Abstract
Objective
We previously demonstrated that a reduced number of CD4+CD25+-regulatory T cells (Tregs) was associated with microvascular dysfunction in hypertension. However, the underlying mechanism by which Tregs regulate vascular endothelial function remains unknown.
Methods and Results
Control and interleukin (IL)-10–/– knockout mice were infused with angiotensin II (400 ng/kg/min) for 2 weeks (hypertensive [HT] and HT-IL-10–/–). Endothelium-dependent relaxation (EDR) in response to acetylcholine was significantly reduced in mesenteric resistance artery (MRA) from HT and HT-IL-10–/– compared with control and IL-10–/– mice. Importantly, the incubation of MRA from HT mice with the conditioned media of cultured Tregs, isolated from control mice, reduced NADPH oxidase activity and improved EDR, whereas no effect was observed in MRA from control mice incubated with the same media. These effects were reversed when MRAs were preincubated with IL-10 antibody or IL-10 receptor antagonist, whereas incubation with transforming growth factor-β receptor antagonist had no effect. The transfer of cultured Tregs, isolated from control mice, into HT-IL-10–/– mice reduced systolic blood pressure (SBP) and NADPH oxidase activity and improved EDR in MRA compared with untreated HT-IL-10–/– mice. In vivo treatment of HT mice with IL-10 (1000 ng/mouse) significantly reduced SBP and NADPH oxidase activity and improved EDR in MRA compared with untreated HT mice. The transfer of cultured Tregs, isolated from IL-10–/– mice, into HT mice did not reduce SBP or NADPH oxidase activity or improve EDR. The incubation of MRA from HT mice with apocynin improved EDR, whereas NADPH oxidase substrate attenuated EDR in MRA from control mice, which was reversed with exogenous IL-10.
Conclusion
These data demonstrate that IL-10 released from Tregs attenuates NADPH oxidase activity, which is a critical process in the improvement of microvascular endothelial function in hypertension, suggesting that Tregs/IL-10 could be a therapeutic target for treatment of vasculopathy in hypertension.
Keywords: hypertension, nitric oxide synthase, vascular biology, vasodilation
Impaired microvascular endothelium-dependent relaxation (EDR) is an important risk factor for vasculopathy and myocardial infarction in cardiovascular diseases, such as hypertension.1 We and other investigators have demonstrated endothelial dysfunction in mesenteric resistance arteries in hypertensive (HT) animal models.2–4 In a previous study, we reported that impaired EDR in coronary arterioles was associated with the induction of inflammation and a decrease in Treg number and interleukin (IL)-10 content in angiotensin II (Ang II)–induced hypertension.5 The transfer of Tregs, isolated from control mice, to HT mice improved EDR in coronary arterioles.4 In the present study, we delineated the mechanism of Treg-mediated improvement in EDR, and we provide direct evidence for the role of Tregs in the regulation of microvascular function.
It has been established that hypertension induced by Ang II infusion increases the expression and activity of NADPH oxidase leading to reactive oxygen species responsible for impairment of endothelial function.6–9
IL-10 is a potent antiinflammatory cytokine that plays a pivotal role in the regulation of immune and inflammatory responses. Indirect evidence indicates that IL-10 has a protective effect on endothelial function in diabetes and hypertension.5,10
Thus, in this study, we demonstrated that treatment with Tregs improves microvascular endothelial function in HT mice by a paracrine effect that involves the release of IL-10. We also delineated the mechanism of IL-10-mediated improvement in endothelial function, which involves alteration in NADPH oxidase activity.
Materials and Methods
General Protocol
All experiments were performed according to the American Guidelines for the Ethical Care of Animals and were approved by Tulane University Health Sciences Center Animal Care and Use Committee. One hundred forty C57BL/6J mice (8-week-old male) and 15 IL-10–/– mice (8-week-old male11) were purchased from the Jackson Laboratory (Bar Harbor, ME), housed in groups of 5, and maintained at a temperature of 23°C with a 12-hour light/dark cycle. Mice were fed on a solid standard diet (Na+ content 0.4%) and water. Seventy control and 5 IL-10–/– mice were euthanized to isolate Tregs and perform experiments on EDR and NADPH oxidase activity. Animals were divided in 2 main groups, control and IL-10–/–. The control group was divided into 4 subgroups: (1) mice were infused with Ang II (400 ng · kg–1 · min–1) using subcutaneous miniosmotic pumps for 2 weeks (HT, n=35); (2) mice received the same dose of Ang II with IL-10 (1000 ng/mouse) for 2 weeks using subcutaneous miniosmotic pumps (HT+IL-10, n=10); (3) mice received the same dose of Ang II using subcutaneous miniosmotic pumps and were injected with Tregs, isolated from IL-10–/– mice (3 injections per week for 2 weeks, HT+Tregs, n=10); and (4) control group (n=15). The IL-10–/– group was divided into 3 subgroups: (1) mice infused with Ang II (400 ng · kg–1 · min–1) using subcutaneous miniosmotic pumps for 2 weeks (HT-IL-10–/–, n=5); (2) mice received the same dose of Ang II with Tregs, isolated from control mice (3 injections per week for 2 weeks, HT+Treg-IL-10–/–, n=5); and (3) IL-10–/– without treatment (n=5).
At the end of treatment, animals were anesthetized with isoflurane, and mesenteric resistance arteries were rapidly harvested, placed in PSS solution (composition in mmol/L: NaCl 118; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4×7H2O 1.2; NaHCO3 25; glucose 11), pH 7.4, and processed appropriately for further study.
Tregs and CD4– T-Cell Isolation
Tregs and CD4– T cells were isolated from cell suspension of spleen as previously described (Supplemental Figure II, available online at http://atvb.ahajournals.org).5 Isolated Tregs and CD4– T cells were then cultured for 24 hours in wells precoated with anti-CD3 (clone 2C11, R&D Systems) in RPMI 1640 with 10% fetal bovine serum, 2 mmol/L glutamine, 10 mmol/L 6-HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin (all from Gibco and Invitrogen) 50 μg of 2-mercaptoethanol (Sigma-Aldrich), and 10 ng/mL human recombinant IL-2 (R&D Systems).
