Abstract
The release of vesicular protons during exocytosis causes a feedback inhibition of Ca2+ channels in photoreceptor terminals; however, the effect of this inhibition on subsequent exocytosis has not been studied. Here we show that a similar L-type Ca2+ channel inhibition occurs in bipolar cell terminals in slices of goldfish retina, and we investigate the effect that this has on subsequent exocytosis with membrane capacitance measurements. We find that transient Ca2+ current inhibition is correlated with exocytosis and modulated by the concentration of extracellular pH buffer. Ca2+ current inhibition is negligible in acutely dissociated terminals, demonstrating the importance of an intact synaptic cleft. The sensitivity of bipolar cell Ca2+ currents to extracellular pH was assessed: channel conductance is reduced and activation is shifted to more positive potentials by acidification. The effect of Ca2+ current inhibition on subsequent exocytosis was investigated by measuring paired-pulse depression. Under conditions in which there is a large amount of inhibition of Ca2+ influx, the degree of paired-pulse depression is significantly reduced. Finally, we show that under physiological (bicarbonate) buffering conditions, pronounced Ca2+ current inhibition occurs after exocytosis (∼60% peak inhibition), which can decrease subsequent exocytosis during single depolarizations. We estimate that exocytosis is accompanied by a transient change in synaptic cleft pH from 7.5 to ∼6.9. We suggest that this effect serves as an activity-dependent modulator of exocytosis at ribbon-type synapses where a large and compact coterie of vesicles can fuse at each active zone.
Keywords: goldfish retina, bipolar cell, presynaptic terminal, Ca2+ current, synaptic ribbon, vesicular pH, paired-pulse depression, exocytosis, membrane capacitance
Introduction
Synaptic vesicles use an H+-ATPase to produce an electrochemical gradient that drives transport of neurotransmitter into the vesicle (Liu and Edwards, 1997). Consequently, vesicular pH is low (pH 5.7 in hippocampal neurons) (Miesenböck et al., 1998). Synaptic activity is associated with a transient decrease in extracellular pH in hippocampal slices (Krishtal et al., 1987) and a loss of vesicular acidification (Miesenböck et al., 1998), consistent with release of protons into the synaptic cleft. Photoreceptors contain Ca2+ channels that are known to be sensitive to extracellular pH. Acidification causes a decrease in conductance and shift in activation to more positive potentials (Barnes and Bui, 1991), which has also been observed for L-type Ca2+ channels in other systems (Hess et al., 1986; Iijima et al., 1986). Light-sensitive postsynaptic responses were found to be pH sensitive, with the likely site of action being photoreceptor Ca2+ channels (Barnes et al., 1993).
Recently, this inhibitory effect of acidification on Ca2+ channels has been shown to be induced by exocytosed protons after transmitter release in cone photoreceptors (DeVries, 2001); however, this study of Ca2+ current inhibition by vesicular protons did not extend to a detailed investigation of the effect on subsequent exocytosis (Traynelis and Chesler, 2001). The size of the bipolar cell response was only slightly potentiated in two of five recordings when the concentration of extracellular pH buffer was increased. Here we investigate whether Ca2+ current inhibition by released vesicular protons leads to subsequent inhibition of exocytosis in a different retinal synaptic terminal: that of the bipolar cell. Bipolar cell and photoreceptor terminals are similar in that transmitter release is activated by slowly inactivating L-type Ca2+ channels (α1F type) (Morgans, 2001) and involves the fusion of large numbers of vesicles at active zones that possess synaptic ribbons (Parsons and Sterling, 2003). Photoreceptor ribbons, however, are larger and less numerous than the bipolar cell ribbons. We have recently developed a preparation that allows recordings of voltage-clamped membrane currents and capacitance measurements to be made directly from the synaptic terminals of bipolar cells in goldfish retinal slices (Palmer et al., 2003). This provides an ideal system for investigating a reciprocal relationship between Ca2+ influx and exocytosis.
We find that voltage-gated Ca2+ currents in bipolar cell terminals are inhibited in a manner that correlates with exocytosis from the terminal and that the inhibition can be modulated by changing the concentration of extracellular pH buffer. In addition, Ca2+ currents in dissociated bipolar cell terminals are strongly inhibited by acidification of the extracellular solution. These results indicate that the mechanism of Ca2+ current inhibition by released vesicular protons demonstrated by DeVries (2001) in cone photoreceptors also occurs in bipolar cell terminals. Furthermore, we significantly extend previous findings by showing that modulation of Ca2+ current inhibition with extracellular pH buffer also modulates subsequent exocytosis from the terminal. Finally, we determine the amount of Ca2+ current inhibition and paired-pulse depression that occur under physiological buffering conditions and estimate the change in synaptic cleft pH that accompanies exocytosis from bipolar cell terminals.
Materials and Methods
Retinal slice preparation. Retinal slices were prepared from goldfish (Carassius auratus) using standard procedures. In brief, isolated retina was treated for 15 min with hyaluronidase (1 mg in 1 ml of medium) to remove vitreous humor, placed ganglion cell layer down on filter paper, and sliced at 250 μm intervals using a Narishige slicer (ST-20; Narishige, Tokyo, Japan). Slices were transferred to the recording chamber and perfused continuously (1 ml/min) with medium comprising (in mm): 120 NaCl, 2.5 KCl, 1.0 MgCl2, 2.5 CaCl2, 12 HEPES, 12 glucose, adjusted to pH 7.45 with NaOH, ∼260 mOsm. NaCl was replaced with Na methane sulfonate for low extracellular Cl- recordings. For experiments requiring high (48 mm) or low (3 mm) extracellular HEPES, osmolarity was maintained by adjusting the concentration of NaCl. For recordings in bicarbonate buffer, HEPES was replaced with NaHCO3 (24 mm), NaCl was reduced to maintain osmolarity (108 mm), and the solution was gassed continuously with 95% O2/5% CO2, pH 7.5. For experiments requiring rapid exchange of extracellular solution, the bath perfusion rate was increased to 3 ml/min. Slice preparation and recordings were performed at room temperature, under normal room light conditions.
Identification of bipolar cell terminals. Slices were viewed with broad-spectrum white-light DIC optics through a 40× water-immersion objective and 1.6× zoom tube (Axioskop; Zeiss) and a CCD camera (Hamamatsu, Tokyo, Japan). Bipolar cell terminals were identified by their size, shape, and position in the slice, as well as depolarization-evoked Ca2+ currents and capacitance responses. A subset of terminals were isolated because of severing of the bipolar cell axon during the slicing procedure; this was determined from the capacitative current response to a -10 mV step from -60 mV (Palmer et al., 2003, their Fig. 1). Terminals fell clearly into two groups. One group was well fit with a double exponential function with a prominent slow component (τs = 1.5 ± 0.1 msec) and had low input resistance (<0.5 GΩ), and the other group was well fit by either a single fast exponential (τf = 98 ± 5 μsec) or a double with only a minor slow component and had high input resistance (>1 GΩ). The first group was classified as intact cells and the second as isolated terminals. This classification was confirmed using Lucifer yellow to image recorded terminals. Intact cells and isolated terminals had baseline membrane capacitance measurements of 9-15 and 3-7 pF, respectively. Only isolated terminals were used for this study.
