Abstract
We show in this study that Salmonella cells, which do not upregulate flagellar gene expression during swarming, also do not increase flagellar numbers per μm of cell length as determined by systematic counting of both flagellar filaments and hooks. Instead, doubling of the average length of a swarmer cell by suppression of cell division effectively doubles the number of flagella per cell. The highest agar concentration at which Salmonella cells swarmed increased from the normal 0.5% to 1%, either when flagella were overproduced or when expression of the FliL protein was enhanced in conjunction with stator proteins MotAB. We surmise that bacteria use the resulting increase in motor power to overcome the higher friction associated with harder agar. Higher flagellar numbers also suppress the swarming defect of mutants with changes in the chemotaxis pathway that were previously shown to be defective in hydrating their colonies. Here we show that the swarming defect of these mutants can also be suppressed by application of osmolytes to the surface of swarm agar. The “dry” colony morphology displayed by che mutants was also observed with other mutants that do not actively rotate their flagella. The flagellum/motor thus participates in two functions critical for swarming, enabling hydration and overriding surface friction. We consider some ideas for how the flagellum might help attract water to the agar surface, where there is no free water.
INTRODUCTION
Swarming bacteria may be divided into two categories: robust swarmers, which can navigate across a hard agar surface (1.5% agar and above), and temperate swarmers, which can swarm only on a softer agar surface (0.5 to 0.8% agar) (1–3). Robust swarmers include polarly flagellated bacteria, which induce peritrichous flagellation upon surface contact, such as Azospirillum, Rhodospirillum, and Vibrio species, as well as the peritrichously flagellated Proteus species (4–6). These bacteria display a hyperflagellated and hyperelongated swarm cell morphology, which is dramatically different from their broth-grown (swimming) counterparts. Transcriptome studies have shown a swarming-specific gene expression program in the robust swarmers (7–10). In polarly flagellated bacteria, frictional forces on the surface are surmised to slow flagellar rotation and signal altered gene expression (11, 12), an observation solidified by experiments demonstrating a direct relationship between motor speed and lateral flagellar (laf) gene transcription (13). How motor speed might be sensed and transduced to activate laf expression is unknown. This phenomenon is apparently unique to polarly flagellated bacteria.
Temperate swarmers include Escherichia coli and Bacillus, Pseudomonas, Rhizobium, Salmonella, Serratia, and Yersinia species. Among these, Bacillus subtilis displays increased flagellar numbers and cell length (14), but this morphology is not as dramatic as that seen in the robust swarmers. In a Bacillus cereus transcriptome, several genes, including flagellar genes, were seen to be differentially regulated on swarm agar (15). In B. subtilis and Proteus mirabilis, the cell envelope has been implicated as a sensor of surface conditions (16, 17). While details of the signaling pathway are unknown, involvement of a response regulator, DegU, in controlling flagellar gene expression in B. subtilis (18, 19) has allowed parallels to be drawn between DegU and the response regulator RcsB in the Rcs signaling pathway, which communicates membrane stress to the genome and influences regulation of a large number of genes, including the flagellar regulon (16).
Gram-negative temperate swarmers do not show a significantly altered swarmer cell morphology, consistent with the absence of changes in flagellar gene regulation as reported for the transcriptomes or flagellar mRNA levels of some of these bacteria (20–23). There are, however, many other changes in gene expression in these bacteria during surface growth, particularly the induction of virulence pathways. However, there is no evidence as yet of specific surface-sensing mechanisms in these bacteria. The changes observed could be a general consequence of altered metabolism related to oxygen availability, rate of diffusion of nutrients to the surface, or cell density-dependent mechanisms.
We report here a methodic analysis of swarmer cell morphology in Salmonella enterica that clearly shows that our earlier descriptions of swarmer cells of Serratia marcescens (24) and E. coli and Salmonella (25) as hyperflagellated were technically incorrect, because we did not take into account the doubling of cell length. This distinction is important, because it clarifies the general confusion regarding whether or not these bacteria “differentiate” during swarming. Combining this analysis with transcriptome data that showed the absence of a swarming-specific gene regulation program in Salmonella (21), we can now say that except for a modest increase in cell length, these bacteria do not differentiate. Going forward, these results will allow us to focus on central issues universal to swarming. Based on the swarming literature, we identify three challenges common to flagellum-mediated motility under laboratory conditions, where bacteria swarm on the surface of agar. These include attracting water, overcoming surface friction, and reducing surface tension (see our accompanying article [3]). Bacteria meet the first challenge by secreting polysaccharides (or other osmolytes), the second challenge by using a variety of mechanisms, including altering surface charge/lubrication with surface-attached polymers such as LPS (lipopolysaccharide) or ECA (enterobacterial common antigen), increasing flagellar numbers (increasing thrust), and employing special stators or stator-associated proteins to increase motor power, and the third challenge by secreting powerful surfactants. Salmonella and E. coli show little or no surfactant activity (26, 27), explaining their fastidious requirement for a special agar surmised to exhibit lower surface tension (1, 28, 29). We focus here on how Salmonella deals with the first two challenges: attracting water and overcoming surface friction.
We show first that increased flagellar numbers enable Salmonella to swarm on harder-than-normal agar and that increasing the expression of FliL protein in conjunction with stator proteins MotAB produces the same result. The FliL protein is essential for swarming in both E. coli and Salmonella and is thought to play a structural role at the basal body (30). In Borrelia burgdorferi, FliL appears to be associated with the stators (31), and genetic data have indicated a role for FliL in increasing stator performance in Rhodobacter sphaeroides (32). We infer that providing more motors (flagella) or increasing motor output (FliL plus MotAB) overcomes the higher friction expected on a harder agar surface.
The connection between flagellar rotation and water availability was first made when Salmonella strains mutated in the chemotaxis (che) pathway, all of which fail to swarm, were demonstrated to be impaired in hydrating their colonies (33); chemotaxis per se was previously shown to not be essential for swarming (34). Wild-type bacteria switch between clockwise (CW) and counterclockwise (CCW) rotation of the motor, whereas che mutants have extreme CW or CCW motor biases, depending on the mutation (35). Swarming was rescued in the che mutants either by spraying a fine mist of water (33) or by restoring a normal motor bias (36). We report here that adding osmolytes to the surface or increasing flagellar numbers also rescues swarming in the che mutants. We consider new ideas to explain these observations.
Overall, this study shows that Salmonella flagella themselves play a central role in meeting the two critical impediments to moving on a surface: the absence of free water and the presence of surface friction.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The strains and plasmids used in this work are listed in Tables 1 and 2. Bacterial cultures were grown in L-broth (LB) base (20 g liter−1). For motility assays, 8 μl of an exponential-phase culture (optical density at 600 nm, ∼0.6) was inoculated onto either swim plates made up of 0.3% Bacto agar (Difco) or swarm plates, ranging from 0.6 to 1% Eiken agar (Eiken Chemical, Tokyo, Japan) supplemented with either 0.5% glucose or arabinose, and grown at 37°C. Additional supplements, such as Ficoll (Sigma), were added to agar prior to cooling, whereas sucrose (Fisher) and glycine betaine (Sigma) were added by pouring solutions onto the surface of preset plates, letting stand for 1 min, draining the solution, and allowing plates to air dry for 15 min before inoculation with bacteria. Antibiotic concentrations were the following: ampicillin (100 μg ml−1), chloramphenicol (20 μg ml−1), tetracycline (10 μg ml−1), and kanamycin (25 μg ml−1).
