Abstract
The pineal gland is a neuroendocrine gland responsible for nocturnal synthesis of melatonin. During early development of the rodent pineal gland from the roof of the diencephalon, homeobox genes of the orthodenticle homeobox (Otx)- and paired box (Pax)-families are expressed and are essential for normal pineal development consistent with the well-established role that homeobox genes play in developmental processes. However, the pineal gland appears to be unusual because strong homeobox gene expression persists in the pineal gland of the adult brain. Accordingly, in addition to developmental functions, homeobox genes appear to be key regulators in postnatal phenotype maintenance in this tissue.
In this paper, we review ontogenetic and phylogenetic aspects of pineal development and recent progress in understanding the involvement of homebox genes in rodent pineal development and adult function. A working model is proposed for understanding the sequential action of homeobox genes in controlling development and mature circadian function of the mammalian pinealocyte based on knowledge from detailed developmental and daily gene expression analyses in rats, the pineal phenotypes of homebox gene-deficient mice and studies on development of the retinal photoreceptor; the pinealocyte and retinal photoreceptor share features not seen in other tissues and are likely to have evolved from the same ancestral photodetector cell.
Keywords: pineal gland, homeobox gene, rodent, brain development, circadian rhythm
1. Introduction: homeobox genes as developmental regulators
Homeobox genes encode a large family of conserved transcription factors; these genes are molecularly defined by the presence of the homeobox, a well-defined 180 bp sequence encoding a definitive DNA-binding homeodomain [1,2]. Homeobox genes are involved in regulation of developmental processes, cellular differentiation and morphogenesis in all metazoans [3]. The initially identified homeobox genes, referred to as Hox genes, are arranged in genomic clusters, the organization of which closely resembles the nested expression patterns along the cranio-caudal axis of the animal [4]. This relation is now the text book example of colinearity; however, no Hox code exists for the most rostral part of the central nervous system, where development is controlled by so-called dispersed homeobox genes including members of the Otx (orthodenticle homeobox), Rax (retina and anterior neural fold homeobox) and Pax (paired-box) families.
Although the general developmental role of homeobox genes is well-established, a growing body of evidence suggests a second function in healthy adult tissues [5,6] primarily including cell types undergoing frequent renewal such as blood cells, endometrium and epithelia of the gastrointestinal tract; in these tissues, homeobox genes appear to be involved in adult cell proliferation and differentiation. However, within recent years persistent and restricted expression of homeobox genes in the postnatal central nervous system especially in the pineal gland has been reported [7–11]. In the following sections, we highlight ontogenetic and phylogenetic aspects of pineal development and recent progress in understanding the roles homeobox genes play in the rodent pineal gland throughout life.
2. Development and circadian function of the mammalian pineal gland
The mammalian pineal gland is a neuroendocrine organ, which converts photoperiodic information into the nocturnal hormonal signal of melatonin [12]. In most mammals, the pineal gland is located directly on the dorsal part of the brain stem at the mesencephalic-diencephalic border, whereas in rodents it is subdivided into a superficial part located on top of the brain just caudal to the hemispheres and a deep part consisting of a collection of pinealocytes located between the habenular and posterior commissures. The superficial and deep parts are connected by a thin pineal stalk (Fig. 1). The parenchyma of the pineal gland consists of the predominant melatonin-producing pinealocytes (about 95%), astrocyte-like interstitial cells (about 4%) and macrophages (< 1%) [13].
Figure 1. Anatomy of the developing pineal gland.
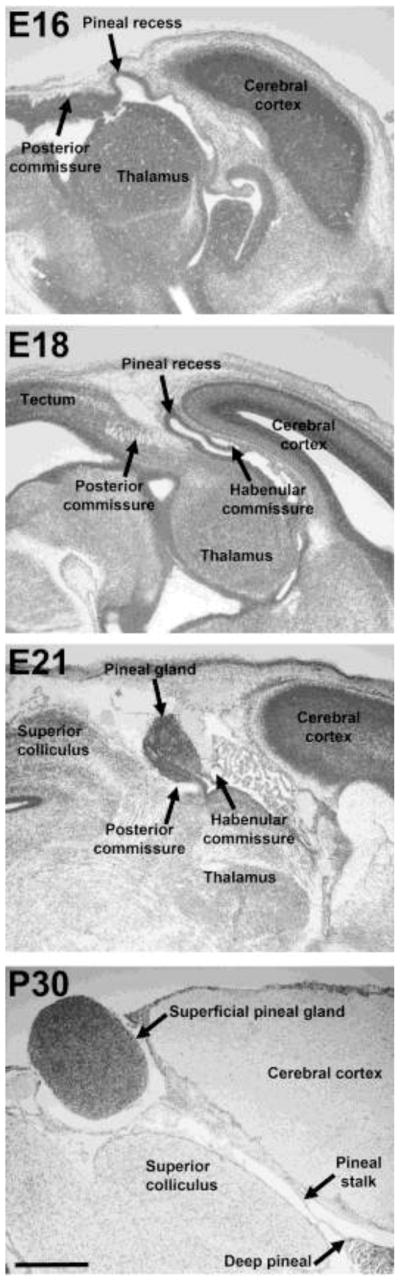
Nissl-stained sagittal sections of the brain including the epithalamus of rats at indicated developmental ages. E, embryonic day; P, postnatal day. Scale bar, 1 mm.
2.1 Cellular development of the rat pineal gland
The pineal gland develops as a tubular evagination, the pineal recess, from the most caudal part of the diencephalic roof between the habenular and posterior commissures (Fig. 1). The pineal evagination appears around embryonic day 16 (E); in the following days of development, the pineal recess elongates (E18), before the lumen gradually disappears. Around birth, the pineal gland appears as a solid organ with a small proximal cleft communicating with the third ventricle (E21), but postnatally, the superficial pineal gland is only connected to the third ventricle via the pineal stalk and the deep pineal gland (P30) [14–16]. Pineal cell proliferation is high in the prenatal gland and ceases rapidly during the two first postnatal weeks [17]. These observations are in line with ultrastructural studies showing that pre- and neonatal pineal cells are undifferentiated pinealoblasts and that differentiation into pinealocytes takes place also during the first two weeks of postnatal life [18,19], thus suggesting that the pinealocytes should be regarded as mature cells afterwards.
2.2 Development of pineal circadian function
The physiological function of the pineal gland is to entrain circadian rhythms to the daily photoperiod via nocturnal secretion of melatonin; melatonin is synthesized from serotonin by sequential action of the pineal enzymes arylalkylamine N-acetyltransferase (AANAT) and acetylserotonin O-methyltransferase (ASMT). The activity of AANAT exhibits large daily changes driving the daily rhythm in pineal melatonin synthesis [20]. The pineal gland is innervated by peripheral ganglia and directly from the central nervous system; the most prominent input to the gland is the dense innervation with sympathetic fibers originating from perikarya in the superior cervical ganglia [21]. The sympathetic nerve terminals in the pineal are located in the perivascular spaces but also penetrate into the pineal parenchyma; however, the sympathetic terminals do not make synapse-like contacts with pinealocytes. These sympathetic fibers constitute the last part of a multisynaptic pathway linking the hypothalamic circadian master clock in the suprachiasmatic nucleus to nocturnal release of norepinephrine from sympathetic nerve terminals in the pineal gland. In the pineal gland, norepinephrine elevates the levels of cyclic AMP, which in turn induces a nighttime increase in AANAT enzyme activity [20]. In the rodent pineal gland, the nocturnal increase in AANAT activity is regulated at transcriptional and posttranslational levels. There is a prominent night-day rhythm (>100-fold) in Aanat transcript abundance [22]. This rhythm is mediated by cis-regulatory cyclic AMP responsive elements (CREs) in the Aanat promoter [23]; transcription is activated by the CRE-binding protein (CREB) upon cyclic AMP-dependent phosphorylation by protein kinase A [24]. Daily rhythms in the pineal transcriptome are broadly driven by this norepinephrine-cyclic AMP mechanism [10]. At the posttranslational level, AANAT activity is controlled by nocturnal cyclic AMP-dependent phosphorylation by protein kinase A; the phosphorylated AANAT reversibly binds 14-3-3 protein and represents the stable and activated form of AANAT [20].
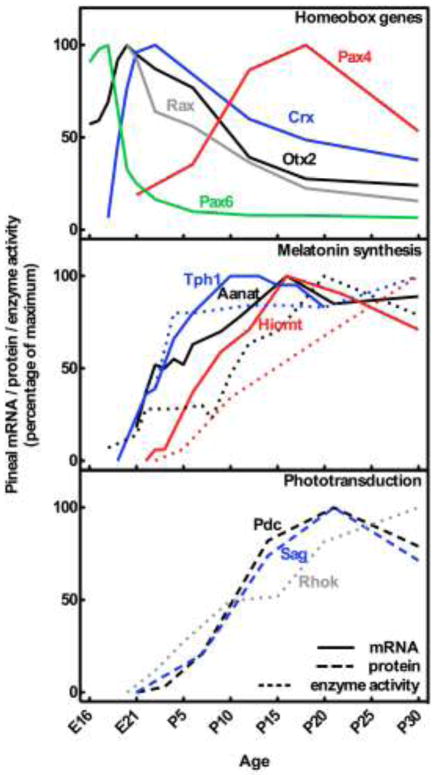
Preceded by the developmental appearance of transcripts encoding the above mentioned melatonin enzymes (Fig. 2), melatonin synthesis is first detectable at P5 in the rat pineal gland [25]. However, day-night rhythms in pineal melatonin synthesis depend on both structural and molecular maturation of the circadian system [26,27], including establishment of the projections terminating with the sympathetic innervation of the pineal. In the rat, sympathetic fibers have been shown to penetrate the pineal parenchyma at P5 [28]; this time point coincides with the first rhythmic expression of the Aanat gene [29] as well as rhythmic AANAT enzyme activity [30]. The amplitude in AANAT activity rapidly increases, and rhythmic pineal melatonin synthesis is detectable between P8 and P11[31,32], thus marking the ontogenetic establishment of mature pineal physiological function.
