Abstract
Background and Purpose
Arterial stiffness is a measure of subclinical cardiovascular disease (CVD) and increases with age. This study examines the association between arterial stiffness and cognitive decline in a cohort of older adults.
Methods
2,488 subjects with baseline measure of arterial stiffness (mean age, 74.2 years; 52.3% women) were prospectively followed over 9 years in the Health, Aging and Body Composition study. Arterial stiffness was measured as pulse wave velocity (PWV) and analyzed in tertiles. Cognitive function was assessed using the Modified Mini-Mental State Exam (3MS) at baseline and repeated at years 3, 5, 8 and 10. Lower 3MS scores indicate worse function. We fit linear mixed models to examine longitudinal changes in cognitive function over the 9 years of follow-up and logistic regression models, restricted to 1,331 participants, to examine cognitive impairment defined as a decrease of ≥5 points after 9 years. We adjusted for socio-demographics, Apoe4 and CVD risk factors.
Results
The annual decrease in 3MS scores was 0.30 points at low PWV (95%CI=−0.37;-0.22), 0.46 points at middle PWV (95%CI=−0.54;-0.39) and 0.45 points at high PWV (95%CI=−0.53;-0.38), from fully-adjusted linear mixed models. In fully-adjusted models, the odds of cognitive impairment after 9 years of follow-up was 40% greater for subjects with middle PWV (OR=1.40; 95% CI=1.03; 1.92) and 59% greater for subjects with high PWV (OR=1.59; 95% CI=1.16; 2.18), compared to low PWV.
Conclusions
High arterial stiffness was modestly associated with cognitive decline and impairment. Interventions to prevent arterial stiffness may be effective in delaying cognitive decline.
Introduction
Arterial stiffness is a measure of subclinical cardiovascular disease risk 1–4 that progressively increases with age.5,6 Arterial stiffness largely contributes to systolic hypertension, the most common form of hypertension among older adults.7,8 Independent of traditional cardiovascular disease risk factors, arterial stiffness has been linked to damages in the central pulse pressure9,10 resulting in cerebral microvascular disease11–13 and changes in the functioning of the frontal-subcortical regions of the brain which may in turn influence cognitive decline.11–15
Cross-sectional studies have demonstrated an association between high PWV and poor cognitive performance.3,16–18 However, the prospective association between PWV and cognitive decline has not yet been established and findings have been inconsistent.8,19–22 For example, results from the Rotterdam study did not provide evidence for an association between PWV and cognitive decline.19 On the contrary, results from the Baltimore Longitudinal Study of Aging (BLSA) suggested significant associations between higher PWV and more rapid cognitive decline on specific cognitive domains such as working memory but not on tests of global cognitive function.8
Using data from the Health, Aging and Body Composition Study (Health ABC), a biracial prospectively-followed cohort of older adults who were initially free of functional limitations, we examined associations between baseline arterial stiffness and change in cognitive function over 9 years of follow-up.
Materials and Methods
Study Population
Participants in this analysis were from Health ABC, a prospective cohort study of 3,075 community-dwelling elders aged 70–79 years at baseline in 1997. Health ABC is a biracial cohort of whites and blacks. Potential participants were either living in Memphis, TN or Pittsburgh, PA. Further details on the study design and recruitment strategy have been published elsewhere.23 Clinical and biologic data were collected annually from year one through year six and biennially through year 10. A total of 2,488 participants had a measurement of arterial stiffness at baseline and thus constituted our analytic cohort.
Assessment of cognitive function
Cognitive function was assessed using the Modified Mini Mental State Examination (3MS), a 100 point assessment of global cognitive function.24 The 3MS has greater sensitivity and specificity and fewer floor and ceiling effects for detecting impairment than other measures such as the Mini Mental State Exam (MMS) and has excellent test-re-test properties.25 Lower scores denote worse cognitive function. The 3MS was administered at baseline (year 1) and in years 3, 5, 8 and 10.
