Abstract
Evidence suggests that the consequences of chronic exposure to stressors extend beyond psychological effects, and that adolescents living in socio-economically disadvantaged neighborhoods may experience an accumulation of exposure to stressors that wears down the physical systems in the body, resulting in hyper-activation of the stress response. This research examines the relationship between exposure to neighborhood stressors and salivary cortisol reactivity in a sample of 163 at-risk African American adolescents (average age 21; 50% female) living in disadvantaged urban neighborhoods. More specifically, the relationship between neighborhood stressors and physiological stress, measured by baseline cortisol and cortisol reactivity is assessed. This research also examines several moderating pathways between exposure to neighborhood disadvantage and cortisol reactivity including substance use, high effort coping, psychological stress and social support. Results indicate that both individual and neighborhood-level factors influence adolescent cortisol. High effort coping and psychological stress were associated with cortisol in the sample, and exposure to neighborhood socio-economic disadvantage resulted in an atypical cortisol response. In addition, neighborhood disadvantage interacted with intra- and interpersonal factors to affect cortisol indirectly. Thus, living in disadvantaged neighborhoods may take a psychological and physiological toll on adolescents, and it also may exert synergistic effects through individual coping and vulnerabilities.
Keywords: youth, neighborhood disadvantage, cortisol, reactivity, stress, risk, protection
Introduction
Adolescents may be exposed to multiple stressors as they transition into emerging adulthood, encountering new responsibilities while interacting with different school, family, work and neighborhood environments (Arnett, 2000; Foster, Hagan, & Brooks-Gunn, 2008). Chronic and cumulative exposure to stressors is associated with psychological stress, poor mental health, and physiological consequences during adolescence (Jackson, Knight, & Rafferty, 2009; Schmeelk-Cone & Zimmerman, 2003), and exposure to stressors during adolescence contributes to poor health outcomes in adulthood (Evans, Kim, Ting, Tesher, & Shannis, 2007). Compared to Whites, African Americans experience more frequent chronic and acute stressors, stressful life events, and trauma, after controlling for their socioeconomic status (Kessler & Neighbors, 1986; Turner & Lloyd, 1995). Researchers suggest that unequal exposure to stressors based on socioeconomic status, race, and ethnicity, may partially explain population patterns of inequities in health and well-being (George & Lynch, 2003; Turner & Lloyd, 1995). Research that contributes to our understanding of exposure to stressors and stress during critical developmental periods, may also contribute to an eventual reduction in health disparities.
While adolescents and young adults are exposed to stressors across multiple contexts, exposure to stressors in the neighborhood may be a particularly important area for research and intervention. Based on socio-ecological models, neighborhoods exert influences on the peer, family and individual levels, and thus have overlapping and complex influences on adolescent health and behavior (Brooks-Gunn, Duncan, Klevanov, & Sealand, 1993). Adolescents from lower socioeconomic status (SES) families, and who live in disadvantaged neighborhoods, are more likely to be exposed to episodic and chronic stressors (Latkin & Curry, 2003; Steptoe & Feldman, 2001). African American youth and young adults may be at highest risk for exposure to stressors because they are represented disproportionately in the most disadvantaged neighborhoods (Browning & Cagney, 2003; Massey, 2004), which in turn puts them at greater risk for exposure to violence, crime and neighborhood social disorder (Brooks-Gunn, Duncan, & Aber, 1997; Browning & Cagney, 2003). The neighborhood context – which reflects larger societal structures including racism, discrimination, residential segregation and concentrated poverty, as well as adolescents’ access to economic resources and opportunity, safety and social support – may help researchers better understand how socio-cultural and economic factors get under the skin during childhood and adolescence, and result in health inequalities later in life.
Neighborhoods and exposure to stressors
One avenue through which neighborhoods may influence health is through a stress pathway. Adolescents and young adults living in disadvantaged neighborhoods may encounter more frequent, severe, and sustained stressful events than those living in more affluent neighborhoods (Latkin & Curry, 2003). They also may experience this exposure over many years of development (i.e., cumulative exposure), as mobility from disadvantaged to more advantaged neighborhoods is limited (Browning & Cagney, 2003; Latkin & Curry, 2003). In addition, children and young adults who grow up in disadvantaged neighborhoods are likely to remain in similar neighborhoods as adults, which may amplify their lifetime exposure to contextual stressors like disadvantage (Garmezy, 1991). Neighborhood disadvantage and its correlates may undermine neighborhood, family, and peer social support systems as well as the personal, social and economic resources that help buffer youth and young adults against the noxious stressors of disadvantage (Latkin & Curry, 2003; Ross, Mirowsky, & Pribesh, 2001). Developing a more thorough understanding of the stress process within the neighborhood context may contribute to programs and policy to reduce lifetime exposure to stressors, stress-related health problems in adulthood and health disparities.
Stressors and stress
The influence of residential context on psychological and mental health has been explored in depth for children, adolescents, and adults (Brooks-Gunn, et al., 1993; Diez Roux & Mair, 2010; Hill, Ross, & Angel, 2005; Latkin & Curry, 2003; Mair et al., 2009; Mayer & Jencks, 1989), and researchers frequently cite disadvantaged neighborhoods as stressful (Latkin & Curry, 2003; Steptoe & Feldman, 2001). Although perceived stress is an important measure of an individual’s psychological response to a stressor, it may not accurately represent an individual’s chronic stress burden, as psychological stress may primarily reflect current and not chronic or cumulative exposure (Worthman & Costello, 2009). In addition, individuals who are chronically exposed to stressful stimuli, as may be the case for youth living in highly disadvantaged neighborhoods, may not perceive high levels of stress due to psychological acclimation to stressors (Worthman & Costello, 2009). While psychological stress measures are valuable indicators of individuals’ current psychological state, physiological biomarkers like cortisol may more adequately represent the connection between chronic exposure to stressors and health, and may therefore enable researchers to study prolonged exposure and the resulting wear and tear on the body that begins in childhood and adolescence (Geronimus, Hicken, Keene, & Bound, 2006; McEwen & Seeman, 1999).
Physiological stress response
When confronted with a stressful situation (stressor), the body initiates a stress response, which is mediated by the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (McEwen & Seeman, 1999). During a stress response, hormones including cortisol and catecolamines are secreted by the body, beginning a cascade of events among receptors and cells (McEwen & Seeman, 1999). Although a stress response to an acute stressor helps regulate the body and maintain allostasis (maintaining homeostasis, or stability through change), repeated challenges to allostasis can damage the feedback loops and wear down the body (McEwen & Seeman, 1999).
Physiological stress biomarkers (e.g., cortisol, norepinepherine) are objective (though not perfect) measures that may reflect cumulative exposure to stressful conditions (Worthman & Costello, 2009). These biomarkers, which may mediate the association between stressful exposures and physiological outcomes, allow researchers to connect social exposures to health. Biomarkers account for individual vulnerabilities and interactions between individuals and their environment, as biomarkers reflect exposures, adaptation and response for an individual (Worthman & Costello, 2009). Worthman & Costello (2009, p. 284) suggest “biomarkers can complement sociocultural analysis by probing biocultural pathways that shape health but lie outside of awareness, everyday observation, or cultural discourse.” More generally, biomarkers may allow researchers to measure the complex socio-cultural and environmental pathway to specific health outcomes.
Cortisol is one of the primary hormones or stress mediators released during a stress response, and it regulates secondary and tertiary outcomes in the body like heart rate and blood pressure (McEwen & Seeman, 1999), which are precursors to many chronic diseases including cardiovascular disease and diabetes. Thus, cortisol is an important biomarker of later disease that may relate back to the stress response and cumulative exposure to stressors. Cortisol, which is largely responsible for regulation of the central nervous, metabolic and immune systems (Miller, Chen, & Zhou, 2007), is related to many health conditions. Chronically elevated cortisol levels are associated with cognitive decline, immunosuppression and insulin resistance (Dowd et al., 2009; Miller, et al., 2007). Despite the emerging and robust evidence of the health effects of cortisol dysregulation, the relationship between exposure to stressors and cortisol is complex. It varies based on study design, time of the day, type of risk, and individual-level factors including genetics, psychology and biology (Burke, Davis, Otte, & Mohr, 2005; Heim, Ehlert, & Hellhammer, 2000; Kudielka, Hellhammer, & Wüst, 2009; Lupien, King, Meaney, & McEwen, 2001). Examining changes in cortisol over time is necessary to help better understand these variations in the stress process.
Several researchers have examined the association between individual-level risk and stress biomarkers (Buske-Kirschbaum et al., 2003; Dowd et al., 2011; Dowd et al., 2009; Gersten, 2008; Gustafsson, Anckarsäter, Lichtenstein, Nelson, & Gustafsson, 2010; Hajat et al., 2010; Heim & Nemeroff, 2001; K. Schmeelk-Cone, Zimmerman, & Abelson, 2003). Researchers also have examined demographic differences in allostatic load based on population-based studies (Geronimus et al., 2006; Peek et al., 2010). Few researchers, however, have examined the association between contextual-level stressors and cortisol. Chen and Paterson (2006) investigated the relationship between neighborhood census indicators and cortisol in a sample of adolescents, and found that individuals in their sample who lived in lower SES neighborhoods had lower basal cortisol levels. Their study, however, used only a single cortisol measurement, which is difficult to interpret in relation to health given the complexity of the cortisol diurnal rhythm (Almeida, McGonagle, & King, 2009). Additional research that explicitly connects contextual exposure to stressors to stress biomarkers is critical in improving our understanding of residential exposures and health; this area of research is largely unexplored. This study will address this gap in the literature, and examine the relationship between neighborhood socio-economic context and physiological stress in youth.
