Abstract
Objective
Test the hypothesis that exercise training increases the contribution of large-conductance, Ca2+-dependent K+ (BKCa) channels to endothelium-mediated dilation in coronary arterioles from collateral-dependent myocardial regions of chronically occluded pig hearts and may function downstream of H2O2.
Methods
An ameroid constrictor was placed around the proximal left circumflex coronary artery to induce gradual occlusion in Yucatan miniature swine. Eight weeks postoperatively, pigs were randomly assigned to sedentary or exercise training (treadmill; 14 wk) regimens.
Results
Exercise training significantly enhanced bradykinin-mediated dilation in collateral-dependent arterioles (~125 μm diameter) compared with sedentary pigs. The BKCa-channel blocker, iberiotoxin alone or in combination with the H2O2 scavenger, polyethylene glycol catalase, reversed exercise training-enhanced dilation in collateral-dependent arterioles. Iberiotoxin-sensitive whole-cell K+ currents (i.e., BKCa-channel currents) were not different between smooth muscle cells of nonoccluded and collateral-dependent arterioles of sedentary and exercise trained groups.
Conclusions
These data provide evidence that BKCa-channel activity contributes to exercise training-enhanced endothelium-dependent dilation in collateral-dependent coronary arterioles despite no change in smooth muscle BKCa-channel current. Taken together, our findings suggest that a component of the bradykinin signaling pathway, which stimulates BKCa channels, is enhanced by exercise training in collateral-dependent arterioles and suggest a potential role for H2O2 as the mediator.
Keywords: ischemia, reactive oxygen species, ischemic heart disease, free radicals
Introduction
Endothelium production of the relatively stable reactive oxygen species, hydrogen peroxide (H2O2), is emerging as an important signaling pathway contributing favorably to physiological responses in various vascular beds. Numerous studies have reported a significant contribution of H2O2 to vasodilation in arterioles from both animal models and humans under control and diseased conditions (13, 25, 31, 33, 44, 53). Recent evidence implicates exercise training as a stimulus that increases the contribution of the superoxide/H2O2 signaling pathway to endothelium-dependent vasodilation with aging (46) and in the underlying setting of coronary artery disease (50). We recently reported that both nitric oxide and the superoxide/H2O2 signaling pathway significantly contribute to exercise training-enhanced endothelium-mediated dilation in collateral-dependent coronary arterioles (53). Specifically, our studies demonstrated that bradykinin-stimulated H2O2 levels were significantly increased in coronary arterioles from exercise trained pigs and that exercise training-enhanced bradykinin-mediated dilation in collateral-dependent arterioles was mediated by H2O2 when compared with sedentary animals. Numerous pathways and effectors have been documented to mediate downstream H2O2-signaling, including arachidonic acid-related pathways (3, 23, 26, 38, 49), cGMP (10), cAMP (23), Kv channels (5, 12, 23, 39, 40, 43) and BKCa channels (3, 4, 28, 47, 55). Based on previous work in diseased human coronary arterioles which demonstrated that H2O2-induced dilation is mediated in part by BKCa channels (28, 55), we were intrigued to explore whether this contribution of BKCa channels to H2O2 signaling was an adaptation of the diseased state since this signaling pathway was not explored previously in non-diseased arterioles and if exercise training-enhanced endothelium-dependent dilation observed previously by our laboratory was attributable to BKCa channel activation.
Despite evidence that BKCa channels play a role in H2O2-induced vasodilation, the role of BKCa channels in exercise training enhanced, endothelium-dependent vasodilation has not been evaluated. Therefore, in the current study, we tested the hypothesis that exercise training increases the contribution of BKCa channels to endothelium-mediated dilation in coronary arterioles from collateral-dependent myocardial regions of chronically occluded pig hearts. A secondary intent was initial exploration into potential interactions of H2O2 and BKCa channels in exercise training-enhanced endothelium-mediated vasodilation.
Materials and Methods
Experimental animals and surgical procedures
All protocols were in accordance with “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” as detailed in the National Institutes of health (NIH) Guide for the Care and Used of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Texas A&M University. Adult female Yucatan miniature swine (Sinclair Research Center, Auxvasse, MO) were surgically instrumented with Ameroid constrictors around the proximal left circumflex coronary (LCX) artery as described previously (16, 20). Animals were preanesthetized with glycopyrrolate (0.004 mg·kg-1, i.m.), midazolam (0.5 mg·kg-1, i.m.) and ketamine (20 mg·kg-1, i.m.), after which surgical anesthesia was induced with 3% isoflurane. Animals were intubated and anesthesia maintained with 2-3% isoflurane, balance O2 during aseptic surgery. During the surgery, animals received the following drugs as necessary: pancuronium (0.1 mg·kg-1; neuromuscular blocker) or vecuronium bromide (0.1 mg·kg-1; neuromuscular blocker) and lidocaine (1 mg·kg-1, i.v.; antiarrythmic). Immediately following surgery, pigs received ketofen (3.0 mg·kg-1, i.v.; NSAID). Prior to surgery and during surgical recovery, animals received either buprenorphine hydrochloride (0.1 mg·kg-1, i.v.; analgesic) or butorphanol tartrate (0.5 mg·kg-1; analgesic) every 3-6 hr, as needed for pain relief. Antibiotics (Naxcel 4 mg·kg-1, i.m.) were administered 24 hours before surgery, immediately prior to surgery and for two days following surgery.
Exercise training procedures
Following eight weeks of postoperative recovery, animals were randomly assigned to sedentary (n=31) or exercise training (n=29) groups. The exercise training group was subjected to a progressive treadmill exercise training program, 5 days/week for 14 weeks. By week 12 of the progressive exercise program, animals were running 85 minutes/day, 5 days/week as described in detail previously (16, 20). Sedentary animals were confined to their pens for the same period. Skeletal muscle citrate synthase activity (48) and heart-to-body weight ratio were measured to evaluate effectiveness of the exercise training regimen (16, 20).
Preparation of coronary arterioles
Following completion of the 14-week exercise training or sedentary protocols, pigs were anesthetized using rompun (2.25 mg·kg-1, i.m.), ketamine (35 mg·kg-1, i.m.) and pentothal sodium (30 mg·kg-1, i.v.), followed by administration of heparin (1000 U·kg-1, i.v.). Pigs were intubated and ventilated with room air and a left lateral thoracotomy was performed in the fourth intercostal space. The heart was removed and placed in iced Krebs bicarbonate buffer (0-4°C) and weighed. Visual examination of the ameroid occluder during dissection of the LCX artery indicated complete occlusion in all animals that were included in this study. Under the dissection microscope, size-matched coronary arterioles (~100-150 μm) were isolated were isolated from subepicardial regions of both the nonoccluded left anterior descending (LAD) artery and the collateral-dependent LCX artery in areas free from infarct. Coronary arterioles for this study were dissected from the midmyocardium and were typically third- or fourth-order branches from the main artery (LCX or LAD).