Table 1.
Strains and the phage used in this study
| Strain or phage | Genotype | Source or reference |
|---|---|---|
| Salmonella strains | ||
| 14028 | Wild-type ATCC strain | 28 |
| JP104 | 14028 Δhin, fljB locked, Cmr | This study |
| JP1165 | 14028 flgE7742::3×HA | This study |
| ST672 | flgE17::Tn10 | J. Lee |
| QW105 | 14028 ΔcheY | 33 |
| QW178 | 14028 ΔmotA | 33 |
| QW215 | 14028 flhDC::tetRA, Tetr | 64 |
| TH16678 | LT2 ΔlrhA ΔydiV252 ΔecnR4::FRT flgE7742::3×HA PflhD7793 | 40 |
| UA74 | 14028 ΔfliL | 30 |
| Phage | ||
| P22 | HT12/4int103 | 36 |
Table 2.
Plasmids used in this study
| Plasmid | Expressed protein | Resistance | Replication origin | Induction | Source or reference |
|---|---|---|---|---|---|
| pBAD18-Kan | Expression vector | Kanamycin | pACYC | Arabinose | 38 |
| pBAD30 | Expression vector | Ampicillin | pACYC | Arabinose | 38 |
| pBAD33 | Expression vector | Chloramphenicol | pACYC | Arabinose | 38 |
| pFlhDC | FlhDC | Chloramphenicol | pACYC | Arabinose | J. Mireles |
| pJP164 | FliL | Ampicillin | pACYC | Arabinose | This study |
| pJP192 | MotAB | Kanamycin | pACYC | Arabinose | This study |
Salmonella was grown under 4 different conditions to prepare for microscopy work. (i) For swim preparations, overnight cultures were subinoculated (1:100) and grown at 37°C for 3 h. Cells were harvested by centrifugation at 3,000 rpm before gentle removal of supernatant and resuspension in an equal volume of 2.5% glutaraldehyde (Fisher Scientific). (ii) Swarm cells were spread plated using the pour method (21), where 5 ml of an exponential-phase culture was poured evenly onto the surface of a swarm plate. After 1 min, the excess was poured off and the plate was allowed to air dry for 15 min before incubation at 37°C. After 3 h, when cells were actively motile, a sterile pipette tip was used to gently lift cells from the surface of the plate before resuspension in 200 μl of glutaraldehyde (2.5%, vol/vol). (iii) Swarm cells from the moving edge of a colony inoculated in the center of the plate were harvested for microscopy, again using a sterile pipette tip to lift cells into the glutaraldehyde solution. (iv) Cells were spread plated on hard agar (1.5%) and prepared for microscopy as detailed for swarm cells.
Plates were hydrated by spraying the agar surface with a fine mist of water as described previously (33). Swarm rates were monitored using two approaches. First, the swarm colony diameter was measured every 30 min after inoculating plates in the center. Second, spread-plated cells were monitored under the microscope (40× magnification) to determine the onset of motility. Typically, 6 biological cultures were evaluated with a large number of replicates prepared. Plates used for observation under the microscope were discarded due to their exposure to air and potential drying of the surface. Other replicates were monitored at progressive time intervals, and the presented data are a consistent representation of the motility onset times observed. The “Swiss cheese” colony morphology was monitored through a similar approach by using plate replicates and regular checks throughout growth. This morphology was identifiable in the wild type between a 1.5-h to 2-h window after incubation began. Photographs were taken at 10× final magnification and are representative of the morphology of 6 biological replicates. Hydration of mutant lawns was measured by gently dropping 1-μl capillary tubes (Drummond Scientific), guided by forceps, onto a 3-h swarming lawn (prepared by the pour plate method). When done correctly, the capillaries stand upright with support from the bacterial lawn. After 3 s, they are lifted off and the height of the fluid inside is measured with a ruler. The procedure was repeated 10 times across different regions of the plate surface, with 3 biological replicates for each strain.
Genetic manipulations.
DNA was isolated and manipulated by conventional methods (37) with regions of interest amplified from the chromosome of wild-type Salmonella 14028, using appropriate oligonucleotides. The coding region of each gene was engineered into the SalI and HindIII restriction sites of either pBAD30 (FlhDC), pBAD33 (FliL), or pBAD18-Kan (MotAB) (38). Deletion of genes was achieved by the one-step mutagenesis procedure (39). Mutations were transferred to fresh backgrounds by P22 transduction where necessary. Mutations were converted to unmarked deletions by using the recombinase system of pCP20 (39). The FljB-locked strain was generated by transduction of a Δhin fljB-ON genotype with a linked Cmr marker from TH5862 (gift from Kelly Hughes) into 14028 to generate JP104. The hemagglutinin (HA)-tagged hook gene (flgE7742::3×HA [40]) was transferred from LT2 (TH16678) to the 14028 background as follows: flgE17::Tn10 was moved from ST672 to the FljB-locked strain JP104 by P22 transduction. The resulting nonmotile strain was transduced to motility from a P22 lysate prepared from TH16678 (flgE7742::3×HA). Tets motile colonies were verified for acquisition of flgE7742::3×HA, which was used for the measurement of hook numbers (JP1165). All engineered chromosomal constructs were verified by PCR amplification and DNA sequencing.
Immunostaining and fluorescence microscopy.
The Salmonella strain used was locked into expressing only FljB flagellin. For flagellar immunostaining, glutaraldehyde-treated cells were fixed on a polylysine-treated coverslip for 15 min prior to treatment with 40 μl of anti-FljB antibody (Difco; 1:100 in phosphate-buffered saline [PBS]) and incubated for 10 min at room temperature before washing with 40 μl of 2% bovine serum albumin (BSA) and incubation with Texas Red-conjugated anti-rabbit antibody (40 μl, 1:100 in PBS; Invitrogen) for an additional 15 min. Between antibody incubation steps, cells were washed four times with 40 μl of PBS. Cells were selected randomly for inclusion in data sets with the only proviso being visual clarity and easy distinction from other cells. For visualization of hooks (flgE7742::3×HA), cells were prepared as described above with fixed cells stained instead with monoclonal anti-hemagglutinin antibodies coupled to Alexa-Fluor 488 (1:100 in PBS; Invitrogen) for 15 min before treatment with 2% BSA. Membrane staining was achieved using FM4-64 (0.5 μg ml−1; Invitrogen) and nucleoid staining using 40 μl of DAPI (4′,6-diamidino-2-phenylindole; 2 μg ml−1 in PBS; Sigma). The stains were allowed to stand for 30 s before washing with 40 μl of PBS.
Fixed/stained cells and flagella were observed with an Olympus BX53 microscope and bright-field illumination with images captured using cellSens standard (version 1.6) from Olympus. Length determination was achieved using cellSens software to provide a scale bar, with 100 cells counted to provide an average length and standard deviations from the means. Fluorescent images to visualize hooks were captured using a Leica SP2 AOBS microscope (using preset filters) with a 63× oil objective at room temperature. Optical z sections were taken every 100 nm and compiled on a single plane by using Leica confocal software (version 2.61) before analysis.
RESULTS
Swarm cell lengths double but flagellar numbers per cell stay the same.
