Abstract
Sigma1 receptors (σ1Rs) are intracellularly mobile chaperone proteins implicated in several disease processes, as well as psychiatric disorders and substance abuse. Here we report that although selective σ1R agonists (PRE-084, (+)-pentazocine) lacked reinforcing effects in drug-naive rats, over the course of 28 experimental sessions, which was more than sufficient for acquisition of cocaine self-administration, responding was not maintained by either σ1R agonist. In contrast, after subjects self-administered cocaine σ1R agonists were readily self-administered. The induced reinforcing effects were long lasting; a response for which subjects had no history of reinforcement was newly conditioned with both σ1R agonists, extinguished when injections were discontinued, and reconditioned when σ1R agonists again followed responses. Experience with food reinforcement was ineffective as an inducer of σ1R agonist reinforcement. Although a variety of dopamine receptor antagonists blocked cocaine self-administration, consistent with its dopaminergic mechanism, PRE-084 self-administration was entirely insensitive to these drugs. Conversely, the σR antagonist, BD1063, blocked PRE-084 self-administration but was inactive against cocaine. In microdialysis studies i.v. PRE-084 did not significantly stimulate dopamine at doses that were self-administered in rats either with or without a cocaine self-administration experience. The results indicate that cocaine experience induces reinforcing effects of previously inactive σ1R agonists, and that the mechanism underlying these reinforcing effects is dopamine independent. It is further suggested that induced σ1R mechanisms may have an essential role in treatment-resistant stimulant abuse, suggesting new approaches for the development of effective medications for stimulant abuse.
Keywords: σ receptor, cocaine, dopamine, drug reinforcement history, reinforcement mechanisms, drug self-administration
INTRODUCTION
Sigma1 receptors (σ1Rs) are intracellular chaperone proteins that translocate from their primary endoplasmic reticulum localization to different subcellular compartments upon agonist actions, and regulate ion channels and G-protein-coupled-receptor signaling (Aydar et al, 2002; Cormaci et al, 2007; Hayashi and Su, 2007). Reports have implicated σ1Rs in various biological functions, and drugs acting at these receptors have been studied for therapeutic effects in cancer, HIV infection, psychiatric disorders, and substance abuse (Katz et al, 2011; Maurice and Su, 2009). σ1Rs are expressed widely, including in dopaminergic brain regions (Hayashi et al, 2010), and drugs acting at these receptors have been shown to regulate dopaminergic function (Nuwayhid and Werling, 2003). Consequently, studies have focused on the interactions between σ1R ligands and psychomotor-stimulant drugs.
σ1R antagonists have been shown to block several cocaine effects that are related to its abuse and excessive intake. For example, the convulsions and lethality produced by cocaine can be blocked by various σR antagonists, including BD1063 and BD1047 (Matsumoto et al, 2001; McCracken et al, 1999). Further, σR antagonists block the locomotor-stimulant effects of cocaine (Katz et al, 2011; Matsumoto, 2009), and cocaine-induced place preferences (Romieu et al, 2000; 2002).
Despite the promising blockade of these effects of cocaine, the effects of σR antagonists in animals self-administering cocaine have been less compelling. For example, over a range of doses sufficient to block other effects of cocaine, BD1047 had little effect on self-administration of cocaine (Martin-Fardon et al, 2007). Our previous studies replicated the lack of antagonism with BD1047, and extended it to various cocaine doses and several σR antagonists (Hiranita et al, 2011b; 2010). The one positive effect of BD1047 was a blockade of the ‘reinstatement' of previously extinguished responding. Nonetheless, taken together the studies of the effects of selective σR antagonists on responding reinforced with cocaine suggest that these drugs are relatively inactive.
In contrast to the minimal effects of σR antagonists on subjects self-administering cocaine, a leftward shift in the cocaine self-administration dose–effect curve was produced by the selective σ1R agonist, PRE-084, and the σ1/2R agonist, DTG (Hiranita et al, 2010). That effect was unusual as σR agonists are often reported to be behaviorally inactive (Maj et al, 1996; Romieu et al, 2002). Drugs that shift the cocaine self-administration dose–effect curve leftward, such as indirect dopamine agonists, typically have their own reinforcing effects (Hiranita et al, 2011b; 2010). The suggested reinforcing effects of selective σR agonists are supported by a previous finding that administration of σR agonists produced dose-dependent stimulation of dopamine levels in the nucleus accumbens shell of rats (Garcés-Ramírez et al, 2011), a brain region involved in the reinforcing effects of drugs of abuse (Pontieri et al, 1995; 1996; Tanda et al, 1997). Further, Hiranita et al (2010) found that both PRE-084 and DTG were in fact self-administered, however, the subjects used in that study had a history of cocaine self-administration. Thus, the purpose of the present studies was to assess the potential reinforcing effects of σ1R agonists in experimentally naive subjects. Further, the mechanisms of the reinforcing effects of σ1R agonists were assessed both pharmacologically and with in vivo microdialysis.
MATERIALS AND METHODS
Details of all procedures are supplied in Supplementary Information. A total of six groups of rats (n=6 for each) were used initially with either cocaine (three groups) or σR agonists (PRE-084, two groups; (+)-pentazocine, one group) self-administration. A final group was studied that was initially trained with food reinforcement.
Self-Administration
Male Sprague-Dawley rats, weighed ∼300 g at the start of the study. Subjects were acclimated to a temperature- and humidity-controlled vivarium for at least 1 week with food and water unrestricted under a 12:12-h light:dark cycle (lights on at 0700 hours). Thereafter weights of rats were maintained at ∼320 g by adjusting daily food rations. Jugular catheters were surgically implanted and subjects were allowed to recover from surgery for ∼7 days.
Experimental sessions were conducted with animals placed in operant-conditioning chambers, which were enclosed within ventilated sound-attenuating cubicles and supplied with masking white noise. Two response levers were located on the front wall on which a downward displacement with a force greater than 20 g defined a response and activated a ‘feedback' relay mounted behind the front wall. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of 45 mg food pellets was mounted midway between the levers. An infusion pump placed above each chamber delivered injections via tubing and a fluid swivel to the subject's catheter that was protected by a surrounding metal spring. Subjects were placed in the chambers daily for sessions that lasted 120 min and started with the illumination of the LEDs above each lever.
