Abstract
Signaling inputs from multiple pathways are essential for the establishment of distinct cell and tissue types in the embryo. Therefore, multiple signals must be integrated to activate gene expression and confer cell fate, but little is known about how this occurs at the level of target gene promoters. During early embryogenesis, Wnt and Nodal signals are required for formation of the Spemann organizer, which is essential for germ layer patterning and axis formation. Signaling by both Wnt and Nodal pathways is required for the expression of multiple organizer genes, suggesting that integration of these signals is required for organizer formation. Here, we demonstrate transcriptional cooperation between the Wnt and Nodal pathways in the activation of the organizer genes Goosecoid (Gsc), Cerberus (Cer), and Chordin (Chd). Combined Wnt and Nodal signaling synergistically activates transcription of these organizer genes. Effectors of both pathways occupy the Gsc, Cer and Chd promoters and effector occupancy is enhanced with active Wnt and Nodal signaling. This suggests that, at organizer gene promoters, a stable transcriptional complex containing effectors of both pathways forms in response to combined Wnt and Nodal signaling. Consistent with this idea, the histone acetyltransferase p300 is recruited to organizer promoters in a Wnt and Nodal effector-dependent manner. Taken together, these results offer a mechanism for spatial and temporal restriction of organizer gene transcription by the integration of two major signaling pathways, thus establishing the Spemann organizer domain.
Keywords: Wnt, Nodal, Organizer, Siamois, Twin, FoxH1, Smad2/3, Signaling, Transcription, Embryo, Xenopus, Gastrula
Introduction
Cells of the vertebrate embryo receive multiple signals that must be integrated to activate developmental gene expression in a robust and specific manner, but how this occurs is not well understood. During Xenopus embryogenesis, Wnt and Nodal signals are required for formation of the Spemann organizer, which is essential for germ layer patterning and axis formation (reviewed in De Robertis et al., 2000). Wnt and Nodal signaling inputs are essential for the expression of several organizer genes, including Goosecoid (Gsc), Cerberus (Cer), and Chordin (Chd) (Agius et al., 2000; Crease et al., 1998; Engleka and Kessler, 2001; Heasman et al., 1994; Hoodless et al., 1999; Miller et al., 1999; Osada and Wright, 1999; Watanabe and Whitman, 1999; Wylie et al., 1996; Yang et al., 2002), suggesting that integrated signaling from these pathways promotes organizer gene transcription. The presence of a Wnt-responsive Proximal Element (PE) and a Nodal-responsive Distal Element (DE) within the Xenopus Gsc promoter suggests that Wnt and Nodal signals may be integrated at the level of transcriptional control (Watabe et al., 1995). The close proximity of the PE and the DE suggests that Wnt and Nodal effectors could interact in activation of Gsc transcription (Watabe et al., 1995). Consistent with this idea, the Cer promoter contains several homeodomain binding sites that mediate a cooperative response to Wnt and Nodal (Yamamoto et al., 2003). Therefore, while the transcription of multiple organizer genes is dependent on both Wnt and Nodal signals, how these signals are functionally integrated is unknown.
Maternal Wnt signals activate expression of two homeodomain proteins, Siamois (Sia) and Twin (Twn), transcriptional activators that are essential for organizer gene expression and axis formation (Bae et al., 2011; Brannon et al., 1997; Brannon and Kimelman, 1996; Carnac et al., 1996; Crease et al., 1998; Fan et al., 1998; Fan and Sokol, 1997; Ishibashi et al., 2008; Kessler, 1997; Kodjabachian and Lemaire, 2001; Kodjabachian and Lemaire, 2004; Laurent et al., 1997; Lemaire et al., 1995). Morpholino knockdown of Sia and Twn together results in a loss of organizer gene expression, including Gsc and Chd, and the absence of dorsal structures (Bae et al., 2011). Overexpression of Sia or Twn in ventral mesoderm induces expression of multiple organizer genes, including Gsc, Cer, and Chd (Kessler, 1997; Kodjabachian and Lemaire, 2001), and Sia and Twn directly regulate transcription of Gsc (Bae et al., 2011; Laurent et al., 1997). Nodal signals through maternal FoxH1 and Smad2/3 to activate expression of mesodermal and organizer genes, including Gsc, Cer, and Chd (Saka et al., 2007; Watanabe and Whitman, 1999). Knockdown of maternal FoxH1 results in a loss of organizer gene expression (Kofron et al., 2004), while expression of a dominant negative Smad2 reduces expression of Gsc, Chd and Cer (Hoodless et al., 1999). FoxH1 directly binds the Gsc promoter (Blythe et al., 2009), suggesting that Gsc is a direct target of Nodal signaling. Active Nodal signaling is required for Sia/Twn mediated expression of Cer and Chd (Crease et al., 1998; Engleka and Kessler, 2001), suggesting that Sia/Twn may cooperate with Nodal in the transcription of these genes. Taken together, these findings suggest that the transcription of multiple organizer genes, including Gsc, Cer, and Chd, is dependent on combined Wnt and Nodal signaling inputs that are integrated at defined promoter elements.
Here, we demonstrate that the Wnt effectors Sia/Twn and Nodal effectors FoxH1 and Smad2/3 cooperate to synergistically activate expression of Gsc, Cer and Chd. Sia/Twn, FoxH1 and Smad2/3 occupy the Gsc, Cer and Chd promoters. Active signaling from both pathways enhances occupancy of these effectors at organizer promoters, suggesting that a transcriptional complex forms at promoters when Wnt and Nodal are active. Sia/Twn or Nodal enhances occupancy of the histone acetyltransferase p300 at organizer promoters, suggesting that recruitment of a common coactivator contributes to organizer gene transcription. Taken together, Wnt and Nodal pathway effectors form a transcriptional complex that synergistically activates expression of multiple organizer genes, providing a common mechanism for the robust transcription of organizer genes in the gastrula.
Materials and methods
Embryo manipulation and microinjection
Xenopus laevis embryos were collected, fertilized, injected, and cultured as previously described (Yao and Kessler, 2001). mRNA templates were pCS2+Siamois (Kessler, 1997), pCS2+Twin (Bae et al., 2011), pCS2+myc-Twin (Bae et al., 2011), pCS2+myc-Siamois (Bae et al., 2011), pCS2+myc-SiaQ191E (Bae et al., 2011; Kessler, 1997), pCS2+myc-FoxH1 (Fast1) (Yaklichkin et al., 2007), and pCS2+Xnr1 (Sampath et al., 1997). This work has been approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC). The University of Pennsylvania is accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC).
Antibody staining and in situ hybridization
For in situ hybridization or immunostaining of bisected embryos, embryos were fixed in MEMFA and bisected in a 30% sucrose/PBS solution. Embryos were processed for in situ hybridization or immunohistochemistry as previously described (Steiner et al., 2006). Templates for in situ probes were pCS2+Sia (Bae et al., 2011), pCS2+Twn (Bae et al., 2011), pCS2+FoxH1 (Fast1) (Yaklichkin et al., 2007), pCS2+Gsc (Yao and Kessler, 2001), pCS2+Chd (Sasai et al., 1994), and pCS2+Cer (Bouwmeester et al., 1996). An affinity-purified polyclonal antibody was used for Smad2/3 staining (Millipore cat 07–408).
Luciferase reporter assay
Luciferase assays were performed as previously described (Kessler, 1997), using 100 pg of pGL3-Gsc(−226)-Luciferase (Watabe et al., 1995) reporter and 10 pg of pGL3-CMV-Renilla as an internal control (Kessler, 1997).