Connecting socio-economic exposures to physiological outcomes
The Transactional Model of Stress and Coping (Lazarus & Folkman, 1984) and the biopsychosocial model (Engel, 1980) help elucidate the mechanism through which exposure to stressors in the neighborhood may result in physiological stress and health. These theories conceptualize the stress response as a process that is dependent on individual’s perception of the stressor(s), demographic and psychological factors, coping processes, behaviors and availability of social support (Folkman, 1984; Wenzel, Glanz, & Lerman, 2002). The biopsychosocial model (Engel, 1980) has been used to guide research on racism and health inequalities (Clark, Anderson, Clark, & Williams, 1999), as well as biological aging and health (Crimmins & Seeman, 2004). It connects exposure to stressors to health via a mediating/moderating pathway that includes psychological, behavioral and physiological factors. The biopsychosocial model supports the hypothesis that an adolescent’s perception of their neighborhood, and general psychological stress, may moderate the relationship between neighborhood stressors (disadvantage) and physiological stress. Perceptions of the neighborhood affect residents’ mental and physical health (Latkin & Curry, 2003; Pearlin, 1989), and these perceptions can increase the fear and anxiety of residents (Ross & Jang, 2000). Thus, youth who perceived more fear of their neighborhood and who live in the most disadvantaged neighborhoods, may experience compounded effects of this interaction on their cortisol reactivity. In this research, we include measures of perceived stress, neighborhood attitudes, and neighborhood fear as important psychological moderating factors.
The Transactional Model of Stress and Coping and the biopsychosocial model also indicate that the relationship between exposure to stressors in the neighborhood and cortisol may be moderated by coping, social support and health behaviors, which are also examined in this research and described in more detail. High effort coping is a prolonged coping process in response to extreme psychosocial stressors and barriers to success (James, 1994), which may be associated with an atypical cortisol response, and may moderate the relationship between neighborhood disadvantage and cortisol. Although high effort coping may help youth overcome stressors through hard work and determination, it also can cause physical damage to the body (James, 1994). Thus, high effort coping may interact with neighborhood disadvantage to increase wear and tear on the body, and thus may amplify cortisol (Geronimus, et al., 2006).
Social support may play a protective role, buffering youth from the psychological and physical results of exposure to stressors (Ensel & Lin, 1991; Lyons, Mickelson, Sullivan, & Coyne, 1998; Thoits, 1995). Cohen and Wills (1985) propose that received support may intervene directly in the neuroendocrine response to stressful stimuli, and then may reduce indirectly, physical reactivity to the stressor, resulting in reduced cortisol reactivity. In addition, social support may reduce psychological reactivity to the stressor or aid in healthy coping, which may then indirectly reduce physical reactivity and resulting physiological markers of stress (Cohen & Wills, 1985). While having more social support is expected to decrease psychological and physiological stress, this protective effect may vary based on the level of neighborhood disadvantage. Extreme disadvantage, or overburdened support networks in disadvantaged neighborhoods, may undermine the beneficial effect of social support (Elliott, 2000; Myers, 2009; Unger, 1985), and so testing the effect of perceived support by neighborhood disadvantage is important in untangling these effects.
Finally, youth living in stressful, disadvantaged neighborhoods may be more likely than youth living in less stressful areas to engage in substance use to relieve stress or escape chronic stressors encountered in daily life (Jackson et al., 2009). Substance use may therefore operate as an avoidant coping method, which may moderate the relationship between contextual stressors and stress (Jackson et al., 2009; Schmeelk-Cone & Zimmerman, 2003), as neighborhood context may differentially affect the relationship between substance use and cortisol based on alcohol availability and exposure to stressors (Jackson et al., 2009). Although substance use may reduce anxiety from exposure to stressors because of its depressive effects on the HPA axis, hormone elevation and activation of the HPA axis also may contribute to physiological stress and allostatic load (Jackson et al., 2009). Physiological consequences of substance use therefore may contribute to health problems for youth as they progress into adulthood, and this risk may be particularly high for youth living in disadvantaged neighborhoods.
Hypotheses
To date, few researchers have examined physiological biomarkers of stress as an endpoint (Hajat, et al., 2010; Kliewer, Reid-Quinones, Shields, & Foutz, 2009; Schmeelk-Cone et al., 2003), and most research in this area focuses only on adult, and particularly older adult, populations. Although researchers focus mainly on adults because they have greater cumulative exposure to stressors than adolescents, Evans et al. (2007) emphasize the importance of studying these biomarkers in youth because of the confluence of critical endocrine and social changes that occur during adolescence. Additionally, few researchers have included neighborhood exposures in their analyses, and none have examined stress and coping or health behavior as a part of the model.
The current study will integrate stress and coping, biopsychosocial, and neighborhood theories to better understand the relationship between neighborhood stressors and cortisol reactivity in African American youth. We focus on African Americans because they are disproportionately represented among low socioeconomic individuals, and in the most disadvantaged neighborhoods. African Americans are exposed to race-specific stressors like racism and discrimination (Estrada-Martínez, Caldwell, Bauermeister, & Zimmerman, , 2012), compounding their exposure to stressors. Research also indicates that African Americans may experience higher rates of psychological distress than Whites (Jackson, et al., 2009; Kessler & Neighbors, 1986), and they also may be physiologically at higher risk for stress reactivity and related health consequences (Geronimus, et al., 2006; Hajat, et al., 2010; Peek, et al., 2010; Jackson, Treiber, Turner, Davis, & Strong, 2004; Skinner, Shirtcliff, Haggerty, Coe, & Catalano, 2011) than their White counterparts. Additionally, by limiting the sample to only African American youth, we avoid confounding in the relationship between neighborhood context and cortisol based on race, which enables us to better isolate the associations between neighborhood stressors and our outcome. Further, the inclusion of only African American youth in our analyses acknowledges within-race variability in our sample.
The first aim of this study is to examine the relationship between intra- and interpersonal factors and physiological stress, measured by cortisol (baseline and reactivity). We hypothesize that promotive interpersonal factors like social support will decrease baseline cortisol, but that substance use, high effort coping, and perceived stress will increase baseline cortisol and result in atypical reactivity for adolescents. Next, we examine the relationship between neighborhood stressors and cortisol. Research that focuses on contextual-level stressors and neuroendocrine biomarkers will help abate some of the confusion in the literature surrounding the importance of psychological versus physiological stress. We hypothesize that greater exposure to neighborhood disadvantage will result in higher baseline cortisol and an atypical cortisol reactivity. Finally, we examine moderating factors because we expect factors like social support, perceived stress, neighborhood perceptions, active coping and substance use to interact with neighborhood stressors and be related differentially to cortisol. Because the neighborhood and physiological literatures are not well connected, there is limited evidence on the relationship between neighborhood, and intra- and interpersonal level interactions and cortisol. Research, however, indicates that psychosocial and behavioral factors may have differential effects on health and well-being based on neighborhood context (Boardman, 2004; Cutrona et al., 2005), as neighborhood stressors may exacerbate intra- and interpersonal risk (Schempf, Strobino, & O'Campo, 2009). Thus, we also examine cross-level interactions, or the moderating pathways between the individual and neighborhood levels on cortisol, as guided by the ecological framework, the Transactional Model of Stress and Coping and the biopsychosocial framework.
Method
Data design and sample
To test the proposed hypotheses, data from the Flint Adolescent Study (FAS) were used (Schmeelk-Cone, et al. 2003; Schmeelk-Cone & Zimmerman, 2003). The FAS is a longitudinal study of 850 youth at risk for substance use and school dropout. Eligible students included ninth graders enrolled in public high schools in an urban city in Michigan, and who had an eighth grade grade point average (GPA) of 3.0 or below upon entering high school. Youth who were diagnosed by the schools with emotional or developmental impairments were excluded from the study sample. Youth self-identified as African American (80%), White (17%) or Bi-racial (3%). Males and females were equally represented in the sample.
The FAS consists of four waves of data collected during the high school years (Waves 1–4; 1994–1997), four waves of data collected after high school (Waves 5–8; 1999–2002), and two waves of data collected in early adulthood (Waves 9–10; 2008–2009). Retention rates were generally high (90% from Waves 1 to 4 and 75% from Waves 4 to 8). Data were collected by structured face-to-face interviews and a self-administered portion, conducted either in a school or alternative community location. The interview included most of the psychosocial variables in the data, as well as information on employment, education, family and friends. A self-administered questionnaire assessed more sensitive information (e.g., substance use, sexual risk behavior, discrimination) and was distributed at the conclusion of each interview in order to facilitate confidentiality. The individual-level data were linked to census data based on geo-coded home address information. This study was approved by the University of Michigan Institutional Review Board (UMIRB# H03-0001309), and informed consent was obtained from all participants prior to data collection.