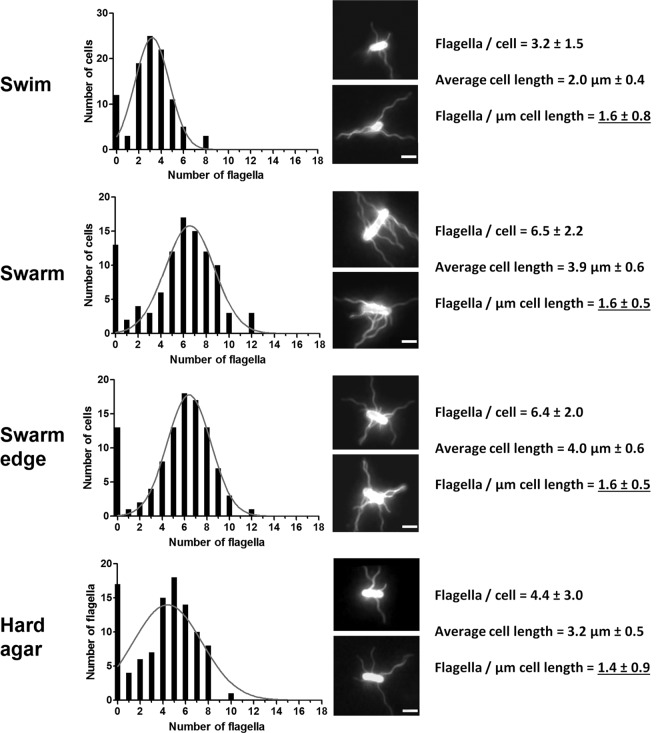
Salmonella enterica strain 14028 (Table 1) was propagated under three different growth conditions, swim (broth), swarm (0.6% agar), and nonswarm/hard (1.5% agar), as described in Materials and Methods. Cells were harvested from broth, from the moving edge of a swarm, or from spread-plated swarm and nonswarm agar at a time when vigorous motility had initiated on swarm agar (3 h after inoculation) (21). Cells were immunostained for visualizing flagella, and cell lengths were simultaneously recorded (Fig. 1). The data showed that the average lengths of swarm cells were twice that of swim cells (4 μm versus 2 μm, respectively), irrespective of whether swarm cells were taken from the edge of a swarm or from spread-plated agar. Cells from nonswarm agar displayed a mixture of short and long cells, and the average length was computed as the intermediate between swim and swarm cell lengths. Flagellar numbers per μm of cell length were similar for all cell types.
Fig 1.
Numbers of flagella and lengths of Salmonella cells under different growth conditions. Cells were prepared for microscopy and chosen for inclusion in the data sets as described in Materials and Methods. Hard agar is also referred to as nonswarm agar in the text. Panels display the distributions of flagella per cell (left), with nonlinear fitting of the Gaussian distribution obtained using Graphpad Prism 5.0 (gray line), to calculate the average number of flagella per cell. Representative examples of immunostained flagella using Texas Red-conjugated antibody are shown (middle). Cell length was measured under bright-field microscopy (right) and used to calculate the distribution of flagella per μm cell length (only the final averages are shown). Bar, 2 μm. n = 100 for all data sets.
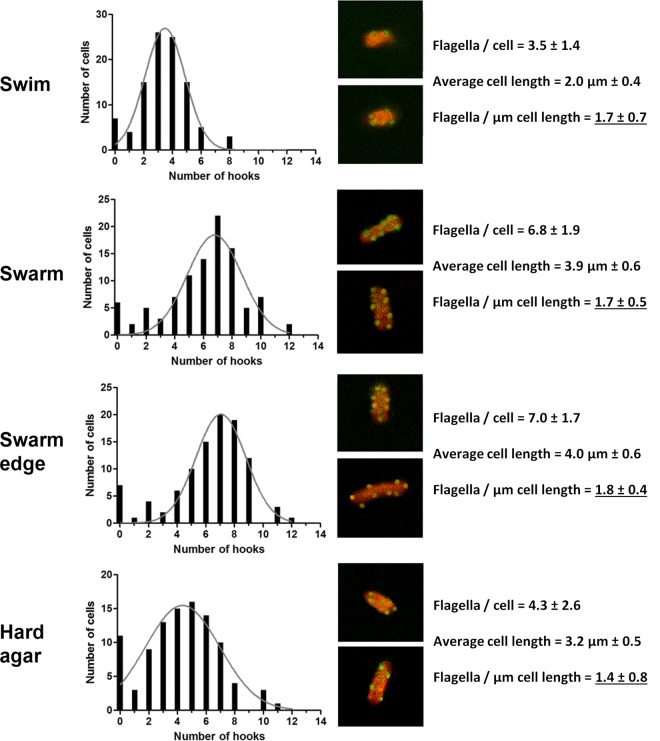
Flagellar filaments are long and tend to break. To ensure that we did not underestimate their numbers, we also counted hooks engineered to encode an HA epitope tag (40); hooks are short and shear resistant (41). Immunostaining with HA antibodies coupled to Alexa-Fluor 488 was used to visualize the hooks in cells propagated under the same conditions as in Fig. 1. Cell lengths were similarly measured. The number of hooks in this strain matched the flagellar numbers remarkably well (Fig. 2). The conclusion with respect to hook numbers per μm of cell length was therefore similar to that derived for flagellar numbers in Fig. 1.
Fig 2.
Numbers of flagellar hooks, estimated by immunostaining. Panel contents are similar to those described for Fig. 1, except that hooks were counted here. Representative examples of immunostained hooks using Alexa-Fluor 488-conjugated antibodies and the cell membrane stain FM4-64 are shown (middle).
To visualize nucleoids, the cells were stained in parallel with the DNA stain DAPI (Fig. 3). Swim cells had one nucleoid, whereas swarm cells had two nucleoids per cell. No constriction of the cell wall was apparent between the two nucleoids in swarm cells, suggesting that these cells get twice as long as swim cells by suppressing cell division. Cells on nonswarm agar showed a mixture of phenotypes: shorter cells had a single nucleoid and longer ones had two nucleoids.
Fig 3.
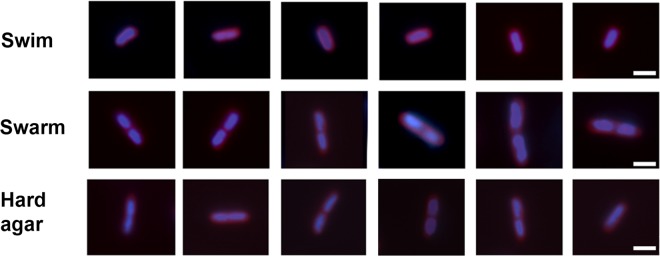
DNA and membrane staining of cells under swim, swarm, and hard agar conditions. Cells were treated with the DNA stain DAPI to visualize nucleoids and the membrane stain FM4-64. Bar, 2 μm. Images are representative of 50 randomly selected cells, as described in Materials and Methods.
We conclude that cell lengths increase on both swarm and nonswarm (hard) agar but that cell lengths are more uniform on swarm agar. The increase in length appears to be a result of suppression of cell division. Flagellar numbers per μm of cell length did not change under any of the three conditions examined.
Increased flagellar numbers allow swarming on harder agar, as do increased FliL levels when combined with increased levels of MotAB stator proteins.