With the exception of studies of pharmacological mechanisms using antagonists, during sessions each right-lever response turned off the LEDs and activated the infusion pump for 10 s (fixed ratio or FR 1 schedule) followed by a 20-s time-out period during which LEDs were off and responding had no scheduled consequences. Drug injections were cocaine (0.32 mg/kg/injection, n=6), PRE-084 (0.32 mg/kg/injection, n=6) or (+)-pentazocine (0.32 mg/kg/injection, n=6). After the time-out, the LEDs were illuminated and the next right-lever response produced an injection. Responses on the left lever were recorded but had no scheduled consequences. This condition remained in effect for 28 experimental sessions.
For the cocaine self-administration group, responses on the left rather than right lever produced injections for the next seven sessions, with all other conditions as in the first 28 sessions. During the subsequent nine sessions, injections and accompanying stimulus changes were discontinued (extinction) with other aspects of the sessions unchanged. Finally, responses on the left lever again produced cocaine injections for five sessions under the FR 1 schedule as described above (reacquisition).
During the initial 28 sessions with PRE-084 or (+)-pentazocine, responding was not maintained by either drug. Subsequently the PRE-084 group was studied with five different doses (0.03–1.0 mg/kg/injection) for 14 sessions each, after which they were allowed to self-administer cocaine (0.32 mg/kg/injection) for 14 sessions under the FR 1 schedule as described above. The (+)-pentazocine group was immediately changed to cocaine self-administration under the FR 1 schedule. After cocaine self-administration, all of the subjects were returned to the FR 1 schedule of PRE-084 or (+)-pentazocine self-administration, and the subsequent series of sessions (change in active lever, extinction, reacquisition) for both groups was as described for the cocaine group.
A separate group of subjects (n=6) were trained with 45-mg food pellets as reinforcement under a FR 1-response schedule of reinforcement otherwise identical to that for drug self-administration. Similarly, the subjects were exposed to sessions of acquisition of lever pressing, followed by an alternation of the lever on which responses produced food, extinction, and reacquisition. After the reacquisition phase, the subjects were catheterized, allowed to respond again for five sessions with food reinforcement, and subsequently allowed to self-administer PRE-084 (0.32 mg/kg/injection) for 28 sessions.
For the studies of pharmacological mechanisms, the procedure was modified. Subjects from the above-described cocaine self-administration experiments, and several experimentally naive subjects (N=18) were trained to self-administer cocaine (0.32 mg/kg/injection). Subsequently, the FR value was increased to five and the session was divided into five 20-min components, each preceded by a 2-min time-out period. This arrangement allowed the assessment of the entire self-administration dose–effect curve in a single session by adjusting infusion volumes and durations. The dose per injection was incremented in the five sequential components in an ascending dose order as follows: no injection (also referred to as extinction, or EXT, because responses had no scheduled consequences), 0.03, 0.10, 0.32, and 1.0 mg/kg/injection for cocaine. A response-independent sample injection of the drug at the corresponding dose was delivered just before the start of each component except the first (Hiranita et al, 2009).
Once performances were stable (see Supplementary Material for more detail), the effects on cocaine self-administration of presession i.p. injections of dopamine receptor antagonists (the dopamine D1-like receptor antagonist SCH 39166, the dopamine D2-like receptor antagonist L-741,626 or the non-selective dopamine and σ receptor antagonist haloperidol) or the preferential σ1R antagonist (BD1063) were assessed. These drugs were also examined in the same rats with PRE-084 (0.03, 0.10, 0.32, and 1.0 mg/kg/injection) substituted for cocaine under otherwise identical conditions. The effects of pre-session treatments on cocaine self-administration were separated by a minimum of 72 h. The antagonists were studied with a mixed order of drugs and doses.
In vivo Microdialysis
Experiments were conducted during the light phase. Under a mixture of ketamine and xylazine (60.0 and 12.0 mg/kg i.p., respectively) anesthesia, concentric dialysis probes were stereotaxically implanted (see Supplementary Information) aimed at the nucleus accumbens shell (uncorrected coordinates from the rat brain atlas of Paxinos and Watson (1998): anterior=+2.0 mm from bregma, lateral=±1.0 mm from bregma, vertical=−7.9 mm from dura), as described previously (Tanda et al, 2005; Tanda et al, 2008; Tanda et al, 1997). Histology results are detailed in Supplementary Information. The experiments were performed on freely moving rats, about 22–24 h after probe implant. Samples (10 μl) were taken every 10 min and immediately analyzed, as detailed in Supplementary Information. After stable dopamine values (less than 10% variability) were obtained for at least three consecutive samples (after about 1 h), rats were injected with increasing doses of PRE-084, spaced 60 min apart. Dopamine was detected in dialysate samples by HPLC coupled with a coulometric detector (5200a Coulochem II, or Coulochem III, ESA, Chelmsford, Massachusetts).
Drugs
Drugs were injected intravenously (cocaine, PRE-084, and (+)-pentazocine) or intraperitoneally (BD 1063, SCH 39166, L-741,626, and haloperidol). Drug pretreatments were administered 5 (BD1063) or 30 min before sessions. The drugs used are fully described in Supplementary Information.