Reverse transcription—Polymerase chain reaction
For RT-PCR analysis, total RNA was isolated using TRIzol (Invitrogen), and cDNA synthesis was performed as described (Wilson and Melton, 1994). The primers for Ef1α were previously described (Agius et al., 2000). cDNAs were amplified using the following QPCR primers: Gsc: F: 5′-CCTCTGGAATAAGAATAAAGACTTGCAC-3′ and R: 5′-CTCTATGTACAGATCCCACATCGT-3′; Cer: F: 5′-CTGAACCACCTGACGCTAATT-GT-3′ and R: 5′-CTGTGCAGTTTGGTGGAAGTTGCT-3′; Chd: F: 5′-CAGCTGCAAAAACATCAAACA-3′ and R: 5′-CAAGTCTTGCAGCAATGTCC-3′.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described (Blythe et al., 2009). Polyclonal anti-myc antibody (Millipore cat 06–549) or anti-Smad2/3 (Millipore cat 07–408) was used for immunoprecipitation. Rabbit IGG (Calbiochem cat #NI01) was used as a control for IP of Smad2/3. QPCR was performed using primers for Gsc, Ef1α or Xmlc2 as previously described (Blythe et al., 2009). Promoter sequence was amplified using the following QPCR primers: Cer promoter: F: 5′-GGAACAGCAAGTCGCTCAGAAACA-3′ and R: 5′-CTCCATCATTCACAAGGCAGACGA-3′; Chd promoter: F: 5′-GCTGAGTCAGGATGCTGTTTCTGAGT-3′ and R: 5′-TGCCCAAGGAAAGTGTCTCTTAACCG-3′.
Results
Wnt and Nodal synergistically activate organizer gene expression
The Gsc promoter contains a Wnt responsive PE and a Nodal responsive DE, and these adjacent response elements are conserved in all vertebrate Gsc promoters (Bae et al., 2011; Watabe et al., 1995). The presence of this pair of conserved response elements in all Gsc promoters suggests that Nodal and Wnt pathway effectors may cooperate in the regulation of Gsc transcription. To assess the interaction of Nodal and the Wnt effectors Sia/Twn in Gsc regulation, we performed luciferase assays in Xenopus animal explants using a Gsc reporter, which contains the DE, PE and minimal promoter (−226 to +1) driving luciferase (Watabe et al., 1995) (Fig. 1). Expression of Xnr1 (Xenopus Nodal-related-1), Sia, or Twn activated expression of the Gsc reporter (6.4-fold, 5.3-fold and 4.7-fold, respectively) (Fig. 1) (Fan and Sokol, 1997; Kessler, 1997; Laurent et al., 1997; Watabe et al., 1995). Coexpression of Sia and Xnr1 or Twn and Xnr1 resulted in a synergistic activation of transcription (48.8-fold for Sia+Xnr1, 36.3-fold for Twn+Xnr1) (Fig. 1). In this case, we have defined synergy as the response to the combined inputs is greater than the sum of the individual inputs; we find that 48.8-fold or 36.3-fold activation of this reporter is greater than Sia+Xnr1 (6.4+5.3) or Twn+Xnr1 (6.4+4.7). We also find that this synergy is greater than that observed when twice the amount (100 pg) of Sia, Twn, or Xnr1 is expressed (8–10-fold) with the reporter (data not shown and (Bae et al., 2011). We note that several doses of Sia, Twn and Xnr1 mRNA were tested for synergistic activation of the Gsc reporter. Synergy was most strongly observed at the doses presented here (50 pg Sia or Twn mRNA plus 50 pg Xnr1 mRNA), but cooperative activation of the Gsc reporter was also observed at lower doses (10–25 pg) of Sia or Twn and Xnr1 mRNA (data not shown). The synergy observed suggests that the interaction of Sia/Twn and Nodal pathway effectors strongly enhances Gsc transcription.
Fig. 1.
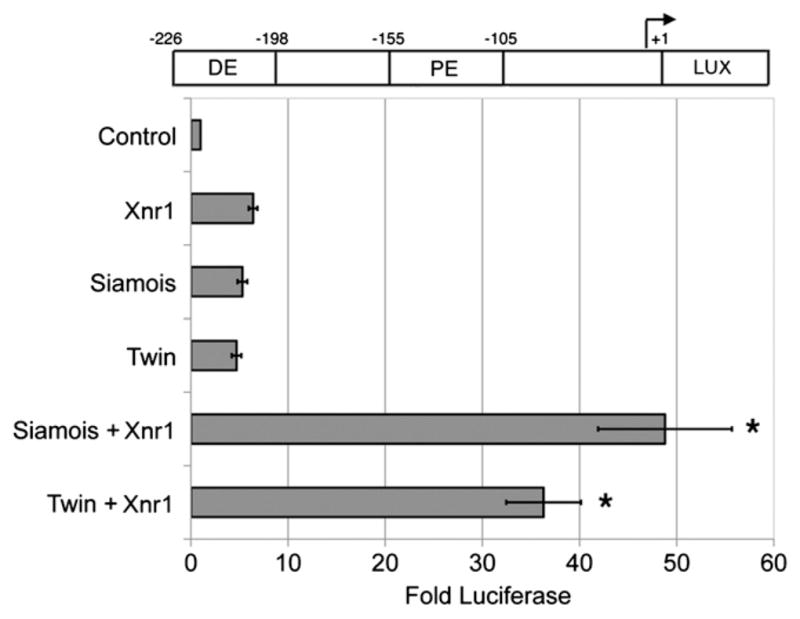
Nodal and Wnt effectors synergistically activate the Gsc promoter. One-cell stage embryos were injected with 50 pg of Sia, Twn or Xnr1 mRNAs, or a mixture of Sia (50 pg) and Xnr1 (50 pg) or Twn (50 pg) and Xnr1 (50 pg). At the two-cell stage plasmid encoding Gsc reporter (100 pg; diagramed at top) was injected with CMV-Renilla Luciferase (10 pg). Animal explants prepared at the blastula stage were assayed for luciferase activity at the midgastrula stage. Values shown are normalized to Renilla luciferase activity, and represent fold activation of reporter activity in the absence of injected mRNAs. The mean and standard error for three independent experiments are presented. * Indicates p value <0.05.
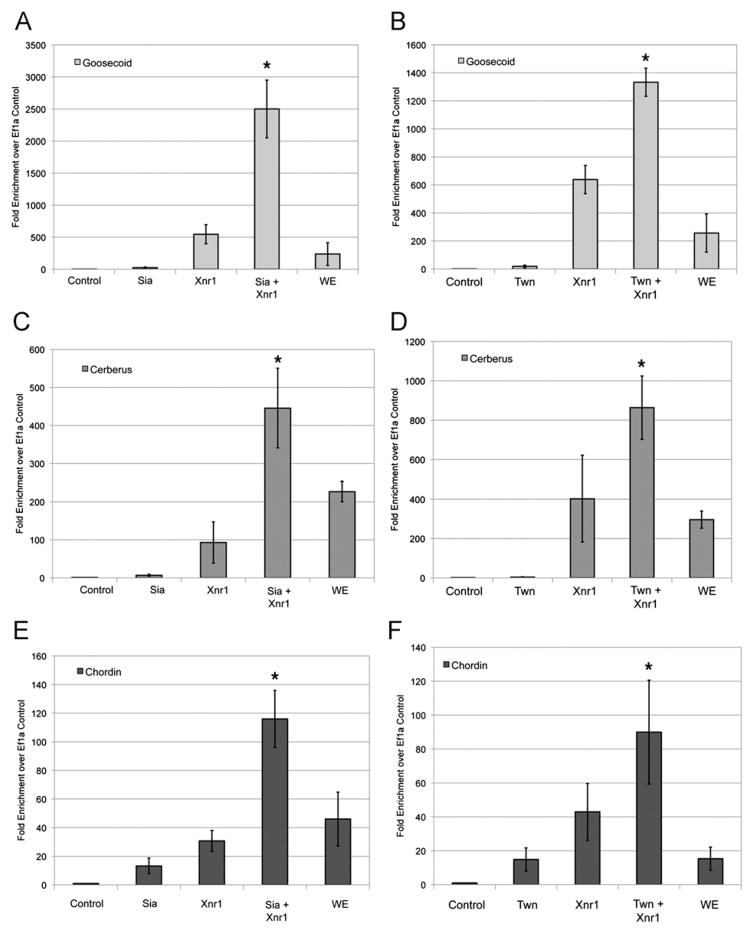
The cooperation between the Wnt effectors Sia/Twn and Nodal signals in the expression of a Gsc reporter suggested that endogenous organizer genes may be cooperatively activated by the Wnt and Nodal pathway signals. To determine whether Wnt and Nodal synergistically activate expression of organizer genes, we performed quantitative reverse transcriptase PCR (QRT-PCR) for Gsc, Cer and Chd transcript in animal explants. Expression of Sia, Twn or Xnr1 alone induced expression of Gsc, Cer and Chd transcripts (7–21.7-fold for Sia, 3–18.5-fold for Twn and 30–638-fold for Xnr1) (Fig. 2A–F). Co-expression of Sia and Xnr1 or Twn and Xnr1 resulted in a synergistic increase in Gsc, Cer and Chd expression (1333–2501-fold for Gsc, 445–865-fold for Cer and 90–115-fold for Chd) (Fig. 2). These data demonstrate a cooperative interaction between Sia/Twn and Nodal in activating transcription of organizer genes.