Only the sixth wave of data collection, which occurred between 2000 and 2001 is used in this analysis. Saliva samples were collected from a subset of youth (N=201) of those who were present in Wave 6 (N=573), who consented to the procedure, and who had not eaten, drank or used tobacco in the hour prior to the collection in Wave 6. Saliva was sampled at three different time points during the 60 minute interview from participants who agreed to the collection, were not pregnant, and were otherwise eligible for the saliva collection. Participants provided saliva samples at the beginning of the interview to assess baseline cortisol, approximately mid-way through the interview (20 minutes after the first sample), and at the end of the interview (30 minutes after the second collection). Samples were placed on ice prior to transportation and assay. Cortisol levels were assayed by Salimetrics, Inc. The current sample consists of 163 African American youth who provided saliva samples in Wave 6 (Mage=21 years (SD=0.63), 50% female), and youth who consented to saliva sampling were not different from the overall Wave 6 sample (see Table 1 for descriptive statistics for the sample).
Table 1.
Sample description for dependent and independent variables
| N | Mean (SD)a | Rangeb | |
|---|---|---|---|
| Cortisol mean | 163 | 0.24 (0.18) | 0–1.0 |
| Cortisol reactivityc | 163 | ||
| Normal (decreasing) | 98 (60.1%) | ||
| Reactive | 32 (19.6%) | ||
| Non-reactive | 33 (20.3%) | ||
| John Henryism | 165 | 4.30 (0.53) | 2.2–5.0 |
| Daily Hassles | 160 | 2.43 (0.60) | 1–3.5 |
| Substance use | 162 | 0.01 (0.78) | −0.7–2.8 |
| Mother support | 155 | 3.93 (1.04) | 1–5 |
| Peer support | 161 | 3.13 (1.04) | 1–5 |
| Relationship support | 161 | 4.42 (0.57) | 2.17–5 |
| Health (self-rated) | 163 | ||
| Healthier than others | 41 (25.2%) | ||
| Same as others | 111 (68.1%) | ||
| Less healthy than others | 11 (6.8%) | ||
| Worry about getting hurt | 162 | ||
| Yes | 33 (20.4%) | ||
| No | 129 (79.6%) | ||
| Fear of neighborhood violence | 163 | ||
| Yes | 65 (39.9%) | ||
| No | 98 (60.1%) |
Note.
Categorical variables are reported as frequency(%)
The range is reported only for continuous variables
Decreasing reactivity defined by decrease in cortisol level from 1st to 3rd sample; increasing reactivity defined by increase in cortisol from 1st to 3rd sample, non-reactive reactivity defined by no change within 0.02 (variance of the change in cortisol) from 1st to 3rd sample
Measures
Cortisol
Cortisol was measured in the thawed saliva samples (Salimetrics, Inc.) using a high-sensitivity salivary cortisol enzyme immunoassay. The three measurements of cortisol (measured as μg/dl) are used in the growth curve analysis to determine the change in an individual’s cortisol during the interview. Saliva samples were taken about 10 minutes into the interview, approximately 22 minutes after the first sample, and then approximately 30 minutes later. All samples were placed on ice and refrigerated. Cortisol values were log transformed to reduce the skewness of the distribution, and the transformed values are used in the analysis. Saliva sampling time was included in the models as a control variable, as the time of day at which the saliva was collected may affect cortisol readings (diurnal patterns).
Demographics
Youth’s sex was coded as 0=female and 1=male. Measures of SES from Wave 1 are included to control for a measure of family socioeconomic status when the participant was a young adolescent, as early life circumstances may be more important predictors of stress than current SES. SES is assigned based on the highest occupational prestige score for either parent, using codes developed by the National Opinion Research Center and then standardized to facilitate interpretation (Nakao & Treas, 1990). The score is assigned based on 20 occupational classifications, ranging from private household work (scored as 29.28) to professional (scored 64.38). The mean prestige score in our sample was 39.07 (SD =9.9), which corresponds to a blue-collar occupation. Maternal education was also included, and was used as a continuous variable that was originally assessed based on a seven-level interval response format. The mean education level in our sample was 4.55 (SD=2.00), which corresponds roughly to an average vocational/training school education (using a scale from 1=less than high school to 9=graduate degree). We included self-reported health status as a covariate and possible confounder of the relationship between exposure to stressors and stress biomarkers. Health status was measured by asking participants to characterize themselves as: healthier than others, equally healthy as others, less healthy as others. Higher values correspond to worse self-reported health. In our sample, 68% of participants rated their health the same as others, and about 25% rated themselves as healthier than others.
Substance use
Substance use is a composite measure that includes items about smoking (number of cigarettes smoked in the past 30 days), alcohol use (composite measure of past 30 day alcohol use, binge drinking over the past two weeks and drinking to get high), and marijuana use (past 30 day marijuana use). Each item or scale was standardized (mean=0, SD=1.00), and the average of the three standardized variables was computed to create a substance use scale (Cronbach’s alpha = 0.62).
Active coping
Active coping was operationalized using the John Henryism measure that was originally developed by James (1983) to assess high effort coping. The measure of John Henryism is an eight-item scale that asks respondents to rate items like: “Hard work is the best possible way for someone to get ahead in life.” These items are rated on a 5-point Likert scale (1=not true to 5=very true) and then averaged to create a measure of high effort coping where higher values signify more coping (Cronbach’s alpha = 0.85).
Social support
Three sources of perceived social support were assessed in this study. Five items focused on emotional support were used from Procidano and Heller’s perceived support scales to assess youths’ support from parents, peers, and other important relationships (Procidano & Heller, 1983).
Mother’s support
Mother’s support was measured by five items using a 5-point Likert Scale. Items include the degree to which the adolescent's mother gives emotional and instrumental support, and the closeness of the mother-youth relationship. The support scale ranges from 1 to 5 and a higher value corresponds to greater levels of social support (Cronbach’s alpha = 0.92).
Peer support
Like maternal support, the peer support measure includes items assessing the same types of emotional and instrumental support, which are averaged to create a composite measure. The scale ranges from 1 to 5 and a higher value corresponds to greater levels of social support (Cronbach’s alpha = 0.91).
Relationship support
Finally, the questionnaire asks the respondent to identify a person in their lives to whom they feel closest, which assesses relationship support. This person is likely to be a significant other, but could be another family member or friend. Relationship support was measured as a mean score of 7 items ranging from 1 to 5. Higher values signify more relationship support (Cronbach’s alpha = 0.83).
Perceived Stress
Daily hassles, which is a measure of perceived stress in the past month was assessed using Cohen and colleagues’ Perceived Stress Scale (PSS) (Cohen, Kamark & Mermelstein, 1983), which was originally a 14-item scale designed to measure subjective (psychological) stress. The scale assesses the degree to which people believe their lives to be “unpredictable, uncontrollable, and overloading” (p. 387) (Cohen et al., 1983). A shortened version of the PSS was used in the FAS; 11 items were included in the survey based on a principal components analysis. The items were averaged and used as a continuous measure. The scale ranges from 1 to 5 and higher values denote more perceived stress (Cronbach’s alpha = 0.82).
Neighborhood perceptions
Fear of violence
Fear of violence assesses whether participants are afraid of violence in their neighborhood. The item was measured on a scale of 1 to 5 with higher values representing more fear. To reduce skewness the variable was dichotomized into a score for participants who reported low levels of fear (below or equal to the median) and another score for those who reported high levels of fear (above the median).
Worry about getting hurt
This item assesses whether participants worry that someone in their neighborhood will physically hurt them. This is also measured using a scale of 1 to 5 where higher values correspond to more worry. A dichotomous measure, dividing participants into two categories of worry based on the sample median was created. Higher values on this item therefore correspond to greater fear that they will be hurt in their neighborhood.
Each neighborhood perception variable was assessed as a single item, and the correlation between these two measures in our sample is 0.50 (p<0.01).
Neighborhood disadvantage
The neighborhood level disadvantage variables were obtained from 2000 US Census data. The census data were linked to the individual data by geocoding techniques. Neighborhood was conceptualized at the census tract level (N=36) for these analyses (Sampson et al., 1997). The following seven census indicators were used based on the percent of families/houses in the census tract: with annual incomes less than $15,000, on public assistance, that are single-headed with children under 18, that are unemployed, detached from the labor force, vacant, and have a head of household with less than a high school education. Higher values on the individual neighborhood disadvantage items denote greater levels of neighborhood disadvantage in the census tract.