RESULTS
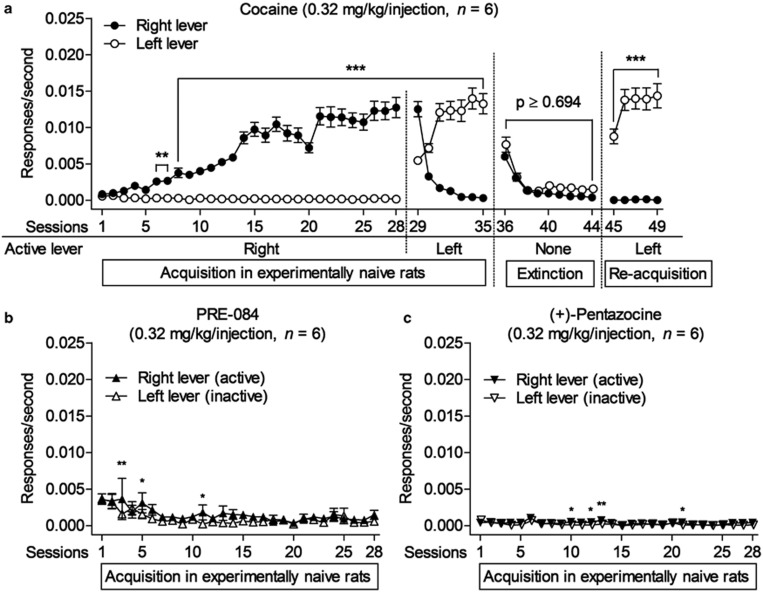
As expected, cocaine self-administration was acquired in drug-naive rats (Figure 1a) when each response produced an injection (a fixed-ratio one-response schedule of reinforcement). Responses on the active (right) lever that produced cocaine injections (0.32 mg/kg/injection) increased in frequency to asymptote over a series of 28 daily 2-h sessions. In contrast, responses on the alternate (left) lever that had no scheduled consequences remained infrequent. During sessions 29−35 cocaine injections were available for presses on the left lever (on which the subjects had no history of reinforcement) instead of the right lever. Responses on the left lever consequently increased and responses on the right lever decreased in frequency. When saline was substituted for cocaine (extinction, sessions 36−44), response rates decreased to low levels. Finally, when cocaine presentation was again dependent on responses on the left lever (sessions 45−49) rate of responding increased again (Figure 1a). A two-way repeated measures ANOVA (lever x sessions) indicated a significant effect of session number (F48,240=104; p<0.001), lever (F1,240=79.2; p<0.001), and their interaction (F48,240=76.3; p<0.001).
Figure 1.
Lack of the reinforcing effects of the selective σ1R agonists in experimentally naive rats compared with the typical acquisition of lever pressing with cocaine injections. (a) When each response on the right lever produced a cocaine injection rates of responding increased whereas rates of responding on the alternate (left) lever, which had no scheduled consequences, remained low. When cocaine injections were available only for responses on the previously inactive (left) lever, responding switched to that lever. Saline substitution decreased responding on both levers to low levels. When cocaine was again available for responses on the left lever, responding increased on that lever. (b, c) Lack of acquisition of (+)-pentazocine or PRE-084 self-administration when each response produced an injection. Response rates on the active lever were not consistently greater than those on the inactive lever throughout the course of 28 experimental sessions. *p<0.05, **p<0.01, ***p<0.001, compared with responding on the inactive lever (post-hoc Bonferroni t-test).
In contrast to cocaine, responding was not maintained in the separate groups of experimentally naive subjects given the opportunity to self-administer the σR agonists, either PRE-084 (Figure 1b) or (+)-pentazocine (Figure 1c) at the doses of 0.32 mg/kg/injection. Rates of responding on the active lever over the course of 28 consecutive sessions did not consistently exceed rates of responding on the inactive lever, and there was no evidence of the increase in frequency of responding seen with the cocaine group. Two-way repeated measures ANOVAs indicated significant effects of session number for PRE-084 (F27,135=2.90; p<0.001) and (+)-pentazocine (F27,135=3.59; p<0.001), but no effects of lever or the interaction of the two (p-values>0.103).
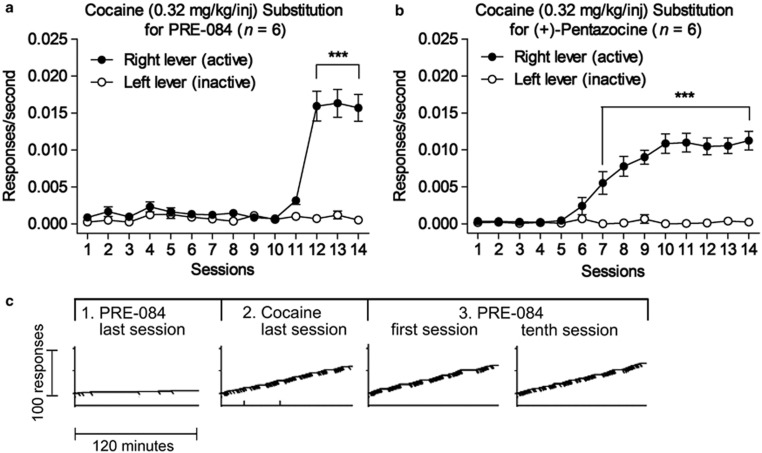
Subjects that failed to self-administer σ1R agonists nonetheless subsequently acquired cocaine self-administration (Figure 2a and b). This acquisition occurred immediately after the sessions shown in Figure 1 for (+)-pentazocine. The two-way repeated measures ANOVA indicated a significant effect of session number (F13,65=46.1; p<0.001), right vs left lever (F1,65=51.5; p<0.001) and the interaction of the two (F13,65=52.6; p<0.001). For PRE-084, another 84 sessions after those shown in Figure 1 were used to assess several higher or lower doses, which were also found to lack reinforcing effects (see below). Significant differences between rates of responding on the right and left levers in these subjects exposed to PRE-084 appeared on the twelfth session of cocaine exposure, whereas those differences appeared after the sixth session in subjects previously exposed to (+)-pentazocine. The difference between these groups may be due to the testing of multiple doses of PRE-084 before exposure to cocaine, which extended their experience in the chamber without reinforcement compared with the group previously exposed to response-contingent (+)-pentazocine injections. Two-way repeated measures ANOVA indicated significant effects of session number (F13,65=33.6; p<0.001), but right vs left lever did not reach significance (F1,65=5.19; p=0.072), although the interaction of lever and session number was significant (F13,65=6.14; p<0.001). Following the acquisition of cocaine self-administration, subjects were again given access to the previously inactive doses of σ1R agonists. In marked contrast to the absence of self-administration before cocaine experience, both σ1R agonists were readily self-administered and this self-administration was stable over the course of 10 daily sessions. Records of performances of an individual subject (Figure 2c) show a lack of PRE-084 self-administration before cocaine exposure, avid cocaine self-administration when that drug was available, self-administration of PRE-084 on the first session in which it was made available immediately after cocaine exposure, and its sustained self-administration at the tenth session of its availability.
Figure 2.