Microvessel cannulation and experimental protocols
Arterioles were cannulated and pressurized for assessment of vascular reactivity, as described in detail previously (18). Arterioles underwent a 1-hour equilibration period during which time the vessels established a stable level of basal tone. Arterioles were further preconstricted with endothelin-1 until a preconstriction level of ~40-70% of maximal diameter was attained. For experiments in which pharmacological antagonists were used, arterioles were pretreated with the inhibitor and then further constricted to the same level (~40-70%) by addition of endothelin-1 until a new steady-state was attained, as described previously (18). Pharmacological inhibitors included the BKCa channel blocker, iberiotoxin (100 nM) and the H2O2 scavenger, polyethylene glycol (PEG)-catalase (1000 U/ml). Concentration-response curves were determined in response to cumulative concentrations of bradykinin. All drug additions were made directly to the tissue bath.
Smooth muscle cell dissociation
All electrophysiology experiments were performed using freshly dispersed arteriolar smooth muscle cells. Coronary arterioles were placed in low-Ca2+ (0.1 mM) physiological buffer containing 1.4 mg/ml papain, 0.4 mg/ml DTT, and 0.4 mg/ml bovine serum albumin. Cells were enzymatically dissociated by incubation in a 37 °C water bath for 30-45 min. The enzyme solution was then replaced with enzyme-free low-Ca2+ solution and the arterioles dispersed with gentle trituration by micropipette for isolation of single smooth muscle cells. Smooth muscle cells were morphologically distinguishable from other cell types in the dispersion, such as endothelial cells and fibroblasts (52). Isolated cells were maintained in low-Ca2+ solution at 4 °C until use (0-4 h).
Whole-cell voltage clamp
K+ currents were obtained from single cells under physiological K+ concentrations using standard whole-cell voltage-clamp techniques as used routinely (17, 21). Cells were superfused with PSS containing the following (in mM): 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, and 10 HEPES, pH 7.4. Heat-polished glass pipettes (2-5 MΩ) were filled with a solution containing the following (in mM): 120 K-Aspartate, 20 KCl, 10 NaCl, 10 EGTA, 10 HEPES, 2 Na2ATP, and 0.5 Tris-GTP, pH 7.1 with KOH. After formation of a GΩ seal, pipette capacitance was canceled and suction applied to achieve whole-cell configuration. Ionic currents were amplified with an Axopatch 200B patch-clamp amplifier. Currents were elicited by 500-ms step depolarizations to potentials ranging from –70 to +100 (in 10 mV increments) from a holding potential of –80 mV. Iberiotoxin-sensitive currents were obtained by subtraction of currents in the presence of iberiotoxin from control currents (difference currents). Currents were low-pass filtered with a cutoff frequency of 2,000 Hz, digitized at 2.5 kHz, and stored on a computer. Data acquisition and analysis were accomplished using pClamp 9.2 software (Axon Instruments). Current densities (pA/pF) were obtained for each cell by normalization of whole-cell current to cell capacitance. Leak subtraction was not performed. Cells were continuously perfused under gravity flow at room temperature (22-25 °C).
Solutions and pharmacological agents
All reagents and pharmacological inhibitors were purchased from Sigma-Aldrich unless otherwise noted. Iberiotoxin was purchased from Anaspec and endothelin-1 from Peninsula Laboratories. Crystallized bovine albumin was purchased from US Biochemicals. Primary antibody catalog numbers and sources are as follows: MnSOD (#SOD-110), ecSOD (#SOD-105), and Cu/ZnSOD (#SOD-100) from Stressgen; catalase (#ab50434) from Abcam, β-actin (#NB400-501) from Novus; Physiological saline solution (pH 7.4) used in cannulated microvessel studies contained (in mM): 145 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 3.0 MOPS, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 1 g/100 ml bovine albumin. Krebs buffer solution for arteriole dissection contained (in mM): 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3 and 0.025 EDTA.
Immunoblots
Coronary arterioles (~100-150 μm diameter; ~10-15 mm total length) were isolated from both the nonoccluded and collateral-dependent myocardial regions, quick-frozen in liquid N2 and stored at -80 °C for later immunoblot analysis. Arterioles were homogenized in 40 μl lysis buffer (20 mM Tris-HCl, 50 μM NaCl, 0.01% Triton-X-100) by freeze-thaw cycles and vortexed ~6-8 times. BCA protein assay kit (Pierce) was used to determine total protein concentration. Arteriole samples (15 μg of total protein) were subjected to 12.5% SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes overnight. PVDF membranes were blocked for 2 hours at room temperature (5% nonfat dry milk in Tris-bufferred saline and 0.1% Tween 20), and incubated with primary antibodies of antioxidant enzymes, ecSOD (1:667), MnSOD (1:3500), Cu/ZnSOD (1:1700), catalase (1:500), and β-actin (1:5000) at 4 °C overnight. After washing, membranes were incubated with the appropriate horseradish peroxidase-conjugated species-specific anti-IgG (1:50,000-1:100,000 depending on primary antibody) for two hours at 25 °C. Peroxidase activity was detected using SuperSignal West Dura Substrate. Scanning densitometry was used to quantify signal density from luminograms. Normalization for potential loading differences was accomplished using ratios of the densitometry signals for proteins of interest to β-actin.
SOD activity assay
Coronary arterioles (~100-150 μm diameter; ~10-15 mm total length) were incubated in PSS in the absence (control) or presence of bradykinin (10 nM) for 5 min at 37 °C. Subsequently, arterioles were homogenized in 20-μl buffer containing (in mM): 10 HEPES, 1 EGTA, 210 mannitol and 70 sucrose, and then centrifuged at 1500 g for 5 min at 4 °C. Supernatant was stored at -80 °C and later analyzed using a SOD activity assay kit per manufacturer's instructions (#706002, Cayman Chemical).
Data presentation and analysis
Citrate synthase, body weight, and heart-to-body weight ratio were compared between animal treatment groups using Student's t-tests. For endothelin-1 preconstriction, data are presented as percent possible constriction, [(DP – DSS) / DP] × 100, where DP is the passive diameter and DSS is the steady-state diameter in the presence of endothelin-1. Dilation responses to bradykinin are presented as the percentage increase in luminal diameter relative to the maximal possible dilation [(DSS-DB) / (DP-DB)]*100 to normalize for differences in initial and passive diameters between vessels. Dss is the steady state diameter attained in response to each bradykinin concentration, DB is the baseline diameter following preconstriction, and DP is the maximal diameter of the arteriole determined by exposure to nitroprusside (100 μM) at the end of each experiment. Bradykinin-mediated dilation and whole-cell K+ currents were evaluated by repeated measures two-way ANOVA and the Greenhouse-Geisser adjustment to control for type I error due to unequal group sizes (29). IC50 values were calculated by hand and confirmed using the method of Alexander et al. (1). Arteriole characteristics and immunoblot data were analyzed using two-way ANOVA. SOD activity was analyzed using one-way or two-way ANOVA, depending on whether two or four treatments were compared. When a significant main effect was detected by ANOVA, mean differences were ascertained using Bonferroni multiple comparison tests. When more than one coronary arteriole from the collateral-dependent or nonoccluded region of a given animal was used in identical protocols, responses were averaged before data analyses were conducted. For all analyses, a P value < 0.05 was considered significant. Data are presented as mean ± S.E.M., and n values in parentheses reflect the number of animals studied.