Analytic strategy
We used a multilevel analysis using HLM 6.0 software (Raudenbush, Bryk & Congdon, 2004) to examine the effect of individual-level risk and promotive factors, and neighborhood-level disadvantage (stressors) on cortisol reactivity. Although HLM handles missing data for cortisol at level-1, it does not handle missing data at levels 2 or 3. To maximize the sample size, we imputed missing data at level-2 (individual level) to ensure that cases with missing demographic data were not dropped from the analysis. Expectation maximization (EM) algorithm (West et al., 2006) in EQS (6.1, Multivariate Software Inc.) was used to impute missing data. We conducted an attrition analysis comparing participants with missing (N=46) and complete data (N=133) to ensure that the imputation did not result in a biased analysis. Based on this analysis no differences between the original or imputed sample were found. All fixed factors that had a meaningful value of zero (e.g., categorical variables and dummy variables) were entered into the models as uncentered variables and all other variables, including the neighborhood disadvantage indicators, were centered around their grand mean. Neighborhood disadvantage indicators were then entered in the model (level-3 model). Each was entered and tested separately due to power limitations. Cross-level interactions between disadvantage and individual risk and promotive factors were also tested. To determine whether the relationship between neighborhood disadvantage and cortisol varied between and within neighborhoods, the error terms at level-3 was allowed to vary on the intercept and slope to account for within neighborhood variation.
Results
Cortisol reactivity (level-1)
Based on a fully unconditional model (FUM), which contains no individual or neighborhood predictors, youth’s initial cortisol levels and average growth rates were positive and associated with cortisol reactivity. Estimation of the neighborhood-level variation indicates significant variability among neighborhoods for initial cortisol levels, χ2(35) = 55.76, p<0.05, and for change in cortisol, χ2(35) = 49.86, p<0.05. In this model, time is centered on zero, which corresponds to the initial cortisol sample collected at the beginning of the interview. The linear time factor is interpreted as the change in cortisol over each sampling time (slope), and for youth in the sample, cortisol increased linearly over time, β = 0.03(0.01), p<0.01. Next, a quadratic time factor was added to the model to determine whether the trajectory of cortisol included a curvilinear component. This term was not significant, however, and was removed from the model. The final level-1 model contained only the intercept (initial cortisol) and the slope (linear change in cortisol) (see Table 3, Model 1).
Table 3.
Coefficients and standard errors for individual, neighborhood, and cross-level effects on baseline cortisol and cortisol reactivity
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| Mean cortisol at baseline (β0) | |||||
| Intercept | 0.156 (0.03)*** |
0.165 (0.03)*** |
0.161 (0.03)*** |
0.158 (0.03)*** |
0.151 (0.03)*** |
| % households in tract not in labor force | -- | -- | -- | -- | 0.324 (0.12)*** |
| Start time | −0.00 (0.00)*** |
−0.00 (0.00)*** |
−0.00 (0.00)*** |
−0.00 (0.00)*** |
−0.00 (0.00)*** |
| SES (continuous) | NS | NS | NS | NS | NS |
| Perceived stress (low) | NS | NS | NS | NS | NS |
| Perceived stress (high) | 0.048 (0.03)* | 0.048 (0.03)* | 0.046 (0.03)* | 0.058 (0.04)* | 0.067 (0.04)* |
| High effort coping (low) | 0.056 (0.03)* | 0.056 (0.03)* | 0.060 (0.03)* | 0.055 (0.03)* | 0.062 (0.03)* |
| High effort coping (high) | 0.070 (0.03)** |
0.038 (0.02)* |
0.070 (0.03)** |
0.066 (0.03)** |
0.066 (0.03)** |
| % in tract on public assistance |
-- | 1.166 (0.65)* | -- | -- | -- |
| % vacant houses in tract | -- | -- | 1.55 (0.75)** | -- | -- |
| % families with < HS Edu | -- | -- | -- | 0.351 (0.18)** |
-- |
| Cortisol reactivity (π1) | |||||
| Intercept | 0.042 (0.01)*** |
0.043 (0.01)*** |
0.045 (0.01)*** |
0.043 (0.01)*** |
0.043 (0.01)*** |
| SES (low) | NS | NS | NS | NS | NS |
| SES (high) | −0.020 (0.01)** |
−0.022 (0.01)** |
−0.024 (0.01)** | −0.023 (0.01)** |
−0.02 (0.01)** |
| Peer support (low) | 0.031 (0.02)** | 0.030 (0.02)** | 0.030 (0.02)** | 0.030 (0.02)** | 0.029 (0.02)* |
| Peer support (high) | NS | NS | NS | NS | NS |
| Perceived stress (low) | −0.025 (0.01)** |
−0.025 (0.01)** |
−0.022 (0.01)* |
−0.026 (0.01)** |
−0.025 (0.01)** |
| Perceived stress (high) | −0.034 (0.01)** | −0.031 (0.01)** |
−0.037 (0.01)*** |
−0.035 (0.01)*** |
−0.033 (0.01)** |
| % in tract earning <$15,000/year |
−0.122 (0.060)** |
-- | -- | -- | -- |
| % in tract on public assistance |
-- | −0.317 (0.15)** |
-- | -- | -- |
| % vacant houses in tract |
-- | -- | −0.312 (0.17)* | -- | -- |
| % families with < HS Edu |
-- | -- | -- | −0.138 (0.04)*** |
-- |
Note. NS = Non-significant. Dashes were used to denote that the variable was not included in the model.
p≤ 0.1,
p≤ 0.05,
p≤0.01
Individual-level model (level-2)
Individual-level fixed effects were included next to determine whether initial cortisol levels (intercept) or change in cortisol (slope) were explained by demographic, risk, promotive, or neighborhood factors that are unique to the individual. Saliva sampling time at level-2 was controlled. Using a step-down modeling approach, all demographic and control variables were entered into the model, and only variables that were associated with the dependent variable were retained (Raudenbush & Bryk, 2002). SES was associated to cortisol reactivity (slope), but not baseline levels. Next, the risk and promotive factors were entered into the model and retained only if significant (see Table 2, Model 1).
Table 2.
Individual and interpersonal influences (fixed effects) on baseline cortisol and cortisol reactivity
| B (SE) | |
|---|---|
| Mean cortisol at baseline (β0) | |
| Intercept | 0.155 (0.03)*** |
| Start time | −0.00 (0.00)*** |
| SES (continuous) | NS |
| Perceived stress (low) | NS |
| Perceived stress (high) | 0.062 (0.04)* |
| High effort coping (low) | 0.056 (0.03)* |
| High effort coping (high) | 0.07 (0.03)** |
| Cortisol reactivity (π1) | |
| Intercept | 0.043 (0.01)*** |
| SES (low) | NS |
| SES (high) | −0.02 (0.01)** |
| Peer support (low) | 0.028 (0.02)* |
| Peer support (high) | NS |
| Perceived stress (low) | −0.026 (0.01)** |
| Perceived stress (high) | −0.034 (0.01)** |
Note. NS =Non-significant
p≤=0.1,
p≤0.05,
p≤0.01
The time at which the first saliva sample was taken was associated with the initial cortisol level for the sample, β= −0.00(0.00), p=0.01, but not with cortisol reactivity. This variable was retained in the model as a control for sampling time. None of the demographic factors were associated with baseline cortisol. Family SES for those adolescents with families in the 75th percentile of SES was associated with cortisol reactivity, such that having a high versus average family SES resulted in a reduction in cortisol reactivity, β = −0.02(0.01), p<0.05. No additional demographic factors were associated with cortisol reactivity, and were therefore dropped from the model.
Next, the intra- and interpersonal risk and promotive factors were entered into the model. In initial exploratory analysis the relationship between the risk and promotive factors and cortisol was curvilinear. Similarly to standard multiple regression, HLM assumes a linear relationship between the predictors and dependent variable, and incorrectly assuming a linear relationship may result in biased results. Thus, a three group categorical variables were created for each risk and promotive factors (e.g., low, average, high) and included the low and high predictors in the model and used the average level as the reference category.
Daily hassles were associated marginally with baseline cortisol such that individuals who reported high versus average levels of hassles had higher baseline cortisol, β = 0.06(0.04), p = 0.09. Youth who reported high versus average levels of high effort coping had higher baseline cortisol than their average coping peers, β = 0.0(0.03), p = 0.018, and those who had low peer support had higher cortisol reactivity than their counterparts with average support, β = 0.03(0.02), p = 0.076. Youth with fewer daily hassles had less cortisol reactivity than those with average daily hassles, β = −0.03(0.01), p = 0.015, as did participants who reported more daily hassles, β = −0.03(0.01), p=0.017. Although addition of the individual-level predictors improved the overall fit of the model, χ2(12) = 27.34, p = 0.01, significant variation in initial cortisol and change in cortisol at the neighborhood level still remained, χ2(35) = 66.49, p = 0.001; χ2(35) = 59.64, p = 0.006].
Neighborhood disadvantage model (level-3)
Neighborhood indicators of social and economic disadvantage were included in the final model to determine whether a portion of the random variation remaining in cortisol reactivity after including level-1 and level-2 variables is attributed to between-neighborhood variations. Each indicator of neighborhood disadvantage was entered individually at level-3 on the intercept and the slope for cortisol to identify differences in initial cortisol levels or change in cortisol based on the level of disadvantage in a neighborhood. Living in a neighborhood with a higher proportion of individuals detached from the labor force resulted in higher baseline cortisol for residents, controlling for cortisol sampling time, SES, perceived stress and high effort coping, β = 0.32(0.12), p = 0.011. The addition of the neighborhood indicator explained more variation in cortisol, χ2(1) = 3.92, p = 0.045. None of the remaining neighborhood disadvantage indicators were related directly to baseline cortisol or cortisol reactivity.