Substitution of cocaine resulted in the acquisition of self-administration in rats that did not self-administer σ1R agonists. (a, b) Acquisition of cocaine (0.32 mg/kg/injection) self-administration when each response produced an injection after extended exposure to PRE-084 (0.32 mg/kg/injection) or after exposure to (+)-pentazocine (0.32 mg/kg/injection). Each point represents the mean ±SEM of six subjects. ***p<0.001, compared with responding on the inactive lever (post-hoc Bonferroni t-test). (c) Examples of actual self-administration performances of a representative subject in real time. Ordinates, cumulative responses; abscissae, time. Each record is from a single 120-min experimental session. Each response on the active lever incrementally stepped the cumulative curve upward and produced a diagonal mark on the cumulative response curve. Vertical marks on the line below the cumulative curve indicate responses on the left (inactive) lever. The first record is from a previously naive subject in the last session with the opportunity to self-administer 0.32 mg/kg/injection of PRE-084. The second record is from that same subject after 14 sessions with the opportunity to self-administer 0.32 mg/kg/injection of cocaine. The third record is from the immediately following session, the first opportunity to self-administer 0.32 mg/kg/injection of PRE-084 after experience with cocaine. The last record shows stable self-administration of PRE-084.
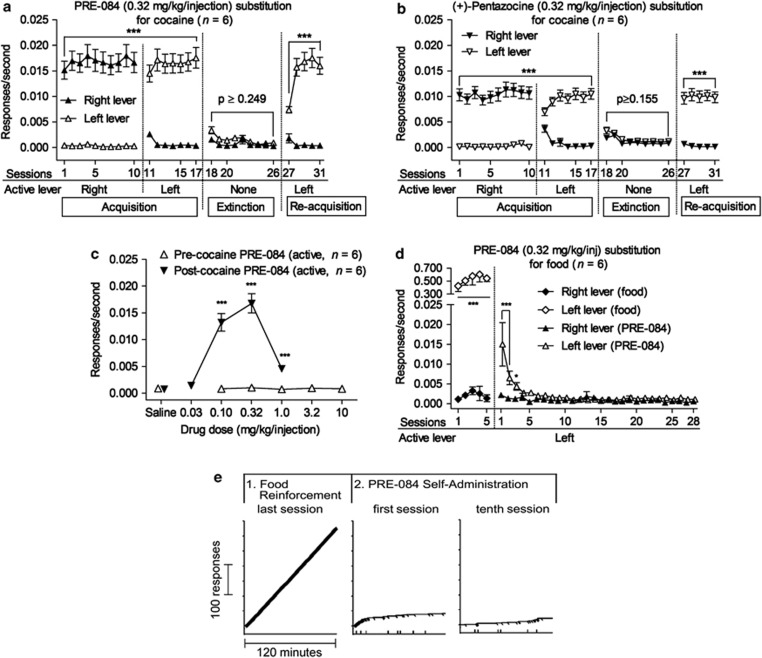
Following cocaine experience, σ1R agonist self-administration was comparable to that of cocaine in all important aspects. When the active lever was switched from the right to the left, responding switched to the newly active lever (Figure 3a snd b). The mean number of infusions of PRE-084 (0.32 mg/kg/injection) over the last three sessions before saline substitution (90.4±7.74) was ∼13-fold higher than that of before cocaine substitution (7.22±2.13). When saline was substituted for either σ1R agonist, self-administration decreased to low levels, and increased again when the σ1R agonist was again made available for self-administration (Figure 3a and b). Two-way repeated measures ANOVA indicated significant effects on response rates maintained by PRE-084 of session number (F30,150=58.2, p<0.001), lever (F1,150=461, p<0.001), and the interaction of the two (F30,150=75.6, p<0.001). Similar outcomes with (+)-pentazocine were obtained for session number (F30,150=51.5, p<0.001), lever (F1,150=12.7, p=0.016), and the interaction of the two (F30,150=71.0, p<0.001). The subsequent testing of different doses of PRE-084 (Figure 3c, filled triangles) showed that its dose–effect curve after experience with cocaine was similar to those seen with other self-administered drugs (Hiranita et al, 2011b; 2009; 2010). The lowest dose of PRE-084 did not maintain self-administration at levels greater than vehicle; with increases in dose, rates of responding increased to their maximum and decreased at the highest dose tested. In contrast, the testing of PRE-084 before cocaine self-administration (Figure 3c, open triangles) indicated that none of these doses were self-administered at levels greater than vehicle up to a dose/injection that was 100-fold greater than an active dose of PRE-084 in cocaine-experienced subjects (Figure 3c, filled triangles). Two-way repeated-measures ANOVA indicated no effect of dose, lever or their interaction on rates of PRE-084 self-administration in cocaine-naive rats (all p-values>0.321), whereas a similar analysis of rates of responding maintained by PRE-084 after experience with cocaine self-administration indicated significance of dose (F4,20=65.4, p<0.001), lever (F1,20=87.3, p<0.001) and their interaction (F4,20=72.2, p<0.001), with post-hoc tests indicating that response rates maintained by doses from 0.1 to 1.0 mg/kg were significantly different (p<0.001) from those maintained by saline injections.
Figure 3.
Selective σ1R agonist self-administration after cocaine experience, but not after experience with food reinforcement. Each point represents the mean ±SEM. (a, b) Self-administration of selective σ1R agonists when each response produced an injection. Reversal of active and inactive levers, extinction, and reacquisition each had the effects expected for a reinforcing agent. (c) Dose–effect curve for PRE-084 self-administration before and after experience with cocaine self-administration. No dose of PRE-084 was self-administered at rates greater than those for saline in cocaine-naive rats. Following cocaine self-administration, the dose–effect curve of PRE-084 self-administration was typical of those obtained with traditional drugs of abuse. (d) A food reinforcement history was not sufficient to induce reinforcing effects of PRE-084. *p<0.05, ***p<0.001, compared with responding on the inactive lever (post-hoc Bonferroni t-test). (e) Performances of a representative subject in real time (details of recording as in figure 2c). The first record is from the last session of responding maintained by food reinforcement. The second record is from the immediately following session, the first opportunity to self-administer 0.32 mg/kg/injection of PRE-084 after experience with food reinforcement, showing the extinction of responding previously maintained by food reinforcement. The 10th sessions confirm no acquisition of PRE-084 self-administration.