Results
Effectiveness of the exercise training program
Efficacy of the 14-wk progressive treadmill exercise training regimen was demonstrated by significant increases in skeletal muscle oxidative enzyme capacity and an increased heart-to-body weight ratio in exercise trained compared with sedentary pigs. Citrate synthase activity increased significantly in the deltoid muscle (44.7 ± 1.5 vs. 37.0 ± 1.0 μmol·min-1·g-1), and the medial (42.0 ± 1.9 vs. 33.6 ± 1.3 μmol·min-1·g-1), lateral (38.9 ± 1.4 vs. 31.8 ± 1.1 μmol·min-1·g-1), and long (36.4 ± 1.5 vs. 28.9 ± 1.2 μmol·min-1·g-1) heads of the triceps brachii muscle in exercise trained (n=29) compared with sedentary (n=31) pigs, respectively. Although body weight was not different between sedentary and exercise trained animals at the time of sacrifice (35.0 ± 1.0 vs. 33.3 ± 1.0 kg, respectively), heart-to-body weight ratio was significantly greater in exercise trained (n=29) compared with sedentary (n=31) pigs (5.4 ± 0.2 vs. 4.4 ± 0.1 g·kg-1, respectively).
Characteristics of arterioles
Maximal (passive) intraluminal diameters (Dmax) of cannulated coronary arterioles measured at 40 mmHg of intraluminal pressure were not significantly different between nonoccluded and collateral-dependent arterioles of sedentary and exercise trained pigs (Table 1). The level of preconstriction (% of maximal intraluminal diameter) was not significantly different between arterioles of all four groups. The concentration of endothelin-1 required to attain this level of preconstriction was not significantly different between arterioles from all four groups (Table 1).
Table 1.
Dimensional characteristics of cannulated coronary arterioles
| n | Maximal diameter (μm) | Preconstriction (%) | ET-1 [nM] | |
|---|---|---|---|---|
| SED nonocc | 39 | 125±4 | 55±3 | 0.35±0.04 |
| EX nonocc | 35 | 127±5 | 61±3 | 0.33±0.04 |
| SED coll-dep | 35 | 127±7 | 57±2 | 0.33±0.04 |
| EX coll-dep | 34 | 129±5 | 61±3 | 0.31±0.03 |
Values are mean ± S.E.M; n indicates number of arterioles studied. Nonocc, nonoccluded; coll-dep, collateral-dependent; SED, sedentary; EX, exercise trained; % preconstriction, % of maximal diameter to which arterioles were preconstricted; ET-1, concentration of endothelin-1 required to obtain % preconstriction. No differences existed.
Bradykinin-mediated dilation
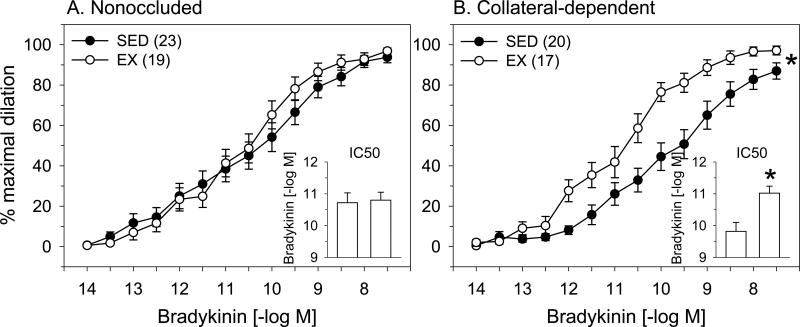
The effects of exercise training on bradykinin-mediated dilation in nonoccluded and collateral-dependent coronary arterioles are presented in Fig 1. Exercise training did not significantly alter concentration-response curves examining bradykinin-mediated dilation in coronary arterioles from the nonoccluded region compared with sedentary pigs (Fig 1A). Furthermore, evaluation of IC50 revealed that sensitivity to bradykinin was not altered by exercise training in the nonoccluded region (Fig 1A, inset). In contrast, exercise training significantly improved bradykinin-mediated dilation in collateral-dependent coronary arterioles compared with those from sedentary pigs (Fig 1B). Exercise training also significantly increased the sensitivity to bradykinin (IC50 values) in arterioles from the collateral-dependent region (Fig 1B, inset). Bradykinin-mediated dilation was significantly impaired in collateral-dependent compared with nonoccluded coronary arterioles of sedentary, but not exercise-trained pigs (Fig 1A vs. Fig 1B). Evaluation of IC50 values revealed that sensitivity to bradykinin was significantly attenuated in arterioles from the collateral-dependent compared with the nonoccluded region of sedentary but not exercise-trained pigs (Fig 1A vs. Fig 1B, insets).
Figure 1. Effect of chronic occlusion and exercise training on bradykinin-mediated vasodilation in porcine coronary arterioles.
Bradykinin-mediated dilation was not significantly altered by exercise training in arterioles of the nonoccluded region (A). In contrast, exercise training significantly enhanced bradykinin-mediated dilation in the collateral-dependent region (B). IC50 values (insets) revealed that sensitivity to bradykinin was not altered by exercise training in the nonoccluded region, but was significantly increased in collateral-dependent arterioles of exercise trained (EX) compared with sedentary (SED) pigs. Bradykinin-mediated dilation and IC50 values (insets) were significantly attenuated in collateral-dependent compared with nonoccluded coronary arterioles of sedentary, but not exercise-trained pigs. Values are means ± S.E.M. of the number of animals in parentheses. A subset of these data has been published previously (53). * P ≤ 0.05.
Contribution of BKCa channels to bradykinin-mediated dilation
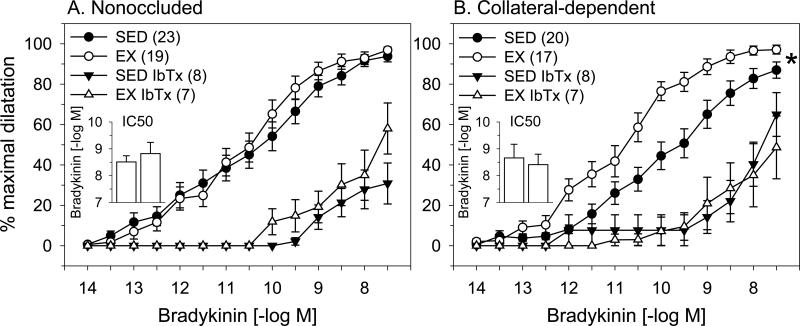
We explored the contribution of BKCa channels to bradykinin-mediated dilation using the selective BKCa channel blocker, iberiotoxin (100 nM). Iberiotoxin pretreatment similarly reduced bradykinin-mediated dilation in arterioles from the nonoccluded region of both sedentary and exercise-trained pigs (Fig 2A). Importantly, BKCa channel blockade abolished the exercise training-enhanced, bradykinin-mediated vasodilation in arterioles from the collateral-dependent region (Fig. 2B). In the presence of iberiotoxin, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (Fig. 2A, inset). Importantly, treatment with iberiotoxin reversed the enhanced sensitivity to bradykinin observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (Fig. 2B, inset).
Figure 2. Effect of BKCa channel blockade on bradykinin-mediated dilation of nonoccluded and collateral-dependent arterioles.
The BKCa channel blocker, iberiotoxin (IbTx; 100 nM), significantly attenuated dilation in nonoccluded (A) and collateral-dependent (B) arterioles of sedentary (SED) and exercise trained (EX) pigs. In the presence of iberiotoxin, dilation in nonoccluded arterioles was similar between sedentary and exercise-trained pigs. In contrast, exercise training-enhanced dilation in collateral-dependent arterioles was reverse in the presence of iberiotoxin. In the presence of iberiotoxin, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (2A, inset). Treatment with iberiotoxin reversed the enhanced sensitivity to bradykinin that was observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (2B, inset). Values are means ± S.E.M. of the number of animals in parentheses. * P ≤ 0.05.