Figure 2. Ontogenetic expression of homeobox gene transcripts (upper part) and gene products involved in melatonin synthesis (middle part) and phototransduction (lower part) in the rat pineal gland.
Curves are based on data from several studies on the developing rat pineal gland: Aanat, arylalkylamine N-acetyltransferase [29,30]; Crx, cone-rod homeobox [7]; Asmt, acetylserotonin O-methyltransferase or hydroxyindole O-methyltransferase [32,121]; Otx2, orthodenticle homeobox 2 [7]; Pax4, paired box 4 [8]; Pax6, paired box 6 [8]; Pdc, phosducin or MEKA [122]; Rax, retina and anterior neural fold homeobox [9]; Rhok, rhodopsin kinase or G protein-coupled receptor kinase 1 [123]; Sag, S-antigen or arrestin [122]; Tph1, tryptophan hydroxylase 1 [28,124].
In addition to control of daily rhythmicity, the sympathetic innervation of the pineal may also have direct developmental implications on the mammalian pinealocyte. Light sensing properties of the neonatal pineal gland are supported by experiments showing that neonatal pinealocytes in culture display rhodopsin immunoreactivity [33] and that exposure to light inhibits melatonin production [34]. Including norepinephrine in the culture medium, thereby mimicking the sympathetic innervation, abolishes the apparent photosensitive capacity of the neonatal pinealocyte [33,34]. This set of observations suggests that the sympathetic innervation of the gland suppresses early photoreceptor-like characteristics of the immature pinealocyte and may be essential for proper pinealocyte maturation.
3. Evolution of the mammalian pinealocyte: pineal and retinal similarities
The capacity to synthesize melatonin appears to have evolved in an ancestral photodetector cell very early in chordate evolution as a detoxification pathway [35,36]. This ancestral photodetector cell is thought to have diverged into two lineages in vertebrates, one being the retinal photoreceptor optimized for efficient phototransduction and the other being the pinealocyte optimized for melatonin synthesis.
The pineal gland of sub-mammalian vertebrates, e.g. fishes, amphibians, reptiles and birds, is a complex photoreceptive organ located directly beneath the skull or as in amphibian and reptilian species with an extracranial location referred to as “the third eye” [37,38]. This extracranial portion, also known as the frontal or parietal organ, is connected to the brain via a pineal nerve and tract. In most sub-mammalian species, the pineal organ is composed of cells endowed with both light-sensing properties [39,40] and nocturnal melatonin production guided by daily oscillations in AANAT activity [41,42]. Light sensing properties of the pineal gland of non-mammalian vertebrates have been interpreted as an earliest phylogenetic evidence of a common ancestral origin of the pinealocyte and the retinal photoreceptor.
Based on ultrastructural studies on the pineal organ of various vertebrates, Collin [43] divided the cells of the pinealocyte lineage into three classes: 1) The true pineal photoreceptor found in anamniotes bear an outer segment consisting of numerous discs connected to an inner segment via a cilium, thus closely resembling the retinal photoreceptor; furthermore, the inner somal part with the perikaryon of the true pineal photoreceptor forms synapse with intrapineal neurons connecting the pineal photoreceptors to the brain via the pineal nerve [44]. 2) The rudimentary pineal photoreceptor, found in most reptile and avian species, is characterized by a less regular light-sensing outer segment and does not synapse with intrapineal neurons. However, secretory granules are often seen in the basal part of these photoreceptors. 3) The mammalian pinealocyte sensu strictu lacks outer and inner segments, but a cilium can be found in some pinealocytes [43,45]. These morphological characteristics presumably reflect a gradual evolutionary transformation of a sensory cell line, like the retinal photoreceptor, into the mammalian secretory pinealocyte without photoreceptive properties [43]. A later revision of the theory of pineal evolution stresses the importance of changes in molecular regulatory mechanisms within the pineal field of the neural plate during vertebrate evolution restricting the development of pineal photoreceptors in mammals [46]; this implies changes in cell fate restriction rather than transformation within a single cell lineage [47].
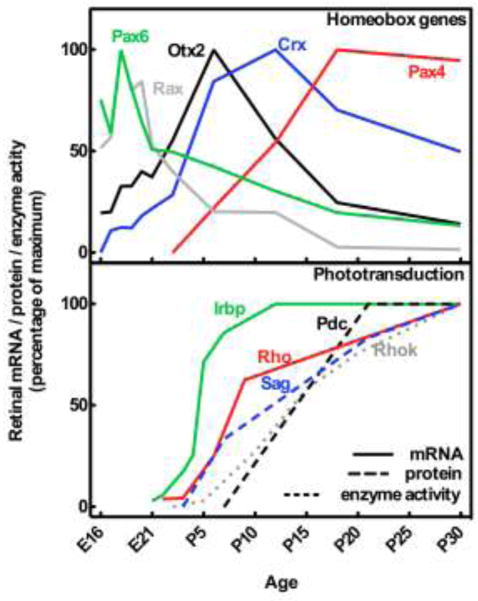
The close relationship between the retinal photoreceptor and the pinealocyte is also supported by the results of ontogenetic studies in mammals. In neonatal rats, a subset of the developing pinealocytes transiently exhibits photoreceptor characteristics, e.g. rudimentary outer segments [48]. This is in accord with gene expression studies showing that the pineal gland of neonatal rats expresses all of the molecular components required for establishing functional phototransduction [49]. The transient existence of a pineal nerve in fetal mammals connecting the pineal and dorsal diencephalon, homologous to the pineal tract and nerve in other vertebrates, also supports a probably rudimentary sensory property of the early mammalian pineal gland [50,51].
Further evidence for a common origin of the pinealocyte and retinal photoreceptor comes from studies showing that numerous gene products otherwise involved in retinal phototransduction are also expressed in the adult mammalian pineal gland (Fig. 2; Fig. 3). These include rhodopsin kinase [52], S-antigen [53], phosducin [54], recoverin [55], interphotoreceptor retinol-binding protein [56], aryl-hydrocarbon-interacting protein-like 1 [10], phosphodiesterase 6B [10] and uncoordinated protein 119 [10]. Whereas rhodopsin expression is either regarded as diffuse or absent in the adult pineal gland [57], the expression of shortwave opsin is similar to that in the retina [10]. Similarly, the retina expresses genes dedicated to melatonin synthesis, albeit at distinctly lower levels than in the pineal gland; the capacity to synthesize melatonin in small quantities is seen in all vertebrates [58–60]. Retinal Aanat expression is localized in photoreceptors [61,62] and exhibits a day-night rhythm [63], but as opposed to the sympathetic neural regulation in the pineal gland, retinal Aanat transcription is driven directly by the endogenous retinal circadian clock via E-boxes in the Aanat promoter [64].
Figure 3. Ontogenetic expression of homeobox gene transcripts (upper part) and gene products involved in phototransduction (lower part) in the rat retina.
Curves are based on data from several studies on the developing rat retina: Crx, cone-rod homeobox [86]; Irbp, interphotoreceptor retinol-binding protein or retinol binding protein 3 [125]; Otx2, orthodenticle homeobox 2 [86]; Pax4, paired box 4 [76]; Pax6, paired box 6 [76]; Pdc, phosducin or MEKA [122]; Rax, retina and anterior neural fold homeobox [9]; Rho, rhodopsin or opsin [126]; Rhok, rhodopsin kinase or G protein-coupled receptor kinase 1 [123]; Sag, S-antigen or arrestin [122]
The most parsimonious explanation of pineal-retinal similarities is that they are due to expression of a common set of homeobox genes, which direct developmental regulatory functions and tissue-specific expression of genes involved in melatonin synthesis and phototransduction in both the retina and the pineal gland (Fig. 2; Fig. 3).
3. Homeobox genes in pineal development
Homeobox genes including members of the Pax- and Otx-families as well as Rax are detectable in the pineal gland. Among these, Otx2 and Pax6 are essential for development of the murine pineal [65,66].
3.1 Pax genes in pineal gland development
Pax6 is extensively expressed in the developing central nervous system [67]. An intriguing aspect of Pax6 biology is its association with eye development throughout the animal kingdom [68]; Pax6 exhibits a diversity of regulatory functions in several cell lineages of the eye [69]. Pax6-deficient mice exhibit an early arrest of eye morphogenesis with a severe reduction in proliferation of retinal progenitor cells [70–72]. Further, Pax6 maintains the multipotent state of retinal progenitors and controls the timing of differentiation [73], thus showing an early developmental role of Pax6 in the retina.