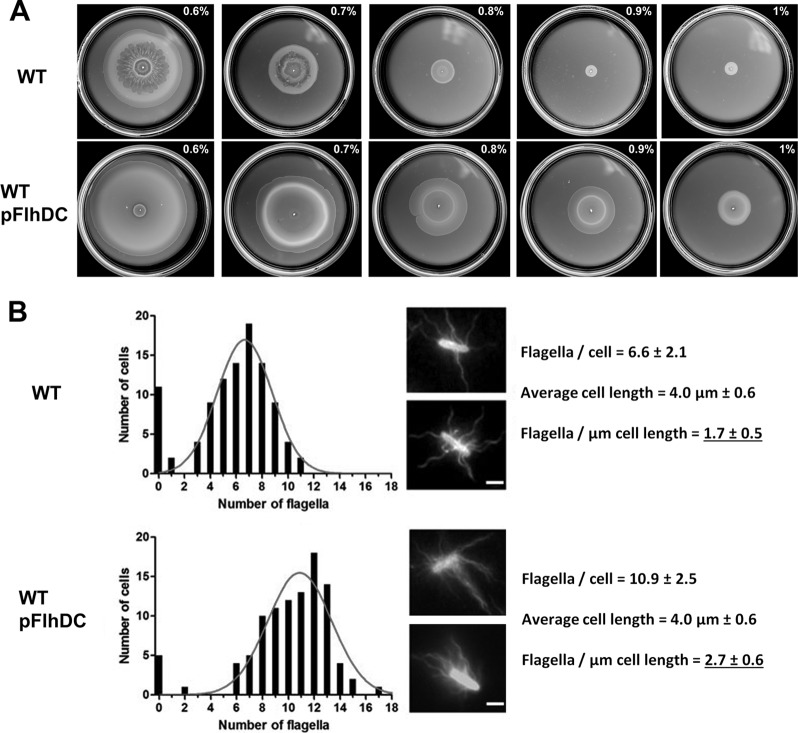
Salmonella swarms within a narrow range (0.5 to 0.6%) of agar concentrations (25). At concentrations beyond this optimal range, motility rapidly falls off, and swarming is completely inhibited at concentrations above 0.8% agar (Fig. 4A, WT). Robust swarmers, which can move over the surface of 1.5% agar, are known to be hyperflagellated (4, 6). To determine if increasing flagellar numbers would allow Salmonella to navigate harder agar surfaces, we introduced into wild-type bacteria plasmid pFlhDC, in which expression of the flagellar master operon flhDC is under the control of the arabinose-inducible PBAD promoter. This master regulon is upregulated 3- to 4-fold in P. mirabilis swarmers (7). Overexpression of FlhDC indeed allowed the bacteria to swarm over agar concentrations as high as 1%. Motility was evident under the microscope at all the agar concentrations shown, although the swarm diameter did not expand as rapidly as on 0.6% agar. On 0.6% agar, the normal 2- to 3-h lag experienced by bacteria prior to initiation of motility was diminished by an hour for the pFlhDC strain (see Fig. S1 in the supplemental material). To determine how many more flagella are made in the pFlhDC strain, these were stained and counted as before. The data showed that flagellar numbers doubled in the pFlhDC strain compared to the wild type (carrying an empty expression vector), but that cell length was not affected (Fig. 4B). A mere doubling of flagella under conditions of flhDC induction from the PBAD promoter was surprising, given the reported 10- to 100-fold increase in protein levels at the arabinose concentrations used (38). We speculate that feedback inhibition of the flagellar regulon via downstream checkpoints may be responsible for the moderate increase in flagellar numbers (42). An flhDC mutant exhibited cell lengths that were similar to the wild type on swarm and nonswarm agar, showing that the motility system is not involved in control of cell length under these conditions (data not shown).
Fig 4.
Salmonella can swarm on higher agar concentrations when flagellar numbers are increased. (A) Wild-type Salmonella with or without a plasmid overexpressing FlhDC was assayed on swarm plates with increasing agar concentrations (0.6 to 1%) supplemented with 0.5% arabinose (inducer) at 37°C. The wild-type strain included an empty plasmid vector as control. The data are representative of three biological replicates, each assayed in triplicate. Plates were photographed at 5 h, when the pFlhDC strain reached the edge of the plate. (B) Flagellar counts and cell length measurements of the strains in panel A (determined as described for Fig. 1).
Increasing flagellar numbers means an increase in the number of motors, effectively increasing the force available for bacteria to move on harder agar surfaces, which likely present higher frictional resistance to motion. We wondered whether we could increase motor force in an alternate manner without increasing flagellar numbers. In Salmonella, FliL is absolutely essential for swarming and has been implicated in improving stator performance in R. sphaeroides (32). Given that the MotAB stators turn over rapidly at the motor (43), we reasoned that increased expression of MotAB in conjunction with increased FliL amounts might increase stator occupancy as well as their dwell time at the motor and/or improve motor output, allowing swarming over harder agar surfaces as well. Figure 5 shows the swarming efficiency of strains with various combinations of plasmids providing increased FlhDC, FliL, and MotAB, all expressed from the arabinose-inducible PBAD promoter. The functionality of these plasmids was confirmed by rescue of their respective genomic mutant phenotypes. We observed that increasing MotAB expression did not improve swarming motility of the wild-type strain whereas increasing FliL expression alone had a moderate effect, up to 0.7 to 0.8% agar (Fig. 5). However, FliL and MotAB together boosted the swarming of the wild-type strain to levels similar to those seen with increased FlhDC alone on plates with 0.6 to 0.9% agar. Similar results were obtained when FliL or MotAB expression was increased concomitantly with FlhDC expression, i.e., MotAB alone did not improve the swarm diameter of the strain expressing FlhDC, but FliL alone boosted the swarming of the FlhDC-expressing strain considerably over all agar concentrations tested. Maximal swarming was observed when all three, FlhDC, MotAB, and FliL, were increased simultaneously, conditions that allowed the bacteria to move out substantially on 1% agar. To determine if numbers of flagella or hooks increased in swarm cells overexpressing FliL and MotAB either alone or together, these were counted as before. The data showed that flagellar/hook numbers and cell lengths did not change under these conditions (see Fig. S2 in the supplemental material).
Fig 5.
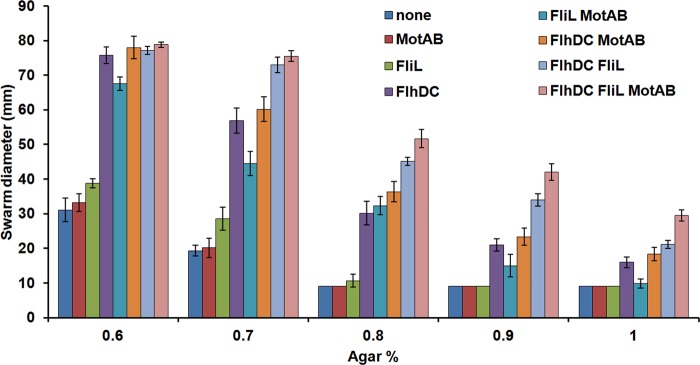
Salmonella can swarm on higher agar concentrations when both FliL and MotAB are overexpressed. Wild-type Salmonella cells transformed with plasmid combinations expressing either no protein (control), FliL, MotAB, or FlhDC were assayed as described for Fig. 4. Plates were photographed after 6 h of incubation at 37°C, with the average swarm diameter for each combination shown. Error bars are standard deviations from the means.