Assessment of arterial stiffness
Pulse Wave Velocity (PWV), a measure of arterial stiffness, was assessed at year 1 examination using non-directional transcutaneous Doppler flow probes (model 810-a, 10 MHZ; Parks Medical Electronics, Aloha, OR). PWV is regarded as the gold standard measure of arterial stiffness and a more direct measure than pulse pressure.26,27 For each participant, three runs, each with a minimum of 10 pairs of simultaneous flow waves from the right carotid and right femoral arteries were recorded and then averaged. The distance between the carotid and femoral arteries was measured above the body surface using a metal tape.28 The time from the R wave on the electrocardiogram to the foot of the pressure wave was also calculated. Both components were then used to calculate PWV as the distance between the carotid and femoral arteries divided by the time differential for the pressure wave to reach both arteries.28 Our measure of PWV shows an interclass correlation of 0.88 between sonographs and 0.84 between readers. The latter was based on repeated PWV measures from a random sample of 14 participants.28 Higher PWV (in cm/sec) indicates greater stiffness of the vessels. Compared to participants with PWV measure, those without PWV measure were younger, had lower education and lower baseline 3MS score but did not differ with regard to cardiovascular risk factors such as Body Mass Index (BMI), prevalence of hypertension and type-2 diabetes.
Covariates
We examined other covariates that were measured during the baseline examination. Participants self-reported their race/ethnicity (white or black), age, sex and years of education that they have completed (less than high school, high school graduate and postsecondary). Participants also reported their alcohol consumption and their current smoking status. Body mass index (BMI in kg/m2) was determined from measured weight and height and calculated as weight in kg/height in meters squared. Levels of lipids including high-density lipoprotein (HDL, mg/dl), low-density lipoprotein (LDL, mg/dl), and triglycerides (mg/dl) were measured from fasting blood drawn at baseline. Collected blood was processed then frozen samples were shipped to Health ABC laboratory for analysis. The presence of type-2 diabetes was ascertained as a combination of self-report of a physician diagnosis, use of diabetes medication, or the following lab value (fasting glucose level ≥126 mg/dl29 or a 2-hour oral glucose tolerance test (OGTT) >200 mg/dL). History of myocardial infarction was ascertained based on self-report of a physician diagnosis or hospitalization. Systolic and diastolic blood pressures were measured twice then averaged. Hypertension was ascertained as a self-report of a physician diagnosis, use of medication, a systolic blood pressure >140 mm Hg, or a diastolic blood pressure >90 mm Hg.30 Mean arterial blood pressure was determined from measured systolic and diastolic blood pressures as diastolic pressure + 1/3 (systolic pressure – diastolic pressure). Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies Depression Scale (CESD) ranging from 0 to 60 with higher scores denoting worse depressive symptoms. Having elevated depressive symptoms was defined as a CES-D score ≥16. The CES-D has high validity and reliability when administered to community-dwelling older adults.31,32
Statistical Analyses
To allow for non-linear response and for ease of interpretation we categorized PWV into tertiles, the main predictor of interest. In bivariate analyses, we used t-test and analysis of variance to examine the distribution of baseline covariates across tertiles of PWV (Table 1). We examined the longitudinal associations between tertiles of PWV and cognitive decline over the 9 years of follow-up using linear mixed models with random slopes and intercepts33,34 (Table 2). In linear mixed models, the outcome includes all available repeated measures of 3MS. We operationalized time as age at time of cognitive assessment which we then grand-mean centered (mean age of 74.2 years). We included a PWV by age interaction to estimate PWV-related cognitive decline. We reported the annual change in the 3MS scores according to tertiles of PWV (estimate and 95%CI). In Figure 1, we illustrated the multivariate-adjusted associations between tertiles of PWV and longitudinal change in 3MS scores over time (as age), based on the results from linear mixed models. We also fit logistic regression models (Table 3) to examine the association between tertiles of PWV and cognitive impairment which we defined as a decrease of 5 or more points on the 3MS between baseline and year 10 exams (N=1,331).35 We reported odds ratios (OR) and 95% confidence intervals (CI). In multivariate models, we adjusted for potential confounders including race/ethnicity, sex, education, APOE4 allele, and traditional cardiovascular disease risk factors such as BMI, type-2 diabetes, hypertension and mean arterial blood pressure. Selection of covariates was based on prior literature as well as the association of covariates with PWV and cognitive function. We conducted all analyses using SAS v.9.2.36
Table 1.
