, leading to Src activation; Src in turn phosphorylates IGF-1R, which recruits Shc; and Shc acts as a translocator to bring ERα to the cytoplasmic membrane. Association of Shc with membrane IGF-1R serves two functions; one is to lead to ERα membrane association, and the other is to activate MAPK in IGF-1R-dependent manner. An extensive discussion is available as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Drs. Margaret A. Shupnik and Raghavendra Mirmira for critical reading of the manuscript and helpful discussions. This research was supported by U.S. Department of Defense Grant DAMD 17-02-1-0610 (to R.X.S.) and National Institutes of Health Grants CA65622 (to R.J.S.) and CA98823 (to R.K.).
Abbreviations: E2, 17β-estradiol; EGFR, epidermal growth factor receptor; ERα, estrogen receptor α; IGF-1R, insulin-like growth factor 1 receptor; MAPK, mitogen-activated protein kinase; siRNA, small inhibitory RNA; ICI, ICI 182,780.
References
- 1.Yager, J. D. (2000) J. Natl. Cancer Inst. Monogr. 27, 67-73. [DOI] [PubMed] [Google Scholar]
- 2.Pietras, R. J., Nemere, I. & Szego, C. M. (2001) Endocrine 14, 417-427. [DOI] [PubMed] [Google Scholar]
- 3.Ho, K. J. & Liao, J. K. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 1952-1961. [DOI] [PubMed] [Google Scholar]
- 4.Collins, P. & Webb, C. (1999) Nat. Med. 5, 1130-1131. [DOI] [PubMed] [Google Scholar]
- 5.Watson, C. S., Campbell, C. H. & Gametchu, B. (1999) Exp. Physiol. 84, 1013-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson, C. S., Norfleet, A. M., Pappas, T. C. & Gametchu, B. (1999) Steroids 64, 5-13. [DOI] [PubMed] [Google Scholar]
- 7.Razandi, M., Pedram, A., Park, S. T. & Levin, E. R. (2003) J. Biol. Chem. 278, 2701-2712. [DOI] [PubMed] [Google Scholar]
- 8.Simoncini, T., Rabkin, E. & Liao, J. K. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 198-203. [DOI] [PubMed] [Google Scholar]
- 9.Song, R. X., McPherson, R. A., Adam, L., Bao, Y., Shupnik, M., Kumar, R. & Santen, R. J. (2002) Mol. Endocrinol. 16, 116-127. [DOI] [PubMed] [Google Scholar]
- 10.Pelicci, G., Lanfrancone, L., Salcini, A. E., Romano, A., Mele, S., Grazia, B. M., Segatto, O., Di Fiore, P. P. & Pelicci, P. G. (1995) Oncogene 11, 899-907. [PubMed] [Google Scholar]
- 11.Dikic, I., Batzer, A. G., Blaikie, P., Obermeier, A., Ullrich, A., Schlessinger, J. & Margolis, B. (1995) J. Biol. Chem. 270, 15125-15129. [DOI] [PubMed] [Google Scholar]
- 12.Ishihara, H., Sasaoka, T., Wada, T., Ishiki, M., Haruta, T., Usui, I., Iwata, M., Takano, A., Uno, T., Ueno, E., et al. (1998) Biochem. Biophys. Res. Commun. 252, 139-144. [DOI] [PubMed] [Google Scholar]
- 13.Bartucci, M., Morelli, C., Mauro, L., Andó, S. & Surmacz, E. (2001) Cancer Res. 61, 6747-6754. [PubMed] [Google Scholar]
- 14.Surmacz, E. & Burgaud, J. L. (1995) Clin. Cancer Res. 1, 1429-1436. [PubMed] [Google Scholar]
- 15.Adam, L., Vadlamudi, R., Kondapaka, S. B., Chernoff, J., Mendelsohn, J. & Kumar, R. (1998) J. Biol. Chem. 273, 28238-28246. [DOI] [PubMed] [Google Scholar]