The self-administration of σ1R agonists in subjects with cocaine experience may result from either a pharmacological action triggered by cocaine or more simply the experience of acquiring lever-pressing behavior. A separate group of experimentally naive rats was trained to lever-press with food reinforcement under a FR 1-response schedule (Supplementary Figure S1). Following that acquisition, the sensitivity of the behavior to reinforcement contingencies was assured with switching the active lever, extinction, and reconditioning (Supplementary Figure S1). After the reacquisition of food-reinforced responding catheters were implanted; subjects recovered and were tested for five daily sessions to ensure stability of food-reinforced responding. Subsequently these subjects were given access to PRE-084 injections (FR 1; 0.32 mg/kg). In contrast to the stable responding maintained by food reinforcement, responding decreased to low levels when PRE-084 self-administration was substituted for food reinforcement (Figure 3d). A two-way repeated-measures ANOVA indicated a significant effect of session number (F27,135=6.78, p<0.001), lever (F1,135=7.86, p=0.038), and the interaction of the two (F27,135=4.28, p<0.001) on response rates. Records of responding from an individual subject (Figure 3e) show avid food-reinforced responding before the opportunity to self-administer PRE-084, and the subsequent extinction of responding during the 28 sessions of access to PRE-084 injections at the dose that maintained responding after experience with cocaine self-administration (Figure 3e and d).
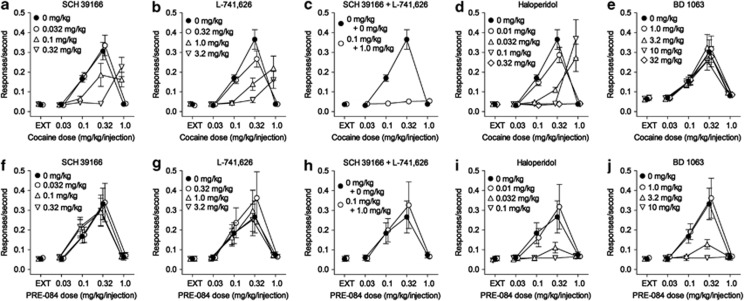
As the self-administration of cocaine is known to result primarily from an inhibition of dopamine uptake (Hiranita et al, 2009; Ritz et al, 1987) and is sensitive to dopamine antagonists (Barrett et al, 2004; Hemby et al, 1996), we compared the effects of dopamine receptor and σ1R antagonists on cocaine and PRE-084 self-administration. The subjects previously trained with cocaine injection under a FR 1-response schedule were studied subsequently under the five-component FR 5 schedule, which allowed the characterization of the effects of pretreatments on a full range of self-administration doses. As expected, injections of both cocaine and PRE-084 (filled symbols in Figure 4, top and bottom rows, respectively) produced bi-phasic dose–effect curves with the drugs being equipotent (maximal self-administration was obtained at 0.32 mg/kg/injection of both drugs). Pre-session treatment with antagonists at either dopamine D1-like (SCH 39166) or D2-like (L-741 626) receptors dose-dependently shifted the cocaine self-administration dose–effect curve to the right (Figure 4a and b). Statistical analysis of results with SCH 39166 indicated significant effects on response rates of cocaine (F4,60=24.6, p<0.001) and antagonist (F3,60=18.5, p<0.001) dose, and their interaction (F12,60=28.8, p<0.001). Similar analysis of results with L-741 626 indicated significant effects on response rates of cocaine (F4,60=26.4, p<0.001) and antagonist (F3,60=33.3, p<0.001) dose, and their interaction (F12,60=26.5, p<0.001). Further, an insurmountable antagonism of cocaine self-administration was produced by a combination of intermediate doses of SCH 39166 and L-741 626 (Figure 4c), with significant effects on response rates of cocaine dose (F4,20=46.5, p<0.001), antagonist treatment (F1,20=91.3, p<0.001), and their interaction (F4,20=60.8, p<0.001). Pretreatment with the non-selective dopamine receptor antagonist, haloperidol shifted the cocaine dose–effect curve to the right with the highest dose producing an insurmountable antagonism across the range of cocaine doses studied (Figure 4d). Statistical analysis of these results indicated significant effects on response rates of cocaine (F4,80=20.6, p<0.001) and haloperidol (F4,60=24.1, p<0.001) dose, and their interaction (F16,80=21.1, p<0.001). Finally, BD 1063 did not produce substantial effects on cocaine self-administration (Figure 4e). However, a two-way repeated measures ANOVA indicated a significant effect of BD 1063 dose (F4,80=10.4, p<0.001). Post-hoc Bonferroni t-tests indicated small but significant effects on rates of responding maintained by injections of 0.32 mg/kg/injection of cocaine with increases produced by 3.2 mg/kg (t=3.59, p=0.002), and decreases at doses of 10 (t=6.93, p<0.001) and 32 (t=3.69, p=0.001) mg/kg of BD 1063. The decreases in response rates were 14.6 and 7.80 percent of control response rates, respectively.
Figure 4.
Sensitivity of cocaine self-administration, and insensitivity of PRE-084 self-administration to dopamine receptor antagonism. Rats were trained to self-administer cocaine (0.032–1.0 mg/kg/injection) under a fixed-ratio five-response schedule of reinforcement with different doses of cocaine available in five components. All antagonists except BD1063 (5 min before sessions) were administered intraperitoneally, 30 min before sessions. Each point represents the mean±SEM of response rates on the active lever. (a–c) Effects of antagonists selective for dopamine D1-like receptors, SCH 39166, D2-like receptors, L-741 626, and the combination of minimally active doses of each. SCH 39166 and L-741 626 shifted the cocaine self-administration dose–effect curve rightward and the combination produced an insurmountable antagonism over the range of tested doses of cocaine. (d) The non-selective dopamine receptor antagonist, haloperidol, produced a dose-related rightward shift in the cocaine self-administration dose–effect curve. (e) The σ1R antagonist, BD1063, did not substantially affect cocaine self-administration. (f–h) The dopamine antagonists and their combination did not substantially affect PRE-084 self-administration. (i) Haloperidol dose-dependently decreased maximal PRE-084 self-administration. (j) BD1063 dose-dependently decreased maximal PRE-084 self-administration.