Baseline characteristics of the study population by tertile of pulse wave velocity, Health, Aging and Body Composition study (N=2,488)
| Pulse Wave Velocity, cm/s* | |||||
|---|---|---|---|---|---|
| Characteristics | Overall | Low | Middle | High | P Value |
| Age, mean (SD), y | 74.2 (2.9) | 73.9 (2.8) | 74.3 (2.9) | 74.4 (2.8) | <0.01 |
| Female, No. (%) | 1302 (52.3) | 481 (58.0) | 425 (51.2) | 396 (47.8) | <0.01 |
| Black | 1002 (40.3) | 296 (35.7) | 348 (41.9) | 358 (43.2) | <0.01 |
| < High School education, No. (%) | 575 (23.1) | 151 (18.2) | 222 (26.8) | 202 (24.4) | <0.01 |
| >1 alcoholic drink/day, No. (%) | 196 (7.9) | 74 (8.9) | 60 (7.2) | 62 (7.5) | 0.38 |
| Current smoker, No. (%) | 254 (10.2) | 75 (9.1) | 89 (10.7) | 90 (10.9) | 0.39 |
| BMI, means (SD), Kg/m2 | 27.4 (4.8) | 26.5 (4.6) | 27.9 (4.8) | 27.8 (4.8) | <0.01 |
| Low Density Lipoprotein, mg/dl | 121.8 (34.7) | 121.3 (33.0) | 121.9 (34.9) | 122.1 (36.1) | 0.87 |
| High Density Lipoprotein, mg/dl | 54.5 (17.0) | 56.7 (16.7) | 53.6 (16.7) | 53.2 (17.5) | <0.01 |
| Triglycerides, mg/dl | 139.9 (84.6) | 134.7 (93.1) | 142.8 (83.7) | 142.0 (76.0) | 0.10 |
| Arterial BP, mean (SD), mm Hg | 93.4 (12.7) | 91.0 (11.7) | 93.4 (12.9) | 95.8 (13.1) | <0.01 |
| Diabetes, No. (%) | 567 (22.8) | 121 (14.6) | 188 (22.7) | 258 (31.1) | <0.01 |
| Myocardial Infarction, No. (%) | 300 (12.1) | 87 (10.5) | 100 (12.1) | 113 (13.6) | 0.15 |
| Hypertension, No. (%) | 1512 (60.8) | 414 (49.9) | 506 (61.0) | 592 (71.4) | <0.01 |
| CESD ≥ 16, No. (%) | 93 (3.7) | 37 (4.5) | 33 (4.0) | 23 (2.8) | 0.18 |
| Apolipoprotein 4, No. (%) | 667 (26.8) | 214 (27.0) | 233 (29.6) | 220 (27.5) | 0.46 |
| 3MS score, mean (SD) | 90.4 (8.1) | 91.2 (7.9) | 90.2 (8.2) | 89.6 (8.2) | <0.01 |
Arterial BP indicates arterial blood pressure; BMI, body mass index; CESD, center for epidemiologic studies depression scale; 3MS: Modified Mini Mental State Examination.
PWV data were log transformed and categorized into tertiles: Low: 5.7cm/s to 6.5cm/s; middle: 6.5cm/s to 6.9cm/s; high: 6.9cm/s to 8.0cm/s
Table 2.