Detailed quantitative in situ hybridization analyses of Pax6 expression in the developing rat pineal gland from E16 into adulthood have revealed a strong prenatal expression peaking around E18 (Fig. 2) [8]. Pax6 transcripts are detectable in the dorsal roof of the diencephalon and the adjacent pretectal area with a remarkable signal in the pineal recess (Fig. 4A), which also exhibits a strong PAX6 immunoreactivity (Fig. 4B), establishing PAX6 as an early marker of pineal development. Pax6-deficient mice exhibit a lack of diencephalic dorsal midline structures, including the pineal recess [66]; mutations in PAX6 results in the absence of the pineal gland in human subjects [74,75]
Figure 4. PAX6 is an early marker of pineal development and is only detectable at low levels in the adult.
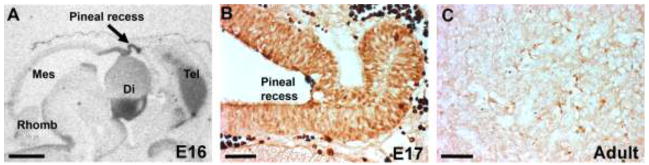
A) In situ hybridization for detection of Pax6 mRNA in a sagittal section of the rat brain at E16. Note the strong signal in the epithelium around the pineal recess (arrow). Di, diencephalon; Mes, mesencephalon; Rhomb, rhombencephalon; Tel, telencephalon. Scale bar, 1 mm. B) Immunohistochemical detection of PAX6 protein in the developing rat pineal gland at E17. Scale bar, 50 μm. The fetal animal was not perfused; therefore, the very dark cells are erythrocytes with a high level of endogenous peroxidase. C) Immunohistochemical detection of PAX6 protein in the pineal gland of a perfusion fixed adult rat. Scale bar, 50 μm. For methodological details, see [76].
The close relationship between the retina and the pineal gland, as well as the known function of Pax6 in retinal development, makes it reasonable to compare the expression pattern of Pax6 in the pineal gland to that of the rat retina [76], in which Pax6 expression peaks in undifferentiated retinal progenitor cells during the last intrauterine days (Fig. 3). At the time of Pax6 dominance in the developing gland, the pineal cells are undifferentiated pinealoblasts [8,18], suggesting that pineal Pax6 in line with the situation in the developing retina controls proliferation and maintenance of differentiation potential of immature pinealocytes in early pineal gland development (Fig. 5A).
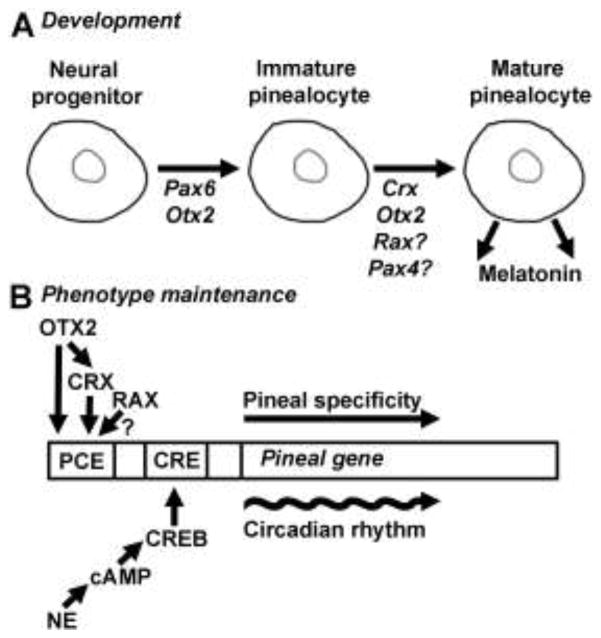
Figure 5. Potential roles of the pineal homeobox genes.
A) Development of the pinealocyte: The proliferation, differentiation and maturation processes of the mammalian pinealocyte are driven by homeodomain transcription factors acting sequentially at different developmental stages. B) Tissue-specific gene expression in the mature pinealocyte: Intrinsic pineal factors in the form of homeodomain proteins control pineal-specific gene expression by binding cis-acting photoreceptor conserved elements (PCEs). On top of this, extrinsic factors in the form of norepinephrine released from sympathetic nerve terminals during nighttime drive cAMP-dependent circadian rhythms in pineal gene expression. For simplicity, only one copy of each cis-element is displayed. The rat Aanat promoter contains at least three PCEs; two of these preferentially bind OTX/CRX, whereas the third might prefer RAX, as indicated by Genomatix promoter analysis. The roles of Pax4 and Rax in the pinealocyte are not yet established, as indicated by question marks (?). CRE, cAMP responsive element; CREB, cAMP responsive element binding protein; NE, norepinephrine; PCE, photoreceptor conserved element, this term refers a group of cis-elements with slightly different core sequences including both OTX2/CRX- and RAX-binding sequences [102].
In both the pineal gland and retina, the prenatal Pax6 expression peak is followed by a rapid perinatal decline (Fig. 2; Fig. 3), which is in line with a terminated developmental role. Notably, Pax6 expression persists in the inner retina [76] and a limited number of pinealocytes are also PAX6-immunopositive in the adult pineal gland (Fig. 4C); however, adult functions of Pax6 in these non-renewable tissues are currently unknown. The PAX6-immunopositive pinealocytes of the mature pineal gland may represent a population of pinealocyte precursors.
The extensive diversity of the pineal transcriptome has been revealed by microarray analysis [10]; among the detected transcripts were Pax4, which is closely related to Pax6, and is essential for development of mature insulin-producing β-cells in the pancreas [77]. Ontogenetic analysis of Pax4 in the rat pineal revealed an expression pattern, which is in marked contrast to that in the pancreatic islets. Pineal Pax4 expression starts around birth and persists into adulthood [8], whereas the gene is strongly expressed early in prenatal pancreatic development [77]. Accordingly, it appears that whereas Pax4 primarily has a developmental role in the pancreas, a regulatory role in the differentiated pinealocyte is more likely in the pineal gland (Fig. 5A). A regulatory function in the mature pinealocyte is supported by the observation that the developmental appearance of pineal Pax4 transcripts is coincident with that of genes required for core pineal functions, e.g. melatonin synthesis (Fig. 2). A similar pattern of expression has been reported in the developing retina of the rat (Fig. 3), where expression is restricted to postnatal photoreceptor cells [76]. Studies on the pancreas have emphasized the antagonizing trans-repressing functions of PAX4 on promoters that are otherwise activated by PAX6. [78]. In mature pinealocytes and retinal photoreceptors, the antagonizing actions of PAX4 therefore may counteract the developmental role of PAX6 to ensure maintenance of the fully differentiated phenotype. Studying the specific function of Pax4 in the rodent pineal gland has been frustrated by the perinatal death of the Pax4 knockout mouse [77] and also the difficulties in analyzing Pax4 in the mouse pineal gland due to very low expression levels, which suggest a limited degree of conservation in pineal Pax4 biology among rodents.
3.2 Otx genes in pineal development
Members of the mammalian Otx-family expressed in the pineal gland include Otx2 and Crx [7,79–81]. Otx2 is expressed from embryonic pre-gastrulation stages and in the developing central nervous system becomes restricted to the pros- and mesencephalic brain regions [79]; Otx2 knockout mice fail to develop rostral head structures [82,83]. As is the case for Pax6, a broad involvement in brain development is accompanied by a central role in eye development [82]. However, within the retina, Otx2 acts at later developmental stages than the progenitor-promoting Pax6 to steer the progenitors towards a photoreceptor fate [65,84]. This division of labor is reflected by the delayed temporal expression of Otx2 as compared to Pax6 (Fig. 3). In contrast to the broad involvement of Otx2 in development of the central nervous system, Crx expression and function is strongly restricted to the pineal gland and retinal photoreceptors [81,80,85]. The Crx-deficient retina displays a reduction in cellular integrity of the outer nuclear layer and absence of photoreceptor outer segments [85]. OTX2 is known to trans-activate Crx [65], which is expressed later in the retinal photoreceptors (Fig. 3) [86] and is believed to induce terminal differentiation of the photoreceptor cells [65,84].
Otx2 expression is detectable in the rat pineal gland at all developmental stages (Fig. 2)[7]; this pattern is in accord with the involvement of Otx2 in both development of the pineal gland [65,87] and maintenance of the pinealocyte phenotype later in life (Fig. 5). A conditional knockout mouse, in which the Otx2 gene is specifically inactivated in pinealocytes and retinal photoreceptor cells, has made it possible to investigate the specific role of Otx2 in these tissues [65]; this mouse exhibits a total lack of pinealocytes and retinal photoreceptors, indicating an essential role for Otx2 in development of both cell types. In this model, the Crx promoter was used to drive tissue-specific Otx2 deletion [65]; since pineal Crx expression in the rat pineal is initiated at E18 [7], well after establishment of the Otx2 expressing pineal recess, the role of Otx2 in initiation and early development of the pineal is not established. However, based on the high expression levels during early pineal development (Fig. 2), it seems appropriate to suggest a role for Otx2 in early pinealocyte specification (Fig. 5A), albeit at a later developmental stage than Pax6. Furthermore, the postnatal lack of pineal gland in the Otx2 conditional knockout indicates a role of Otx2 in promoting survival of immature pinealocytes.