In contrast, the self-administration of PRE-084 was insensitive to the selective dopamine receptor antagonists either alone or in combination at doses that were effective against cocaine (Figure 4f–h). Statistical analyses indicated nonsignificant effects of SCH 39166 dose (F3,60=1.55, p=0.243), significant effects of L-741 626 dose (F3,60=3.32, p=0.049) that were a reflection of small increases in response rates with L-741 626 pretreatment (Figure 4g), and nonsignificant (F1,20=1.85, p=0.232) effects of the combination of the two dopamine antagonists. In contrast, haloperidol which also possesses σR antagonist effects (Hayashi et al, 2007), produced a dose-related blockade of the self-administration of PRE-084. Statistical analysis of results with haloperidol indicated significant effects on response rates of PRE-084 (F4,60=6.57, p=0.002), haloperidol dose (F3,60=6.74, p=0.004), and their interaction (F12,60=5.61, p<0.001). In addition, the preferential σ1R-antagonist, BD1063, similar to haloperidol, dose-dependently decreased the maximal rates of self-administration of PRE-084 at doses that were inactive against cocaine self-administration (Figure 4j). A two-way repeated-measures ANOVA of these effects on response rates indicated significant effects of cocaine (F4,60=11.6, p<0.001), BD1063 dose (F3,60=10.4, p<0.001), and their interaction (F12,60=9.77, p<0.001).
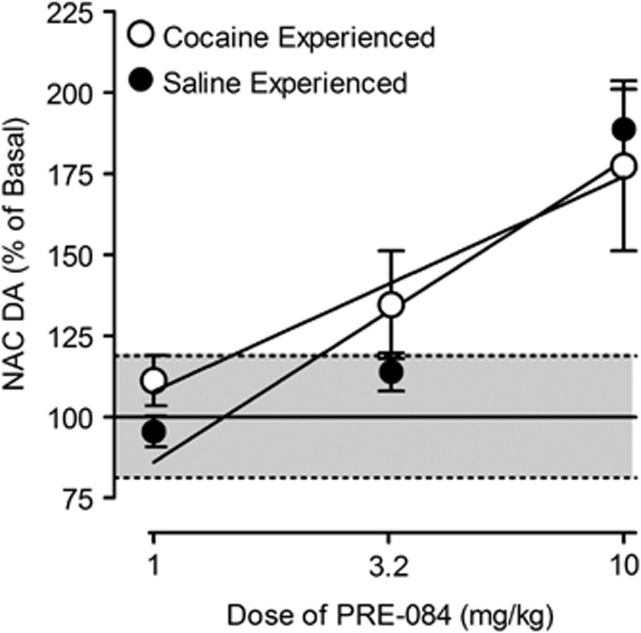
A group of rats that had self-administered PRE-084 after cocaine self-administration were implanted with probes aimed at the nucleus accumbens shell and administered successive increasing doses of PRE-084 (Figure 5). The effects of PRE-084 in these rats were compared with its effects in a second group that had an opportunity to self-administer PRE-084 after the opportunity to self-administer saline. PRE-084 produced dose-dependent increases in dopamine concentrations in both groups of subjects (Figure 5; F3,18=19.3; p<0.001), with only those at the 10.0 mg/kg dose significant (t=6.69; p<0.053). Although there was a trend, there were no significant differences in basal dopamine concentrations in the two groups (t=−2.12; p=0.08) and a single regression line best described the dose–effect curves (F2,20=0.664; p=0.526).
Figure 5.
Dose-dependent effects of PRE-084 on extracellular levels of dopamine in the nucleus accumbens shell. Ordinates: extracellular dopamine levels as a percentage of baseline during the 30-min period of time after cumulative drug administration. Abscissae: dose of drug in mg/kg, log scale. Each point represents the mean effect ±SEM determined in four rats. The average basal dopamine values in 10 μl samples of dialysates from the nucleus accumbens shell were 16.2 ±1.46 fmoles (±SEM) and 29.7±2.79 for rats, respectively, with and without cocaine experience, which did not significantly differ in the two groups (t=−2.12; p=0.08).
DISCUSSION
The results of the present study indicate that a history of cocaine self-administration triggers σ1R-mediated reinforcing effects that were absent in subjects without that particular experience with cocaine. The induction of reinforcing effects of σ1R agonists was not due simply to the perseveration of previously reinforced behavior, as a history of food reinforcement was an insufficient precondition for σ1R-mediated reinforcing effects. Further, the effect was a qualitative change from a virtual absence of reinforcing efficacy to enduring reinforcing effects comparable to those of cocaine. Once the reinforcing effects were induced behavior was amenable to all characteristic modifications by contingencies of reinforcement: responding followed from one lever to the other when the consequent injection became available only on the previously inactive lever; responding was extinguished by eliminating σ1R agonist injections; and the extinguished response was reconditioned with the reintroduction of the contingency. These varied outcomes showing sensitivity to the contingencies of reinforcement occurred over the course of 30-some daily sessions with no indication of a waning of the reinforcing effects of either selective σ1R agonist.
Several previous findings suggest mechanisms that underlie the present induction of reinforcing effects of σ1R agonists. Cocaine exposure can increase levels of σ1R mRNA and protein. These effects occur in brain regions implicated in drug reinforcement and can be blocked by the σR antagonist, BD1063 (Liu et al, 2005; Liu and Matsumoto, 2008). Further, the antagonism by BD1063 suggests that the upregulation of σ1Rs is triggered by direct actions of cocaine at σ1Rs, and cocaine has reported affinity for σ1Rs (Garcés-Ramírez et al, 2011; Hayashi et al, 2007; Hiranita et al, 2011b; Sharkey et al, 1988). These findings suggest that the present induction of a reinforcing effect of σ1R agonists is due to repeated agonist actions at σ1Rs produced by cocaine. However, at variance with this hypothesis are findings that repeated agonist actions produced by the selective σ1R agonist, igmesine, failed to upregulate σ1Rs (Meunier et al, 2006; O′Connell et al, 1996), suggesting that some action of cocaine in addition to its action at σ1Rs is necessary for σ1R upregulation.