Endothelial Cell Culture
Primary cultured microvascular endothelial cells were used as previously described.12 Cells were stimulated with (1) NADPH oxidase substrate (NADPH, 100 μmol/L) with and without IL-10 (5 ng/mL); (2) NADPH in the presence of p38 mitogen-activated protein (MAP) kinase inhibitor (2-(4-chlorophenyl)-4-(4-fluorophenyl)-5-pyridin-4-yl-1,2-dihydropyrazol-3-one, 10 μmol/L, Calbiochem) and JAK I inhibitor (2-(1,1-dimethylethyl)-9-fluoro-3,6-dihydro-7H-benz[h]-imidaz[4,5-f]isoquinolin-7-one, Pyridone 6, P6, DBI, 10 μmol/L, Calbiochem), added 30 minutes before NADPH addition with or without IL-10.
Vascular Reactivity
In Vitro Experiments
Mesenteric resistance arteries were isolated, carefully cleaned of fat and connective tissue, and cut into rings (2 mm in length). Mesenteric resistance arteries (100 to 120 μm in diameter) from control and HT mice were mounted in a small-vessel dual-chamber myograph for measurement of isometric tension. Two steel wires (25 μm) were introduced through the lumen of the mesenteric resistance artery (MRA) and mounted according to the method described by Mulvany and Halpern.13 After a 30-minute equilibration period in PSS solution bubbled with carbogen at 37°C and pH 7.4, MRAs were stretched to their optimal lumen diameter for active tension development. Internal diameter (100 to 120 μm) of all microvessels was set to a pretension corresponding to an intraluminal pressure of 80 mm Hg. After a second 30-minute equilibration period, the vessels were exposed to phenylephrine (PE) (10–4 mol/L), and the presence of functional endothelium was assessed by the ability of acetylcholine (ACh) (10–6 mol/L) to induce relaxation. After pre-contraction with PE (10–4 mol/L) and steady maximal contraction (the lumen diameter and the contraction in response to PE were similar in all groups of mice), cumulative concentration response curves were obtained for ACh (1×10–8 to 3×10–5 mol/L).
After a wash period, rings were incubated for 30 minutes with the conditioned media of Tregs or of CD4– T cells or with complete media alone, and then dose-response curves in response to ACh (1×10–8 to 3×10–5 mol/L) were performed after precontraction of mesenteric resistance arteries rings with 10–4 mol/L PE. In other experiments, Treg conditioned media and MRAs were pretreated with an antibody against IL-10 (5 μg/mL), IL-10 receptor antagonist (5 μg/mL), and transforming growth factor-β (TGF-β) receptor antagonist (SB 431542, 10 μmol/L) respectively.
To evaluate the direct effect of IL-10, MRAs with and without endothelium from HT and control groups were used. After a stabilization period in PSS solution, MRAs were precontracted with PE, and a dose-response curve for relaxation in response to exogenous IL-10 (5, 10, and 60 ng) was obtained.
To determine the role of NADPH oxidase in the impaired EDR in HT mice, MRAs were incubated with 2 types of NADPH oxidase inhibitors (apocynin, 100 μmol/L; gp91 ds-tat, 3 μmol/L) for 30 minutes, and then EDR responses to ACh were studied after precontraction with PE. The apocynin concentration used in the present study makes the inhibitor less specific for NADPH.14 Therefore, we used another NADPH inhibitor (gp91 ds-tat) to strengthen our results. To determine the effect of IL-10 on NADPH oxidase activity, EDR responses to ACh were determined in the presence or absence of the substrate of NADPH oxidase (100 μmol/L) with or without IL-10 (5 nmol/L).
In Vivo Experiments
Another series of experiments was carried out using MRA from mice infused with Ang II with or without Treg transfer or IL-10 infusion (HT, HT-IL-10–/–, HT+IL-10, IL-10–/–, HT+Tregs, and HT-IL-10–/–+Tregs). Mice were trained for tail cuff measurements 1 week before starting the experiments. Systolic blood pressure (SBP) was measured in conscious mice using the CODA tail-cuff blood pressure system (Kent Scientific Torrington). Systolic arterial blood pressure measurements were performed at the same time (between 9 am and 11 am) to avoid the influence of the circadian cycle, and the value of SBP was obtained by estimating the average reading of 8 measurements for a single trial. The tail cuff procedure accurately measures SBP but underestimates diastolic blood pressure. Also, changes in time of day; ambient conditions; operator handling of each animal; or subtle behavioral differences between groups, strains, or individual animals can introduce experimental variability to the measurement.
At the end of treatment period, mice were anesthetized with isoflurane, and the MRAs were cleaned and cut into rings to study reactivity, NADPH oxidase activity, and endothelial nitric oxide synthase (eNOS) phosphorylation levels. After a stabilization period in PSS solution, EDR in response to ACh (1×10–8 to 3×10–5 mol/L) was determined in PE-precontracted rings.
NADPH Oxidase Activity
Superoxide anion levels generated by NADPH oxidase activity were measured in lysates of MRA using lucigenin chemiluminescence. Briefly, lysates were prepared in a sucrose buffer containing 50 mmol/L KH2PO4, 1 mmol/L EGTA, and 150 mmol/L sucrose, pH 7.0, in an homogenizer (Bullet Blender, Next Advance, Inc). The lysates supernatants were collected and stored at –80°C. To measure NADPH oxidase activity, a volume of 100 μL of each lysate was used in a total volume of 1 mL of PBS buffer containing lucigenin (5 μmol/L) and NADPH (100 μmol/L) and then preheated at 37°C. Blank samples were prepared using 100 μL of sucrose buffer. Lucigenin activity was measured every 30 seconds for a 10-minute period in a luminometer (Turner Bio-Systems 20/20 single-tube luminometer). Superoxide anions generated by NADPH oxidase were also measured in mouse endothelial cells. The area under the curve was used to quantify chemiluminescence results.15 Data are expressed as relative light units normalized to protein content (μg of protein). As a negative control for NADPH oxidase activity, we used cells incubated with apocynin (100 μmol/L) before the addition of NADPH oxidase substrate.