Combination of PEG-catalase and iberiotoxin in bradykinin-mediated dilation
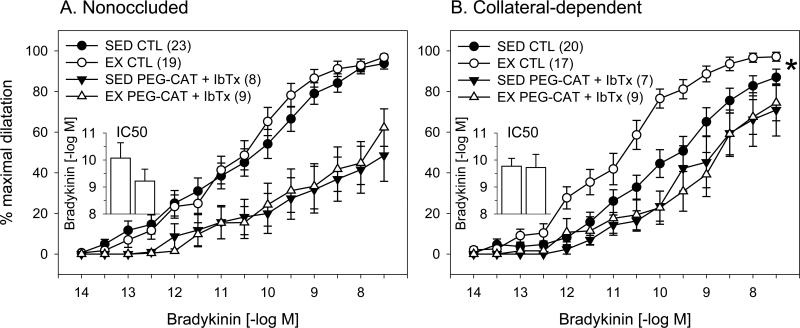
We previously reported that the H2O2 scavenger, PEG-catalase, reversed the exercise training-enhanced, bradykinin-mediated vasodilation in arterioles from the collateral-dependent region (53), similar to that observed with iberiotoxin in the current study. To explore a potential interaction of H2O2 and BKCa, we examined the combination of PEG-catalase and iberiotoxin on bradykinin-mediated dilation (Fig. 3). Our findings demonstrate that the combination of these pharmacological agents similarly attenuated bradykinin-mediated dilation in arterioles from the nonoccluded regions of sedentary and exercise trained pigs (Fig. 3A). In arterioles from the collateral-dependent region, PEG-catalase plus iberiotoxin reversed the exercise training-enhanced, bradykinin-mediated dilation (Fig. 3B). Importantly, the combination of PEG-catalase and iberiotoxin did not produce greater inhibition of bradykinin-mediated dilation than each of the antagonists alone. In fact, simultaneous inhibition of H2O2 and BKCa channels showed a tendency to attenuate bradykinin-mediated dilation to a lesser extent than each of the antagonists alone. Moreover, in collateral-dependent arterioles of sedentary pigs, dilation was significantly enhanced during combined antagonism (Fig 3B) compared with iberiotoxin alone (Fig 2B). Nevertheless, under both conditions, dilation was significantly attenuated compared with control conditions. We previously reported that vehicle control studies with an equivalent concentration of PEG (2.5 mg/ml) in the tissue bath did not alter bradykinin-mediated dilation (41).
Figure 3. Effect of combined BKCa channel blockade and scavenging of H2O2 on bradykinin-mediated dilation in nonoccluded and collateral-dependent arterioles.
Combined pretreatment with iberiotoxin and the H2O2 scavenger, PEG-catalase (PEG-CAT; 1000 U/ml), significantly attenuated dilation in nonoccluded (A) and collateral-dependent (B) arterioles of sedentary (SED) and exercise trained (EX) pigs. In the presence of iberiotoxin plus PEG-CAT, dilation in nonoccluded arterioles was similar between sedentary and exercise-trained pigs. In contrast, exercise training-enhanced dilation in collateral-dependent arterioles was reverse in the presence of the combined inhibitors. In the presence of iberiotoxin plus PEG-CAT, IC50 values were similar in arterioles from the nonoccluded region of sedentary and exercise-trained pigs (3A, inset). Treatment with iberiotoxin plus PEG-CAT reversed the enhanced sensitivity to bradykinin that was observed under control conditions in arterioles from the collateral-dependent region of exercise-trained pigs (3B, inset). Values are means ± S.E.M. of the number of animals in parentheses. * P ≤ 0.05.
Whole-cell K+ channel current
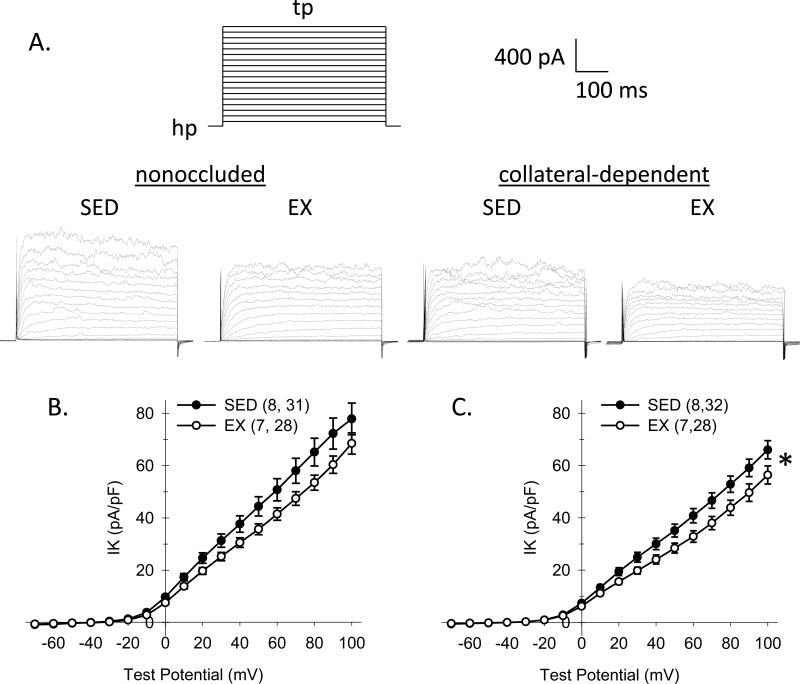
We also determined the effects of chronic occlusion and exercise training on coronary arteriolar smooth muscle K+ channel currents. We hypothesized that exercise training would increase BKCa channel current in smooth muscle cells of collateral-dependent coronary arterioles, a potential downstream effector of the H2O2 signaling pathway. As presented in Fig. 4A, currents were elicited by 500-ms step depolarizations to potentials ranging from -70 to +100 mV from a holding potential of -80 mV. Representative traces for currents of cells from nonoccluded and collateral-dependent arterioles of sedentary and exercise-trained pigs are shown in Fig. 4A. For current-voltage relationships in Fig. 4B and 4C, we plotted the mean value of outward current for last 100 ms of each test potential normalized to cell membrane capacitance (pA/pF). Cell capacitance was not significantly different between smooth muscle cells from nonoccluded and collateral-dependent regions of sedentary (10.5 ± 0.7 and 11.3 ± 0.6 pF, respectively) and exercise trained (10.6 ± 0.7 and 11.8 ± 0.7 pF, respectively) pigs. Comparison of the current-voltage relationships (I-V) revealed that smooth muscle cells of arterioles from exercise trained pigs displayed significantly reduced whole cell K+ channel currents in the collateral-dependent myocardial region (Fig. 4C) and showed a strong tendency (P=0.07) for reduced K+ channel currents in the nonoccluded region (Fig. 4B). Furthermore, K+ channel currents were significantly decreased in cells from collateral-dependent compared with nonoccluded regions in both sedentary and exercise-trained pigs.