- 16.Dews, M., Prisco, M., Peruzzi, F., Romano, G., Morrione, A. & Baserga, R. (2000) Endocrinology 141, 1289-1300. [DOI] [PubMed] [Google Scholar]
- 17.Parrizas, M., Gazit, A., Levitzki, A., Wertheimer, E. & LeRoith, D. (1997) Endocrinology 138, 1427-1433. [DOI] [PubMed] [Google Scholar]
- 18.Jensen, E. V. & Jordan, V. C. (2003) Clin. Cancer Res. 9, 1980-1989. [PubMed] [Google Scholar]
- 19.Kousteni, S., Chen, J. R., Bellido, T., Han, L., Ali, A. A., O'Brien, C. A., Plotkin, L., Fu, Q., Mancino, A. T., Wen, Y., et al. (2002) Science 298, 843-846. [DOI] [PubMed] [Google Scholar]
- 20.Zhu, Y., Bond, J. & Thomas, P. (2003) Proc. Natl. Acad. Sci. USA 100, 2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanker, K. S., Prokscha, G. W. & Blumel, G. (1981) J. Cancer Res. Clin. Oncol. 100, 135-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mesbah, M., Nemere, I., Papagerakis, P., Nefussi, J. R., Orestes-Cardoso, S., Nessmann, C. & Berdal, A. (2002) J. Bone Miner. Res. 17, 1588-1596. [DOI] [PubMed] [Google Scholar]
- 23.Li, L., Haynes, M. P. & Bender, J. R. (2003) Proc. Natl. Acad. Sci. USA 100, 4807-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards, R. G., DiAugustine, R. P., Petrusz, P., Clark, G. C. & Sebastian, J. (1996) Proc. Natl. Acad. Sci. USA 93, 12002-12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards, R. G., Walker, M. P., Sebastian, J. & DiAugustine, R. P. (1998) J. Biol. Chem. 273, 11962-11969. [DOI] [PubMed] [Google Scholar]
- 26.Kahlert, S., Nuedling, S., van Eickels, M., Vetter, H., Meyer, R. & Grohe, C. (2000) J. Biol. Chem. 275, 18447-18453. [DOI] [PubMed] [Google Scholar]
- 27.Lennartsson, J., Blume-Jensen, P., Hermanson, M., Ponten, E., Carlberg, M. & Ronnstrand, L. (1999) Oncogene 18, 5546-5553. [DOI] [PubMed] [Google Scholar]
- 28.Adams, T. E., Epa, V. C., Garrett, T. P. & Ward, C. W. (2000) Cell. Mol. Life Sci. 57, 1050-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valentinis, B. & Baserga, R. (2001) Mol. Pathol. 54, 133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simoncini, T., Hafezi-Moghadam, A., Brazil, D. P., Ley, K., Chin, W. W. & Liao, J. K. (2000) Nature 407, 538-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castoria, G., Migliaccio, A., Bilancio, A., Di Domenico, M., de Falco, A., Lombardi, M., Fiorentino, R., Varricchio, L., Barone, M. V. & Auricchio, F. (2001) EMBO J. 20, 6050-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filardo, E. J., Quinn, J. A., Bland, K. I. & Frackelton, A. R., Jr. (2000) Mol. Endocrinol. 14, 1649-1660. [DOI] [PubMed] [Google Scholar]
- 33.Nelson, K. G., Takahashi, T., Bossert, N. L., Walmer, D. K. & McLachlan, J. A. (1991) Proc. Natl. Acad. Sci. USA 88, 21-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hom, Y. K., Young, P., Wiesen, J. F., Miettinen, P. J., Derynck, R., Werb, Z. & Cunha, G. R. (1998) Endocrinology 139, 913-921. [DOI] [PubMed] [Google Scholar]
- 35.Wiesen, J. F., Young, P., Werb, Z. & Cunha, G. R. (1999) Development (Cambridge, U.K.) 126, 335-344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.