Fig. 2.
Nodal and Wnt effectors synergistically activate organizer gene transcription. Analysis of Gsc (A), (B), Cer (C), (D) or Chd (E), (F) transcript expression in animal cap explants in response to injection of (A), (C), (E) 50 pg Sia, 50 pg Xnr1, or 50 pg Sia and 50 pg Xnr1 or (B), (D), (F) 50 pg Twn, 50 pg Xnr1, or 50 pg Twn and 50 pg Xnr1. Animal explants were analyzed by quantitative RT-PCR at the gastrula stage for the expression of Gsc, Chd or Cer normalized to Ef1α. Control represents uninjected animal explants and WE represents intact embryos. * Indicates p<0.05 as compared to the Sia, Twn and Xnr1 conditions. The mean and standard error for six independent experiments is presented. Identical reactions without reverse transcriptase served as negative control (data not shown).
Expression patterns of Wnt and Nodal effectors support a cooperative interaction of these effectors in endogenous organizer gene expression (Blumberg et al., 1991; Bouwmeester et al., 1996; Chen et al., 1996; Germain et al., 2000; Saka et al., 2007; Sasai et al., 1994; Schohl and Fagotto, 2002). As previously reported, Nodal signaling is broadly active within the mesodermal and endodermal germ layers at the blastula stage. To confirm this, we examined the expression of the Nodal effectors Smad2/3 and FoxH1 by immunohistochemistry or in situ hybridization of bisected blastula and gastrula stage embryos. Total Smad2/3 protein is present throughout the embryo, and is distributed in distinct nuclear puncta in the larger vegetal cells of blastula and gastrula embryos (Fig. S1A–C). As Smad2/3 is thought to signal only when localized to the nucleus, this Smad2/3 localization is consistent with active Nodal signaling in the vegetal half of the blastula and gastrula embryo, when organizer gene expression is activated (Saka et al., 2007; Schohl and Fagotto, 2002; Skirkanich et al., 2011). Smad2/3 protein is also observed in the ectodermal and mesodermal cells of the early embryo (Fig. S1A–C). For these smaller cells, it is unclear whether the distribution is nuclear, cytoplasmic or both. Transcripts of FoxH1 are ubiquitously distributed at the blastula and gastrula stages (Fig. S1D–F), and are observed in the marginal zone of the embryo, where the organizer will form. The Wnt effectors Sia and Twn are expressed in the dorsal marginal zone prior to gastrulation (Fig. S1G,H), and at the dorsal blastopore lip at the early gastrula stage (Fig. S1I,J). Gsc, Cer and Chd are expressed at the dorsal blastopore lip and expression extends to the blastocoel floor in the deep marginal zone (Fig. S1K–P). Therefore, Wnt and Nodal effector distribution overlaps in a region that corresponds to the domain of organizer gene expression.
Wnt and Nodal pathway effectors occupy organizer promoters
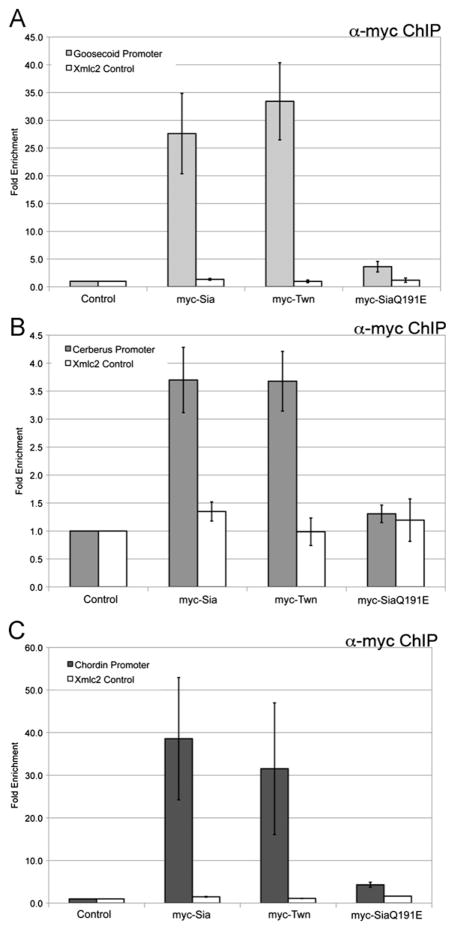
The apparent cooperation between Wnt and Nodal pathways in organizer gene expression suggests that pathway effectors directly bind these promoters to activate transcription. For Gsc, well defined Wnt and Nodal response elements are present within the promoter, with only 43 bp of separation. We predict that Wnt and Nodal pathway effectors will occupy their respective response elements at the Gsc promoter in the early gastrula, allowing for possible physical and functional interactions. To determine whether Sia, Twn and Nodal pathway effectors directly regulate Gsc, whole embryo chromatin immunoprecipitation (ChIP) was performed in the early gastrula. Myc-Sia or myc-Twn expressing embryos were collected and fixed at early gastrula stage (stage 10.25). Immunoprecipitation was performed for the myc-tag and quantitative PCR (QPCR) assessed recovery of the Gsc promoter, using primers designed to amplify the −226 Gsc promoter region. Sia and Twn occupy the Gsc promoter (Fig. 3A) (Bae et al., 2011) and do not occupy genomic Xmlc2, demonstrating direct regulation of Gsc by Sia and Twn (Fig. 3A). Sia binds the Gsc promoter with specificity, as a DNA-binding inactive form of Sia (SiaQ191E) did not occupy the Gsc promoter (Fig. 3A) (Bae et al., 2011).
Fig. 3.
Wnt pathway effectors occupy organizer gene promoters. Genomic regions recovered by chromatin immunoprecipitation (ChIP) from embryos injected with 50 pg myc-Sia, 50 pg myc-Twn or 50 pg of a DNA-binding inactive Sia (myc-SiaQ191E) were evaluated by quantitative PCR (QPCR) for the (A) Gsc, (B) Cer, or (C) Chd promoters. Immunoprecipitation using anti-myc antibody was performed on uninjected embryos (Control). The mean fold enrichment (normalized to uninjected samples) and standard error for five independent experiments is presented. The white bars represent QPCR for genomic Xmlc2 as a control.