The pineal gland of the Crx knockout develops normally [85,88], suggesting that Crx, in contrast to the situation in the retina, is not essential for pineal gland development. However, double knockout studies suggest that the apparent integrity of the pineal gland is due to complementation by Otx2 [87]. As indicated above, pineal expression of Crx starts and peaks later than that Otx2 (Fig. 2); this is in agreement with the trans-activating function of OTX2 on the Crx-promoter [65] and a later role of Crx in pineal development as compared to Otx2. Crx expression peaks around birth and remains high in the first postnatal weeks supporting a role in terminal differentiation of the mature pinealocyte (Fig. 2; Fig. 5), as is the case in the retinal photoreceptor. Notably, CRX itself trans-activates and confers tissue-specific expression of a number of genes involved in phototransduction and melatonin synthesis in the pineal gland and retina [80,81,85,88–91] (see section 4). In both tissues, the developmental appearance of Crx transcripts immediately precedes that of melatonin- and vision-related gene products with clearly defined physiological roles (Fig. 2; Fig. 3).
The similarity in sequential expression patterns of each homeobox gene in the pineal gland and retina indicates that these genes may have conserved roles in development of each tissue. In this regard, the appearance and peak of Pax6 transcripts before Otx2 and Crx support current knowledge on Pax6 functions in retinal progenitors, the role of Otx2 in promoting photoreceptor precursors as well as Otx2 and Crx in differentiating photoreceptors. In general, the appearance and peak in homeobox gene expression is delayed in the retina as compared to the pineal gland (compare Fig. 2 and Fig. 3); this is consistent with the observation that mature pineal function, e.g. melatonin synthesis, is developed prior to functional retinal phototransduction in the rodent [25,92]. This difference in physiological maturation is reflected by the developmental expression patterns of genes involved in melatonin synthesis and phototransduction in the pineal gland and retina, respectively.
3.3 Rax: pineal developmental aspects
Rax is another homeobox gene widely expressed in the developing forebrain controlling development of rostral brain structures [93,94]. Rax is essential for eye morphogenesis and maintains proliferative activity of retinal progenitors [95]; however, dual roles also involving regulation of postnatal photoreceptor-specific gene expression have been reported [96,97].
Rax expression has been reported in the rat pineal gland [9,10,98]. The developmental profile of Rax in the pineal gland shows a late onset in its expression (Fig. 2) as compared to expression in the retina (Fig. 3)[9]. Also, whereas Otx2 and Pax6 are widely expressed in the dorsal mesencephalic and diencephalic areas with expression being progressively restricted to the pineal [7,8], Rax expression is strongly initiated at E20 in the pineal gland well after establishment of the pineal anlage (Fig. 1). Therefore, it seems unlikely that Rax is required for early development of the pineal gland, as is the case in the retina; rather, it would appear that Rax is involved in terminal differentiation and maintenance of pinealocyte phenotype (Fig. 5). RAX has been shown to trans-activate Otx2 during retinal photoreceptor determination [99]; however, given the temporal expression profiles of these genes in the rodent pineal (Fig. 2), a similar relationship in immature pinealocytes is unlikely. The Rax knockout mouse fails to develop the forebrain [93]; thus making analysis of the pineal gland impossible. However, a recently developed conditional knockout mouse, in which Rax is specifically deleted in the pineal gland and retinal photoreceptors [99,100], may shed light on the function of Rax in pineal development. It has previously been pointed out that a truly pineal-specific and pineal-defining homeobox gene has not been identified [101]; in this regard, the temporal sequence rather than the final composition of homeobox gene products may be the defining feature of the mammalian pinealocyte restricting the cell towards a pinealocyte fate rather than a photoreceptor phenotype. This interpretation would be in line with the previously mentioned proposed theory of pinealocyte evolution depending on cell fate restriction in the pineal area of the neural plate [46,47]. Thus, the absence of a developmental initiator, such as Rax, early in pineal development may prevent the immature pinealocyte from establishing photoreceptor features.
4. Homeobox genes in the mature pinealocyte
As indicated in the introduction, gene products encoded by a number of homeobox genes have been identified in the mature pineal gland [7–11]. Among these, members of the Otx-family are involved in conferring tissue-specific melatonin synthesis, whereas other homeobox genes also display daily variations.
4.1 Tissue-specific melatonin synthesis
Melatonin synthesis is restricted to pinealocytes and the retinal photoreceptor cells. Pineal and photoreceptor-specific gene expression is directed by cis-regulatory photoreceptor conserved elements (PCE; consensus TAATT/C[102]); several PCE copies are found in the promoters of mammalian pineal and photoreceptor-specific genes [81,89,91,103,104]. Different subtypes of PCEs bind both OTX2/CRX and RAX proteins though with slightly different preferences and affinities [96]. In the zebrafish, PCEs are essential for the CRX-related OTX5 transcription factor in mediating expression of pineal specific genes including Aanat [105,106]. Thus, in regulation of pineal gene expression the PCE motif conferring tissue-specificity seems to be a highly conserved feature from fishes to mammals, whereas the norepinephrine-responsive CRE is a mammalian feature for generating rhythmic pineal gene expression replacing the clock-driven E-box in non-mammalian vertebrates (Fig. 5B). Notably, in regulation of tissue-specific Aanat expression in the mammalian pineal gland, an E-box sequence has been shown to inhibit ectopic Aanat expression, thus introducing another level of tissue-specificity control [107].
OTX2 and CRX are persistently expressed in the pineal gland of the adult rat (Fig. 2; Fig. 6) [7]; this is also the case in retinal photoreceptor cells [86]. OTX2 and CRX have been shown to be involved in transcriptional regulation of the two last enzymes in melatonin synthesis [88]; direct trans-acting PCE-dependent links, e.g. promoter binding and activation, have been identified between OTX2 and Asmt [108] as well as CRX and Aanat [85,88,89] and Asmt [90]. Since both transcription factors seem to act by binding the same PCEs, the division of labor between CRX and OTX2 is unclear, and is further complicated by the fact that both transcription factors trans-activate Crx itself (Fig. 5B) [65]. The Crx knockout displays only a reduced pineal expression of Aanat [88], suggesting that OTX2 or other factors compensate for the lack of CRX. Notably, OTX2 and CRX are present in both the superficial and deep pineal gland (Fig. 6). The molecular machinery for melatonin synthesis, that is expression of Aanat and Asmt [109,110], is also present in the deep pineal gland (Fig. 6), which is in line with the common ontogenetic origin of these structures (Fig. 1).
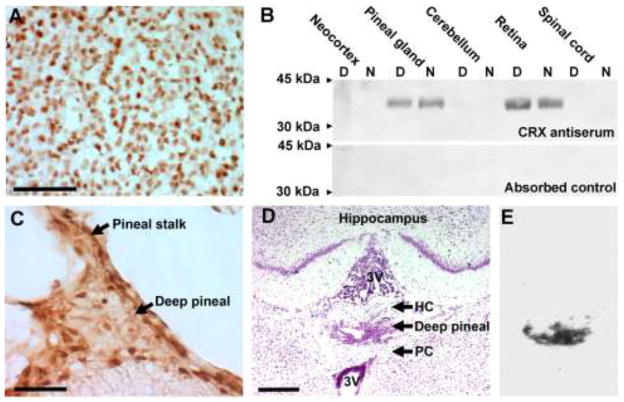
Figure 6. Persistent presence of OTX2 and CRX proteins in the pineal gland of the adult rat.
Continuous expression is also detectable in the deep pineal gland, which is also a source of melatonin synthesis as indicated by expression of Aanat in this structure. A) Immunohistochemical demonstration of OTX2 protein in the rat superficial pineal gland. Scale bar, 100 μm. B) Western blot analysis of CRX protein distribution in the central nervous system of the adult rat. Rat CRX protein (NP_068627) has a predicted molecular weight of 32.4 kDa. The antiserum was raised in rabbit against a peptide corresponding to position 284–299 on rat CRX protein. The lower blot represents a pre-absorption specificity-control. D, daytime sample; N, nighttime sample. C) Immunohistochemical demonstration of OTX2 protein in the rat deep pineal gland. Scale bar, 50 μm. D) Anatomical demonstration of the deep pineal gland in a Nissl-stained coronal section of the adult rat brain. 3V, third ventricle; HC, habenular commissure; PC, posterior commissure. Scale bar, 500 μm. E) Expression of Aanat in the deep pineal as demonstrated by radiochemical in situ hybridization. An autoradiograph of same section shown in (D) is displayed. For methodological details, see [7,10,76,86].
RAX has been shown in vitro to at least partly exert its function by cooperating with CRX to ensure high expression of photoreceptor genes [96]. Although experimental data in pineal systems are lacking, we hypothesize that RAX acting on PCEs in pineal promoters may also generate high expression of pineal-specific genes in synergistic cooperation with CRX and OTX2 in the mature pinealocyte (Fig. 5B). From a developmental point of view, the expression of Otx2, Crx and Rax peaks perinatally, thus temporally correlated with the first detectable expression of genes involved in melatonin synthesis (Fig. 2); this supports a role for these transcription factors in controlling development of the physiologically mature pinealocyte (Fig. 5A).
In summary, the two-fold rhythms detected for Crx, Otx2 and Rax in the pineal gland [7,9,10,89] do not imply that these genes are not involved in controlling the rhythmic nature of genes that display marked circadian rhythms in the pineal gland, such as Aanat exhibiting a 150-fold daily rhythm [22,20] (Fig. 7). Although the proposed synergistic action of CRX and RAX may generate a more than two-fold rhythm in PCE-based trans-activating potential, we suggest a model where homeodomain proteins acting on PCEs confer tissue-specificity, whereas the norepinephrine-induced increase in cyclic AMP generates CRE-based circadian rhythmicity (Fig. 5B).
Figure 7. Daily expression patterns of rhythmic homeobox genes and Aanat in the rat pineal gland.