Cross-level interactions between neighborhood disadvantage and fixed effects
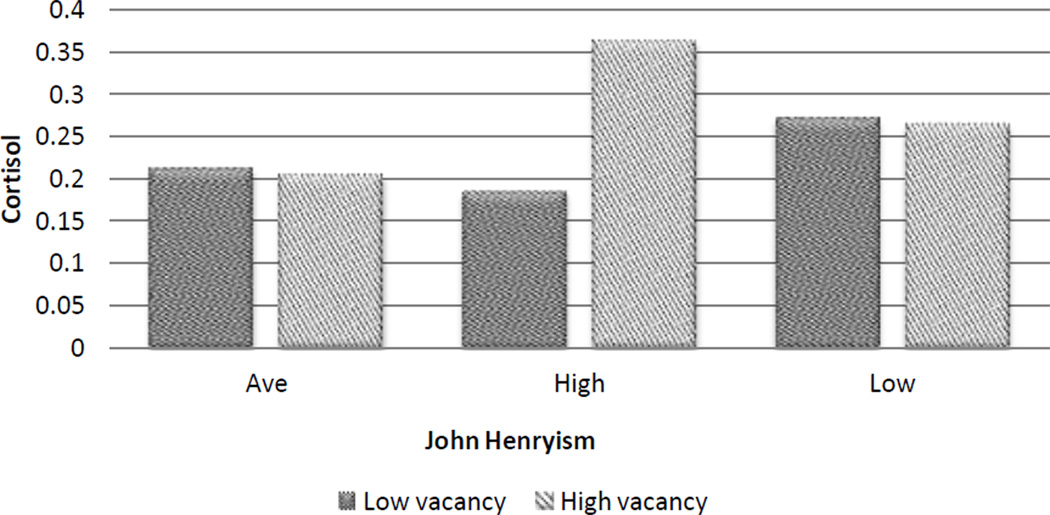
Lastly, we examined the relationship between cross-level interactions and cortisol. Adolescents living in neighborhoods with a high proportion of vacant houses, and who demonstrated a higher level of John Henryism compared to average levels, had higher baseline cortisol than their average high effort coping peers living in neighborhood with an average number of vacant houses, β = 1.55(0.75), p = 0.043 (Figure 1). In addition, youth who reported high John Henryism, and who lived in neighborhoods with the largest percentage of residents with less than a high school education, had the highest baseline cortisol, β = 0.351(0.18), p = 0.054.
Figure 1.
Baseline cortisol by coping and neighborhood vacancy
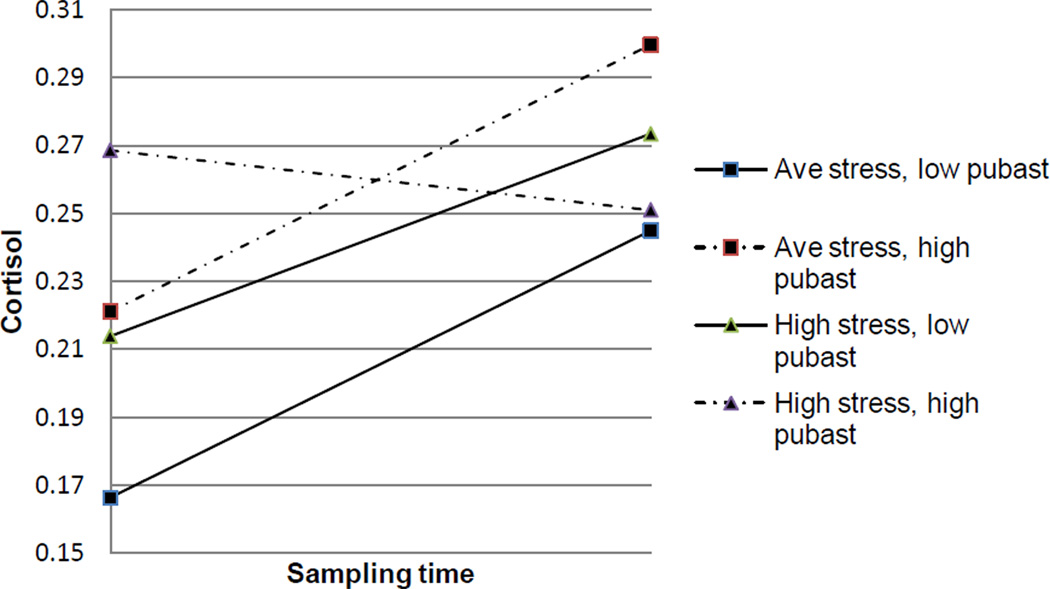
Neighborhood-level indicators of disadvantage also interacted with an individual-level factor to influence cortisol reactivity. Adolescents who lived in neighborhoods with a high proportion of households earning less than $15,000 per year, and who also reported a high number of daily hassles, had reduced cortisol reactivity compared to their counterparts with an average number of hassles living in neighborhoods with average household poverty, β = −0.122(0.06), p = 0.030. Similarly, those youth living in neighborhoods with a higher proportion of households using public assistance (Figure 2), and who reported high levels of daily hassles, had reduced cortisol reactivity compared to their counterparts with an average number of daily hassles and who lived in neighborhoods at the mean for the percentage of families on public assistance, β = −0.317(0.15), p = 0.036. The same relationships emerged for neighborhood-level education, β = -0.138(0.04), p = 0.002.
Figure 2.
Cortisol reactivity by perceived stress and public assistance
Discussion
Exposure to stressors in the neighborhood context during childhood and adolescence may translate into poor health during adulthood (Elliott, 2000; Pickett & Pearl, 2001), yet the biological pathways that connect these exposures to disease are still not well understood. This research contributes to the continuously growing body of literature of neighborhood effects on health, by addressing the association between neighborhood disadvantage and cortisol for African American youth living in urban areas. To date, only a handful of researchers have connected neighborhood context with cortisol as a physiological biomarker for stress (Chen & Paterson, 2006; Do et al., 2011; Dulin-Keita, Casazza, Fernandez, Goran, & Gower, 2012; Kliewer, et al., 2009), and of these studies, only one includes neighborhood and individual risk (Kliewer, et al., 2009). The results of this study support the biopsychosocial model, theories of stress and coping, and neighborhood models as they relate to neighborhood disadvantage and cortisol. Youth living in more stressful neighborhoods, as measured by socio-economic disadvantage, have atypical cortisol patterns compared to individuals living in neighborhoods with fewer stressors (less disadvantage). In addition, the relationships between coping and cortisol, and between perceived stress and cortisol, are moderated by the degree of neighborhood disadvantage. This supports the biopsychosocial model and Transactional Theory of Stress and Coping, which reveal complex relationships between exposure to stressors, stress, and health that include psychological, coping and physiological processes. Although only some aspects of neighborhood disadvantage are associated with cortisol, the results are consistent in the direction of their relationship with baseline cortisol and cortisol reactivity. Similarly, the synergistic interaction between neighborhood stressors and individual-level predictors consistently indicate that both high effort coping and perceived stress are dependent on the level of neighborhood disadvantage in their effect on cortisol. While our results may not be generalizable to other populations, they support the salient role of neighborhood context in the stress process and physiological biomarkers of stress for youth, which is critical to understand as the transitioning from adolescence into adulthood occurs.
Interpreting the relationships between exposure to stressors and cortisol, and between cortisol and health is not well understood or straightforward. What is normal for cortisol is dependent on the specific stressor; the quantity and duration of the exposure; individual demographic, health, behavioral factors, vulnerability; and methods of assessment (Dowd et al., 2011; Miller, et al., 2007). Despite this uncertainty, researchers believe that elevated morning cortisol indicates an unhealthy response to stressors (Dowd et al., Almeida, et al., 2009; 2009), and that blunted cortisol reactivity also suggests an atypical stress response (Almeida, et al., 2009; Dowd et al., 2009). Controlling for sampling time, we find that individuals who live in neighborhoods where more people are disconnected from the labor force have higher baseline cortisol. This is consistent with past research, adding to our confidence in the overall results (Fernald & Gunnar, 2009; Lupien, et al., 2001; van Eck, Berkhof, Nicolson, & Sulon, 1996).