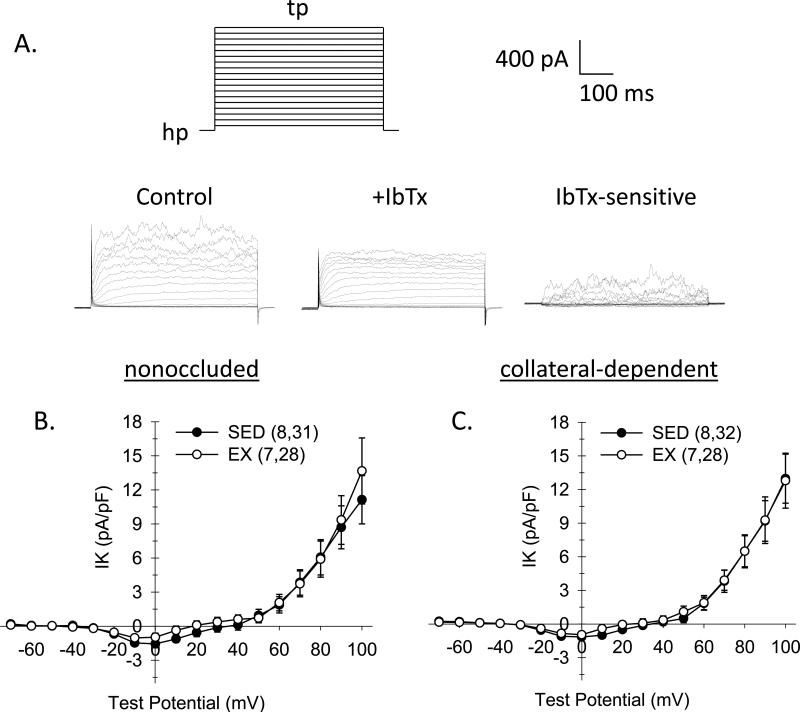
Figure 4. Effect of chronic occlusion and exercise training on whole-cell K+ current in coronary arteriolar smooth muscle cells.
A. As illustrated by the voltage template, currents were elicited by 500-ms step depolarizations (tp) to potentials ranging from –70 to +100 (in 10 mV increments) from a holding potential (hp) of –80 mV. Representative current traces for whole-cell K+ current of cells from nonoccluded and collateral-dependent arterioles of sedentary and exercise-trained pigs. B and C. Comparison of I-V relationships obtained by plotting mean current at the end of the steps as a function of the indicated test potential. Whole-cell K+ current was significantly diminished in cells from exercise-trained pigs from both nonoccluded (B) and collateral-dependent (C) arterioles. K+ current was also significantly reduced in cells from collateral-dependent compared with nonoccluded arterioles of both sedentary and exercise trained animals. Numbers in parentheses indicate number of pigs, cells. Values are means ± S.E.M. of the number of cells in parentheses; *P≤0.05.
Iberiotoxin-sensitive K+ channel current
Based on our finding that the BKCa channel blocker, iberiotoxin, abolished the exercise training-enhanced, bradykinin-mediated vasodilation in arterioles from the collateral-dependent region, we determined iberiotoxin-sensitive K+ channel currents in smooth muscle cells isolated from the nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Iberiotoxin-sensitive currents were obtained by subtraction of currents in the presence of iberiotoxin from control currents (difference currents) (Fig. 5A). Iberiotoxin-sensitive whole-cell K+ currents were not different between smooth muscle cells of nonoccluded and collateral-dependent arterioles of sedentary and exercise trained groups, indicating that whole-cell BKCa channel currents were not altered by occlusion or exercise training (Figs. 5B and 5C).
Figure 5. Effect of chronic occlusion and exercise training on iberiotoxin-sensitive K+ channel currents in coronary arteriolar smooth muscle cells.
A. Iberiotoxin-sensitive K+ channel currents were obtained by subtraction of currents in the presence of iberiotoxin (100 nM) from control currents from cells of both nonoccluded and collateral-dependent arterioles of sedentary and exercise trained pigs. B. Comparison of iberiotoxin-sensitive I-V relationships obtained by plotting mean iberiotoxin (100 nM)-sensitive current at the end of the steps as a function of the indicated step potential. Values are means ± S.E.M. of the number of cells in parentheses; No significant differences existed.
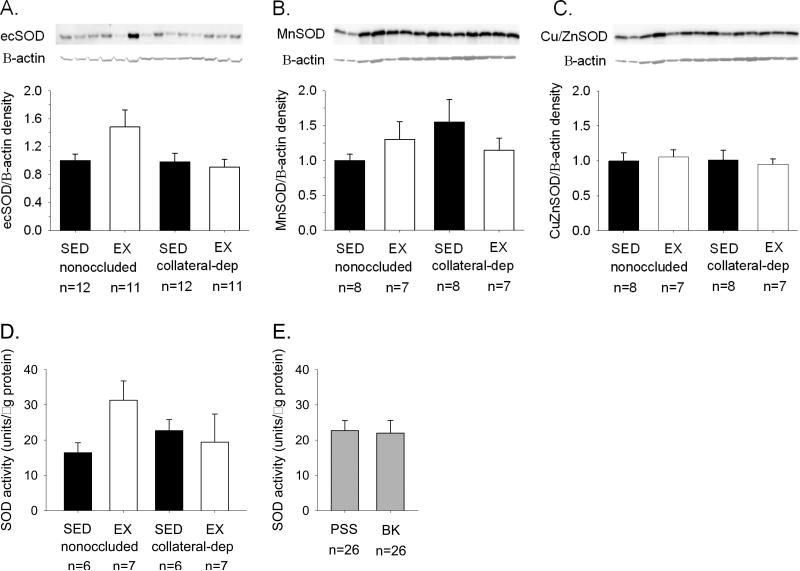
SOD protein levels and SOD activity assay
We examined protein levels of the three SOD isoforms, ecSOD, MnSOD, and Cu/ZnSOD. Representative immunoblots are displayed for ecSOD (Fig. 6A), MnSOD (Fig. 6B), and Cu/ZnSOD (Fig. 6C), as well as β-actin for all immunoblots as loading control, using arterioles from the nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Immunoblot analyses revealed no significant effect of occlusion or exercise training on arteriolar protein levels of the SOD isoform family. Furthermore, SOD activity assay revealed that total SOD enzyme activity was not altered by chronic occlusion or exercise training (Fig. 6D). Bradykinin treatment of arterioles did not alter SOD enzyme activity compared with control (PSS) conditions (Fig. 6D).
Figure 6. Effect of chronic occlusion and exercise training on superoxide dismutase protein and activity levels in coronary arterioles.
Immunoblot analyses of the superoxide dismutase (SOD) family of isoforms revealed that neither ecSOD (A), MnSOD (B), nor Cu/ZnSOD (C) protein levels were significantly altered by occlusion or exercise training. Protein was quantified by densitometry analysis, normalized to β-actin, and expressed relative to the density of nonoccluded arterioles of sedentary (SED) pigs. Evaluation of SOD activity also demonstrated no significant different between arteriolar treatment groups. Furthermore, bradykinin (BK; 10 nM) treatment had no effect on SOD activity compared with control (PSS; conditions). EX, exercise-trained. Values are means ± S.E.M. of the number of animals indicated.