Comparisons of rats actively self-administering methamphetamine and those passively receiving the drug at the same doses and frequencies (ie, ‘yoked' controls) have also shown increases in midbrain σ1R protein, σ1R mRNA levels in hippocampus, and σ1R increases in the olfactory bulb (Hayashi et al, 2010; Stefanski et al, 2004), and in addition a comparative downregulation of dopamine D2 autoreceptors (Stefanski et al, 1999). More recently the σ1R increases in the olfactory bulb have been shown to result in activation of extracellular signal-regulated kinase and attenuation of protein kinase A (Hayashi et al, 2010). Further, σ1Rs in the olfactory bulb were found to be colocalized with dopamine D1 receptors (Hayashi et al, 2010). Moreover, a linkage between D1 and σ1 receptors is further supported by studies suggesting that these proteins can form heterodimers (Navarro et al, 2010) and that the upregulation of σ1Rs by cocaine administration in vivo does not occur in mice with a genetic deletion of D1 receptors (Zhang et al, 2005). Thus, current evidence suggests that initial activation of dopaminergic effects, likely involving D1 receptors, may be critical for the triggering an upregulation of σ1Rs, which in turn may be involved in the induction of σ1R-agonist reinforcing effects.
Once established, the reinforcing effects of the selective σ1R agonists were independent of dopaminergic mechanisms. Administration of i.v. doses of PRE-084 that maintain self-administration behavior did not elicit any significant stimulation of dopamine levels in the accumbens shell in rats that self-administered cocaine and PRE-084 or in rats that did not self-administer PRE-084 and were never exposed to cocaine. Significant increases in extracellular dopamine in the nucleus accumbens produced by PRE-084 were obtained at doses 18–30 times greater than those self-administered, and produced less stimulation of dopamine levels than cocaine (Garcés-Ramírez et al, 2011). Several previous studies suggested some dopaminergic activity induced by σ1R agonists. For example increases in dopamine concentrations in striata of rats after administration of σ1R agonists have been detected using in vivo microdialysis (Gudelsky, 1995; Patrick et al, 1993). However, more recent studies examining the selective σ1R agonist, PRE-084, indicated that it was significantly less potent than cocaine (Garcés-Ramírez et al, 2011), whereas the drugs PRE-084 and cocaine were equipotent in self-administration. Additionally, the effects of PRE-084 on dopamine in the nucleus accumbens were not antagonized by the σR antagonist, BD1063 (Garcés-Ramírez et al, 2011), indicating that in contrast to self-administration, the high-dose effects of PRE-084 on dopamine were not σR mediated.
In contrast to cocaine, the self-administration of PRE-084 was insensitive to pretreatments with dopamine receptor antagonists. Further, in this and previous studies (Hiranita et al, 2011b; 2010; Martin-Fardon et al, 2007), self-administration of cocaine was insensitive to pretreatment with σ1R antagonists, whereas the self-administration of the σ1R agonist, PRE-084, in the present study was dose-dependently blocked by σ1R antagonists. A lack of substantive dopaminergic mediation of the effects of σR agonists is further supported by a failure of either PRE-084 or DTG to substitute for cocaine in rats trained to discriminate cocaine from saline injections (Hiranita et al, 2011a), a procedure in which a number of indirect dopaminergic agonists fully substitute for cocaine (Li et al, 2006; Witkin et al, 1991). Importantly, the cocaine-discrimination procedure involves regular cocaine injections, further indicating that administration of cocaine alone does not induce pharmacological responses to σR agonists similar to those of cocaine. Finally, a previous study reported comparable stimulation of locomotor activity by methamphetamine in σ1R-knockout mice and their wild-type controls (Fontanilla et al, 2009). The present results, together with these published findings, suggest pharmacologically distinct mechanisms of stimulant drugs and σ1R agonists, and importantly, minimal if any involvement of dopamine neurotransmission before and after the reinforcing effects of the σ1R agonists are triggered by cocaine.
Given the substantial effects of dopamine receptor antagonists on cocaine self-administration in the present and previous studies (Barrett et al, 2004; Hemby et al, 1996), and the recognized role of dopamine systems in varied effects of cocaine (eg, Ritz et al, 1987; Pontieri et al, 1995, van Rossum and Hurkmans, 1964; Heikkila et al, 1975), it may seem puzzling that experience with cocaine induces a dopamine-independent reinforcing mechanism. However, dopamine-independent aspects of reinforcing mechanisms have been reported (Hemby et al, 1996), and specific behavioral and pharmacological histories have been shown to produce qualitative and profound changes in the behavioral effects of drugs (eg, Barrett, 1977; Collins and Woods, 2007; Glowa and Barrett, 1983; Young and Woods, 1981).
Understanding the pharmacological and behavioral mechanisms underlying the induction by cocaine of reinforcing effects of σ1R agonists is in its beginning stages. A previous study indicated that similar subjective (interoceptive) effects of cocaine and σR agonists appears unlikely as a contributing factor; σR agonists did not substitute for the discriminative-stimulus effects of cocaine (Hiranita et al, 2011a). An account involving behavioral momentum or perseveration has insufficient explanatory power as food-reinforced responding, at least under the current conditions that closely paralleled those used with cocaine, was ineffective as an inducer of reinforcing effects of σ1R agonists. That reinforcing effects of σR agonists were not induced by a history of food reinforcement suggest that the effect in some way involves the pharmacology of reinforcing drugs. Ongoing experiments are examining the specificity of the drug reinforcer.
There is no lack of hypotheses regarding pharmacological and behavioral mechanisms that may be involved in the current effect. Several previous studies have documented a capacity of dopamine D2-like receptor agonists to enhance rates of a response that produced a previously neutral stimulus that was subsequently paired with cocaine injections (Collins and Woods, 2007, 2009; see also Hill, 1970). A modulation by the σ1R agonists of the conditioned-reinforcing effects of stimuli that previously accompanied cocaine injections remains to be pursued in future studies.
The present triggering of reinforcing effects of previously inactive drugs through a history of cocaine self-administration may have a critical role in the documented resistance of stimulant abuse to various attempts at medical treatment (Gorelick et al, 2004; Vocci et al, 2005a; Vocci and Elkashef, 2005b), particularly treatments targeting dopamine systems. Numerous reports exist in the literature of drugs including selective σ1R antagonists that failed to selectively alter the self-administration of cocaine (Hemby et al, 1996; Hiranita et al, 2011b 2010; Martin-Fardon et al, 2007). Despite this, a recent study demonstrated that drugs targeting both σRs and the dopamine transporter show preclinical indications of efficacy as potential cocaine-abuse treatments (Hiranita et al, 2011b). Thus, the present results may suggest that the induction of other reinforcement mechanisms may contribute to the well-known intractability of stimulant abuse, and point to novel targets for development of combination chemotherapies to combat stimulant dependence.