Our results support the biopsychosocial model of stress. Although only neighborhood-level labor force participation is associated directly with baseline cortisol, we find that youth who reported high, compared to average, John Henryism, and who also live in neighborhoods with the highest degree of disadvantage, have higher baseline cortisol levels than their peers living in less disadvantaged neighborhoods. This relationship holds true for neighborhood vacant housing, education and public assistance. Thus, adolescents who constantly strive to overcome a challenge and who persist in their coping, and live in neighborhoods with the highest disadvantage, may be most at risk for the negative effects of the physiological stress response. Researchers have found that adolescents who engage in active coping in response to uncontrollable stressors actually may have worse health outcomes (Gonzales, 2001). Our results suggest that this may be the case for adolescents living in disadvantaged neighborhoods, as these youth may encounter multiple stressors in their neighborhood related to disadvantage, but also family and school stressors that may be affected or compounded by neighborhood disadvantage (Jeanne Brooks-Gunn, et al., 1993; Cook, Herman, Phillips, & Settersten, 2002). These results support previous research that finds that African Americans who have low SES and high levels of John Henryism, have an elevated risk of hypertension and higher diastolic blood pressure (James, Keenan, Strogatz, Browning, & Garrett, 1992; James et al. 1987). Although research on high effort coping and weathering is typically restricted to older populations, the moderating effect of high effort coping in our research suggests that adolescents also may be psychologically and physiologically sensitive to cumulative exposure to stressors in the neighborhood. In light of the multiple stressors to which adolescents and young adults are exposed as they transition into early adulthood (Arnett, 2000), understanding the mechanisms that connect exposure to stressors and cortisol may be particularly critical during this period of development.
Although youth who live in the most disadvantaged neighborhoods have higher levels of cortisol on average, other researchers have reported that exposure to higher neighborhood or individual socio-economic disadvantage results in decreased cortisol levels (Chen & Paterson, 2006; Dowd et al., 2011; Dulin-Keita, et al., 2012; Kliewer, et al., 2009). Dulin-Keita et al. (2012) note that while exposure to acute stressors may result in increased cortisol levels, chronic exposure may be associated with lower cortisol secretion. These inconsistent associations may be indicative of the complexity involved in assessing and understanding cortisol, as cortisol levels vary based on sampling time (Burke, et al., 2005), age of the participants (Lupien, et al., 2001), the type of stressor (Heim, et al., 2000) and numerous factors that result in intra- and inter- individual variability (e.g., age, diet and genetic factors) (Kudielka, et al., 2009). In addition, factors like mobility, cumulative exposure to neighborhood stressors, and interpersonal factors like personality and coping, may be related to cortisol and contribute to variation between studies. These discrepant results suggest that a more detailed examination of dose of exposure and additional mediating and moderating factors could help tease apart inter-study discrepancies.
Our research indicates that youth who report more perceived stress, have a blunted cortisol response during the interview. This is suggestive of the physiological desensitization of individuals who are chronically exposed to stressors. Ganzel et al. (2010, p. 134) refer to the long term wear on the body due to exposure to stressors (expressed as a blunted cortisol response in this research) as “the cost of physiological accommodation to environmental demand.” When the body is forced to fight constantly against demands (i.e., neighborhood disadvantage), and the physiological stress response is chronically activated, the natural stress defense system begins to wear down, resulting in a desensitized or blunted response, and eventual harm the body. Youth living in disadvantaged neighborhoods are likely to have grown up in similar environments (Garmezy, 1991), and therefore, may be more likely than more transient adults to experience this desensitization to stressors. Our results are consistent with much of the reactivity literature, which finds that individuals who experience chronic stressors or laboratory and interview stressors, experience a blunted cortisol response (Buske-Kirschbaum, et al., 2003; Chandola, Heraclides, & Kumari, 2010; Do, et al., 2011; Hajat, et al., 2010; Hankin, Badanes, Abela, & Watamura, 2010; Suglia et al., 2010).
We also find, however, that youth who report low levels of perceived stress have a blunted cortisol response. These individuals may report low psychological stress because they have become desensitized to stressors, but despite low perceived stress, they may experience physiological symptoms to living in disadvantaged neighborhoods. Several researchers consider the idea of u-shaped associations between risk and an outcome (Bowling, Barber, Morris, & Ebrahim, 2006; Fergus & Zimmerman, 2005; Gary, Stark, & LaVeist, 2007; Gustafsson, et al., 2010), and this type of model is referred to as the challenge model in resiliency theory (Garmezy, Masten, & Taylor, 1984). Considering our results, the challenge model implies that low levels of perceived stress will result in less cortisol reactivity, but more moderate perceptions of stress (our reference group) will result in greater, or more normal reactivity because the adolescents already have been inoculated to psychological risk by experiencing low levels of perceived stress (Fergus & Zimmerman, 2005; Zimmerman & Brenner, 2009). Adolescents who report the highest levels of perceived stress, however, are also hypothesized to have blunted cortisol reactivity, as too much exposure to risk – regardless of inoculation – may be too difficult for youth to overcome (Zimmerman & Brenner, 2009). The curvilinear relationship between perceived stress and cortisol reactivity indicates that future research could include more thorough investigation of the relationship between varying degrees of psychological stress and cortisol reactivity. It is also possible that different measures of psychological stress may yield different results, as the daily hassles measure used in this analysis most likely assesses current feelings of stress.
Finally, we find that adolescents, who report higher psychological stress and live in disadvantaged neighborhoods, have a consistently diminished cortisol response to the interview compared to their counterparts in less disadvantaged neighborhoods. This may suggest that living in a disadvantaged neighborhood increases the risk of physiological harm to the body beyond psychological factors alone, even as early in life as late adolescence. Programs and policies aimed at reducing stress and improving coping resources might focus specifically on the period when youth are about to transition into adult roles. This stress-provoking developmental phase (Arnett, 2000) is also a period where increased vulnerability to psychological stress may be reflected in physiological systems in the body, setting the scene for poor health in adulthood. Additionally, helping emerging adults develop adequate coping skills during this period of instability and increased vulnerability to stress, may help foster resiliency and reduce their risk of developing physiological consequences as they move into adulthood.
We do not find evidence to support an association between social support and cortisol, substance use and cortisol, and neighborhood fear and cortisol. Although stress and coping theories identify social support as a critical intermediary in the stress process, it may not relate to physiological stress in the same way. It is also possible that our measures of social support and neighborhood fear assess more immediate support and perceptions, and that cortisol reflects accumulation of exposure to stressors during childhood and early adolescence. Despite research indicating a relationships between substance use and blunted cortisol reactivity (Kudielka, et al., 2009), we do not find support for this relationship. Most of the physiological stress research that includes substance use as a covariate also includes lifetime or abuse measures of substance use, and it is therefore possible that we are not able to detect a relationship between substance use and cortisol using past 30 day substance use measures. The results suggest that research that considers various risk and promotive factors over time and beginning earlier in adolescence, and their changing effect on cortisol during late adolescence and early adulthood would be useful.
Although this research suggests a critical link between neighborhood context and physiological stress, several limitations should be noted. First, the measurement of cortisol reactivity used in this study, while preferable to a single measure of baseline cortisol or an average cortisol measure, may not be ideal for assessing the diurnal rhythm of cortisol in its entirety. Cortisol is best assessed over multiple time points each day, beginning at wakeup and extending to bedtime, over several days (Dowd et al., 2009). By examining cortisol reactivity over three closely-spaced sampling times, we are unable to assess the cortisol awakening response, multiple reactions to stress throughout the day, or the general decline of stress during the day. Despite this limitation, assessing cortisol over three time points captures the cortisol response, or reactivity, which enables us to characterize individuals’ response to a stressor based on pre- and post-stress cortisol levels. While there is precedent for our method of measuring cortisol reactivity (Fernald & Gunnar, 2009; Hankin, et al., 2010; Kliewer, et al., 2009), researchers could consider more longitudinal measurement of both cortisol and exposure to stressors.
Another limitation of this research is our measurement of neighborhood. First, we define neighborhoods based on census tract designations, which may not correspond to residents’ perceptions of their neighborhood. Census tracts are rather large, and contain an average of around 4,000 people (U.S. Census Bureau, 2001), but residents are likely to define their neighborhood as a much smaller area. In addition, characterizing exposure to stressors in the neighborhood with a measure of neighborhood socio-economic disadvantage may fail to capture more important structural, social and physical aspects of the neighborhood, which residents may identify with better than census-based indicators of disadvantage. Research that includes measures of crime and violence; physical neighborhood conditions and disorder; and neighborhood social capital and cohesion, has the potential to recognize additional stressors to which residents are regularly exposed and may relate more closely to cortisol. Despite this limitation, using a measure of socio-economic disadvantage is more likely to underestimate the effect of neighborhood context on cortisol rather than produce an inflated result. Therefore, researchers who more thoroughly assess neighborhood effects on cortisol may find stronger effects than those found in this study.
These limitations notwithstanding, our study makes several new contributions to our understanding of neighborhood stressors and physiological response at a critical state of development. Only few researchers, for example, include relevant covariates (Chen & Paterson, 2006; Do, et al., 2011) related to neighborhood exposures and cortisol despite theoretical connections between neighborhood exposures, individual behavior, coping, psychological factors and stress among youth. This research fills a critical gap in the literature because it suggests that risk and promotive factors and cross-level interactions may intervene in the relationship between neighborhoods and physiological stress in adolescents. While researchers have found that promotive factors increase resilience in youth (Masten, Best, & Garmezy, 1990), the relationship between these factors and cortisol is less clear, as physiological expressions of stress may be less mutable regardless of coping, or may be equally influenced by risk and promotive factors as hypothesized in resiliency theory. Our results also suggest that neighborhoods have an independent effect on cortisol, and possibly health, through a stress mechanism. Our support for the biopsychosocial model of stress adds to our understanding the effect of exposure to stressors during adolescence and early adulthood, which may set the foundation for health later in life (Evans, et al., 2007; Goodman, 2005; Murali & Chen, 2005)
Acknowledgements
This research was funded by the National Institute on Drug Abuse, Grant No. DA07484. The research reported here does not necessarily reflect the views or policies of the National Institute on Drug Abuse.