Dissociated bipolar cell terminal preparation. Goldfish retinal bipolar cells were acutely isolated as described previously (Heidelberger and Matthews, 1992). In brief, pieces of isolated retina were treated with hyaluronidase (1 mg in 1 ml of medium) (Sigma, St. Louis, MO) to remove vitreous humor, followed by treatment with papain (10 mg in 1 ml of medium) (Fluka, Milwaukee, WI) and mechanical dissociation with a small-bore Pasteur pipette. Cells were plated and viewed with DIC optics through a 40× water-immersion objective and 1.6× zoom tube (Axioskop; Zeiss), and a CCD camera (Hamamatsu). Isolated bipolar cell terminals were identified by their size and shape, as well as depolarization-evoked Ca2+ currents and capacitance responses. Recordings were made in extracellular medium comprising (in mm): 120 NaCl, 2.5 KCl, 1.0 MgCl2, 2.5 CaCl2, 10 HEPES, 12 glucose, adjusted to desired pH with NaOH, ∼260 mOsm. Cells were dissociated in low Ca2+ solution (0.5 mm). Bath solution was exchanged at a continuous rate of 3-4 ml/min. Recordings were made at room temperature (22-24°C).
Electrophysiology. Whole-cell voltage-clamp recordings were obtained using 5-8 MΩ patch pipettes pulled from thick-walled borosilicate glass (World Precision Instruments, Sarasota, FL) using a Narishige puller (model PP-830). Pipettes were coated with wax to reduce pipette capacitance and electrical noise and filled with solution comprising (in mm): 115 Cs gluconate, 25 HEPES, 10 TEA-Cl, 3 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA, adjusted to pH 7.2 with CsOH, ∼270 mOsm. Cs gluconate was replaced with CsCl for high intracellular Cl- recordings. After gaining whole-cell access, series resistance was typically 10-15 MΩ, and leak current was <50 pA at a holding potential of -60 mV. Data acquisition was controlled by “Pulse” software (Heka, Lambrecht, Germany), and signals were recorded via a double EPC-9 (Heka) patch-clamp amplifier. Sampling rates and filter settings were 10 and 3 kHz, respectively. Capacitance measurements were performed by the “sine + DC” method. In brief, a 1 kHz sinusoidal voltage command (30 mV peak to peak) was added to the holding potential of -60 mV, and the resulting current was analyzed at two orthogonal phase angles by the EPC-9 lock-in amplifier. These signals, together with the DC current, were used to generate values for membrane capacitance, membrane conductance, and series conductance (Gillis, 2000). Off-line analysis was performed with “IgorPro” software (Wavemetrics, Lake Oswego, OR).
Analysis. Pooled data are expressed as mean ± SEM. Statistical significance was assessed with paired and unpaired Student's t tests as appropriate, with p < 0.05 considered significant. The increase in membrane capacitance, ΔCm, evoked by membrane depolarization, was measured as ΔCm = Cm(response) - Cm(baseline), where Cm (baseline) was the average Cm value during the 100 msec before the depolarizing step, and Cm (response) was the average Cm value measured over 50 or 100 msec after the step, starting 40 msec after repolarization to allow time for all evoked conductances to have decayed.
For assessment of the effect of extracellular pH on Ca2+ currents in dissociated bipolar cell terminals, voltage ramps from -60 to +50 mV and voltage steps from -60 to 0 mV were delivered before and after changing the bath solution from pH 7.5 to a test pH between 6.0 and 8.0 and back to pH 7.5. Recorded currents were leak-subtracted by a standard P/4 protocol, which did not introduce noticeable changes to Ca2+ current kinetics or current-voltage (I-V) relationship. For cells that exhibited Ca2+ current rundown, a correction factor was applied to the test pH currents that was derived from a linear extrapolation of the Ca2+ current rundown from its initial to final amplitude in pH 7.5. Normalized Ca2+ current activation curves were obtained by dividing the mean I-V relationship for each pH by the Ca2+ driving force (V - Vrev) according to Ohm's law, where Vrev is the reversal potential for the Ca2+ current. These activation curves were then best fit (using IgorPro software) with a normalized Boltzmann function F(V) = A/{1 + exp[-(V - V1/2)/m]}, where A (set to 1 in the normalized curves), V1/2 (the midpoint voltage), and m (the slope factor: m = kT/ze) are fitting constants. With T = 22-24°C (room temperature), the value of kT/e was ∼25.5 mV. The fit parameter m varied from 6.57 to 5.23 mV, so z (the valence of the gating charge) varied from 3.9 to 4.9. Fits to the Hill equation were done using IgorPro software and the function F(pH) = Base + (Max - Base)/[1 + pH1/2/pH)n]. In Figure 6b, the values for the solid line (0 mV) of Base, Max, n, and pH1/2 are 0.18, 1.9, 11.6, and 7.6, and for the dashed line (-10 mV) they are 0.017, 1.3, 26, and 7.2, respectively.
Figure 6.
pH sensitivity of ICa in bipolar cell terminals. a, ICa evoked by 200 msec depolarizations from -60 to 0 mV in a dissociated bipolar cell terminal. Extracellular pH was changed from 7.5 to 6.5 and back to control via a complete exchange of the bath solution. Currents are leak subtracted. The small inward current observed after repolarization is caused by activation of Ca2+-dependent Cl- channels (Okada et al., 1995). b, Solid symbols indicate mean ICa amplitude evoked by depolarizations to 0 mV as a function of pH. Each measurement was normalized to the current at pH 7.5 and corrected for rundown of ICa during the experiment (n = 3-7 for each point; error bars represent SEM). Data were fit with a Hill equation (pH1/2 = 7.6). Open symbols indicate mean ICa amplitude evoked by depolarizations to -10 mV, obtained from the I-V relationship at each pH (no leak subtraction; n = 3-4 for each point). Data were fit with a Hill equation (pH1/2 = 7.2). c, ICaI-V relationship measured with a depolarizing ramp from -60 to +50 mV in the same terminal as a at pH 7.5 and 6.5. ICa activation is shifted to a more positive potential at lower pH. Note the smooth rise in the I-V curve at negative potentials indicating good voltage-clamp control of the terminal. d, Boltzmann-fit activation curves derived from the average I-V relationships at each pH. Half-activation potentials of these curves are -22 mV (pH 8.0; n = 4), -16 mV (pH 7.5; n = 10), -10 mV (pH 7.0; n = 3), -2 mV (pH 6.5; n = 2), and 9 mV (pH 6.0; n = 4).
Drug application. Drugs were bath applied in the perfusing medium. 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX), dl-2-amino-5-phosphonopentanoic acid (dl-AP5), and dl-threo-β-benzyloxyaspartate (TBOA) were obtained from Tocris (Bristol, UK). Picrotoxin, strychnine, and all other chemicals and salts were obtained from Sigma.
Results
Paired-pulse depression of exocytosis in bipolar cells
Goldfish retina contains a class of bipolar cells that depolarize to light (ON-type) and have an unusually large synaptic terminal (Witkovsky and Dowling, 1969; Saito and Kujiraoka, 1982). These cells have been classified morphologically as mixed bipolar (Mb) cells because they receive both rod and cone input. The Mb cell synaptic terminal (8-15 μm in diameter) (Ishida et al., 1980; Sherry and Yazulla, 1993) contains a large number of glutamatergic synaptic vesicles (500,000-750,000) (von Gersdorff et al., 1996) and displays a slowly inactivating L-type Ca2+ current (Heidelberger and Matthews, 1992). Ca2+ influx through L-type channels triggers exocytosis of synaptic vesicles (Tachibana et al., 1993) that can be measured as an increase in terminal membrane capacitance (ΔCm) (von Gersdorff and Matthews, 1994).
