Summary
Helicases catalytically unwind duplex DNA or RNA using energy derived from the hydrolysis of nucleoside triphosphates and are attractive drug targets because they are required for viral replication. This review discusses methods for helicase identification, classification and analysis, and presents an overview of helicases that are necessary for the replication of human pathogenic viruses. Newly developed methods to analyze helicases, coupled with recently determined atomic structures, have led to a better understanding of their mechanisms of action. The majority of this research has concentrated on enzymes encoded by the herpes simplex virus (HSV) and the hepatitis C virus (HCV). Helicase inhibitors that target the HSV helicase–primase complex comprised of the UL5, UL8 and UL52 proteins have recently been shown to effectively control HSV infection in animal models. In addition, several groups have reported structures of the HCV NS3 helicase at atomic resolutions, and mechanistic studies have uncovered characteristics that distinguish the HCV helicase from related cellular proteins. These new developments should eventually lead to new antiviral medications.
Helicases are ubiquitous biological machines that separate a double helix made of DNA or RNA. Nucleic acid strands must be separated to allow cellular proteins to access, read or rearrange genetic information. In humans, several debilitating inherited disorders are linked to genetic defects in helicase genes, including Bloom's, Werner's and Rothmund-Thomson's syndromes.1 Decades of experiments with model viruses have shown that without a functional helicase, a virus can no longer infect or destroy host cells. Although progress in exploiting viral helicases as antiviral drug targets has been slow, the last 5 years has seen impressive progress toward understanding the mechanism of helicase action on a molecular level. As a result, two groups recently described a series of compounds that inhibit a helicase encoded by herpes simplex virus (HSV) and thereby decrease disease severity in animal models.2
Because all viruses synthesize their genomes in a template-dependent manner, they all require a helicase in addition to a DNA or RNA polymerase (Fig. 1). In viruses with duplex DNA genomes, like HSV, a replicative helicase provides one strand for continual leading strand synthesis and another strand for discontinuous lagging strand synthesis. In viruses with single-stranded DNA or RNA genomes, a helicase is required to displace single-stranded genomes after replication so they can be packaged into new viral particles. Helicases are usually associated with one strand of a duplex and are functionally classified according to the polarity of that strand. The helicase in Figure 1 surrounds the leading strand template and travels in a 3′ to 5′ direction like the polymerase. Such a 3′–5′ helicase requires a 3′ single-stranded DNA (ssDNA) tail in order to unwind duplex DNA. If the helicase were associated primarily with the other strand in Figure 1, it would require a 5′ ssDNA tail and would be classified as a 5′–3′ helicase.
Fig. 1.

Role of helicase in viral replication. Helicases unwind duplex DNA or RNA intermediates formed during viral replication in a reaction driven by energy derived from the hydrolysis of nucleoside triphosphates.
Viral helicase identification and characterization
Hundreds of putative helicases have been identified based on the presence of certain sequence motifs in viral genomes. These motifs are summarized in Table I, but their presence alone is not always indicative of helicase activity. The biochemical assays described below are still necessary to identify and properly characterize a potential helicase.
Table 1. Classification of viral helicasesa.
| SUPERFAMILY 1 | SUPERFAMILY 2 | SUPERFAMILY 3 | FAMILY 4 | |
|---|---|---|---|---|
| Motif Ib |
|
|
|
|
| Motif II | +++DExo |

|

|
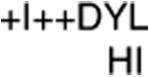
|
| Motif III | +++GDxoQ |

|
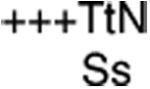
|
|
| Motif IV | xx+xooxR |

|
|
|
| Motif V |
|
|
||
| Motif VI |

|
QxxGRxxR | ||
| Human DNA virus (Protein) | Herpesvirus (UL5) | Herpesvirus (UL9) Poxvirus (NPH-II) |
Papillomavirus (E1) | |
| Human RNA virus (Protein) | Alphavirus (NSP2) Rubella virus (p220) Coronavirus (p66HEL) |
Flavivirus (NS3) Hepatitis C virus (NS3) |
Poliovirus (2C) Hepatitis A virus (2C) Rhinovirus (2C) |
|
| Model Helicases (Organism) | Rep (E. coli) UvrD (E. coli) RecBCD (E. coli) PcrA (Bacillus stearo.) Dda (Bacteriophage T4) |
elF4a (human) PriA (E. coli) RecQ (E. coli) UvrB (E. coli) UvsW (phage T4) |
RuvB (E. coli) MCM proteins (human) T-antigen (SV40) |
DnaB (E. coli) Gp4 (phage T7) Gp41 (phage T4) |
Consensus signature motifs are as described by Hall and Matson62
Invariant amino acids are designated by capital letters and conserved residues are noted with lower case letters. A“+” signifies any hydrophobic residue, and an “o” designates any hydrophilic amino acid. An “x” represents any residue.