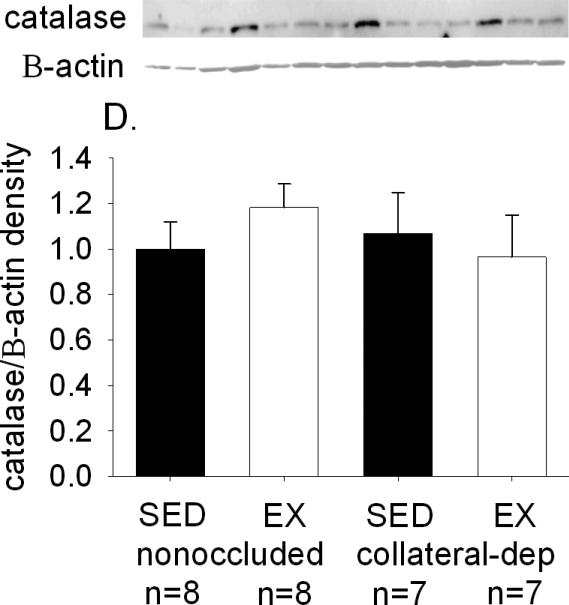
Catalase protein levels
Immunoblot analysis was used to determine if potential differences in catalase protein levels may have altered the degree or rate of decomposition of H2O2 between arteriole treatment groups. Figure 7 provides a representative immunoblot of catalase, as well as β-actin as loading control, using arterioles from the nonoccluded and collateral-dependent regions of sedentary and exercise-trained pigs. Immunoblot analysis revealed no significant effect of occlusion or exercise training on arteriolar protein levels of catalase.
Figure 7. Effect of chronic occlusion and exercise training on catalase protein levels in coronary arterioles.
Immunoblot analyses demonstrated that catalase protein levels were not significantly altered by occlusion or exercise training. Protein was quantified as in Figure 6 legend. SED, sedentary; EX, exercise-trained. Values are means ± S.E.M. of the number of animals in parentheses. No significant differences existed.
Discussion
In the current study, we report the novel finding that blockade of BKCa channels corrects exercise training-enhanced endothelium-dependent dilation in collateral-dependent arterioles. In contrast, whole-cell iberiotoxin-sensitive K+ (BKCa) channel currents were not altered directly by exercise training or occlusion, suggesting that a component of the signaling pathway linking bradykinin with BKCa channels is upregulated in collateral-dependent arterioles of exercise trained pigs. Previously, we reported that the H2O2 scavenger, PEG-catalase, also corrected the exercise training-enhanced bradykinin-mediated dilation in collateral-dependent arterioles (53). Thus, we tested the effect of combined scavenging of H2O2 and inhibition of BKCa channels and found that the combination of these antagonists did not attenuate bradykinin-mediated dilation to a greater extent than each of the antagonists alone. Our findings suggest that H2O2 and BKCa channels operate sequentially to produce bradykinin-mediated dilation.
The selective BKCa channel blocker, iberiotoxin, significantly attenuated bradykinin-mediated dilation in arterioles from both the nonoccluded and collateral-dependent regions of exercise-trained and sedentary animals. Notably, iberiotoxin abolished the exercise training-enhanced, bradykinin-mediated dilation in collateral-dependent arterioles. We previously reported that the H2O2 scavenger, PEG-catalase, abolished the difference in bradykinin-mediated dilation between collateral-dependent coronary arterioles in exercise trained and sedentary pigs (53). Based on our findings that PEG-catalase and iberiotoxin each reversed the exercise training-enhanced, bradykinin-mediated dilation in collateral-dependent arterioles, we examined the effects of the combination of H2O2 scavenging and BKCa channel blockade to begin to explore the interaction of these two mechanisms. Combination of PEG-catalase and iberiotoxin reversed the exercise training-enhanced, bradykinin-mediated dilation in collateral-dependent arterioles. Importantly, the combination of PEG-catalase and iberiotoxin did not produce greater inhibition of bradykinin-mediated dilation than each of the antagonists alone, and may provide evidence that H2O2 and BKCa channels operate sequentially to stimulate bradykinin-mediated dilation. These results are consistent with previous studies which have demonstrated that H2O2 produces membrane hyperpolarization by stimulating BKCa in different tissues and cell types (3, 4, 24, 49). Taken together, these data suggest that the observed exercise training-enhanced, endothelium-dependent vasodilation in collateral-dependent coronary arterioles is mediated via H2O2-induced activation of BKCa channels and subsequent hyperpolarization.
It is also noteworthy that the combination of H2O2 scavenging and BKCa channel blockade showed a tendency to attenuate bradykinin-mediated dilation to a lesser extent than each of the antagonists alone; this was especially evident in the collateral-dependent arterioles of both sedentary and exercise-trained pigs. In fact, in the collateral-dependent arterioles of sedentary pigs, dilation was significantly enhanced during combined antagonism (iberiotoxin plus PEG-catalase; Fig 3B) versus iberiotoxin alone (Fig 2B); nonetheless under both conditions, dilation was significantly reduced compared with control conditions. Although difficult to reconcile, these findings suggest the interesting possibility that combined inhibition of multiple signaling mechanisms may remove an inhibitory effect of these components on other pathways of vasodilation. For instance, controversial evidence implicates inhibitory and stimulatory roles for H2O2 upon eNOS activity, as discussed previously (27). Interestingly, we have previously reported increased eNOS and p-eNOS (Ser1179) protein levels in coronary arterioles from collateral-dependent arterioles of sedentary and exercise-trained pigs (53). Theoretically, if H2O2 acts to inhibit eNOS activity in porcine coronary arterioles, the removal of the inhibitory effect with PEG-catalase treatment may permit increased nitric oxide production and subsequently enhanced dilation in the presence of combined inhibition of BKCa channels and scavenging of H2O2 compared with BKCa channel blockade alone.
We also report the novel finding that both occlusion and exercise training decreased whole-cell K+ currents in coronary arteriolar smooth muscle cells. K+ currents in cells of arterioles from the collateral-dependent region were significantly decreased in both sedentary and exercise-trained pigs. Furthermore, K+ currents in cells of the collateral-dependent arterioles of exercise-trained animals were significantly reduced compared with those from sedentary pigs, while those from cells of the nonoccluded region showed a strong tendency to be reduced. Taken together, these data indicate that both occlusion and exercise training resulted in a reduction in whole cell K+ currents, which was additive in the presence of occlusion plus exercise training. Previous studies in smooth muscle cells from coronary arteries and arterioles from both control and hypercholesterolemic pigs revealed no effect of exercise training on whole-cell K+ currents (8, 18, 54), whereas exercise training decreased K+ currents in coronary smooth muscle cells from conduit-sized arteries, but not arterioles, of diabetic, dyslipidemic pigs (34, 35).
Most relevant to our cannulated microvessel studies, we compared iberiotoxin-sensitive K+ currents to determine adaptations in BKCa channel currents of arteriolar smooth muscle cells of nonoccluded and collateral-dependent arterioles from sedentary and exercise trained pigs. Results from these studies demonstrated that iberiotoxin-sensitive K+ currents were not altered by either chronic occlusion or exercise training. Therefore, despite reductions in whole-cell K+ channel currents by exercise training and occlusion, neither perturbation influenced BKCa channel currents. Despite this finding, blockade of BKCa channels in cannulated arterioles reversed the exercise training-enhanced bradykinin-mediated dilation in collateral-dependent arterioles. Taken together, our data suggest the new concept that a component of the cellular signaling pathway between the bradykinin receptor and the BKCa channel is altered by exercise training in collateral-dependent arterioles. Indeed, we have reported previously that bradykinin-stimulated H2O2 production is increased in the endothelium of arterioles from exercise-trained pigs and that scavenging of H2O2 reversed the exercise training-enhanced bradykinin-mediated dilation in collateral-dependent arterioles (53), similar to the effect of iberiotoxin in our current study. Taken together, these data support a role for increased agonist-stimulated BKCa channel activity via increased H2O2 production in collateral-dependent arterioles of exercise-trained pigs, although additional signaling molecules may also be enhanced by exercise training.