Annual change in the Modified Mini Mental State Exam according to tertile of pulse wave velocity from linear mixed models, Health Aging and Body Composition study
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| PWV tertile | |||
| Low | −0.32 (−0.40; −0.25) | −0.31 (−0.39; −0.24) | −0.30 (−0.37; −0.22) |
| Middle | −0.49 (−0.56; −0.41) | −0.47 (−0.55; −0.40) | −0.46 (−0.54; −0.39) |
| High | −0.45 (−0.53; −0.37) | −0.45 (−0.53; −0.38) | −0.45 (−0.53; −0.38) |
PWV indicates pulse wave velocity
Model 1 was age-adjusted and included PWV by age interactions; Model 2 was additionally adjusted for race, sex and education; Model 3 was additionally adjusted for APOE4, body mass index, high density lipoprotein, diabetes, hypertension, and mean arterial blood pressure
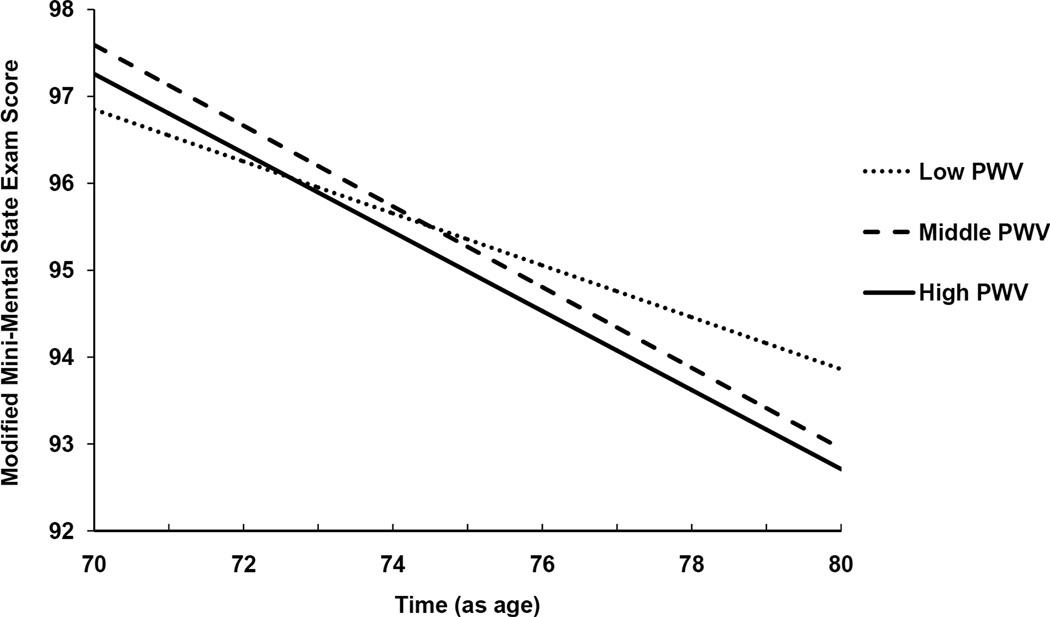
Figure 1.
Multivariate adjusted longitudinal changes in performance on the Modified Mini Mental State Exam (3MS) as a function of tertiles of Pulse Wave Velocity (PWV, in centimeters per second). The overall p-value for tertiles of PWV by age interaction is <0.01. These associations were assessed in linear mixed models, adjusted for age, sex, race/ethnicity, education, Apoe4, body mass index, diabetes, hypertension, and mean arterial blood pressure. Lower scores on the 3MS indicate worse cognitive performance.
Table 3.
Risk of cognitive impairment† on the Modified Mini Mental State Exam by tertile of pulse wave velocity after 9 years of follow-up, Health Aging and Body Composition study
| Model 1* | Model 2* | Model 3* | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| PWV tertile | |||
| Low (ref) | 1 | 1 | 1 |
| Middle | 1.50 (1.13; 1.99) | 1.34 (0.99; 1.79) | 1.40 (1.03; 1.92) |
| High | 1.63 (1.22; 2.19) | 1.48 (1.09; 2.00) | 1.59 (1.16; 2.18) |
PWV indicates pulse wave velocity
Model 1 was unadjusted; Model 2 adjusted for age, sex, race, and education; Model 3 was additionally adjusted for APOE4, body mass index, high density lipoprotein, diabetes, hypertension, and mean arterial blood pressure
Cognitive impairment is defined as a decline of 5 or more points
Results
Higher PWV was associated with greater age at enrollment, being male, black, having less education, higher BMI, lower HDL, higher mean arterial blood pressure, type-2 diabetes, myocardial infarction, hypertension, and lower cognitive score (Table 1).