ATPase assays
The simplest helicase assays involve measuring the rate of ATP hydrolysis catalyzed by the protein in the presence and absence of DNA or RNA. Because ATP is the fuel for helicase action, its hydrolysis is always dramatically stimulated by the presence of nucleic acids in the assay. Standard ATPase assays measure ATP hydrolysis using thin-layer chromatography3 or colorimetric assays,4 or by coupling the reaction to NADH oxidation using pyruvate kinase and lactate dehydrogenase.5 A new, very sensitive, continuous ATPase assay utilizing a coumarin-labeled phosphate-binding protein has also been developed.6
Binding assays
A second basic property of a helicase is its ability to bind DNA. The most commonly used assay for DNA binding is a gel shift assay in which bound and free radiolabeled DNA are separated using nondenaturing polyacrylamide gel electrophoresis (PAGE). However, this assay sometimes underestimates substrate affinity because binding can be perturbed during electrophoresis. Preferred binding assays include filter binding assays7 or assays that monitor changes in protein (or nucleic acid) absorbance8 or fluorescence9 upon binding. Other methods used to detect ligand binding to helicases include circular dichroism, isothermal titration calorimetry and NMR spectroscopy.10
Unwinding assays
Helicase-catalyzed DNA (or RNA) unwinding is more difficult to measure than either ATP hydrolysis or nucleic acid binding because a double helix frequently anneals more rapidly than unwinding can be observed. The first and still most sensitive unwinding assay utilizes a radiolabeled oligonucleotide annealed to a circular ssDNA bacteriophage genome.11,12 When this substrate is incubated with a putative helicase, the oligonucleotide is gradually displaced. To measure the rate of displacement, the reaction products are separated on native PAGE after the reaction is terminated at various times. Similar assays can be performed using two short synthetic oligonucleotides. In unwinding assays, the strand that contains the single strand tail necessary for helicase loading is called the template strand and the other (radiolabeled) strand is called the release strand. A DNA trap is often included in unwinding assays that is designed to prevent reannealing of the release and template strands.13 Newer helicase assays, classified as strand-capture assays, detect unwinding using methods more amenable to automation than PAGE. One method to capture and measure helicase reaction products is a scintillation proximity assay (SPA). The radiolabeled ssDNA products are captured in an SPA by coating the beads with a complementary strand of DNA.14,15 Another strand-capture assay, termed a helicase ELISA, uses an antigen-labeled release strand instead of a radiolabeled release strand.16 Electrochemiluminescence has also been used to develop a highly sensitive strand capture assay.17
All of the above assays detect unwound products only after the reaction is terminated and, therefore, cannot be used to continually monitor helicase action. Continuous assays that directly monitor the rates of helicase action measure either fluorescence resonance energy transfer (FRET) or the displacement of dyes that selectively bind duplex DNA. In most FRET assays, one strand is labeled with a donor fluorophore, and the other is labeled with a quenching acceptor fluorophore.18 When the probes are in close proximity, as they are when covalently attached to the 3′- and 5′-ends of annealed DNA strands, FRET reduces the observed fluorescence of the donor; upon strand dissociation, the observed signal increases. FRET assays are readily scaled-down for use in high-throughput screens.19 In fluorescence dye displacement assays, DNA dyes are used to label DNA. The resulting fluorescence decreases as the helicase unwinds the duplex. Dye displacement assays performed under the microscope have been used to visualize the action of an individual helicase as it unwinds a single DNA molecule.20
Translocation assays
Helicases not only act on duplex molecules, but they also move along single stranded nucleic acids. The ability of a helicase to translocate on an ssDNA template was first demonstrated using the bacteriophage T7 gene 4 protein (which contains both helicase and primase activity) by carefully analyzing the frequency of primase recognition site usage on a circular ssDNA template.21 A more direct method to assay directionality is to anneal two different release strand oligonucleotides to a single template strand. The direction and rate of helicase movement can be determined by evaluating when each strand is released under single turnover conditions.22 Another assay that has been used to examine the translocation directionality of viral helicases measures the ability of a helicase to displace proteins bound to ssDNA.23,24
Classification of viral helicases
Comparison of the protein sequences of various known helicases has enabled the identification of several consensus “signature” motifs (Table I). Gorbalenya and Koonin25 have used this information to classify helicases into families and superfamilies. A superfamily contains several related families and is thus a broader evolutionary classification. A sampling of clinically relevant viral helicases whose activities have been experimentally confirmed is provided in Table I. In addition, Table I lists relevant helicases from model organisms whose properties could be used to help guide future studies of the viral proteins.
Superfamily 1
The two largest helicase groups, Superfamily 1 (SF1) and Superfamily 2 (SF2), are also the most closely related. Proteins in these two groups share many sequence and structural similarities. Within SF1 there are several families of closely related proteins. For example, in one SF1 family, the protein members are similar to the DNA repair protein UvrD of Escherichia coli, and the proteins in another family are similar to the Dda helicase from phage T4. Two protein families within SF1 contain helicases from pathogenic human viruses. The first family is comprised of proteins similar to the product of the UL5 gene of HSV, and the second is comprised of proteins from RNA viruses.