Given the similar Wnt-dependent expression patterns of Gsc, Cer, and Chd in the gastrula, it seems likely that Sia/Twn may also directly regulate the transcription of Cer and Chd. For Cer, functional Sia/Twn response elements have previously been identified within the proximal promoter sequence (Yamamoto et al., 2003), and primers were designed to amplify this region from immunoprecipitated embryo extracts. For Chd, we sought to identify such a Sia/Twn response element, and were successful in identifying a Sia-responsive element within the Chd proximal promoter (Fig. S2). Primers were designed to amplify the Sia-responsive region of the Chd promoter, to quantify recovery from ChIP extracts. Consistent with direct regulation of Cer and Chd transcription, Sia and Twn occupy the Cer and Chd promoters in the previously identified regions (Fig. 3B,C), while SiaQ191E does not (Fig. 3B,C). We note that a wide dosage range of myc-Sia or myc-Twn mRNA (1–150 pg) was initially tested, and occupancy of both Sia and Twn at the Gsc, Cer, and Chd promoters was observed at doses as low as 1 pg (data not shown), suggesting that Sia/Twn occupancy at these promoters is quite robust. Taken together, Sia/Twn likely mediate the transcriptional response of multiple organizer genes to Wnt signals.
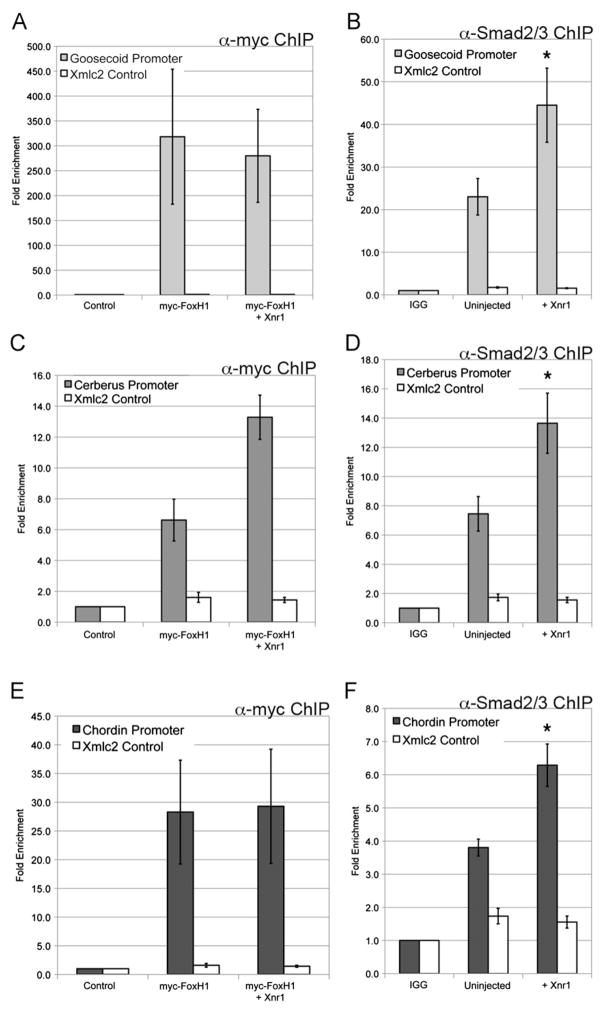
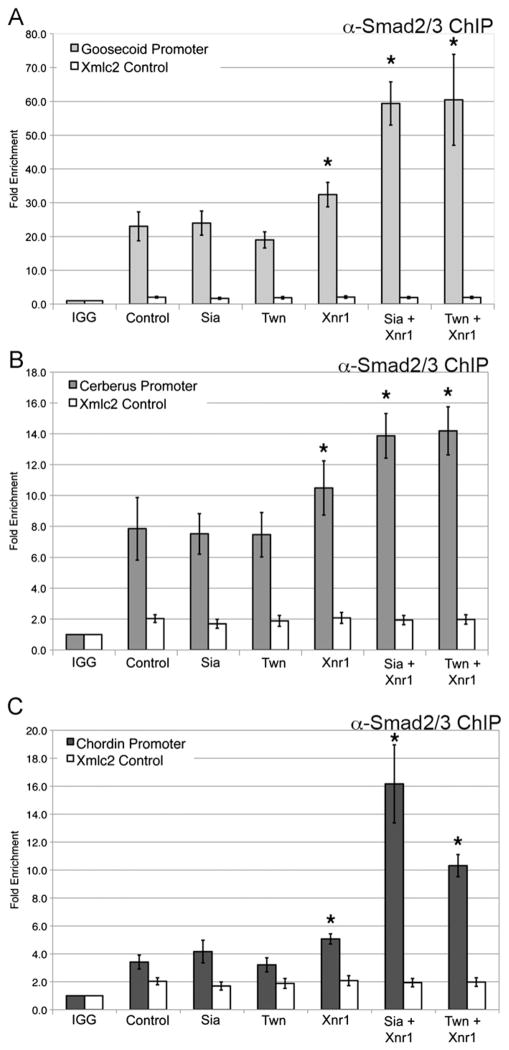
Gsc, Cer and Chd expression in the organizer is also dependent on Nodal signaling (Agius et al., 2000; Engleka and Kessler, 2001), and both the Gsc and Cer promoters contain defined Nodal response elements (Yamamoto et al., 2003). The Chd promoter contains several putative FoxH1 binding sites (Fig. S2C), but a defined Nodal-response element has not yet been identified. ChIP analyses were performed to determine whether Nodal effectors occupy the Gsc, Cer and Chd promoters, using the same primers previously described. Myc-FoxH1 occupied the Gsc, Cer and Chd promoters, both in the absence and presence of Xnr1 (Fig. 4A, C, E) (Blythe et al., 2009). We note that FoxH1 occupancy at organizer promoters was observed using expression of as little as 25 pg myc-FoxH1 mRNA (data not shown). Using an antibody specific for endogenous Smad2/3, we found that Smad2/3 occupied these promoters at elevated levels in response to Xnr1 (Fig. 4B, D, F). This suggests that the Wnt effectors Sia/Twn and the Nodal effectors FoxH1/Smad2/3 occupy sites with close proximity in organizer promoters, as the same primer sets were used to amplify genomic regions recovered from both Sia/Twn and FoxH1/Smad2/3 chromatin immunoprecipitations. The results demonstrate that both Wnt and Nodal effectors are present at the organizer gene promoters in the early gastrula, consistent with direct regulation by Wnt and Nodal.
Fig. 4.
Nodal pathway effectors occupy organizer gene promoters. (A), (C), (E) Genomic regions recovered by ChIP for myc-FoxH1, or myc-FoxH1 coexpressed with Xnr1 (myc FoxH1+Xnr1) were evaluated by QPCR for the (A) Gsc, (C) Cer, or (E) Chd promoters. Immunoprecipitation using anti-myc antibody was performed on uninjected embryos (Control). The data presented represent three independent experiments. (B), (D), (F) Genomic regions recovered by ChIP for endogenous Smad2/3 in uninjected embryos or embryos expressing Xnr1 mRNA (+Xnr1) were evaluated by QPCR for the (B) Gsc, (D) Cer, or (F) Chd promoters. Rabbit IGG added to uninjected embryo extract serves as a negative control (IGG). The mean fold enrichment (normalized to uninjected samples) and standard error for three independent experiments is presented. The white bars represent QPCR for genomic Xmlc2 as a control. * Indicates p value <0.05 as compared to uninjected embryos.
Taken together, these results demonstrate that the Gsc, Cer and Chd promoters have a similar functional organization, with a Nodal responsive element within the same region as the Sia/Twn response element. The close proximity of the Nodal and Wnt response elements in each promoter (Fig. 1 and Fig. S2) (Watabe et al., 1995; Yamamoto et al., 2003), and the location of the response elements within 250 bp of the start site of transcription (this study; (Watabe et al., 1995; Yamamoto et al., 2003) strongly argue for functional conservation of organizer gene promoters in mediating the response to Wnt and Nodal. Furthermore, the presence of both Wnt and Nodal effectors in close proximity at these promoters suggests potential physical and functional interactions that mediate the synergistic response to combined Wnt and Nodal inputs.