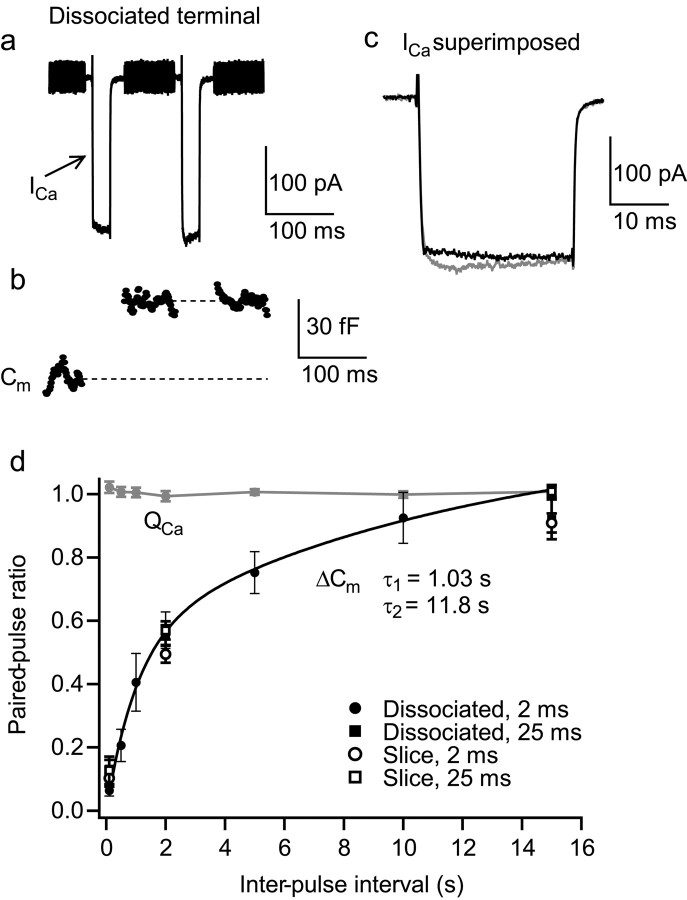
To identify Ca2+ current inhibition associated with exocytosis, the pronounced paired-pulse depression of exocytosis exhibited by Mb cells was exploited. Paired-pulse depression in dissociated terminals is not caused by depression of Ca2+ influx and requires ∼15 sec for full recovery (von Gersdorff and Matthews, 1997; Gomis et al., 1999). An example is shown in Figure 1a of the membrane current and capacitance responses to a pair of 25 msec depolarizations from -60 to -10 mV, with an interpulse interval of 100 msec. The first depolarization evoked an inward Ca2+ current (ICa) (Fig. 1a,c) and ΔCm (Fig. 1b), whereas the second depolarization evoked ICa of similar magnitude (Fig. 1a,c) but no ΔCm (Fig. 1b), reflecting depression of exocytosis. The amount of depression was reduced as the interpulse interval increased, as shown in Figure 1d. Mean paired-pulse ratio (second pulse/first pulse) was measured for pulses of 2 and 25 msec duration in both acutely dissociated terminals and terminals embedded in retinal slices. A bi-exponential best-fit of the dissociated terminal 2 msec pulse data gives recovery time constants of 1.03 and 11.8 sec. Very similar rates of recovery from paired-pulse depression were observed with 2 and 25 msec pulses and between dissociated terminals and terminals in retinal slices (Fig. 1d).
Figure 1.
Paired-pulse depression of exocytosis in bipolar cells. a, The membrane current evoked by a pair of 25 msec voltage steps from -60 to -10 mV (100 msec interval) in a dissociated isolated terminal. Rapidly activating, nondesensitizing inward Ca2+ currents (ICa) were evoked. The fast voltage sinewave used to measure membrane capacitance (Cm) was not delivered during the depolarizations. The intracellular solution contained Cs gluconate; the extracellular solution contained 12 mm HEPES. b, The corresponding Cm trace for the terminal in a. ΔCm was evoked by the first depolarization but not the second; the terminal exhibited strong paired-pulse depression of exocytosis. Baseline Cm for this terminal was 4.2 pF. c, First and second pulse ICa superimposed on an expanded time scale (first black, second gray; also applies to subsequent figures). d, Mean paired-pulse ratio (second pulse/first pulse) of ΔCm and Ca2+ influx (QCa) for pairs of 2 msec depolarizations in dissociated terminals with interpulse intervals ranging from 100 msec to 15 sec. Each point is an average of values from at least six terminals. The black line is a bi-exponential fit of the ΔCm ratio values with time constants of 1.03 sec (43%) and 11.8 sec (57%). Also shown are the mean ΔCm ratios with interpulse intervals of 100 msec, 2 sec, and 15 sec for pairs of 25 msec depolarizations in dissociated terminals, and for both 2 and 25 msec depolarizations in terminals in retinal slices (n = 4-16). The magnitude and rate of recovery from depression were similar under all conditions.
Paired-pulse depression is most readily explained by the selective depletion of vesicles docked close to Ca2+ channels (Burrone and Lagnado, 2000) and can be overcome by increasing Ca2+ influx to access more distant vesicles. For example, a stimulation protocol comprising a pair of 2 msec depolarizations followed by a 25 msec depolarization (100 msec intervals) evokes mean ΔCm values of 56 ± 5, 6 ± 1, and 18 ± 4 fF, respectively (n = 7 dissociated terminals). The total amount of release to this protocol (80 ± 7 fF) is not significantly different from that evoked by a single 25 msec depolarization delivered 20 sec later (77 ± 7 fF; n = 7), suggesting that these stimuli access the same total pool of vesicles. These data are also inconsistent with a refractory period or adaptation process causing depression after release, as has been suggested to occur at some synapses (Korn et al., 1984; Hsu et al., 1996; Bellingham and Walmsley, 1999). A bi-exponential recovery from paired-pulse depression has also been observed in hair cells (Moser and Beutner, 2000), which also have synaptic ribbons. Interestingly, a recent study of ON-EPSCs in retinal ganglion cells that were evoked by paired pulses of light also shows a bi-exponential recovery from paired-pulse depression with a remarkably similar time course (e.g., ≈80% recovery after 5 sec) (Akopian, 2003).
Ca2+ current inhibition is associated with exocytosis in retinal slices
ICa evoked by the first and second depolarizations of a pair were compared to investigate differences associated with exocytosis. In dissociated terminals, the second ICa was sometimes observed to be slightly larger than the first (Fig. 1c). In terminals in retinal slices, however, the difference was much more pronounced. A transient upward deflection was consistently observed during the first ICa that was not present during the second ICa (Fig. 2c). This upward deflection was isolated as a difference current by subtraction of the second from the first ICa (Fig. 2d). It was observed in the presence of antagonists of ionotropic GABA (50 μm picrotoxin), glycine (1 μm strychnine), and glutamate (5-10 μm NBQX, 100 μm dl-AP5) receptors (n = 12), which block activation of reciprocal inhibitory amacrine cell synapses (Hartveit, 1999). The upward deflection was also insensitive to the glutamate transporter inhibitor TBOA (30-100 μm, n = 9) (Fig. 2e) and unaffected by bath perfusion of a high concentration of L-glutamate (1 mm; n = 4) or reversal of the Cl- gradient across the terminal membrane (20 mm extracellular, 125 mm intracellular; n = 8) (Fig. 2f). The upward deflection is therefore not a glutamate- or GABA-evoked current or other Cl- conductance and is most likely to reflect transient inhibition of ICa, similar to that reported by DeVries (2001) in photoreceptors. The inhibition appears to be associated with exocytosis because of the correlation with ΔCm during paired-pulse stimulation. A similar correlation was found by DeVries (2001) between cone Ca2+ current inhibition and the postsynaptic bipolar cell response. The size of the cone difference current and the bipolar cell response recovered from paired-pulse depression with a similar time course.