In other experiments, mice were infused with Ang II for 2 weeks. Then, isolated MRAs were incubated with IL-10 (10 pg/mL) for 15, 30, and 60 minutes, and NADPH oxidase activity was then determined.
Western Blot Analysis
The phosphorylation and expression of eNOS, p38 MAP kinase, and JAK-I were determined using specific antibodies (1:1000 dilution, Cell Signaling Technology, Inc) as previously described.12,16
Real-Time Polymerase Chain Reaction
IL-10 receptor mRNA levels were determined in MRA from control and IL-10–/– infused or not infused with Ang II and untreated or treated with Tregs isolated from control mice for 2 weeks. Total RNA was obtained using the RNeasy Fibrous Tissue Mini Kit (Qiagen) according to the manufacturer's recommendations. A total of 1 μg of DNase I-treated RNA was reverse transcribed into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) with random hexamers in a 20-μL reaction. Polymerase chain reaction was performed in duplicate for each sample using 1 μL of cDNA as a template, 1× TaqMan Universal PCR Master Mix (Applied Biosystems), and 10× of TaqMan Gene Expression Assays (Applied Biosystems) in a 20-μL reaction. Assays-on-Demand (Applied Biosystems) of TaqMan fluorescent real-time polymerase chain reaction primers and probes were used for IL-10 ra (Mm00434151_m1) and 18S rRNA (Hs99999901_s1), which was used as endogenous control to normalize results. Quantitative real-time PCR was carried out in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems) using the following conditions: 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Relative mRNA levels were determined using the 2-ΔΔCt method. Results are expressed as the relative expression of mRNA in treated mice compared with untreated mice.
Drugs
PE hydrochloride, ACh chloride, apocynin, NADPH, IL-10, and Ang II were obtained from Sigma-Aldrich. The gp91 ds-tat was purchased from Anaspec, and SB 431542 was purchased from Tocris Bioscience. Stock solutions of drugs were prepared in ultrapure water and stored at –20°C, and appropriate dilutions were made on the day of the experiment.
Statistical Analysis
Data are expressed as mean±SEM. Concentration-response curves were analyzed using GraphPad Prism 4.0 software. The responses to ACh and IL-10 are expressed as percentages of PE contraction. Statistical calculations for significant differences were performed using the Student t test and 2-way ANOVA to analyze dose response in multiple groups. Significance was accepted at P<0.05.
Results
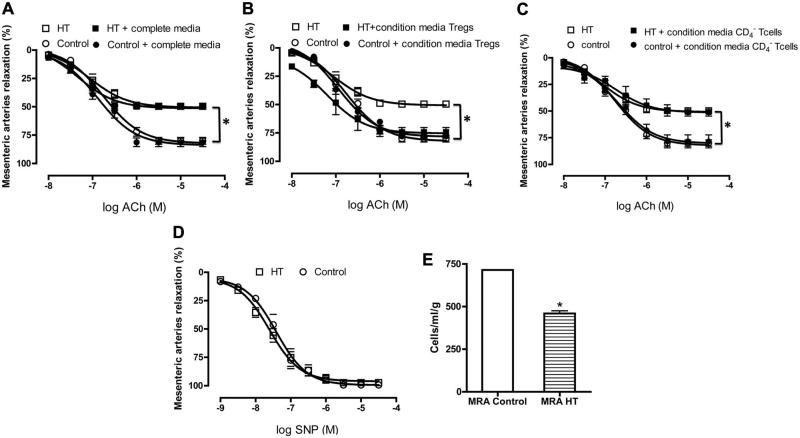
To determine whether Tregs directly regulate resistance artery endothelial function in hypertension, we first examined EDR in MRA in response to dose response of ACh in control and HT mice. EDR in MRA was significantly attenuated in HT mice compared with control (Figure 1A to 1C). We observed that incubation of MRA, from HT mice, in the conditioned media from Tregs significantly improved EDR (Figure 1A), and no effect on the response to ACh was observed when MRAs were incubated with complete media alone (Figure 1B). Furthermore, the specificity of the effect of Tregs on endothelial function was determined by incubating MRA from HT mice with conditioned media from CD4– T cells. The results revealed no effect on EDR (Figure 1C). MRA from control mice were also incubated with the conditioned media from Tregs or CD4– T cells or with complete media, and the data revealed no effect on EDR (Figure 1A to 1C). We did not detect any reactivity problems in endothelium-independent relaxation assessed in response to a nitric oxide donor in MRA (sodium nitroprusside) (Figure 1D). It was also observed that Treg number was reduced in MRA from the HT group compared with the control group (Figure 1E).
Figure 1.
Endothelium-dependent relaxation in mesenteric resistance arteries from control and hypertensive (HT) mice incubated with conditioned media from CD4+CD25+-regulatory T cells (Tregs) (A) (n=5, *P<0.05 for HT vs HT+Tregs or control±Tregs), complete media (B) (n=5, *P<0.05 for HT and HT+complete media vs control±complete media), and conditioned media of CD4– T cells (C) (n=5, *P<0.05 for HT vs HT+conditioned media from CD4– T cells or control±conditioned media of CD4– T cells). D, Endothelium-independent relaxation of mesenteric resistance arteries from control and HT mice (n=5, P>0.05, not significant). E, Treg number from mesenteric resistance artery (MRA) of control and HT mice (n=4); *<0.05 for MRA control vs MRA HT. ACh indicates acetylcholine.