Results from linear mixed models of the associations between tertiles of PWV and change in cognitive scores over the 9 years of follow-up are presented in Table 2. All models included PWV tertiles, age and PWV by age interactions indicating PWV-related cognitive decline. We presented the results as annual change in the 3MS score associated with tertiles of PWV. In model 1, the annual decrease in 3MS scores was 0.32 points for participants with low PWV (95%CI=−0.40; −0.25), annual decrease of 0.49 points at middle PWV (95%CI=−0.56; −0.41) and an annual decrease of 0.45 points at high PWV (95%CI=−0.53; −0.37). Adjusting for socio-demographics (model 2), Apoe4 allele and CVD risk factors (model 3) slightly attenuated the associations but remained significant.
The multivariable-adjusted associations between tertiles of PWV and age-related cognitive decline are illustrated in Figure 1, based on results from linear mixed models. For example over 9 years of follow-up, participants with the high and middle tertiles of PWV experienced an average decline in 3MS scores of about 4.5 points versus 2.9 points for those with the lowest tertile of PWV.
We performed exploratory analysis to examine whether higher PWV was associated with decline on specific cognitive domains of the 3MS (data not shown). We created composite scores pertaining to four cognitive domains: memory, executive function, language, and visuo-spatial. Our findings suggested that higher PWV was modestly associated with greater decline on visuo-spatial and language tasks (p<0.05) but not on executive function or memory tasks.
Results from logistic regression models of the associations between tertiles of PWV and odds of cognitive impairment after 9 years of follow-up included a total of 1,331 participants who completed both baseline and year 10 cognitive assessments (Table 3). Of the 1,331 participants, a total of 409 (30.7%) experienced a decrease of 5 or more points in their 3MS score at year 10 and thus are considered cognitively impaired. In unadjusted models and compared to subjects with low PWV, those with middle PWV had 50% greater odds of cognitive impairment (OR=1.50; 95% CI=1.13; 1.99) and those with high PWV had 63% greater odds of cognitive impairment (OR=1.63; 95% CI=1.22; 2.19). Adjusting for socio-demographics (model 2), Apoe4 allele and cardiovascular risk factors (model 3) slightly attenuated the associations but remained significant. In fully-adjusted models and compared to participants with low PWV, those with middle PWV had 40% greater odds of cognitive impairment (OR=1.40; 95% CI=1.03; 1.91) and those with high PWV had 59% greater odds of impairment (OR=1.59; 95% CI=1.16; 2.19).
Discussion
Our findings confirm that higher arterial stiffness, as measured by PWV, is associated with faster rates of cognitive decline over 9 years of follow-up and with greater odds of cognitive impairment among community-dwelling older adults, beyond traditional cardiovascular risk factors such as BMI, type-2 diabetes, hypertension, and mean arterial blood pressure.
Several pathways linking arterial stiffness and cognitive decline have been postulated. First, when arteries undergo stiffness they often result in damages to pressure pulsatility. The increase in central pulse pressure results in hemodynamic stress in the heart as well as in high-flow end-organs to which it is transmitted such as the brain. The high levels of central pulse pressure in the brain result in structural changes and dysfunction to its microcirculation.9,11 Second, high pulse pressures may result in structural changes to cerebral blood vessels which may in turn interfere with the transport of important nutrients to the brain as well as interfere with the clearance of toxic byproducts out of the brain.37 Third, recent brain imaging studies have increasingly linked arterial stiffness to cerebral microvascular disease and changes in the functioning of the frontal-subcortical regions of the brain, such as white matter hyperintensities, which in turn are associated with cognitive impairment.11–15 Finally, arterial stiffness has also been demonstrated as an independent predictor of CVD events and CVD risk factors1–4 which in turn are important predictors of cognitive decline.
Our study findings were consistent with prior studies demonstrating a cross-sectional association between arterial stiffness and cognitive impairment.3,16–18 Importantly however, only a handful studies documented the longitudinal associations between arterial stiffness and cognitive decline and reported inconsistent conclusions.8,19–22 Our study findings were inconsistent with those among older adults of the Rotterdam study which did not provide evidence for a prospective association between PWV and cognitive decline, measured across 2 study time points only.19 Our findings were in accordance with those from the BLSA study (N=582) which found significant associations between higher PWV and more rapid cognitive decline on the Blessed Information Memory Concentration Test (working memory test). However, results from the BLSA study did not provide evidence for an association between PWV and tests of global cognitive function.8 Previous results from a sub sample (N=552) of older adults in Health ABC provided an association between higher PWV and greater cognitive decline on the psychomotor speed only but not on tests of memory and global cognitive function.21 Finally, recent findings from the PARTAGE study, among 873 institutionalized patients aged 80 and above living in France and in Italy, showed an association between higher tertile of PWV and greater cognitive decline on the Mini-Mental Status Examination over 1-year follow-up only.22 Further results from our exploratory analyses suggested that higher PWV, suggestive of increased vascular risk, may be more associated with deteriorations in language and visual-spatial tasks than in memory or executive function tasks.