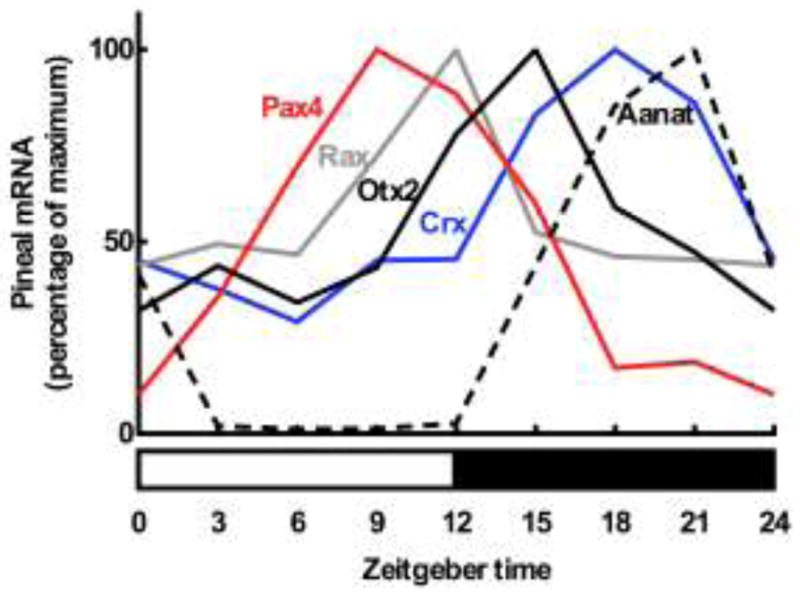
Data are adapted from several studies. Lower horizontal bar reflects the daily lighting conditions (light, white part; darkness, black part). Data were obtained by radiochemical in situ hybridization followed by densitometric quantitation on sections of the rat pineal gland. Aanat, arylalkylamine N-acetyltransferase (Rohde K, Møller M and Rath MF, in preparation); Crx, cone-rod homeobox (Rohde K, Møller M and Rath MF, in preparation); Otx2, orthodenticle homeobox 2 (Rohde K, Møller M and Rath MF, in preparation); Pax4, paired box 4; Rax, retina and anterior neural fold homeobox [9]. For methodological details, see [7,8,10].
4.2 Daily rhythms in pineal homeobox gene expression
A large number of transcripts display daily rhythms in the rat pineal gland [10]. Pax4, Crx, Otx2 and Rax are rhythmic in the pineal (Fig. 7)[8,9], whereas Pax6 is expressed at the same levels throughout the day [7,8]. Rax and Crx display moderate two-fold rhythms in the pineal gland and are both arrhythmic in the retina [9,89]; Pax4 exhibits a larger six-fold rhythm in both tissues [8,76]. These are not clear-cut day-night phenomena as is the case for Aanat in the rodent pineal gland [22] (Fig. 7): Pax4 expression peaks during daytime, Rax expression is high at the day-night transition, the peak in Otx2 appear early in the night, and only the expression of Crx appears in the middle of the night preceding the nocturnal peak in Aanat. As indicated above, RAX and CRX/OTX2 acting through PCEs may in concert modulate, fine-tune or even boost the daily expression profile of pineal-specific genes displaying cyclic AMP/CRE dependent rhythms. This interpretation is consistent with the sequence of peaks in the daily expression of Rax, Otx2, Crx and Aanat (Fig. 7).
As described above, a multisynaptic pathway passing through the superior cervical ganglia controls pineal functions, including the daily rhythm in melatonin synthesis [12,13]. Removal of these ganglia blocks sympathetic neural stimulation of the gland. The rhythmic expression of Pax4 in the pineal gland is abolished by ganglionectomy, but sustained high levels of the Pax4 transcript are detectable [8]. The Pax4 rhythm is driven by nocturnal suppression of transcript levels via norepinephrine acting through cyclic AMP. This inverse relationship between norepinephrine and Pax4 transcript levels is in marked contrast to the inducing effect of adrenergic stimulation on pineal levels of the Aanat transcript, which is reduced to constitutive daytime values by superior cervical ganglionectomy [22]. On the other hand, midday and midnight pineal expression of Otx2 is not affected by ganglionectomy [7]. These observations suggest that although the input of norepinephrine to the gland is important for rhythmic gene expression, the intrinsic non-rhythmic expression of homeobox genes in the pineal is not influenced by the sympathetic input to the gland.
5. Future directions
A number of pineal homeobox genes, in addition to Pax, Otx and Rax, have been reported and analyzed to variable degree. Among these, the brain-specific homeobox (Bsx) is expressed in the pineal gland of the mouse [11], and the Bsx knockout exhibits a hypoplastic pineal gland, suggesting a cell proliferative or cell survival function of Bsx in rodent pineal gland development [111]. Contrarily, in the Xenopus pineal organ, Bsx promotes photoreceptor fate while restricting pineal cell proliferation [112], thus suggesting a relatively late role for this gene in development of the Xenopus pineal photoreceptor. Certain LIM homeobox (Lhx) genes also exhibit a conserved pineal-specific pattern of expression as evidenced by investigations in several vertebrate species including the mouse [113]. Other only sporadically described homeobox genes expressed in the rodent pineal gland include ALX homeobox 4 (Alx4) [98] and msh homeobox 1 (Msx1)[10]. Detailed comparative ontogentic analyses of these homeodomain transcription factors will help to elucidate the transcriptional network underlying pineal gland development and adult physiology.
The content of this paper has been restricted to homeobox genes. Drawing on knowledge on development of the retinal photoreceptor, other transcriptional regulators such as the basic helix-loop-helix transcription factors may in combination with homeodomain proteins also be important in development and mature function of the pineal gland. Among these, the basic helix-loop-helix transcription factor NeuroD has been shown to modulate transcription in both the developing and mature rodent pineal gland [114,115]. The zebrafish pineal organ has been used as a model for understanding neuronal cell fate determination; these studies have revealed the involvement of the BMP (bone morphogenic protein) signaling pathway in defining pineal neuronal subtypes and positioning the epiphyseal expressing domain of the homeobox gene floating head [116,117], which is required for zebrafish pineal development [118]. In specification of pineal cell identity, floating head acts via basic loop helix downstream targets including NeuroD [119]. Similar mechanisms may be involved in development of the mammalian pineal gland.
Recent data show that the microRNA miRNA-483 suppresses pineal Aanat expression and exhibits an inverse developmental expression pattern with high levels early in development [120], thus suggesting that maturation of pineal physiology in addition to a specific composition of homeodomain transcription factors may also involve a timely controlled relief from suppressing microRNAs.
Acknowledgments
This work was supported by the Danish Medical Research Council, the Lundbeck Foundation, the Novo Nordisk Foundation and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
References
- 1.McGinnis W, Garber RL, Wirz J, Kuroiwa A, Gehring WJ. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984;37 (2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nature reviews Genetics. 2005;6(12):893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 3.Slack JM, Holland PW, Graham CF. The zootype and the phylotypic stage. Nature. 1993;361(6412):490–492. doi: 10.1038/361490a0. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68 (2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- 5.Morgan R. Hox genes: a continuation of embryonic patterning? Trends in genetics: TIG. 2006;22(2):67–69. doi: 10.1016/j.tig.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi Y, Hamada J, Murakawa K, Takada M, Tada M, Nogami I, Hayashi N, Nakamori S, Monden M, Miyamoto M, Katoh H, Moriuchi T. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Experimental cell research. 2004;293 (1):144–153. doi: 10.1016/j.yexcr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Rath MF, Munoz E, Ganguly S, Morin F, Shi Q, Klein DC, Møller M. Expression of the Otx2 homeobox gene in the developing mammalian brain: embryonic and adult expression in the pineal gland. Journal of neurochemistry. 2006;97(2):556–566. doi: 10.1111/j.1471-4159.2006.03773.x. [DOI] [PubMed] [Google Scholar]
- 8.Rath MF, Bailey MJ, Kim JS, Ho AK, Gaildrat P, Coon SL, Møller M, Klein DC. Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: nocturnal down-regulation is mediated by adrenergic-cyclic adenosine 3′,5′-monophosphate signaling. Endocrinology. 2009;150(2):803–811. doi: 10.1210/en.2008-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rohde K, Klein DC, Møller M, Rath MF. Rax: developmental and daily expression patterns in the rat pineal gland and retina. Journal of neurochemistry. 2011;118(6):999–1007. doi: 10.1111/j.1471-4159.2011.07385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey MJ, Coon SL, Carter DA, Humphries A, Kim JS, Shi Q, Gaildrat P, Morin F, Ganguly S, Hogenesch JB, Weller JL, Rath MF, Møller M, Baler R, Sugden D, Rangel ZG, Munson PJ, Klein DC. Night/day changes in pineal expression of >600 genes: central role of adrenergic/cAMP signaling. The Journal of biological chemistry. 2009;284(12):7606–7622. doi: 10.1074/jbc.M808394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cremona M, Colombo E, Andreazzoli M, Cossu G, Broccoli V. Bsx, an evolutionary conserved Brain Specific homeoboX gene expressed in the septum, epiphysis, mammillary bodies and arcuate nucleus. Gene expression patterns: GEP. 2004;4 (1):47–51. doi: 10.1016/s1567-133x(03)00151-0. [DOI] [PubMed] [Google Scholar]
- 12.Klein DC, Bailey MJ, Carter DA, Kim JS, Shi Q, Ho AK, Chik CL, Gaildrat P, Morin F, Ganguly S, Rath MF, Møller M, Sugden D, Rangel ZG, Munson PJ, Weller JL, Coon SL. Pineal function: impact of microarray analysis. Molecular and cellular endocrinology. 2010;314(2):170–183. doi: 10.1016/j.mce.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Møller M, Baeres FM. The anatomy and innervation of the mammalian pineal gland. Cell and tissue research. 2002;309(1):139–150. doi: 10.1007/s00441-002-0580-5. [DOI] [PubMed] [Google Scholar]