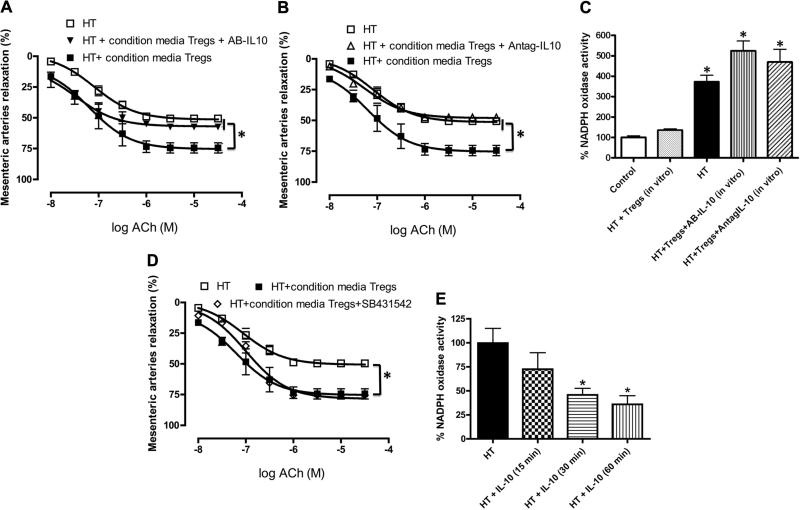
To elucidate whether IL-10 released by Tregs is an important factor in the improvement in endothelium-dependent MRA relaxation, we examined the effect of IL-10 antibody (5 μg/mL) and IL-10 receptor antagonist (5 μg/mL). The results of these experiments revealed that the IL-10 antibody and the IL-10 receptor antagonist completely blocked the improvement in EDR of MRA incubated with conditioned media from Tregs (Figure 2A and 2B). Figure 2C illustrates an increase in NADPH oxidase activity in MRA from HT mice compared with MRA from control mice and HT mice incubated with Treg conditioned media. This effect was reversed when MRAs were incubated with the IL-10 antibody or IL-10 receptor antagonist.
Figure 2.
A, Endothelium-dependent relaxation in mesenteric resistance arteries from hypertensive (HT) mice with and without conditioned media from CD4+CD25+-regulatory T cells (Tregs) alone or in combination with specific antibody against interleukin (IL)-10 (AB-IL10). n=5, *P<0.05 for HT or HT+Tregs vs HT+Tregs+AB-IL-10. B, Endothelium-dependent relaxation of mesenteric resistance arteries from HT mice with and without conditioned media from Tregs alone or in combination with IL-10 receptor antagonist (Antag-IL-10). n=5, *P<0.05 for HT or HT+Tregs vs HT+Tregs+Antag-IL-10. C, NADPH oxidase activity in mesenteric resistance arteries from HT mice, incubated with or without conditioned media from Tregs alone, in combination with specific antibody against IL-10 (AB-IL-10) or with IL-10 receptor antagonist (Antag-IL-10), from control. n=5, *P<0.05 for HT or HT+Tregs+AB-IL-10 or HT+Tregs+Antag-IL-10 vs HT+Tregs or control. D, Endothelium-dependent relaxation in mesenteric resistance arteries from HT mice with and without conditioned media from Tregs alone or in combination with transforming growth factor-β receptor antagonist (SB 431542). n=5, *P<0.05 for HT or HT+Tregs vs HT+Tregs+SB. E, NADPH oxidase activity in mesenteric resistance arteries from HT mice, incubated with or without IL-10 (10 pg/mL) for 15-, 30-, and 60-minute periods. n=5, *P<0.05 for HT vs HT+IL-10 (30 minutes) or HT+IL-10 (60 minutes). ACh indicates acetylcholine.
To determine whether TGF-β is involved in EDR in MRA, we incubated arteries with the TGF-β receptor antagonist (SB 431542) for 1 hour, and the results revealed no effect on MRA relaxation (Figure 2D), even at high concentration of the TGF-β receptor inhibitor (data not shown).
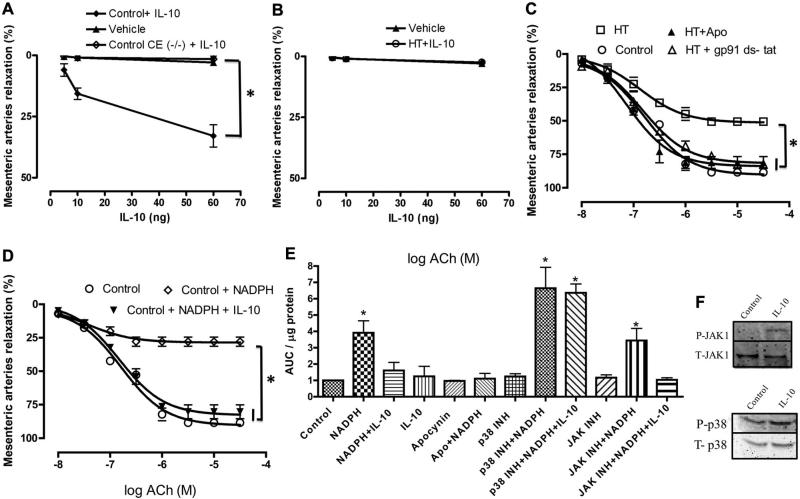
In addition, we also determined whether IL-10 itself could be a relaxing factor. Figure 3A and 3B illustrates that IL-10 can act as a very weak relaxing factor, producing 20% of maximum relaxation in control mice at very high doses, with no effect on tension in HT mice.
Figure 3.
A, Relaxation of mesenteric resistance arteries from control mice, with and without endothelial cells, in response to exogenous interleukin (IL)-10 or vehicle. n=5, *P<0.05 for IL-10 vs vehicle. B, Relaxation of mesenteric resistance arteries from hypertensive (HT) mice in response to exogenous IL-10 or vehicle. n=5. C, Endothelium-dependent relaxation of mesenteric resistance arteries from control and HT mice incubated with and without apocynin (Apo) or gp91 ds-tat. n=5, *P<0.05 for HT vs HT+apocynin or control or HT+gp91 ds-tat. D, Endothelium-dependent relaxation of mesenteric resistance arteries from control mice incubated with or without NADPH oxidase substrate or NADPH oxidase substrate+IL-10. n=5, *P<0.05 for control+NADPH oxidase substrate vs control or control+NADPH oxidase substrate+IL-10. E, NADPH oxidase activity in microvascular endothelial cells incubated with vehicle, IL-10 (5 ng/mL), or NADPH (100 μmol/L) alone or in the presence of IL-10, inhibitors of p38 (10 μmol/L) or JAK1 (10 μmol/L), or apocynin (300 μmol/L). *P<0.05 vs control. F, Western blot analysis showing phosphorylated (P) JAK1, total (T) JAK1, P-p38, and T-P38 in microvascular endothelial cells incubated with vehicle and IL-10 (5 ng/mL). ACh indicates acetylcholine; CE, control endothelium removal.