Wnt and Nodal effectors interact at organizer promoters
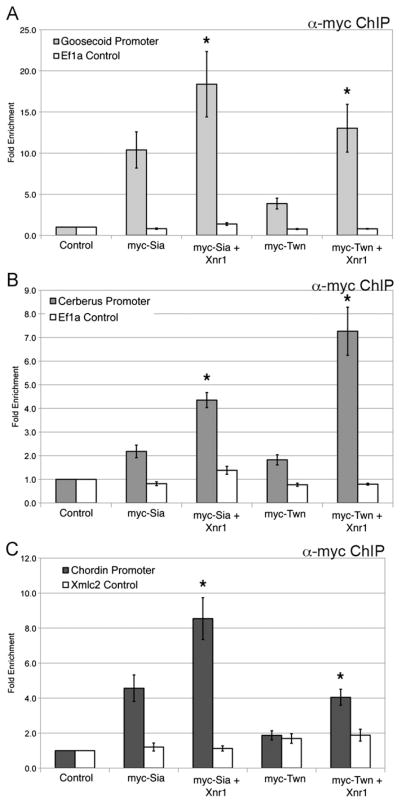
The synergistic transcriptional response to Nodal and Wnt may reflect the formation of a transcriptional complex that enhances effector occupancy and/or activity. To assess the possible interactions of Nodal and Wnt effectors, we examined occupancy of Sia or Twn in response to Nodal signals. Sia and Twn occupancy at the Gsc, Cer and Chd promoters is significantly enhanced (2–4-fold) with addition of Xnr1 (Fig. 5). We note that the influence of Nodal signaling on Sia and Twn occupancy was examined at increasing doses of Sia and Twn (1–50 pg). At lower expression levels of Sia or Twn (1–25 pg), enhanced occupancy is observed in response to Xnr1 (Fig. 5 and data not shown), while at higher expression levels of Sia and Twn (50 pg; Figs. 1–3) the already strong occupancy was not enhanced. To determine if Nodal signaling is required for Sia/Twn occupancy at organizer promoters, myc-Sia or myc-Twn were coexpressed with Cerberus-short (Cer-S), an extracellular inhibitor of Nodal signaling (Piccolo et al., 1999). Although embryos expressing Cer-S fail to gastrulate, no change in occupancy was observed for either myc-Sia or myc-Twn at the Gsc, Cer and Chd promoters in the presence of Cer-S, suggesting that Nodal signaling is not required for Sia/Twn occupancy at organizer genes (data not shown). Rather, we hypothesize that Sia/Twn occupancy is enhanced with active Nodal signaling, resulting in enhanced transcriptional output when both Wnt and Nodal pathway effectors are bound to target gene promoters.
Fig. 5.
Siamois/Twin occupancy at organizer promoters is enhanced by Nodal signaling. Genomic regions recovered by ChIP for 10 pg myc-Sia, 10 pg myc-Sia and 50 pg Xnr1, 10 pg myc-Twn, or 10 pg myc-Twn and 50 pg Xnr1 were evaluated by QPCR for the (A) Gsc, (B) Cer or (C) Chd promoters. The white bars represent QPCR for genomic EF1α as a control. The mean fold enrichment (normalized to uninjected samples) and standard error for eight independent experiments is presented.
To determine whether Sia/Twn influence the occupancy of the Nodal effectors FoxH1 or Smad2/3 at organizer promoters, chromatin immunoprecipitation for Smad2/3 or FoxH1 was examined in response to Sia or Twn in the presence or absence of Xnr1 (Fig. 6). Smad2/3 occupied the Gsc, Cer, and Chd promoters in control embryos, and occupancy was not increased in response to Sia or Twn alone (Fig. 6). As previously demonstrated in Fig. 4, Smad2/3 occupancy at organizer promoters was increased in response to Xnr1 expression (Fig. 6). However, Smad2/3 occupancy was further increased (2–3-fold) at each promoter when Sia or Twn was coexpressed with Xnr1 (Fig. 6). FoxH1 occupancy at the organizer promoters was not increased in response to Sia or Twn (data not shown). We note that the increase in Smad2/3 occupancy is observed at doses of Sia+Xnr1 or Twn+Xnr1 (50 pg each) that result in synergistic activation of the Gsc luciferase reporter (Fig. 1). Taken together, the results indicate that Smad2/3 interacts with Sia and Twn at organizer promoters, and this interaction results in enhancement of occupancy of Sia, Twn and Smad2/3. This enhanced occupancy likely reflects the formation of a stable transcriptional complex containing both Wnt and Nodal effectors, as well as other co-regulatory proteins. Assembly of such a complex at organizer promoters may account for the synergistic activation of transcription in response to Wnt and Nodal signals.
Fig. 6.
Smad2/3 occupancy at organizer promoters is enhanced by Siamois/Twn and Nodal. Genomic regions recovered from ChIP for endogenous Smad2/3 in uninjected embryos (Control), or embryos expressing 50 pg Sia, 50 pg Twn or 50 pg Xnr1, or combinations of 50 pg Sia and 50 pg Xnr1 or 50 pg Twn and 50 pg Xnr1 were evaluated by QPCR for the (A) Gsc, (B) Cer, or (C) Chd promoters. The white bars represent QPCR for genomic Xmlc2 as a control. Smad2/3 association with the promoters is significantly enhanced (*p value <0.05) in the presence of Xnr1 as compared to uninjected embryos. Smad2/3 association with the promoters is further enhanced (*p value <0.05) in the presence of Sia and Xnr1 or Twn and Xnr1 as compared to Sia, Twn, or Xnr1 alone. The mean fold enrichment (normalized to uninjected samples) and standard error for six independent experiments is presented.
Wnt and Nodal effectors recruit p300 to organizer gene promoters
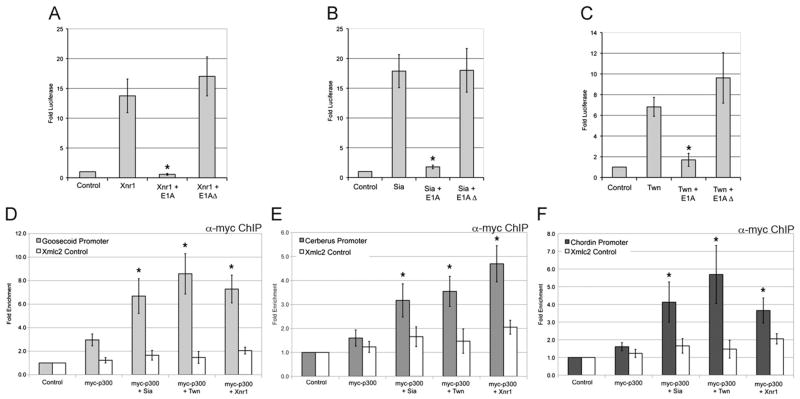
The transcription complex that forms at organizer gene promoters may include common coactivators recruited in response to both Wnt and Nodal signals. The histone acetyltransferase, p300, is a widely employed coactivator recruited to target gene promoters by many transcriptional regulatory proteins, where it modifies chromatin, promoter-specific factors, or both, to active transcription (reviewed in Bedford et al., 2010). In the Xenopus gastrula, interference with p300 function using adenoviral E1A inhibited Gsc and Chd expression (Kato et al., 1999). Consistent with a role in organizer gene expression, p300 binds to and acetylates Smad2/3 and results in enhanced transcription in response to Nodal (Inoue et al., 2007; Ross et al., 2006; Tu and Luo, 2007). To verify a functional interaction between Sia/Twn, Nodal and p300, we examined the requirement for p300 activity in Sia/Twn or Nodal-mediated activation of the Gsc reporter. While Xnr1, Sia or Twn strongly activated the Gsc promoter (7–17-fold activation), co-expression of E1A, an inhibitor of p300 function, strongly inhibited that response (~2-fold activation), while E1AΔ2-36, which lacks the p300 interaction domain, did not decrease transcriptional activity (Fig. 7A–C) (Frisch and Mymryk, 2002). Consistent with a role for p300 in the synergistic activation of Gsc, preliminary results suggest that inhibition of p300 activity by E1A also inhibited the synergy observed with coexpression of Sia and Xnr1 (data not shown). These results demonstrate that p300 is a required co-regulator in the transcriptional activation of Gsc by Nodal and Sia/Twn.