UL5 is part of the three-protein replicative helicase primase complex (UL5/ UL8/UL52) that is the target of recently reported HSV drug candidates.2 The primase function, which provides RNA primers for lagging strand synthesis, is part of the UL52 protein. The precise function of UL8, the third component of the HSV helicase-primase complex, is unclear, but it likely helps coordinate events at the replication fork. Mutagenesis of the conserved motifs in the UL5 protein results in defects in viral replication and the ability of the complex to unwind DNA and hydrolyze ATP.26
The largest protein family in SF1 contains proteins from positive sense RNA viruses, the vast majority of which infect plants. Helicase activity has been confirmed for only a few of these proteins isolated from animal viruses. The first RNA virus helicase activity identified in this group was that of Semliki Forest virus nonstructural protein 2.27 Semliki Forest virus is a prototype alphavirus in the Togaviridae family and is related to rubella virus. The second SF1 RNA virus family protein demonstrated to be a helicase was obtained from the 229E strain of human coronavirus.28 Coronavirus infections lead to colds and sometimes to severe respiratory disease and pneumonia. Helicase activity for a third protein in this family has been identified in the equine arteritis virus nonstructural protein 10.29
Several helicases in SF1 have been studied in detail and can be used as models to understand the action of viral helicases. The first structure reported for any helicase was that of the PcrA protein from Bacillus stearothermophilus.30 PcrA is a homologue of the E. coli Rep helicase, one of the most extensively studied model helicases. Before structural data was available, Rep helicase was believed to function as a dimer that rolled along DNA as the individual subunits altered between conformations that preferentially bound single-stranded and double-stranded DNA. The structure of Rep later confirmed a dimeric protein containing two subunits in different conformations.31 Unlike Rep, PcrA crystallizes as a monomer, but PcrA assumes different conformations in the presence and absence of ATP.30,32 Alterations between these conformations have been proposed to allow the monomeric PcrA helicase to crawl along DNA like an inchworm. Conformational changes necessary for either the “rolling model” or the “inchworm model” are likely regulated by ATP binding and hydrolysis.
Superfamily 2
Less progress has been reported in developing drugs targeting SF2 helicases, in part because the proteins in this family are more closely related to a number of critical host factors. The largest family in SF2 contains proteins that are similar to the cellular transcription factor eIF-4A33 and contain the signature Asp-Glu-Ala-Asp in motif 2, which is frequently referred to as the DEAD-box motif. Another SF2 family contains proteins with an Asp-Glu-Ala-His (DEAH-box) sequence in motif 2 and are related to the RNA splicing complex factor Prp16.34 Because these two SF2 families contain important cellular proteins, viral SF2 helicases might be considered less attractive drug targets than helicases with more distant relationships to cellular proteins. Nevertheless, SF2 includes three evolutionarily distinct viral helicase families, each containing important human pathogens. The first family contains proteins from viruses in the Herpesviridae family, the second contains Poxviridae proteins and the third contains proteins from Flavi-viridae.
The HSV helicase in SF2 is the UL9 protein. UL9 binds as a dimer in a sequence-specific manner and unwinds primarily at viral origins of replication. When bound to DNA, UL9 rapidly hydrolyzes ATP, but even under optimal conditions, UL9 displays only modest helicase activity. The conserved helicase motifs in UL9 are necessary for viral replication and this helicase activity.35
A second group of intriguing SF2 helicases contains proteins encoded by the poxviruses, the prototype being the NPH-II helicase from vaccinia virus. Careful kinetic analyses have revealed that NPH-II unwinds about six base pairs in each catalytic cycle36 and can dislodge RNA-binding proteins.37 Although the vaccinia protein itself would not necessarily be a drug target, similar proteins exist in related viruses like smallpox.
All viruses in Flaviviridae encode nonstructural peptides that possess the SF2 helicase motifs. Flaviviridae contains the genera Flavivirus, Pestivirus and Hepacivirus, all of which contain important animal pathogens. All these viruses encode one large polyprotein that is processed into structural and nonstructural proteins. The Flaviviridae helicase resides in nonstructural protein 3 (NS3) together with a protease that is responsible for processing much of the viral polyprotein. The NS3 helicase has been characterized from dengue virus,38 West Nile virus,39 yellow fever virus,40 bovine viral diarrhea virus,41 hepatitis C virus42 and hepatitis G virus.43
The HCV helicase has been one of the most intensely studied viral helicases, and the resulting structural and mechanistic insights warrant further discussion. Three groups have independently reported structures of the HCV helicase domain.44–46 The HCV helicase is a Y-shaped molecule composed of three domains separated by two deep clefts. One of the structures45 contains a DNA poly(U) oligonucleotide that is bound in the cleft separating domains 1 and 2 from domain 3 (Fig. 2). In the HCV-DNA complex, a sulfate ion binds where the β-phosphate of ATP binds to other helicases. All of the conserved sequence motifs are in the first two domains, and cluster around the cleft separating domains 1 and 2. Domain 3 forms a novel structure not seen in other helicases.