- 14.Calvo J, Boya J. Postnatal evolution of the rat pineal gland: light microscopy. Journal of anatomy. 1984;138 ( Pt 1):45–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo J, Boya J. Embryonic development of the rat pineal gland. The Anatomical record. 1981;200(4):491–500. doi: 10.1002/ar.1092000412. [DOI] [PubMed] [Google Scholar]
- 16.Calvo J, Boya J. Ultrastructural study of the embryonic development in the rat pineal gland. The Anatomical record. 1981;199(4):543–553. doi: 10.1002/ar.1091990410. [DOI] [PubMed] [Google Scholar]
- 17.Calvo JL, Boya J, Carbonell AL, Garcia-Maurino JE. Cell proliferation in the developing rat pineal gland. A bromodeoxyuridine immunohistochemical study. Histology and histopathology. 2000;15 (4):1005–1010. doi: 10.14670/HH-15.1005. [DOI] [PubMed] [Google Scholar]
- 18.Calvo J, Boya J. Postnatal development of cell types in the rat pineal gland. Journal of anatomy. 1983;137 (Pt 1):185–195. [PMC free article] [PubMed] [Google Scholar]
- 19.Møller M. The ultrastructure of the human fetal pineal gland. I. Cell types and blood vessels. Cell and tissue research. 1974;152 (1):13–30. doi: 10.1007/BF00224208. [DOI] [PubMed] [Google Scholar]
- 20.Klein DC. Arylalkylamine N-acetyltransferase: “the Timezyme”. The Journal of biological chemistry. 2007;282(7):4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 21.Møller M. Introduction to mammalian pineal innervation. Microscopy research and technique. 1999;46(4–5):235–238. doi: 10.1002/(SICI)1097-0029(19990815/01)46:4/5<235::AID-JEMT1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Roseboom PH, Coon SL, Baler R, McCune SK, Weller JL, Klein DC. Melatonin synthesis: analysis of the more than 150-fold nocturnal increase in serotonin N-acetyltransferase messenger ribonucleic acid in the rat pineal gland. Endocrinology. 1996;137 (7):3033–3045. doi: 10.1210/endo.137.7.8770929. [DOI] [PubMed] [Google Scholar]
- 23.Baler R, Covington S, Klein DC. The rat arylalkylamine N-acetyltransferase gene promoter. cAMP activation via a cAMP-responsive element-CCAAT complex. The Journal of biological chemistry. 1997;272 (11):6979–6985. doi: 10.1074/jbc.272.11.6979. [DOI] [PubMed] [Google Scholar]
- 24.Roseboom PH, Klein DC. Norepinephrine stimulation of pineal cyclic AMP response element-binding protein phosphorylation: primary role of a beta-adrenergic receptor/cyclic AMP mechanism. Molecular pharmacology. 1995;47 (3):439–449. [PubMed] [Google Scholar]
- 25.Klein DC, Namboodiri MA, Auerbach DA. The melatonin rhythm generating system: developmental aspects. Life sciences. 1981;28 (18):1975–1986. doi: 10.1016/0024-3205(81)90644-5. [DOI] [PubMed] [Google Scholar]
- 26.Seron-Ferre M, Mendez N, Abarzua-Catalan L, Vilches N, Valenzuela FJ, Reynolds HE, Llanos AJ, Rojas A, Valenzuela GJ, Torres-Farfan C. Circadian rhythms in the fetus. Molecular and cellular endocrinology. 2012;349(1):68–75. doi: 10.1016/j.mce.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki S, Yoshikawa T, Biscoe EW, Numano R, Gallaspy LM, Soulsby S, Papadimas E, Pezuk P, Doyle SE, Tei H, Sakaki Y, Block GD, Menaker M. Ontogeny of circadian organization in the rat. Journal of biological rhythms. 2009;24(1):55–63. doi: 10.1177/0748730408328438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Håkanson R, Lombard des Gouttes MN, Owman C. Activities of tryptophan hydroxylase, dopa decarboxylase, and monoamine oxidase as correlated with the appearance of monoamines in developing rat pineal gland. Life sciences. 1967;6 (24):2577–2585. doi: 10.1016/0024-3205(67)90107-5. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer M, Stehle JH. Ontogeny of a diurnal rhythm in arylalkylamine-N-acetyltransferase mRNA in rat pineal gland. Neuroscience letters. 1998;248 (3):163–166. doi: 10.1016/s0304-3940(98)00356-5. [DOI] [PubMed] [Google Scholar]
- 30.Ellison N, Weller JL, Klein DC. Development of a circadian rhythm in the activity of pineal serotonin N-acetyltransferase. Journal of neurochemistry. 1972;19 (5):1335–1341. doi: 10.1111/j.1471-4159.1972.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 31.Tamarkin L, Reppert SM, Orloff DJ, Klein DC, Yellon SM, Goldman BD. Ontogeny of the pineal melatonin rhythm in the Syrian (Mesocricetus auratus) and Siverian (Phodopus sungorus) hamsters and in the rat. Endocrinology. 1980;107 (4):1061–1064. doi: 10.1210/endo-107-4-1061. [DOI] [PubMed] [Google Scholar]
- 32.Ribelayga C, Gauer F, Pevet P, Simonneaux V. Ontogenesis of hydroxyindole-O-methyltransferase gene expression and activity in the rat pineal gland. Brain research Developmental brain research. 1998;110 (2):235–239. doi: 10.1016/s0165-3806(98)00114-x. [DOI] [PubMed] [Google Scholar]
- 33.Araki M. Cellular mechanism for norepinephrine suppression of pineal photoreceptor-like cell differentiation in rat pineal cultures. Developmental biology. 1992;149 (2):440–447. doi: 10.1016/0012-1606(92)90298-u. [DOI] [PubMed] [Google Scholar]
- 34.Tosini G, Doyle S, Geusz M, Menaker M. Induction of photosensitivity in neonatal rat pineal gland. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11540–11544. doi: 10.1073/pnas.210248297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein DC. The 2004 Aschoff/Pittendrigh lecture: Theory of the origin of the pineal gland--a tale of conflict and resolution. Journal of biological rhythms. 2004;19(4):264–279. doi: 10.1177/0748730404267340. [DOI] [PubMed] [Google Scholar]
- 36.Klein DC. Evolution of the vertebrate pineal gland: the AANAT hypothesis. Chronobiology international. 2006;23(1–2):5–20. doi: 10.1080/07420520500545839. [DOI] [PubMed] [Google Scholar]
- 37.Eakin RM. The Third Eye. University of California Press; Berkeley: 1973. [Google Scholar]
- 38.Vollrath L, Möllendorff Wv, Bargmann W, Oksche A. Handbuch der mikroskopischen Anatomie des Menschen, vol/begr von Wilhelm von Möllendorff Fortgef von Wolfgang Bargmann Hrsg von A Oksche; Bd 6. Springer; Berlin: 1981. The pineal organ. [u.a.] [Google Scholar]
- 39.Deguchi T. Rhodopsin-like photosensitivity of isolated chicken pineal gland. Nature. 1981;290 (5808):706–707. doi: 10.1038/290706a0. [DOI] [PubMed] [Google Scholar]
- 40.Meissl H, Ekström P. Photoreceptor responses to light in the isolated pineal organ of the trout, Salmo gairdneri. Neuroscience. 1988;25 (3):1071–1076. doi: 10.1016/0306-4522(88)90060-7. [DOI] [PubMed] [Google Scholar]
- 41.Binkley S, Riebman JB, Reilly KB. Timekeeping by the pineal gland. Science. 1977;197 (4309):1181–1183. doi: 10.1126/science.897660. [DOI] [PubMed] [Google Scholar]
- 42.Falcon J, Guerlotte JF, Voisin P, Collin JP. Rhythmic melatonin biosynthesis in a photoreceptive pineal organ: a study in the pike. Neuroendocrinology. 1987;45 (6):479–486. doi: 10.1159/000124778. [DOI] [PubMed] [Google Scholar]
- 43.Collin JP. Differentiation and regression of cells of the sensory line in the epiphysis cerebri. In: Wolstenholme GEW, editor. The pineal gland. Churchill-Livingstone; Edinburg: 1971. pp. 79–125. [Google Scholar]
- 44.Kappers JA. Survey of the Innervation of the Epiphysis Cerebri and the Accessory Pineal Organs of Vertebrates. Progress in brain research. 1965;10:87–153. doi: 10.1016/s0079-6123(08)63448-2. [DOI] [PubMed] [Google Scholar]
- 45.Oksche A. Sensory and glandular elements of the pineal organ. In: Wolstenholme GEW, editor. The Pineal Gland. Churchill-Livingstone; Edinburg: 1971. pp. 127–146. [Google Scholar]
- 46.Ekström P, Meissl H. Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2003;358(1438):1679–1700. doi: 10.1098/rstb.2003.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mano H, Fukada Y. A median third eye: pineal gland retraces evolution of vertebrate photoreceptive organs. Photochemistry and photobiology. 2007;83(1):11–18. doi: 10.1562/2006-02-24-IR-813. [DOI] [PubMed] [Google Scholar]
- 48.Zimmerman BL, Tso MO. Morphologic evidence of photoreceptor differentiation of pinealocytes in the neonatal rat. The Journal of cell biology. 1975;66 (1):60–75. doi: 10.1083/jcb.66.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackshaw S, Snyder SH. Developmental expression pattern of phototransduction components in mammalian pineal implies a light-sensing function. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17 (21):8074–8082. doi: 10.1523/JNEUROSCI.17-21-08074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Møller M, Møllgard K, Kimble JE. Presence of a pineal nerve in sheep and rabbit fetuses. Cell and tissue research. 1975;158 (4):451–459. doi: 10.1007/BF00220212. [DOI] [PubMed] [Google Scholar]