Incubation of isolated MRA from HT mice with IL-10 reduced NADPH oxidase activity within 30 minutes of incubation (Figure 2E).
To further delineate the mechanism by which IL-10 improves microvascular endothelial function in hypertension, we first incubated MRA from HT mice with apocynin and gp 91 ds-tat (NADPH oxidase inhibitors). Our results revealed that the inhibition of NADPH oxidase activity significantly improved EDR in MRA from HT mice (Figure 3C). We also examined the relationship between NADPH oxidase activity and EDR. In these experiments, the incubation of MRA from control mice with NADPH oxidase substrate produced a significant attenuation in the EDR in response to ACh (Figure 3D). Importantly, the effect of NADPH oxidase substrate was reversed when MRAs were incubated with IL-10 (Figure 4D).
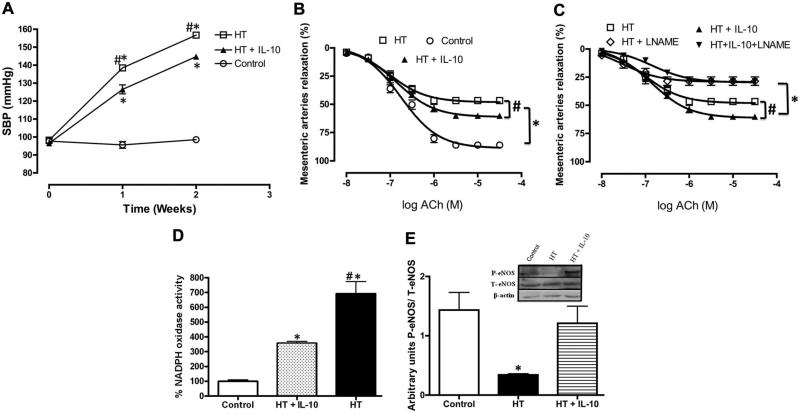
Figure 4.
A, Systolic blood pressure from control and hypertensive (HT) mice treated in vivo with interleukin (IL)-10 for 2 weeks. n=5, *P<0.05 for control vs HT or HT+IL-10, #P<0.05 for HT vs HT+IL-10. B and C, Endothelium-dependent relaxation in mesenteric resistance arteries from HT mice treated in vivo with exogenous IL-10 for 2 weeks incubated with or without l-NG-Nitroarginine methyl ester (l-NAME). n=5, *P<0.05 for HT or HT+IL-10 vs HT+l-NAME or HT+IL-10+l-NAME, #P<0.05 for HT vs HT+IL-10. D, NADPH oxidase activity in mesenteric resistance arteries from control and HT mice treated in vivo with exogenous IL-10 for 2 weeks. n=5, *P<0.05 for control vs HT or HT+IL-10, #P<0.05 for HT vs HT+IL-10. E, Western blot analysis and quantitative data showing phosphorylated (P) endothelial nitric oxide synthase (eNOS), total (T) eNOS and β-actin in mesenteric resistance arteries from HT, HT+ IL-10 and control. ACh indicates acetylcholine.
Moreover, we measured NADPH oxidase activity in primary cultured endothelial cells incubated with NADPH oxidase substrate for 30 minutes with and without IL-10 (5 ng/mL) and observed a significant increase in NADPH oxidase activity compared with control and after incubation with IL-10. The effect of NADPH on NADPH oxidase activity was significantly reduced when cells were prestimulated with IL-10 (Figure 3E). The inhibitory effect of IL-10 was reduced with the p38 MAP kinase inhibitor but not with the JAK-1 inhibitor (Figure 3E). Western blot analysis revealed that IL-10 increased p38 MAP kinase and JAK-1 phosphorylation (Figure 3F).
To further elucidate the mechanism of the effect of Tregs/IL-10 on the regulation of microvascular endothelial function, control mice were infused with Ang II with and without exogenous IL-10 for 2 weeks. IL-10 treatment significantly reduced SBP in mice infused with Ang II (Figure 4A). Figure 4B and 4C shows the improvement of EDR in MRA from HT mice infused with IL-10 compared with HT mice. The inhibition of eNOS significantly reduced EDR in MRA from both groups (Figure 4C). These results were associated with an increase in eNOS phosphorylation and a decrease in NADPH oxidase activity in HT mice with IL-10 compared with HT without IL-10 treatment (Figure 4D and 4E) We did not observe any change in total eNOS expression (Figure 4E).
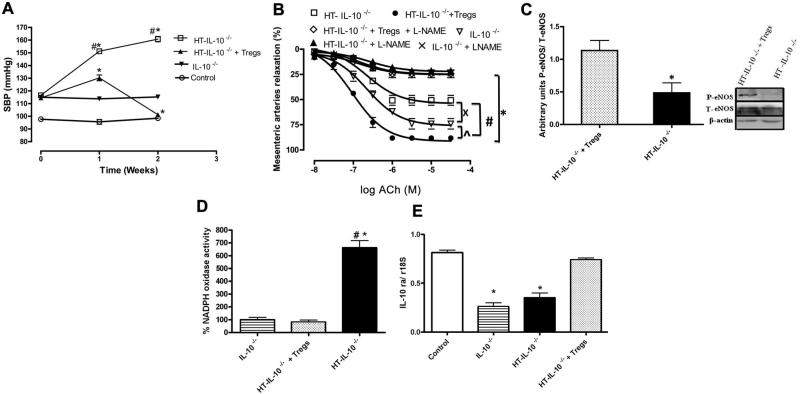
In another series of experiments, isolated Tregs from control mice, when transferred into HT IL-10–/– mice, significantly reduced SBP and NADPH oxidase activity and improved the EDR in MRA associated with enhanced eNOS phosphorylation and IL-10 receptor expression (Figure 5A to 5E).