Fig. 2.
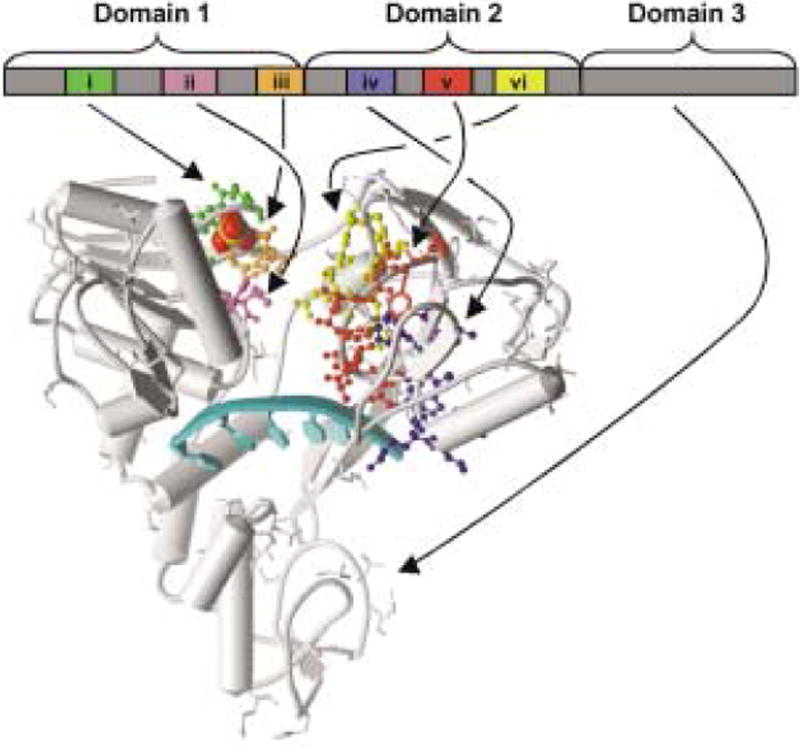
Structure of the hepatitis C virus helicase DNA complex. Schematic representation of NS3 helicase bound to a polyU ssDNA (arrow) with a sulfate ion (space-fill) bound in the putative ATP binding site. Conserved motifs are shown as colored ball/sticks. Coordinates are from Protein Data Bank entry 1A1V.45
In the HCV helicase, motifs I, II and III are present in Domain 1. Motif I is a Walker-type nucleotide-binding site that forms a phosphate-binding loop (P-loop), which likely surrounds the phosphates of ATP (as seen in related proteins).31,32 By similar analogy, the conserved Asp in the second SF2 motif likely coordinates a metal ion necessary to precisely position ATP in the binding site.32 Motif III is located on a loop that connects Domain 1 to Domain 2, and may help couple ATP hydrolysis to a conformational change that leads to translocation along RNA. Domain 2 is structurally similar to Domain 1 and could help transmit conformational changes that occur upon NTP hydrolysis at the P-loop to help separate the double helix. SF2 motifs IV, V and VI reside in domain 2, complete the ATP binding cleft and make key contacts with the bound DNA. Noteworthy nucleic acid contacts come from the first Thr in motif V (T411) and an analogous conserved Thr in Domain 1 (T269), which form hydrogen bonds with the phosphate backbone of the bound nucleic acid.
When they reported the structure of HCV helicase bound to an oligonucleotide, Kim et al.45 proposed a model for helicase action that formed the basis for the inchworm model mentioned above in reference to the PcrA helicase.32 They proposed that in the absence of ATP, HCV helicase assumes an open conformation tightly bound to the DNA (or RNA) with a residue in Domain 3 (W501) intercalated between the nucleotides like a bookend. When ATP binds, the cleft is proposed to close in a change analogous to the domain closure visualized in PcrA structures.32 When the cleft closes, W501 releases its grip and slides one or two base pairs. After hydrolysis, W501 again grips the 3′ end of the nucleic acid and the inchworm widens. The net result is a translocation of the protein in a 3′ to 5′ direction.
Superfamily 3 and Family 4
SF3 and F4 helicases are fundamentally different from SF1 and SF2 helicases. The well-characterized helicases in these groups do not function as monomers (or dimers), but rather as hexamers.47 Model helicases such as the SV40 T-antigen (SF3) and the phage T7 gene 4 protein (F4) have been shown to form ring structures that encircle one strand of DNA and exclude the other DNA strand. A hexameric helicase likely acts as a rotary motor that travels in one direction along one strand to separate it from the complementary strand, as diagramed in Figure 1. Although no F4 helicases from pathogenic human viruses have been reported, an SF3 helicase from human papillomavirus has been extensively studied both mechanistically and structurally.48 This protein could be an important target for treatments of this class of tumor viruses. Many pathogenic RNA viruses, such as poliovirus, hepatitis A virus and rhinovirus, encode proteins that contain SF3 helicase motifs, but a true helicase activity has not yet been demonstrated for any of these proteins. These proteins, such as the poliovirus 2C protein49 and the Norwalk-like virus p41 enzyme,50 likely utilize the helicase motor function for some purpose other than duplex unwinding.