Prior literature suggests that blacks have greater arterial stiffness,38 higher prevalence of hypertension39 and exhibit lower rates of blood pressure control40 than non-Hispanic whites. While prior epidemiological studies such as BLSA included whites and blacks, their small sample size with available PWV measurement (N=582) may have hindered their statistical power to examine any racial/ethnic-related disparity. As an exploratory analysis, we examined whether the association between arterial stiffness and cognitive decline differed for our white (n=1,486) and black (n=1,002) participants. Although blacks had lower cognitive scores at baseline than whites, the rates of cognitive decline associated with arterial stiffness did not differ by race/ethnicity (p-value >0.05). Future studies need to confirm our findings.
Our study is the first population-based study to examine the association between arterial stiffness and cognitive decline in a large cohort of community-dwelling older adults who underwent more than two repeated cognitive assessments and over a long period of follow-up. Furthermore, our study extends the literature by having examined for racial/ethnic-related disparity in the longitudinal association of arterial stiffness and cognitive decline and which other studies did not examine, possibly due to lack of statistical power. As such, our study has several strengths including the large sample size of a biracial community-dwelling cohort of older adult whites and blacks who were very well-functioning at baseline. Participants in this study were prospectively followed over a long study period with repeated measures of cognitive testing. Finally, PWV is regarded as the gold standard for measuring arterial stiffness and thus provides a great strength to our study.26,27 Our study has some limitations that are worth noting. PWV was only measured at baseline which limited our ability to examine the associations of concurrent changes in PWV and cognitive function over time. Given the longitudinal nature of the study, there was attrition due to mortality or other competing risks. Premature mortality, particularly among those with the highest PWV tertile, may have resulted in an attenuation of our results (dropout due to mortality was 37%, 45% and 49% in participants with low, middle and high tertile of PWV, respectively). Finally, given that our cohort was very-well functioning at baseline, potential ceiling effects may have hindered our ability to detect much change in cognitive scores over time.
Summary/Conclusions
In this cohort of community-dwelling older adults, we provided evidence of an association between higher arterial stiffness and greater cognitive decline and impairment, beyond traditional cardiovascular risk factors. PWV may therefore constitute a useful and non-invasive measure in predicting the risk of cognitive decline among older adults. Our results from this longitudinal study suggest that interventions to prevent arterial stiffness may be effective in delaying cognitive decline. Further prospective studies need to confirm our longitudinal results of the association of arterial stiffness and cognitive decline in older adults.