Figure 2.
ICa in bipolar cell terminals exhibits an outward component associated with exocytosis. a, ICa evoked by a pair of 25 msec voltage steps from -60 to -10 mV (100 msec interval) in a terminal in a retinal slice. The intracellular solution contained Cs gluconate; the extracellular solution contained 50 μm picrotoxin, 1 μm strychnine, 5 μm NBQX, 100 μmdl-AP5, and 12 mm HEPES. b, ΔCm evoked by the depolarizations in a, showing paired-pulse depression. c, The first and second pulse ICa from a superimposed on an expanded time-scale. A transient outward component was observed during the first but not the second current. d, The difference current obtained by subtracting second pulse ICa from first pulse ICa. e, Superimposed first and second pulse ICa and difference current obtained as above but for a terminal with 30 μm TBOA added to the extracellular solution. The difference current is not caused by glutamate transporter activation. f, The first and second pulse ICa and difference current obtained as above but for a terminal with a reversed Cl- gradient across the membrane (20 mm extracellular, 125 mm intracellular). The difference current is not mediated by a Cl- channel. It is most likely to result from a transient inhibition of ICa.
The relationship between ICa inhibition and exocytosis was further tested by using the rundown in exocytosis that occurs during the course of whole-cell recordings from bipolar cell terminals (Palmer et al., 2003). Pairs of 25 msec depolarizations (100 msec interval) were delivered to terminals every 20 sec. For each pair of stimuli, the size of the upward deflection (Iinhibition) was measured as the peak of the difference current (Fig. 3a,b) and compared with first pulse ΔCm (Fig. 3c). In all terminals, Iinhibition as well as ΔCm gradually decreased during the recording, and the rate of rundown was very similar for both parameters (Fig. 3d). Thus, after 280 sec, ΔCm was 22 ± 8% and Iinhibition was 10 ± 6% of the value evoked by the first stimulus (n = 5). Inhibition of ICa and exocytosis are therefore correlated over both short and long time periods. Interestingly, recordings of Ca2+ currents in mammalian cones in intact retina show a very similar inhibitory component that disappears after ∼5 min of whole-cell dialysis (Taylor and Morgans, 1998).
Figure 3.
Correlated rundown of Iinhibition and ΔCm during recordings from bipolar cell terminals. a, Paired 25 msec depolarizations taken from three time points during a recording. First and second pulse ICa are superimposed. The extracellular solution contained 12 mm HEPES. b, Iinhibition, obtained by subtracting second pulse from first pulse ICa, for the depolarizations in a. Iinhibition decreases during the recording. d, ΔCm evoked by the paired depolarizations in a. First pulse ΔCm decreases during the recording. e, Mean first pulse ΔCm (gray circles) and Iinhibition (black squares), normalized to the first stimulus, during the course of five recordings. ΔCm and Iinhibition run down at a very similar rate. The labels i, ii, and iii relate the currents in a with the time points in d.
Comparison of Iinhibition time course with rate of exocytosis
If inhibition of ICa is occurring as a consequence of exocytosis, the two processes should occur sequentially with a similar time course during the depolarization. DeVries (2001) observed that the cone difference current had kinetics similar to the postsynaptic bipolar cell response. In bipolar cell terminals, the rate of exocytosis can be measured more directly with ΔCm. First, the time course of ICa inhibition was determined. Pairs of 25 msec depolarizations to -10 mV were delivered in the presence of ionotropic GABA, glycine, and glutamate receptor antagonists (50 μm picrotoxin, 1 μm strychnine, 10 μm NBQX, 100 μm dl-AP5) and the glutamate transporter inhibitors TBOA (30 μm) or dl-threo-3-hydroxyaspartate (THA) (300 μm). Iinhibition was obtained by subtracting the second pulse from first pulse ICa and averaged for four terminals (Fig. 4a). In a different set of slice terminals, the rate of release during a 25 msec depolarization to -10 mV was calculated from the ΔCm evoked by depolarizations of 1, 2, 5, 10, and 50 msec duration (Fig. 4b). Release rate and Iinhibition during the depolarization were then compared (Fig. 4c). Release rate was greatest during the first milliseconds and then decreased with a time constant of 1.04 msec (Fig. 4c), which is similar to previous findings (1.5 msec for steps to 0 mV) (Mennerick and Matthews, 1996). The peak of Iinhibition occurred within 2 msec of the start of the depolarization, and the decay was well fit by a double-exponential function with time constants of 0.73 msec (68%) and 7.6 msec (32%) (Fig. 5c). The onset of the inhibition of ICa was therefore similar to the initial rate of exocytosis from the terminal, with a delay of ∼1 msec. The fast decay time constant of Iinhibition, i.e., fast recovery of ICa, was similar to the decrease in the rate of exocytosis during the depolarization. The degree of ICa inhibition may therefore be tightly linked to the rate of exocytosis; however, the slow decay time constant indicates that an additional process delays the recovery of ICa. The reason for the initial downward deflection of Iinhibition is currently unknown.
Figure 4.
Time course of exocytosis and Iinhibition during a depolarization. a, Mean Iinhibition (n = 4 terminals) obtained from paired 25 msec depolarizations in the presence of 50 μm picrotoxin, 1 μm strychnine, 5 μm NBQX, 100 μmdl-AP5, 30 μm TBOA/300 μm THA, and 12 mm HEPES. b, Mean ΔCm evoked by 1, 2, 5, 10, and 50 msec depolarizations from -60 to -10 mV, in a different set of slice terminals (n = 9). Solid line is a double exponential best fit of the data. c, Rate of exocytosis during a 25 msec depolarization, obtained from points in b and fit with a single exponential, plotted with Iinhibition from a, for comparison of time course. The rise and fast decay time constant (τfast) of Iinhibition were similar to the rise and decay (τexo) of release rate, but the decay of Iinhibition also exhibited a slower component (τslow).
Figure 5.
Modulation of Iinhibition by extracellular pH buffer. a, ICa evoked by a pair of 25 msec depolarizations to -10 mV (100 msec interval) in two terminals, in the presence of a fourfold higher (48 mm) or fourfold lower (3 mm) than standard concentration of extracellular HEPES. b, ΔCm evoked by the depolarizations in a. A similar amount of exocytosis was observed in 3 and 48 mm HEPES. c, First and second pulse ICa from a superimposed. Iinhibition was suppressed in 48 mm HEPES but greatly potentiated in 3 mm HEPES. d, Mean Qinhibition (second pulse Ca2+ charge minus first pulse Ca2+ charge) in the presence of 48 mm (n = 6), 12 mm (n = 8), and 3 mm (n = 5) extracellular HEPES. (Error bars represent SEM. *p < 0.05).