Helicase inhibitors, drug candidates and prospects
Groups from both Bayer51 and Boehringer Ingelheim52 have reported that new drug candidates designed to inhibit the HSV helicase complex reduce viral replication and disease severity in animal models. The structures of some of these novel compounds are shown in Figure 3. These compounds were identified from high-throughput helicase assays as recently reviewed elsewhere.2,53 Unlike acyclovir and related drugs, the new anti-HSV compounds are not nucleoside analogues, although they all share a thiazole ring structure. The first anti-HSV thiazole compound reported in the scientific literature, T-157602, displayed little cytotoxic effects but was a potent helicase, primase and ATPase inhibitor with an IC50 in the micromolar range. T-157602 also inhibited viral replication in cell culture (no animal studies were reported).54 The newer compounds are either thiazolyl pheny1- containing drugs like compound BILS-179 BS52 or thiazole urea derivatives like BAY-57-129351 (Fig. 3). Both BAY-57-129351,55 and BILS-179 BS52 are more effective than T-157602 in vitro, in cell culture and in animal models. Each acts by inhibiting the heterotrimeric complex of the HSV proteins UL5, UL8 and UL52,51,52 possibly by locking the helicase on ssDNA and thus preventing the processive action of DNA unwinding. However, exactly how the new HSV inhibitors function is still unclear because mechanistic and structural details of the protein-inhibitor interactions are not yet available.
Fig. 3.

Inhibitors of herpes simplex virus replication. Structures of the nucleoside analogue drug acyclovir, the 2-amino thiazole derivative T-157602 identified by Tularik Inc.,54 the Boehringer Ingelheim compound BILS-179 BS52 and the inhibitor BAY-57-1293 reported by Bayer.51
Although the HSV work has clearly validated helicases as drug targets, less progress has been made with other viruses. For example, no drug candidates have yet been reported that target the HCV helicase, although several groups have been intensely pursuing this goal for nearly a decade. One reason may be the lack of a robust system to cultivate HCV in cells or convenient animal models to evaluate HCV drug candidates. Several groups have reported specific HCV helicase inhibitors,56 but most are nucleotide analogues, which might also influence cellular SF2 helicases. For example, L-β-dCTP, L-β-dTTP,57 ribavirin triphosphate58 and imidazo[4,5-d]pyridazine nucleosides58 each have been reported to inhibit nucleic acid unwinding by the HCV helicase.
The lessons learned from developing HSV inhibitors must be applied to aid the development of drugs targeting other helicases. It is highly unlikely that compounds that inhibit helicase by competing with ATP, ADP or Pi will provide valuable lead compounds, as they could also interact with a variety of critical host factors. Because of the highly conserved nature of the helicase motor function, inhibitors targeting helicases can be potent inhibitors of cellular proteins. For example, nucleoside-based HSV inhibitors of the HSV helicase-primase complex were also potent inhibitors of the human primase complex.59
In order to effectively target viral helicases, differences between viral and host proteins will need to be defined, as is being done with the HCV helicase. Notable differences between the HCV helicase and cellular DEAD-box proteins include nucleic acid and NTP preferences.60 Comparison of the HCV structure with that of other SF1 and SF2 helicases has revealed unique features of the HCV helicase that could provide targets for antiviral drugs.61 Only two of the three domains of the HCV helicase are similar to other helicases, and most of Domain 3 (Fig. 2) forms a novel domain that is not seen in related enzymes. It is possible that residues in Domain 3 confer distinguishing characteristics on the HCV helicase. Once the differences between HCV helicase and related cellular SF2 helicases are clarified, noncompetitive inhibitors or allosteric regulators that bind far from the conserved helicase motifs should be more easily identified.
Conclusion
Helicases are complex proteins, and much more research will need to be done to understand how they manipulate genomes. The latest arsenal of helicase assays should help elucidate these mechanisms. Any resulting knowledge will undoubtedly aid antiviral drug development.
Acknowledgments
This work was supported by an AASLD Liver Scholar Award from the American Liver Foundation.