- 51.Møller M. Presence of a pineal nerve (nervus pinealis) in the human fetus: a light and electron microscopical study of the innervation of the pineal gland. Brain research. 1978;154 (1):1–12. doi: 10.1016/0006-8993(78)91046-6. [DOI] [PubMed] [Google Scholar]
- 52.Somers RL, Klein DC. Rhodopsin kinase activity in the mammalian pineal gland and other tissues. Science. 1984;226 (4671):182–184. doi: 10.1126/science.6091271. [DOI] [PubMed] [Google Scholar]
- 53.Korf HW, Møller M, Gery I, Zigler JS, Klein DC. Immunocytochemical demonstration of retinal S-antigen in the pineal organ of four mammalian species. Cell and tissue research. 1985;239 (1):81–85. doi: 10.1007/BF00214906. [DOI] [PubMed] [Google Scholar]
- 54.Reig JA, Yu L, Klein DC. Pineal transduction. Adrenergic----cyclic AMP-dependent phosphorylation of cytoplasmic 33-kDa protein (MEKA) which binds beta gamma-complex of transducin. The Journal of biological chemistry. 1990;265 (10):5816–5824. [PubMed] [Google Scholar]
- 55.Korf HW, White BH, Schaad NC, Klein DC. Recoverin in pineal organs and retinae of various vertebrate species including man. Brain research. 1992;595 (1):57–66. doi: 10.1016/0006-8993(92)91452-k. [DOI] [PubMed] [Google Scholar]
- 56.van Veen T, Katial A, Shinohara T, Barrett DJ, Wiggert B, Chader GJ, Nickerson JM. Retinal photoreceptor neurons and pinealocytes accumulate mRNA for interphotoreceptor retinoid-binding protein (IRBP) FEBS letters. 1986;208 (1):133–137. doi: 10.1016/0014-5793(86)81547-2. [DOI] [PubMed] [Google Scholar]
- 57.Korf HW, Foster RG, Ekström P, Schalken JJ. Opsin-like immunoreaction in the retinae and pineal organs of four mammalian species. Cell and tissue research. 1985;242 (3):645–648. doi: 10.1007/BF00225432. [DOI] [PubMed] [Google Scholar]
- 58.Gern WA, Ralph CL. Melatonin synthesis by the retina. Science. 1979;204 (4389):183–184. doi: 10.1126/science.432640. [DOI] [PubMed] [Google Scholar]
- 59.Pang SF, Yu HS, Suen HC, Brown GM. Melatonin in the retina of rats: a diurnal rhythm. The Journal of endocrinology. 1980;87 (1):89–93. doi: 10.1677/joe.0.0870089. [DOI] [PubMed] [Google Scholar]
- 60.Coon SL, Del Olmo E, Young WS, Klein DC. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87) The Journal of clinical endocrinology and metabolism. 2002;87 (10):4699–4706. doi: 10.1210/jc.2002-020683. [DOI] [PubMed] [Google Scholar]
- 61.Niki T, Hamada T, Ohtomi M, Sakamoto K, Suzuki S, Kako K, Hosoya Y, Horikawa K, Ishida N. The localization of the site of arylalkylamine N-acetyltransferase circadian expression in the photoreceptor cells of mammalian retina. Biochemical and biophysical research communications. 1998;248(1):115–120. doi: 10.1006/bbrc.1998.8916. [DOI] [PubMed] [Google Scholar]
- 62.Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell and tissue research. 2004;315(2):197–201. doi: 10.1007/s00441-003-0822-1. [DOI] [PubMed] [Google Scholar]
- 63.Sakamoto K, Ishida N. Circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neuroscience letters. 1998;245 (2):113–116. doi: 10.1016/s0304-3940(98)00189-x. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain research Molecular brain research. 2000;81 (1–2):43–50. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 65.Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nature neuroscience. 2003;6(12):1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 66.Estivill-Torrus G, Vitalis T, Fernandez-Llebrez P, Price DJ. The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mechanisms of development. 2001;109 (2):215–224. doi: 10.1016/s0925-4773(01)00527-5. [DOI] [PubMed] [Google Scholar]
- 67.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113 (4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 68.Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes to cells: devoted to molecular & cellular mechanisms. 1996;1 (1):11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- 69.Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: A multi-level regulator of ocular development. Progress in retinal and eye research. 2012 doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354(6354):522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 71.Grindley JC, Davidson DR, Hill RE. The role of Pax-6 in eye and nasal development. Development. 1995;121 (5):1433–1442. doi: 10.1242/dev.121.5.1433. [DOI] [PubMed] [Google Scholar]
- 72.Philips GT, Stair CN, Young Lee H, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Developmental biology. 2005;279(2):308–321. doi: 10.1016/j.ydbio.2004.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105 (1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell TN, Free SL, Williamson KA, Stevens JM, Churchill AJ, Hanson IM, Shorvon SD, Moore AT, van Heyningen V, Sisodiya SM. Polymicrogyria and absence of pineal gland due to PAX6 mutation. Annals of neurology. 2003;53(5):658–663. doi: 10.1002/ana.10576. [DOI] [PubMed] [Google Scholar]
- 75.Abouzeid H, Youssef MA, ElShakankiri N, Hauser P, Munier FL, Schorderet DF. PAX6 aniridia and interhemispheric brain anomalies. Molecular vision. 2009;15:2074–2083. [PMC free article] [PubMed] [Google Scholar]
- 76.Rath MF, Bailey MJ, Kim JS, Coon SL, Klein DC, Møller M. Developmental and daily expression of the Pax4 and Pax6 homeobox genes in the rat retina: localization of Pax4 in photoreceptor cells. Journal of neurochemistry. 2009;108(1):285–294. doi: 10.1111/j.1471-4159.2008.05765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 78.Smith SB, Ee HC, Conners JR, German MS. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Molecular and cellular biology. 1999;19 (12):8272–8280. doi: 10.1128/mcb.19.12.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358(6388):687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- 80.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91 (4):531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 81.Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA, Zack DJ. Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron. 1997;19 (5):1017–1030. doi: 10.1016/s0896-6273(00)80394-3. [DOI] [PubMed] [Google Scholar]
- 82.Matsuo I, Kuratani S, Kimura C, Takeda N, Aizawa S. Mouse Otx2 functions in the formation and patterning of rostral head. Genes & development. 1995;9 (21):2646–2658. doi: 10.1101/gad.9.21.2646. [DOI] [PubMed] [Google Scholar]
- 83.Acampora D, Mazan S, Lallemand Y, Avantaggiato V, Maury M, Simeone A, Brulet P. Forebrain and midbrain regions are deleted in Otx2−/− mutants due to a defective anterior neuroectoderm specification during gastrulation. Development. 1995;121 (10):3279–3290. doi: 10.1242/dev.121.10.3279. [DOI] [PubMed] [Google Scholar]
- 84.Swaroop A, Kim D, Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nature reviews Neuroscience. 2010;11(8):563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nature genetics. 1999;23(4):466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 86.Rath MF, Morin F, Shi Q, Klein DC, Møller M. Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Experimental eye research. 2007;85(1):65–73. doi: 10.1016/j.exer.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 87.Koike C, Nishida A, Ueno S, Saito H, Sanuki R, Sato S, Furukawa A, Aizawa S, Matsuo I, Suzuki N, Kondo M, Furukawa T. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Molecular and cellular biology. 2007;27(23):8318–8329. doi: 10.1128/MCB.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rovsing L, Clokie S, Bustos DM, Rohde K, Coon SL, Litman T, Rath MF, Møller M, Klein DC. Crx broadly modulates the pineal transcriptome. Journal of neurochemistry. 2011;119(2):262–274. doi: 10.1111/j.1471-4159.2011.07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li X, Chen S, Wang Q, Zack DJ, Snyder SH, Borjigin J. A pineal regulatory element (PIRE) mediates transactivation by the pineal/retina-specific transcription factor CRX. Proceedings of the National Academy of Sciences of the United States of America. 1998;95 (4):1876–1881. doi: 10.1073/pnas.95.4.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bernard M, Dinet V, Voisin P. Transcriptional regulation of the chicken hydroxyindole-O-methyltransferase gene by the cone-rod homeobox-containing protein. Journal of neurochemistry. 2001;79 (2):248–257. doi: 10.1046/j.1471-4159.2001.00555.x. [DOI] [PubMed] [Google Scholar]
- 91.Livesey FJ, Furukawa T, Steffen MA, Church GM, Cepko CL. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Current biology: CB. 2000;10 (6):301–310. doi: 10.1016/s0960-9822(00)00379-1. [DOI] [PubMed] [Google Scholar]
- 92.Weidman TA, Kuwabara T. Development of the rat retina. Investigative ophthalmology. 1969;8 (1):60–69. [PubMed] [Google Scholar]