Figure 5.
A, Systolic blood pressure in interleukin (IL)-10–/– and hypertensive (HT) IL-10–/– mice treated with CD4+CD25+-regulatory T cells (Tregs), isolated from control mice, for 2 weeks. n=5, *P<0.05 for IL-10–/– vs HT- IL-10–/– or HT+Tregs- IL-10–/–, #P<0.05 for HT vs HT+IL-10. B, Endothelium-dependent relaxation of mesenteric resistance arteries with or without l-NG-Nitroarginine methyl ester (l-NAME) from IL-10–/– and HT IL-10–/– mice treated with Tregs, isolated from control mice, for 2 weeks. n=5, *P<0.05 for IL-10–/– vs HT- IL-10–/– or HT+Tregs- IL-10–/–, #P<0.05 for HT- IL-10–/– vs HT+Tregs- IL-10–/–. C, Western blot analysis and quantitative data showing phosphorylated (P) endothelial nitric oxide synthase (eNOS), total (T) eNOS, and β-actin in mesenteric resistance arteries from IL-10–/–, HT-IL-10–/–, and HT+Tregs- IL-10–/–. D, NADPH oxidase activity in mesenteric resistance arteries from IL-10–/– and HT IL-10–/– mice treated in vivo with Tregs, isolated from control mice, for 2 weeks. n=5, *P<0.05 for IL-10–/– vs HT - IL-10–/–, #P<0.05 for IL-10–/– vs HT+Tregs-IL-10–/–. E, IL-10 receptor mRNA in mesenteric resistance artery (MRA) from IL-10–/– and HT IL-10–/– mice treated with Tregs, isolated from control mice, for 2 weeks. n=5, *P<0.05 for IL-10–/– and HT - IL-10–/– vs HT+Tregs- IL-10–/–. ACh indicates acetylcholine.
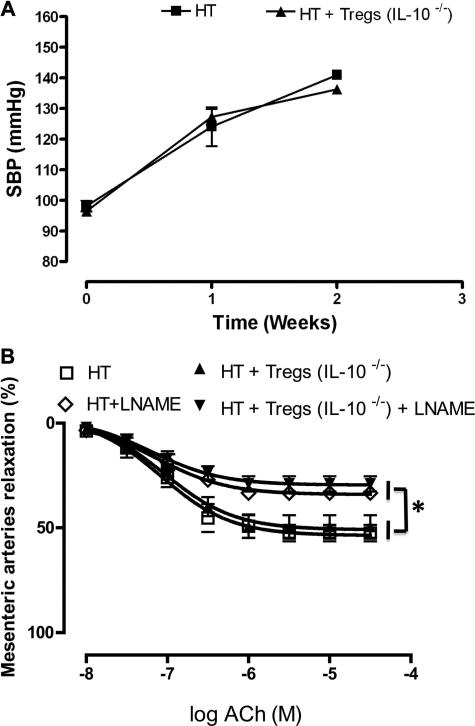
The importance of IL-10 in the regulation of microvascular EDR was also observed with Tregs isolated from IL-10–/– mice and then transferred to HT mice. The experiments revealed no changes in SBP and EDR (Figure 6).
Figure 6.
A, Systolic blood pressure in control and hypertensive (HT) mice treated with CD4+CD25+-regulatory T cells (Tregs), isolated from interleukin (IL)-10–/– mice, for 2 weeks. n=5. B, Endothelium-dependent relaxation in mesenteric resistance arteries from control and HT mice treated with Tregs, isolated from IL-10–/– mice, for 2 weeks. n=5. SBP indicates systolic blood pressure; *P<0.05 for HT and HT+Tregs (IL-10–/–) vs HT+l-NG-Nitroarginine methyl ester (l-NAME) and HT+Tregs (IL-10–/–)+ l-NAME. ACh, acetylcholine.
Supplemental Figure I is a schematic diagram summarizing how Tregs regulate microvascular endothelial function.
Discussion
The present study identifies CD4+CD25+ regulatory T cells (Tregs) and its cytokine IL-10 as novel targets for the treatment of microvascular endothelial dysfunction in hypertension. We demonstrated that IL-10 released by Tregs reduces NADPH oxidase activity, increases eNOS activity, and subsequently improves EDR in resistance arteries from HT mice. We also ascertained that HT mice treated with IL-10 significantly reduced SBP and NADPH oxidase activity and improved EDR in resistance arteries. Importantly, the transfer of Tregs, isolated from control mice, into HT IL-10–/– mice reduced SBP and NADPH oxidase activity and improved EDR in resistance arteries, whereas the transfer of Tregs isolated from IL-10–/– knockout mice had no effect in HT mice. These results suggest that Treg-IL-10 plays an important role in the regulation of microvascular endothelial function.
Hypertension, with its cardiovascular complications, is the leading cause of morbidity and mortality in the world.17 The connection between hypertension and impaired microvascular endothelial function is well established.18,19 Endothelial dysfunction is associated with an imbalance between nitric oxide bioavailability and reactive oxygen species production. One source of oxidative stress in the vascular system is NADPH oxidase. The superoxide anions that are excessively generated interact with NO and form peroxynitrite, which reduces NO bioavailability, resulting in impaired eNOS-dependent relaxation.10,20 It has been reported that Ang II-induced hypertension enhances the generation of superoxide anions by increasing NADPH oxidase activity, which causes vascular endothelial dysfunction.21–24
Tregs play an important role in the immune system, maintaining immunologic unresponsiveness to self-antigens and suppressing excessive immune responses deleterious to the host.25,26 Recent studies illustrated a relationship between Tregs and vascular wall function in cardiovascular diseases.5,27–30 Additionally, an association between the immune system and hypertension has been reported.31,32 We recently demonstrated that an increase in apoptotic Tregs is responsible for the induction of vascular inflammation and impaired EDR in coronary arterioles in hypertension.5 The transfer of Tregs, isolated from control mice, into HT mice reduces arterial blood pressure and improves coronary arteriolar endothelial function.5,33 However, the underlying molecular mechanisms of how Tregs improve microvascular endothelial function remain largely unknown.