The contribution of BKCa channels to coronary blood flow under in vivo conditions has been explored in a number of studies with conflicting results. Recent studies in swine suggest that BKCa channels do not contribute to local metabolic coronary vasodilation during exercise (7) or to coronary reactive hyperemia (6). On the other hand, Kv channels play a significant role in the reactive hyperemic response (6). In contrast, other studies of BKCa channel blockade using tetraethylammonium (TEA) in swine suggest that BKCa channels contribute negligibly to coronary blood flow under resting conditions but the contribution of these channels increased during exercise when metabolic demands were enhanced (32). However, potential effects of TEA as an autonomic ganglionic blocker, as discussed previously (7) and as an effective blocker of voltage-gated K+ (Kv) channels in porcine coronary arterioles (17), in addition to its effects on BKCa channels, obscures the interpretation of these data (32). In open chest dogs, intracoronary infusion of the BKCa channel blockers iberiotoxin, charybdotoxin, or TEA demonstrated no effect on coronary blood flow under non-ischemic conditions but the contribution of these channels was significant during acute experimental ischemia, with BKCa channel blockade resulting in reduced blood flow and impaired contractile function (37). Discrepancies in the outcome of these studies may be attributable to variations in animal models, metabolic demands, degree of ischemia, delivery of K+ channel blockers (intravenous vs. intracoronary) or other experimental variations. Additionally, we postulate that mechanisms contributing to control of coronary blood flow in our porcine model of chronic occlusion may differ from that observed in nonoccluded animals. Indeed, previous studies have reported that mechanisms underlying exercise-induced coronary vasodilation in chronically occluded (51) pigs differ from that observed in control pigs (2). Thus, in vivo studies examining the contribution of H2O2 and BKCa channels to exercise-induced coronary vasodilation in the chronically occluded pig is warranted.
Based on our whole-cell voltage clamp experiments, we postulate that voltage-gated K+ (Kv) channels are the specific subfamily of channels that were altered by occlusion and exercise training in the present study. We base this speculation on our finding that iberiotoxin-sensitive (BKCa) channels were not altered by either perturbation. Furthermore, the contribution of KATP channels to whole-cell currents was minimized by inclusion of 2 mM ATP in the pipette solution. In addition, others have reported that H2O2 stimulates Kv channels in canine coronary smooth muscle cells (39). While our cannulated microvessel studies demonstrate a remarkable contribution of BKCa channels to endothelium-dependent vasodilation in the porcine microcirculation, this does not rule out a potential role for Kv channels in this model also. Indeed, we have previously demonstrated that Kv channels contribute to resting tone in small coronary arteries isolated from our porcine model of chronic occlusion and exercise training that most marked in collateral-dependent arteries of exercise-trained pigs (19).
Previous studies have reported that H2O2 signaling contributes to endothelium-dependent bradykinin-mediated dilation in coronary arterioles of heart disease patients (13, 25, 31, 33, 44, 53). Furthermore, our recent studies revealed that exercise training produces adaptations in H2O2 signaling that correct impaired endothelium-dependent dilation in coronary arterioles from ischemic hearts (53). Based on these findings, we were interested in determining potential sources of increased H2O2 after exercise training in our porcine model of ischemic heart disease. Exercise training has been shown to produce increases in protein levels of vascular ecSOD (15) and Cu/ZnSOD (41, 42) as well as SOD activity (41, 42), suggesting that increased antioxidant defenses are developed with prolonged training. In our studies, both ecSOD protein and SOD activity showed a tendency to be increased by exercise training in arterioles from the nonoccluded region compared with the other treatment groups, however, these adaptations did not attain statistical significance. We have previously reported that bradykinin-stimulated arteriolar H2O2 levels are increased after exercise training (53), however, SOD activity was not altered by bradykinin treatment in our studies. Based on these data, we speculate that under agonist-stimulated conditions, more substrate (i.e., superoxide) may be generated in the collateral-dependent arterioles of exercise trained compared with sedentary pigs. Thus, if SOD activity was substrate-limited rather than rate-limited, more H2O2 may result under these conditions, despite no change in SOD enzymatic activity with occlusion and exercise training. On the other hand, there is evidence in the literature that H2O2 can be produced directly from NADPH oxidases (specifically NOX4) in vascular cells (30, 45), potentially providing a direct source of increased H2O2 observed in our previous studies, and thus circumventing SOD enzyme activity for the production of enhanced H2O2 in our model.
Limitations
Our data demonstrate that combined inhibition of BKCa channels and scavenging of H2O2 (iberiotoxin plus PEG-catalase) did not produce greater inhibition of bradykinin-mediated dilation than BKCa channel blockade (iberiotoxin) alone. However, because of the profound effect of iberiotoxin alone on bradykinin-mediated dilation, it is difficult to discern the effects of the additional antagonist (PEG-catalase) on the dilatory response. If in fact H2O2 does produce vasodilation via a completely separate mechanism from BKCa channels, treatment with PEG-catalase would have had little additional inhibitory effect on vasodilation because of nearly complete inhibition of dilation in the presence of iberiotoxin alone, especially at lower bradykinin concentrations. Furthermore, although our findings provide evidence that a component of the signaling pathway between bradykinin activation of its endothelial-specific receptors and activation of the BKCa channel, rather than BKCa channel current, is altered in arterioles from the collateral-dependent myocardial region by exercise training, evaluation of potential pathways was not determined and will be the subject of future studies.
Conclusions and Perspectives
A large body of evidence shows that exercise training protects against coronary heart disease in human patients and animal models, and relates to improved survival following an ischemic event in humans (9, 14, 22, 36). However, the underlying mechanisms of these protective effects have not been fully elucidated (11). Evaluation of the H2O2 signaling pathway, as well as potential target K+ channels in endothelium-dependent dilation is important to the understanding of hemodynamic changes and local vasoregulatory mechanisms in ischemic conditions in response to exercise training. Our present studies provide new evidence that BKCa channels contribute to exercise training-enhanced endothelium-dependent vasodilation in ischemic heart disease. Despite no effect of occlusion or exercise training on whole-cell BKCa channel currents, reversal of the exercise training-enhanced, bradykinin-mediated dilation in collateral-dependent arterioles with iberiotoxin suggests a key role for altered regulation of BKCa channels in this exercise-induced adaptation. Taken together with our recent studies (53), we speculate that H2O2 and BKCa channels may function together to increase vasodilation in collateral-dependent coronary arterioles and improve blood flow to the compromised myocardial region. Therefore, our novel findings reveal that a H2O2/BKCa channel signaling pathway, complementary to our previous reports of exercise training-enhanced nitric oxide signaling, may play a critical role in the adaptive responses in coronary artery disease after exercise training.