Frictional forces are expected to be greater on harder agar. Because FliL is required for motility on a surface, we also assessed whether FliL contributes to swimming in a viscous medium, since viscosity represents liquid friction (44). The free-swimming speed of a fliL mutant is ∼20% lower than that of the wild type in motility buffer, and this is reflected in a proportionately smaller swim diameter of the fliL mutant compared to the wild-type strain (30) (see Fig. S3A in the supplemental material). As viscosity was gradually increased through addition of Ficoll, the difference in the swim diameters of the two strains grew larger (see Fig. S3A). As observed on swarm plates, overexpression of FliL and MotAB increased the swim diameter of the wild-type strain in both normal (no Ficoll) and 15% Ficoll swim plates (see Fig. S3B).
We conclude that increased flagellar numbers allow Salmonella to swarm on harder agar because of the resulting increase in motor power. Increased FliL along with the MotAB stator proteins has an equivalent effect in the absence of increased flagellar numbers. The positive effect of FliL on motility was also seen during swimming through a medium of higher viscosity, which offers greater resistance to movement. We surmise, therefore, that FliL works with MotAB to counter friction by increasing the power output of existing motors.
Increased flagellar numbers alone are sufficient to suppress the swarming defect of chemotaxis mutants, as are osmolytes.
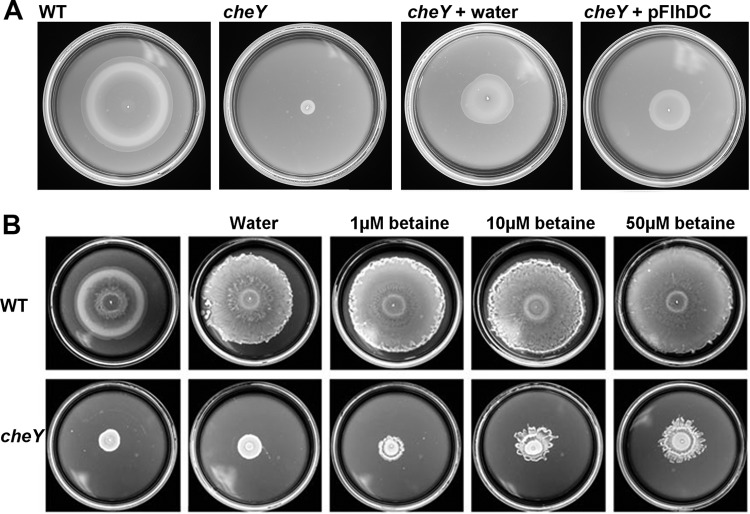
All che mutants of Salmonella are nonswarming (25, 34), because they are unable to hydrate their colonies (33). In our earlier study, spraying a mist of water over a lawn of spread-plated che mutant cells restored swarming motility (33). We could reproduce this observation under the experimental setup where cells were inoculated in the center and allowed to swarm out (Fig. 6A; only a representative cheY mutant is shown). Although motility was initially vigorous, the colony did not expand at the same rate as the wild type, likely because the mutant was more sensitive to the effects of drying when plates were incubated at 37°C. Overexpression of FlhDC in the che mutant strains rescued motility in a manner similar to spraying the plate with water.
Fig 6.
Water, osmolytes and increased FlhDC expression all rescue the swarming defect of che mutants. (A) Salmonella strains were inoculated at the center of 0.6% swarm plates supplemented with 0.5% arabinose and incubated at 37°C for 6 h. In the plate with water, a fine mist of water was sprayed on the plate 10 min prior to inoculation, as described in Materials and Methods. (B) Plates were rinsed with 5 ml of the indicated solution concentrations of betaine, the excess solution was poured off, and plates were allowed to air dry before inoculation. Growth conditions were the same as those described for panel A, except that plates were supplemented with 0.5% glucose.
If water can rescue the che defect, then so should osmolytes, whose property is to attract water. We tested several different categories of osmolytes: amino acids, sugars, and salts. For this experiment, plates were rinsed with 5 ml of the osmolyte, the excess solution was poured off, and the plate was allowed to air dry before inoculation with bacteria. Of the osmolytes tested (glycine betaine, sucrose, and NaCl), glycine betaine and sucrose showed better rescue of motility in the che mutants than NaCl. Data with glycine betaine are shown in Fig. 6B, and the sucrose data are shown in Fig. S4 of the supplemental material. Although the osmolytes rescued motility when observed under the microscope, the che mutant colony did not expand at the same rate as the wild type, as also seen in Fig. 6A, with spraying a mist of water. The “frilly” appearance of the colony edge in all cases where solutions (even plain water [Fig. 6B, top row]) were added to the surface is likely due to perturbation of the surface by these manipulations; swarm colony morphologies can be very sensitive to small changes in surface conditions.
The osmolytes tested increased the swarm colony diameter of the wild type as well (Fig. 6B, top row). Flagella were counted for wild-type cells from the swarming edge on agar supplemented with 50 μM betaine or 250 μM sucrose; the numbers were similar to the no-osmolyte control shown in Fig. 1 (data not shown). Both osmolytes showed improved swarming on 0.7% agar for the wild type but did not support swarming on higher agar concentrations (see Fig. S5 in the supplemental material). Addition of either osmolyte did not change bacterial growth profiles in broth (data not shown).
In summary, addition of water or of osmolytes rescued the swarming defect of che mutants, as did increasing flagellar numbers. While addition of osmolytes can be reconciled as increasing colony hydration, why increasing flagellar numbers has the same effect can only be speculated upon (see Discussion).
Flagellar rotation is important for hydrating the swarm colony.
The hydration defect of che mutants reported by Wang et al. (33) was initially deduced from the dryer colony morphology of the mutants as observed by eye and by the flat featureless landscape of che mutant colonies under the microscope, in contrast with the three-dimensional topography of a wild-type colony, which shows striking high and low regions, dubbed “Swiss cheese” morphology (33). A strong correlation between colony hydration and colony morphology was observed by measuring the amount of fluid that entered microcapillaries dropped vertically on the colony; with che mutants, only half the fluid retained by a wild-type colony was found (see Fig. 3 in reference 33). The Swiss cheese morphology reported for wild-type spread-plated cells was clearly identified in this study as well (Fig. 7A). In our hands, although not prominent, a faint Swiss cheese landscape could also be discerned in the cheY mutant lawn. The fliL mutant showed a morphology that was similar to that of the cheY mutant (Fig. 7A). In contrast, the motA colony was completely flat and featureless. This was supported with measurements of fluid retention in each of these strains (Fig. 7B), where again the cheY colony retained half the fluid retained by the wild type and the fliL colony showed a similar fluid level to the cheY mutant, whereas a motA colony showed 3-fold less fluid than the wild type. Thus, compared to the wild type, colonies of all three nonswarming mutants, cheY, fliL, and motA, showed morphologies indicative of a lack of hydration. We know that the motA mutant cannot rotate its filaments and that flagellar filaments in the fliL mutant break on swarm agar, contingent on rotation (30). Therefore, the flagella will eventually stop rotating in the fliL mutant.
Fig 7.