Biographies
Allison Brenner is a research fellow at the University of Michigan in the Department of Epidemiology. She received her doctorate in Health Behavior and Health Education from the University of Michigan School of Public Health. Her major research interests include adolescent risk and promotion, neighborhood context and exposure to stressors in the neighborhood, and psychological and physiological stress and their influence on racial health disparities.
Jose Bauermeister is the John G. Searle Assistant Professor in Health Behavior and Health Education, and Director of the Sexuality & Health Lab (SexLab) at the University of Michigan School of Public Health. He received his doctorate in Health Behavior and Health Education from the University of Michigan, School of Public Health. His primary research interests focus on sexuality and health, and interpersonal prevention and health promotion strategies for high-risk adolescents and young adults. He is Principal Investigator of several projects examining HIV/AIDS risk among young men who have sex with men (YMSM) and other health-related disparities among sexual minorities.
Marc Zimmerman is Chair and Professor in the department of Health Behavior and Health Education at the University of Michigan School of Public Health. He received his Ph.D. in Psychology, Personality, and Social Ecology from University of Illinois. Dr. Zimmerman's research focuses on adolescent health, resiliency and empowerment theory. He examines how positive factors in adolescent's lives help them overcome risks they face. His research includes analysis of adolescent resiliency for risks associated with alcohol and drug use, violent behavior, precocious sexual behavior, and school failure. He is also studying developmental transitions and longitudinal models of change.
Dr. Caldwell is an Associate Professor in Health Behavior and Health Education at the University of Michigan School of Public Health, as well as Director of the Center for Research on Ethnicity, Culture, and Health. She received her Ph.D. in Social Psychology from the University of Michigan. Her research focuses on intergenerational family relationships, discrimination, youth violence, the mental health of African American and Caribbean Black adolescents, and research methods within Black communities.
Footnotes
Allison Brenner is the corresponding author and all co-authors listed below have contributed substantially to the analysis, data collection, or drafting of this manuscript, and all are aware that this manuscript has been resubmitted for review at the Journal of Youth and Adolescence. AB conceived of the study, performed the data analysis, and drafted the manuscript; JB assisted with portions of the data analysis, participated in the drafting of the manuscript, and the revisions; MZ participated in the design of the analysis, drafting of the manuscript and the revisions; CC helped with the conceptualization of the developmental literature and assisted with revisions. All authors read and approved the final manuscript.
The authors declare that they have no conflict of interest.
Contributor Information
Allison B. Brenner, Epidemiology, University of Michigan School of Public Health
Marc A. Zimmerman, Health Behavior and Health Education, University of Michigan School of Public Health
Jose A. Bauermeister, Health Behavior and Health Education, University of Michigan School of Public Health
Cleopatra H. Caldwell, Health Behavior and Health Education, University of Michigan School of Public Health
References
- Almeida D, McGonagle K, King H. Assessing daily stress processes in social surveys by combining stressor exposure and salivary cortisol. Biodemography and Social Biology. 2009;55(2):219–237. doi: 10.1080/19485560903382338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55(5):469–480. [PubMed] [Google Scholar]
- Boardman JD. Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Social Science and Medicine. 2004;58(12):2473–2483. doi: 10.1016/j.socscimed.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Bowling A, Barber J, Morris R, Ebrahim S. Do perceptions of neighbourhood environment influence health? Baseline findings from a British survey of aging. Journal of epidemiology and community health. 2006;60(6):476–483. doi: 10.1136/jech.2005.039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan G, Klevanov P, Sealand N. Do neighborhoods influence child and adolescent development? The American Journal of Sociology. 1993;99(2):353–395. [Google Scholar]
- Brooks-Gunn J, Duncan GJ, Aber JL. Context and Consequences for Children. Vol. 1. New York City: Russell Sage Foundation; 1997. [Google Scholar]
- Browning C, Cagney K. Moving beyond poverty: neighborhood structure, social processes, and health. Journal of Health and Social Behavior. 2003;44(4):552–571. [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum, von Auer K, Krieger S, Weis S, Rauh W, Hellhammer D. Blunted cortisol responses to psychosocial stress in asthmatic children: A general feature of Atopic Disease? Psychosomatic Medicine. 2003;65:806–810. doi: 10.1097/01.psy.0000095916.25975.4f. [DOI] [PubMed] [Google Scholar]
- Chandola T, Heraclides A, Kumari M. Psychophysiological biomarkers of workplace stressors. Neuroscience and Biobehavioral Reviews. 2010;35(1):51–57. doi: 10.1016/j.neubiorev.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Paterson LQ. Neighborhood, family, and subjective socioeconomic status: How do they relate to adolescent health? Health Psychology. 2006;25(6):704–714. doi: 10.1037/0278-6133.25.6.704. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson N, Clark V, Williams D. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54(10):805–816. doi: 10.1037//0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen J, Kamark T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98(2):310–357. [PubMed] [Google Scholar]
- Cook T, Herman M, Phillips M, Settersten RJ. Some ways in which neighborhood, nuclear families, friendship groups, and schools jointly affect changes in early adolescent development. Child Development. 2002;73(4):1283–1309. doi: 10.1111/1467-8624.00472. [DOI] [PubMed] [Google Scholar]
- Crimmins E, Seeman T. Integrating biology into the study of health disparities. Population and Development Review. 2004;30:89–107. (Supplement: Aging, Health and Public Policy) [Google Scholar]
- Cutrona CE, Russell DW, Brown PA, Clark LA, Hessling RM, Gardner KA. Neighborhood Context, personality, and stressful life events as predictors of depression among African American women. Journal of Abnormal Psychology. 2005;114(1):3–15. doi: 10.1037/0021-843X.114.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals New York Academy of Sciences. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, et al. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The Multi-Ethnic Study of Atherosclerosis. Health & Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J, Ranjit N, Do D, Young E, House J, Kaplan G. Education and levels of salivary cortisol over the day in US adults. Annals of Behavioral Medicine. 2011;41(1):13–20. doi: 10.1007/s12160-010-9224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd J, Simanek A, Aiello A. Socio-economic status, cortisol and allostatic load: a review of the literature. International Journal of Epidemiology. 2009;38:1297–1309. doi: 10.1093/ije/dyp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighbourhoods matter? Neighbourhood disorder and long-term trends in serum cortisol levels. Journal of Epidemiology and Community Health. 2012;66(1):24–29. doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. The stress process in neighborhood context. Health & Place. 2000;6(4):287–299. doi: 10.1016/s1353-8292(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Engel G. The clinical application of the biopsychosocial model. The American Journal of Psychiatry. 1980;137(5):535–544. doi: 10.1176/ajp.137.5.535. [DOI] [PubMed] [Google Scholar]
- Ensel WM, Lin N. The life stress paradigm and psychological distress. Journal of Health and Social Behavior. 1991;32(4):321–341. [PubMed] [Google Scholar]
- Estrada-Martínez L, Caldwell C, Bauermeister J, Zimmerman M. Stressors in multiple life-domains and the risk for externalizing and internalizing behaviors among African Americans during emerging adulthood. Journal of Youth and Adolescence. :1–13. doi: 10.1007/s10964-012-9778-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43(2):341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- Fergus S, Zimmerman MA. Adolescent resilience: A framework for understanding healthy development in the face of risk. Annual Review in Public Health. 2005;26:399. doi: 10.1146/annurev.publhealth.26.021304.144357. [DOI] [PubMed] [Google Scholar]
- Fernald LCH, Gunnar MR. Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science and Medicine. 2009;68(12):2180–2189. doi: 10.1016/j.socscimed.2009.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. Personal control and stress and coping processes: A theoretical analysis. Journal of Personality and Social Psychology. 1984;46(4):839–852. doi: 10.1037//0022-3514.46.4.839. [DOI] [PubMed] [Google Scholar]
- Foster H, Hagan J, Brooks-Gunn J. Growing up fast: Stress exposure and subjective "weathering" in emerging adulthood. Journal of Health and Social Behavior. 2008;49(2):162–177. doi: 10.1177/002214650804900204. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological Review. 2010;117(1):134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmezy N. Resiliency and vulnerability to adverse developmental outcomes associated with poverty. The American Behavioral Scientist. 1991;34(4):416–430. [Google Scholar]
- Garmezy N, Masten AS, Taylor R. The study of stress and competence in children: A building block for developmental psychopathology. Child Development. 1984;55(1):97. [PubMed] [Google Scholar]
- Gary TL, Stark SA, LaVeist TA. Neighborhood characteristics and mental health among African Americans and whites living in a racially integrated urban community. Health & Place. 2007;13(2):569–575. doi: 10.1016/j.healthplace.2006.06.001. [DOI] [PubMed] [Google Scholar]