Acknowledgments
Sources of funding
This work was supported by the American Heart Association/American Stroke Association /American Brain Foundation Lawrence M. Brass, M.D. Stroke Research Postdoctoral Fellowship (Dr. Zeki Al Hazzouri) and by the National Institute on Aging (NIA) grant number AG031155 (Dr. Yaffe). This work was also supported by NIA contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Adina Zeki Al Hazzouri: None
Anne B Newman:
Research Grant: Health Aging and Body Composition Study- NIA Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106
Eleanor Simonsick: None
Kaycee M Sink: None
Kim Sutton Tyrrell: None
Nora Watson: None
Suzanne Satterfield:
Research Grant: UCSF subcontract for NIA-N01-AG-6-2106
University of Pittsburgh subcontract for NINR-R01-NR012459
Tamara Harris: None
Kristine Yaffe:
Research Grant: National Institutes of Health; Alzheimer's Association; AHAF; Department of Defense
Honoraria: Novartis Inc
Consultant/Advisory Board: OSMB for Takeda; National Institute on Aging; Pfizer
References
- 1.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer's Abeta. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 2.Johnson TE. Recent results: biomarkers of aging. Exp. Gerontol. 2006;41:1243–1246. doi: 10.1016/j.exger.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marioni RE, Strachan MW, Reynolds RM, Lowe GD, Mitchell RJ, Fowkes FG, et al. Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:710–713. doi: 10.2337/db09-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am. J. Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 7.Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am. J. Hypertens. 2004;17:39S–48S. doi: 10.1016/j.amjhyper.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J. Appl. Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 11.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 12.Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke; a journal of cerebral circulation. 2009;40:1229–1236. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- 13.Ohmine T, Miwa Y, Yao H, Yuzuriha T, Takashima Y, Uchino A, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:75–81. doi: 10.1291/hypres.31.75. [DOI] [PubMed] [Google Scholar]
- 14.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol. Aging. 2002;23:421–431. doi: 10.1016/s0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara M, Matsumura K, Ansai T, Takata Y, Sonoki K, Akifusa S, et al. Prediction of cognitive function by arterial stiffness in the very elderly. Circ J. 2006;70:756–761. doi: 10.1253/circj.70.756. [DOI] [PubMed] [Google Scholar]
- 17.Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J. Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- 18.Triantafyllidi H, Arvaniti C, Lekakis J, Ikonomidis I, Siafakas N, Tzortzis S, et al. Cognitive impairment is related to increased arterial stiffness and microvascular damage in patients with never-treated essential hypertension. Am. J. Hypertens. 2009;22:525–530. doi: 10.1038/ajh.2009.35. [DOI] [PubMed] [Google Scholar]
- 19.Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke; a journal of cerebral circulation. 2007;38:888–892. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 20.Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J. Hypertens. 2007;25:1035–1040. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- 21.Watson NL, Sutton-Tyrrell K, Rosano C, Boudreau RM, Hardy SE, Simonsick EM, et al. Arterial Stiffness and Cognitive Decline in Well-Functioning Older Adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2011;66:1336–1342. doi: 10.1093/gerona/glr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, et al. Pulse Wave Velocity is Associated With 1-Year Cognitive Decline in the Elderly Older than 80 Years: The PARTAGE Study. J Am Med Dir Assoc. 2012;13:239–243. doi: 10.1016/j.jamda.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol. Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the MMSE and 3MS. Arch. Clin. Neuropsychol. 2005;20:485–503. doi: 10.1016/j.acn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J. Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 28.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 29.The American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- 30.American Heart Association. High blood pressure AHA recommendations. [Accessed March 19, 2010]; http://www.americanheart.org/presenter.jhtml?identifier=4623.
- 31.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol. Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman JA, Mast BT, Miles T, Markides KS. Vascular risk and depression in the Hispanic Established Population for the Epidemiologic Study of the Elderly (EPESE) Int. J. Geriatr. Psychiatry. 2009;24:409–416. doi: 10.1002/gps.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear and Mixed Models. Second ed. New York: Wiley; 2008. [Google Scholar]
- 34.West BT, Kathleen WB, Andrzej GT. Linear Mixed Models: A practical guide using statistical software. Taylor & Francis Group, LLC; 2007. pp. 273–328. [Google Scholar]
- 35.Andrew MK, Rockwood K. A five-point change in Modified Mini-Mental State Examination was clinically meaningful in community-dwelling elderly people. J. Clin. Epidemiol. 2008;61:827–831. doi: 10.1016/j.jclinepi.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 36.SAS Institute. SAS statistical software, release 9.2. Cary, NC: 2005. [computer program] [Google Scholar]
- 37.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke; a journal of cerebral circulation. 2009;40:S40–S44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heffernan KS, Jae SY, Fernhall B. Racial differences in arterial stiffness after exercise in young men. Am. J. Hypertens. 2007;20:840–845. doi: 10.1016/j.amjhyper.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, et al. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) Am. J. Hypertens. 2004;17:963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Delgado J, Jacobs EA, Lackland DT, Evans DA, de Leon CF. Differences in blood pressure control in a large population-based sample of older african americans and non-Hispanic whites. J. Gerontol. A. Biol. Sci. Med. Sci. 2012;67:1253–1258. doi: 10.1093/gerona/gls106. [DOI] [PMC free article] [PubMed] [Google Scholar]