References
- 1.Nakayama H. RecQ family helicases: Roles as tumor suppressor proteins. Oncogene. 2002;21:9008–21. doi: 10.1038/sj.onc.1205959. [DOI] [PubMed] [Google Scholar]
- 2.Crumpacker CS, Schaffer PA. New anti-HSV therapeutics target the helicase- primase complex. Nat Med. 2002;8:327–8. doi: 10.1038/nm0402-327. [DOI] [PubMed] [Google Scholar]
- 3.Wong I, Moore KJ, Bjornson KP, Hsieh J, Lohman TM. ATPase activity of Escherichia coli Rep helicase is dramatically dependent on DNA ligation and protein oligomeric states. Biochemistry. 1996;35:5726–34. doi: 10.1021/bi952959i. [DOI] [PubMed] [Google Scholar]
- 4.Lam AMI, Keeney D, Eckert PQ, Frick DN. Hepatitis C virus NS3 ATPase/helicases from different genotypes exhibit variations in enzymatic properties. J Virol. 2003;77:3950–61. doi: 10.1128/JVI.77.7.3950-3961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preugschat F, Averett DR, Clarke BE, Porter DJT. A steady-state and pre-steady-state kinetic analysis of the NTPase activity associated with the hepatitis C virus NS3 helicase domain. J Biol Chem. 1996;271:24449–57. doi: 10.1074/jbc.271.40.24449. [DOI] [PubMed] [Google Scholar]
- 6.Dillingham MS, Wigley DB, Webb MR. Demonstration of unidirectional single-stranded DNA translocation by PcrA helicase: Measurement of step size and translocation speed. Biochemistry. 2000;39:205–12. doi: 10.1021/bi992105o. [DOI] [PubMed] [Google Scholar]
- 7.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: Application to protein-nucleic acid interactions. Proc Natl Acad Sci USA. 1993;90:5428–32. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locatelli GA, Spadari S, Maga G. Hepatitis C virus NS3 ATPase/helicase: An ATP switch regulates the cooperativity among the different substrate binding sites. Biochemistry. 2002;41:10332–42. doi: 10.1021/bi026082g. [DOI] [PubMed] [Google Scholar]
- 9.Levin MK, Patel SS. Helicase from hepatitis C virus, energetics of DNA binding. J Biol Chem. 2002;277:29377–85. doi: 10.1074/jbc.M112315200. [DOI] [PubMed] [Google Scholar]
- 10.Sarver RW, Rogers JM, Stockman BJ, et al. Physical methods to determine the binding mode of putative ligands for hepatitis C virus NS3 helicase. Anal Biochem. 2002;309:186–95. doi: 10.1016/s0003-2697(02)00301-9. [DOI] [PubMed] [Google Scholar]
- 11.Matson SW, Tabor S, Richardson CC. The gene 4 protein of bacteriophage T7. Characterization of helicase activity. J Biol Chem. 1983;258:14017–24. [PubMed] [Google Scholar]
- 12.LeBowitz J, McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986;261:4738–48. [PubMed] [Google Scholar]
- 13.Nanduri B, Eoff RL, Tackett AJ, Raney KD. Measurement of steady-state kinetic parameters for DNA unwinding by the bacteriophage T4 Dda helicase: Use of peptide nucleic acids to trap single-stranded DNA products of helicase reactions. Nucleic Acids Res. 2001;29:2829–35. doi: 10.1093/nar/29.13.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyono K, Miyashiro M, Taguchi I. Detection of hepatitis C virus helicase activity using the scintillation proximity assay system. Anal Biochem. 1998;257:120–6. doi: 10.1006/abio.1998.2560. [DOI] [PubMed] [Google Scholar]
- 15.Hicham Alaoui-Ismaili M, Gervais C, Brunette S, et al. A novel high throughput screening assay for HCV NS3 helicase activity. Antiviral Res. 2000;46:181–93. doi: 10.1016/s0166-3542(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CC, Hwang LH, Huang YW, Chi WK, Chu YD, Chen DS. An ELISA for RNA helicase activity: Application as an assay of the NS3 helicase of hepatitis C virus. Biochem Biophys Res Commun. 1998;253:594–9. doi: 10.1006/bbrc.1998.9813. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Schwartz G, O'Donnell M, Harrison RK. Development of a novel helicase assay using electrochemiluminescence. Anal Biochem. 2001;293:31–7. doi: 10.1006/abio.2001.5093. [DOI] [PubMed] [Google Scholar]
- 18.Bjornson KP, Amaratunga M, Moore KJ, Lohman TM. Single-turnover kinetics of helicase-catalyzed DNA unwinding monitored continuously by fluorescence energy transfer. Biochemistry. 1994;33:14306–16. doi: 10.1021/bi00251a044. [DOI] [PubMed] [Google Scholar]
- 19.Earnshaw DL, Moore KJ, Greenwood CJ, et al. Time-resolved fluorescence energy transfer DNA helicase assays for high throughput screening. J Biomol Screen. 1999;4:239–48. doi: 10.1177/108705719900400505. [DOI] [PubMed] [Google Scholar]
- 20.Bianco PR, Brewer LR, Corzett M, et al. Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature. 2001;409:374–8. doi: 10.1038/35053131. [DOI] [PubMed] [Google Scholar]