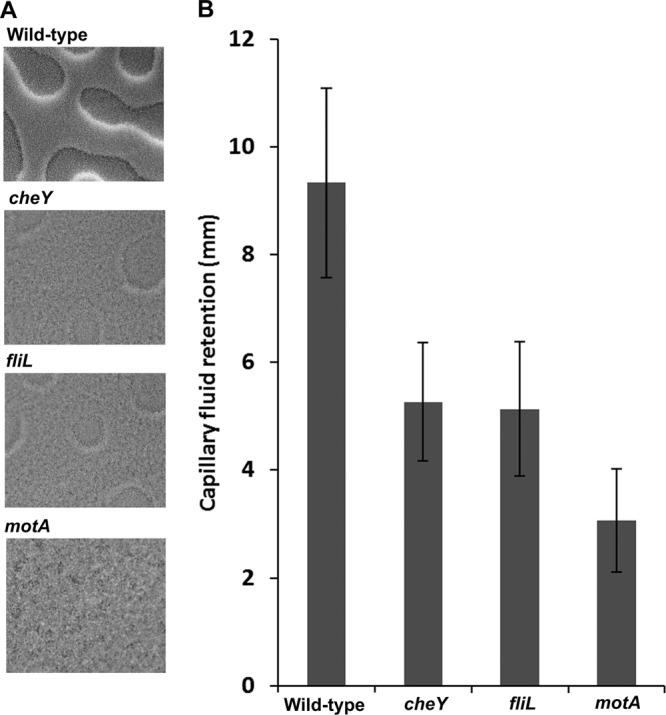
Lawn morphologies and hydration of the wild type and cheY, fliL, and motA mutants propagated on swarm agar. Cultures were spread plated onto 0.6% swarm agar supplemented with 0.5% glucose by the pour method as described in Materials and Methods and incubated at 37°C. (A) Colony morphology photographs were taken immediately prior to the onset of motility in the wild-type strain (2 h in this experiment) with a camera attached to an Olympus BX-53. Magnification, ×10. (B) Comparison of fluid retention levels in 3-h lawns of the indicated strains by the capillary drop method, as described in Materials and Methods (33). The height in the capillary (in mm) is indicated on the y axis. Data are representative of 3 plates (biological replicates), with each sampled from 10 points across the surface. Error bars indicate standard deviations from the means.
We conclude that the ability to both rotate flagella and switch rotation direction is important for hydrating a swarm colony.
DISCUSSION
Swarming is the fastest mode of bacterial surface navigation (1). It is an important phenomenon to study for many different reasons, including the fact that surface-adapted cells are more pathogenic and more resistant to antimicrobials (3). Understanding swarming strategies, which are as varied as the bacteria that utilize them, is important because they help us understand the different ways in which bacteria can adapt successfully to a surface niche, acquire pathogenic potential, and respond to environmental signals that regulate swarming (3).
Robust swarming has been observed by microbiologists ever since it became a common practice to culture bacteria on solid substrates (45). Temperate swarming was discovered in our laboratory in S. marcescens, E. coli, and Salmonella (24, 25) and has since been observed in many bacteria, both Gram positive and Gram negative (1, 14). The precedent set in robust swarmers for a differentiated swarm cell phenotype and the observation that in polarly flagellated bacteria the flagellum controls differentiation by “sensing” the surface and inducing peritrichous flagella have been tendentious in interpreting the morphology and behavior of temperate swarmers. The evidence against a one-size-fits-all scenario came when a large study comparing the transcriptomes of Salmonella on swim (broth), swarm, and nonswarm surfaces showed that the largest differences were between broth and surface conditions, not between swarm and nonswarm surface conditions (21). Importantly, these studies found no change in flagellar gene expression profiles under swim versus swarm conditions, leading us to reexamine our original description of swarm cells as hyperflagellated (25). Our preliminary experiments counting flagella and basal bodies in swim and swarm cells revealed no differences in these numbers per cell, reported as unpublished data in a perspective on swarming (29). In this study, we have performed a systematic analysis of flagellar numbers. We discuss below these and other new findings.
Swarm cell morphology.
Analysis of multiple parameters—cell length, flagella, hooks, and nucleoids—showed that Salmonella swarm cells only appear to be hyperflagellated; in fact, their flagellar numbers per unit cell do not increase (Fig. 1 and 2). The doubling of cell length is due to suppression of cell division, as evidenced by two nucleoids per swarm cell, with no apparent cell wall constriction at the mid-cell division site (Fig. 3). Similar binucleoid swarmer cells were observed for B. subtilis (14). Suppression of cell division appears to be a surface-related phenomenon, because it is seen on both swarm and nonswarm (hard) agar. This surface phenomenon is unrelated to the motility regulon, because an flhDC mutant shows the same cell length distribution as the wild type on swarm agar. We note that mutation of flhD alone has been reported to affect cell division in E. coli grown in LB broth (46, 47).
Transcriptome studies in Salmonella have not revealed any changes in expression of cell division genes (21), which is reasonable given the modest increase in swarm cell length. However, even in the robust swarmer V. parahaemolyticus, which shows substantial cell elongation, transcriptome studies have not revealed swarming-specific changes in cell division genes (8), suggesting that posttranscriptional controls may regulate this phenotype. A possible function of increased cell length in robust swarmers is to accommodate increased flagellar numbers. The Salmonella case, where flagellar numbers do not increase, suggests that elongation serves some other role. Shape is known to influence friction, and a study of nanorods shows that rods are better than spheres at reducing friction (48). Increased length may facilitate minimization of the fluid drag associated with moving objects, or the lengthwise alignment of cells into rafts (3). Swarming rafts of E. coli were observed to move forward more consistently and to be deflected less easily (49). Rafts are also expected to be more effective in breaking the surface tension of liquid for forward motion, because they collectively generate a larger torque. Thus, both increased flagellar numbers, which increase the power strokes for moving against friction, and increased cell lengths are likely adaptations for overriding surface friction and tension. The modest change in Salmonella swarm cell morphology must reflect the lower surface friction on softer agar.
FliL assists MotAB stators in promoting swarming on harder agar.
If flagellar numbers provide more power to move against increased friction, then overproduction of flagella should allow Salmonella to swarm on harder agar surfaces. This was indeed seen to be the case (Fig. 4). Interestingly, increasing FliL and MotAB levels together was equivalent to increasing flagellar numbers for enabling Salmonella to swarm on harder agar (Fig. 5). FliL has been surmised to provide structural support to the basal body in Salmonella, and it has been implicated in generating higher torque, because bacterial swimming speeds are reduced in the absence of FliL (30). Several lines of evidence indicate that FliL is associated with stators in other bacteria (31). In R. sphaeroides, the complete loss of swimming in a fliL mutant could be rescued by suppressor mutations in the stator component MotB (32), in a region where similar mutations in E. coli increase the proton motive force (PMF) through the stators (50). Our data are consistent with a stator-related function for FliL (Fig. 5; see also Fig. S3 in the supplemental material). We note that overexpression of FliL and MotAB did not increase flagellar numbers, nor did it increase cell length (see Fig. S2 in the supplemental material). The observation that overexpression of both MotAB and FliL is required to promote swarming on harder agar may hint at a strong relationship between the two proteins, with FliL facilitating stator recruitment, stator stability, PMF flow, or perhaps a combination of all these. This is in addition to the established structural role FliL provides, a function of heightened importance considering that the enhanced torque needed to drive swarming presumably puts greater stress on the flagellum. Even though no direct measurements of surface friction on different agar surfaces are available, the data presented in this study, where increased FliL promoted both swarming on harder agar and swimming against increasing viscosity (liquid friction [44]), suggest that frictional forces are stronger on harder agar. For Salmonella, the swarming fluid likely does not have a higher viscosity than water, because swimming and swarming speeds are similar in the closely related bacterium E. coli (49). Therefore, FliL is likely employed to override frictional forces stemming from charge interactions or drag resistance of the fluid accompanying motion of bacteria over the agar surface. Increasing flagella on the robust swarmer P. mirabilis allowed it to swim through more viscous media (51). Taken together, these data suggest that at least one function of higher flagellar numbers in robust swarmers is to provide more thrust to overcome higher frictional resistance on harder agar.