Fig. 7.
Recruitment of p300 to organizer gene promoters by Wnt and Nodal pathway effectors. (A)–(C) At the one-cell stage the animal pole was injected with Xnr1 (A), Sia (B) or Twn (C), either alone or together with full length E1A or E1AΔ2-36 as a negative control. Two-cell embryos were injected with the Gsc reporter (100 pg) and CMV-Renilla Luciferase (10 pg) plasmids. Animal explants prepared at the blastula stage were assayed for luciferase activity at the midgastrula stage. Values shown are normalized to Renilla luciferase activity, and represent fold activation of reporter activity in the absence of injected mRNAs. The mean and standard error for three independent experiments are presented. *Indicates p value <0.05 as compared to Xnr1, Sia or Twn activation of Gsc reporter. (D)–(F) Genomic regions recovered by ChIP for myc-p300 (4 ng plasmid injected) either alone or coexpressed with 150 pg GST-Sia or 150 pg GST-Twn or 50 pg Xnr1 were evaluated by QPCR for the (D) Gsc, (E) Cer, or (F) Chd promoters. The white bars represent QPCR for genomic Xmlc2 as a control. The mean fold enrichment (normalized to uninjected samples) and standard error for six independent experiments is presented.*Indicates p<0.05 when compared to myc-p300 alone.
The requirement for p300 in transcriptional activation of the Gsc promoter suggests that p300 is recruited to organizer promoters by Wnt and Nodal effectors. To examine p300 occupancy at organizer promoters, a myc-tagged form of Xenopus p300 was expressed alone or in combination with Sia, Twn or Xnr1. While p300 alone had low occupancy at the Gsc, Cer and Chd promoters, occupancy was significantly increased (2–4-fold) in the presence of Sia, Twn or Xnr1 (Fig. 7D–F). The activation domains of Sia and Twn are within the N-terminal regions of the proteins (data not shown). When Sia or Twn lacking the activation domain are coexpressed with p300, we observe very little p300 occupancy at the Gsc, Cer and Chd promoters (Fig. S3), suggesting that the Sia/Twn activation domain plays an essential role in p300 occupancy at organizer promoters. Therefore, both Wnt and Nodal pathway effectors mediate recruitment of p300 to organizer gene promoters. The recruitment of a common coactivator by both Wnt and Nodal effectors may contribute to the synergistic activation of organizer genes in response to Wnt and Nodal signaling inputs.
Discussion
Formation of the Spemann organizer is dependent on both Wnt and Nodal signals, which are active in the presumptive organizer domain in the blastula prior to the onset of organizer gene transcription (reviewed in De Robertis et al., 2000). Cells within this domain receive both Wnt and Nodal signals, and integrate these inputs to generate temporally and spatially specific transcriptional responses. In the work presented here, we demonstrate that the Wnt and Nodal signaling inputs are received directly at multiple organizer gene promoters, and the physical and functional interactions among the pathway effectors result in strong transcriptional activation of organizer genes. Transcriptional integration is accomplished by the assembly of a stable activating complex, containing Sia, Twn, FoxH1, Smad2/3, p300 and other components, at the promoters of Gsc, Cer, Chd, and likely additional organizer genes. We propose that in the late blastula, cells receiving both Wnt and Nodal inputs integrate these signals at the level of organizer gene promoters, establishing a discrete transcriptional domain that results in the formation of the Spemann organizer.
Functional conservation of Wnt and Nodal response elements in organizer promoters
The Wnt and Nodal pathways cooperate to activate transcription of the organizer genes Gsc, Cer, and Chd utilizing adjacent Wnt and Nodal responsive cis-regulatory elements present in the proximal promoters close to the start site of transcription (this study; Watabe et al., 1995; Yamamoto et al., 2003). Functional conservation of these promoters is apparent in the sequence of the response elements, the proximity of the two elements, and their distance from the start site of transcription. The Sia/Twn response is mediated by defined P3 elements present in each of the promoters (this study; Bae et al., 2011; Laurent et al., 1997; Watabe et al., 1995; Yamamoto et al., 2003). Elements mediating the FoxH1-dependent response to Nodal signals have been identified in close proximity to the Sia/Twn elements of each promoter, but are less conserved in sequence (Fig. S2; Labbe et al., 1998; Zhou et al., 1998). For Gsc, Cer and Chd, the two response elements are in close proximity and are separated by no more than 43 bp (Fig. S2C; Watabe et al., 1995; Yamamoto et al., 2003). And in each case, the pair of response elements has a strikingly similar location within 250 bp of the start site of transcription (−226 for Gsc, −216 for Cer, and −211 for Chd) (this study; Watabe et al., 1995; Yamamoto et al., 2003). These similar features of three organizer gene promoters argue for functional conservation in mediating the transcriptional response to Wnt and Nodal signaling inputs.
Wnt and Nodal effectors synergistically activate organizer gene transcription
At enhancer regions, multiple bound transcription factors may interact to synergistically activate a strong transcriptional output. A number of mechanisms may account for synergy, including cooperative binding to regulatory elements, cooperative recruitment of coactivators, as well as alterations in DNA conformation or nucleosome deposition (reviewed in Levine, 2010). The synergy in activation of Gsc, Cer, and Chd may reflect one or several of these mechanisms. While it remains unclear whether cooperative binding is occurring among the Wnt and Nodal effectors, our data clearly demonstrate that the steady state binding of transcriptional effectors is increased when both Wnt and Nodal pathway effectors occupy these promoters (Figs. 5 and 6). This suggests that the presence of Sia/Twn with FoxH1 and Smad2/3 at organizer gene promoters facilitates enhanced occupancy, which is suggestive of cooperative binding.
The common coactivator and lysine acetyltransferase, p300, is recruited to organizer gene promoters in response to both the Wnt and Nodal pathways (Fig. 7D–F). The role that p300 plays in the synergistic transcription of organizer genes in response to Wnt and Nodal is not yet understood. Overexpression of p300 alone has no apparent phenotype (data not shown), suggesting that increasing p300 levels does not alter expression of target genes. Our results demonstrate a requirement for p300 activity in the expression of a Gsc reporter, as well as increased occupancy of p300 at organizer promoters in the presence of Sia/Twn or Nodal signals (Fig. 7). However, we do not observe further enhancement of p300 occupancy in response to the combination of Wnt and Nodal (data not shown). Perhaps p300 provides a permissive function for transcription, while other recruited coactivators provide an activating function (reviewed in Bedford et al., 2010). Similarly, p300 could be acting as a scaffolding protein, either stabilizing a transcriptional complex of both Wnt and Nodal effectors, or allowing effectors to interact with other coactivators and/or the basal transcriptional machinery (reviewed in Bedford et al., 2010). p300 has also been shown to acetylate transcription factors and histones (reviewed in Bedford et al., 2010). The combined effects of Wnt and Nodal inputs could enhance p300 enzymatic activity, resulting in more extensive modification of local histones or transcription factors and increased transcription. In the context of organizer gene expression, changes in histone H3K9/14 or H4K5/8/12/16 acetylation have not been observed in response to Wnt or Nodal signals (data not shown). However, p300 is also known to modify other lysine residues in histone tails, such as H3K18/27 (Jin et al., 2011), as well as transcription factors (reviewed in Bedford et al., 2010). Activated Smad2/3 is acetylated by p300, which increases transcriptional activity (Inoue et al., 2007; Ross et al., 2006; Tu and Luo, 2007). Preliminary results indicate that Sia is acetylated (data not shown), however, it is unclear what role acetylation might play in Sia-dependent transcription, or whether other Nodal or Wnt effectors might be acetylated in a signal-dependent manner.