- 21.Tabor S, Richardson CC. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc Natl Acad Sci USA. 1981;78:205–9. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco PR, Kowalczykowski SC. Translocation step size and mechanism of the RecBC DNA helicase. Nature. 2000;405:368–72. doi: 10.1038/35012652. [DOI] [PubMed] [Google Scholar]
- 23.Morris PD, Raney KD. DNA helicases displace streptavidin from biotin-labeled oligonucleotides. Biochemistry. 1999;38:5164–71. doi: 10.1021/bi9822269. [DOI] [PubMed] [Google Scholar]
- 24.Morris PD, Byrd AK, Tackett AJ, et al. Hepatitis C virus NS3 and simian virus 40 T antigen helicases displace streptavidin from 5′-biotinylated oligonucleotides but not from 3′- biotinylated oligonucleotides: Evidence for directional bias in translocation on single-stranded DNA. Biochemistry. 2002;41:2372–8. doi: 10.1021/bi012058b. [DOI] [PubMed] [Google Scholar]
- 25.Gorbalenya AE, Koonin EV. Helicases: Amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–29. [Google Scholar]
- 26.Boehmer PE, Lehman IR. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–84. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 27.Gomez de Cedron M, Ehsani N, Mikkola ML, Garcia JA, Kaariainen L. RNA helicase activity of Semliki Forest virus replicase protein NSP2. FEBS Lett. 1999;448:19–22. doi: 10.1016/s0014-5793(99)00321-x. [DOI] [PubMed] [Google Scholar]
- 28.Seybert A, Hegyi A, Siddell SG, Ziebuhr J. The human coronavirus 229E superfamily 1 helicase has RNA and DNA duplex-unwinding activities with 5′-to-3′ polarity. RNA. 2000;6:1056–68. doi: 10.1017/s1355838200000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seybert A, van Dinten LC, Snijder EJ, Ziebuhr J. Biochemical characterization of the equine arteritis virus helicase suggests a close functional relationship between arterivirus and coronavirus helicases. J Virol. 2000;74:9586–93. doi: 10.1128/jvi.74.20.9586-9593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanya HS, Bird LE, Brannigan JA, Wigley DB. Crystal structure of a DExx box DNA helicase. Nature. 1996;384:379–83. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 31.Korolev S, Hsieh J, Gauss GH, Lohman TM, Waksman G. Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP. Cell. 1997;90:635–47. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 32.Velankar SS, Soultanas P, Dillingham MS, Subramanya HS, Wigley DB. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 33.Caruthers JM, Johnson ER, McKay DB. Crystal structure of yeast initiation factor 4A, a DEAD-box RNA helicase. Proc Natl Acad Sci USA. 2000;97:13080–5. doi: 10.1073/pnas.97.24.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wagner JD, Guthrie C. The DEAH-box splicing factor Prp16 unwinds RNA duplexes in vitro. Curr Biol. 1998;8:441–51. doi: 10.1016/s0960-9822(98)70178-2. [DOI] [PubMed] [Google Scholar]
- 35.Marintcheva B, Weller SK. A tale of two HSV-1 helicases: Roles of phage and animal virus helicases in DNA replication and recombination. Prog Nucleic Acid Res Mol Biol. 2001;70:77–118. doi: 10.1016/s0079-6603(01)70014-1. [DOI] [PubMed] [Google Scholar]
- 36.Jankowsky E, Gross CH, Shuman S, Pyle AM. The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature. 2000;403:447–51. doi: 10.1038/35000239. [DOI] [PubMed] [Google Scholar]
- 37.Jankowsky E, Gross CH, Shuman S, Pyle AM. Active disruption of an RNA-protein interaction by a DExH/D RNA helicase. Science. 2001;291:121–5. doi: 10.1126/science.291.5501.121. [DOI] [PubMed] [Google Scholar]
- 38.Matusan AE, Pryor MJ, Davidson AD, Wright PJ. Mutagenesis of the dengue virus type 2 NS3 protein within and outside helicase motifs: Effects on enzyme activity and virus replication. J Virol. 2001;75:9633–43. doi: 10.1128/JVI.75.20.9633-9643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borowski P, Niebuhr A, Mueller O, et al. Purification and characterization of West Nile virus nucleoside triphosphatase (NTPase)/helicase: Evidence for dissociation of the NTPase and helicase activities of the enzyme. J Virol. 2001;75:3220–9. doi: 10.1128/JVI.75.7.3220-3229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warrener P, Tamura JK, Collett MS. RNA-stimulated NTPase activity associated with yellow fever virus NS3 protein expressed in bacteria. J Virol. 1993;67:989–96. doi: 10.1128/jvi.67.2.989-996.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warrener P, Collett MS. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J Virol. 1995;69:1720–6. doi: 10.1128/jvi.69.3.1720-1726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwong AD, Kim JL, Lin C. Structure and function of hepatitis C virus NS3 helicase. Curr Top Microbiol Immunol. 2000;242:171–96. doi: 10.1007/978-3-642-59605-6_9. [DOI] [PubMed] [Google Scholar]