Acknowledgments
These studies were supported by research funds from the National Institutes of Health, R01-HL064931. The authors greatly appreciate the technical and surgical expertise of Mildred Mattox and excellent technical contributions of Jeff Bray and Erin Ashmore.
References
- 1.Alexander B, Browse DJ, Reading SJ, Benjamin IS. A simple and accurate mathematical method for calculation of the EC50. J Pharmacol Toxicol Methods. 1999;41:55–58. doi: 10.1016/s1056-8719(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 2.Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res. 1994;28:119–124. doi: 10.1093/cvr/28.1.119. [DOI] [PubMed] [Google Scholar]
- 3.Barlow RS, El-Mowafy AM, White RE. H2O2 opens BKCa channels via the PLA2-arachidonic acid signaling cascade in coronary artery smooth muscle. Am J Physiol Heart Circ Physiol. 2000;279:H475–H483. doi: 10.1152/ajpheart.2000.279.2.H475. [DOI] [PubMed] [Google Scholar]
- 4.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 5.Berwick ZC, Moberly SP, Kohr MC, Morrical EB, Kurian MM, Dick GM, Tune JD. Contribution of voltage-dependent K+ and Ca2+ channels to coronary pressure-flow autoregulation. Basic Res Cardiol. 2012;107:264. doi: 10.1007/s00395-012-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borbouse L, Dick GM, Payne GA, Berwick ZC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Metabolic syndrome reduces the contribution of K+ channels to ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2010;298:H1182–H1189. doi: 10.1152/ajpheart.00888.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Alloosh M, Bratz IN, Sturek M, Tune JD. Contribution of BKCa channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298:H966–H973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowles DK, Laughlin MH, Sturek M. Exercise training increases K+-channel contribution to regulation of coronary arterial tone. J Appl Physiol. 1998;84:1225–1233. doi: 10.1152/jappl.1998.84.4.1225. [DOI] [PubMed] [Google Scholar]
- 9.Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- 10.Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3',5'-cyclic monophosphate. Am J Physiol. 1991;261:L393–L398. doi: 10.1152/ajplung.1991.261.6.L393. [DOI] [PubMed] [Google Scholar]
- 11.Calvert JW. Cardioprotective effects of nitrite during exercise. Cardiovasc Res. 2011;89:499–506. doi: 10.1093/cvr/cvq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294:H2371–H2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- 13.Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse-cerebral arteries. Cardiovasc Res. 2007;73:73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Freimann S, Scheinowitz M, Yekutieli D, Feinberg MS, Eldar M, Kessler-Icekson G. Prior exercise training improves the outcome of acute myocardial infarction in the rat. Heart structure, function, and gene expression. J Am Coll Cardiol. 2005;45:931–938. doi: 10.1016/j.jacc.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 15.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol. 1999;87:1948–1956. doi: 10.1152/jappl.1999.87.5.1948. [DOI] [PubMed] [Google Scholar]
- 17.Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol. 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 18.Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. J Appl Physiol. 2008;105:1761–1771. doi: 10.1152/japplphysiol.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol. 2006;290:H1128–H1135. doi: 10.1152/ajpheart.00973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol. 2000;278:H1984–H1992. doi: 10.1152/ajpheart.2000.278.6.H1984. [DOI] [PubMed] [Google Scholar]
- 21.Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H568–H576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 22.Hull SS, Jr., Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- 23.Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- 24.Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol. 2002;283:H406–H411. doi: 10.1152/ajpheart.00007.2002. [DOI] [PubMed] [Google Scholar]
- 25.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr., Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol. 2009;29:739–745. doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu DH, Chen YM, Liu Y, Hao BS, Zhou B, Wu L, Wang M, Chen L, Wu WK, Qian XX. Ginsenoside Rb1 reverses H2O2-induced senescence in human umbilical endothelial cells: involvement of eNOS pathway. J Cardiovasc Pharmacol. 2012;59:222–230. doi: 10.1097/FJC.0b013e31823c1d34. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011;108:566–573. doi: 10.1161/CIRCRESAHA.110.237636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- 30.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, Mukai Y, Hirakawa Y, Akaike T, Takeshita A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol. 2003;23:1224–1230. doi: 10.1161/01.ATV.0000078601.79536.6C. [DOI] [PubMed] [Google Scholar]
- 32.Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006;291:H2090–2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- 33.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:E31–E40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 34.Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288:H1233–H1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- 35.Mokelke EA, Hu Q, Song M, Toro L, Reddy HK, Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol. 2003;95:1179–1193. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- 36.Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2:1207–1210. doi: 10.1016/s0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 37.Node K, Kitakaze M, Kosaka H, Minamino T, Hori M. Bradykinin mediation of Ca2+-activated K+ channels regulates coronary blood flow in ischemic myocardium. Circulation. 1997;95:1560–1567. doi: 10.1161/01.cir.95.6.1560. [DOI] [PubMed] [Google Scholar]
- 38.Oltman CL, Kane NL, Miller FJ, Jr., Spector AA, Weintraub NL, Dellsperger KC. Reactive oxygen species mediate arachidonic acid-induced dilation in porcine coronary microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H2309–H2315. doi: 10.1152/ajpheart.00456.2003. [DOI] [PubMed] [Google Scholar]
- 39.Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr., Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H1404–H1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- 40.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr., Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. Am J Physiol Heart Circ Physiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 41.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–H1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- 42.Rush JWE, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol. 2000;279:H2068–H2076. doi: 10.1152/ajpheart.2000.279.5.H2068. [DOI] [PubMed] [Google Scholar]
- 43.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol. 2006;26:2614–2621. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 44.Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2003;285:H2345–H2354. doi: 10.1152/ajpheart.00458.2003. [DOI] [PubMed] [Google Scholar]
- 45.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol. 2009;587:3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 1997;28:2290–2295. doi: 10.1161/01.str.28.11.2290. [DOI] [PubMed] [Google Scholar]
- 48.Srere PA. Citrate synthase. In: John ML, editor. Methods in Enzymology. Academic Press; 1969. pp. 3–11. [Google Scholar]
- 49.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and - independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 50.Thengchaisri N, Shipley R, Ren Y, Parker J, Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol. 2007;27:791–798. doi: 10.1161/01.ATV.0000258416.47953.9a. [DOI] [PubMed] [Google Scholar]
- 51.Traverse JH, Kinn JW, Klassen C, Duncker DJ, Bache RJ. Nitric oxide inhibition impairs blood flow during exercise in hearts with a collateral-dependent myocardial region. J Am Coll Cardiol. 1998;31:67–74. doi: 10.1016/s0735-1097(97)00437-3. [DOI] [PubMed] [Google Scholar]
- 52.Wagner-Mann C, Hu Q, Sturek M. Multiple effects of ryanodine on intracellular free Ca2+ in smooth muscle cells from bovine and porcine coronary artery: modulation of sarcoplasmic reticulum function. Br J Pharmacol. 1992;105:903–911. doi: 10.1111/j.1476-5381.1992.tb09076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie W, Parker JL, Heaps CL. Effect of exercise training on nitric oxide and superoxide/H2O2 signaling pathways in collateral-dependent porcine coronary arterioles. J Appl Physiol. 2012;112:1546–1555. doi: 10.1152/japplphysiol.01248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Jones AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1553–H1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- 55.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res. 2012;110:471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]