ICa inhibition is modulated by extracellular pH buffer
The inhibition of ICa recently described in photoreceptors (DeVries, 2001) was greatly reduced by addition of the pH buffer HEPES (20 mm) to the extracellular medium. We therefore investigated the effect of changing the HEPES concentration on ICa in bipolar cell terminals. Pairs of 25 msec depolarizations to -10 mV were delivered in the presence of 3 or 48 mm extracellular HEPES (Fig. 5a,b) (standard solution contained 12 mm HEPES; pH and osmolarity were maintained between solutions). Because of the effects of rundown, only the first few responses obtained after gaining whole-cell access were compared between terminals. The HEPES concentration had no direct effect on Ca2+ influx because the average amplitude of second pulse ICa was not significantly different in 3 mm (188 ± 13 pA; n = 5) and 48 mm (211 ± 16 pA; n = 6) HEPES. In addition, the average ΔCm evoked by the first pulse was not significantly different in 3 mm (57 ± 9 fF; n = 5) and 48 mm (54 ± 9 fF; n = 6) HEPES. However, changing the extracellular HEPES concentration had a dramatic effect on the first pulse inhibition of ICa (Fig. 5c). The amount of inhibition (Qinhibition) was quantified by subtracting first pulse from second pulse Ca2+ charge. Qinhibition was 264 ± 22 fC in standard 12 mm HEPES (n = 8), 73 ± 22 fC in 48 mm HEPES (n = 6; p < 0.05), and 805 ± 192 fC in 3 mm HEPES (n = 5; p < 0.05) (Fig. 5d). This sensitivity to extracellular HEPES is consistent with inhibition of ICa in bipolar cell terminals by a transient increase in extracellular proton concentration after exocytosis.
Extracellular acidification inhibits ICa
To determine the pH sensitivity of voltage-gated Ca2+ channels in bipolar cell terminals, ICa was recorded in dissociated terminals in the presence of extracellular solutions of varying pH. Step depolarizations (200 msec) to 0 mV were delivered at pH 7.5 and compared with pH 6.0, 6.5, 7.0, or 8.0 in the same terminal by fast exchange of bath solution, followed by a return to pH 7.5 (Fig. 6a). ICa amplitude was found to be strongly pH dependent, with acidification from pH 7.5 to 6.0 causing inhibition of 71 ± 3% (n = 5) (Fig. 6b). The effect of pH on the voltage sensitivity of ICa was assessed using voltage ramps from -60 to +50 mV in the presence of extracellular solutions of varying pH. Both ICa activation and the peak current were shifted to more positive potentials in low pH solution (Fig. 6c). The mean I-V relationships obtained at each pH were used to generate normalized activation curves and fit with a Boltzmann function (Fig. 6d). The half-activation voltages revealed that acidification from pH 7.5 to 6.0 caused a +25 mV shift in Ca2+ channel activation [pH 7.5: -16 mV (n = 10); pH 6.0: +9 mV (n = 4)]. Thus, bipolar cell terminal Ca2+ currents are highly sensitive to changes in extracellular pH.
Extracellular HEPES modulates paired-pulse depression of release
To determine whether inhibition of ICa by released vesicular protons causes subsequent inhibition of exocytosis from the terminal, brief depolarizations were used to maximize the degree of inhibition. The average ΔCm evoked by a 2 msec depolarization was measured in the presence of 3 or 48 mm extracellular HEPES. The amount of exocytosis in 3 mm HEPES (23 ± 3 fF; n = 8) tended to be less than in 48 mm HEPES (31 ± 4 fF; n = 8), but this difference was not significant. It is likely that the variability in depolarization-evoked ΔCm between terminals makes it difficult to detect subtle effects of HEPES. Paired-pulse depression of release was therefore used as a more sensitive measure of exocytosis.
Paired-pulse depolarizations of 2, 5, and 10 msec duration (100 msec interval) were delivered in the presence of 3 or 48 mm extracellular HEPES. The Ca2+ charge (QCa) ratio and ΔCm ratio (second pulse/first pulse) were measured for each pair of pulses. In 48 mm HEPES, the QCa ratio was close to 1 for all pulse durations (2 msec: 1.03 ± 0.01; 5 msec: 1.05 ± 0.01; 10 msec: 1.04 ± 0.01; n = 8) because there was little inhibition of first pulse ICa (Fig. 7a,e). The ΔCm ratio was close to zero (2 msec: 0.11 ± 0.02; 5 msec: 0.06 ± 0.02; 10 msec: 0.05 ± 0.03; n = 8), reflecting almost complete depression of release to the second pulse (Fig. 7b,f). In contrast, with 3 mm HEPES, the QCa ratio was >1 (2 msec: 1.22 ± 0.05; 5 msec: 1.39 ± 0.08; 10 msec: 1.30 ± 0.06; n = 8; p < 0.01 compared with 48 mm HEPES), attributable to inhibition of first pulse ICa (Fig. 7c,e). This effect was accompanied by a significant increase in the ΔCm ratio (2 msec: 0.41 ± 0.07; 5 msec: 0.43 ± 0.07; 10 msec: 0.23 ± 0.03; n = 8; p < 0.01 compared with 48 mm HEPES), indicating a reduction in the amount of paired-pulse depression of exocytosis (Fig. 7d,f). The suppression of Ca2+ influx that follows exocytosis in the presence of low extracellular pH buffer therefore enables subsequent, identical depolarizations to evoke greater Ca2+ influx and further release from the terminal.
Figure 7.
Modulation of paired-pulse depression of exocytosis by pH buffer. a, ICa evoked by a pair of 5 msec depolarizations (100 msec interval) in the presence of 48 mm extracellular HEPES, first and second pulse superimposed. b, ΔCm evoked by the depolarizations in a. No exocytosis was observed after the second pulse. c, ICa evoked by the same stimulus protocol in a different terminal in the presence of 3 mm extracellular HEPES. d, ΔCm evoked by the depolarizations in c. The second pulse induced exocytosis. e, The average QCa ratios (second pulse/first pulse) for paired depolarizations of 2, 5, and 10 msec duration in the presence of 3 mm (n = 8) and 48 mm (n = 8) extracellular HEPES. The QCa ratios were significantly greater in 3 than 48 mm extracellular HEPES (*p < 0.01). f, The average ΔCm ratios (second pulse/first pulse) for the paired depolarizations in e. The ΔCm ratios were significantly greater in 3 than 48 mm HEPES (*p < 0.01), indicating a reduction in paired-pulse depression of exocytosis.