- 93.Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387(6633):603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 94.Furukawa T, Kozak CA, Cepko CL. rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proceedings of the National Academy of Sciences of the United States of America. 1997;94 (7):3088–3093. doi: 10.1073/pnas.94.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Regulation of vertebrate eye development by Rx genes. The International journal of developmental biology. 2004;48(8–9):761–770. doi: 10.1387/ijdb.041878tb. [DOI] [PubMed] [Google Scholar]
- 96.Kimura A, Singh D, Wawrousek EF, Kikuchi M, Nakamura M, Shinohara T. Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor-specific gene expression. The Journal of biological chemistry. 2000;275 (2):1152–1160. doi: 10.1074/jbc.275.2.1152. [DOI] [PubMed] [Google Scholar]
- 97.Pan Y, Martinez-De Luna RI, Lou CH, Nekkalapudi S, Kelly LE, Sater AK, El-Hodiri HM. Regulation of photoreceptor gene expression by the retinal homeobox (Rx) gene product. Developmental biology. 2010;339(2):494–506. doi: 10.1016/j.ydbio.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asbreuk CH, van Schaick HS, Cox JJ, Smidt MP, Burbach JP. Survey for paired-like homeodomain gene expression in the hypothalamus: restricted expression patterns of Rx, Alx4 and goosecoid. Neuroscience. 2002;114 (4):883–889. doi: 10.1016/s0306-4522(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 99.Muranishi Y, Terada K, Inoue T, Katoh K, Tsujii T, Sanuki R, Kurokawa D, Aizawa S, Tamaki Y, Furukawa T. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31(46):16792–16807. doi: 10.1523/JNEUROSCI.3109-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muranishi Y, Terada K, Furukawa T. An essential role for Rax in retina and neuroendocrine system development. Development, growth & differentiation. 2012;54(3):341–348. doi: 10.1111/j.1440-169X.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- 101.Maronde E, Stehle JH. The mammalian pineal gland: known facts, unknown facets. Trends in endocrinology and metabolism: TEM. 2007;18(4):142–149. doi: 10.1016/j.tem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Appelbaum L, Gothilf Y. Mechanism of pineal-specific gene expression: the role of E-box and photoreceptor conserved elements. Molecular and cellular endocrinology. 2006;252(1–2):27–33. doi: 10.1016/j.mce.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 103.Chen Q, Lee JW, Nishiyama K, Shadrach KG, Rayborn ME, Hollyfield JG. SPACRCAN in the interphotoreceptor matrix of the mouse retina: molecular, developmental and promoter analysis. Experimental eye research. 2003;76 (1):1–14. doi: 10.1016/s0014-4835(02)00273-7. [DOI] [PubMed] [Google Scholar]
- 104.Gaildrat P, Møller M, Mukda S, Humphries A, Carter DA, Ganapathy V, Klein DC. A novel pineal-specific product of the oligopeptide transporter PepT1 gene: circadian expression mediated by cAMP activation of an intronic promoter. The Journal of biological chemistry. 2005;280(17):16851–16860. doi: 10.1074/jbc.M414587200. [DOI] [PubMed] [Google Scholar]
- 105.Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nature genetics. 2002;30(1):117–121. doi: 10.1038/ng793. [DOI] [PubMed] [Google Scholar]
- 106.Appelbaum L, Anzulovich A, Baler R, Gothilf Y. Homeobox-clock protein interaction in zebrafish. A shared mechanism for pineal-specific and circadian gene expression. The Journal of biological chemistry. 2005;280(12):11544–11551. doi: 10.1074/jbc.M412935200. [DOI] [PubMed] [Google Scholar]
- 107.Humphries A, Wells T, Baler R, Klein DC, Carter DA. Rodent Aanat: intronic E-box sequences control tissue specificity but not rhythmic expression in the pineal gland. Molecular and cellular endocrinology. 2007;270(1–2):43–49. doi: 10.1016/j.mce.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Dinet V, Girard-Naud N, Voisin P, Bernard M. Melatoninergic differentiation of retinal photoreceptors: activation of the chicken hydroxyindole-O-methyltransferase promoter requires a homeodomain-binding element that interacts with Otx2. Experimental eye research. 2006;83(2):276–290. doi: 10.1016/j.exer.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 109.Ribelayga C, Gauer F, Pevet P, Simonneaux V. Distribution of hydroxyindole-O-methyltransferase mRNA in the rat brain: an in situ hybridisation study. Cell and tissue research. 1998;291 (3):415–421. doi: 10.1007/s004410051011. [DOI] [PubMed] [Google Scholar]
- 110.Garidou ML, Bartol I, Calgari C, Pevet P, Simonneaux V. In vivo observation of a non-noradrenergic regulation of arylalkylamine N-acetyltransferase gene expression in the rat pineal complex. Neuroscience. 2001;105 (3):721–729. doi: 10.1016/s0306-4522(01)00197-x. [DOI] [PubMed] [Google Scholar]
- 111.McArthur T, Ohtoshi A. A brain-specific homeobox gene, Bsx, is essential for proper postnatal growth and nursing. Molecular and cellular biology. 2007;27(14):5120–5127. doi: 10.1128/MCB.00215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D’Autilia S, Broccoli V, Barsacchi G, Andreazzoli M. Xenopus Bsx links daily cell cycle rhythms and pineal photoreceptor fate. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6352–6357. doi: 10.1073/pnas.1000854107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seidah NG, Barale JC, Marcinkiewicz M, Mattei MG, Day R, Chretien M. The mouse homeoprotein mLIM-3 is expressed early in cells derived from the neuroepithelium and persists in adult pituitary. DNA and cell biology. 1994;13 (12):1163–1180. doi: 10.1089/dna.1994.13.1163. [DOI] [PubMed] [Google Scholar]
- 114.Munoz EM, Bailey MJ, Rath MF, Shi Q, Morin F, Coon SL, Møller M, Klein DC. NeuroD1: developmental expression and regulated genes in the rodent pineal gland. Journal of neurochemistry. 2007;102(3):887–899. doi: 10.1111/j.1471-4159.2007.04605.x. [DOI] [PubMed] [Google Scholar]
- 115.Ochocinska MJ, Munoz EM, Veleri S, Weller JL, Coon SL, Pozdeyev N, Iuvone PM, Goebbels S, Furukawa T, Klein DC. NeuroD1 is required for survival of photoreceptors but not pinealocytes: Results from targeted gene deletion studies. Journal of neurochemistry. 2012 doi: 10.1111/j.1471-4159.2012.07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126 (22):4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- 117.Quillien A, Blanco-Sanchez B, Halluin C, Moore JC, Lawson ND, Blader P, Cau E. BMP signaling orchestrates photoreceptor specification in the zebrafish pineal gland in collaboration with Notch. Development. 2011;138(11):2293–2302. doi: 10.1242/dev.060988. [DOI] [PubMed] [Google Scholar]
- 118.Masai I, Heisenberg CP, Barth KA, Macdonald R, Adamek S, Wilson SW. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron. 1997;18 (1):43–57. doi: 10.1016/s0896-6273(01)80045-3. [DOI] [PubMed] [Google Scholar]
- 119.Cau E, Wilson SW. Ash1a and Neurogenin1 function downstream of Floating head to regulate epiphysial neurogenesis. Development. 2003;130 (11):2455–2466. doi: 10.1242/dev.00452. [DOI] [PubMed] [Google Scholar]
- 120.Clokie SJ, Lau P, Kim HH, Coon SL, Klein D. Micro RNAs in the pineal gland: mir-483 regulates melatonin synthesis by targeting arylalkylamine N-Acetyltransferase. The Journal of biological chemistry. 2012 doi: 10.1074/jbc.M112.356733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sugden D, Klein DC. Regulation of rat pineal hydroxyindole-O-methyltransferase in neonatal and adult rats. Journal of neurochemistry. 1983;40 (6):1647–1653. doi: 10.1111/j.1471-4159.1983.tb08138.x. [DOI] [PubMed] [Google Scholar]
- 122.Babila T, Schaad NC, Simonds WF, Shinohara T, Klein DC. Development of MEKA (phosducin), G beta, G gamma and S-antigen in the rat pineal gland and retina. Brain research. 1992;585 (1–2):141–148. doi: 10.1016/0006-8993(92)91199-o. [DOI] [PubMed] [Google Scholar]
- 123.Ho AK, Somers RL, Klein DC. Development and regulation of rhodopsin kinase in rat pineal and retina. Journal of neurochemistry. 1986;46 (4):1176–1179. doi: 10.1111/j.1471-4159.1986.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 124.Besancon R, Chouaf L, Jouvet A, Sliwinski S, Belin MF, Fevre-Montange M. Developmental expression of tryptophan hydroxylase mRNAs in the rat pineal gland: an in situ hybridization study. Brain research Molecular brain research. 1995;29 (2):253–262. doi: 10.1016/0169-328x(94)00256-e. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Fernandez F, Healy JI. Early expression of the gene for interphotoreceptor retinol-binding protein during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. The Journal of cell biology. 1990;111 (6 Pt 1):2775–2784. doi: 10.1083/jcb.111.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Treisman JE, Morabito MA, Barnstable CJ. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Molecular and cellular biology. 1988;8 (4):1570–1579. doi: 10.1128/mcb.8.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]