Role for flagella in hydrating the agar surface.
Agar is extremely adsorptive, and there is no free water at the surface (52). For flagella to enable motion, water is critical. The most important task for swarming bacteria therefore is to attract water to the surface. P. mirabilis is the only bacterium that is known to secrete polysaccharides and osmolytes (53, 54) that have been implicated in colony hydration (55, 56). No specific osmolytes have been directly identified in other bacteria (3).
We showed in this study that osmolytes applied to the surface of swarm agar suppress the swarming defect of che mutants (Fig. 6; see also Fig. S4 in the supplemental material). Osmolyte addition is expected to stimulate the flow of water from the agar to the surface. Therefore, suppression of the swarming defect of che mutants with osmolytes is consistent with their demonstrated hydration defect (33). In addition to artificial hydration, the che mutant phenotype can be suppressed by mutations or conditions that suppress the extreme motor bias of the chemotaxis mutants (34, 36). The new observation reported in this study is that increasing flagellar numbers also rescues the swarming defect of che mutants (Fig. 6). We consider below a couple of models reconciling all of these observations.
New models.
We had earlier suggested a mechanical model where the flagellar filaments on surface-stranded cells initially stick to the dry agar and the ability to reverse flagellar motor direction helps the flagella to unstick (33). Freely rotating flagella strip LPS off their neighbors, and the released LPS penetrates the agar and acts as an osmolyte. However, added LPS does not rescue the che mutant defect (28). We now propose the following modification to this model: once flagella unstick and are free to rotate, they facilitate extraction of more water from the agar by capillary action induced by continuous displacement of the surface fluid by rotating flagellar bundles. When flagellar numbers are artificially increased by FlhDC overexpression, the probability that some flagella will not stick increases. Biophysical methods will be needed to test the proposed roles of flagellar reversals in unsticking, or of the capillary action of rotating flagella in bringing up water from the agar.
In an alternate model, we propose that when flagella stick to a dry surface and rotation is restricted, the stalled motor generates a signal activating osmolyte secretion. This model draws from observations in Caulobacter crescentus, where restriction of flagellar rotation activates immediate export of hold-fast polysaccharide by a posttranslational mechanism (57). The ability to switch rotor conformations is somehow important in signal propagation. When water arrives, the initial “dry” signal dissipates. Continued hydration is promoted, as in the first model, through capillary action generated by rotating flagella. In this model, overexpression of FlhDC may increase osmolyte production through alternate metabolic pathways, given that flagellar master regulators control the expression of other regulators (16, 42), which in turn control a large number of genes, including those for sugar transport and general metabolism in different bacteria (46, 58, 59). The model can be tested by using new mass spectrometry methodologies to directly detect secreted metabolites within bacterial colonies (60, 61), correlating their presence to a stalled, nonswitching, or rotating motor. In both models, the dry colony morphologies (absence of the “Swiss cheese” appearance) of fliL and motA mutants are explained by a role for flagellar rotation in hydration (Fig. 7). A role for flagellar motion in pumping colony fluid outward from the edge of a swarming colony boundary, thereby facilitating wetting of the dry terrain ahead, has been proposed for E. coli (62).
The effects of motor bias and of flagellar rotation in colony hydration were also observed with B. subtilis, where the swarming colony of a CW-biased chemotaxis mutant was poorly hydrated, as measured by the slow diffusion of MgO beads on the colony surface compared to a wild type or a CCW-biased mutant (27). CCW-biased mutants will form flagellar bundles, while CW-biased mutants are not expected to do so (63), explaining the swarming phenotypes of these mutants. These data support the model where capillary action of the rotating bundle draws water from the agar. MgO beads were completely immobile on a nonflagellate B. subtilis mutant (27). Because both the CW-biased and the nonflagellate mutant were poorly hydrated despite copious amounts of surfactant produced by these mutants, these results also demonstrate that surfactants are not osmolytes.
Summary.
In contrast to bacteria swimming in water, bacteria swarming on an agar surface encounter an absence of free water and strong frictional forces. One strategy to overcome friction is to increase the propulsive force on cells by increasing flagellar numbers. Salmonella cells do not employ this strategy. Instead, they likely use FliL to stabilize and increase the power output of existing motors. The importance of flagellar rotation/bias in hydrating the swarm colony was revealed by the dry colony morphologies of che, fliL, and mot mutants. We have suggested two models to explain how flagellar rotation enables colony hydration. It is apparent that flagella serve varied roles directed at overcoming the impediments to moving on a surface.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kelly Hughes (University of Utah) and Marc Erdhardt (University of Freiburg) for providing strains and for helpful discussions.
This work was supported by NIH grant GM57400.
Footnotes
Published ahead of print 21 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.02064-12.
REFERENCES
- 1. Harshey RM. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249–273 [DOI] [PubMed] [Google Scholar]
- 2. Kearns DB. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8:634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Partridge JD, Harshey RM. 2013. Swarming: flexible roaming plans. J. Bacteriol. 195:909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCarter LL. 2004. Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7:18–29 [DOI] [PubMed] [Google Scholar]
- 5. Allison C, Hughes C. 1991. Bacterial swarming: an example of prokaryotic differentiation and multicellular behaviour. Sci. Prog. 75:403–422 [PubMed] [Google Scholar]
- 6. Rather PN. 2005. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7:1065–1073 [DOI] [PubMed] [Google Scholar]
- 7. Pearson MM, Rasko DA, Smith SN, Mobley HL. 2010. Transcriptome of swarming Proteus mirabilis. Infect. Immun. 78:2834–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gode-Potratz CJ, Kustusch RJ, Breheny PJ, Weiss DS, McCarter LL. 2011. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 79:240–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gode-Potratz CJ, McCarter LL. 2011. Quorum sensing and silencing in Vibrio parahaemolyticus. J. Bacteriol. 193:4224–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trimble MJ, McCarter LL. 2011. Bis-(3′-5′)-cyclic dimeric GMP-linked quorum sensing controls swarming in Vibrio parahaemolyticus. Proc. Natl. Acad. Sci. U. S. A. 108:18079–18084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belas R, Simon M, Silverman M. 1986. Regulation of lateral flagellar gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarter L, Hilmen M, Silverman M. 1988. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell 54:345–351 [DOI] [PubMed] [Google Scholar]
- 13. Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. 1996. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol. Microbiol. 20:693–699 [DOI] [PubMed] [Google Scholar]
- 14. Kearns DB, Losick R. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581–590 [DOI] [PubMed] [Google Scholar]