Paired-pulse depression with physiological pH buffer
To investigate the degree of paired-pulse depression of release under more physiological pH buffering conditions, experiments were performed with HEPES-free, bicarbonate-buffered extracellular solution (24 mm NaHCO3, 95% O2/5% CO2, pH 7.5). The average amplitude of second pulse ICa under these conditions (184 ± 28 pA; n = 6) was not significantly different from that with 3 or 48 mm HEPES, indicating that the pH buffering conditions do not directly affect Ca2+ currents in bipolar cell terminals. The average ΔCm values evoked by 2 and 25 msec depolarizations were 22 ± 3 fF (n = 7) and 53 ± 10 fF (n = 6), respectively, not significantly different from the values in 3 or 48 mm extracellular HEPES. A relatively large amount of inhibition of first pulse ICa was observed in the presence of bicarbonate buffer. For example, pairs of 25 msec depolarizations gave a Qinhibition of 662 ± 128 pC (n = 6) (Fig. 8a), close to the value in 3 mm HEPES. The QCa ratio for pairs of 2 msec depolarizations was 1.26 ± 0.05 and the ΔCm ratio was 0.30 ± 0.06 (n = 7) (Fig. 8b,c). This ΔCm ratio in bicarbonate buffer is significantly larger than that measured in 12 mm HEPES (0.10 ± 0.03; n = 7; p < 0.05) or 48 mm HEPES but not significantly different from 3 mm HEPES, suggesting that 3 mm HEPES more closely mimics physiological pH buffering in the synaptic cleft.
Figure 8.
Iinhibition and paired-pulse depression in physiological pH buffer. a, ICa and ΔCm evoked by a pair of 25 msec depolarizations to -10 mV (100 msec interval) in the presence of HEPES-free, bicarbonate-buffered extracellular solution. Inhibition of first pulse ICa was prominent. b, ICa and ΔCm evoked by a pair of 2 msec depolarizations in the presence of bicarbonate-buffered extracellular solution. Exocytosis was evoked by the second pulse. c, Average QCa and ΔCm ratios for paired 2 msec depolarizations in the presence of bicarbonate buffer (n = 7), plotted for comparison with the values in 3 and 48 mm extracellular HEPES (from Fig. 8). Both the QCa and ΔCm ratios were significantly greater in bicarbonate buffer than 48 mm HEPES but not significantly different from 3 mm HEPES (*p < 0.05).
pH buffering modulates exocytosis evoked by single depolarizations
The amount of exocytosis evoked by the first of a pair of depolarizations was found to be not significantly affected by pH buffering when compared between different sets of terminals. To eliminate interterminal variability, exocytosis was measured in the same terminal under different pH buffering conditions. Rapid exchange of extracellular solution was required because of rundown of exocytosis during recordings (Fig. 3). A pair of 5 msec depolarizations was first delivered in the presence of 24 mm bicarbonate-buffered extracellular solution and then delivered again, 30 sec later, in the presence of 48 mm HEPES. As shown in Figure 9a, a large amount of inhibition of first pulse ICa was observed in the presence of bicarbonate buffer, but, as expected, the inhibition was greatly reduced after perfusion of 48 mm HEPES (QCa = 0.54 pC in bicarbonate, 1.13 pC in 48 mm HEPES). In addition, ΔCm evoked by the first pulse was larger in the presence of 48 mm HEPES (52 fF in bicarbonate, 69 fF in 48 mm HEPES) (Fig. 9b), indicating that released vesicular protons can inhibit further exocytosis during single depolarizations. Consistent with the demonstrated effects of pH buffering on paired-pulse depression, the second depolarization evoked less exocytosis in 48 mm HEPES than bicarbonate buffer. Consequently, the total amount of exocytosis was very similar under both buffering conditions. A similar increase in ΔCm evoked by single 5 msec depolarizations after switching from bicarbonate to 48 mm HEPES buffered solutions was observed in five additional terminals. ΔCm was on average 42 ± 11 fF in bicarbonate buffer and 81 ± 21 fF in 48 mm HEPES (p < 0.05).
Figure 9.
Modulation of exocytosis evoked by single depolarizations by pH buffer. a, ICa evoked by a pair of 5 msec depolarizations to -10 mV (100 msec interval) in the presence of 24 mm bicarbonate-buffered extracellular solution (left). ICa evoked by the same stimulation protocol in the same terminal after perfusion of extracellular solution containing 48 mm HEPES (right). Inhibition of first pulse ICa was greatly reduced by 48 mm HEPES. b, ΔCm evoked by the paired depolarizations in a (solid symbols represent bicarbonate; open symbols represent 48 mm HEPES). First pulse ΔCm was potentiated after perfusion of 48 mm HEPES, indicating that ICa inhibition can reduce exocytosis during single depolarizations. Rapid exchange of bath solution enabled rundown of ΔCm to be overcome. The total ΔCm to the pair of depolarizations was very similar in both pH buffering conditions. The large (∼85%) peak inhibition of ICa (a) indicates that the pH in the synaptic cleft transiently decreased from 7.5 to 6.5 in this example.
Synaptic cleft acidification after exocytosis
The decay of Iinhibition in the presence of bicarbonate buffer, obtained from a double-exponential fit of the difference current as in Figure 4, was similar to that in 12 mm HEPES. Mean fast and slow time constants were 905 μsec and 7.4 msec, respectively (n = 5; 731 μsec and 7.6 msec for 12 mm HEPES). The peak inhibition of ICa during a 25 msec depolarization in the presence of bicarbonate buffer was 61 ± 5% (n = 11). From the results in Figure 6b (dashed line), this would result from a transient acidification in the vicinity of the Ca2+ channels from pH 7.5 to ∼6.9. The peak inhibition occurred 2.1 ± 0.2 msec after the start of the depolarization. Approximately 75% of the exocytosis during a 25 msec depolarization occurs within the first 2 msec (Fig. 4b), which equates to ΔCm of 40 fF for these recordings. Assuming a vesicle capacitance of 26 aF (von Gersdorff et al., 1996), this corresponds to the release of ∼1550 vesicles, or ∼30 vesicles per ribbon-type active zone with 55 ribbons per terminal (von Gersdorff et al., 1996). If each active zone and postsynaptic target function as an independent “synaptic cleft,” the release of ∼30 vesicles causes extracellular acidification of 0.6 pH units under physiological buffering conditions. This is sufficient to cause significant inhibition of further Ca2+ influx and to modulate exocytosis from the terminal. The speed of the modulation implies that Ca2+ channels must be located within nanometers of vesicle fusion sites, which agrees with recent evidence that hot spots of Ca2+ influx coincide with the location of synaptic ribbons (Morgans, 2001; Zenisek et al., 2003).
Discussion
This study demonstrates that exocytosis is modulated by released vesicular protons in retinal bipolar cell terminals. The modulation occurs via an inhibition of presynaptic Ca2+ currents, as described previously by DeVries (2001) in photoreceptor terminals. We have shown that in bipolar cell terminals the degree of Ca2+ channel inhibition correlates with the amount of exocytosis, measured as an increase in membrane capacitance, and the inhibition is greatly potentiated by low concentrations of extracellular pH buffer, consistent with inhibition of Ca2+ channels by released vesicular protons. Furthermore, the pH buffer concentration significantly affects the amount of paired-pulse depression of exocytosis in bipolar cell terminals. For example, depression was reduced from 94% in high pH buffer to 57% in low pH buffer for 5 msec depolarizations.
Ca2+ current inhibition by released protons was found to reduce exocytosis evoked by single depolarizations when two buffering conditions were compared in the same terminal; however, the effect was small relative to the inhibition of ICa and was not significant between groups of terminals recorded in either high or low concentrations of HEPES. This is likely to be attributable to the rapidly decreasing rate of release during sustained depolarizations (Fig. 4) (Mennerick and Matthews, 1996). Thus, a large amount of release occurs during the first millisecond, further Ca2+ influx is inhibited, but this modulates a much lower release rate and so has little effect on the total ΔCm. The small effect of pH buffer on exocytosis evoked by single depolarizations is similar to that reported by DeVries (2001), who observed only a slight increase in postsynaptic responses in two of five recordings in the presence of high pH buffer. Moreover, this increase may have been caused by actions of protons on either presynaptic or postsynaptic targets. By using ΔCm rather than postsynaptic responses to monitor exocytosis, we are able to exclude inhibitory actions of protons on glutamate receptors (Tang et al., 1990; Ihle and Patneau, 2000) as a mechanism for the observed effects.