It is difficult to relate our experimental induction of organizer gene expression with combinations of Sia/Twn and Nodal to the natural activation of these genes in the intact embryo. We hypothesize that the temporal and spatial restriction of organizer gene expression is due, at least in part, to the presence of Sia, Twn and Nodal effectors in the cells of the organizer. However, the increase in organizer gene expression observed in response to Sia+Xnr1 or Twn+Xnr1 is much greater than the endogenous expression levels of Gsc, Chd or Cer in the whole embryo (Fig. 2). Similarly, expression of the Gsc-luciferase reporter in dorsal blastomeres results in an approximately 10-fold increase in luciferase activity (data not shown), which is much lower than the nearly 36–48-fold induction observed in response to Sia+Xnr1 or Twn+Xnr1. We hypothesize that an increase in ectopic axis formation would be observed in response to low doses of Sia+Xnr1 or Twn+Xnr1, but we were unable to obtain consistent results. This issue might be more clearly addressed by timed loss of function experiments to specifically inhibit Sia/Twn or Nodal activity during organizer formation. It also seems likely that a number of other transcription factors, such as specific repressors of organizer gene expression may be involved in the formation of the organizer domain.
Conserved and non-conserved aspects of organizer gene regulation
In this work we define a molecular mechanism for the transcriptional integration of Wnt and Nodal signals at organizer gene promoters in the Xenopus gastrula. We further propose that this mechanism is likely utilized in multiple vertebrate species to establish the organizer transcriptional domain. Support for the conservation of this mechanism across vertebrates comes from regulatory similarities in organizer formation, organizer gene expression and organizer gene promoter structure (reviewed in De Robertis et al., 2000). Wnt and Nodal signals are essential for organizer gene expression and organizer formation in Xenopus, zebrafish, chick and mouse (Boettger et al., 2001; Conlon et al., 1994; De Robertis et al., 2000; Liu et al., 1999). The functional organization of organizer gene promoters is also conserved to an extent. Most strikingly in the case of Gsc, highly conserved DE and PE elements are present in the Xenopus, zebrafish, chick, mouse, and human Gsc genes (Bae et al., 2011; Watabe et al., 1995). For Cer, conserved response elements are present in Xenopus, zebrafish and mouse, but their organization differs among species (Yamamoto et al., 2003). For Chd, the available genomic information is insufficient for a conclusive comparison. The effectors of Nodal signaling, FoxH1 and Smad2/3, are also utilized in the control of organizer gene transcription in these vertebrate systems (Boettger et al., 2001; Conlon et al., 1994; Hoodless et al., 2001; Nomura and Li, 1998; Waldrip et al., 1998; Weinstein et al., 1998; Zhou et al., 1993).
In contrast to these many conserved features of organizer gene regulation, Sia and Twn are only found in amphibian species, and not in other vertebrates. Given that Wnt inputs and the PE element are conserved across species (Bae et al., 2011; De Robertis et al., 2000; Heasman, 2006; Watabe et al., 1995), it is likely that functional homologs of Sia/Twn, mediating the Wnt-dependent transcriptional activation via the PE, exist in other vertebrate species. Alternatively, Sia/Twn may serve a regulatory function that is unique to organizer gene regulation in Xenopus; if this is the case, conservation of the PE may reflect distinct regulatory requirements among species. It should be noted that Sia/Twn are not the only species-specific regulators of organizer formation. In zebrafish, the transcriptional repressor bozozok is a direct target of the Wnt pathway, is expressed early in organizer formation, and is essential for organizer gene expression and organizer formation (Fekany et al., 1999; Koos and Ho, 1999; Shimizu et al., 2000; Solnica-Krezel and Driever, 2001; Yamanaka et al., 1998). However, as is the case for Sia/Twn, no vertebrate orthologs of bozozok have been identified. Whether functional homologs of Sia/Twn and bozozok exist in other species or whether these factors carry out species-specific regulatory functions remains to be seen. Given the dramatically different sizes and developmental rates for vertebrate embryos, and the non-autonomous function of the organizer, temporal and spatial constraints for organizer formation may differ among species. The non-conserved regulatory components found in Xenopus and zebrafish may be necessary for the unique regulatory demands of organizer formation in distinct species.
A number of important aspects of organizer gene regulation remain undefined. The full composition and structure of the activating protein complex, which forms at organizer gene promoters, is yet to be defined. How the Wnt and Nodal pathway effectors interact physically, what modifications occur in response to cofactor recruitment, and how together these result in enhanced, yet spatially restricted transcriptional output, are important mechanistic questions to pursue. Our results offer a molecular mechanism for the initiation of organizer gene expression in a spatially and temporally precise manner. However, organizer gene expression is a dynamic process with changing regulatory inputs as development proceeds. Within 60 min of the initiation of organizer gene expression it is likely that promoter occupancy and regulatory complex composition changes dramatically as the initiation phase gives way to the maintenance phase or cell lineage specification. Whether the mechanism we propose for the initiation of organizer gene expression is broadly applicable to the many known organizer genes, and across species as well, will require genome wide analyses of effector occupancy, coregulator recruitment, and chromatin modification in several vertebrate species. Ongoing studies such as these will provide profound mechanistic insight at the interface of transcriptional control and embryonic pattern formation.
Conclusion
Cells within the organizer domain receive Wnt and Nodal signals and integrate these signals to generate temporally and spatially specific transcriptional responses. Wnt and Nodal inputs are directly received at multiple organizer gene promoters, and functional interactions among pathway effectors result in strong transcriptional activation of organizer genes. Integration of these signals is accomplished by assembly of an activating complex, consisting of Sia, Twn, FoxH1, Smad2/3, and p300 at the Gsc, Cer, and Chd promoters. In the late blastula, cells receiving both Wnt and Nodal inputs integrate these signals at the level of organizer gene promoters, thus establishing a temporally and spatially distinct transcription domain, resulting in formation of the Spemann organizer.