- 15. Salvetti S, Faegri K, Ghelardi E, Kolsto AB, Senesi S. 2011. Global gene expression profile for swarming Bacillus cereus bacteria. Appl. Environ. Microbiol. 77:5149–5156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patrick JE, Kearns DB. 2012. Swarming motility and the control of master regulators of flagellar biosynthesis. Mol. Microbiol. 83:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgenstein RM, Rather PN. 2012. Role of the Umo proteins and the Rcs phosphorelay in the swarming motility of the wild type and an O-antigen (waaL) mutant of Proteus mirabilis. J. Bacteriol. 194:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsueh YH, Cozy LM, Sham LT, Calvo RA, Gutu AD, Winkler ME, Kearns DB. 2011. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol. Microbiol. 81:1092–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calvio C, Osera C, Amati G, Galizzi A. 2008. Autoregulation of swrAA and motility in Bacillus subtilis. J. Bacteriol. 190:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tolker-Nielsen T, Christensen AB, Holmstrom K, Eberl L, Rasmussen TB, Sternberg C, Heydorn A, Molin S, Givskov M. 2000. Assessment of flhDC mRNA levels in Serratia liquefaciens swarm cells. J. Bacteriol. 182:2680–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q, Frye JG, McClelland M, Harshey RM. 2004. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52:169–187 [DOI] [PubMed] [Google Scholar]
- 22. Overhage J, Bains M, Brazas MD, Hancock RE. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 190:2671–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tremblay J, Deziel E. 2010. Gene expression in Pseudomonas aeruginosa swarming motility. BMC Genomics 11:587 doi:10.1186/1471-2164-11-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alberti L, Harshey RM. 1990. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J. Bacteriol. 172:4322–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harshey RM, Matsuyama T. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. U. S. A. 91:8631–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen BG, Turner L, Berg HC. 2007. The wetting agent required for swarming in Salmonella enterica serovar Typhimurium is not a surfactant. J. Bacteriol. 189:8750–8753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Be'er A, Harshey RM. 2011. Collective motion of surfactant-producing bacteria imparts superdiffusivity to their upper surface. Biophys. J. 101:1017–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toguchi A, Siano M, Burkart M, Harshey RM. 2000. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J. Bacteriol. 182:6308–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harshey RM. 2010. Swarming adventures, p 163–172 In Maloy SM, Casadesus J, Hughes K. (ed), The lure of bacterial genetics: a tribute to John Roth. American Society for Microbiology, Washington, DC [Google Scholar]
- 30. Attmannspacher U, Scharf BE, Harshey RM. 2008. FliL is essential for swarming: motor rotation in absence of FliL fractures the flagellar rod in swarmer cells of Salmonella enterica. Mol. Microbiol. 68:328–341 [DOI] [PubMed] [Google Scholar]
- 31. Motaleb MA, Pitzer JE, Sultan SZ, Liu J. 2011. A novel gene inactivation system reveals altered periplasmic flagellar orientation in a Borrelia burgdorferi fliL mutant. J. Bacteriol. 193:3324–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suaste-Olmos F, Domenzain C, Mireles-Rodriguez JC, Poggio S, Osorio A, Dreyfus G, Camarena L. 2010. The flagellar protein FliL is essential for swimming in Rhodobacter sphaeroides. J. Bacteriol. 192:6230–6239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. 2005. Sensing wetness: a new role for the bacterial flagellum. EMBO J. 24:2034–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burkart M, Toguchi A, Harshey RM. 1998. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 95:2568–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manson MD. 1992. Bacterial motility and chemotaxis. Adv. Microb. Physiol. 33:277–346 [DOI] [PubMed] [Google Scholar]
- 36. Mariconda S, Wang Q, Harshey RM. 2006. A mechanical role for the chemotaxis system in swarming motility. Mol. Microbiol. 60:1590–1602 [DOI] [PubMed] [Google Scholar]
- 37. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, New York, NY [Google Scholar]
- 38. Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singer HM, Erhardt M, Steiner AM, Zhang MM, Yoshikami D, Bulaj G, Olivera BM, Hughes KT. 2012. Selective purification of recombinant neuroactive peptides using the flagellar type III secretion system. mBio. 3(3):e00115–12 doi:10.1128/mBio.00115-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosu V, Hughes KT. 2006. σ28-dependent transcription in Salmonella enterica is independent of flagellar shearing. J. Bacteriol. 188:5196–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chevance FF, Hughes KT. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leake MC, Chandler JH, Wadhams GH, Bai F, Berry RM, Armitage JP. 2006. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature 443:355–358 [DOI] [PubMed] [Google Scholar]
- 44. Walker JS. 2003. Physics: an introduction, 2nd ed. Prentice-Hall Inc., Upper Saddle River, NJ [Google Scholar]
- 45. Henrichsen J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pruss BM, Matsumura P. 1996. A regulator of the flagellar regulon of Escherichia coli, flhD, also affects cell division. J. Bacteriol. 178:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pruss BM, Markovic D, Matsumura P. 1997. The Escherichia coli flagellar transcriptional activator flhD regulates cell division through induction of the acid response gene cadA. J. Bacteriol. 179:3818–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Akbulut M, Belman N, Golan Y, Israelachvili J. 2006. Frictional properties of confined nanorods. Adv. Mater. 18:2589–2592 [Google Scholar]
- 49. Darnton NC, Turner L, Rojevsky S, Berg HC. 2010. Dynamics of bacterial swarming. Biophys. J. 98:2082–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hosking ER, Vogt C, Bakker EP, Manson MD. 2006. The Escherichia coli MotAB proton channel unplugged. J. Mol. Biol. 364:921–937 [DOI] [PubMed] [Google Scholar]
- 51. Tuson HH, Copeland MF, Carey S, Sacotte R, Weibel DB. 2013. Flagellum density regulates Proteus mirabilis swarmer cell motility in viscous environments. J. Bacteriol. 195:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Banaha M, Daerr A, Limat L. 2009. Spreading of liquid drops on agar gels. Eur. Phys. J. Spec. Top. 166:185–188 [Google Scholar]
- 53. Gygi D, Rahman MM, Lai HC, Carlson R, Guard-Petter J, Hughes C. 1995. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 17:1167–1175 [DOI] [PubMed] [Google Scholar]
- 54. Lahaye E, Aubry T, Fleury V, Sire O. 2007. Does water activity rule P. mirabilis periodic swarming? II. Viscoelasticity and water balance during swarming. Biomacromolecules 8:1228–1235 [DOI] [PubMed] [Google Scholar]
- 55. Rauprich O, Matsushita M, Weijer CJ, Siegert F, Esipov SE, Shapiro JA. 1996. Periodic phenomena in Proteus mirabilis swarm colony development. J. Bacteriol. 178:6525–6538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stahl SJ, Stewart KR, Williams FD. 1983. Extracellular slime associated with Proteus mirabilis during swarming. J. Bacteriol. 154:930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. 2012. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol. Microbiol. 83:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pruss BM, Liu X, Hendrickson W, Matsumura P. 2001. FlhD/FlhC-regulated promoters analyzed by gene array and lacZ gene fusions. FEMS Microbiol. Lett. 197:91–97 [DOI] [PubMed] [Google Scholar]
- 59. Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM. 2010. FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar Typhimurium. J. Bacteriol. 192:6261–6270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watrous J, Roach P, Alexandrov T, Heath BS, Yang JY, Kersten RD, van der Voort M, Pogliano K, Gross H, Raaijmakers JM, Moore BS, Laskin J, Bandeira N, Dorrestein PC. 2012. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. U. S. A. 109:E1743–E1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoefler BC, Gorzelnik KV, Yang JY, Hendricks N, Dorrestein PC, Straight PD. 2012. Enzymatic resistance to the lipopeptide surfactin as identified through imaging mass spectrometry of bacterial competition. Proc. Natl. Acad. Sci. U. S. A. 109:13082–13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turner L, Zhang R, Darnton NC, Berg HC. 2010. Visualization of flagella during bacterial swarming. J. Bacteriol. 192:3259–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berg HC. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 [DOI] [PubMed] [Google Scholar]
- 64. Wang Q, Zhao Y, McClelland M, Harshey RM. 2007. The RcsCDB signaling system and swarming motility in Salmonella enterica serovar Typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J. Bacteriol. 189:8447–8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.