In bipolar cell terminals, examination of paired-pulse depression revealed significant effects of released vesicular protons on exocytosis. In low extracellular pH buffer, Ca2+ influx during the first pulse is inhibited by released protons, but the second pulse Ca2+ current is less inhibited because of vesicle depletion. Consequently there is greater Ca2+ influx during the second than the first depolarization, which overcomes paired-pulse depression and evokes further release. Hence Ca2+ current inhibition by released vesicular protons has the net effect of reducing depression of exocytosis.
The results in bicarbonate buffer indicate the extent to which this mechanism occurs under physiological conditions. Ca2+ current inhibition by vesicular protons was prominent with 24 mm bicarbonate, and the degree of paired-pulse depression of release was closer to that measured in 3 mm than 12 or 48 mm HEPES. The apparently low buffering power of bicarbonate (pKa 6.1) compared with HEPES (pKa 7.5) may be attributable to exclusion of the enzyme carbonic anhydrase from the synaptic cleft and the resulting reduction in the effective pKa of bicarbonate (DeVries, 2001). To obtain an estimate of the change in synaptic cleft pH after exocytosis with bicarbonate buffer, we first measured the sensitivity of bipolar cell Ca2+ currents to extracellular solutions of varying pH in dissociated terminals. Acidification caused a decrease in conductance and positive shift in voltage-dependent activation, with a sensitivity very similar to that reported in photoreceptors (Barnes and Bui, 1991) and smooth muscle cells (Klockner and Isenberg, 1994). From the Ca2+ current inhibition and ΔCm observed after exocytosis in physiological pH buffer, we estimate that synaptic cleft acidification of ∼0.6 pH units results from the rapid release of ∼30 vesicles at each of 55 active zones in a terminal. This represents the pH change seen by the Ca2+ channels, which are probably located very close to sites of exocytosis (Raviola and Raviola, 1982). DeVries (2001) compared the voltage shift in photoreceptor Ca2+ current activation after exocytosis with the shift evoked by changing extracellular pH to estimate a synaptic cleft acidification of 0.2 pH units. The higher value obtained in this study is likely to result from various factors, including stronger depolarization, weaker intracellular Ca2+ buffering, and differences in synaptic function and geometry between the two cell types.
The rapid rise and fast decay component of Ca2+ current inhibition in bipolar cell terminals were observed to closely reflect the rate of exocytosis with a delay of ∼1 msec, consistent with a rapid effect of released protons on Ca2+ channels (Prod'hom et al., 1987). The additional slower component to the decay of the inhibition (τ ∼7.5 msec) is likely to represent the rate of clearance of protons from the synaptic cleft by buffering and diffusion. This rate was similar in the presence of 12 mm HEPES and 24 mm bicarbonate buffers. By contrast, the decay of the equivalent difference current in photoreceptors was well fit by a single exponential (τ = 1.8 msec) (DeVries, 2001).
Bipolar cells generally respond to light with graded changes in membrane potential around the Ca2+ channel activation voltage (Saito et al., 1979; Ashmore and Falk, 1980). Depolarization in the physiological range to approximately -30 mV is likely to release approximately half as many vesicles as depolarization to -10 mV (Burrone and Lagnado, 2000) and result in synaptic cleft acidification from pH 7.5 to ∼7.2. We have observed that acidification to pH 7.0 at -30 mV causes a 3.4-fold decrease in Ca2+ channel conductance and from this estimate an approximate twofold decrease at pH 7.2. Bipolar cell terminal Ca2+ influx is therefore likely to be halved by proton-mediated feedback during physiological depolarizations. The effect of this is likely to depend on the bipolar cell response kinetics. ON-bipolar cells exhibit either a sustained (tdecay ∼4.0 sec) or more transient (tdecay ∼0.4 sec) depolarization in response to step illumination (Awatramani and Slaughter, 2000). During sustained depolarization, continuous exocytosis will occur at a low rate, resulting in a steady proton concentration in the synaptic cleft. Ca2+ influx will be tonically reduced and a state of equilibrium is likely to be reached between exocytosis and inhibition. This will reduce depletion of the releasable pool of synaptic vesicles during the depolarization and may enable the bipolar cell to respond more strongly to subsequent increases in stimulation intensity. In transient bipolar cells, the observed proton-mediated feedback effect of reducing paired-pulse depression may be more important. This mechanism would enable maintained responsiveness to high-frequency illumination and may aid in the perception of movement.
Other systems exist at bipolar cell synapses that exert more indirect negative feedback. For example, reciprocal amacrine cell synapses counteract bipolar cell depolarization via activation of ionotropic GABA receptors in the terminal (Tachibana and Kaneko, 1987; Lukasiewicz and Werblin, 1994; Hartveit, 1999). Bipolar cell terminals also contain glutamate transporters with a large associated anion current (Palmer et al., 2003), which will tend to hyperpolarize the terminal after exocytosis, and metabotropic glutamate receptors that inhibit transmitter release (Awatramani and Slaughter, 2001), as well as Ca2+-activated K+ (Sakaba et al., 1997) and Cl- currents (Okada et al., 1995; Protti et al., 2000). It remains to be determined how inhibition of Ca2+ currents by vesicular protons interacts with these systems and with vesicle depletion to determine bipolar cell output.
The inhibition of Ca2+ channels by released protons may also function to reduce Ca2+ influx during periods when exocytosis is depressed by other mechanisms, thereby reducing the metabolic demands of Ca2+ clearance from the terminal. Ca2+ clearance is mediated predominantly by a cell membrane Ca2+ ATPase and a Na+-Ca2+ exchanger (Kobayashi and Tachibana, 1995; Zenisek and Matthews, 2000). The activity of cell membrane Ca2+ pumps is reported to be pH dependent (inhibited by extracellular alkalization) (Dipolo and Beaugé, 1982), so acidification of the synaptic cleft after exocytosis may act to speed the decay of the intracellular Ca2+ transient (Kobayashi and Tachibana, 1995).
Protons released from exocytosed vesicles are likely to exert effects in the synaptic cleft in addition to their actions on Ca2+ channels and pumps. For example, AMPA and NMDA glutamate receptors, which are present in postsynaptic amacrine and ganglion cells, are modulated by extracellular protons (Tang et al., 1990; Ihle and Patneau, 2000), and glutamate transporter-associated anion currents in bipolar cell terminals are potentiated in low extracellular pH buffer (Palmer et al., 2003). Vesicular protons may therefore be a significant modulator of synaptic function. It is at present unknown whether exocytosed protons cause Ca2+ current inhibition or other effects at conventional (i.e., non-ribbon) central synapses. At such synapses, exocytosis is evoked by rapidly deactivating Ca2+ currents in response to invading action potentials and normally involves the release of only one or a few vesicles at each active zone (Meyer et al., 2001). The resulting change in extracellular pH is therefore likely to be much smaller than at ribbon synapses.