The importance of Tregs in suppressing inappropriate inflammation has become increasingly clear in the past several years.34 The release of the antiinflammatory IL-10 by Tregs could be an important mechanism by which Tregs can reverse cardiovascular diseases such as atherosclerosis and vascular dysfunction in hypertension.
Our data provide evidence that Tregs release IL-10, which plays an important role in the improvement of microvascular EDR. Our data are in agreement with a previous study showing the protective effect of IL-10 in experimental hypertension.35,36
Subsequently, we determined whether IL-10 itself is a vasorelaxing factor and whether it rescues eNOS phosphorylation in hypertension. We studied EDR in response to a wide range of doses of IL-10. Thus, IL-10 induced weak EDR (5 ng IL-10 induced 2% of maximum relaxation). The small relaxing effect of IL-10 was abolished in hypertension. In our previous study, the in vivo IL-10 level measured was 5 pg.5 Therefore, IL-10 most likely functions to rescue eNOS phosphorylation and subsequently EDR.
It has been shown that hypertension is associated with an increase in oxidative stress,9,37 which is in part responsible for vascular endothelial dysfunction.38,39 We found that the incubation of resistance arteries from control mice with NADPH oxidase substrate reduced EDR, an effect that was reversed when arteries were preincubated with the NADPH oxidase substrate and IL-10. NADPH oxidase activity was significantly higher in resistance arteries from HT mice, which is in agreement with previous studies.40 Interestingly, resistance arteries from HT mice incubated with the conditioned media from Tregs displayed a significant reduction in NADPH oxidase activity, which was reversed with the IL-10 antibody and the IL-10 receptor antagonist. These results indicate that IL-10 reduces oxidative stress through the inhibition of NADPH oxidase activity, suggesting that IL-10 is an important element in the regulation of vascular endothelial function by the balancing redox reactions, and this concept is supported by previous studies.35,36 Our in vivo results are supported by our in vitro studies using primary cultured endothelial cells isolated from resistance arteries. The inhibitory effect of IL-10 on NADPH oxidase activity was by a p38 MAP kinase–dependent and JAK-1-independent mechanism.
To further study the effect of IL-10 on endothelial dysfunction and NADPH oxidase activity, we performed in vivo experiments in which control mice were infused with Ang II with and without IL-10. Thus, SBP and NADPH oxidase activity were significantly reduced in HT mice treated with IL-10. These responses were associated with an increase in eNOS phosphorylation and improved EDR. These in vivo data emphasize the pathophysiological relevance of IL-10 in the regulation of vascular endothelial function. Our studies are in agreement with previous studies reporting that IL-10 attenuates vascular oxidative stress and improves endothelial function in diabetes, and protects against the development of atherosclerosis and thrombosis.41–43 In addition, several studies suggest that IL-10 inhibits the expression of proinflammatory cytokines, including IL-6 and tumor necrosis factor-α, and the activation of transcription factors such as nuclear factorκB, which are important mediators of vascular endothelial dysfunction.43–45 Previous in vitro studies using large arteries subjected to Ang II with or without a high dose of IL-10 (300 nmol/L) for 24 hours prevented endothelial dysfunction.46 Our findings are supported by previous studies35,36 and indicate that IL-10 reduces oxidative stress and improves microvascular endothelial function in hypertension.
To extend our results, we transferred isolated Tregs from control mice into IL-10–/– mice infused with Ang II. The results of these studies showed a reduction in SBP and NADPH oxidase activity, associated with a rescue of eNOS phosphorylation and EDR. Treg transfer into IL-10–/– mice infused with Ang II significantly reduced SBP and rescued EDR. We determined that there was an increased expression of IL-10 receptor, indicating that IL-10 receptor expression is regulated by IL-10. Thus, IL-10 receptor upregulation could explain the beneficial effect of Tregs in IL-10–/– mice. Interestingly, the transfer of Tregs from IL-10–/– mice into HT mice did not alter SBP or microvascular EDR, providing support for the role of IL-10 in the regulation of endothelial function.
In conclusion, the present study provides novel and significant insight into the basic mechanism of how Tregs control resistance artery endothelial function. We have demonstrated that Tregs release IL-10, which reduces NADPH oxidase activity by a p38 MAP kinase–dependent mechanism, rescues eNOS phosphorylation, and improves EDR in hypertension. Thus, Tregs/IL-10 are a potential target for treating microvascular complications in hypertension.
Supplementary Material
Acknowledgments
Sources of Funding
We acknowledge grant support from National Institutes of Health (1R01HL095566; PI: Dr Matrougui) and (5R01HL097111; PI: Dr Trebak).
Footnotes
Drs Kassan and Galan share first authorship.
Disclosures
None.