- 43.Gwack Y, Yoo H, Song I, Choe J, Han JH. RNA-stimulated ATPase and RNA helicase activities and RNA binding domain of hepatitis G virus nonstructural protein 3. J Virol. 1999;73:2909–15. doi: 10.1128/jvi.73.4.2909-2915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao N, Hesson T, Cable M, et al. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–7. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]
- 45.Kim JL, Morgenstern KA, Griffith JP, et al. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: The crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 46.Cho HS, Ha NC, Kang LW, et al. Crystal structure of RNA helicase from genotype 1b hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J Biol Chem. 1998;273:15045–52. doi: 10.1074/jbc.273.24.15045. [DOI] [PubMed] [Google Scholar]
- 47.Patel SS, Picha KM. Structure and function of hexameric helicases. Annu Rev Biochem. 2000;69:651–97. doi: 10.1146/annurev.biochem.69.1.651. [DOI] [PubMed] [Google Scholar]
- 48.Wilson VG, West M, Woytek K, Rangasamy D. Papillomavirus E1 proteins: Form, function, and features. Virus Genes. 2002;24:275–90. doi: 10.1023/a:1015336817836. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez PL, Carrasco L. Poliovirus protein 2C contains two regions involved in RNA binding activity. J Biol Chem. 1995;270:10105–12. doi: 10.1074/jbc.270.17.10105. [DOI] [PubMed] [Google Scholar]
- 50.Pfister T, Wimmer E. Polypeptide p41 of a Norwalk-like virus is a nucleic acid-independent nucleoside triphosphatase. J Virol. 2001;75:1611–19. doi: 10.1128/JVI.75.4.1611-1619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleymann G, Fischer R, Betz UA, et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat Med. 2002;8:392–8. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 52.Crute JJ, Grygon CA, Hargrave KD, et al. Herpes simplex virus helicase-primase inhibitors are active in animal models of human disease. Nat Med. 2002;8:386–91. doi: 10.1038/nm0402-386. [DOI] [PubMed] [Google Scholar]
- 53.Kleymann G. Novel agents and strategies to treat herpes simplex virus infections. Expert Opin Investig Drugs. 2003;12:165–83. doi: 10.1517/13543784.12.2.165. [DOI] [PubMed] [Google Scholar]
- 54.Spector EC, Liang L, Giordano H, Sivaraja M, Peterson MG. Inhibition of herpes simplex virus replication by a 2-amino thiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J Virol. 1998;72:6979–87. doi: 10.1128/jvi.72.9.6979-6987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Betz UA, Fischer R, Kleymann G, Hendrix M, Rubsamen-Waigmann H. Potent in vivo antiviral activity of the herpes simplex virus primase-helicase inhibitor BAY 57-1293. Antimicrob Agents Chemother. 2002;46:1766–72. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borowski P, Schalinski S, Schmitz H. Nucleotide triphosphatase/helicase of hepatitis C virus as a target for antiviral therapy. Antiviral Res. 2002;55:397–412. doi: 10.1016/s0166-3542(02)00096-7. [DOI] [PubMed] [Google Scholar]
- 57.Locatelli GA, Gosselin G, Spadari S, Maga G. Hepatitis C virus NS3 NTPase/helicase: Different stereoselectivity in nucleoside triphosphate utilisation suggests that NTPase and helicase activities are coupled by a nucleotide-dependent rate limiting step. J Mol Biol. 2001;313:683–94. doi: 10.1006/jmbi.2001.5088. [DOI] [PubMed] [Google Scholar]
- 58.Borowski P, Lang M, Niebuhr A, et al. Inhibition of the helicase activity of HCV NTPase/helicase by 1-beta-D- ribofura-nosyl-1,2,4-triazole-3-carboxamide-5′- triphosphate (ribavirin- TP) Acta Biochim Pol. 2001;48:739–44. [PubMed] [Google Scholar]
- 59.Crute JJ, Lehman IR, Gambino J, et al. Inhibition of herpes simplex virus type 1 helicase-primase by (dichloroanilino)purines and -pyrimidines. J Med Chem. 1995;38:1820–5. doi: 10.1021/jm00010a027. [DOI] [PubMed] [Google Scholar]
- 60.Du MX, Johnson RB, Sun XL, Staschke KA, Colacino J, Wang QM. Comparative characterization of two DEAD-box RNA helicases in superfamily II: Human translation-initiation factor 4A and hepatitis C virus non-structural protein 3 (NS3) helicase. Biochem J. 2002;363:147–55. doi: 10.1042/0264-6021:3630147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singleton MR, Wigley DB. Modularity and specialization in superfamily 1 and 2 helicases. J Bacteriol. 2002;184:1819–26. doi: 10.1128/JB.184.7.1819-1826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hall MC, Matson SW. Helicase motifs: The engine that powers DNA unwinding. Mol Microbiol. 1999;34:867–77. doi: 10.1046/j.1365-2958.1999.01659.x. [DOI] [PubMed] [Google Scholar]
- 63.Kadare G, Haenni AL. Virus-encoded RNA helicases. J Virol. 1997;71:2583–90. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