In conclusion, the results of this study demonstrate a novel form of synaptic modulation that involves exocytosed vesicular protons acting via inhibition of presynaptic Ca2+ influx to reduce short-term synaptic depression. This mechanism may be a characteristic feature of ribbon-type synapses. The possibility that released vesicular protons act via multiple targets to modulate synaptic function throughout the nervous system is ripe for exploration (Bianchi and Driscoll, 2002).
Footnotes
This work was supported by National Institutes of Health/National Eye Institute and Pew Biomedical Research Scholar grants. We thank Drs. Ed McCleskey, Mark Connor, and Craig Jahr for valuable discussions.
Correspondence should be addressed to Dr. H. von Gersdorff, The Vollum Institute, Oregon Health and Science University, 3181 Southwest Sam Jackson Park Road, Portland, OR 97239. E-mail: vongersd@ohsu.edu.
M. J. Palmer's present address: Medical Research Council Centre for Synaptic Plasticity, University of Bristol, BS8 1TD, UK.
Copyright © 2003 Society for Neuroscience 0270-6474/03/2311332-10$15.00/0
References
- Akopian A ( 2003) Differential modulation of light-evoked On- and Off-EPSCs by paired-pulse stimulation in salamander retinal ganglion cells. Brain Res 967: 235-246. [DOI] [PubMed] [Google Scholar]
- Ashmore JF, Falk G ( 1980) Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol (Lond) 300: 115-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM ( 2000) Origin of transient and sustained responses in ganglion cells of the retina. J Neurosci 20: 7087-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM ( 2001) Intensity-dependent, rapid activation of presynaptic metabotropic glutamate receptors at a central synapse. J Neurosci 21: 741-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Bui Q ( 1991) Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J Neurosci 11: 4015-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F ( 1993) Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA 90: 10081-10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B ( 1999) A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron 23: 159-170. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M ( 2002) Protons at the gate: DEG/EnaC ion channels help us feel and remember. Neuron 34: 337-340. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L ( 2000) Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci 20: 568-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH ( 2001) Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron 32: 1107-1117. [DOI] [PubMed] [Google Scholar]
- Dipolo R, Beaugé L ( 1982) The effect of pH on Ca2+ extrusion mechanisms in dialyzed squid axons. Biochim Biophys Acta 688: 237-245. [DOI] [PubMed] [Google Scholar]
- Gillis KD ( 2000) Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Eur J Physiol 439: 655-664. [DOI] [PubMed] [Google Scholar]
- Gomis A, Burrone J, Lagnado L ( 1999) Two actions of calcium regulate the supply of releasable vesicles at the ribbon synapse of retinal bipolar cells. J Neurosci 19: 6309-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E ( 1999) Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J Neurophysiol 81: 2923-2936. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G ( 1992) Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol (Lond) 447: 235-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW ( 1986) Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol 88: 293-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S-F, Augustine GJ, Jackson MB ( 1996) Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron 17: 501-512. [DOI] [PubMed] [Google Scholar]
- Ihle EC, Patneau DK ( 2000) Modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Mol Pharmacol 58: 1204-1212. [DOI] [PubMed] [Google Scholar]
- Iijima T, Ciani S, Hagiwara S ( 1986) Effects of the external pH on Ca channels: experimental studies and theoretical considerations using a two-site, two-ion model. Proc Natl Acad Sci USA 83: 654-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Stell WK, Lightfoot DO ( 1980) Rod and cone inputs to bipolar cells in goldfish retina. J Comp Neurol 191: 315-335. [DOI] [PubMed] [Google Scholar]
- Klockner U, Isenberg G ( 1994) Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J Gen Physiol 103: 665-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tachibana M ( 1995) Ca2+ regulation in the presynaptic terminals of goldfish retinal bipolar cells. J Physiol (Lond) 483: 79-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS, Burnod Y, Triller A ( 1984) Regulation of efficacy at central synapses. J Neurosci 4: 125-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Osipchuk YV, Shelest TN, Smirnoff SV ( 1987) Rapid extracellular pH transients related to synaptic transmission in rat hippocampal slices. Brain Res 436: 352-356. [DOI] [PubMed] [Google Scholar]
- Liu Y, Edwards RH ( 1997) The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci 20: 125-156. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Werblin FS ( 1994) A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci 14: 1213-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Matthews G ( 1996) Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron 17: 1241-1249. [DOI] [PubMed] [Google Scholar]
- Meyer AC, Neher E, Schneggenburger R ( 2001) Estimation of quantal size and number of functional active zones at the calyx of Held synapse by nonstationary EPSC variance analysis. J Neurosci 21: 7889-7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE ( 1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192-195. [DOI] [PubMed] [Google Scholar]
- Morgans CW ( 2001) Localization of the α1F calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 42: 2414-2418. [PubMed] [Google Scholar]
- Moser T, Beutner D ( 2000) Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA 97: 883-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Horiguchi H, Tachibana M ( 1995) Ca2+-dependent Cl- current at the presynaptic terminals of goldfish retinal bipolar cells. Neurosci Res 23: 297-303. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H ( 2003) Synaptic activation of presynaptic glutamate transporters in nerve terminals. J Neurosci 23: 4831-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T, Sterling P ( 2003) Synaptic ribbon: conveyor belt or safety belt? Neuron 37: 379-382. [DOI] [PubMed] [Google Scholar]
- Prod'hom B, Pietrobon D, Hess P ( 1987) Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature 329: 243-246. [DOI] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, von Gersdorff H ( 2000) Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron 25: 215-227. [DOI] [PubMed] [Google Scholar]
- Raviola E, Raviola G ( 1982) Structure of the synaptic membranes in the inner plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J Comp Neurol 209: 233-248. [DOI] [PubMed] [Google Scholar]
- Saito T, Kujiraoka T ( 1982) Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol 205: 161-170. [DOI] [PubMed] [Google Scholar]
- Saito T, Kondo H, Toyoda J-I ( 1979) Ionic mechanisms of two types of on-center bipolar cells in the carp retina. J Gen Physiol 73: 73-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaba T, Ishikane H, Tachibana M ( 1997) Ca2+-activated K+ current at presynaptic terminals of goldfish retinal bipolar cells. Neuroscience Res 27: 219-228. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Yazulla S ( 1993) Goldfish bipolar cells and axon terminal patterns: a Golgi study. J Comp Neurol 329: 188-200. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A ( 1987) γ-Aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc Natl Acad Sci USA 84: 3501-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M ( 1993) Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci 13: 2898-2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Dichter M, Morad M ( 1990) Modulation of the N-methyl-d-aspartate channel by extracellular H+ Proc Natl Acad Sci USA 87: 6445-6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Morgans C ( 1998) Localization and properties of voltage-gated calcium channels in cone photoreceptors of Tupaia belangeri. Vis Neurosci 15: 541-552. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Chesler M ( 2001) Proton release as a modulator of presynaptic function. Neuron 32: 960-962. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G ( 1994) Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature 367: 735-739. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G ( 1997) Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci 17: 1919-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P ( 1996) Evidence that vesicles on the synaptic ribbon of retinal bipolar cells can be rapidly released. Neuron 16: 1221-1227. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Dowling JE ( 1969) Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat 100: 60-82. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G ( 2000) The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron 25: 229-237. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W ( 2003) Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci 23: 2538-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]