References
- 1.Tzemos N, Lim PO, MacDonald TM. Valsartan improves endothelial dysfunction in hypertension: a randomized, double-blind study. Cardiovasc Ther. 2009;27:151–158. doi: 10.1111/j.1755-5922.2009.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matrougui K, Maclouf J, Levy BI, Henrion D. Impaired nitric oxide- and prostaglandin-mediated responses to flow in resistance arteries of hypertensive rats. Hypertension. 1997;30:942–947. doi: 10.1161/01.hyp.30.4.942. [DOI] [PubMed] [Google Scholar]
- 3.Lominadze D, Joshua IG, Schuschke DA. Blood flow shear rates in arterioles of spontaneously hypertensive rats at early and established stages of hypertension. Clin Exp Hypertens. 2001;23:317–328. doi: 10.1081/ceh-100102670. [DOI] [PubMed] [Google Scholar]
- 4.Tanko LB, Matrougui K. Can we apply results from large to small arteries? Circ Res. 2002;90:e68. doi: 10.1161/01.res.0000013737.02527.15. [DOI] [PubMed] [Google Scholar]
- 5.Matrougui K, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Houston MC. Nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Prog Cardiovasc Dis. 2005;47:396–449. doi: 10.1016/j.pcad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Kitiyakara C, Wilcox CS. Antioxidants for hypertension. Curr Opin Nephrol Hypertens. 1998;7:531–538. doi: 10.1097/00041552-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Gunnett CA, Heistad DD, Faraci FM. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: role of superoxide. Diabetes. 2002;51:1931–1937. doi: 10.2337/diabetes.51.6.1931. [DOI] [PubMed] [Google Scholar]
- 11.Bristol IJ, Mahler M, Leiter EH, Sundberg JP. Il10tm1Cgn, an interleukin-10 gene targeted mutation. [May 1, 2011];JAX Notes. 1997 (471) http://jaxmice.jax.org/jaxnotes/archive/471a.html.
- 12.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 15.Fortuño A, Bidegain J, Robador PA, Hermida J, López-Sagaseta J, Beloqui O, Díez J, Zalba G. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension. 2009;54:744–750. doi: 10.1161/HYPERTENSIONAHA.109.129353. [DOI] [PubMed] [Google Scholar]
- 16.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beetz N, Harrison MD, Brede M, Zong X, Urbanski MJ, Sietmann A, Kaufling J, Barrot M, Seeliger MW, Vieira-Coelho MA, Hamet P, Gaudet D, Seda O, Tremblay J, Kotchen TA, Kaldunski M, Nusing R, Szabo B, Jacob HJ, Cowley AW, Jr, Biel M, Stoll M, Lohse MJ, Broeckel U, Hein L. Phosducin influences sympathetic activity and prevents stress-induced hypertension in humans and mice. J Clin Invest. 2009;119:3597–3612. doi: 10.1172/JCI38433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matrougui K, Loufrani L, Heymes C, Levy BI, Henrion D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- 19.Park JB, Schiffrin EL. Effects of antihypertensive therapy on hypertensive vascular disease. Curr Hypertens Rep. 2000;2:280–288. doi: 10.1007/s11906-000-0011-5. [DOI] [PubMed] [Google Scholar]
- 20.Gunnett CA, Heistad DD, Berg DJ, Faraci FM. IL-10 deficiency increases superoxide and endothelial dysfunction during inflammation. Am J Physiol Heart Circ Physiol. 2000;279:H1555–H1562. doi: 10.1152/ajpheart.2000.279.4.H1555. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation: contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virdis A, Colucci R, Fornai M, Duranti E, Giannarelli C, Bernardini N, Segnani C, Ippolito C, Antonioli L, Blandizzi C, Taddei S, Salvetti A, Del Tacca M. Cyclooxygenase-1 is involved in endothelial dysfunction of mesenteric small arteries from angiotensin II-infused mice. Hypertension. 2007;49:679–686. doi: 10.1161/01.HYP.0000253085.56217.11. [DOI] [PubMed] [Google Scholar]
- 23.Dal-Ros S, Bronner C, Schott C, Kane MO, Chataigneau M, Schini-Kerth VB, Chataigneau T. Angiotensin II-induced hypertension is associated with a selective inhibition of endothelium-derived hyperpolarizing factor-mediated responses in the rat mesenteric artery. J Pharmacol Exp Ther. 2009;328:478–486. doi: 10.1124/jpet.108.145326. [DOI] [PubMed] [Google Scholar]
- 24.Puddu P, Puddu GM, Zaca F, Muscari A. Endothelial dysfunction in hypertension. Acta Cardiol. 2000;55:221–232. doi: 10.2143/AC.55.4.2005744. [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 30. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 27.Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann Med. 2009;41:224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]
- 28.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 29.Gallego J, Martinez Vila E, Munoz R. Patients at high risk for ischemic stroke: identification and actions. Cerebrovasc Dis. 2007;24:49–63. doi: 10.1159/000107379. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi: 10.1007/s00281-009-0143-x. [DOI] [PubMed] [Google Scholar]
- 31.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes-Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension. 2011;57:1167–1175. doi: 10.1161/HYPERTENSIONAHA.110.162917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 35.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinsley JH, South S, Chiasson VL, Mitchell BM. Interleukin-10 reduces inflammation, endothelial dysfunction, and blood pressure in hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R713–R719. doi: 10.1152/ajpregu.00712.2009. [DOI] [PubMed] [Google Scholar]
- 37.Vasdev S, Gill VD, Singal PK. Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Exp Clin Cardiol. 2006;11:206–216. [PMC free article] [PubMed] [Google Scholar]
- 38.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol. 2003;14:2783–2789. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- 40.Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40:504–510. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- 41.Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 42.Gunnett CA, Berg DJ, Faraci FM, Feuerstein G. Vascular effects of lipopolysaccharide are enhanced in interleukin-10-deficient mice. Stroke. 1999;30:2191–2195. doi: 10.1161/01.str.30.10.2191. [DOI] [PubMed] [Google Scholar]
- 43.Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP. IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2576–2581. doi: 10.1161/ATVBAHA.107.153080. [DOI] [PubMed] [Google Scholar]
- 45.Raines EW, Garton KJ, Ferri N. Beyond the endothelium: NF-κB regulation of smooth muscle function. Circ Res. 2004;94:706–708. doi: 10.1161/01.RES.0000125646.08156.4D. [DOI] [PubMed] [Google Scholar]
- 46.Zemse SM, Hilgers RH, Webb RC. Interleukin-10 counteracts impaired endothelium-dependent relaxation induced by ANG II in murine aortic rings. Am J Physiol Heart Circ Physiol. 2007;292:H3103–H3108. doi: 10.1152/ajpheart.00456.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.