- George LK, Lynch SM. Race differences in depressive symptoms: A dynamic perspective on stress exposure and vulnerability. Journal of Health and Social Behavior. 2003;44(3):353–369. Special Issue: Race, Ethnicity, and Mental Health. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. "Weathering" and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersten O. Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Social Science & Medicine. 2008;66(3):507–519. doi: 10.1016/j.socscimed.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales On the limits of coping: Interaction between stress and coping for inner-city adolescents. Journal of Adolescent Research. 2001;16(4):372–395. [Google Scholar]
- Goodman E. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosomatic Medicine. 2005;67(1):9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35(9):1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, et al. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2010;35(6):932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LS, Abela JRZ, Watamura SE. Hypothalamic pituitary adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68(5):484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hill TD, Ross CD, Angel RJ. Neighborhood disorder, psychophysiological distress, and health. Journal of Health Social Behavior. 2005;46(2):170–186. doi: 10.1177/002214650504600204. [DOI] [PubMed] [Google Scholar]
- Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: Chronic stress, the HPA axis, and physical and mental health disparities over the life course. American Journal of Public Health. 2009;99(12):1–7. doi: 10.2105/AJPH.2008.143446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. John Henryism and the health of African Americans. Culture of Medicine and Psychiatry. 1994;18:163–182. doi: 10.1007/BF01379448. [DOI] [PubMed] [Google Scholar]
- James S, Keenan N, Strogatz D, Browning S, Garrett J. Socioeconomic status, John Henryism, and blood pressure in black adults. American Journal of Epidemiology. 1992;135(1):59–67. doi: 10.1093/oxfordjournals.aje.a116202. [DOI] [PubMed] [Google Scholar]
- James SA, Hartnett SA, Kalsbeek WD. John Henryism and blood pressure differences among black men. Journal of Behavioral Medicine. 1983;6(3):259–278. doi: 10.1007/BF01315113. [DOI] [PubMed] [Google Scholar]
- James SA, Strogatz DS, Wing SB, Ramsey DL. Socioeconomic status, John Henryism, and hypertension in Blacks and Whites. American Journal of Epidemiology. 1987;126(4):664–673. doi: 10.1093/oxfordjournals.aje.a114706. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Neighbors HW. A new perspective on the relationship among race, social class, and psychological distress. Journal of Health and Social Behavior. 1986;27(2):107–115. [PubMed] [Google Scholar]
- Kliewer W, Reid-Quinones K, Shields BJ, Foutz L. Multiple risks, emotion regulation skill, and cortisol in low-income African American youth: A prospective study. Journal of Black Psychology. 2009;35(1):24–43. [Google Scholar]
- Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Latkin C, Curry A. Stressful neighborhoods and depression: A prospective study of the impact of neighborhood disorder. Journal of Health and Social Behavior. 2003;44(1):34–44. [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, Appraisal and Coping. New York: Springer; 1984. [Google Scholar]
- Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortsiol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lyons R, Mickelson K, Sullivan M, Coyne J. Coping as a communal process. Journal of Social and Personal Relationships. 1998;15(5):579–605. [Google Scholar]
- Mair C, Diez Roux AV, Shen M, Shea S, Seeman T, Echeverria S, et al. Cross-sectional and longitudinal associations of neighborhood cohesion and stressors with depressive symptoms in the Multiethnic Study of Atherosclerosis. Annals of Epidemiology. 2009;19(1):49–57. doi: 10.1016/j.annepidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey DS. Segregation and stratification: A biosocial perspective. Du Bois Review. 2004;1(1):7–25. [Google Scholar]
- Masten AS, Best KM, Garmezy N. Resilience and development: Contributions from the study of children who overcome adversity. Development and Psychopathology. 1990;2:425. [Google Scholar]
- Mayer S, Jencks C. Growing up in poor neighborhoods: How much does it matter? Science. 1989;243(4897):1441–1445. doi: 10.1126/science.243.4897.1441. [DOI] [PubMed] [Google Scholar]
- McEwen B, Seeman T. Protective and damaging effects of mediators of stress. Annals New York Academy of Science. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Murali R, Chen E. Exposure to violence and cardiovascular and neuroendocrine measures in adolescents. Annals of Behavioral Medicine. 2005;30(2):155–163. doi: 10.1207/s15324796abm3002_8. [DOI] [PubMed] [Google Scholar]
- Myers H. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. Journal of Behavioral Medicine. 2009;32:9–19. doi: 10.1007/s10865-008-9181-4. [DOI] [PubMed] [Google Scholar]
- Nakao K, Treas J. Computing 1989 occupational prestige scores. Chicago: National Opinion Research Center; 1990. [Google Scholar]
- Pearlin L. The sociological study of stress. Journal of Health and Social Behavior. 1989;30(3):241–256. [PubMed] [Google Scholar]
- Peek MK, Cutchin M, Salinas J, Sheffield K, Eschbach K, Stowe R, et al. Allostatic load among non-Hispanic whites, non-Hispanic blacks, and people of Mexican origin: effects of ethnicity, nativity, and acculturation. American Journal of Public Health. 2010;100(5):940. doi: 10.2105/AJPH.2007.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett K, Pearl M. Multilevel analysis of neighbourhood socioeconomic context and health outocmes: A critical review. Journal of Epidemiology and Community Health. 2001;55(2):111–122. doi: 10.1136/jech.55.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procidano M, Heller K. Measures of perceived social from friends and from family: Three validation studies. Journal of Community Psychology. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Raudenbush S, Bryk A, Congdon R. HLM 6 for Windows (Version 6) Lincolnwood: Scientific International Software, Inc.; 2004. [Google Scholar]
- Repetto PB, Zimmerman MA, Caldwell CH. A longitudinal study of the relationship between depressive symptoms and alcohol use in a sample of inner-city black youth. Journal of Studies on Alcohol. 2004;65(2):169–178. doi: 10.15288/jsa.2004.65.169. [DOI] [PubMed] [Google Scholar]
- Ross CE, Jang S. Neighborhood disorder, fear, and mistrust: The buffering role of social ties with neighbors. American Journal of Community Psychology. 2000;28(4):401–420. doi: 10.1023/a:1005137713332. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J, Pribesh S. Powerlessness and the amplification of threat: Neighborhood disadvantage, disorder, and mistrust. American Sociological Review. 2001;66(4):568–591. [Google Scholar]
- Sampson RJ, Raudenbush S, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Schempf A, Strobino D, O'Campo P. Neighborhood effects on birthweight: An exploration of psychosocial and behavioral pathways in Baltimore, 1995–1996. Social Science and Medicine. 2009;68(1):100–110. doi: 10.1016/j.socscimed.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeelk-Cone K, Zimmerman M, Abelson J. The buffering effects of active coping on the relationship between SES and cortisol among African American young adults. Behavioral Medicine. 2003;29(2):85–94. doi: 10.1080/08964280309596061. [DOI] [PubMed] [Google Scholar]
- Schmeelk-Cone KH, Zimmerman M. A longitudinal analysis of stress in African American youth: predictors and outcomes of stress trajectories. Journal of Youth and Adolescence. 2003;32(6):419–430. [Google Scholar]
- Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and Psychopathology. 2011;23(Special Issue 04):1167–1186. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Annals of Behavioral Medicine. 2001;23(3):177–185. doi: 10.1207/S15324796ABM2303_5. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Staudenmayer J, Cohen S, Enlow MB, Rich-Edwards JW, Wright RJ. Cumulative stress and cortisol disruption among Black and Hispanic pregnant women in an urban cohort. Psychological Trauma: Theory, Research, Practice, and Policy. 2010;2(4):326–334. doi: 10.1037/a0018953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits P. Stress, coping and social support processes: Where are we? What next? The Journal of Health and Social Behavior. 1995;35:53–79. [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Lifetime trauma and mental health: The significance of cumulative adversity. Journal of Health and Social Behavior. 1995;36:360–376. [PubMed] [Google Scholar]
- U.S. Census Bureau GD. Cartiographic Boundary Files. 2001, 2011 Retrieved January 30, 2012, from http://www.census.gov/geo/www/cob/tr_metadata.html.
- Unger DG. The importance of neighbors: The social, cognitive, and affective components of neighboring. American Journal of Community Psychology. 1985;13(2):139–169. [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosomatic Medicine. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Wenzel L, Glanz K, Lerman C. Stress, coping and health behavior. In: Glanz K, Rimer B, Lewis F, editors. Health Behavior and Health Education. 3rd ed. San Francisco: Jossey-Bass; 2002. pp. 210–239. [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models. 1st ed. Boca Raton: Taylor & Francis, Inc.; 2006. [Google Scholar]
- Worthman CM, Costello J. Tracking biocultural pathways in population health: The value of biomarkers. Annals of Human Biology. 2009;36(3):281–297. doi: 10.1080/03014460902832934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Brenner A. Resilience in Adolescence: Overcoming neighborhood disadvantage. In: Reich J, Zautra A, Hall J, editors. Handbook of Adult Resilience: Concepts, Methods, and Applications. New York: Guilford Press; 2009. pp. 283–308. [Google Scholar]