Acknowledgments
We thank Patty Ballerstadt for administrative assistance and Drs Tsung-Ping Su, Teruo Hayashi, James H. Woods, and James E. Barrett for advice on these studies and the preparation of the manuscript. The work reported herein was supported by the Intramural Research Program of the National Institute on Drug Abuse.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Portions of this manuscript were presented at the annual meeting of International Study Group Investigating Drugs as Reinforcers (Scottsdale, AZ; June 2010), the annual meeting of Experimental Biology (Washington, DC; April 2011), and the Early Career Investigators Poster Session (cosponsored by NIDA/NIAAA/APA, and APA Divisions 28 and 50), American Psychological Association, 119th Annual Meeting (Washington, DC; August 2011). An abstract describing these results was included in the program materials of the canceled annual meeting of the Japanese Pharmacological Society(Yokohama, Japan; March 2011)
Supplementary Material
References
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47 (Suppl 1:256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Barrett JE. Behavioral history as a determinant of the effects of d-amphetamine on punished behavior. Science. 1977;198:67–69. doi: 10.1126/science.408925. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behav Pharmacol. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormaci G, Mori T, Hayashi T, Su TP. Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: implication for neuroplasticity. J Pharmacol Exp Ther. 2007;320:202–210. doi: 10.1124/jpet.106.108415. [DOI] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcés-Ramírez L, Green J, Hiranita T, Kopajtic T, Mereu M, Thomas A, et al. Sigma receptor agonists: receptor binding and effects on mesolimbic dopamine neurotransmission assessed by microdialysis in rats. Biol Psychiatry. 2011;69:208–217. doi: 10.1016/j.biopsych.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Barrett JE. Drug history modifies the behavioral effects of pentobarbital. Science. 1983;220:333–335. doi: 10.1126/science.6682244. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Effects of sigma receptor ligands on the extracellular concentration of dopamine in the striatum and prefrontal cortex of the rat. Eur J Pharmacol. 1995;286:223–228. doi: 10.1016/0014-2999(95)00415-8. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Justinova Z, Hayashi E, Cormaci G, Mori T, Tsai SY, et al. Regulation of sigma-1 receptors and endoplasmic reticulum chaperones in the brain of methamphetamine self-administering rats. J Pharmacol Exp Ther. 2010;332:1054–1063. doi: 10.1124/jpet.109.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of [3H]dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Hill RT.1970Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulationIn: Costa E,, Garattini S, (eds).Amphetamines and Related Compounds Raven Press: New York; 781–795. [Google Scholar]
- Hiranita T, Soto P, Tanda G, Katz J. Lack of cocaine-like discriminative-stimulus effects of σ receptor agonists in rats. Behav Pharmacol. 2011a;22:525–530. doi: 10.1097/FBP.0b013e328349ab22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, et al. Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and {σ} receptors. J Pharmacol Exp Ther. 2011b;339:662–677. doi: 10.1124/jpet.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther. 2009;329:677–686. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. J Pharmacol Exp Ther. 2010;332:515–524. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T, et al. A role for sigma receptors in stimulant self administration and addiction. Pharmaceuticals. 2011;4:880–914. doi: 10.3390/ph4060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. J Pharmacol Exp Ther. 2006;317:1088–1096. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. Cocaine up-regulates Fra-2 and sigma-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmacol Exp Ther. 2005;314:770–779. doi: 10.1124/jpet.105.084525. [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther. 2008;327:187–195. doi: 10.1124/jpet.108.141051. [DOI] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G. Some behavioral effects of 1,3-di-o-tolylguanidine, opipramol and sertraline, the sigma site ligands. Polish J Pharmacol. 1996;48:379–395. [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol. 2009;2:351–358. doi: 10.1586/ecp.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting sigma1 receptors produce anti-cocaine effects in mice. Eur J Pharmacol. 2001;419:163–174. doi: 10.1016/s0014-2999(01)00968-2. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Meunier J, Demeilliers B, Celerier A, Maurice T. Compensatory effect by sigma1 (σ1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav Brain Res. 2006;166:166–176. doi: 10.1016/j.bbr.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Aymerich M, Marcellino D, McCormick PJ, Mallol J, et al. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Nat Acad Sci USA. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Sigma1 receptor agonist-mediated regulation of N-methyl-D-aspartate-stimulated [3H]dopamine release is dependent upon protein kinase C. J Pharmacol Exp Ther. 2003;304:364–369. doi: 10.1124/jpet.102.043398. [DOI] [PubMed] [Google Scholar]
- O′Connell AW, Earley B, Leonard BE. The sigma ligand JO 1784 prevents trimethyltin-induced behavioural and sigma-receptor dysfunction in the rat. Pharmacol Toxicol. 1996;78:296–302. doi: 10.1111/j.1600-0773.1996.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Patrick SL, Walker JM, Perkel JM, Lockwood M, Patrick RL. Increases in rat striatal extracellular dopamine and vacuous chewing produced by two sigma receptor ligands. Eur J Pharmacol. 1993;231:243–249. doi: 10.1016/0014-2999(93)90456-r. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Sidney, Australia: Academic Press; 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Nat Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. Involvement of the sigma1 receptor in the cocaine-induced conditioned place preference. Neuroreport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. Cocaine binding at sigma receptors. Eur J Pharmacol. 1988;149:171–174. doi: 10.1016/0014-2999(88)90058-1. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–135. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, Katz JL. Effects of 4′-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther. 2005;313:613–620. doi: 10.1124/jpet.104.080465. [DOI] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, Katz JL. Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem. 2008;106:147–157. doi: 10.1111/j.1471-4159.2008.05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- van Rossum JM, Hurkmans JAThM. Mechanism of action of psychomotor stimulant drugs: significance of dopamine in locomotor stimulant action. Int J Neuropharmacol. 1964;3:227–239. doi: 10.1016/0028-3908(64)90012-7. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005a;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005b;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257:706–713. [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther. 1981;218:720–727. [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, et al. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–1454. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.