Supplementary Material
Acknowledgments
We are grateful to Doug Epstein, Jon Epstein and Peter Klein for critical reading of the manuscript. This work was supported by a grant from the NIH (T32-HD007516) to C.D.R., by grants from the NIH (R01-HD43996) and the James D. Shaw and Dorothy Shaw Fund of the Greater Milwaukee Foundation to M.D.S., and by grants from the NIH (R01-GM64768) and NSF (IOS-0718961) to D.S.K.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2012.05.018.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Reid CD, Kessler DS. Siamois and Twin are redundant and essential in formation of the Spemann organizer. Dev Biol. 2011;352:367–381. doi: 10.1016/j.ydbio.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford DC, Kasper LH, Fukuyama T, Brindle PK. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics. 2010;5(9–15) doi: 10.4161/epi.5.1.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Wright CV, De Robertis EM, Cho KW. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- Blythe SA, Reid CD, Kessler DS, Klein PS. Chromatin immunoprecipitation in early Xenopus laevis embryos. Dev Dyn. 2009;238:1422–1432. doi: 10.1002/dvdy.21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Knoetgen H, Wittler L, Kessel M. The avian organizer. Int J Dev Biol. 2001;45:281–287. [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D. A beta-catenin/XTcf-3 complex binds to the Siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev. 1997;11:2359–2370. doi: 10.1101/gad.11.18.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Kimelman D. Activation of Siamois by the Wnt pathway. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development. 1996;122:3055–3065. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubock MJ, Whitman M. A transcriptional partner for MAD proteins in TGF-beta signalling. Nature. 1996;383:691–696. doi: 10.1038/383691a0. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Crease DJ, Dyson S, Gurdon JB. Cooperation between the activin and Wnt pathways in the spatial control of organizer gene expression. Proc Nat Acad Sci USA. 1998;95:4398–4403. doi: 10.1073/pnas.95.8.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleka MJ, Kessler DS. Siamois cooperates with TGFbeta signals to induce the complete function of the Spemann–Mangold organizer. Int J Dev Biol. 2001;45:241–250. [PubMed] [Google Scholar]
- Fan MJ, Gruning W, Walz G, Sokol SY. Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc Nat Acad Sci USA. 1998;95:5626–5631. doi: 10.1073/pnas.95.10.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MJ, Sokol SY. A role for Siamois in Spemann organizer formation. Development. 1997;124:2581–2589. doi: 10.1242/dev.124.13.2581. [DOI] [PubMed] [Google Scholar]
- Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Mymryk JS. Adenovirus-5 E1A: paradox and paradigm. Nat Rev Mol Cell Biol. 2002;3:441–452. doi: 10.1038/nrm827. [DOI] [PubMed] [Google Scholar]
- Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and under-expression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana JL. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. doi: 10.1101/gad.881501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoodless PA, Tsukazaki T, Nishimatsu S, Attisano L, Wrana JL, Thomsen GH. Dominant-negative Smad2 mutants inhibit activin/Vg1 signaling and disrupt axis formation in Xenopus. Dev Biol. 1999;207:364–379. doi: 10.1006/dbio.1998.9168. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, Onozaki K, Hayashi H. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hanafusa H, Matsumoto K, De Robertis EM, Kuroda H. Expression of Siamois and Twin in the blastula Chordin/Noggin signaling center is required for brain formation in Xenopus laevis embryos. Mech Dev. 2008;125:58–66. doi: 10.1016/j.mod.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, Wang C, Brindle PK, Dent SY, Ge K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30:249–262. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Shi Y, He X. Neuralization of the Xenopus embryo by inhibition of p300/CREB-binding protein function. J Neurosci. 1999;19:9364–9373. doi: 10.1523/JNEUROSCI.19-21-09364.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Nat Acad Sci USA. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodjabachian L, Lemaire P. Siamois functions in the early blastula to induce Spemann’s organiser. Mech Dev. 2001;108:71–79. doi: 10.1016/s0925-4773(01)00484-1. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L, Lemaire P. Role of Siamois before and during Gastrulation. In: Stern C, editor. Gastrulation: From Cells to Embryo. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2004. pp. 609–617. [Google Scholar]
- Kofron M, Puck H, Standley H, Wylie C, Old R, Whitman M, Heasman J. New roles for FoxH1 in patterning the early embryo. Development. 2004;131:5065–5078. doi: 10.1242/dev.01396. [DOI] [PubMed] [Google Scholar]
- Koos DS, Ho RK. The nieuwkoid/dharma homeobox gene is essential for bmp2b repression in the zebrafish pregastrula. Dev Biol. 1999;215:190–207. doi: 10.1006/dbio.1999.9479. [DOI] [PubMed] [Google Scholar]
- Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L. Smad2 and Smad3 positively and negatively regulate TGF beta-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbacher U, Cho KW. The Xenopus homeobox gene twin mediates Wnt induction of goosecoid in establishment of Spemann’s organizer. Development. 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Levine M. Transcriptional enhancers in animal development and evolution. Curr Biol. 2010;20:R754–R763. doi: 10.1016/j.cub.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–437. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Leyns L, Bhattacharyya S, Grunz H, Bouwmeester T, De Robertis EM. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Cheung E, Petrakis TG, Howell M, Kraus WL, Hill CS. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 2006;25:4490–4502. doi: 10.1038/sj.emboj.7601332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka Y, Hagemann AI, Piepenburg O, Smith JC. Nuclear accumulation of Smad complexes occurs only after the midblastula transition in Xenopus. Development. 2007;134:4209–4218. doi: 10.1242/dev.010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development. 1997;124:3293–3302. doi: 10.1242/dev.124.17.3293. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schohl A, Fagotto F. Beta-catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yamanaka Y, Ryu SL, Hashimoto H, Yabe T, Hirata T, Bae YK, Hibi M, Hirano T. Cooperative roles of Bozozok/Dharma and Nodal-related proteins in the formation of the dorsal organizer in zebrafish. Mech Dev. 2000;91:293–303. doi: 10.1016/s0925-4773(99)00319-6. [DOI] [PubMed] [Google Scholar]
- Skirkanich J, Luxardi G, Yang J, Kodjabachian L, Klein PS. Transcription before the midblastula transition is required for mesendoderm induction in Xenopus. Dev Biol. 2011;357:478–491. doi: 10.1016/j.ydbio.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, Driever W. The role of the homeodomain protein Bozozok in zebrafish axis formation. Int J Dev Biol. 2001;45:299–310. [PubMed] [Google Scholar]
- Steiner AB, Engleka MJ, Lu Q, Piwarzyk EC, Yaklichkin S, Lefebvre JL, Walters JW, Pineda-Salgado L, Labosky PA, Kessler DS. FoxD3 regulation of Nodal in the Spemann organizer is essential for Xenopus dorsal mesoderm development. Development. 2006;133:4827–4838. doi: 10.1242/dev.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu AW, Luo K. Acetylation of Smad2 by the co-activator p300 regulates activin and transforming growth factor beta response. J Biol Chem. 2007;282:21187–21196. doi: 10.1074/jbc.M700085200. [DOI] [PubMed] [Google Scholar]
- Waldrip WR, Bikoff EK, Hoodless PA, Wrana JL, Robertson EJ. Smad2 signaling in extraembryonic tissues determines anterior–posterior polarity of the early mouse embryo. Cell. 1998;92:797–808. doi: 10.1016/s0092-8674(00)81407-5. [DOI] [PubMed] [Google Scholar]
- Watabe T, Kim S, Candia A, Rothbacher U, Hashimoto C, Inoue K, Cho KW. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Whitman M. FAST-1 is a key maternal effector of mesoderm inducers in the early Xenopus embryo. Development. 1999;126:5621–5634. doi: 10.1242/dev.126.24.5621. [DOI] [PubMed] [Google Scholar]
- Weinstein M, Yang X, Li C, Xu X, Gotay J, Deng CX. Failure of egg cylinder elongation and mesoderm induction in mouse embryos lacking the tumor suppressor smad2. Proc Nat Acad Sci USA. 1998;95:9378–9383. doi: 10.1073/pnas.95.16.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Melton DA. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr Biol. 1994;4:676–686. doi: 10.1016/s0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Wylie C, Kofron M, Payne C, Anderson R, Hosobuchi M, Joseph E, Heasman J. Maternal beta-catenin establishes a ‘dorsal signal’ in early Xenopus embryos. Development. 1996;122:2987–2996. doi: 10.1242/dev.122.10.2987. [DOI] [PubMed] [Google Scholar]
- Yaklichkin S, Steiner AB, Lu Q, Kessler DS. FoxD3 and Grg4 physically interact to repress transcription and induce mesoderm in Xenopus. J Biol Chem. 2007;282:2548–2557. doi: 10.1074/jbc.M607412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Hikasa H, Ono H, Taira M. Molecular link in the sequential induction of the Spemann organizer: direct activation of the cerberus gene by Xlim-1, Xotx2, Mix. 1, and Siamois, immediately downstream from Nodal and Wnt signaling. Dev Biol. 2003;257:190–204. doi: 10.1016/s0012-1606(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tan C, Darken RS, Wilson PA, Klein PS. Beta-catenin/Tcf-regulated transcription prior to the midblastula transition. Development. 2002;129:5743–5752. doi: 10.1242/dev.00150. [DOI] [PubMed] [Google Scholar]
- Yao J, Kessler DS. Goosecoid promotes head organizer activity by direct repression of Xwnt8 in Spemann’s organizer. Development. 2001;128:2975–2987. doi: 10.1242/dev.128.15.2975. [DOI] [PubMed] [Google Scholar]
- Zhou S, Zawel L, Lengauer C, Kinzler KW, Vogelstein B. Characterization of human FAST-1, a TGF beta and activin signal transducer. Mol Cell. 1998;2:121–127. doi: 10.1016/s1097-2765(00)80120-3. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.